Abstract
Purpose
Negative physical functioning outcomes including incontinence and erectile dysfunction are relatively common following radical prostatectomy (RP) and are associated with treatment regret and compromised quality of life (QOL). The role that treatment regret may have in influencing the association between prostate-specific QOL (i.e., sexual, urinary, bowl functioning) and general QOL following RP has not been examined.
Method
This study examined the associations of treatment regret, general QOL (SF-36 Physical (PCS) and Mental Health (MCS) composite scores) and prostate-specific QOL (PCQOL sexual, urinary, bowl functioning, and cancer worry subscales) in 95 men who underwent RP for prostate cancer.
Results
Multiple regression analyses indicated that poorer sexual and urinary functioning was associated with poorer MCS. Additionally, men with lower sexual and urinary functioning reported greater treatment regret. Treatment regret was also associated with lower MCS. Finally, treatment regret partially mediated the effects of both sexual and urinary functioning on MCS.
Conclusions
These findings suggest that regardless of a patient’s prostate-specific QOL, reducing treatment regret may improve mental health following RP. Though there are limited options to alter patients’ sexual or urinary functioning following RP, treatment regret may be a modifiable contributor to post-surgical adjustment and QOL.
Keywords: treatment regret, prostate cancer, psychosocial adjustment, QOL
INTRODUCTION
Prostate cancer is the most common cancer in men, aside from skin cancer, with 220,000 newly diagnosed men and about 30,000 deaths from prostate cancer each year in the United States [1]. Treatment options for prostate cancer include active surveillance, radiation therapy, hormone therapy, or radical prostatectomy (RP). Each of these treatment choices are associated with similar cancer-related and quality of life (QOL) outcomes [2-5]. Negative physical functioning outcomes are relatively common following RP, with 29 to 89% of men experiencing erectile dysfunction, up to 61% reporting decreased sexual drive, and 4 to 33% of men experiencing incontinence over 1 year after surgery [6,5]. Poor sexual and urinary functioning following RP is associated with distress and compromised QOL [5,4,7]. Despite the high prevalence of physical dysfunction associated with RP, men tend to report relatively low treatment regret, with about 80% of men stating that they would make the same treatment choice again [8,3,9,2,10,11]. However, Herr et al. [12] found that rates of RP-related regret increased over time, with only 17% reporting regret within 3 years after surgery and 47% of men reporting regret 5 years after RP.
Treatment regret may be associated with lower income, less education, older age, and higher biopsy Gleason score [11,3,2], though these findings are not consistent [8,13]. Additionally, pre-surgical incontinence and cardiovascular disease have been shown to predict greater treatment regret [11,10]. Post-surgical physical functioning, both general and prostate-specific (e.g., poorer sexual, urinary, and bowel functioning) is also associated with greater treatment regret [8,10,11,14,3]. Though treatment regret has been associated with greater psychological distress and poorer QOL [15,16], the contribution of treatment regret to general QOL over and above that of prostate-specific QOL has yet to be determined. Additionally, treatment regret has not been yet been examined as a mediator of the relationship between prostate-specific QOL and general QOL. Treatment regret as a mediator would suggest that, though it is difficult to reduce physical symptoms following RP, it may be possible to improve long term QOL by reducing treatment regret.
The Present Study
The present study is a secondary analysis of a randomized controlled trial (RCT) assessing the QOL impact of a brief pre-surgical stress management intervention (SM) compared to supportive attention (SA) or standard care (SC) on men undergoing RP for prostate cancer. Previously published findings from this dataset demonstrated that men in SM reported significantly reduced distress prior to surgery compared to men in SA and SC and also had significantly higher physical QOL (Physical Component Scale on the SF-36) 1 year following surgery compared to men in SC group [17]. Interestingly, there were no group differences in mental health-related QOL, prostate-specific QOL, or treatment regret at any time-point. In the current study, we hypothesized that 1 year after surgery 1) physical and mental health-related QOL would be positively associated with prostate-specific QOL; 2) treatment regret would be negatively associated with physical and mental health-related QOL and prostate-specific QOL; 3) treatment regret would mediate the relationship of prostate-specific QOL and physical and mental health-related QOL, such that treatment regret would account for some of the association between poor urinary, sexual, and bowel health and physical and mental health-related QOL.
METHOD
Participants
Participants were men with early-stage prostate cancer who were undergoing RP at one of three hospitals within the Texas Medical Center. Patients were included if they were 18 years of age or older, undergoing RP, English speaking, and able to come to the medical center four times prior to their surgery or live within 100 miles of the Texas Medical Center. Patients were excluded if they had any other surgeries in the preceding year, a major illness likely to limit survival to less than two years, medical conditions likely to affect outcome measures such as an autoimmune disease, endocrine abnormalities, chronic pain problems, or a current or past drug and alcohol dependence, had any major psychiatric diagnoses, were undergoing psychiatric or psychological counseling, or had severe cognitive dysfunction detected by the Mini-Mental State Exam [18]. These exclusion criteria were used to increase the homogeneity of the sample, in an attempt to strengthen the primary study’s power to detect statistical differences among the intervention groups. There were a total of 95 participants who completed all measures at baseline and 1 year post surgery.
Procedures
Two hundred twenty-one men were screened for eligibility for the 3-arm RCT of brief pre-surgical SM compared to SA or SC. Forty-two men refused, 15 were ineligible, and 5 withdrew after consent but prior to randomization. Thus, 159 men were randomized to 1 of the 3 study conditions. Details of the study procedures have been reported elsewhere [19,17]. Briefly, after obtaining informed consented, self-report data was collected from patients at 3 weeks before surgery (baseline) and 12 months post-surgery. The pre-surgical SM intervention consisted of two 90-minute individual sessions that were cognitive-behavioral in nature, with a focus on relaxation (e.g., diaphragmatic breathing and guided imagery), and other coping skills (e.g., problem solving, seeking social support, and having realistic surgery and recovery expectations). Patients in the SA group participated in a semi-structured interview focused on their psychosocial and medical history for two 90-minute sessions (discussions about how and when they were diagnosed with prostate cancer, other medical issues and family history; discussion of any fears and concerns; clinician used reflective listening and provided empathy but did not teach specific skills). Patients in the SC group received no intervention, but completed assessments at the same time points. There were no differences between study conditions on any of the variables that are the focus of the present paper (i.e., mental health-related QOL, prostate-specific QOL, or treatment regret) aside from physical aspects of QOL (SF-36 PCS), which was reported as higher for men in the SM group compared to the SC group 1 year after surgery [17]. We controlled for the possible effect of group assignment in all analyses, but ultimately examined the association of study variables across groups. Ninety-five participants completed the follow up measures relevant to this study 1 year after surgery. This study was approved by the Institutional Review Board of all participating hospitals.
Measures
To assess treatment regret, participants responded to 7 items related to the frequency with which they had thoughts about how their current situation could have turned out more positively had they made a different treatment decision (see Appendix A for full measure). Responses were on a 5-point scale ranging from 1 (never) to 5 (very frequently). In this study sample, the internal reliability was high (Cronbach’s alpha = 0.92).
The Medical Outcomes Study 36-item Short Form Health Survey (SF-36 [20]) was used to assess general health-related QOL. It assesses QOL in several domains including physical functioning, bodily pain, general health perceptions, vitality, social functioning, role-emotional, and mental health. In this study sample, the internal reliability for each of these subscales was acceptable (0.79-.90). There are two component scores derived from the subscales that measure mental health (MCS) and physical functioning (PCS).
The Prostate Cancer Quality of Life Scale (PCQOL) is a 52-item instrument with 10 domain scales: function, limitations, and bother for each of urinary, bowel and sexual issues as well as a scale assessing worry/anxiety about having prostate cancer and treatment [21]. Internal consistency has been established for the PCQOL (alphas 0.70-0.90). As strong convergence of the function, limitation, and bother scales within each of the urinary, sexual and bowel categories have been noted in other studies [21], these subscales were combined to form four subscales of urinary function, sexual function, bowel function, and cancer worry. In the present sample, these four scales demonstrated acceptable internal reliability with Chronbach’s alphas of 0.94, 0.89, 0.68, and 0.87, respectively.
Analyses
Descriptive statistics were computed. We examined whether baseline demographic (age, ethnicity, marital status, education), clinical (Prostate Specific Antigen (PSA), stage of disease), or group (SM, SA, SC) characteristics were related to general QOL (MCS and PCS), prostate-specific QOL (4 subscales of the PCQOL) and treatment regret 1 year after surgery. To determine the association between prostate-specific QOL and general QOL, the 4 subscales of PCQOL were regressed onto the 2 component scores of the SF-36 (MCS and PCS), covarying for intervention group and the respective baseline measures. As age and surgery type were associated with sexual functioning, analyses involving sexual functioning covaried for these variables. Additionally, education was associated with cancer worry, so analyses involving cancer worry covaried for education. The Bonferroni method was used to correct for the 8 regression analyses (4 subscales of PCQOL regressed onto PCS and MCS), and alpha was set at 0.006. To determine the association between QOL and treatment regret, each QOL measure (2 subscales of SF-36 and 4 subscales of PCQOL) was regressed onto treatment regret, covarying for intervention group and the respective baseline measures. The Bonferroni method was used to correct for the 6 regression analyses, and alpha was set to 0.008. Mediation of the association between prostate-specific QOL on general QOL by treatment regret at 1 year after surgery was explored using the PROC REG procedure following the criteria outlined by Baron and Kenny [22]. G*Power was used to determined that, given a total of 95 participants, we were able to declare as statistically significant a medium effect size (Cohen’s f 2 > 0.15) assuming 80% power and a two-sided significance level of 0.05 [23,24]. Cohen suggested that f 2 effect sizes of 0.02, 0.15, and 0.35 are termed small, medium, and large, respectively [23].
RESULTS
Sample Characteristics
Measurements were obtained for 56% of the sample at 1 year (n = 95). There were no significant differences in demographic, medical, or QOL (SF-36 or PCQOL) scores at any time point between those who completed baseline and 1 year assessments and those who dropped out of the study by 1 year follow up (all p’s > 0.29), indicating that men who dropped out of the study likely did not differ on any measures relevant to the present study from men who completed all measures. The demographic and medical characteristics are summarized in Table 1. The associations between demographic variables and each of the study variables of interest (2 component scales SF-36, 4 subscales of PCQOL, and treatment regret) were examined to identify potential confounding variables to include as covariates in the analyses. Age (β = −0.137, p = 0.053) and non-nerve sparing surgery (β = 0.214, p = 0.040) were associated with poorer sexual functioning, and higher education was associated with less cancer worry (β = 0.214, p = 0.040). No demographic or medical variables were associated with treatment regret. Thus, age and surgery type were entered as covariates in analyses involving sexual functioning, and education was entered as a covariate in analyses involving cancer worry. As previously reported, there were intervention group differences in PCS ratings 1 year after surgery (p = 0.004) [17], but there were no group differences in MCS, any PCQOL subscales, or treatment regret at baseline or 1 year follow up. Nevertheless, group assignment was entered as a covariate for all analyses to control for any potential bias.
Table 1.
Demographic Information
| Characteristic | No. (%) or M (SD) | |
|---|---|---|
| Mean Age | 60.9 (6.6) | |
|
| ||
| Ethnicity | ||
| White | 124 | 86% |
| African American | 21 | 14% |
|
| ||
| Married | 135 | 87% |
| Divorced | 20 | 13% |
|
| ||
| Education | ||
| High School | 31 | 20% |
| Some College | 36 | 23% |
| College Graduate | 59 | 37% |
| Graduate Degree | 31 | 20% |
|
| ||
| Mean Prostate Specific Antigen | 6.7(5.3) | |
|
| ||
| Stage of Disease | ||
| I | 20 | 13% |
| II | 116 | 74% |
| III | 20 | 13% |
|
| ||
| Type of Surgery | ||
| Non-nerve sparing | 37 | 24% |
| Nerve Sparing | 101 | 67% |
| Nerve Graft | 14 | 9% |
Descriptive information on PCS, MCS, PCQOL subscales, and treatment regret at baseline and 1 year post surgery can be found in Table 2. T-tests indicate that sexual (t (88) = 11.24, p < 0.0001) and urinary (t (96) = 8.01, p < 0.0001) function, and cancer related worry (t (98) = −7.17, p < 0.0001) decreased from baseline to 1 year post surgery, while bowel function did not change (p > 0.60). Though men reported relatively infrequent rates of treatment regret (M = 11.08, SD = 5.81), 57% of participants reported having had at least some thoughts of regret regarding their prostate cancer treatment decision. The items receiving highest ratings were, “If only I hadn’t had the surgery, I wouldn’t be having any of the sexual difficulties I do now,” and “If only I hadn’t had the surgery, I wouldn’t be having any of the urinary control problems I do now.”
Table 2.
QOL and Treatment Regret Descriptive Statistics at Baseline and 1 Year Post-Surgery
| Variable | Baseline (N=154) M (SD) |
1 Year (N=95) M (SD) |
t | p |
|---|---|---|---|---|
| SF-36 | ||||
| PCS | 51.99 (6.89) | 49.82 (9.32) | 2.05 | 0.043 |
| MSC | 53.92 (7.89) | 53.99 (7.78) | 0.42 | 0.676 |
|
| ||||
| PCQOL | ||||
| Sexual Function | 235.20 (45.48) | 178.91 (43.99) | 11.24 | <.0001 |
| Urinary Function | 284.15 (24.58) | 239.39 (53.0) | 8.01 | <.0001 |
| Bowl Function | 246.28 (12.58) | 243.88 (20.44) | 1.27 | 0.2083 |
| Cancer Worry | 58.08 (31.92) | 77.56 (24.45) | −7.17 | <.0001 |
|
| ||||
| Treatment Regret | n/a | 11.08 (5.81) | n/a | n/a |
Note:Higher scores on the SF-36 Scales indicate better health, higher scores on the Prostate Cancer QOL scales indicate better functioning, and higher Treatment Regret scores indicate greater treatment regret.
Association between PCQOL and General QOL
We examined how general QOL (MCS and PCS) 1 year after surgery varied as a function of prostate-specific QOL (PCQOL). Results indicated that poorer sexual (β = 0.32, p = 0.003) and urinary (β = 0.321, p < 0.001) functioning was associated with poorer MCS. Poorer bowel function was associated with poorer PCS (β = 0.306, p = 0.001). Cancer worry was not associated with general QOL.
Association between QOL and Treatment Regret
We next examined how treatment regret 1 year after surgery varied as a function of QOL-related variables measured 1 year after surgery. Results of regression analyses can be seen in Table 3. Men who reported lower sexual (β = −0.41, p < 0.001) and urinary (β = −0.40, p < 0.001) functioning and greater cancer worry (β = −0.57, p < 0.001) reported greater treatment regret. Additionally, men who reported lower MCS reported greater treatment regret (β = −0.55, p < 0. 001). Reports of bowel function and PCS were not associated with treatment regret (p’s > 0.155).
Table 3.
QOL-related Variables Regressed onto Treatment Regret
| Variable | β | p |
|---|---|---|
| SF-36 | ||
| PCS | −0.17 | 0.155 |
| MSC | −0.55 | <0.001 |
|
| ||
| PCQOL | ||
| Sexual Function | −0.41 | <0.001 |
| Urinary Function | −0.40 | <0.001 |
| Bowl Function | −0.05 | 0. 632 |
| Cancer Worry | −0.57 | <0.001 |
Analyses involving sexual function controlled for age and type of surgery (non-nerve sparing, nerve sparing, or nerve-graft), analyses involving cancer worry controlled for education level, and all analyses controlled group assignment and the respective baseline predictor.
Mediation Analyses
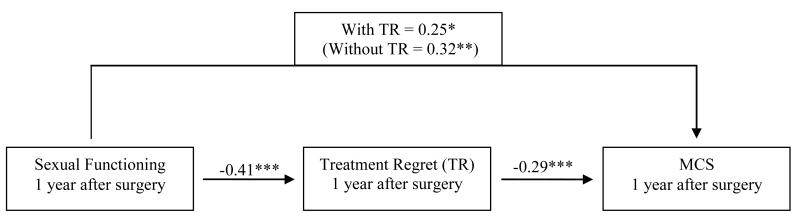
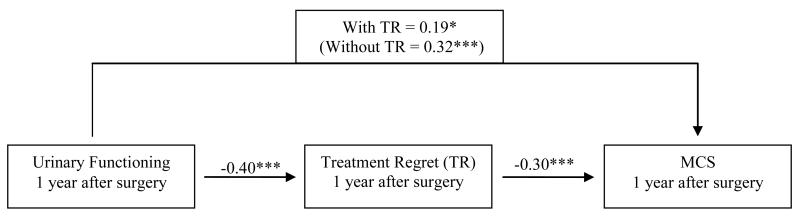
Baron and Kenny’s [22] method was used to determine whether treatment regret mediated association between prostate-specific QOL and general QOL. Specifically, we sought to determine whether treatment regret mediated the effects of sexual and urinary functioning on MCS, as these variables met the initial criteria of mediation such that 1) sexual and urinary functioning were associated with MCS, 2) sexual and urinary functioning were associated with treatment regret, and 3) treatment regret was associated with MCS. When both sexual functioning and treatment regret were regressed onto MCS, treatment regret remained significantly associated with MCS (β = −0.29, p = 0.001), and the association of sexual functioning on MCS was reduced by about 7% (β = 0.25, p = 0.013), indicating partial mediation (see Figure 1). Similarly, when both urinary functioning and treatment regret were regressed onto MCS, treatment regret remained significantly associated with MCS (β = −0.30, p = 0.001) and the association of urinary function on MCS was reduced by about 13% (β = 0.19, p = 0.024), indicating partial mediation (see Figure 2).
Figure 1. Treatment regret (TR) mediates the effect of sexual functioning on mental health-related QOL (MCS) at 1 year.
Note. Values on each path are standardized β values. Regression coefficients taken from regression analyses controlling for age, type of surgery (non-nerve sparing, nerve sparing, or nerve-graft), group assignment, baseline sexual functioning and baseline MCS.
*p < 0.05, **p < 0.01, ***p <0 .001
Figure 2. Treatment regret (TR) mediates the effect of urinary functioning on mental health-related QOL (MCS) at 1 year.
Note. Values on each path are standardized β values. Regression coefficients taken from regression analyses controlling for group assignment, baseline urinary functioning and baseline MCS.
*p < 0.05, **p <0 .01, ***p < 0.001
DISCUSSION
Results are consistent with previous research indicating an association between prostate-specific QOL and general QOL. Specifically, poorer urinary and sexual functioning and greater cancer worry were associated with poorer mental health-related QOL. Additionally, findings support previous research indicating that patients experience a decline in sexual and urinary function 1 year after RP [5,6], but report decreased cancer-related worry 1 year after RP [25]. A novel aspect of this study was identifying the mediating role of treatment regret on the relationship between prostate-specific QOL with mental health-related QOL. Our hypothesis that treatment regret accounted for some of the association between both urinary and sexual functioning and mental health-related QOL was confirmed. This finding is supported by previous research linking treatment regret to poorer QOL, particularly in the mental health domain [26,15,27]. While other studies have found treatment regret to be associated with relatively fixed demographic (e.g., lower income, less education, and older age [11,3,2]) and medical (e.g., higher biopsy Gleason score, pre-surgical incontinence [11,10], and post-surgical physical functioning [8,10,11,14,3]) factors, the present study indicates that there is an association between treatment regret and mental health-related QOL regardless of the patient’s demographic and medical conditions state. Interestingly, this suggests that, irrespective of a patient’s prostate-specific QOL (e.g., urinary and sexual functioning), reducing his treatment regret may improve his mental health following radical prostatectomy. This finding is particularly important because, though there are limited options to alter patients’ sexual or urinary functioning following prostate surgery, treatment regret may be a modifiable contributor to post-surgical adjustment and QOL.
It is important to note that the present study is a secondary analysis of a 3-arm randomized controlled trail, in which a pre-surgical intervention was delivered. The SM intervention was designed to reduce pre-surgical stress and improve post-surgical QOL through cognitive-behavioral coping skills, and the SA intervention was designed to control for face-to-face time with a psychologist Though the SM intervention did improve physical functioning-related QOL [17], it was not associated with improvements in mental health-related QOL or treatment regret. Several studies suggest that pre-surgical interventions designed to increase patient competence in communicating with their doctor [28] and improve patient knowledge about potential negative outcomes and management of those outcomes [29] have led to reduced treatment regret following RP. Similarly, patients who reported greater understanding of potential treatment complications [13] and greater self-efficacy for managing prostate symptoms [30] reported less regret following treatment for prostate cancer. It is possible that the intervention delivered in the present study would have been more effective at reducing treatment regret had it focused more on fostering patient self-efficacy at communicating with their providers and managing negative treatment outcomes. These strategies could be combined with other psychosocial and medical interventions (e.g., sexual counseling, urinary treatments) to further improve patient’s physical and psychological outcomes [31].
There are several limitations to recognize in this study. First, the majority of participants were white, non-Hispanic, married and highly educated. Thus, future research is needed to test the generalizability of these findings to more diverse populations. Additionally, participants reported relatively high levels of mental health at study entry compared with normative data. Lastly, treatment regret was only assessed at the 12 month time point, rather than after the surgery but before the 12 month follow up (e.g., at a 6 month follow up), making it impossible to establish directions of effect.
Sexual and urinary problems following prostate surgery are common, largely irreversible, and associated with poorer mental health [4,5,7,6]. Some of the association between sexual and urinary problems and mental health can be attributed to treatment regret. Thus, treatment regret is an important component of post-surgical mental health that may be responsive to or prevented by intervention[28,29]. Future research to further examine the contribution of treatment regret to QOL as well as methods to reducing treatment regret are necessary to develop methods to improve QOL for patients after this common and complicated procedure.
AKNOWLEDGEMENTS
This project was supported by a research grant from the National Institutes of Health/National Cancer Institute (RO1 MH59432), the National Institutes of Health (CA016672), and a cancer prevention fellowship for Chelsea Gilts from the National Cancer Institute (R25T CA57730, Shine Chang, PhD, Principle Investigator). We are indebted to Dr. Andy Baum who helped to start it all. We thank Drs. Richard Babaian, Louis Pisters, and Brian Miles for opening up this trial to their patients, Dr. Danielle Carr for study oversight, and Adoneca Fortier for helping with data collection.
APPENDIX A. Treatment Regret Scale
Please rate the frequency with which you have had each of the below thoughts in the past month.

If only I hadn’t had the surgery, I wouldn’t be having any of the sexual difficulties I do now.
If only I hadn’t had the surgery, I wouldn’t be having any of the urinary control problems I do now.
If only I hadn’t had the surgery, my life would be much better right now.
If only I had been given more information about the side effects of the surgery, I would have chosen a different treatment.
If only I had decided on a different treatment option, I wouldn’t be having the problems I do now.
If only I had chosen a different treatment for my prostate cancer, I’d be doing much better now.
If only I knew then what I know now about the surgery, I would never have had the surgery and wouldn’t be having the problems I do now.
Footnotes
CONFLICT OF INTEREST
The authors have no conflict of interest to disclose. Approval of this paper has been provided by all authors. The authors have full control of all primary data and agree to allow the journal to review the data if requested.
Contributor Information
Chelsea D. Gilts, Department of Clinical Psychology, University of Houston, 126 Heyne Building, Houston, TX 77204.
Lorenzo Cohen, Integrative Medicine Program, Department of General Oncology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 426, Houston, Texas 77030.
Curtis A. Pettaway, Department of Urology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 1373, Houston, Texas 77030.
Patricia A. Parker, Department of Behavioral Science, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 1330, Houston, Texas 77030.
REFERENCES
- 1.Group USCSW . United States Cancer Statistics: 1999-2007 Incidence and Mortality Web-based Report. 2010. [Google Scholar]
- 2.Sidana A, Hernandez DJ, Feng Z, Partin AW, Trock BJ, Saha S, Epstein JI. Treatment decision-making for localized prostate cancer: What younger men choose and why. The Prostate. 2012;72:58–64. doi: 10.1002/pros.21406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schroeck FR, Krupski TL, Sun L, Albala DM, Price MM, Polascik TJ, Robertson CN, Tewari AK, Moul JW. Independent predictors for dissatisfaction with and regret of treatment choice after radical prostatectomy. Journal of Urology. 2008;179(4 - supplement):109. [Google Scholar]
- 4.Steineck G, Helgesen F, Adolfsson J, Dickman PW, Johansson J-E, Norlen BJ, et al. Quality of life after radical prostatectomy or watchful waiting. New England Journal of Medicine. 2002;347(11):790–796. doi: 10.1056/NEJMoa021483. [DOI] [PubMed] [Google Scholar]
- 5.Kyrdalen AE, Dahl AA, Hernes E, Smastuen MC, Fossa SD. A national study of adverse effects and global quality of life among candidates for curative treatment for prostate cancer. BJU Int. 2012 doi: 10.1111/j.1464-410X.2012.11198.x. doi:10.1111/j.1464-410X.2012.11198.x. [DOI] [PubMed] [Google Scholar]
- 6.Penson DF, Litwin MS. Quality of life after treatment for prostate cancer. Current Urology Reports. 2003;4:185–195. doi: 10.1007/s11934-003-0068-1. [DOI] [PubMed] [Google Scholar]
- 7.Penson DF, Feng Z, Kuniyuki A, et al. General quality of life 2 years following treatment for prostate cancer: What influences outcomes? Results from the Prostate Cancer Outcomes Study. Journal of Clinical Oncology. 2003;21:1147–1154. doi: 10.1200/JCO.2003.07.139. [DOI] [PubMed] [Google Scholar]
- 8.Davison BJ, Goldenberg SL. Decisional regret and quality of life after participating in medical decision-making for early-stage prostate cancer. BJU International. 2003;91(1):14–17. doi: 10.1046/j.1464-410x.2003.04005.x. doi:10.1046/j.1464-410X.2003.04005.x. [DOI] [PubMed] [Google Scholar]
- 9.Sebesta M, Cespedes RD, Luhman E, Optenberg S, Thompson IM. Questionnaire-based outcomes of urinary incontinence and satisfaction rates after radical prostatectomy in a national study population. Urology. 2002;60(6):1055–1058. doi: 10.1016/s0090-4295(02)01989-1. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen PL, Chen MH, Hoffman KE, Chen RC, Hu JC, Bennett CL, Kattan MW, Sartor O, Stein K, D’Amico AV. Cardiovascular comorbidity and treatment regret in men with recurrent prostate cancer. BJU Int. 2012;110(2):201–205. doi: 10.1111/j.1464-410X.2011.10709.x. doi:10.1111/j.1464-410X.2011.10709.x. [DOI] [PubMed] [Google Scholar]
- 11.Lavery HJ, Levinson AW, Hobbs AR, Sebrow D, Mohamed NE, Diefenbach MA, Samadi DB. Baseline functional status may predict decisional regret following robotic prostatectomy. J Urol. 2012;188(6):2213–2218. doi: 10.1016/j.juro.2012.08.016. doi:10.1016/j.juro.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Herr HW. Quality of life of incontinent men after radical prostatectomy. The Journal of Urology. 1994;151(3):652–654. doi: 10.1016/s0022-5347(17)35038-3. [DOI] [PubMed] [Google Scholar]
- 13.Lin Y. Treatment decision regret and related factors following radical prostatectomy. Cancer Nursing. 2011;34:5–417. doi: 10.1097/NCC.0b013e318206b22b. [DOI] [PubMed] [Google Scholar]
- 14.Hu JC, Kwan L, Saigal CS, Litwin MS. Regret in men treated for localized prostate cancer. The Journal of Urology. 2003;169(6):2279–2283. doi: 10.1097/01.ju.0000065662.52170.6f. [DOI] [PubMed] [Google Scholar]
- 15.Gilbar O, Hevroni A. Counterfactuals, coping strategies and psychological distress among breast cancer patients. Anxiety, Stress & Coping: An International Journal. 2007;20(4):383–392. doi: 10.1080/10615800701384439. [DOI] [PubMed] [Google Scholar]
- 16.Clark JA, Wray NP, Ashton CM. Living With Treatment Decisions: Regrets and Quality of Life Among Men Treated for Metastatic Prostate Cancer. Journal of Clinical Oncology. 2001;19(1):72–80. doi: 10.1200/JCO.2001.19.1.72. [DOI] [PubMed] [Google Scholar]
- 17.arker PA, Pettaway CA, Babaian RJ, Pisters LL, Miles B, Fortier A, Wei Q, Carr DD, Cohen L. The effects of a presurgical stress management intervention for men with prostate cancer undergoing radical prostatectomy. Journal of Clinical Oncology. 2009;27(19):3169–3176. doi: 10.1200/JCO.2007.16.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatry Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 19.Cohen L, Parker PA, Vence L, Savary C, Kentor D, et al. Presurgical stress management improves post-operative immune function in men with prostate cancer undergoing radical prostatectomy. Psychosomatic Medicine. 2011;73(3):218–225. doi: 10.1097/PSY.0b013e31820a1c26. [DOI] [PubMed] [Google Scholar]
- 20.Ware J, Sherbourne C. The MOS 36-item shortform health survey. Medical Care. 1992:30. [PubMed] [Google Scholar]
- 21.Giesler R, Miles B, Cowen M, Kattan M. Assessing quality of life in men with clinically localized prostate cancer: Development of a new instrument for use in multiple settings. Quality of Life Research. 2000;9(6):645–665. doi: 10.1023/a:1008931703884. doi:10.1023/a:1008931703884. [DOI] [PubMed] [Google Scholar]
- 22.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 23.Cohen S, Mermelstein R, Kamarck T, Hoberman H. Measuring the functional components of social support. Social support: Theory, research, and applications. Martinus Nijhoff; The Hague, The Netherlands: 1985. [Google Scholar]
- 24.Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 25.Dale W, Bilir P, Han M, Meltzer D. The role of anxiety in prostate carcinoma. Cancer. 2005;104(3):467–478. doi: 10.1002/cncr.21198. doi:10.1002/cncr.21198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parker PA, Middleton MS, Kulik JA. Counterfactual thinking and quality of life among women with silicone breast implants. Journal of Behavioral Medicine. 2002;25(4):317–335. doi: 10.1023/a:1015828914643. [DOI] [PubMed] [Google Scholar]
- 27.Davis C, Lehman D, Wortman C, Silver R, Thompson S. The undoing of traumatic life events. Personality and Social Psychology Bulletin. 1995;21:109–124. [Google Scholar]
- 28.Mishel MH, Germino BB, Lin L, Pruthi RS, Wallen EM, Crandell J, Blyler D. Managing uncertainty about treatment decision making in early stage prostate cancer: A randomized clinical trial. Patient Education and Counseling. 2009;77(3):349–359. doi: 10.1016/j.pec.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Kinsella J, Acher P, Ashfield A, Chatterton K, Dasgupta P, Cahill D, Popert R, O’Brien T. Demonstration of erectile management techniques to men scheduled for radical prostatectomy reduces long-term regret: a comparative cohort study. British Journal of Urology International. 2012;109(2):254–258. doi: 10.1111/j.1464-410X.2011.10237.x. [DOI] [PubMed] [Google Scholar]
- 30.Goh AC, Kowalkowski MA, Bailey J,DE, Kazer MW, Knight SJ, Latini DM. Perception of cancer and inconsistency in medical information are associated with decisional conflict: a pilot study of men with prostate cancer who undergo active surveillance. British Journal of Urology International. 2011 doi: 10.1111/j.1464-410X.2011.10791.x. doi:10.1111/j.1464-410X.2011.10791.x. [DOI] [PubMed] [Google Scholar]
- 31.Schover LR, Canada AL, Yuan Y, Sui D, Neese L, Jenkins R, Rhodes MM. A randomized trial of Internet-based versus traditional sexual counseling for couples after localized prostate cancer treatment. Cancer. 2012;118(2):500–509. doi: 10.1002/cncr.26308. doi:10.1002/cncr.26308. [DOI] [PMC free article] [PubMed] [Google Scholar]