Abstract
The use of radiation therapy has been linked to an increased risk of cardiovascular disease. To understand the mechanisms underlying radiation-induced vascular dysfunction, we employed two models. First, we examined the effect of X-ray irradiation on vasodilation in rabbit carotid arteries. Carotid arterial rings were irradiated with 8 or 16 Gy using in vivo and ex vivo methods. We measured the effect of acetylcholine-induced relaxation after phenylephrine-induced contraction on the rings. In irradiated carotid arteries, vasodilation was significantly attenuated by both irradiation methods. The relaxation response was completely blocked by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one, a potent inhibitor of soluble guanylate cyclase. Residual relaxation persisted after treatment with L-Nω-nitroarginine (L-NA), a non-specific inhibitor of nitric oxide synthase (NOS), but disappeared following the addition of aminoguanidine (AG), a selective inhibitor of inducible NOS (iNOS). The relaxation response was also affected by tetraethylammonium, an inhibitor of endothelium-derived hyperpolarizing factor activity. In the second model, we investigated the biochemical events of nitrosative stress in human umbilical-vein endothelial cells (HUVECs). We measured iNOS and nitrotyrosine expression in HUVECs exposed to a dose of 4 Gy. The expression of iNOS and nitrotyrosine was greater in irradiated HUVECs than in untreated controls. Pretreatment with AG, L-N6-(1-iminoethyl) lysine hydrochloride (a selective inhibitor of iNOS), and L-NA attenuated nitrosative stress. While a selective target of radiation-induced vascular endothelial damage was not definitely determined, these results suggest that NO generated from iNOS could contribute to vasorelaxation. These studies highlight a potential role of iNOS inhibitors in ameliorating radiation-induced vascular endothelial damage.
Keywords: vascular endothelium, radiation injuries, inducible nitric oxide synthase, nitrotyrosine
INTRODUCTION
The vascular endothelium mediates vasodilation via nitric oxide (NO), prostacyclin, and endothelium-derived hyperpolarizing factor (EDHF) [1–3]. In vascular smooth-muscle cells, NO stimulates soluble guanylate cyclase (sGC) to increase the levels of cyclic guanosine monophosphate, leading to vasodilation [4, 5]. In addition to controlling vascular tone, NO and prostacyclin prevent vascular occlusion and ischemia by inhibiting platelet aggregation.
NO is synthesized from three different isoforms of the nitric oxide synthase (NOS): neuronal NOS (nNOS, NOS1), endothelial NOS (eNOS, NOS3), and inducible NOS (iNOS, NOS2) [5, 6]. The constitutive isoforms, nNOS and eNOS, are calcium-dependent. NO generated by these isoforms is responsible for neurotransmission and vascular regulation [7, 8]. In contrast to the constitutive isoforms, iNOS is calcium-independent and activated by external stimuli such as inflammatory cytokines and oxidative stress [9, 10]. The calcium-independent isoform generates a larger amount of NO over longer periods compared to the constitutive NOS isoforms [8]. Ionizing radiation increases the risk of cardiovascular disease prevalence [11, 12]. Loss of NO-dependent relaxation and decrease of eNOS activity have been suggested as underlying mechanisms of radiation-induced vascular dysfunction [13–16]. Several reports have shown radiation-induced iNOS activation in various tissues, including the vascular endothelium [17–19]. Once iNOS is activated, the NO it produces then reacts with the superoxide anion, resulting in an increase in peroxynitrite, a potent oxidizing agent [20, 21].
Aminoguanidine (AG) selectively inhibits iNOS. AG has been shown to reduce radiation-induced nitrosative stress [22–24]. Although increased iNOS expression has been demonstrated in vascular endothelial cells, the consequent increase of peroxynitrite production and inhibitory effects of AG on this pathway in vascular endothelium have not yet been studied. In the current study, we examined (i) the appearance of impaired vasodilation after exposure to megavoltage X-ray irradiation in rabbit carotid arteries, (ii) the production of iNOS and peroxynitrite in human umbilical-vein endothelial cells (HUVECs), and (iii) the effect of AG and other inhibitors on these responses.
MATERIALS AND METHODS
Agents
Phenylephrine (PE), acetylcholine (ACh), L-Nω-nitroarginine (L-NA), 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), AG, L-N6-(1-iminoethyl)lysine hydrochloride (L-nil), and tetraethylammonium (TEA) were purchased from Sigma-Aldrich (St Louis, MO, USA).
Preparation of carotid arterial rings
Animal experiments were approved by the Institutional Animal Care and Use Committee of the Armed Forces Medical Research Institute (AFMRI-10-IACUC-01). Both common carotid arteries were obtained from male New Zealand white rabbits purchased from Samtako Bio Korea (Osan, Republic of Korea). The rabbits were euthanized by exsanguinations after anesthetization with inhaled dimethyl ether. The carotid arteries were placed in cold physiological salt solution (normal Tyrode solution: 135 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, and 5 mM glucose) adjusted to pH 7.4.
Two irradiation methods were used for the carotid arteries. The first was whole-body irradiation followed by carotid artery excision (in vivo method). A source axis distance technique with opposing anterior–posterior fields was used. A dose of 8 Gy or 16 Gy at a rate of 4.1 Gy/min was administered at mid-depth of the rabbits in prone position. Intramuscular injection of acepromazine (1 mg/kg) was administered for sedation before irradiation. The rabbits were sacrificed 20 h after irradiation. The second method was irradiation of the excised carotid artery (ex vivo method). A source surface distance technique was used. The prescribed dose was either 8 Gy or 16 Gy at a rate of 3.9 Gy/min and the minimum set-up margin was 2 cm in all directions. The dose selection and study times were based on previous studies [14, 16, 25]. The lower dose of 8 Gy was selected because it is between the dose prescribed by Soloviev et al. (6 Gy) and the dose suggested to be lethal in 50% of animals by Gratwohl et al. (10–12 Gy). A higher dose of 16 Gy was selected to enhance radiation-induced vascular dysfunction. A linear accelerator (LINAC, Varian Medical System, Palo Alto, CA, USA) producing 6-MV X-rays was used. Irradiated arteries were cut into rings of 2-mm thickness and placed into 20-ml organ baths filled with normal Tyrode solution. The organ baths were maintained at 37°C and equilibrated with a gas mixture of 95% O2 and 5% CO2. Arterial rings were suspended by a pair of stainless steel hooks under a resting tension of 2 g.
Measurement of tension
A computerized automated isometric transducer system (LabChart and Scope v6; AD Instruments, Heidelberg, Germany) was used for recording the tension of the rings. After an equilibration period of at least 60 min, high K+ solution (the same composition as normal Tyrode solution, but with 70 mM NaCl and 70 mM KCl) was administered two or three times to achieve reproducible contractile responses. Contraction was induced by 10 µM of PE, followed by relaxation by 10 µM of ACh. The ACh-induced relaxation was compared in healthy and irradiated vessels. The measurement of tension on arterial rings was conducted as described previously [26]. To investigate the underlying mechanisms of the impaired vasodilation, 100 µM of L-NA, 10 µM of ODQ, 100 µM of AG, or 1 mM of TEA were added to the organ baths and incubated for at least 20 min. Changes in relaxant responses to ACh were examined as above.
Cell treatment and Western blot analysis
HUVECs were acquired from Lonza (Allendale, NJ, USA) and cultured in endothelial cell growth medium (EGM®, Lonza). The cells were cultured in 25 cm2 flasks at 37°C under 5% CO2 in air. Cells from the second subculture were used for the experiment. The cells were serum-starved using endothelial basal medium (EBM®) 3 h before irradiation, treated with 1 mM AG, L-nil, or L-NA 1 h before irradiation, then irradiated with a dose of 4 Gy at 2.7 Gy/min using a LINAC machine producing 4-MV X-rays.
At 1.5, 3 or 6 h after irradiation, cells were lysed using RIPA buffer containing protease inhibitor cocktail at 4°C for 30 min. The protein lysates were fractionated on 8% sodium dodecyl sulfate polyacrylamide gels and transferred to polyvinylidine difluoride filter membranes. The membranes were blocked for 1 h with 5% skim milk prepared in a mixture of Tris-buffered saline and 0.5% Tween-20 (TBS-T). Membranes were incubated overnight at 4°C with a primary antibody against iNOS (sc-651), eNOS (sc-653), or nitrotyrosine (sc-55256), which was diluted as recommended by the manufacturer (Santa Cruz Biotechnology, Inc., CA, USA). After washing with TBS-T, incubation with goat anti-rabbit antibody (sc-2054) followed. Blots were visualized by chemiluminescence and quantified by densitometry.
Statistical analysis
Data from the experiments are shown as mean ± SEM. The number of preparations was indicated by n. The Wilcoxon signed-rank test was used to determine statistical significance in the relaxation responses of arterial rings, as well as in the expression of iNOS and nitrotyrosine in HUVECs. SAS software (SAS 9.1.3; SAS Institute Inc., Cary, NC, USA) was used for statistical analysis. P values less than 0.05 were considered statistically significant.
RESULTS
Effect of irradiation on vascular responsiveness
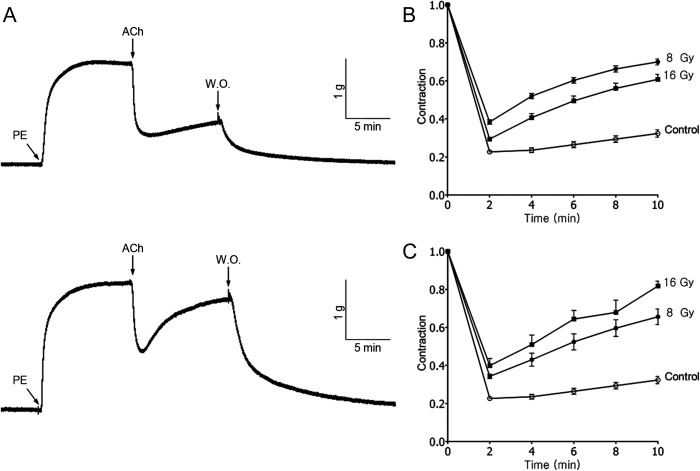
To examine the effects of irradiation on vascular responsiveness, irradiated and untreated carotid arteries were contracted by PE (10 µM) and then relaxed by ACh (10 µM). Figure 1A shows representative records of vascular responsiveness in non-irradiated (upper) and irradiated (8 Gy, lower) carotid artery. ACh-induced relaxation was converted to percentage of PE-induced contraction. ACh produced a maximal relaxation of 77.4 ± 1.1% (n = 46) in non-irradiated carotid artery (Fig. 1B). When irradiated by in vivo methods, vascular responsiveness of the carotid artery decreased to 61.6 ± 1.2% (n = 24, P < 0.0001) and 70.6 ± 1.1% (n = 26, P = 0.0001) following exposure to 8 Gy and 16 Gy, respectively (Fig. 1B). By ex vivo methods, vascular responsiveness decreased to 65.7 ± 1.2% (n = 24, P < 0.0001) and 60.1 ± 3.8% (n = 16, P < 0.0001) after 8 Gy and 16 Gy of irradiation, respectively (Fig. 1C). There was a dose-dependent response relationship in the carotid arteries irradiated by the ex vivo method, whereas the in vivo method showed some discrepancy. These results clearly show that irradiation impairs the ACh-induced vasodilation of carotid arteries.
Fig. 1.
Effects of 6-MV X-irradiation on ACh (10 µM)-induced vasorelaxation after contraction evoked by PE (10 µM). (A) Original recording of relaxation of non-irradiated (upper) and irradiated (8 Gy, lower) carotid arterial rings of rabbit. The effect of (B) in vivo and (C) ex vivo irradiation on relaxation response. Each point represents the mean ± SEM. Relaxation responses were measured every 2 min after administration of ACh for 10 min.
The underlying mechanisms of radiation-induced impaired vasodilation
To investigate the underlying mechanisms of radiation-induced impaired vasodilation, we examined the effects of L-NA (a non-specific inhibitor of NOS), ODQ (a potent inhibitor of sGC), AG (a selective inhibitor of iNOS), TEA (a potassium channel blocker), and the combined application of L-NA and AG on carotid artery relaxation after exposure to radiation.
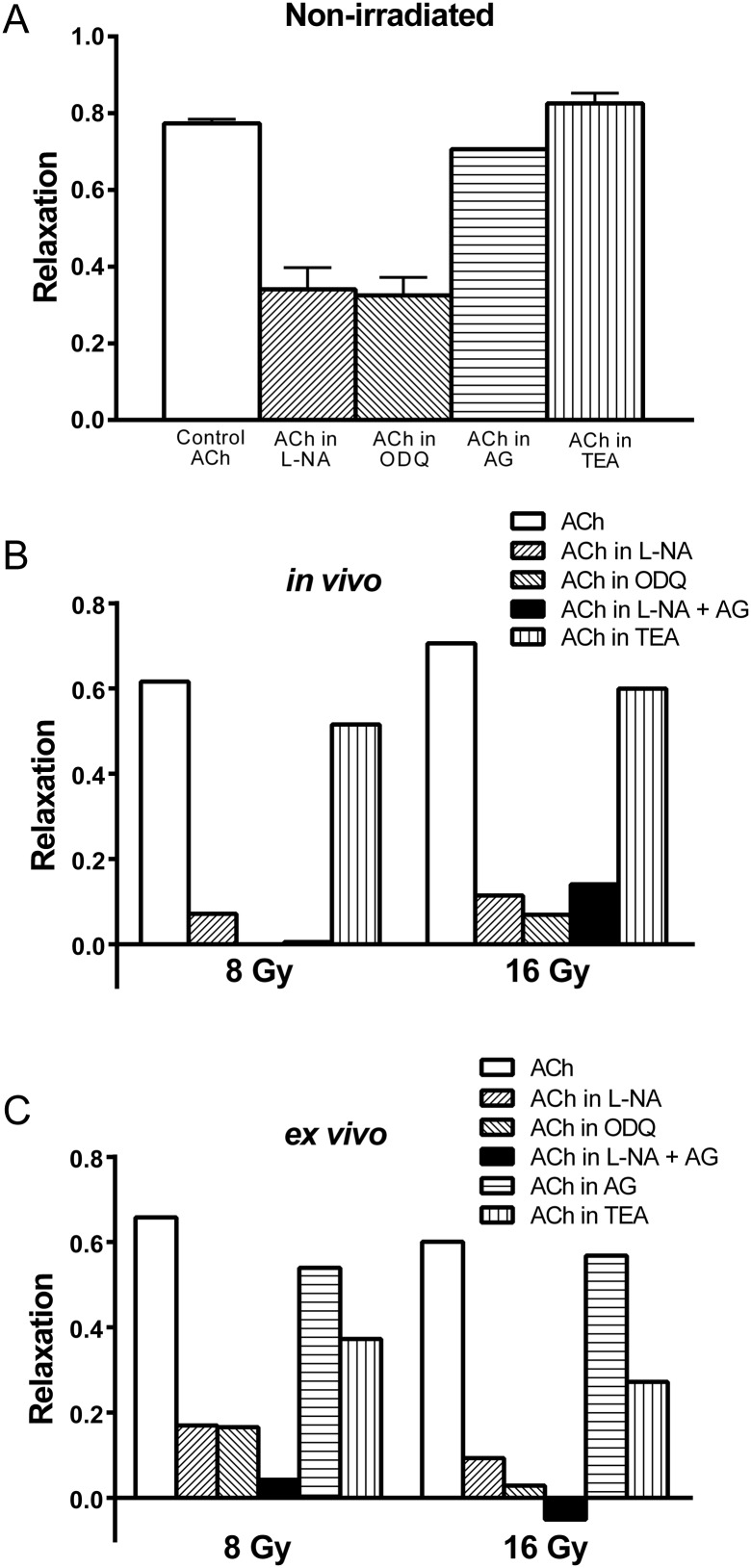
In the non-irradiated carotid artery, treatment with L-NA or ODQ similarly decreased maximum relaxation to 34.1 ± 5.6% (n = 11, P < 0.0001) and 32.5 ± 4.7% (n = 14, P < 0.0001), respectively (Fig. 2A). Neither AG nor TEA altered the responses (P = 0.1624 and 0.2240, respectively). In the irradiated carotid artery, ODQ completely abolished the relaxation response in the 8 Gy in vivo and 16 Gy ex vivo groups (Fig. 2B and C). This observation was not seen in the irradiated carotid artery treated with L-NA. The differences in the responses between L-NA and ODQ were significant by the Wilcoxon signed-rank test (P = 0.0024). However, when AG treatment was used in combination with L-NA, the relaxation response of the irradiated carotid artery was similar to that seen with ODQ alone. The difference in relaxation response between ODQ-treated and AG + L-NA-treated arteries was not significant (P = 0.6523). AG alone did not alter the relaxation response significantly.
Fig. 2.
Maximum relaxation responses to Ach (10 µM) in the carotid artery contracted by PE (10 µM) observed 20 h after irradiation. (A) Effect of L-NA (100 µM), ODQ (10 µM), AG (100 µM), and TEA (1 mM) on non-irradiated arterial rings. L-NA and ODQ similarly decreased maximum relaxation responses. Effects of the drugs on arterial rings irradiated by (B) the in vivo method and (C) the ex vivo method.
In the non-irradiated carotid artery, TEA did not affect ACh-induced relaxation; the maximal relaxation was 82.6 ± 2.7% (n = 4). Interestingly, TEA attenuated the relaxation response in the irradiated carotid artery (P = 0.0024). EDHF, which also mediates endothelium-dependent relaxation, involves the opening of K+ conductance that can be abolished by potassium-channel blockers such as TEA [3]. Therefore, these results suggest that exclusive selectivity between the NO and EDHF pathways could not be determined in terms of radiation-induced vascular endothelial damage. In addition, the difference in the relaxation responses between L-NA and ODQ, which was diminished by the addition of AG, suggests that NO generated from iNOS might contribute to vasorelaxation in irradiated arteries.
Induction of iNOS and nitrotyrosine by irradiation in HUVECs
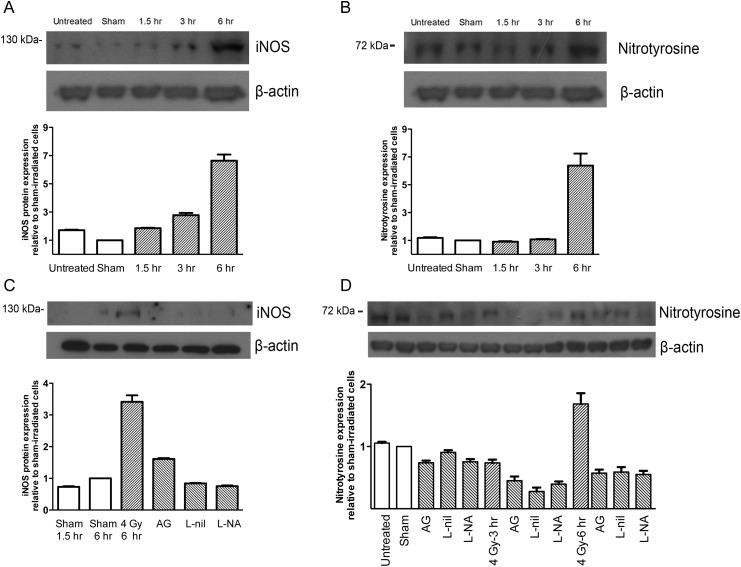
To investigate the relationship between iNOS and radiation-induced vascular dysfunction, we examined whether radiation induced the expression of iNOS, eNOS, or nitrotyrosine (a biomarker for peroxynitrite) in HUVECs. HUVECs were exposed to 4 Gy X-ray irradiation and the expression levels of iNOS, eNOS, and nitrotyrosine were examined over time. As seen in Fig. 3A, radiation increased the expression of iNOS in a time-dependent manner, peaking at 6 h. The density of the band reflecting the iNOS protein level was 6.64 ± 0.44-fold greater (n = 8, P = 0.0078) relative to the sham-irradiated control. In contrast, the radiation did not increase the expression of eNOS (data not shown). As seen in Fig. 3B, radiation similarly increased the expression of nitrotyrosine by 6.38 ± 0.86-fold (n = 8, P = 0.0078) at 6 h after irradiation. Pretreatment with an iNOS selective inhibitor (AG or L-nil) or a non-specific NOS inhibitor (L-NA) decreased iNOS expression at 6 h after irradiation (Fig. 3C). Nitrotyrosine expression was also attenuated by pretreatment with these inhibitors (Fig. 3D). These results indicate that radiation induces the expression of iNOS and nitrotyrosine, but not eNOS, in HUVECs. Further, the increase of iNOS and nitrotyrosine expression by radiation could be suppressed by iNOS inhibition.
Fig. 3.
The effect of irradiation on iNOS and nitrotyrosine expression. HUVECs were exposed to 4 Gy of 6-MV X-ray irradiation. The expression levels of (A) iNOS protein, and (B) nitrotyrosine, were measured by Western blot. Similarly, the expression of (C) iNOS, and (D) nitrotyrosine, was measured after a 1-h pretreatment with inhibitors. Values in the graph represent the relative densities compared to sham-irradiated control cells. Representative bands are shown on top. Untreated cells were kept in the incubator.
DISCUSSION
The most distinctive feature of radiation-induced vascular endothelial damage is impairment of NO-mediated vasodilation [13–16]. In the present study, we examined the mechanism of radiation-induced vasodilation impairment in rabbit carotid arterial rings. In the experiments measuring vascular relaxation responses, we used two irradiation methods and two prescription doses. Although ex vivo irradiation could not fully reflect the process of radiation-induced damage, a dose response of impaired vasodilation was shown in ex vivo-irradiated rings. Our results suggest that both in vivo and ex vivo irradiation could be helpful in understanding radiation-induced vascular damage.
The main differences between our study and those of others were in the time interval and the type of radiation. First, we investigated the relaxation responses at an earlier time-point compared with previous reports. Maximum relaxation responses to ACh (10 µM) in irradiated arteries were >50%, which were greater than those observed after a longer time interval [16]. This result clearly shows that differences in radiation-induced impairment of vasodilation can be observed at earlier time-points. In addition to the time interval, we used 4 or 6 MV of X-ray irradiation generated from LINAC at higher dose rates, whereas previous studies used a cobalt-60 source delivering radiation at lower dose rates [13–16]. Considering the use of higher dose rates of X-rays in medical practices, our study design might better represent therapeutic or accidental exposure to radiation in clinical settings.
Interestingly, our results clearly showed the inhibitory effects of L-NA on vasodilation in irradiated rings. The addition of AG to L-NA treatment completely inhibited the remaining residual vasodilation. However, in contrast to our findings, Soloviev et al. reported that L-NA did not affect the responses in irradiated vessels [16]. This discrepancy may be due to the use of a different radiation source or time of measurement. Another possible explanation could be the non-specific activity of L-NA [27, 28]. We found an inhibitory effect of ODQ on relaxation responses in irradiated arteries, suggesting the existence of iNOS activity, even in the presence of L-NA. In addition, the relaxation responses were partially affected by TEA, an inhibitor of EDHF. Therefore, exclusive selectivity between the NO and EDHF pathways could not be determined. We also showed increased expression of iNOS in irradiated HUVECs. These data are in accordance with a previous study that showed radiation-induced iNOS expression in bovine aortic endothelial cells [19]. Induction of iNOS subsequently leads to the continuous generation of NO [19]. The production of NO contributes to vasorelaxation and endothelial dysfunction through formation of peroxynitrite, a potent reactive oxygen radical [29, 30]. In the current study, we measured nitrotyrosine levels as an indicator of peroxynitrite. Radiation increased the level of nitrotyrosine in HUVECs, suggesting increased peroxynitrite. However, there is no direct evidence that formation of nitrotyrosine is a specific biomarker of peroxynitrite production [31]. Furthermore, other reactive nitrogen species may be involved.
Radiotherapy (RT) has been shown to increase the risk of cardiovascular disease [32–35]. Carotid artery stenosis is increased in patients who have received neck irradiation for head-and-neck cancer [32, 33]. Increased risk of ischemic heart disease and cardiac death has also been observed following RT for left-sided breast cancer [34, 35]. Inhibitors of iNOS or NOS significantly reduced expression of iNOS and nitrotyrosine, in agreement with previous studies [23]. Because the inhibition of iNOS has a protective effect on normal tissues such as the salivary gland [23], further study is required to clarify the role of iNOS inhibition in radiation-induced cardiovascular disease.
One limitation of the present study is the inconsistency of relaxation responses in the presence of a range of agents in a variety of experimental settings (Fig. 2). We believe that standardized experimental settings could eliminate this limitation. We observed a modest, yet significant inhibitory effect of AG on vasorelaxation. Although the amount of time post-irradiation (20 h) was relatively shorter than that observed in previous studies, it was still later than the time peak of iNOS expression (6 h), which is indicative of NO generation. This time-gap might explain the impaired vascular relaxation in irradiated arteries despite the increased expression of iNOS, and the potential for another mechanism of impairment exists.
CONCLUSION
In conclusion, we showed radiation-induced impairment of vasodilation in rabbit carotid arteries in both in vivo and ex vivo models. In addition, we observed radiation-induced expression of iNOS and nitrotyrosine in HUVECs, which was inhibited by AG, L-nil, and L-NA. The potential role of these inhibitors in ameliorating radiation-induced vascular endothelial damage should be investigated in future studies.
FUNDING
This work was supported by the Korean Military Medical Research Project funded by the Republic of Korea Ministry of National Defense [ROK-MND-2010-KMMRP-020].
ACKNOWLEDGEMENTS
We specially thank the following investigators who participated in this study, along with the authors: M.T. Kim, MD for preparation and measuring the tension of arterial rings; S. Kim, MD, PhD and W.J. Park, MD, PhD for Western blot analysis; S.G. Joo and J.S. Kim, PhD for X-ray irradiation by LINAC; and Cpt H.J. Shin for support and cooperation with administrative tasks.
REFERENCES
- 1.Vanhoutte PM. Endothelium and control of vascular function. State of the Art lecture. Hypertension. 1989;13:658–67. doi: 10.1161/01.hyp.13.6.658. [DOI] [PubMed] [Google Scholar]
- 2.Vane JR, Anggard EE, Botting RM. Regulatory functions of the vascular endothelium. N Engl J Med. 1990;323:27–36. doi: 10.1056/NEJM199007053230106. [DOI] [PubMed] [Google Scholar]
- 3.Vanhoutte PM. Endothelium-dependent hyperpolarizations: the history. Pharmacol Res. 2004;49:503–8. doi: 10.1016/j.phrs.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Ignarro LJ, Buga GM, Wood KS, et al. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987;84:9265–9. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–6. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 6.Xu WM, Liu LZ. Nitric oxide: from a mysterious labile factor to the molecule of the Nobel Prize. Recent progress in nitric oxide research. Cell Res. 1998;8:251–8. doi: 10.1038/cr.1998.25. [DOI] [PubMed] [Google Scholar]
- 7.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–42. [PubMed] [Google Scholar]
- 8.Nathan C, Xie QW. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994;78:915–8. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 9.Anggard E. Nitric oxide: mediator, murderer, and medicine. Lancet. 1994;343:1199–206. doi: 10.1016/s0140-6736(94)92405-8. [DOI] [PubMed] [Google Scholar]
- 10.Nathan C, Xie QW. Regulation of biosynthesis of nitric oxide. J Biol Chem. 1994;269:13725–8. [PubMed] [Google Scholar]
- 11.Yamada M, Wong FL, Fujiwara S, et al. Noncancer disease incidence in atomic bomb survivors, 1958–1998. Radiat Res. 2004;161:622–32. doi: 10.1667/rr3183. [DOI] [PubMed] [Google Scholar]
- 12.Ivanov VK, Maksioutov MA, Chekin SY, et al. The risk of radiation-induced cerebrovascular disease in Chernobyl emergency workers. Health Phys. 2006;90:199–207. doi: 10.1097/01.HP.0000175835.31663.ea. [DOI] [PubMed] [Google Scholar]
- 13.Menendez JC, Casanova D, Amado JA, et al. Effects of radiation on endothelial function. Int J Radiat Oncol Biol Phys. 1998;41:905–13. doi: 10.1016/s0360-3016(98)00112-6. [DOI] [PubMed] [Google Scholar]
- 14.Qi F, Sugihara T, Hattori Y, et al. Functional and morphological damage of endothelium in rabbit ear artery following irradiation with cobalt60. Br J Pharmacol. 1998;123:653–60. doi: 10.1038/sj.bjp.0701654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugihara T, Hattori Y, Yamamoto Y, et al. Preferential impairment of nitric oxide-mediated endothelium-dependent relaxation in human cervical arteries after irradiation. Circulation. 1999;100:635–41. doi: 10.1161/01.cir.100.6.635. [DOI] [PubMed] [Google Scholar]
- 16.Soloviev AI, Tishkin SM, Parshikov AV, et al. Mechanisms of endothelial dysfunction after ionized radiation: selective impairment of the nitric oxide component of endothelium-dependent vasodilation. Br J Pharmacol. 2003;138:837–44. doi: 10.1038/sj.bjp.0705079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacNaughton WK, Aurora AR, Bhamra J, et al. Expression, activity and cellular localization of inducible nitric oxide synthase in rat ileum and colon post-irradiation. Int J Radiat Biol. 1998;74:255–64. doi: 10.1080/095530098141645. [DOI] [PubMed] [Google Scholar]
- 18.Gorbunov NV, Pogue-Geile KL, Epperly MW, et al. Activation of the nitric oxide synthase 2 pathway in the response of bone marrow stromal cells to high doses of ionizing radiation. Radiat Res. 2000;154:73–86. doi: 10.1667/0033-7587(2000)154[0073:aotnos]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 19.Hirakawa M, Oike M, Masuda K, et al. Tumor cell apoptosis by irradiation-induced nitric oxide production in vascular endothelium. Cancer Res. 2002;62:1450–7. [PubMed] [Google Scholar]
- 20.Crow JP, Beckman JS. Reactions between nitric oxide, superoxide, and peroxynitrite: footprints of peroxynitrite in vivo. Adv Pharmacol. 1995;34:17–43. doi: 10.1016/s1054-3589(08)61079-0. [DOI] [PubMed] [Google Scholar]
- 21.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–37. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 22.Giaid A, Lehnert SM, Chehayeb B, et al. Inducible nitric oxide synthase and nitrotyrosine in mice with radiation-induced lung damage. Am J Clin Oncol. 2003;26 doi: 10.1097/01.COC.0000077940.05196.86. e67–72. [DOI] [PubMed] [Google Scholar]
- 23.Hanaue N, Takeda I, Kizu Y, et al. Peroxynitrite formation in radiation-induced salivary gland dysfunction in mice. Biomed Res. 2007;28:147–51. doi: 10.2220/biomedres.28.147. [DOI] [PubMed] [Google Scholar]
- 24.Huang EY, Wang FS, Lin IH, et al. Aminoguanidine alleviates radiation-induced small-bowel damage through its antioxidant effect. Int J Radiat Oncol Biol Phys. 2009;74:237–44. doi: 10.1016/j.ijrobp.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 25.Gratwohl A, John L, Baldomero H, et al. FLT-3 ligand provides hematopoietic protection from total body irradiation in rabbits. Blood. 1998;92:765–9. [PubMed] [Google Scholar]
- 26.Kim MT, Park WJ, Kim S, et al. Involvement of calmodulin kinase II in the action of sulphur mustard on the contraction of vascular smooth muscle. Basic Clin Pharmacol Toxicol. 2010;108:28–33. doi: 10.1111/j.1742-7843.2010.00623.x. [DOI] [PubMed] [Google Scholar]
- 27.Miller MJ, Thompson JH, Liu X, et al. Failure of L-NAME to cause inhibition of nitric oxide synthesis: role of inducible nitric oxide synthase. Inflamm Res. 1996;45:272–6. doi: 10.1007/BF02280990. [DOI] [PubMed] [Google Scholar]
- 28.Ciftci I, Dilsiz A, Aktan TM, et al. Effects of nitric oxide synthase inhibition on intestinal damage in rats with experimental necrotizing enterocolitis. Eur J Pediatr Surg. 2004;14:398–403. doi: 10.1055/s-2004-821105. [DOI] [PubMed] [Google Scholar]
- 29.Stoclet JC, Muller B, Gyorgy K, et al. The inducible nitric oxide synthase in vascular and cardiac tissue. Eur J Pharmacol. 1999;375:139–55. doi: 10.1016/s0014-2999(99)00221-6. [DOI] [PubMed] [Google Scholar]
- 30.Gunnett CA, Lund DD, McDowell AK, et al. Mechanisms of inducible nitric oxide synthase-mediated vascular dysfunction. Arterioscler Thromb Vasc Biol. 2005;25:1617–22. doi: 10.1161/01.ATV.0000172626.00296.ba. [DOI] [PubMed] [Google Scholar]
- 31.Halliwell B. What nitrates tyrosine? Is nitrotyrosine specific as a biomarker of peroxynitrite formation in vivo? FEBS Lett. 1997;411:157–60. doi: 10.1016/s0014-5793(97)00469-9. [DOI] [PubMed] [Google Scholar]
- 32.Lam WW, Leung SF, So NM, et al. Incidence of carotid stenosis in nasopharyngeal carcinoma patients after radiotherapy. Cancer. 2001;92:2357–63. doi: 10.1002/1097-0142(20011101)92:9<2357::aid-cncr1583>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 33.Brown PD, Foote RL, McLaughlin MP, et al. A historical prospective cohort study of carotid artery stenosis after radiotherapy for head and neck malignancies. Int J Radiat Oncol Biol Phys. 2005;63:1361–7. doi: 10.1016/j.ijrobp.2005.05.046. [DOI] [PubMed] [Google Scholar]
- 34.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 35.Paszat LF, Vallis KA, Benk VM, et al. A population-based case-cohort study of the risk of myocardial infarction following radiation therapy for breast cancer. Radiother Oncol. 2007;82:294–300. doi: 10.1016/j.radonc.2007.01.004. [DOI] [PubMed] [Google Scholar]