Abstract
Despite the potential devastating health consequences of intense total-body irradiation, and the decades of research, there still remains a dearth of safe and effective radiation countermeasures for emergency, radiological/nuclear contingencies that have been fully approved and sanctioned for use by the US FDA. Vitamin E is a well-known antioxidant, effective in scavenging free radicals generated by radiation exposure. Vitamin E analogs, collectively known as tocols, have been subject to active investigation for a long time as radioprotectors in patients undergoing radiotherapy and in the context of possible radiation accidents or terrorism scenarios. Eight major isoforms comprise the tocol group: four tocopherols and four tocotrienols. A number of these agents and their derivatives are being investigated actively as radiation countermeasures using animal models, and several appear promising. Although the tocols are well recognized as potent antioxidants and are generally thought to mediate radioprotection through ‘free radical quenching’, recent studies have suggested several alternative mechanisms: most notably, an ‘indirect effect’ of tocols in eliciting specific species of radioprotective growth factors/cytokines such as granulocyte colony-stimulating factor (G-CSF). The radioprotective efficacy of at least two tocols has been abrogated using a neutralizing antibody of G-CSF. Based on encouraging results of radioprotective efficacy, laboratory testing of γ-tocotrienol has moved from a small rodent model to a large nonhuman primate model for preclinical evaluation. In this brief review we identify and discuss selected tocols and their derivatives currently under development as radiation countermeasures, and attempt to describe in some detail their in vivo efficacy.
Keywords: Countermeasures, mice, radiation, tocopherols, tocotrienols, vitamin E
INTRODUCTION
Radioactive sources have been used globally for decades for a wide variety of purposes, including, but not limited to, diagnostic and therapeutic medicine, nuclear-generated energy, engineering and construction, sterilization of food and other products. As a consequence, on a global level, radioactive sources are quite plentiful, often loosely maintained and inventoried, and, unfortunately, at times are poorly secured. The current reality is that there are only minimal barriers that terrorists would face in attempting to acquire radioactive materials from these widely ranging sources. The radioactive materials needed to build a ‘dirty bomb’ can be found in almost any country in the world, and more than 100 countries may not have adequate regulatory control and monitoring programs necessary to prevent or even detect the theft of these materials [1]. Such ‘intended’ events, or those ‘unintended’ accidental events, that involve release or dispersal of massive amounts of radioactive material are an undeniable, potentially catastrophic possibility [2]. The number of individuals who would be affected and in need of medical care after a large-scale event, such as activation of an improvised nuclear device, would be high [3]. Therefore, without question, there is an urgent need to be better prepared than we are currently for such radiological/nuclear contingencies, regardless of whether they are intentional or otherwise.
Acute radiation syndrome (ARS) is characterized by the differential response of the body's vital organ systems to various intensities of radiation exposure. Clinical and pathophysiological details of these ARS-associated responses have been reported elsewhere [4]. However, in terms of discussing medical countermeasures for ARS, a brief overview of this disease complex is given below. There are at least three distinct subsyndromes—hematopoietic, gastrointestinal, and neuro/cerebrovascular—that are dependent upon the radiation exposure's total dose, dose rate and duration, as well as upon the quality of the radiation itself [5, 6]. Each of the subsyndromes follows a similar, three-phase, clinical pattern: (i) prodromal phase occurring during the initial period (i.e. less than an hour to a day or so) following exposure; (ii) a latent phase, which shortens with increasing intensity of exposure, and (iii) the manifest or ‘illness’ phase. The prodromal phase is characterized by the onset of nausea, vomiting and malaise. The time of nausea and vomiting directly relates to the radiation dose the individual has received. The latent phase follows the prodromal phase, and is when the exposed individual will be relatively symptom-free. The length of the latent phase can be quite variable, depending on the exposure intensity and setting and ranges in time from a short couple of hours or days, to longer periods of several weeks to a month or more. Compared to the longer latency of the hematopoietic syndrome, the latency of the gastrointestinal syndrome is relatively short (lasting a few days to a week), and it is exceedingly short for the neurovascular syndrome (generally only a few hours).
The illness phase presents with clinical symptoms associated with the manifest pathologies of the major organ systems injured (marrow, intestine, or neurologic and vascular systems). Individuals receiving ∼1.5 Gy or greater of acute whole-body irradiation will experience a decline in bone marrow function and pancytopenia with a magnitude incrementally increasing with increasing exposure intensity. The time of onset of blood cytopenias and the extent of marrow suppression vary considerably, but are clearly in relation to the intensity and duration of radiation exposure. Significant changes within the peripheral blood profiles of exposed individuals can be observed within hours to a few days following irradiation. Blood levels of lymphocytes decline the most rapidly and erythrocytes the least rapidly. Other blood cell types, e.g. leukocyte subsets in general, but neutrophils and monocytes specifically, decline more slowly than lymphocytes.
The gastrointestinal syndrome is generally elicited by much higher exposure levels (e.g. 6–10 Gy) than those that elicit the hematopoietic syndrome. However, lower radiation doses may also cause gastrointestinal syndrome under certain circumstances. Manifestation of the gastrointestinal syndrome has serious clinical implications, especially when accompanied by a marked reduction in the bone marrow's blood-forming capacity and the suppression of the body's innate immune response. The clinical phase of the gastrointestinal syndrome tends to occur earlier than does the hematopoietic syndrome, but often the two syndromes will overlap.
The neuro/cerebrovascular syndrome is associated with very high, acute doses of radiation (i.e. generally in considerable excess of 10 Gy). After a short latency period, the clinical course is marked by a steadily deteriorating state of consciousness with eventual coma and death. Convulsions also may occur [7]. The neuro/cerebrovascular syndrome is intractable by nature due to the current lack of suitable preventive or therapeutic measures. In contrast, individuals receiving lower radiation doses that result in the hematopoietic and gastrointestinal syndromes are more likely to be successfully managed clinically by interventions with suitable radiation countermeasures. Therefore, these two syndromes are specific targets for the development of novel countermeasures.
It is now well recognized that free radicals formed by the radiolysis of cellular aqueous milieu, and their interaction with one another and with oxygen, are primary mediators of radiation injury [4]. Most forms of ionizing radiation cause the production of reactive oxygen species through hydrolysis of water. These include superoxide, hydrogen peroxide, and hydroxyl radicals (the most reactive form). Such reactive oxygen species induced by ionizing radiation can initiate oxidative cellular injury, as well as activating intracellular signaling pathways and stimulating cytochrome c release from mitochondria leading to apoptosis (programmed cell death). This understanding has placed emphasis on the search for antioxidant agents that are suitable as radiation countermeasures [8–10]. Although endogenous antioxidant systems (glutathione, thioredoxin, superoxide dismutase, and catalase) normally inhibit the deleterious effects of reactive oxygen species, these systems may be overwhelmed in irradiated cells. Exogenously supplemented antioxidants, or agents that stimulate endogenous antioxidant systems within cells, have shown promise in terms of suppressing the harmful effects of irradiation. If present in the cells at the time of radiation exposure, such antioxidants may protect cells from radiation damage by scavenging reactive oxygen species before they act on cellular components. A variety of reducing agents, such as vitamin E analogs, polyphenols, thiols and superoxide dismutase mimetics have been described as potential radiation countermeasures in the recent past [8, 10].
Vitamin E
Natural products with human health benefits have become attractive targets for research [11]. For both prevention and therapy of human diseases, such compounds are common in our diets and, hence, are often perceived as being more ‘natural’ and better suited for medicinal purposes than ‘unnatural’ synthetic analogs due to being well tolerated and minimally toxic even at the upper ranges of dietary intake. By and large, such compounds are absorbed and processed easily by our body. Vitamins are prominent among natural compounds considered beneficial for human health [12]. Vitamin E is well known for its established health benefits, including antioxidant, neuroprotective, and anti-inflammatory properties [13]. Vitamin E has emerged as an essential, fat-soluble nutrient that functions as an antioxidant in the human body. It is essential because the body cannot manufacture its own vitamin E, so foods and supplements must provide it. At present, vitamin E represents a generic term for all tocopherols and their derivatives with naturally occurring and biologically active stereoisomeric compounds of α-tocopherol (AT) [14–16].
Tocopherols and tocotrienols
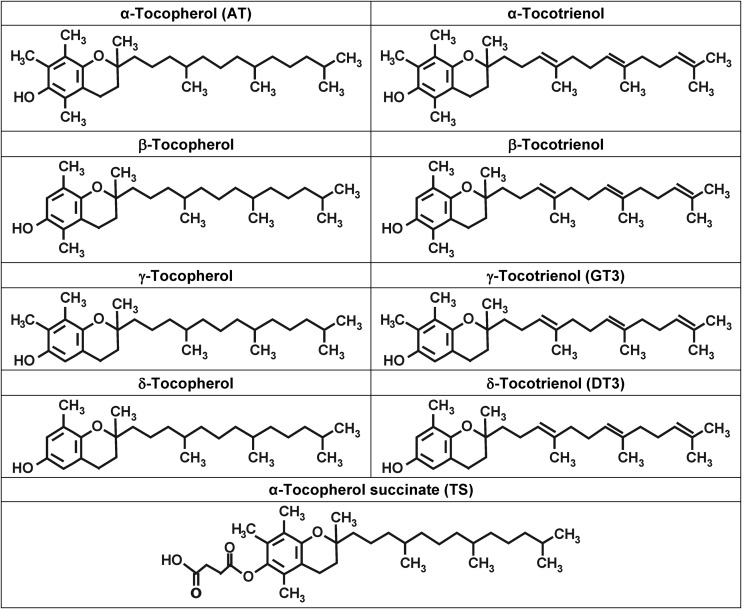
Vitamin E represents a family of compounds that is divided into two subgroups called tocopherols and tocotrienols, which act as important antioxidants that regulate peroxidation reactions and control free-radical production within the body [17, 18]. This family of compounds has eight different isoforms belonging to two categories: four saturated analogs (α, β, γ and δ) called tocopherols, and four unsaturated analogs referred to as tocotrienols (Fig. 1). These eight components are collectively known as tocols. Tocopherols and tocotrienols share common structural features of a chromanol ring and a 15-carbon tail at the C-2 position derived from homogenistate and phytyl diphosphate, respectively. Tocotrienols differ structurally from tocopherols by the presence of three trans-double bonds in the hydrocarbon tail. The isomeric forms of tocopherol and tocotrienol are distinguished by the number and location of methyl groups on the chromanol rings. Recent studies suggest that both the molecular and therapeutic targets of the tocotrienols are distinct from those of the tocopherols. While the tocopherols have been investigated extensively, relatively little is known about the tocotrienols. The abundance of AT in the human body has led biologists to neglect the non-tocopherol vitamin E molecules as topics for basic and clinical research. Recent developments suggest a serious reconsideration of this conventional wisdom is warranted. The latest developments in vitamin E research clearly indicate that members of the vitamin E family are not redundant with respect to their biological functions [13]. α-, γ-, and δ-tocotrienol have emerged as tocol molecules with functions in maintaining health and treating disease that are clearly distinct from that of tocopherols [19]. Current knowledge indicates prudent investigation of the less well-known isoforms is important, enabling intelligent selection of suitable molecules for studies.
Fig. 1.
Chemical structures of tocopherols, tocotrienols and tocopherol succinate.
Although tocotrienols were discovered five decades ago, the majority of their biological properties have been revealed only in the last decade. The anti-inflammatory, antioxidant, and cholesterol-lowering properties of tocotrienols can prevent cancer, diabetes, and cardiovascular and neurodegenerative diseases [20]. The isoforms of tocotrienols, which differ in their number of methyl groups, also differ in their biological activities. While several investigations have suggested that α-tocotrienol is highly neuroprotective [21], it has been demonstrated that δ-tocotrienol (DT3) is effective in targeting prostate cancer stem cell-like populations [22], and DT3 was also found to be effective against pancreatic carcinoma [23]. Earlier studies suggest that there may be as much as a 30-fold difference in the ability of α-, γ-, and δ-tocotrienol to inhibit cholesterol biosynthesis [24].
Tocols and their derivatives have been evaluated, sometimes comparatively, for their radioprotective properties at our institute as well as at several other institutions. The majority of these studies have been conducted with AT, the most commonly used vitamin E supplement and the most abundant vitamin E isoform in human and animal tissues [9, 25, 26]. During the last decade, tocotrienol research has gained substantial momentum. As stated above, for radioprotective efficacy, DT3 and γ-tocotrienol (GT3) are comparable and appear to be even better than other tocols [27–30]. Here we discuss selected and promising tocols and their derivatives under development as radiation countermeasures.
Comparison of tocopherols and tocotrienols
Tocopherols and tocotrienols differ in that the latter contain three double bonds in their isoprenoid side chain while the former do not [21]. Although more than 30 000 papers have been published on tocopherols, less than 600 papers deal with tocotrienols, with the majority of those published within the last decade. There are a limited number of publications ( < 50) about radioprotective efficacy of these agents, and almost all of these publications appeared during the last ten years.
Relative abundance in different food
Although tocopherols and tocotrieniols can be found in, and isolated from, a wide variety of foods (e.g. grains, legumes, seeds, etc.), the abundance of these two major classes of tocols in these foods differ, often significantly [12, 16]. For example, tocopherols are relatively abundant in corn, wheat and soybeans, whereas tocotrienols show greater abundance in barley, oats, palm and rice bran (Table 1). Tocotrienols are present in a range of unrelated plant groups and are almost exclusively found in seeds and fruits.
Table 1.
Natural sources of AT and tocotrienols (mg/100 gm) being developed as radiation countermeasures
| Source | AT | DT3 | GT3 |
|---|---|---|---|
| Palm oil | 20.5 | 7.2 | 32.3 |
| Rice bran | 23.6 | 5.3 | |
| Wheat germ | 2.4 | 11.8 | 49.3 |
| Barley | 67 | 5 | |
| Oat | 18 | 5 | 5 |
| Coconut oil | 0.5 | 0.6 | |
| Palm kernel oil | 2.1 | ||
| Soyabean | 10 | ||
| Soya bean oil | 26.4 | 59.3 | |
| Safflower oil | 38.7 | ||
| Peanut oil | 2.1 | ||
| Cocoa butter | 0.2 | 1.7 | 17 |
| Olive oil | |||
| Sunflower | 67 | ||
| Maize | 28.2 | 0.6 | 16.1 |
| Rapeseed | 20.2 |
Metabolism and other related biologic activities
Both tocopherols and tocotrienols are metabolized through ω-hydroxylation followed by five cycles of β-oxidation [31]. Tocotrienols possess better neureoprotective, anticancer and cholesterol lowering properties compared to tocopherols [32]. In two interesting studies, it has been shown that tocotrienols, but not tocopherols, inhibit the activation of pp60 (c-Src, a key regulator of glutamate-induced neuronal cell death) [21], and protect the neurons from glutamate-induced 12-lipoxygenase (12-Lox) activation [33]. The oral administration of tocotrienols but not tocopherols block tumor-induced angiogenesis [34]. Tocotrienols down-regulate vascular endothelial growth factor (VEGF) receptor expression in human umbilical vein endothelial cells (HUVEC) and block VEGF signaling. Tocotrienols are converted to tocopherols in vivo and the rate of conversion is species dependent. Though the conversion of tocotrienols to tocopherols is higher in human and chicken compared to swine, only a small percentage of tocotrienols is converted to tocopherols, which may not be biologically relevant [35–37].
Antioxidant properties of tocols
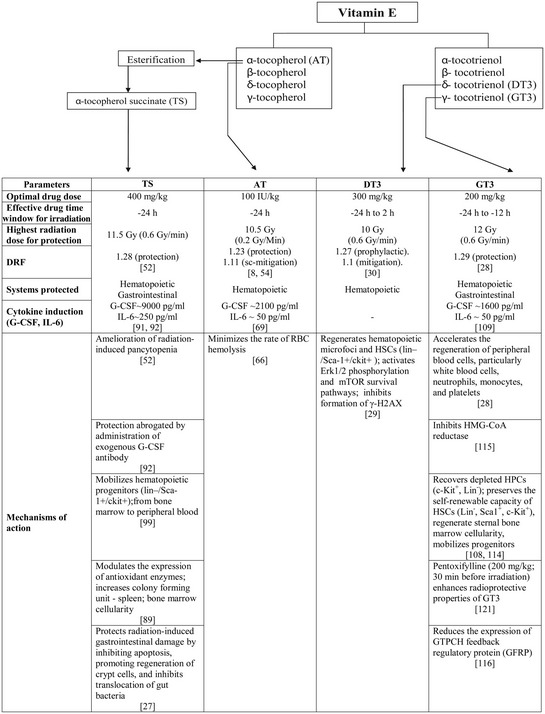
A selected number of studies have demonstrated that tocotrienols have superior antioxidant activity compared with tocopherols [24, 38–40]. One study reported that the antioxidant potential of tocotrienols is 1600 times more than that of AT [41]. Other reports suggesting that the tocotrienol's superior antioxidant efficacy may lie in the unsaturation in the aliphatic tail, which facilitates easier penetration into the tissue. Although the above studies suggest that tocotrienols have stronger antioxidant properties than tocopherols, other studies were not able to demonstrate a difference between tocopherols and tocotrienol in antioxidant potential [42]. There is no unanimity regarding whether the intrinsic antioxidant abilities of tocotrienols are different from those of tocopherols, or whether the different effects of tocopherols and tocotrienols in tissue may be due to differences in uptake. The data from the existing literature suggest that the reducing ability and radical chain-breaking activity of tocols depend on the circumstances under which the assays are performed. Tocopherol succinate (TS), DT3 and GT3 have comparable radioprotective efficacy, but all three appear to be better than AT, thus suggesting that better antioxidant activity may not necessarily translate into better radioprotective efficacy (Table 2). The alternative explanation might be that these tocols might not be significantly different in terms of their inherent antioxidant activities.
Table 2.
Summary of radioprotective efficacy of AT (α-tocopherol), TS (α-tocopherol succinate), DT3 (δ-tocotrienol) and GT3 (γ-tocotrienol)
 |
Effects of tocols on 3-hydroxy-3-methylglutaryl-coenzyme A reductase
3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase is a regulatory enzyme for cholesterol biosynthesis and it modulates serum cholesterol levels. In laboratory animals, supplementation of tocopherols in the diet raises blood serum levels of both total and low-density lipoprotein cholesterol levels, whereas supplementation of tocotrienols lowers serum total and low-density lipoprotein cholesterol levels [43]. Alpha tocotrienol inhibits HMG-CoA reductase while AT exhibits either inhibitory or stimulatory effect on liver HMG-CoA reductase [43–45]. DT3 and GT3 inhibit HMG-CoA reductase by inducing its degradation in the ubiquitin-proteasome pathway [46]. This is a mechanism different from common HMG-CoA reductase inhibitors such as statins that inhibit the rate-limiting step in the sterol biochemical pathway and the conversion of HMG-CoA to mevalonate [47]. These results provide a plausible mechanism for the hypercholesterolemic activity of tocotrienols that has been observed in both animals and humans.
Several mechanisms could account for potency differences in efficacy of tocopherols and tocotrienols:
the chromanol ring of tocotrienols may interact more efficiently with the lipid bilayer than that of tocopherols;
based on structural differences, tocotrienols may be more uniformly distributed in the lipid bilayer;
the initial rate of cellular uptake of alpha tocotrienol is 70 times higher than that of AT [48];
tocotrienols may have a higher recycling efficiency [41].
When administered orally to mice, tocotrienols appear faster in the blood plasma but at lower levels compared with tocopherols [49]. Faster appearance of a drug in circulation and sustained release at lower levels suggest the early- and long-acting nature of the drug. These important attributes may make tocotrienols better therapeutic agents than tocopherols.
Radiation countermeasures: radioprotectors, mitigators and therapeutics
Although the search for suitable radiation countermeasures has been going on for the last 50 years, no safe and effective radiation countermeasure has been approved by the US Food and Drug Administration (US FDA) for ARS. This situation has prompted intensified investigation to identify a new generation of countermeasures. In this communication we discuss promising radiation countermeasures being developed from tocols.
Radioprotectors are agents administered before exposure to prevent radiation-induced cellular and molecular damage [50]. Radiation mitigators are drugs administered shortly after radiation exposure that accelerate recovery or repair injury caused by radiation. Radiation therapeutics or treatments are agents administered after symptoms appear to stimulate repair or regeneration of damaged tissues/organ systems. All agents in the above three categories are collectively known as radiation countermeasures [8, 9, 51]. Numerous candidate countermeasures have been identified and investigated, and are at various stages of development [8–10, 25, 26].
In this communication, we briefly summarize recent progress with various agents grouped under tocols (mainly AT, DT3, GT3 and TS) (Fig. 1 and Table 2). Current research in radiation countermeasures focuses on radioprotectors as well as on mitigators and treatments. Radioprotectants will be useful for personnel expected to be at risk of exposure, such as military personnel (Special Operations or National Guard individuals preparing for a mission with a possibility of radiation exposure) and first responders, as well as civilians exposed to fallout fields during evacuation from radiological disaster areas. Given the likely breakdown in transportation during a mass-casualty scenario, such exposures may be significant. Radiomitigators and therapeutics will be useful in mass-casualty scenarios resulting from nuclear accidents or terrorist attacks involving nuclear or radiological devices.
The route of administration and the time of administration relative to time of radiation exposure are important parameters for comparing various drugs and selecting a radiation countermeasure for advance development. Optimally, the radiation countermeasure should be effective orally and can be administered as late as possible after radiation exposure. All tocols tested for radioprotective efficacy have demonstrated protection against radiation exposure when administered subcutaneously 24 h before radiation exposure [28, 52–54]. Tocotrienols may show higher cell uptake, but lower oral bioavailability [49]. High concentrations of tocopherols may reduce the uptake of tocotrienols.
The magnitude of protection against radiation damage is expressed as the dose reduction factor (DRF) or dose modification factor (DMF). The DRF is calculated by dividing the radiation LD50 (lethal dose, 50%) for drug-treated, irradiated animals by the LD50 for irradiated animals treated only with the vehicle used to administer the drug. The LD50 is based on a probit analysis, typically using at least three radiation doses that do not result in all or none mortality. For example, the DRF for a 30-day survival in the mouse quantifies protection of the hematopoietic system and is probably the most useful measure for comparing the efficacy against ARS [55, 56]. Because the probit regression lines are not always parallel, it is necessary to perform the complete DRF analysis. Even when using DRF, comparison may be difficult because the DRF can vary as a function of species, strain, age, gender, vehicle, route of administration, and the time of administration relative to time of radiation exposure. In meaningfully comparing the DRF of different drugs, one needs to consider the relative toxicity of the drug doses being used. The therapeutics index of a drug is defined as the ratio of drug LD50 to the effective dose of the drug. The larger the therapeutic index, the safer the drug will be for human [9].
FDA's Animal Efficacy Rule
Efficacy studies of radiation countermeasures in humans cannot be conducted because it would be unethical to deliberately expose healthy human volunteers to lethal or permanently damaging biological, chemical, radiological or nuclear substances. Accordingly, the US FDA Animal Efficacy Rule (21CFR 314) allows approval of new drug products that have been studied for their safety and efficacy in ameliorating or preventing serious or life-threatening conditions caused by exposure to lethal or permanently disabling toxic biological, chemical, radiological or nuclear substances, based on animal efficacy studies and phase I safety trials using healthy volunteers.
Animal models are used to define a developmental pathway for licensure, or to provide supportive data for drug approval or emergency use authorization of radiation countermeasures [57, 58]. The criteria of the FDA Animal Efficacy Rule relevant to animal model development are as follows:
The animal model and the mechanism of action of radiation exposure on the specific organ system must be well characterized. How the radiation countermeasure affects that mechanism must be reasonably well understood.
The effect of the countermeasure is demonstrated in more than one animal species expected to react to radiation with a response predictive of the human response to radiation and its treatment.
The experimental endpoint is clearly related to the desired benefit in humans, generally the enhancement of survival or prevention of major morbidity.
The pharmacokinetics and pharmacodynamics of the countermeasure or other relevant information in the animal models and humans allows selection of an effective countermeasure dose in humans.
Tocols as radiation countermeasures: chemical and biological nature and mechanisms of action
There is significant interest in developing tocols as radioprotectors because of their potent antioxidant properties, free radical scavenging ability, lack of performance-degrading toxicity, and the presumed chemical benefit of suppressing chronic radiation-induced fibrosis in some organ systems. The naturally occurring tocols comprise α-, β-, δ- and γ-tocopherol and α-, β-, δ-, and γ-tocotrienol [19]. Some of these agents have been shown to induce high levels of cytokines, particularly, granulocyte colony-stimulating factor [8].
AT
There have been an ample number of previous reports demonstrating the radioprotective efficacy of tocols [54, 59–65]. In this regard, we demonstrated that the administration of tocols subcutaneously, either 1 h before or 15 min after irradiation (dose rate 0.2 Gy/min), significantly increased the 30 day survival of mice. At a dose of 100 international units/kg of tocols administered 15 min after irradiation, the DRF was 1.1 [66]. When tocols were injected 1 h before 60Co γ-irradiation at a higher dose rate of 1 Gy/min, the DRF was 1.06. The DRF differences of the above two experiments may be due to differences in the dose rates of radiation exposure, in addition to differences in the treatment schedule (i.e. time of drug administration in relation to irradiation). There is a report that radiation-induced lipid peroxidation is greater after a lower dose rate of radiation compared to that after a higher dose rate of irradiation [61]. When administered orally, either before or after 1 Gy radiation exposure, tocols at 25 mg/kg significantly reduced the frequencies of micronuclei and chromosomal aberrations in mouse bone marrow cells [64]. These anti-cytoclastic effects of tocols administration probably have greater impact on late-arising, radiation-induced pathologies than on the manifestation of ARS.
Administration of AT immediately after irradiation increased the number of hematopoietic colony-forming units in the spleen of mice [67]. At 400 mg/kg of AT, enhanced mouse protection was achieved when administered subcutaneously 24 h before 60Co γ-irradiation (0.6 Gy/min) [54]. The ‘time-window’ for such AT-elicited radioprotection ranged from 20–24 h, while the pharmacokinetics of AT in blood suggested bimodal maxima at 24 h and at 4 h. In these studies, the DRF was estimated to be 1.23 with a 95% confidence limit ranging from 1.19–1.28 [68]. The requirement of administration 24 h prior to irradiation suggests induction of other factors responsible for protection. Keeping in mind such mechanisms, various tocopherol analogs were studied for induction of radioprotective cytokines and growth factors in response to treatment, and TS was found to stimulate granulocyte colony-stimulating factor (G-CSF) to the greatest extent [69].
The radiomitigative potential of AT was first observed in 1978 when an aqueous preparation was used intraperitoneally [62]. AT's radiomitigative effect in mice was linked to enhanced cell-mediated immunity [63].
α-tocopherol-mono-glucoside
A novel water-soluble derivative of AT (2-(α-D-glucopyranosyl) methyl-2,5,7,8-tetramethylchroman-6-ol, known as α-tocopherol-mono-glucoside (TMG)), was derived by substituting the linear carbon chain with a glucopyranosyl moiety, and was demonstrated to have better antioxidant activity [70, 71]. Due to TMG's long hydrophobic phytyl side chain, the mobility of tocols and its free radical scavenging activity are limited to a cell's lipid membrane. TMG is a water-soluble derivative of tocols and has been investigated for its radioprotective efficacy using various in vitro and in vivo models. TMG was found to protect DNA from radiation-induced strand breaks. It also protected thymine glycol formation induced by γ-radiation. TMG was nontoxic to mice when administered orally up to 7 g/kg. The drug's LD50 through the intraperitoneal route was 1.15 g/kg. TMG offered protection against whole-body γ-radiation-induced lethality in mice [72]. Embryonic mortality resulting from ionizing radiation exposure (2 Gy) was reduced by 75% when TMG (0.6 g/kg) was administered intraperitoneally. TMG has also been reported to reduce the radiation-induced aberrant metaphases and micronucleated erythrocytes in Swiss albino mice when administered before radiation exposure [73].
When TMG was injected intraperitoneally at a dose of 600 mg/kg within 10 min after 60Co γ-radiation exposure (1.6 Gy/min), it protected the mice and its DRF was 1.09 [74]. TMG administration was reported to enhance hematopoietic recovery in mice exposed to X-rays [75]. Another study has reported TMG effectiveness in preventing radiation-induced bone marrow damage in mice [76]. It also inhibited the formation of γ-radiation-induced DNA single-strand breaks in plasmid pBR322 [77]. There are several reports highlighting the mechanism of action of TMG [78, 79].
α-tocopherol succinate
α-tocopherol succinate (TS) is the hemisuccinate ester derivative of AT. TS has been shown to be a promising antitumor agent due to its abilities to inhibit proliferation and induce apoptosis in a variety of human malignant cell lines, while being relatively less active towards normal cells [80–85]. TS has been reported as the most effective form of tocols, compared with AT, alpha-tocopheryl acetate and alpha-tocopheryl nicotinate, in inducing differentiation, inhibition of proliferation and apoptosis in cancer cells, depending upon its concentration [86]. Additionally, TS enhances radiation-induced chromosomal damage in cancer cells, while normal cells are not affected either in vitro or in vivo [80, 87, 88]. TS significantly protected mice against lethal doses of 60Co γ-radiation when administered 24 h before irradiation (DRF 1.28). TS stimulated circulating G-CSF, with a peak at 24 h after drug injection, and TS also stimulated G-CSF message in bone marrow cells at 12 and 24 h after injection. Further, TS significantly reduced thrombocytopenia, neutropenia and monocytopenia. TS had no significant effect on lymphocytes, indicating that its radioprotective effects may be restricted to the myeloid cell compartments rather than the lymphoid compartments of the lymphohematopoietic system [52]. TS modulated the expression of antioxidant enzymes and inhibited expression of oncogenes in mice after irradiation [89]. TS also increased colony-forming unit-spleen (CFU-S) numbers and bone marrow cellularity in irradiated mice. These results provide additional support for the observed radioprotective efficacy of TS, as well as insight into its mechanisms of action.
Our recent results demonstrate that, when administered 24 h before radiation exposure, TS enhanced survival in a significant number of mice irradiated with sufficiently high doses of 60Co γ-radiation to cause the gastrointestinal syndrome. In this regard, TS was shown to protect the intestinal tissue of irradiated mice, specifically in terms of maintenance of crypt and villi number, villus length and mitotic figures. TS treatment decreased the number of TUNEL (terminal deoxynucleotidyl transferase mediated dUTP nick end labeling)- and PUMA- (p53 upregulated modulator of apoptosis)-positive cells and increased 5-bromo-2'-deoxyuridine- (BrdU)-positive cells in jejunum compared to vehicle-treated mice. Further, TS inhibited gut bacterial translocation to the heart, spleen and liver in irradiated mice [27]. Our data indicate that TS protects mice from radiation-induced gastrointestinal damage by inhibiting apoptosis and promoting regeneration of crypt cells, and inhibits translocation of gut bacteria. Possible mechanisms might include: a generalized stabilization of cytoplasmic membrane gut epithelia, or more specifically, the stabilization of their junctional complexes. Recently, we demonstrated that TS injection significantly decreased the number of CD68-positive cells, DNA damage (comet assay) and apoptotic cells in irradiated mice, as judged by various apoptotic pathway markers (BAX, caspase 3, and cleaved poly(ADP-ribose) polymerase-positive cells) [90]. TS treatment also increased proliferating cells in irradiated mice, as determined by cleaved poly(ADP-ribose) polymerase (cPARP) and phospho-histone H3 (pH3) protein expression as a mitotic marker. These results further support our contention that TS protects mice against lethal doses of ionizing radiation by inhibiting radiation-induced apoptosis and DNA damage while enhancing cell proliferation.
TS also induced very high levels of G-CSF and keratinocyte-derived chemokine (KC) production in peripheral blood 24 h after subcutaneous administration. When TS-injected mice were administered a neutralizing antibody to G-CSF, there was complete neutralization of G-CSF in circulating blood, and the protective effect of TS was significantly abrogated [91, 92]. Histopathology of jejunum from TS-injected and irradiated mice demonstrated protection of gastrointestinal tissue from radiation-induced apoptosis, yet protection was clearly suppressed by administration of a G-CSF neutralizing antibody, suggesting that the induction of G-CSF resulting from TS administration is responsible for protection from 60Co γ-radiation injury. The above results suggest that TS may merit further development using appropriate large animal models for the purpose of seeking US FDA drug approval as a safe and effective pharmacologic countermeasure for humans at risk to potentially lethal effects of acute radiation exposure.
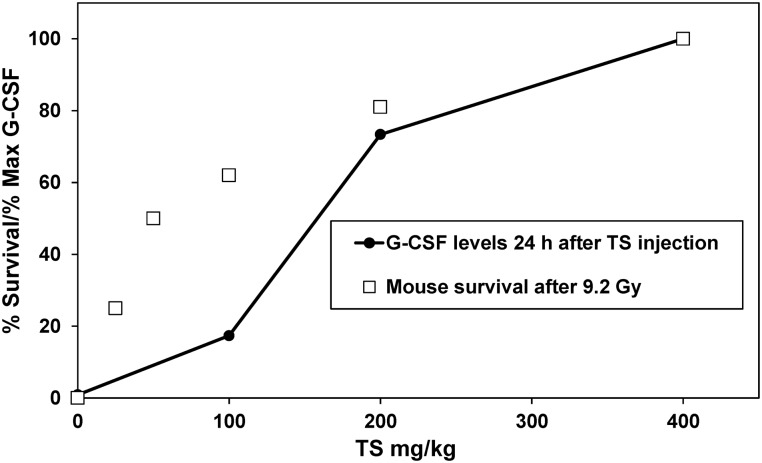
We have observed that mouse protection by TS against radiation injury is highly drug dose-dependent. Rising TS drug levels not only limit lipid peroxidation and destabilization of vital cell membranes following acute radiation exposure, but also serve to stimulate high levels of G-CSF. Thus G-CSF can be used as an efficacy biomarker for the radioprotective efficacy of TS, as can the more conventional post-exposure monitoring of the blood leukocyte response. This is particularly interesting since TS-induced G-CSF levels reach the highest levels at 24 h after injection, and TS demonstrates optimal radioprotective efficacy when administered 24 h before radiation exposure. Data presented in Fig. 2 demonstrate a correlation between mouse protection and levels of G-CSF in peripheral circulation.
Fig. 2.
Correlation between mouse protection against 60Co γ-irradiation and G-CSF induction. Different doses of TS were administered subcutaneously 24 h before 60Co γ-irradiation and the 30-day survival was scored. In another set of experiments, different doses of TS were administered subcutaneously and serum G-CSF was measured by Luminex at different time-points after TS injection.
There are several reports demonstrating the radioprotective efficacy of G-CSF against ionizing radiation in animal models [93–97]. G-CSF has been shown to be efficacious against lower doses of ionizing radiation but failed to protect animals when higher doses of radiation were used. These studies suggested that cytokine therapy with G-CSF increases survival through the induction of earlier recovery of neutrophils in lethally irradiated experimental animals. Clinical reports on G-CSF indicate that severe life-threatening side effects are uncommon; however, transient non-life-threatening bone pain is a rather commonly noted side effect [98].
We had earlier hypothesized that TS would stimulate a G-CSF-induced mobilization of bone marrow progenitor cells into the peripheral circulation. This hypothesis was clearly confirmed using several different approaches [99]. First, a direct fluorescence flow cytometric approach was used to identify and phenotype the putative, mobilized hematopoietic stem cells (HSC) in question [92]. In this study, both TS and AMD3100 (a blood cell mobilizing adjuvant), administered either separately or in combination, enhanced the rate of HSC mobilization, as evidenced by elevated numbers of circulating blood cells in treated mice that bore ‘signature-like’ surface markers of primitive, marrow-repopulating progenitors; i.e. Sca-1+ c-Kit+ lineage negative cells [99]. Second, we evaluated the efficacy of whole blood obtained from TS-treated mice for protection against γ-irradiation to compare with blood obtained from G-CSF-treated mice. All mice that were irradiated but which had received no transfusion died. Survival was significantly higher in groups where mice received blood from TS-treated animals [100]. Further, our results demonstrated that infusions of HSC-enriched, peripheral blood fractions of mononuclear cells (PBMC) from TS-injected mice greatly improved chances of extended survival of lethally irradiated mice [100]. The transfused cells act secondarily as a bridging therapy for irradiated mice while their own immune system recovers from the radiation-induced damage. Further, our recent results demonstrated that infusion of whole blood or PBMC from TS- and AMD3100-injected mice significantly improved survival of mice receiving still higher, gastrointestinal-syndrome-eliciting radiation doses. Histopathology and immunostaining of jejunum from these irradiated and TS- and AMD3100-mobilized PBMC-transfused mice revealed significant protection of gastrointestinal tissue from radiation injury [101]. We also observed that the infusion of PBMC from TS- and AMD3100-injected mice significantly inhibited apoptosis, increased cell proliferation in the analyzed tissues of recipient mice, and inhibited bacterial translocation to various organs compared to mice receiving cells from vehicle-mobilized cells [102]. This study further supports our contention that the infusion of TS-mobilized progenitor-containing PBMC acts as a bridging therapy by inhibiting radiation-induced apoptosis, enhancing cell proliferation, and inhibiting bacterial translocation in irradiated mice.
A simpler, improved protocol for the clinical management of individuals suffering ARS-associated hematopoietic and/or gastrointestinal syndromes might well be in the offing if the above results with TS administration can be replicated using larger animal models of ARS (i.e. in either canine, minipig and/or nonhuman primate models).
DT3
As stated above, AT is the most investigated form of tocols, and recent studies suggest that tocotrienols also possess potent antioxidant activity [30, 103, 104]. DT3 has demonstrated antioxidant activity greater than that of γ- and α-tocotrienol in the membrane system while protecting primary neuronal cells against glutamate toxicity [18, 104]. Such powerful antioxidant activity made DT3 another promising candidate for evaluation as a radiation countermeasure. Plasma concentration of DT3 reached ∼195 µM (Cmax) 1 h after injection (Tmax), and was eliminated from plasma 12 h later in mice [105]. A single subcutaneous injection of DT3 before or after 60Co γ-irradiation significantly protected mice in a 30-day survival experiment. DT3 was effective at a wide dose range of 18.75–400 mg/kg [29, 30]. The DRF values for radioprotective treatment (24 h before irradiation) with 150 and 300 mg/kg were 1.19 and 1.27, respectively. For radiomitigation treatment with 150 mg/kg of DT3 administered 2 h after irradiation, the DRF was 1.1. When DT3 was administered at 300 mg/kg dose 24 h before irradiation, it significantly reduced radiation-induced cytopenia, suggesting its stimulatory effects on hematopoietic recovery [30]. Recently, it was demonstrated that DT3 reduced activation of caspase-8, caspase-3 and caspase-7, while increasing autophagy-related beclin-1 expression in irradiated bone marrow cells [105].
The mechanism of DT3-mediated radioprotection may be due to stimulation of the extracellular signal-related kinase (Erk) activation associated with the mammalian target of the rapamycin (mTOR) survival pathway. DT3 has been reported to increase cell survival and regeneration of hematopoietic microfoci and lineage−/Sca-1+/c-Kit+ stem and progenitor cells in irradiated mouse bone marrow cells. DT3 also protected CD34+ cells from radiation-induced damage [29]. DT3 activated Erk 1/2 phosphorylation and inhibited γ-H2AX foci. Further, DT3 upregulated mTOR and phosphorylation of its downstream effector 4EBP-1. These changes were associated with activation of the mRNA translation regulator eIF4E and ribosomal protein S6. These findings suggest that DT3 protects mouse bone marrow and human CD34+ cells from radiation-induced injury through Erk activation associated with the mTOR survival pathway.
GT3
As stated above, GT3 is a potent inhibitor of HMG-CoA reductase [106, 107], and has received great attention in recent years. Its antioxidant activity is a compelling reason to evaluate it for radioprotective efficacy. At a dose of 100 and 200 mg/kg administered 24 h before 60Co γ-irradiation, GT3 significantly protected mice against radiation doses as high as 11.5 Gy. Its dose reduction factor as a radioprotector (24 h before irradiation, 200 mg/kg dose) was 1.29. GT3 treatment accelerated hematopoietic recovery, as judged by higher numbers of total white blood cells, neutrophils, monocytes, platelets and reticulocytes in peripheral blood [28], and enhanced hematopoietic progenitors [108] in bone marrow of irradiated mice. GT3-treated irradiated mice had higher numbers of colony-forming cells, more regenerative microfoci for myeloid and megakaryocytes, higher cellularity in bone marrow, and reduced frequency of micronucleated erythrocytes compared to vehicle-treated mice [108].
To investigate the effects of GT3 on the hematopoietic system, Kulkarni et al. measured various cytokines and growth factors by cytokine array and Luminex in a mouse model [109]. GT3 treatment resulted in significant induction of G-CSF in mice. G-CSF levels increased markedly within 12–24 h after GT3 administration. Time-course analysis demonstrated that G-CSF was induced transiently after GT3 injection, and returned to background levels by 48 h after GT3 administration. Interleukin-6 (IL-6) followed a similar pattern of stimulation in response to GT3 administration, though the titer of IL-6 was significantly lower than that of G-CSF. The peak of IL-6 was observed at an earlier time-point compared to G-CSF. Survival studies with GT3 suggested the most efficacious time for drug administration was 24 h prior to irradiation. This may be due to induction of key hematopoietic cytokines in that time-window. These results also suggest a possible role of GT3-induced G-CSF stimulation in protection from radiation-induced neutropenia and cytopenia. Using four radiation countermeasures (including GT3), we have demonstrated that the use of G-CSF antibody abrogates radioprotective efficacy [91, 92, 110–113]. Using different animal models (mice, nonhuman primates and canines), we recently demonstrated that G-CSF and IL-6 may serve as efficacy biomarkers for selected radiation countermeasures [111]. GT3 was recently found to be effective in mobilizing progenitors in peripheral blood, as shown previously with TS in mice [114].
Because GT3 inhibits HMG-CoA reductase, it was of interest to evaluate whether HMG-CoA reductase inhibition plays a role in the radioprotection afforded by GT3. It was demonstrated that GT3 decreased radiation-induced vascular oxidative stress, an effect that was reversible by mevalonate (the product of reaction catalyzed by HMG-CoA) [115]. GT3 also reduced intestinal radiation injury and accelerated the recovery of soluble markers of endothelial function [115]. HMG-CoA reductase inhibitors mediate their pleiotropic effects via endothelial nitric oxide synthase that needs the cofactor 5,6,7,8-tetrahydrobiopterin. Radiation exposure decreased tetrahydrobiopterin in lungs, which was reversed by GT3 administration. Both GT3 and tetrahydrobiopterin supplementation reduced post-irradiation vascular peroxynitrite production [116]. GT3 also ameliorated endothelial cell apoptosis and reduced endothelial cell guanosin triphosphate cyclohydrolase 1 (GTPCH) feedback regulatory protein (GFRP) levels and GFRP-GTPCH binding by decreasing transcription of the GFRP gene.
There are several reports in preclinical and clinical studies that combined treatment with tocols and the methylxanthine derivative pentoxyfylline (phosphodiesterase inhibitor) is effective in reducing and even reversing radiation-induced cardiac, lung, intestinal and dermal injury [59, 117–120]. The majority of these reports studied the effects of the above combination on radiation-induced fibrosis, a late effect of irradiation, and there is very little known about the effects of this combination on acute radiation injury. Berbee et al. reported significantly improved survival of mice against 60Co γ-irradiation with combined treatment of GT3 and pentoxyfylline compared with either GT3 or pentoxyfylline administered alone [121]. The GT3 and pentoxyfylline combination protected all mice against radiation doses as high as 12 Gy. GT3 plus pentoxyfylline improved bone marrow colony-forming units, spleen colony counts, and platelet recovery compared to GT3 alone. There was no benefit of the combination in ameliorating intestinal injury or vascular peroxynitrite production [121].
Based on such encouraging findings, GT3 has been selected by our institute as the most promising agent of the tocols for development as a radiation countermeasure. Currently, GT3 is being investigated for its pharmacokinetics, pharmacodynamics, and efficacy against 60Co γ-irradiation using a nonhuman primate model.
Relative efficacy of tocols as potential countermeasures and amifostine
The US FDA has approved amifostine for preventing radiation injury in the salivary glands of head and neck cancer patients receiving radiotherapy, to reduce xerostomia [8]. Amifostine is a phosphorothioate that is not taken into cells until it is dephosphorylated by alkaline phosphatase [122]. Once dephosphorylated, the agent freely diffuses into cells and can act as a free radical scavenger. Additional potential mechanisms of protection have been described, including induction of hypoxia through increased oxygen use and condensation of DNA [123]. Phosphorothioates are effective radioprotective agents when administered orally to mice but are not effective in nonhuman primates. Amifostine has been used intraperitoneally, intramuscularly and intravenously in various studies. The DRF of amifostine with a dose of 500 mg/kg given intraperitoneally in mice has been reported to be 2.7 for hematopoietic death and 1.8 for gastrointestinal death [9]. These values can be used as a standard for comparing other radioprotective agents. Usually amifostine was used shortly before radiation exposure, say 15 min before irradiation. As presented in Table 2, the DRF of all tocols tested for radioprotection through subcutaneous route administered 24 h prior to radiation exposure in mice is from 1.2–1.3. The amifostine therapeutic index is low, and high levels of survival were observed only at doses very close to their maximum tolerated dose. The therapeutic index of tocols is comparatively higher. Further, the performance-decrementing activity of amifostine is significant, even under relatively low drug-dosing regimens that are marginally survival promoting [124]; such negative, performance-decrementing effects are not generally attributable to tocols. The radioprotective efficacy of the majority of tocols has been shown to be mediated through G-CSF and there is no such information available for amifostine.
CONCLUSION
Disappointment with tocopherols, based on two large randomized controlled clinical trials for the prevention of prostate cancer [125], is growing as the trials fail to meet expectations. Similar negative results were reported earlier with vitamin E when its effect was examined on cardiovascular events and cancer in a randomized clinical trial [126]. Although these findings are clearly disappointing, they suggest to researchers that some tocols, particularly TS and tocotrienols, might have greater health benefits.
Our results demonstrate that TS has the potential to protect tissues from radiation injury beyond the hematopoietic system by improving structural integrity, inhibiting apoptosis, and enhancing cell proliferation in gastrointestinal tissue in mice exposed to high doses of 60Co γ-radiation. TS did not protect mice when administered as an injury-mitigator after irradiation [27], so it may be useful only as a radioprotector. TS will be of use to the military where there are potential incidences of exposure. Furthermore, TS could also be used in limited situations where the potential for exposure will continue long after a nuclear accident and new personnel are deployed. TS has been used subcutaneously 24 h before irradiation in mice at a dose of 400 mg/kg, a dose consistent with recent published results [27, 89]. Protection provided by TS is of great significance because the radiation dose used in this study is equivalent to a highly lethal dose for humans. TS appears to be an attractive radiation countermeasure candidate with no known toxicity. It is a candidate for further development as a radiation countermeasure in large animals such as canines, minipigs or nonhuman primates, and ultimately for use in humans.
We also propose a new strategy to treat individuals (e.g. military personnel) who are at high risk for acute, high-dose ionizing radiation exposure [100, 101]. TS may be administered to select military personnel preparing for special missions having an increased risk of excessive high-radiation exposures. Blood samples (either whole blood or PBMC) of these individuals can be collected prior to a mission and stored frozen. If, during the course of the mission, such an acute, high-dose exposure event were to occur, the exposed individual could be removed from the field of operation, transported back to a medical facility and given a therapeutic transfusion of his/her own previously stored blood/PBMC sample. Because of the simple nature of this protocol, we believe it to be logistically feasible.
There are a number of major advantages that make TS-mobilized progenitors ideal for the treatment of patients/casualties with ARS: (i) TS-mobilized progenitor therapy is by all accounts essentially nontoxic and exceedingly well tolerated; (ii) TS-mobilized progenitor therapy clearly allows for a broader treatment range (in terms of the extent of radiation exposure) and is therapeutic for both the hematopoietic and gastrointestinal subsyndromes of ARS; (iii) TS is an inexpensive product easily available from vendors; (iv) TS is stable at room temperature and suitable for storage; (v) TS can replace currently used G-CSF for progenitor mobilization in the clinic. Together, these characteristics make TS-mobilized progenitors a suitable candidate as a bridging therapy for acute radiation victims that can be administered in the field with minimal infrastructure requirements. With further preclinical development in large animals, we may be able to provide an appropriate protocol in the near future for the clinical management of individuals suffering from exposure to high doses of ionizing radiation.
Recent developments with tocotrienols, particularly DT3- and GT3, are encouraging. Both have demonstrated significant and comparable radioprotective efficacy in mice. There are recent publications demonstrating the mechanism of action of these agents. Based on the promising results in mice, GT3 is being evaluated in nonhuman primates for pharmacokinetics, pharmacodynamics, safety and efficacy. More preclinical studies in long-lived large animal models of ARS (NHP, canine or minipig) and clinical studies are needed to fully realize their potential and facilitate advanced development of these radiation countermeasures.
FUNDING
The research project for tocopherol succinate was supported by the Armed Forces Radiobiology Research Institute intramural research program (RAB2CZ and RBB2GQ), and the GT3 project was supported by the Defense Threat Reduction Agency (CBM.RAD.01.10-AR-005).
ACKNOWLEDGEMENTS
The opinions or assertions contained herein are the private views of the authors and are not necessarily those of the Armed Forces Radiobiology Research Institute, the Uniformed Services University of the Health Sciences, or the Department of Defense. The authors are thankful to Mark H. Whitnall for helpful discussions, Ana Posarac and Elizabeth Ducey for graphics, and Pankaj K. Singh for literature searches.
REFERENCES
- 1.Singh VK, Seed TM. Radiation effects. In: Roy MJ, editor. Physician's Guide to Terrorist Attack. Totowa: Humana Press; 2003. pp. 339–62. [Google Scholar]
- 2.Carter AB, May MM, Perry WJ. The day after: action following a nuclear blast in a U.S. city. Washington Quarterly. 2007;30:19–32. [Google Scholar]
- 3.Benjamin GC, McGeary M, McCutchen SR. Washington, DC: The National Academies Press; 2009. Assessing Medical Preparedness to Respond to a Terrorist Nuclear Event: Workshop Report. [PubMed] [Google Scholar]
- 4.Hall EJ, Giaccia AJ. Radiobiology for the Radiobiologist. Philadelphia, PA: Lippincott Williams and Wilkins; 2006. [Google Scholar]
- 5.Dainiak N. Hematologic consequences of exposure to ionizing radiation. Exp Hematol. 2002;30:513–28. doi: 10.1016/s0301-472x(02)00802-0. [DOI] [PubMed] [Google Scholar]
- 6.Gusev IA, Guskova AK, Mettler FA. Medical Management of Radiation Accidents. Boca Raton, FL: CRC Press; 2001. [Google Scholar]
- 7.Glasstone S, Dolan PJ. The Effects of Nuclear Weapons. Washington, DC: Department of Army, Headquarters; 1977. [Google Scholar]
- 8.Singh VK, Ducey EJ, Brown DS, et al. A review of radiation countermeasure work ongoing at the Armed Forces Radiobiology Research Institute. Int J Radiat Biol. 2012;88:296–310. doi: 10.3109/09553002.2012.652726. [DOI] [PubMed] [Google Scholar]
- 9.Weiss JF, Landauer MR. History and development of radiation-protective agents. Int J Radiat Biol. 2009;85:539–73. doi: 10.1080/09553000902985144. [DOI] [PubMed] [Google Scholar]
- 10.Dumont F, Le Roux A, Bischoff P. Radiation countermeasure agents: an update. Expert Opin Ther Pat. 2010;20:73–101. doi: 10.1517/13543770903490429. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal SS, Singh VK. Immunomodulators: a review of studies on Indian medicinal plants and synthetic peptides. Part I: Medicinal plants. Proc Ind Nat Sci Acad B. 1999;65:179–204. [Google Scholar]
- 12.Papas A. The Vitamin Factor. New York, NY: Harper Perennial, Harper-Collins Publishers Inc; 1999. [Google Scholar]
- 13.Nesaretnam K. Multitargeted therapy of cancer by tocotrienols. Cancer Lett. 2008;269:388–95. doi: 10.1016/j.canlet.2008.03.063. [DOI] [PubMed] [Google Scholar]
- 14.Traber MG, Packer L. Vitamin E: beyond antioxidant function. Am J Clin Nutr. 1995;62:1501–9S. doi: 10.1093/ajcn/62.6.1501S. [DOI] [PubMed] [Google Scholar]
- 15.Traber MG, Sies H. Vitamin E in humans: demand and delivery. Annu Rev Nutr. 1996;16:321–47. doi: 10.1146/annurev.nu.16.070196.001541. [DOI] [PubMed] [Google Scholar]
- 16.Papas A. Vitamin E: tocopherols and tocotrienols. In: Papas AM, editor. Antioxidant Status, Diet, Nutrition, and Health. Boca Raton: CRC Press; 1999. pp. 189–210. [Google Scholar]
- 17.Palozza P, Simone R, Picci N, et al. Design, synthesis, and antioxidant potency of novel alpha-tocopherol analogues in isolated membranes and intact cells. Free Radic Biol Med. 2008;44:1452–64. doi: 10.1016/j.freeradbiomed.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Palozza P, Verdecchia S, Avanzi L, et al. Comparative antioxidant activity of tocotrienols and the novel chromanyl-polyisoprenyl molecule FeAox-6 in isolated membranes and intact cells. Mol Cell Biochem. 2006;287:21–32. doi: 10.1007/s11010-005-9020-7. [DOI] [PubMed] [Google Scholar]
- 19.Sen CK, Khanna S, Roy S. Tocotrienols: Vitamin E beyond tocopherols. Life Sci. 2006;78:2088–98. doi: 10.1016/j.lfs.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aggarwal BB, Sundaram C, Prasad S, et al. Tocotrienols, the vitamin E of the 21st century: its potential against cancer and other chronic diseases. Biochem Pharmacol. 2010;80:1613–31. doi: 10.1016/j.bcp.2010.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sen CK, Khanna S, Roy S, et al. Molecular basis of vitamin E action. Tocotrienol potently inhibits glutamate-induced pp60(c-Src) kinase activation and death of HT4 neuronal cells. J Biol Chem. 2000;275:13049–55. doi: 10.1074/jbc.275.17.13049. [DOI] [PubMed] [Google Scholar]
- 22.Luk SU, Yap WN, Chiu YT, et al. Gamma-tocotrienol as an effective agent in targeting prostate cancer stem cell-like population. Int J Cancer. 2011;128:2182–91. doi: 10.1002/ijc.25546. [DOI] [PubMed] [Google Scholar]
- 23.Hussein D, Mo H. d-delta-Tocotrienol-mediated suppression of the proliferation of human PANC-1, MIA PaCa-2, and BxPC-3 pancreatic carcinoma cells. Pancreas. 2009;38:e124–36. doi: 10.1097/MPA.0b013e3181a20f9c. [DOI] [PubMed] [Google Scholar]
- 24.Pearce BC, Parker RA, Deason ME, et al. Hypocholesterolemic activity of synthetic and natural tocotrienols. J Med Chem. 1992;35:3595–606. doi: 10.1021/jm00098a002. [DOI] [PubMed] [Google Scholar]
- 25.Weiss JF, Landauer MR. Radioprotection by antioxidants. Ann N Y Acad Sci. 2000;899:44–60. [PubMed] [Google Scholar]
- 26.Weiss JF, Landauer MR. Protection against ionizing radiation by antioxidant nutrients and phytochemicals. Toxicology. 2003;189:1–20. doi: 10.1016/s0300-483x(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 27.Singh PK, Wise SY, Ducey EJ, et al. Alpha-tocopherol succinate protects mice against radiation-induced gastrointestinal injury. Radiat Res. 2012;177:133–45. doi: 10.1667/rr2627.1. [DOI] [PubMed] [Google Scholar]
- 28.Ghosh SP, Kulkarni S, Hieber K, et al. Gamma-tocotrienol, a tocol antioxidant as a potent radioprotector. Int J Radiat Biol. 2009;85:598–606. doi: 10.1080/09553000902985128. [DOI] [PubMed] [Google Scholar]
- 29.Li XH, Fu D, Latif NH, et al. Delta-tocotrienol protects mouse and human hematopoietic progenitors from gamma-irradiation through extracellular signal-regulated kinase/mammalian target of rapamycin signaling. Haematologica. 2010;95:1996–2004. doi: 10.3324/haematol.2010.026492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Satyamitra M, Kulkarni S, Ghosh SP, et al. Hematopoietic recovey and amelioration of radiation-induced lethality by the vitamin E isoform, delta-tocotrienol. Radiat Res. 2011;175:736–45. doi: 10.1667/RR2460.1. [DOI] [PubMed] [Google Scholar]
- 31.Birringer M, Pfluger P, Kluth D, et al. Identities and differences in the metabolism of tocotrienols and tocopherols in HepG2 cells. J Nutr. 2002;132:3113–8. doi: 10.1093/jn/131.10.3113. [DOI] [PubMed] [Google Scholar]
- 32.Das S, Das M, Das DK. Vitamin E isomers, tocotrienols, in cardioprotection In: Wartson RR, Preedy VR, editors. . Tocotrienols: Vitamins Beyond Tocopherols. Boca Raton: CRC Press; 2009. pp. 285–93. [Google Scholar]
- 33.Khanna S, Roy S, Slivka A, et al. Neuroprotective properties of the natural vitamin E alpha-tocotrienol. Stroke. 2005;36:2258–64. doi: 10.1161/01.STR.0000181082.70763.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyazawa T, Tsuzuki T, Nakagawa K, et al. Antiangiogenic potency of vitamin E. Ann N Y Acad Sci. 2004;1031:401–4. doi: 10.1196/annals.1331.057. [DOI] [PubMed] [Google Scholar]
- 35.Qureshi AA, Sami SA, Salser WA, et al. Synergistic effect of tocotrienol-rich fraction (TRF(25)) of rice bran and lovastatin on lipid parameters in hypercholesterolemic humans. J Nutr Biochem. 2001;12:318–29. doi: 10.1016/s0955-2863(01)00144-9. [DOI] [PubMed] [Google Scholar]
- 36.Qureshi AA, Sami SA, Salser WA, et al. Dose-dependent suppression of serum cholesterol by tocotrienol-rich fraction (TRF25) of rice bran in hypercholesterolemic humans. Atherosclerosis. 2002;161:199–207. doi: 10.1016/s0021-9150(01)00619-0. [DOI] [PubMed] [Google Scholar]
- 37.Qureshi AA, Peterson DM, Hasler-Rapacz JO, et al. Novel tocotrienols of rice bran suppress cholesterogenesis in hereditary hypercholesterolemic swine. J Nutr. 2001;131:223–30. doi: 10.1093/jn/131.2.223. [DOI] [PubMed] [Google Scholar]
- 38.Kamal-Eldin A, Appelqvist LA. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids. 1996;31:671–701. doi: 10.1007/BF02522884. [DOI] [PubMed] [Google Scholar]
- 39.Pearce BC, Parker RA, Deason ME, et al. Inhibitors of cholesterol biosynthesis. 2. Hypocholesterolemic and antioxidant activities of benzopyran and tetrahydronaphthalene analogues of the tocotrienols. J Med Chem. 1994;37:526–41. doi: 10.1021/jm00030a012. [DOI] [PubMed] [Google Scholar]
- 40.Serbinova E, Kagan V, Han D, et al. Free radical recycling and intramembrane mobility in the antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Free Radic Biol Med. 1991;10:263–75. doi: 10.1016/0891-5849(91)90033-y. [DOI] [PubMed] [Google Scholar]
- 41.Serbinova EA, Packer L. Antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Methods Enzymol. 1994;234:354–66. doi: 10.1016/0076-6879(94)34105-2. [DOI] [PubMed] [Google Scholar]
- 42.Muller L, Theile K, Bohm V. In vitro antioxidant activity of tocopherols and tocotrienols and comparison of vitamin E concentration and lipophilic antioxidant capacity in human plasma. Mol Nutr Food Res. 2010;54:731–42. doi: 10.1002/mnfr.200900399. [DOI] [PubMed] [Google Scholar]
- 43.Khor HT, Ng TT. Effects of administration of alpha-tocopherol and tocotrienols on serum lipids and liver HMG CoA reductase activity. Int J Food Sci Nutr. 2000;(51 Suppl):S3–11. [PubMed] [Google Scholar]
- 44.Qureshi AA, Pearce BC, Nor RM, et al. Dietary alpha-tocopherol attenuates the impact of gamma-tocotrienol on hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in chickens. J Nutr. 1996;126:389–94. doi: 10.1093/jn/126.2.389. [DOI] [PubMed] [Google Scholar]
- 45.Parker RA, Pearce BC, Clark RW, et al. Tocotrienols regulate cholesterol production in mammalian cells by post-transcriptional suppression of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J Biol Chem. 1993;268:11230–8. [PubMed] [Google Scholar]
- 46.Song BL, DeBose-Boyd RA. Insig-dependent ubiquitination and degradation of 3-hydroxy-3-methylglutaryl coenzyme a reductase stimulated by delta- and gamma-tocotrienols. J Biol Chem. 2006;281:25054–61. doi: 10.1074/jbc.M605575200. [DOI] [PubMed] [Google Scholar]
- 47.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–30. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 48.Saito Y, Yoshida Y, Nishio K, et al. Characterization of cellular uptake and distribution of vitamin E. Ann N Y Acad Sci. 2004;1031:368–75. doi: 10.1196/annals.1331.047. [DOI] [PubMed] [Google Scholar]
- 49.Tsuzuki W, Yunoki R, Yoshimura H. Intestinal epithelial cells absorb gamma-tocotrienol faster than alpha-tocopherol. Lipids. 2007;42:163–70. doi: 10.1007/s11745-007-3021-0. [DOI] [PubMed] [Google Scholar]
- 50.Stone HB, Moulder JE, Coleman CN, et al. Models for evaluating agents intended for the prophylaxis, mitigation and treatment of radiation injuries. Report of an NCI Workshop, December 3–4, 2003. Radiat Res. 2004;162:711–28. doi: 10.1667/rr3276. [DOI] [PubMed] [Google Scholar]
- 51.Seed TM. Radiation protectants: current status and future prospects. Health Phys. 2005;89:531–45. doi: 10.1097/01.hp.0000175153.19745.25. [DOI] [PubMed] [Google Scholar]
- 52.Singh VK, Brown DS, Kao TC. Tocopherol succinate: a promising radiation countermeasure. Int Immunopharmacol. 2009;9:1423–30. doi: 10.1016/j.intimp.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 53.Berbee M, Hauer-Jensen M. Novel drugs to ameliorate gastrointestinal normal tissue radiation toxicity in clinical practice: what is emerging from the laboratory? Curr Opin Support Palliat Care. 2012;6:54–59. doi: 10.1097/SPC.0b013e32834e3bd7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kumar KS, Srinivasan V, Toles R, et al. Nutritional approaches to radioprotection: vitamin E. Mil Med. 2002;167:57–9. [PubMed] [Google Scholar]
- 55.Yuhas JM, Storer JB. Chemoprotection against three modes of radiation death in the mouse. Int J Radiat Biol Relat Stud Phys Chem Med. 1969;15:233–7. doi: 10.1080/09553006914550411. [DOI] [PubMed] [Google Scholar]
- 56.Brown DQ, Graham WJ, III, MacKenzie LJ, et al. Can WR-2721 be improved upon? Pharmacol Ther. 1988;39:157–68. doi: 10.1016/0163-7258(88)90057-5. [DOI] [PubMed] [Google Scholar]
- 57.Nightengale SL, Prasher JM, Simonson S. Emergency use authorization (EUA) to enable use of needed products in civilian and military emergencies, United States. Emerg Infect Dis. 2002;7:1046–55. doi: 10.3201/eid1307.061188. [DOI] [PubMed] [Google Scholar]
- 58.U.S. Food and Drug Administration. Guidance for Industry, Animal Models – Essential Elements to Address Efficacy under the Animal Rule. Silver Spring, MD: CBER; 2009. [Google Scholar]
- 59.Boerma M, Roberto KA, Hauer-Jensen M. Prevention and treatment of functional and structural radiation injury in the rat heart by pentoxifylline and alpha-tocopherol. Int J Radiat Oncol Biol Phys. 2008;72:170–7. doi: 10.1016/j.ijrobp.2008.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Empey LR, Papp JD, Jewell LD, et al. Mucosal protective effects of vitamin E and misoprostol during acute radiation-induced enteritis in rats. Dig Dis Sci. 1992;37:205–14. doi: 10.1007/BF01308173. [DOI] [PubMed] [Google Scholar]
- 61.Konings AW, Damen J, Trieling WB. Protection of liposomal lipids against radiation induced oxidative damage. Int J Radiat Biol Relat Stud Phys Chem Med. 1979;35:343–50. doi: 10.1080/09553007914550411. [DOI] [PubMed] [Google Scholar]
- 62.Malick MA, Roy RM, Sternberg J. Effect of vitamin E on post irradiation death in mice. Experientia. 1978;34:1216–7. doi: 10.1007/BF01922966. [DOI] [PubMed] [Google Scholar]
- 63.Roy RM, Petrella M, Shateri H. Effects of administering tocopherol after irradiation on survival and proliferation of murine lymphocytes. Pharmacol Ther. 1988;39:393–5. doi: 10.1016/0163-7258(88)90089-7. [DOI] [PubMed] [Google Scholar]
- 64.Sarma L, Kesavan PC. Protective effects of vitamins C and E against gamma-ray-induced chromosomal damage in mouse. Int J Radiat Biol. 1993;63:759–64. doi: 10.1080/09553009314552161. [DOI] [PubMed] [Google Scholar]
- 65.Srinivasan V, Jacobs AJ, Simpson SA, et al. Radioprotection by vitamin E: effects on hepatic enzymes, delayed type hypersensitivity and postirradiation survival of mice In: Meyskens FL Jr, Prasad KN, editors. . Modulation and Mediation of Cancer by Vitamins. Basel: Karger; 1983. pp. 119–31. [Google Scholar]
- 66.Srinivasan V, Weiss JF. Radioprotection by vitamin E: injectable vitamin E administered alone or with WR-3689 enhances survival of irradiated mice. Int J Radiat Oncol Biol Phys. 1992;23:841–5. doi: 10.1016/0360-3016(92)90657-4. [DOI] [PubMed] [Google Scholar]
- 67.Bichay TJ, Roy RM. Modification of survival and hematopoiesis in mice by tocopherol injection following irradiation. Strahlenther Onkol. 1986;162:391–9. [PubMed] [Google Scholar]
- 68.Seed T, Kumar S, Whitnall M, et al. New strategies for the prevention of radiation injury: possible implications for countering radiation hazards of long-term space travel. J Radiat Res (Tokyo) 2002;(43 Suppl):S239–44. doi: 10.1269/jrr.43.s239. [DOI] [PubMed] [Google Scholar]
- 69.Singh VK, Shafran RL, Jackson WE, III, et al. Induction of cytokines by radioprotective tocopherol analogs. Exp Mol Pathol. 2006;81:55–61. doi: 10.1016/j.yexmp.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 70.Murase H, Yamauchi R, Kato K, et al. Synthesis of a novel vitamin E derivative, 2-(alpha-D-glucopyranosyl) methyl-2,5,7,8-tetramethylchroman-6-ol, by alpha-glucosidase-catalyzed transglycosylation. Lipids. 1997;32:73–8. doi: 10.1007/s11745-997-0011-6. [DOI] [PubMed] [Google Scholar]
- 71.Murase H, Moon JH, Yamauchi R, et al. Antioxidant activity of a novel vitamin E derivative, 2-(alpha-D glucopyranosyl)methyl-2,5,7,8-tetramethylchroman-6-ol. Free Radic Biol Med. 1998;24:217–25. doi: 10.1016/s0891-5849(97)00221-9. [DOI] [PubMed] [Google Scholar]
- 72.Nair CK, Devi PU, Shimanskaya R, et al. Water soluble vitamin E (TMG) as a radioprotector. Indian J Exp Biol. 2003;41:1365–71. [PubMed] [Google Scholar]
- 73.Satyamitra M, Devi PU, Murase H, et al. In vivo radioprotection by alpha-TMG: preliminary studies. Mutat Res. 2001;479:53–61. doi: 10.1016/s0027-5107(01)00135-x. [DOI] [PubMed] [Google Scholar]
- 74.Satyamitra M, Uma Devi P, Murase H, et al. In vivo postirradiation protection by a vitamin E analog, alpha-TMG. Radiat Res. 2003;160:655–61. doi: 10.1667/rr3077. [DOI] [PubMed] [Google Scholar]
- 75.Cherdyntseva N, Shishkina A, Butorin I, et al. Effect of tocopherol-monoglucoside (TMG), a water-soluble glycosylated derivate of vitamin E, on hematopoietic recovery in irradiated mice. J Radiat Res (Tokyo) 2005;46:37–41. doi: 10.1269/jrr.46.37. [DOI] [PubMed] [Google Scholar]
- 76.Ueno M, Inano H, Onoda M, et al. Modification of mortality and tumorigenesis by tocopherol-mono-glucoside (TMG) administered after X irradiation in mice and rats. Radiat Res. 2009;172:519–24. doi: 10.1667/RR1695.1. [DOI] [PubMed] [Google Scholar]
- 77.Rajagopalan R, Wani K, Huilgol NG, et al. Inhibition of gamma-radiation induced DNA damage in plasmid pBR322 by TMG, a water-soluble derivative of vitamin E. J Radiat Res (Tokyo) 2002;43:153–9. doi: 10.1269/jrr.43.153. [DOI] [PubMed] [Google Scholar]
- 78.Kapoor S, Mukherjee T, Kagiya TV, et al. Redox reactions of tocopherol monoglucoside in aqueous solutions: a pulse radiolysis study. J Radiat Res (Tokyo) 2002;43:99–106. doi: 10.1269/jrr.43.99. [DOI] [PubMed] [Google Scholar]
- 79.Nair CK, Salvi V, Kagiya TV, et al. Relevance of radioprotectors in radiotherapy: studies with tocopherol monoglucoside. J Environ Pathol Toxicol Oncol. 2004;23:153–60. doi: 10.1615/jenvpathtoxoncol.v23.i2.80. [DOI] [PubMed] [Google Scholar]
- 80.Kumar B, Jha MN, Cole WC, et al. D-alpha-tocopheryl succinate (vitamin E) enhances radiation-induced chromosomal damage levels in human cancer cells, but reduces it in normal cells. J Am Coll Nutr. 2002;21:339–43. doi: 10.1080/07315724.2002.10719232. [DOI] [PubMed] [Google Scholar]
- 81.Neuzil J, Svensson I, Weber T, et al. Alpha-tocopheryl succinate-induced apoptosis in Jurkat T cells involves caspase-3 activation, and both lysosomal and mitochondrial destabilisation. FEBS Lett. 1999;445:295–300. doi: 10.1016/s0014-5793(99)00141-6. [DOI] [PubMed] [Google Scholar]
- 82.Neuzil J, Weber T, Schroder A, et al. Induction of cancer cell apoptosis by alpha-tocopheryl succinate: molecular pathways and structural requirements. FASEB J. 2001;15:403–15. doi: 10.1096/fj.00-0251com. [DOI] [PubMed] [Google Scholar]
- 83.Neuzil J, Zhao M, Ostermann G, et al. Alpha-tocopheryl succinate, an agent with in vivo anti-tumour activity, induces apoptosis by causing lysosomal instability. Biochem J. 2002;362:709–15. doi: 10.1042/0264-6021:3620709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Neuzil J. Vitamin E succinate and cancer treatment: a vitamin E prototype for selective antitumour activity. Br J Cancer. 2003;89:1822–6. doi: 10.1038/sj.bjc.6601360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kang YH, Lee E, Choi MK, et al. Role of reactive oxygen species in the induction of apoptosis by alpha-tocopheryl succinate. Int J Cancer. 2004;112:385–92. doi: 10.1002/ijc.20424. [DOI] [PubMed] [Google Scholar]
- 86.Prasad KN, Kumar B, Yan XD, et al. Alpha-tocopheryl succinate, the most effective form of vitamin E for adjuvant cancer treatment: a review. J Am Coll Nutr. 2003;22:108–17. doi: 10.1080/07315724.2003.10719283. [DOI] [PubMed] [Google Scholar]
- 87.Shanker M, Gopalan B, Patel S, et al. Vitamin E succinate in combination with mda-7 results in enhanced human ovarian tumor cell killing through modulation of extrinsic and intrinsic apoptotic pathways. Cancer Lett. 2007;254:217–26. doi: 10.1016/j.canlet.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 88.Wang XF, Dong L, Zhao Y, et al. Vitamin E analogues as anticancer agents: lessons from studies with alpha-tocopheryl succinate. Mol Nutr Food Res. 2006;50:675–85. doi: 10.1002/mnfr.200500267. [DOI] [PubMed] [Google Scholar]
- 89.Singh VK, Parekh VI, Brown DS, et al. Tocopherol succinate: modulation of antioxidant enzymes and oncogene expression, and hematopoietic recovery. Int J Radiat Oncol Biol Phys. 2011;79:571–8. doi: 10.1016/j.ijrobp.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 90.Singh VK, Singh PK, Wise SY, et al. Radioprotective properties of tocopherol succinate against ionizing radiation in mice. J Radiat Res. 2013;54:210–20. doi: 10.1093/jrr/rrs088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Singh PK, Wise SY, Ducey EJ, et al. Radioprotective efficacy of tocopherol succinate is mediated through granulocyte-colony stimulating factor. Cytokine. 2011;56:411–21. doi: 10.1016/j.cyto.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 92.Singh VK, Brown DS, Kao TC. Alpha-tocopherol succinate protects mice from gamma-radiation by induction of granulocyte-colony stimulating factor. Int J Radiat Biol. 2010;86:12–21. doi: 10.3109/09553000903264515. [DOI] [PubMed] [Google Scholar]
- 93.Patchen ML, MacVittie TJ, Solberg BD, et al. Survival enhancement and hemopoietic regeneration following radiation exposure: therapeutic approach using glucan and granulocyte colony-stimulating factor. Exp Hematol. 1990;18:1042–8. [PubMed] [Google Scholar]
- 94.MacVittie TJ, Monroy RL, Patchen ML, et al. Therapeutic use of recombinant human G-CSF (rhG-CSF) in a canine model of sublethal and lethal whole-body irradiation. Int J Radiat Biol. 1990;57:723–36. doi: 10.1080/09553009014550891. [DOI] [PubMed] [Google Scholar]
- 95.Tanikawa S, Nose M, Aoki Y, et al. Effects of recombinant human granulocyte colony-stimulating factor on the hematologic recovery and survival of irradiated mice. Blood. 1990;76:445–9. [PubMed] [Google Scholar]
- 96.Schuening FG, Storb R, Goehle S, et al. Effect of recombinant human granulocyte colony-stimulating factor on hematopoiesis of normal dogs and on hematopoietic recovery after otherwise lethal total body irradiation. Blood. 1989;74:1308–13. [PubMed] [Google Scholar]
- 97.Farese AM, Cohen MV, Katz BP, et al. Filgrastim improves survival in lethally irradiated nonhuman primates. Radiat Res. 2013;179:89–100. doi: 10.1667/RR3049.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gabrilove JL, Jakubowski A, Scher H, et al. Effect of granulocyte colony-stimulating factor on neutropenia and associated morbidity due to chemotherapy for transitional-cell carcinoma of the urothelium. N Engl J Med. 1988;318:1414–22. doi: 10.1056/NEJM198806023182202. [DOI] [PubMed] [Google Scholar]
- 99.Singh VK, Singh PK, Wise SY, et al. Mobilized progenitor cells as a bridging therapy for radiation casualties: a brief review of tocopherol succinate-based approaches. Int Immunopharmacol. 2011;11:842–47. doi: 10.1016/j.intimp.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 100.Singh VK, Brown DS, Kao TC, et al. Preclinical development of a bridging therapy for radiation casualties. Exp Hematol. 2010;38:61–70. doi: 10.1016/j.exphem.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 101.Singh VK, Wise SY, Singh PK, et al. Alpha-tocopherol succinate- and AMD3100-mobilized progenitors mitigate radiation-induced gastrointestinal injury in mice. Exp Hematol. 2012;40:407–17. doi: 10.1016/j.exphem.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 102.Singh VK, Wise SY, Singh PK, et al. Alpha-tocopherol succinate-mobilized progenitors improve intestinal integrity after whole body irradiation. Int J Radiat Biol. 2013 doi: 10.3109/09553002.2013.762137. doi:10.3109/09553002.2013.762137, Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 103.Sen CK, Khanna S, Rink C, et al. Tocotrienols: the emerging face of natural vitamin E. Vitam Horm. 2007;76:203–61. doi: 10.1016/S0083-6729(07)76008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sen CK, Khanna S, Roy S. Tocotrienol: the natural vitamin E to defend the nervous system? Ann N Y Acad Sci. 2004;1031:127–42. doi: 10.1196/annals.1331.013. [DOI] [PubMed] [Google Scholar]
- 105.Satyamitra M, Ney P, Graves J, III, et al. Mechanism of radioprotection by delta-tocotrienol: pharmacokinetics, pharmacodynamics and modulation of signalling pathways. Br J Radiol. 2012;85:e1093–103. doi: 10.1259/bjr/63355844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Qureshi AA, Burger WC, Peterson DM, et al. The structure of an inhibitor of cholesterol biosynthesis isolated from barley. J Biol Chem. 1986;261:10544–50. [PubMed] [Google Scholar]
- 107.Baliarsingh S, Beg ZH, Ahmad J. The therapeutic impacts of tocotrienols in type 2 diabetic patients with hyperlipidemia. Atherosclerosis. 2005;182:367–74. doi: 10.1016/j.atherosclerosis.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 108.Kulkarni S, Ghosh SP, Satyamitra M, et al. Gamma-tocotrienol protects hematopoietic stem and progenitor cells in mice after total-body irradiation. Radiat Res. 2010;173:738–47. doi: 10.1667/RR1824.1. [DOI] [PubMed] [Google Scholar]
- 109.Kulkarni SS, Cary LH, Gambles K, et al. Gamma-tocotrienol, a radiation prophylaxis agent, induces high levels of granulocyte colony-stimulating factor. Int Immunopharmacol. 2012;14:495–503. doi: 10.1016/j.intimp.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 110.Grace MB, Singh VK, Rhee JG, et al. 5-AED enhances survival of irradiated mice in a G-CSF-dependent manner, stimulates innate immune cell function, reduces radiation-induced DNA damage and induces genes that modulate cell cycle progression and apoptosis. J Radiat Res. 2012;53:840–53. doi: 10.1093/jrr/rrs060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Krivokrysenko VI, Shakhov AN, Singh VK, et al. Identification of granulocyte colony-stimulating factor and interleukin-6 as candidate biomarkers of CBLB502 efficacy as a medical radiation countermeasure. J Pharmacol Exp Ther. 2012;343:497–508. doi: 10.1124/jpet.112.196071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Singh VK, Fatanmi OO, Singh PK, et al. Role of radiation-induced granulocyte colony-stimulating factor in recovery from whole body gamma-irradiation. Cytokine. 2012;58:406–14. doi: 10.1016/j.cyto.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 113.Kulkarni S, Singh PK, Ghosh S, et al. Granulocyte-colony stimulating factor antibody abrogates radioprotective efficacy of gamma-tocotrienol, a promising radiation countermeasure. Cytokine. 2013 doi: 10.1016/j.cyto.2013.03.009. doi:10.1016/j.cyto.2013.03.009, Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 114.Ray S, Kulkarni SS, Chakraborty K, et al. Mobilization of progenitor cells into peripheral blood by gamma-tocotrienol: a promising radiation countermeasure. Int Immunopharmacol. 2013;15:557–64. doi: 10.1016/j.intimp.2012.12.034. [DOI] [PubMed] [Google Scholar]
- 115.Berbee M, Fu Q, Boerma M, et al. Gamma-tocotrienol ameliorates intestinal radiation injury and reduces vascular oxidative stress after total-body irradiation by an HMG-CoA reductase-dependent mechanism. Radiat Res. 2009;171:596–605. doi: 10.1667/RR1632.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Berbee M, Fu Q, Boerma M, et al. Reduction of radiation-induced vascular nitrosative stress by the vitamin E analog gamma-tocotrienol: evidence of a role for tetrahydrobiopterin. Int J Radiat Oncol Biol Phys. 2011;79:884–91. doi: 10.1016/j.ijrobp.2010.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Delanian S, Porcher R, Balla-Mekias S, et al. Randomized, placebo-controlled trial of combined pentoxifylline and tocopherol for regression of superficial radiation-induced fibrosis. J Clin Oncol. 2003;21:2545–50. doi: 10.1200/JCO.2003.06.064. [DOI] [PubMed] [Google Scholar]
- 118.Delanian S, Porcher R, Rudant J, et al. Kinetics of response to long-term treatment combining pentoxifylline and tocopherol in patients with superficial radiation-induced fibrosis. J Clin Oncol. 2005;23:8570–9. doi: 10.1200/JCO.2005.02.4729. [DOI] [PubMed] [Google Scholar]
- 119.Hille A, Christiansen H, Pradier O, et al. Effect of pentoxifylline and tocopherol on radiation proctitis/enteritis. Strahlenther Onkol. 2005;181:606–14. doi: 10.1007/s00066-005-1390-y. [DOI] [PubMed] [Google Scholar]
- 120.Misirlioglu CH, Demirkasimoglu T, Kucukplakci B, et al. Pentoxifylline and alpha-tocopherol in prevention of radiation-induced lung toxicity in patients with lung cancer. Med Oncol. 2007;24:308–11. doi: 10.1007/s12032-007-0006-z. [DOI] [PubMed] [Google Scholar]
- 121.Berbee M, Fu Q, Garg S, et al. Pentoxifylline enhances the radioprotective properties of gamma-tocotrienol: differential effects on the hematopoietic, gastrointestinal and vascular systems. Radiat Res. 2011;175:297–306. doi: 10.1667/RR2399.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bacq ZM. The amines and particularly cysteamine as protectors against roentgen rays. Acta Radiol. 1954;41:47–55. doi: 10.3109/00016925409175832. [DOI] [PubMed] [Google Scholar]
- 123.Citrin D, Cotrim AP, Hyodo F, et al. Radioprotectors and mitigators of radiation-induced normal tissue injury. Oncologist. 2010;15:360–71. doi: 10.1634/theoncologist.2009-S104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Landauer MR, Davis HD, Kumar KS, et al. Behavioral toxicity of selected radioprotectors. Adv Space Res. 1992;12:273–83. doi: 10.1016/0273-1177(92)90117-g. [DOI] [PubMed] [Google Scholar]
- 125.Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lonn E, Bosch J, Yusuf S, et al. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA. 2005;293:1338–47. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]