Abstract
Purpose: We evaluated the relationship between dosimetric parameters (DPs) and the incidence of radiation pneumonitis (RP) and investigated the feasibility of a proposed treatment planning technique with the potential of reducing RP in esophageal cancer patients treated with definitive chemoradiotherapy using extended fields. Patients and Methods: A total of 149 patients with locally advanced esophageal cancer were prospectively enrolled for extended-field radiotherapy (EFRT) to three-field regional lymphatics between September 2004 and June 2009. We retrospectively reviewed 86 consecutive patients who were treated with a total dose of 50.4 Gy (plus an optional 9 Gy boost) and were available for dose-volume analysis. Lung DPs of patients in the Grade 0–1 RP (RPG≤1) group and the Grade 2–5 RP (RPG≥2) group were compared. We compared the proposed plan with the conventional plan to 50.4 Gy on DPs for each case. Results: Of these 86 patients, 10 (12%) developed RPG≥2 (Grade 2, n = 2 patients; Grade 3, n = 3; Grade 4, n = 3; Grade 5, n = 2). The patients in the RPG≤1 group showed significantly lower (P < 0.05) V5 and V10 values for the whole lung compared with those in the RPG≥2 group. There were two advantages gained from the proposed plan for V5 (<55%) and V10 (< 37%) values and the conformity of the PTV. Conclusion: The increase in the volume of the lung exposed to low doses of EFRT was found to be associated with the incidence of RP. Our proposed plan is likely to reduce the incidence of RP.
Keywords: radiation pneumonitis, chemoradiation, esophageal cancer, dose-volume histogram, extended-field, dosimetric parameter
INTRODUCTION
Radiation pneumonitis (RP) is one of the most common dose-limiting toxicities in thoracic radiotherapy, especially in the era of extended-field radiation therapy (EFRT) for intrathoracic esophageal radiotherapy, which is more likely to induce RP. While three-field (neck, mediastinum, and abdomen) dissection in radical surgery has already been established as an effective technique for intrathoracic esophageal cancer, EFRT should be considered more for clinical use, since it is a credible method in definitive chemoradiotherapy (CRT). Recently developed 3-D treatment-planning systems facilitate the quantitative study of related dosimetric parameters (DPs). Many studies have demonstrated the usefulness of DPs such as the mean lung dose (MLD) and the percentage volume of the lung receiving more than a threshold dose (e.g. V20) for evaluating the risk of RP [1–7]. However, most of these studies have included patients with different demographics or tumor stages, or patients undergoing different methods of treatment [8]. In addition, reported DPs related to RP have been poorly reproducible because the clinical factors used for radiotherapy are diverse. Moreover, most of these studies have addressed lung cancer in which the optimal dose is usually > 60 Gy [1–4]. There have been few studies of dosimetric analysis with consideration for the volume of the radiation field in esophageal cancer, especially with regards to EFRT in which the optimal dose is generally <50.4 Gy. In dosimetric analysis, there is a potential for the detection of important DPs for RP by fixing some of the variable clinical factors. We therefore focused on correlations between DPs in computed tomography (CT)-based treatment plans and the incidence of RP for esophageal cancer patients treated with EFRT, applying a uniform radiation field by reference to anatomical landmarks.
In this retrospective study, we limited the evaluation of DPs as predictors of RP to patients who prospectively received uniform extended-field irradiation with chemotherapy. Additionally, we investigated the feasibility of the proposed treatment-planning technique with respect to the potential of reducing the incidence of RP.
MATERIALS AND METHODS
Patients
We retrospectively reviewed the medical charts and radiation records of 149 patients with esophageal squamous cell carcinoma who received concurrent CRT at Keiyukai Sapporo Hospital between September 2004 and June 2009. The initial criteria for inclusion in the analysis were as follows: age ≤80 years, concomitant chemotherapy consisting of 5-fluorouracil (5-FU) and cisplatin (CDDP), no previous chemotherapy or radiotherapy, and no surgery. Of the 149 candidate patients, 134 satisfied these clinical inclusion criteria. Among the 134 patients, 48 were ineligible for this analysis for the following reasons: 16 due to deviation from typical fields in the clinical target volume (CTV) and planning target volume (PTV) for reasons such as bone metastasis and lung metastasis, 22 due to the use of a CT dataset different from the initial one for the off-cord planning because of the inability to combine the two sequential plans for dose-volume histograms (DVH) analysis accurately, eight due to deviation from the scheduled prescribed dose because of the cessation of the radiation therapy, and two due to the use of wedge fields. A total of 86 patients were thus eligible for analysis. We obtained approval from our institutional review board for this study.
Chemotherapy
All patients received concurrent 5-FU and CDDP chemotherapy with EFRT. The chemotherapy consisted of two courses of protracted infusion of 5-FU (750 mg/m2/day) and a 1-h infusion of CDDP (20 mg/m2/day) on Days 1–5 and 28–32. Thirteen patients who received only one course due to blood toxicities were eligible for this study.
Treatment planning for actual treatment
CT images for treatment planning were acquired using 5-mm slice thickness. Pinnacle3 version 8.0h (ADAC, Philips, CA) was used as the treatment-planning system. The CTV was delineated (by two radiation oncologists) as the whole thoracic esophagus including the gross primary tumor and the regional lymphatics. Concerning intrathoracic esophageal squamous cell carcinoma, the incidence of lymph node metastasis in the bilateral deep cervical regions was reported to be 17–35% in extended esophagectomy with three-field lymph node dissection [9]. Therefore, we electively included the bilateral lower cervical lymph node regions as a part of the three-field lymphatic region. Additionally, we included the lymphatic areas along the lesser gastric curvature and celiac lymph nodal area in the abdomen. The inferior border of the CTV was extended to the lymph nodal area along the common hepatic artery. After 39.6 Gy, the CTV was contracted to the primary tumor and elective lymphatic region. The PTV was defined by expanding the CTV with an isotropic margin of 1.0 cm. The calculation was performed using a grid size of 4 mm with the adaptive convolution algorithm.
In the treatment plan, 1.8 Gy per fraction with 10-MV X-rays were used. The total dose was 50.4 Gy in 28 fractions with a conventional beam arrangement: anterior-posterior (AP) and posterior-anterior (PA) fields up to 39.6 Gy followed by off-cord oblique fields. The dose was prescribed to the ICRU reference point, which was usually the isocenter located in the centroid of the PTV. Boost irradiation of 9 Gy in 5 fractions to the primary tumor and involved lymph nodes was adopted for 70 patients after 50.4 Gy EFRT using two opposite oblique beams in principle, and using four cross-fire type beams on four patients.
Planning simulation
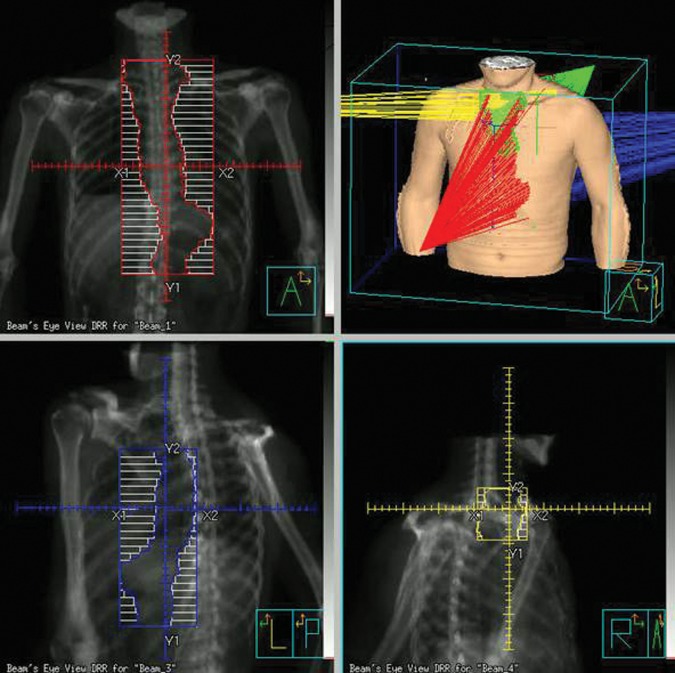
For the proposed plan, which we call the ‘E-plan’ as a nominal designation of the treatment plan for EFRT, 86 conventional plans were recalculated with prescribed doses of 50.4 Gy in 28 fractions using 10-MV X-ray beams in order to allow them to be compared with the conventional plans for actual treatment. In the E-plan, in addition to AP-PA fields, two oblique beams were targeted to the higher portion and lower portion around the curvature in the upper thoracic spine to avoid the spinal cord, as shown in Fig. 1. The beam angle and weighting were optimized to minimize exposure to the whole lung and cord without reductions of the PTV coverage. Gantry angles for the upper right anterior oblique (RAO) beam ranged from 275–290°, and from 130–145° for the lower left posterior oblique (LPO) beam. A dose prescription of 1.8 Gy per fraction for the AP-PA and lower oblique fields was calculated using the same reference point as for the conventional plan. Another dose prescription for the upper oblique field was defined at another reference point ranging from 0.15–0.25 Gy in order to compensate for the shortage of the dose in the upper part of the PTV. The reference point in the upper part of the PTV was irradiated to 1.8 Gy.
Fig. 1.
Beam alignment in the proposed E-plan. Note the addition of the upper right anterior oblique (RAO) beam (yellow) and the lower left posterior oblique (LPO) beam (blue).
Evaluation of radiation pneumonitis
Patients were separated into a Grade 0–1 RP (RPG≤1) group and a Grade 2–5 RP (RPG≥2) group using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.0, which provides clearer clinical explanations than version 3.0. We also used two adverse events from the respiratory thoracic and mediastinal disorders from the NCI-CTCAE version 4.0: pneumonitis and pulmonary fibrosis. The grade of RP was defined by clinical evaluation of CT images and the natural course of respiratory disease within 3 months after RP occurrence. Even if the patients had passed away after therapy from RP, they were not categorized into the Grade 5 group, because the results of RP treatment were sometimes modified with other clinical factors after initial steroid treatment. Each patient follow-up status was observed in our outpatient clinic every 2 months within a year after definitive CRT. CT or positron emission tomography (PET)/CT was performed every 4 months during the 2–3 years after definitive CRT, with or without gastroesophagoscopy. Once we checked RPG≥2 out of all the patients with RP conserving the symptoms or image findings more than Grade1 RP, we continued follow-up for them by X-ray photograph or CT and C-reactive protein weekly.
Dosimetric parameters
The percentage volume of the whole lung receiving at least 5–40 Gy (V5–V40) and MLD values were obtained from DVHs for each patient to compare the DPs in the RPG≤1 group and RPG≥2 group. In addition to the whole lung, the DVHs of the PTV and spinal cord were analyzed to compare the DPs between the conventional plan and the E-plan. PTV coverage was assessed numerically by calculating the V95 and V105 from the DVHs, which represent the percentage volume of the PTV that receives at least 95% and 105% of the prescribed dose, respectively.
Statistical analysis
The survival time was calculated from the date of treatment initiation, to that of death from any cause or to the last date of confirmation of survival. The two-year survival rate was estimated using the Kaplan-Meier method. Fisher's exact test, Mann-Whitney's U test and logistic regression analysis were used for the correlation between the incidence of RPG≥2 and each parameter. A paired Student's t-test was used to compare the difference in the DPs of the PTV and organ at risk (OAR) parameters derived from each plan in the same patient. Two-tailed values of P < 0.05 were defined as having statistical significance. SPSS version 20 (SPSS Inc., Chicago, IL) and JMP version 9.0 (SAS Institute Inc., Cary, NC) were used in these analyses.
RESULTS
Patient characteristics
Of the 149 patients who received EFRT with chemotherapy, 11 patients (7%) developed RPG≥2 (Grade 2 RP in three patients, Grade 3 RP in three, Grade 4 RP in three, and Grade 5 RP in two). Of the 86 patients, 10 (12%) who met the criteria for enrolment developed RPG≥2 (Grade 2 RP in two patients, Grade 3 RP in three, Grade 4 RP in three, and Grade 5 RP in two). Only three patients had detectable Grade 1 RP with or without mild respiratory symptoms or slight changes in their CT images. One of the three patients categorized into the Grade 3 RP had presented with bronchiolitis obliterans organizing pneumonia (BOOP)-type pneumonitis and finally died from adult respiratory distress syndrome.
The characteristics of the 86 patients are shown in Table 1. There were 77 males and 9 females with a median age of 66 years (range, 48–80 years). With a median follow-up period of 15.8 months, the median survival time was 18.3 months (95% CI, 11.6–25.2 months); the 2-year survival rate was 41.5% (standard error, 5.4%). The total dose was 50.4 Gy (n = 16; 19%) or 59.4 Gy (n = 70; 81%). However, the total dose was not a statistically significant factor for RPG≥2, although patients who received a total of 50.4 Gy EFRT did not develop RPG≥2. For those 10 patients with RPG≥2, the median duration to onset was 3.4 months (range, 1.6–8.2 months) from the initiation of radiotherapy. There was no significant difference in the median volume of the PTV between the RPG≤1 group and the RPG≥2 group (RPG≤1 group, 1022 cm3; RPG≥2 group, 1093 cm3; P = 0.34). There was no difference in the median volume of the whole lung (RPG≤1 group, 3662 cm3; RPG≥2 group, 3423 cm3; P = 0.74). There were also no significant differences in the other clinical factors listed in Table 1.
Table 1.
Patient characteristics
| Characteristics | Radiation pneumonitis |
P–value (Fisher's) | ||
|---|---|---|---|---|
| All patients | Grade 0–1 | Grade 2–5 | ||
| (n = 86) | (n = 76) | (n = 10) | ||
| Gender, n (%) | 0.59 | |||
| Male | 77 (90%) | 67 (88%) | 10 (100%) | |
| Female | 9 (10%) | 9 (12%) | 0 (0%) | |
| Age (y) | 0.089 | |||
| Median (Range) | 66 (48–80) | 66 (48–80) | 72 (61–80) | |
| ≤66 y | 43 (50%) | 41 (54%) | 2 (20%) | |
| > 66 y | 43 (50%) | 35 (46%) | 8 (80%) | |
| Smoking history | 0.83 | |||
| Current smoker | 42 (49%) | 38 (50%) | 4 (40%) | |
| Quit smoking >0.5 y before | 36 (42%) | 31 (41%) | 5 (50%) | |
| Nonsmoker | 8 (9%) | 7 (9%) | 1 (10%) | |
| Tumor portion, n (%) | 0.23 | |||
| Ut | 33 (38%) | 31 (41%) | 2 (20%) | |
| Mt | 43 (50%) | 35 (46%) | 8 (80%) | |
| Lt | 7 (8%) | 7 (9%) | 0 (0%) | |
| Other | 3 (4%) | 3 (4%) | 0 (0%) | |
| T stage, n (%) | 0.30 | |||
| T1 | 10 (12%) | 9 (12%) | 1 (10%) | |
| T2 | 6 (7%) | 4 (5%) | 2 (20%) | |
| T3 | 41 (48%) | 38 (50%) | 3 (30%) | |
| T4 | 29 (33%) | 25 (33%) | 4 (40%) | |
| N stage, n (%) | 1.0 | |||
| N0 | 7 (8%) | 6 (8%) | 1 (10%) | |
| N1 | 79 (92%) | 70 (92%) | 9 (90%) | |
| M stage, n (%)a | 0.51 | |||
| M0 | 52 (60%) | 47 (62%) | 5 (50%) | |
| M1lym | 34 (40%) | 29 (38%) | 5 (50%) | |
| Chemotherapy, n (%) | 0.17 | |||
| 1 course | 13 (15%) | 10 (13%) | 3 (30%) | |
| 2 courses | 73 (85%) | 66 (87%) | 7 (70%) | |
| Total dose, n (%) | 0.11 | |||
| 50.4 Gy | 16 (19%) | 16 (21%) | 0 (0%) | |
| 59.4 Gy | 70 (81%) | 60 (79%) | 10 (100%) | |
| PTV length (cm) | 0.74 | |||
| Median (Range) | 30.5 (24.5–39.1) | 30.5 (24.6–39.1) | 31.1 (24.5–34.0) | |
| ≤30 cm | 43 (50%) | 37 (49%) | 6 (40%) | |
| >30 cm | 43 (50%) | 39 (51%) | 4 (40%) | |
| PTV volume (cc) | 0.34 | |||
| Median (Range) | 1032 (582–1524) | 1022 (610–1524) | 1093 (582–1366) | |
| ≤1000 cc | 39 (45%) | 36 (47%) | 3 (30%) | |
| >1000 cc | 47 (55%) | 40 (53%) | 7 (70%) | |
| Lung volume (cc) | 0.74 | |||
| Median (Range) | 3591 (2032–6636) | 3662 (2156–6636) | 3423 (2032–4632) | |
| ≤3600 cc | 43 (50%) | 37 (49%) | 6 (60%) | |
| >3600 cc | 43 (50%) | 39 (51%) | 4 (40%) | |
Ut = Upper thoracic; Mt = Middle thoracic; Lt = Lower thoracic; Other = multicentric tumors; PTV = planning target volume. aUICC 6th.
Dosimetric parameters
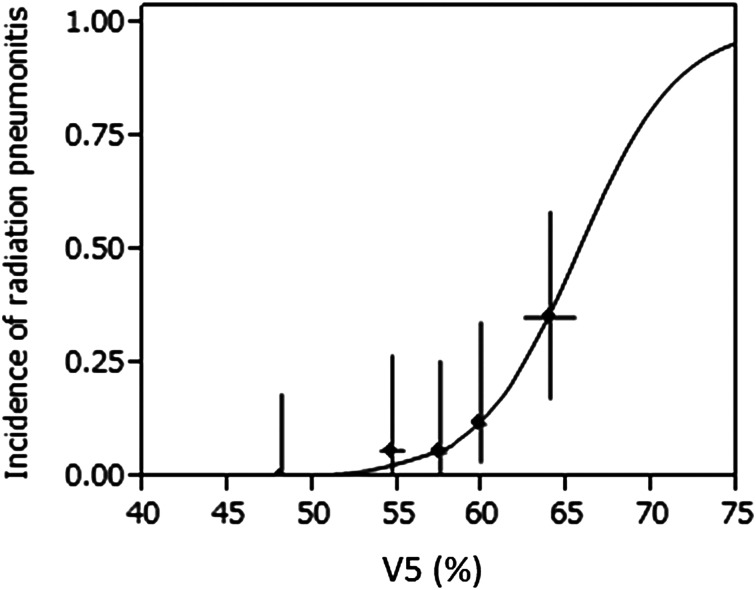
The V5–V40 (in 5 Gy increments) and MLD values for the 86 patients who received 50.4 Gy EFRT are shown in Table 2a. The DPs for the 70 patients who received 59.4 Gy are shown in Table 2b. For both doses, the results showed that the V5 and V10 values were significantly associated with RPG≥2 (P < 0.05) but the V15–V40, and MLD values were not. In addition, as shown in Table 3, the logistic regression analysis yields the same results as the other statistical analysis, except with respect to the MLD, V15, and V20 values at 59.4 Gy. Figure 2 shows the relationship between the incidence of RPG≥2 and V5 at 50.4 Gy based on logistic regression analysis. The association between V5 value and RPG≥2 was the most significant correlation in all analyses.
Table 2.
Comparison of dosimetric parameters for patients according to severity of radiation pneumonitis
| (a) 50.4 Gy | All patients (n = 86) |
Grade 0–1 (n = 76) |
Grade 2–5 (n = 10) |
P-value (U test) |
|---|---|---|---|---|
| Mean ± S.D. | Mean ± S.D. | Mean ± S.D. | ||
| MLD (Gy) | 12.6 ± 1.5 | 12.5 ± 1.4 | 13.4 ± 1.5 | 0.13 |
| V5 (%) | 56.8 ± 5.6 | 56.0 ± 5.3 | 62.9 ± 4.9 | 0.001 |
| V10 | 41.1 ± 4.9 | 40.5 ± 4.7 | 45.5 ± 4.4 | 0.006 |
| V15 | 27.2 ± 4.4 | 26.9 ± 4.3 | 29.5 ± 5.3 | 0.17 |
| V20 | 18.8 ± 3.6 | 18.8 ± 3.5 | 19.4 ± 4.5 | 0.49 |
| V25 | 15.7 ± 3.2 | 15.7 ± 3.1 | 16.2 ± 4.0 | 0.48 |
| V30 | 13.6 ± 2.9 | 13.5 ± 2.8 | 14.0 ± 3.7 | 0.52 |
| V35 | 11.7 ± 2.6 | 11.6 ± 2.5 | 12.0 ± 3.3 | 0.54 |
| V40 | 9.0 ± 2.3 | 9.0 ± 2.2 | 9.3 ± 2.7 | 0.65 |
| (b) 59.4 Gy |
All patients (n = 70) |
Grade 0–1 (n = 60) |
Grade 2–5 (n = 10) |
P-value (U test) |
| Mean ± S.D. | Mean ± S.D. | Mean ± S.D. | ||
| MLD (Gy) | 16.0 ± 1.7 | 15.8 ± 1.6 | 17.0 ± 2.1 | 0.11 |
| V5 (%) | 63.0 ± 5.5 | 62.0 ± 4.8 | 69.4 ± 4.9 | 0.001 |
| V10 | 46.1 ± 4.7 | 45.3 ± 4.4 | 50.6 ± 4.6 | 0.003 |
| V15 | 39.9 ± 4.5 | 39.4 ± 4.2 | 43.2 ± 4.9 | 0.065 |
| V20 | 34.3 ± 4.5 | 33.8 ± 4.1 | 37.2 ± 5.9 | 0.075 |
| V25 | 22.6 ± 4.3 | 22.4 ± 4.0 | 23.8 ± 5.8 | 0.37 |
| V30 | 16.7 ± 3.7 | 16.7 ± 3.6 | 16.9 ± 4.4 | 0.54 |
| V35 | 13.9 ± 3.1 | 13.9 ± 3.0 | 14.2 ± 3.9 | 0.54 |
| V40 | 11.4 ± 2.8 | 11.3 ± 2.7 | 11.6 ± 3.3 | 0.63 |
MLD = mean lung dose, U test = Mann-Whitney's U test, Vn = the percentage volume of lung receiving at least n Gy of radiation.
Table 3.
Dosimetric parameters that may affect the risk of radiation pneumonitis
| 50.4 Gy |
59.4 Gy |
|||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P-value | Odds ratio | 95% CI | P-value | |
| MLD (Gy) | 1.00 | 1.00–1.01 | 0.066 | 1.00 | 1.00–1.01 | 0.043 |
| V5 (%) | 1.41 | 1.17–1.80 | <0.001 | 1.51 | 1.22–2.06 | <0.001 |
| V10 | 1.30 | 1.10–1.62 | 0.001 | 1.39 | 1.13–1.83 | 0.001 |
| V15 | 1.15 | 0.98–1.36 | 0.081 | 1.28 | 1.08–1.62 | 0.004 |
| V20 | 1.05 | 0.85–1.30 | 0.63 | 1.20 | 1.03–1.46 | 0.022 |
| V25 | 1.05 | 0.84–1.33 | 0.65 | 1.08 | 0.92–1.28 | 0.34 |
| V30 | 1.05 | 0.84–1.33 | 0.65 | 1.02 | 0.84–1.22 | 0.86 |
| V35 | 1.06 | 0.82–1.37 | 0.65 | 1.09 | 0.85–1.43 | 0.50 |
| V40 | 1.06 | 0.79–1.42 | 0.69 | 1.04 | 0.81–1.33 | 0.78 |
MLD = mean lung dose, CI = confidence interval, Vn = the percentage volume of lung receiving at least n Gy of radiation.
Fig. 2.
Incidence of radiation pneumonitis (RP) and V5 at 50.4 Gy. The solid curve represents the fit of the logistic model to the data. The solid dots represent the observed incidence of RP in 5 subgroups (for each; n = 17 or 18) plotted at the mean value of V5. The horizontal error bars represent the 95% confidence interval of the mean V5 in each group. The vertical error bars represent the 95% confidence interval of the incidence of RP by the score method.
Comparison of the conventional plan and the proposed E-plan technique
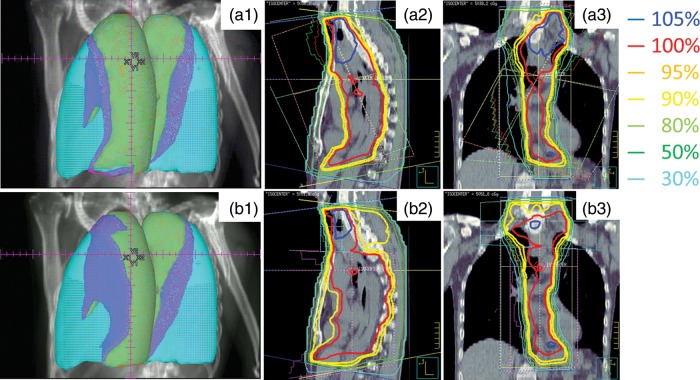
Table 4 shows the results of the comparison of DPs between the conventional plan and the proposed E-plan in 86 patients. Figure 3 shows the configuration of the V5 and V10 areas in the whole lung for a conventional plan (a1) and an E-plan (b1), and the isodose distributions on sagittal and coronal images for a conventional plan (a2 and a3) and an E-plan (b2 and b3) from a single representative patient. Figure 4 shows the DVHs of the conventional and the E-plan for 50.4 Gy EFRT in the same patient as shown in Fig. 3. With respect to the coverage of the PTV, the V95 and V105 values in the E-plan were significantly higher than those in the conventional plan (P < 0.001). The mean dose of PTV in the E-plan was increased by 0.3 Gy compared with the conventional plan, and this increase was statistically significant (P < 0.001). Table 4 also shows a comparison of the DPs of the lung and cord. The E-plan had clear advantages with respect to the differences between the V5, V10, V15 and MLD values (P < 0.001). However, the E-plan was disadvantageous with respect to the values of V30, V35 and V40. The maximum dose to the spinal cord was not significantly different between the two planning techniques.
Table 4.
Comparison of dosimetric parameters for the conventional plan and proposed E-plan
| n = 86 | Conventional plan (Mean ± S.D.) | E-plan (Mean ± S.D.) | P-value |
|---|---|---|---|
| PTV | |||
| Mean dose (Gy) | 50.0 ± 1.4 | 50.3 ± 1.3 | <0.001 |
| V95 (%) | 77.2 ± 7.6 | 83.0 ± 4.3 | <0.001 |
| V105 | 16.8 ± 8.3 | 7.5 ± 7.4 | <0.001 |
| Lung | |||
| MLD (Gy) | 12.6 ± 1.5 | 12.2 ± 1.6 | <0.001 |
| V5 (%) | 56.8 ± 5.6 | 53.9 ± 6.2 | <0.001 |
| V10 | 41.1 ± 4.9 | 34.6 ± 5.1 | <0.001 |
| V15 | 27.2 ± 4.4 | 25.5 ± 4.3 | <0.001 |
| V20 | 18.8 ± 3.6 | 18.9 ± 3.5 | 0.441 |
| V25 | 15.7 ± 3.2 | 15.8 ± 3.2 | 0.318 |
| V30 | 13.6 ± 2.9 | 13.7 ± 2.9 | 0.001 |
| V35 | 11.7 ± 2.6 | 11.9 ± 2.6 | <0.001 |
| V40 | 9.0 ± 2.3 | 9.7 ± 2.3 | <0.001 |
| Spinal cord | |||
| Maximum dose (Gy) | 48.0 ± 1.5 | 48.1 ± 1.2 | 0.369 |
PTV = planning target volume, MLD = mean lung dose, V95% and V105% represent percentage volume of the PTV receiving at least 95% and 105% of the prescribed dose, respectively.
Fig. 3.
Configuration of the V5 and V10 areas in the whole lung for a conventional plan (a1) and an E-plan (b1), and the isodose distributions on sagittal and coronal images for a conventional plan (a2 and a3) and an E-plan (b2 and b3) for a single representative patients. Conventional plan case (V5 = 56.6%, V10 = 42.4%), (b1) E-plan case (V5 = 41.5%, V10 = 25.7%), light blue = whole lung, blue = V5 area, green = V10 area.
Fig. 4.
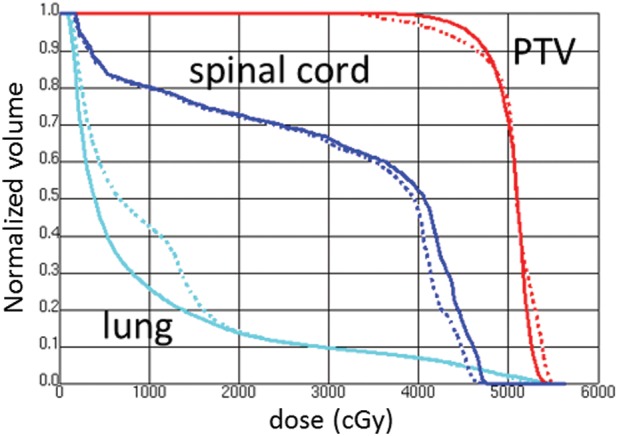
Example of the dose-volume histograms for the conventional plan and proposed E-plan. In the DVHs, solid line = E-plan, dashed line = conventional plan.
DISCUSSION
The results of this cohort study show an important relationship between a mechanism of RP induction and comprehensive wide mediastinal irradiation. In this study, several clinical factors such as fractionated size, anatomical landmarks, total dose, and chemotherapy were fixed. We found small but statistically reproducible differences in the V5 and V10 values between the group with and without RPG≥2. All 10 patients who developed RPG≥2 were treated with up to 59.4 Gy. However, the total dose was not a significant clinical factor for RPG≥2 because 81% of all patients had a 9 Gy boost after 50.4 Gy EFRT in this study. This result may come from the difference in population size, and the significant difference might have been shown between two kinds of the prescribed doses if the population size in the 50.4 Gy group were increased. However, the variation pattern of the mean difference between 50.4 Gy and 59.4 Gy in the RPG≥2 group was almost consistent with that in the RPG≤1 group, as shown in Fig. 5. That is, the difference of the total dose with or without the boost irradiation of 9 Gy is considered insignificant in the incidence of RPG≥2. From Table 2 and Table 3, the V5 and V10 values were found to be associated with RPG≥2 at either 50.4 Gy or 59.4 Gy, but the MLD value was not associated with RPG≥2 at 50.4 Gy EFRT. MLD is an established DP for the prediction of RP in several reports whose prescribed doses were almost all > 60 Gy [1, 2, 4, 6]. We consider that the DPs in the low-dose levels such as V5 and V10 values are prediction factors for RP in limited cases such as the analysis of the DVH at 50.4 Gy EFRT. In other words, we suggest that the incidence of RPG≥2 is affected not only by the MLD value but also by the DPs at low-dose levels.
Fig. 5.
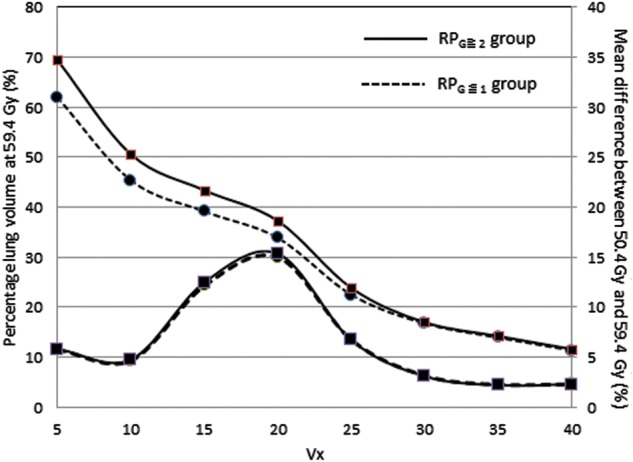
The percentage volume of the whole lung at 59.4 Gy and the variation patterns of the mean difference between 50.4 Gy and 59.4 Gy in the RPG≥2 group and the RPG≤1 group.
To date, many studies have shown that DPs can predict the risk of RP [1–7]. However, most of these studies have involved lung cancer patients treated with various types of radiation fields for peripheral and mediastinal regions [1–4]. On the other hand, there have been few studies on dosimetric analysis of esophageal cancer patients with definitive CRT. Asakura et al. performed a retrospective study of 37 patients with definitive CRT and found that all DVH parameters (i.e. V5–V50 and MLD values) were significantly associated with RPG≥2 [5]. Similarly, Zhu et al. published their results for 56 patients with or without CRT and found that the V30 value was predictive of late lung toxicity [6]. In that report, they also found that V5–V40 and MLD values were significantly associated with the occurrence of late lung injury. These dosimetric analyses for lung injury were not consistent with our results on DPs because they use various types of radiation doses and fields. On the other hand, Wang et al. reported that the volume of lung spared exposure doses < 5 Gy was the strongest factor associated with postoperative pulmonary complications for 110 esophageal cancer patients treated with CRT followed by surgery [7]. They also found that the V5 value was the only factor significantly associated with an incidence of pulmonary complications. Although there were no descriptions of the target volume in their report, the reasons for the significance of the V5 value include that wide thoracic irradiation similar to EFRT could have been used with ≤ 50.4 Gy for advanced cases from their clinical information. We recommend that in clinical analyses on DPs for RP, the following radiation field information related to the PTV is necessary: wide mediastinal irradiation with ≤ 50 Gy, and localized irradiation with ≥ 60 Gy. Our dataset is more homogeneous than these cohort studies in terms of dose to anatomical structures.
In this analysis, RPG≥2 was diagnosed in 12% of all patients. Although the incidence of RPG≥2 was comparatively low compared to other reports on esophageal cancer or lung cancer with CRT [1, 3, 5–7], that of severe RP (Grade 3, 4, and 5) was higher (three, three and two patients, respectively). The reason for this tendency was considered to be that the lung volume receiving the low-dose was broadened by EFRT compared to general thoracic irradiation. If the incidence of RPG≥2 is 12% as it was in this study, the predictive values in V5 and V10 obtained by the inverse prediction method based on the logistic regression model as shown in Fig. 2 were 59.7% (95% CI, 55.5–62.1%) and 43.2% (95% CI, 37.5–46.2%), respectively. We recommend that the V5 and V10 values should be limited to less than 55% and 37% at 50.4Gy, respectively.
For these reasons, we propose a new planning technique for esophageal cancer treatment using extended fields to reduce the incidence of RPG≥2. The proposed E-plan has two advantages over the conventional plan. The first is that the dose conformity, such as V95 and V105 for PTV, is improved in the E-plan. Compared with the conventional plan technique, the E-plan technique makes it easier to spare the spinal cord while maintaining dose conformity in the PTV. The second advantage is that the mean dose and low-dose region in the lung are usually decreased compared to the conventional plan. From our results, the E-plan significantly decreased the DPs such as V5, V10, V15 and MLD, although it increased V30, V35 and V40. This result may have been caused by the LPO beam, which was irradiated to 50.4 Gy; i.e. the E-plan increased the dose delivered to the left lung, rather than decreasing the dose delivered to the right lung, which does not receive a dose from the RAO beam in the conventional plan. From the results shown in Table 4, V5 and V10 values were decreased below the lower bound of the 95% confidence interval compared to the conventional plan using the E-plan technique. Therefore, our proposed E-plan is likely to be useful in clinical practice in terms of reducing the incidence of RP.
With respect to planning techniques, several researchers have recently reported that an intensity-modulated radiotherapy (IMRT) plan is superior to a 3D conformal radiotherapy (3D-CRT) plan for patients with lung cancer or upper esophageal cancer, because an IMRT plan can spare the lung and spinal cord and improve the target dose conformity [10–12]. However, their reports have also shown that the V5 and V10 values in the IMRT plan were higher than those of the 3D-CRT plan [10, 11]. Therefore, in terms of our study, an IMRT plan may not be the optimal method to reduce the incidence of RP. We must make an effort to reduce RP for expansive mediastinal radiotherapy including EFRT, considering the volume for the lung exposed to low-dose irradiation. We have recently begun a clinical study using 3D-CRT for reducing the peripheral lung dose in our institution [13]. As there are few reports suggesting a relationship between low-dose level of lung DVH and respiratory toxicities, further clinical investigation of this topic will be needed.
ACKNOWLEDGEMENTS
The authors thank Kenneth Lee Sutherland (Assistant Professor, Division of Medical Physics, Hokkaido University Graduate School of Medicine) for his support in this study. Results from this study were presented at the 22nd Japanese Society of Therapeutic Radiology and Oncology (JASTRO) Annual Meeting, Chiba, 2010.
REFERENCES
- 1.Barriger RB, Fakiris AJ, Hanna N, et al. Dose-volume analysis of radiation pneumonitis in non-small-cell lung cancer patients treated with concurrent cisplatinum and etoposide with or without consolidation docetaxel. Int J Radiat Oncol Biol Phys. 2010;78:1381–6. doi: 10.1016/j.ijrobp.2009.09.030. [DOI] [PubMed] [Google Scholar]
- 2.Yorke ED, Jackson A, Rosenzweig KE, et al. Correlation of dosimetric factors and radiation pneumonitis for non-small-cell lung cancer patients in a recently completed dose escalation study. Int J Radiat Oncol Biol Phys. 2005;63:672–82. doi: 10.1016/j.ijrobp.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 3.Tsujino K, Hirota S, Endo M, et al. Predictive value of dose-volume histogram parameters for predicting radiation pneumonitis after concurrent chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys. 2003;55:110–5. doi: 10.1016/s0360-3016(02)03807-5. [DOI] [PubMed] [Google Scholar]
- 4.Hernando ML, Marks LB, Bentel GC, et al. Radiation-induced pulmonary toxicity: a dose-volume histogram analysis in 201 patients with lung cancer. Int J Radiat Oncol Biol Phys. 2001;51:650–9. doi: 10.1016/s0360-3016(01)01685-6. [DOI] [PubMed] [Google Scholar]
- 5.Asakura H, Hashimoto T, Zenda S, et al. Analysis of dose-volume histogram parameters for radiation pneumonitis after definitive concurrent chemoradiotherapy for esophageal cancer. Radiother Oncol. 2010;95:240–4. doi: 10.1016/j.radonc.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Zhu SC, Shen WB, Liu ZK, et al. Dosimetric and clinical predictors of radiation-induced lung toxicity in esophageal carcinoma. Tumori. 2011;97:596–602. doi: 10.1177/030089161109700510. [DOI] [PubMed] [Google Scholar]
- 7.Wang SL, Liao Z, Vaporciyan AA, et al. Investigation of clinical and dosimetric factors associated with postoperative pulmonary complications in esophageal cancer patients treated with concurrent chemoradiotherapy followed by surgery. Int J Radiat Oncol Biol Phys. 2006;64:692–9. doi: 10.1016/j.ijrobp.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Rodrigues G, Lock M, Souza DD, et al. Prediction of radiation pneumonitis by dose-volume histogram parameters in lung cancer: a systematic review. Radiother Oncol. 2004;71:127–38. doi: 10.1016/j.radonc.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Tachibana M, Kinugasa S, Yoshimura H, et al. Extended esophagectomy with 3-field lymph node dissection for esophageal cancer. Arch Surg. 2003;138:1383–9. doi: 10.1001/archsurg.138.12.1383. [DOI] [PubMed] [Google Scholar]
- 10.Murshed H, Liu HH, Liao Z, et al. Dose and volume reduction for normal lung using intensity-modulated radiotherapy for advanced-stage non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;58:1258–67. doi: 10.1016/j.ijrobp.2003.09.086. [DOI] [PubMed] [Google Scholar]
- 11.Yom SS, Liao Z, Liu HH, et al. Initial evaluation of treatment-related pneumonitis in advanced-stage non-small-cell lung cancer patients treated with concurrent chemotherapy and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2007;68:94–102. doi: 10.1016/j.ijrobp.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 12.Fu W, Wang L, Zhou Z, et al. Comparison of conformal and intensity-modulated techniques for simultaneous integrated boost radiotherapy of upper esophageal carcinoma. World J Gastroenterol. 2004;10:1098–102. doi: 10.3748/wjg.v10.i8.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myojin M, Tanabe S, Hosokawa M, et al. Does 3-D CRT plan become a clinical relevant factor to radiation pneumonitis risk in patients with esophageal cancer treated with definitive chemoradiotherapy? Int J Radiat Oncol Biol Phys. 2011;81:S321. [Google Scholar]