Abstract
Alternative splicing of mRNA precursors enables one gene to produce multiple protein isoforms with differing functions. Under normal conditions, this mechanism is tightly regulated in order for the human genome to generate proteomic diversity sufficient for the functional requirements of complex tissues. When deregulated, however, cancer cells take advantage of this mechanism to produce aberrant proteins with added, deleted, or altered functional domains that contribute to tumorigenesis. Here we discuss aspects of alternative splicing misregulation in cancer, focusing on splicing events affected by deregulation of regulatory splicing factors and also recent studies identifying mutated components of the splicing machinery.
Introduction
The vast majority of protein-coding genes in humans contain multiple exons. Splicing of mRNA precursors (pre-mRNA), the removal of introns and the joining of flanking exons, is a fundamental step in the production of the encoded protein. Although the splicing of individual exons must be precise, the selection of exons to be included in the final mRNA allows a certain degree of plasticity. Alternative use of exons, or alternative splicing (AS), enables a single gene to produce multiple mRNA variants. More than 90% of human genes produce transcripts that are alternatively spliced (1, 2), and 60% of the splice variants encode distinct protein isoforms (3). Protein isoforms of a given gene can have different or even opposing functions (4, 5). Thus, AS is considered to be a major mechanism for generating proteomic diversity (6).
Regulation of AS is tightly controlled during normal tissue differentiation (7, 8). Misregulation of AS can lead to production of aberrant protein isoforms, which may contribute to diseases including cancer. Genome-wide studies have revealed more than 15,000 tumor-associated splice variants in a wide variety of cancers (9–11). Computational analysis of tumor-associated splice variants indicates that AS occurs with genes involved in almost every aspect of cancer cell biology, including proliferation, differentiation, cell cycle control, metabolism, apoptosis, motility, invasion, and angiogenesis (9). In a functional screen of selected splice variants, it was found that 10% (4 out of 41 tested) AS events specific to breast and/or ovarian cancers contribute to cancer cell survival (12). Although the functional significance of cancer-specific AS events is still largely unexplored, the link between aberrant AS and cancer has been established (4, 13, 14).
Aberrant AS events often reflect abnormalities in splicing regulation. Pre-mRNA splicing is generally regulated by cis-acting splicing sequences in primary transcripts and trans-acting splicing factors that bind these RNA sequences (15). Alterations in protein levels and activity of regulatory splicing factors, mutations in cis-acting splicing sequences, and mutations in the core components of the splicing machinery itself may result in aberrant AS in cancer and contribute to many cancer phenotypes. Here we discuss recent studies on the misregulation of AS in cancer. For more insights into the importance and mechanisms of AS regulation in health and disease, the reader is referred to several excellent reviews (4, 5, 14, 15).
AS Patterns in Cancer
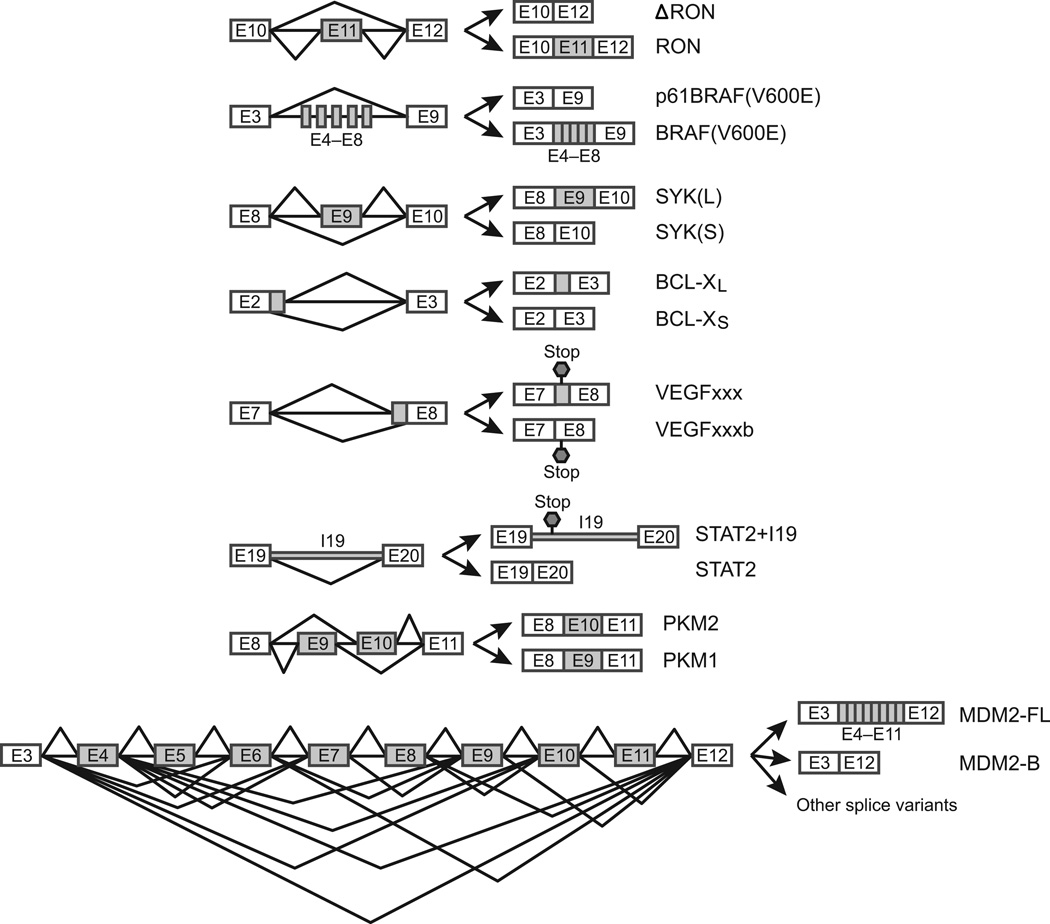
AS patterns in cancer cells reflect those found in normal cells. Global analysis of more than 15,000 cancer-specific splice variants in 27 types of cancers shows that the average number of cancer-specific splice variants per gene is smaller than that of tissue-specific splice variants in 35 normal tissues (1.51 vs. 1.99)(9). This is expected, because tissue-specific splice variants are required for generating the necessary proteomic complexities of human tissues and the splice variants have undergone extensive natural selection during the course of evolution (16, 17). Cancer-specific splice variants, which may bestow survival advantages to cancer cells, often result in rapid death of the human subject that harbors the cancer, and therefore are selected against rather than selected for at the organismal level. Regardless, cancer-specific AS includes all of the five main AS patterns observed in normal tissues: cassette exons, alternative 5′ splice sites (ss), alternative 3′ ss, intron retention, and mutually exclusive exons (Figure 1), suggesting that cancer cells and differentiated cells use fundamentally similar splicing mechanisms. To illustrate the AS patterns that cancer cells use to gain survival advantage, we describe below an exemplary set of functionally important AS events (Figure 1).
Figure 1.
Examples of AS patterns in cancer. The main AS patterns in cancer cells include cassette exons (skipping of one exon, skipping of multiple exons, and exon inclusion), alternative 5’ ss, alternative 3’ ss, intron retention, and mutually exclusive exons. Specific examples discussed in the text are shown.
Cassette exons—skipping of one exon
The RON gene encodes a tyrosine kinase receptor for macrophage-stimulating protein (MSP). Under normal conditions, RON is involved in cell mobility and invasion in response to MSP binding (18, 19). A splice isoform, ΔRON, which lacks exon 11, is overexpressed in a number of cancers (20). Skipping of exon 11 results in the deletion of an extracellular domain that affects the proteolytic maturation of the protein. The truncated ΔRON is constitutively active (even in the absence of its ligand) and promotes cancer invasiveness (21).
Cassette exons—skipping of multiple exons
BRAF is a proto-oncogene encoding the serine/threonine-protein kinase BRAF, which regulates the MAPK/ERK signaling pathway. An often fatal mutation (V600E) was found in more than half of the patients with malignant melanomas (22). Effective treatment can involve the use of BRAF inhibitors such as PLX4032, to which BRAF(V600E) is sensitive. However, skipping of exons 4–8 during splicing of BRAF(V600E) transcripts results in an in-frame deletion of the N-terminal RAS-binding domain. The truncated enzyme is insensitive to the inhibitors and therefore confers melanoma cells resistance to the drugs (23).
Cassette exons—exon inclusion
SYK, or spleen tyrosine kinase, functions as a tumor suppressor in breast cancer (24), but acts as an oncogene in T-cell lymphomas (25), chronic leukemias (26) and head and neck carcinomas (27). This paradox had not been explained until recently when Prinos et al. found that SYK expresses two distinct splice isoforms, a longer SYK(L) and shorter SYK(S) isoform (12). SYK(L), which includes exon 9 and is found in many cancers, promotes cell survival and tumor malignancy. Switching SYK(L) to SYK(S), which lacks exon 9, induces apoptosis of ovarian cancer cells, while a switch in the opposite direction, which can be induced by epidermal growth factor, leads to cancer cell growth.
Alternative 5’ ss
AS of BCL-X pre-mRNA is the best-known example of this pattern. BCL-X belongs to the BCL-2 protein family, whose members form hetero- or homodimers that act as anti- or pro-apoptotic regulators both in health and in disease (28). BCL-X produces two splice isoforms, BCL-XL and BCL-XS, through the alternative use of two competing 5′ ss in exon 2 (29). The longer isoform BCL-XL has anti-apoptotic effects and is overexpressed in various cancer types (30–32). In contrast, the shorter isoform BCL-XS is pro-apoptotic and is down-regulated in cancer (33).
Alternative 3’ ss
VEGF (vascular endothelial growth factor) is a mitogen that stimulates angiogenesis required for tumor growth (34, 35). Pre-mRNA of VEGF contains eight exons, with two competing 3′ ss in exon 8. Alternative use of the 3′ ss leads to production of two families of VEGF isoforms (36). Selection of the proximal 3′ ss produces one family of isoforms called VEGFxxx, where xxx indicates the number of amino acids on the protein. When the distal 3′ ss is used, VEGF produces the other isoform family VEGFxxxb. These two isoform families have opposing functions: VEGFxxx isoforms are pro-angiogenic, and are overexpressed in a number of tumors, whereas VEGFxxxb isoforms are anti-angiogenic and downregulated in tumors (36). It is believed that the opposing functions are caused by the distinct C-termini produced by alternative use of the 3′ ss. The C-terminus of VEGFxxxb (for example VEGF165b) fails to bind its receptor, neurophilin 1, which is required for full activation of the VEGF-signaling transduction (37).
Intron retention
STAT2 (signal transducer and activator of transcription 2) is a transcription factor and a main component of the JAK (Janus kinase)/STAT signaling pathway (38). Upon interferon (IFN) stimulation, STAT2 dimerizes with STAT1 and the heterodimer translocates to the nucleus and activates transcription of IFN responsive genes. Through this pathway, IFN induces apoptosis of cancer cells (38). IFN is used as a treatment for many cancers, and is most effective on hematological malignancies (39). However, cancer cells frequently develop resistance to IFN. Du et al. discovered that IFN resistant cells produce a STAT2 splice variant containing intron 19 (40). This retained intron introduces a stop codon before the Src homology 2 domain, leading to disruption of STAT dimerization.
Mutually exclusive exons
PKM (pyruvate kinase M) is a metabolic enzyme that catalyzes the last step of glycolysis, and AS of PKM pre-mRNA is critical for tumor metabolism. Tumor cells have long been known for their use of massive amounts of glucose and production of large quantities of lactate, even in the presence of oxygen (aerobic glycolysis or the Warburg effect) (41). Aerobic glycolysis produces ATP less efficiently, but it is believed to promote accumulation of glycolytic intermediates that are channeled to biosynthesis pathways for making new tumor cells. It is now clear that the switch between aerobic glycolysis and oxidative phosphorylation is at least partly achieved by AS of PKM pre-mRNA. PKM has two mutually exclusive exons: exon 9 (E9) and exon 10 (E10). AS of these exons results in production of two isoforms: the adult isoform PKM1, which includes E9 but not E10, and the embryonic isoform PKM2, which includes E10 but not E9 (42). PKM2 is ubiquitously expressed in tumors, while PKM1 is expressed in differentiated tissues, such as muscle and brain (42–44). Replacing PKM2 with PKM1 in tumor cells reduced lactate production and increased oxidative phosphorylation. When the cells were injected into nude mice, tumor growth was greatly inhibited (44).
Complex splicing patterns
MDM2 (Mouse double minute 2 homolog) is a negative regulator of the p53 tumor suppressor (45). The MDM2 gene has 12 exons, and AS of its pre-mRNA involves skipping of one or more exons and use of several cryptic splice sites, leading to production of at least 40 splice variants in various tumors and normal tissues (46). Full-length MDM2 binds to p53 and acts as an ubiquitin ligase and facilitates proteasomal degradation of p53 (47, 48). The functions of most MDM2 splice isoforms are unclear. At least four of the splice isoforms (MDM2-A, -B, -C, and -D) in human cancers lack part of the p53 binding domain, and therefore are unable to bind to and degrade p53 (49). Interestingly, the most frequently expressed tumor isoform, MDM2-B, binds to full-length MDM2 and sequesters it, leading to accumulation of p53. However, the increased p53 activity contrasts with the transforming ability of MDM2-B (49), consistent with a more complex view of MDM2 function (50).
As described above, and in many other instances not discussed here, aberrant AS in cancer enables individual genes to produce distinct protein isoforms with deleted, added, or altered domains. This in turn brings about different or even opposing functions that contribute to a variety of cancer cell activities such as growth, apoptosis, invasiveness, drug resistance, angiogenesis, and metabolism.
Misregulation of AS in Cancer by Regulatory Splicing Factors
Splicing regulation is essentially the process of selecting splice sites in pre-mRNA transcripts. This process is generally directed by cis-acting regulatory sequences and trans-acting RNA binding proteins (RBPs) (15). Well-studied RBPs include two families: serine/arginine-rich (SR) proteins and heterogeneous nuclear ribonucleoproteins (hnRNPs) (15, 51–55). SR proteins bind to exonic splicing enhancers (ESEs) and intronic splicing enhancers (ISEs) and usually promote exon inclusion. In contrast, hnRNPs bind to exonic splicing silencers (ESSs) and intronic splicing silencers (ISSs) and in most cases lead to exon skipping (15, 51–55). Thus, one major mechanism of AS misregulation is through alterations in the levels and activity of RBPs. Below we describe a few RBPs that have been implicated in misregulation of AS in cancer.
SRSF1 (formerly known as ASF/SF2) is perhaps the best-known SR protein, and is involved in both constitutive and regulated splicing as well as other cellular processes. It is upregulated in various human tumors and its induced overexpression leads to transformation of mammary epithelial cells and immortal rodent fibroblasts, suggesting that it may be a proto-oncogene (56, 57). SRSF1 affects AS of many target pre-mRNAs, some of which are known to contribute to tumorigenesis. It binds to an ESE in exon 12 of RON and promotes skipping of exon 11 to produce ΔRON, which enhances cancer invasiveness (20). Overexpression of SRSF1 leads to inclusion of exon 12a of BIN1 (a tumor suppressor gene), and the resulting isoform loses tumor-suppressor activity due to its inability to interact with MYC (56). In addition, SRSF1 promotes the production of isoform 2 of S6K1 through AS, and overexpression of this isoform is able to transform NIH3T3 cells (56). SRSF1 also modulates AS of MNK2 pre-mRNA: overexpression of SRSF1 results in production of the MNK2b isoform, promoting MAPK-independent phosphorylation of the eukaryotic initiation factor eIF4E, which enhances cap-dependent translation and may contribute to oncogenic transformation (56). It has also been shown that SRSF1 can interact directly with mTOR to facilitate phosphorylation of the translation inhibitor 4E-BP, leading to 4E-BP release from eIF4E and activation of translation (58). Recently, Anczukow et al. found that SRSF1 stimulates production of isoform BIM γ1, which lacks exons 2 and 3 of BIM (a pro-apoptotic BCL-2 family member), and concomitantly downregulates production of BIN1+13, a BIN1 isoform that includes exon 13 (57). Expression of BIM γ1 increased acinar size and decreased apoptosis whereas expression of isoform BIN1+13 did the opposite. Therefore, it was proposed that BIM γ1 upregulation and BIN1+13 downregulation combined contributes to SRSF1-induced tumorigenesis (57). Recently, it was shown that SRSF1 is regulated by MYC: MYC directly binds to two non-canonical E-boxes in the SRSF1 promoter and activates its transcription. Knockdown of MYC downregulates SRSF1 expression in lung cancer cell lines (59).
SRSF3 (formerly SRp20) is another SR protein that has been implicated in misregulation of AS in cancer. It is overexpressed in human ovarian, lung, breast, stomach, skin, bladder, colon, liver, thyroid, and kidney cancers (57, 60). Overexpression of SRSF3 leads to transformation of rodent fibroblasts, suggesting that it is a proto-oncogene (60). Knockdown of SRSF3 results in apoptosis of a variety of cancer cells (57, 60, 61). It was shown that knockdown of SRSF3 led to skipping of exon 8 of homeodomain-interacting protein kinase-2 (HIPK2), an antioncogene that induces tumor cell apoptosis. Full-length HIPK2 binds to an E3 ubiquitin ligase (Siah-1) and is constantly degraded, whereas the isoform HIPK2 Δe8, which lacks 27 amino acids, loses the ability to bind Siah-1 and therefore is resistant to protein degradation. HIPK2 Δe8 still retains antioncogenic activity and therefore induces apoptosis (61). Tang et al. provided evidence that SRSF3 also regulates AS of p53. Binding of SRSF3 to exon i9 of p53 inhibits production of isoform p53β. Downregulation of SRSF3 induces p53β production and promotes cellular senescence (62). Finally, Wang et al. identified an SRSF3 binding site in exon 10 of PKM pre-mRNA and showed that knockdown of SRSF3 resulted in an ∼20% switch from PKM2 to PKM1 and reduced lactate production (63).
hnRNP A1 and hnRNP A2 are two structurally and functionally related hnRNPs that likely play a role in cancer. Both proteins are overexpressed in a wide variety of cancers (42, 64, 65). RNAi-mediated knockdown of hnRNP A1 and A2 (A1/A2) together results in apoptosis in cancer cells, but not in normal cells, suggesting that the two proteins are important for cancer cell growth (64). Golan-Gerstl et al. showed that overexpression of hnRNP A2 in NIH3T3 cells induces skipping of RON exon 11 and production of ΔRON. Knockdown of RON inhibited the hnRNP A2-mediated transformation (65). A recent genome-wide analysis of AS events using high-throughput sequencing of RNA isolated by crosslinking immunoprecipitation (HITS-CLIP) showed that hnRNP A1 and A2 each potentially regulates more than 2,000 AS events (66). One important finding is that A1/A2 share many (one third) common targets, one of which is PKM. As mentioned above, deregulation of PKM AS is known to be critical for glucose metabolism in tumor cells. David et al. showed that A1/A2 together with another hnRNP protein, PTB, bind to sequences flanking exon 9 of PKM pre-mRNA and repress E9 inclusion and promote E10 inclusion (42). Expression levels of A1/A2 and PTB were found to correlate perfectly with the ratios of PKM2/PKM1 in a number of normal brain and glioma samples. Knockdown of A1/A2 and PTB, or of c-Myc, which drives their expression, results in switching from PKM2 to PKM1 (42). Consistent with these findings, another study showed that A1/A2 and PTB knockdown leads to a decrease of lactate production in a glioblastoma (GBM) cell line (43). Chen et al. further demonstrated how mechanistically the protein levels of A1/A2 and PTB determine the outcome of PKM AS: at high levels, A1/A2 and PTB predominantly bind to sites in and around exon 9 to repress E9 inclusion, but when their levels are reduced, their binding shifts to sites flanking exon 10, preventing E10 inclusion (67).
PTB (also known as hnRNP I) is an hnRNP that binds polypyrimidine-rich intronic elements and in most cases represses the inclusion of the regulated exon (68). It has been shown that PTB is upregulated in ovarian cancer and gliomas (42, 69, 70). Knockdown of PTB suppresses ovarian tumor cell growth and invasiveness in vitro (70). However, overexpression of PTB in immortalized or normal cells does not enhance proliferation, anchorage-independent growth, or invasion (71), suggesting that PTB may play a necessary, but not transforming, role in tumorigenesis. PTB is known to regulate several AS events that are relevant to cancer. Binding of PTB to an ISS element in the FGFR-1 transcript leads to skipping of the α exon and production of isoform FGFR-1β (72). This truncated receptor has higher affinity for FGF-1 (73), and might facilitate malignant progression of astrocytic tumors (74). PTB also regulates AS of USP5, a deubiquitinating enzyme whose knockdown can lead to accumulation of p53 (75). Two USP5 isoforms can be generated by use of alternative 5′ ss in exon 15. In GBM, high levels of PTB inhibit the proximal 5′ ss and use of the distal 5′ ss produces USP5 isoform 2. Switching isoform 2 to isoform 1 using antisense oligonucleotides inhibited growth and migration of two GBM cell lines (69). Genome-wide studies of PTB-regulated AS events in HeLa cells using HITS-CLIP showed that PTB not only represses but also activates exon inclusion, depending on whether its binding sites are located within and upstream of or downstream of the regulated exons (68, 76). The functional significance of these PTB-regulated AS events has not been examined, although one of the PTB targets is an ISS element upstream of exon 9 of PKM (68). As discussed earlier, PTB binds to this ISS and, together with hnRNP A1/A2, regulates PKM AS (42). The fact that tumor cells overexpress and recruit three different hnRNP proteins to regulate PKM AS reinforces the importance of producing PKM2 in tumor cells.
hnRNP H has recently been implicated in oncogenesis through the misregulation of AS of both IG20/MADD and RON pre-mRNAs (77). hnRNP H is upregulated in gliomas and binds to an ESS in exon 16 of IG20/MADD, leading to skipping of exon 16 and production of the MADD isoform, which is necessary and sufficient for cell survival (77). RNAi-mediated knockdown of hnRNP H reverses AS, producing the exon 16-containing IG20 isoform, and results in cell death of both U373 glioma and HeLa cells, possibly through IG20-triggered caspase 8 activation (78). In addition, hnRNP H binds to a similar ESS in exon 11 of RON and leads to skipping of exon 11 and production of ΔRON, which promotes cell invasiveness (77).
In the above examples, misregulation of AS occurs in the absence of genetic mutations and, in many cases, without changes in the overall levels of the alternatively spliced transcripts. Switching from one isoform to another is regulated by the levels and activity of RBPs, either individually or in combination. A recent proteomic study revealed as many as 860 RBPs in human (79). However, only a couple of dozens are well studied. A genome-wide analysis shows that each of six tested RBPs binds multiple sites and more than half of all AS events are regulated by multiple RBPs (66). It remains a difficult challenge to determine how these hundreds of RBPs cooperate and coordinately regulate the tens of thousands of normal AS events that are required for tissue differentiation (16, 17, 80). Any misregulation in this process may generate aberrant AS that leads to serious consequences, such as cancer.
Mutations in the Core Splicing Machinery and Cancer
The above studies all describe how changes in the intracellular levels of splicing regulatory proteins can contribute to cancer. What was lacking, however, were any examples of mutations in genes encoding splicing proteins that either cause or contribute to neoplastic transformation. A number of recent studies, though, have identified mutations affecting components of the core splicing machinery that play critical roles in neoplasia. Given the importance of these findings to our appreciation of the role of splicing in cancer, we discuss these studies in some detail.
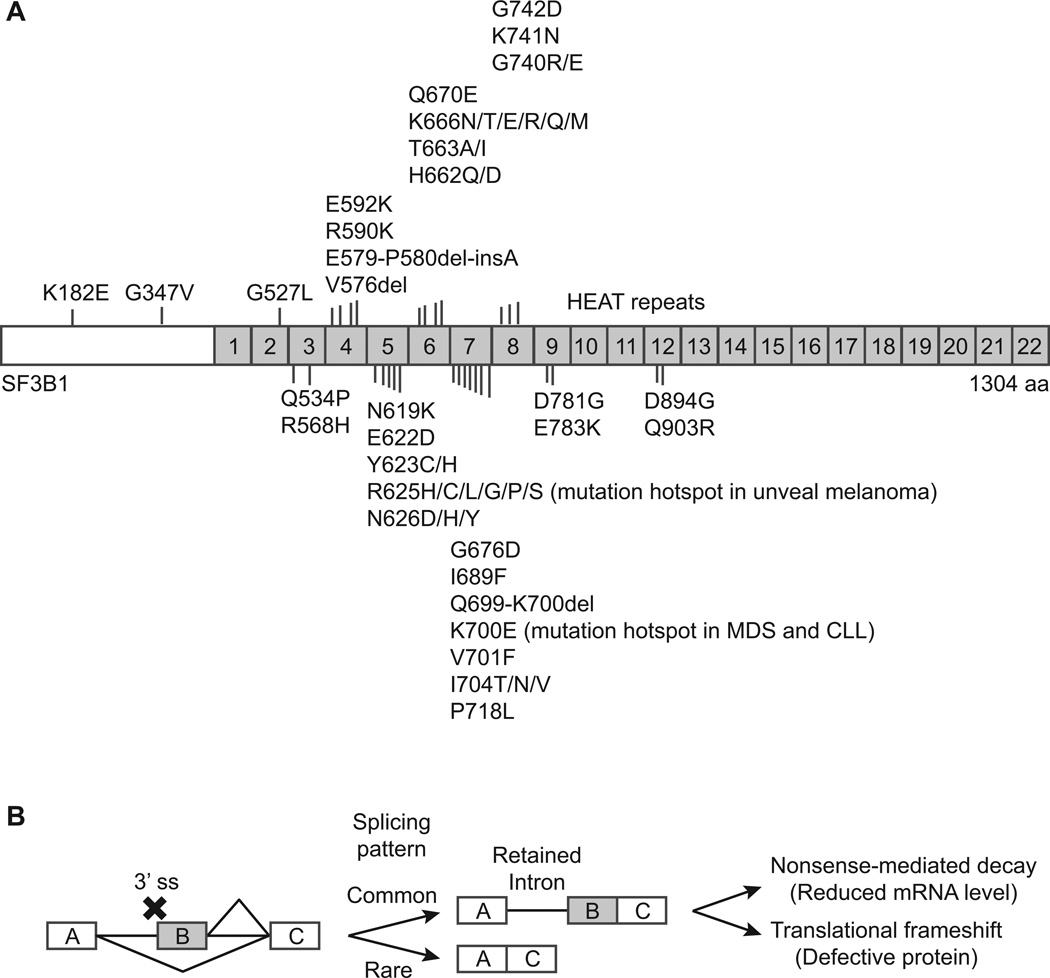
In 2011, Yoshida et al. reported recurrent somatic mutations in the genes encoding components of the RNA splicing machinery in Myelodysplastic syndromes (MDS), a diverse group of myeloid neoplasms characterized by an abnormality in myeloid blood cell production and propensity of progression into acute myeloid leukemia (81). The most frequently mutated genes encode splicing factors SF3B1, U2AF1, SRSF2, and ZRSR2. Mutational frequencies for SF3B1 are particularly high in refractory anemia with ring sideroblasts (RARS) and RARS with thrombocytosis (RARS-T), ranging from 64–83% (81–83). SF3B1 is also frequently mutated in Chronic Lymphocytic Leukemia (CLL) (84–86), as well as in uveal melanoma (87, 88). All SF3B1 mutations are heterozygous, and none are nonsense mutations or introduce a frameshift (Figure 2A). The mechanism through which the splicing factor mutations misregulate RNA splicing and subsequently lead to disease is still unknown. Here we offer our perspectives.
Figure 2.
Splicing factor mutations and aberrant splicing patterns. A, distribution of mutations in SF3B1. B, aberrant splicing with defects in 3’ ss recognition. Outcomes of defects in 3’ splice recognition or utilization are diagrammed.
SF3B1, U2AF1, SRSF2, and ZRSR2 are all involved in the selection of splice sites at the 3′ end of introns. Mutations in these genes most likely reflect defects in 3′ ss recognition during RNA splicing. As shown in Figure 2, defects in 3′ ss recognition (but with normal 5′ ss recognition) can result in two 5′ ss competing for one 3′ ss, an AS pattern that resembles alternative 5′ ss. A frequent outcome of alternative 5′ ss is the selection of the 5′ ss proximal to the downstream 3′ ss (89). As a result, the final mRNA product often has retained introns (Figure 2B). In support of this speculation, Yoshida et al. showed that expression of mutant U2AF1 results in large scale (∼5%) intron retention in HeLa cells (81). Because introns are rich with stop codons, retained introns frequently introduce into the mRNA premature termination codons (PTCs) that activate nonsense-mediated mRNA decay (NMD), which was observed in the HeLa cells expressing mutant U2AF1 (81). It must be noted that not every intron is retained by 5%, but instead some introns are retained while others are not. For example, intron 59 of BIRC6 pre-mRNA is mostly retained, but no retention is found in intron 58 or intron 60 of the same gene. This ″all-or-nothing″ (technically ″more-or-less″) splicing pattern implies that there is intron sequence specificity for mutant U2AF1. Because most of the mRNAs with retained introns are rapidly degraded by NMD, it is difficult to identify sequence conservation in those degraded introns. Therefore, NMD inhibitors may be useful to help identify the targets of the mutated splicing factors.
Several studies have begun to examine the role of SF3B1 in MDS. Visconte et al. showed that knockdown of SF3B1 in K562 cells resulted in retention of introns (90). However, SF3B1 knockdown did not produce ring sideroblasts (RS), possibly because K562 cells are not able to differentiate along the erythroid lineage. Indeed, in healthy human bone marrow cells, RS formation was induced by meayamycin, an SF3B1 inhibitor (90). However, this SF3B1 “haploinsufficiency” hypothesis cannot explain the absence of nonsense and frameshift mutations in SF3B1, which suggests that the mutated protein likely maintains structural integrity, but with altered function. RNA sequencing analysis of samples from one healthy donor and two patients with SF3B1 mutations revealed that 130 genes were differentially expressed, of which 94% (an unusually high percentage) had lower expression in patients (90). One explanation for this is that some introns in these genes are retained and the mRNA is rapidly degraded through NMD. Nevertheless, none of these downregulated genes is involved in mitochondrial function or related to the RS phenotype. This may reflect the choice of control. Visconte et al. used total bone marrow cells from a healthy donor as a control, which contains a mixture of all types of blood cells with unknown cell ratios, making a complex gene expression profile. When purified CD34+ cells were used as a control, Papaemmanuil et al. found that key genes in the mitochondrial pathways are downregulated in MDS patients with SF3B1 mutations (83). In particular, the mitochondrial gene ABCB7 is consistently downregulated in RARS patients, suggesting that it may be a key mediator of ineffective erythropoiesis of RARS (91). Indeed, Nikpour et al. recently demonstrated that reduced expression of ABCB7 in normal bone marrow markedly reduced erythroid differentiation and growth with accumulation of mitochondrial ferritin, a phenotype similar to that observed in intermediate RARS erythroblasts (92). It remains to be determined how SF3B1 mutations result in downregulation of ABCB7, although it is possible that intron retention followed by NMD contributes.
The link between the splicing gene mutations and clonal expansion of hematopoietic stem cells remains unclear. Expression of mutant U2AF1 leads to death, rather than promoting growth, of both HeLa cells and TF-1 cells in vitro (81). This unexpected result might reflect the fact that the outcome of splicing defects may depend on certain cellular contexts, as knockdown of SF3B1 in K562 does not induce the RS phenotype, while inhibition of SF3B1 in bone marrow cells does (90). Another unexpected result is that mutant U2AF1 impairs the reconstitution capability, rather than promoting clonal expansion, of mouse CD34+ cells (81). A possible explanation for this stems from the perhaps unexpected differences between mouse and human AS. Recent studies showed that mouse AS is drastically different from human AS (16, 17). Even though human and mouse splicing factors are almost identical (SF3B1 and SRSF2 are 100%, U2AF1 96% and ZRSR2 82% identical between human and mouse), only one quarter of human AS events were observed in mouse (16). Therefore, using mouse models to study human diseases that reflect changes in AS, or aberrant splicing more generally, may be misleading.
Several other lines of evidence also suggest that the effects of spliceosomal gene mutations may be dependent of cellular contexts. For example, MDS patients with SF3B1 mutations generally have a favorable prognosis while SF3B1 mutations in CLL correlate with poor overall survival and resistance to chemotherapy (85, 86, 93–95). Unlike in adult MDS, spliceosomal mutations are rare in pediatric MDS and juvenile myelomonocytic leukemia (96). In unveal melanoma SF3B1 mutations are frequent (87, 88), but none of the 85 cutaneous melanomas has an SF3B1 mutation (97). This cellular-context dependency of effects provides an opportunity for developing anti-tumor drugs: tumor cells and normal cells are known to have different cellular contexts; therefore, modulating the activities of spliceosomal proteins will likely yield different, even opposing, effects. Indeed, spliceosome modulators such as sudemycins, pladienolide B, FR901464 and its derivative spliceostatin A (SSA), have potent toxicity to tumor cell lines, but display little toxicity to normal cells (98–100). It has been shown that FR901464 and SSA bind to SF3b complex and promote retention of intron 1 of p27, a cyclin-dependent kinase (CDK) inhibitor. Translation of the intron-1 containing pre-mRNA leads to production of a C-terminal truncated protein isoform p27*, which is resistant to proteasomal degradation and inhibits CDK2 kinase activity, thereby inhibiting cell growth (101). SSA treatment also leads to intron retention in VEGF and results in reduction of VEGF levels (possibly by NMD), inhibiting cancer cell angiogenesis (102). Although the exact mechanism of selective tumor cytotoxicity remains to be fully explored, one explanation is that growth of cancer cells often relies on oncogenic protein isoforms (arising from AS), which are lacking in normal cells.
Conclusions
It has become clear that aberrant pre-mRNA AS is a major contributor to cancer phenotypes. With the rapid advances in high-throughput RNA sequencing technologies, more cancer-specific AS events will likely be discovered. However, our understanding of the misregulation of AS in cancer lags far behind. The past decades have implicated only a handful of RBPs in this process. It remains a challenge to study systematically how the likely hundreds of RBPs (as well as components of the core splicing machinery) coordinately regulate tens of thousands of AS events in normal tissues and how they misregulate AS in cancer. Nevertheless, therapeutic intervention targeting either the cancer-specific AS events themselves or the splicing factors that misregulate them is promising. Given that cancer cells utilize AS mechanism to gain survival advantages, it also will be important in the future to explore AS regulation in still greater depth to find ways to combat cancer.
Significance.
An increasing body of evidence indicates that aberrant splicing of mRNA precursors leads to production of aberrant proteins that contribute to tumorigenesis. Recent studies show that alterations in cellular concentrations of regulatory splicing factors and mutations in components of the core splicing machinery provide major mechanisms of misregulation of mRNA splicing in cancer. A better understanding of this misregulation will potentially reveal a group of novel drug targets for therapeutic intervention.
Acknowledgments
Grant Support
This work is supported by a grant (R01GM048259 to J.L. Manley) from the National Institutes of Health. J. Zhang is the recipient of an American Brain Tumor Association Basic Research Fellowship in Honor of Denise Kimball.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors' Contributions
Conception and design: J. Zhang, J.L. Manley
Writing, review, and/or revision of the manuscript: J. Zhang, J.L. Manley
References
- 1.Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang L, Mayr C, et al. Alternative isoform regulation in human tissue transcriptomes. Nature. 2008;456:470–476. doi: 10.1038/nature07509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan Q, Shai O, Lee LJ, Frey BJ, Blencowe BJ. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat Genet. 2008;40:1413–1415. doi: 10.1038/ng.259. [DOI] [PubMed] [Google Scholar]
- 3.Leoni G, Le Pera L, Ferre F, Raimondo D, Tramontano A. Coding potential of the products of alternative splicing in human. Genome Biol. 2011;12:R9. doi: 10.1186/gb-2011-12-1-r9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.David CJ, Manley JL. Alternative pre-mRNA splicing regulation in cancer: pathways and programs unhinged. Genes & Dev. 2010;24:2343–2364. doi: 10.1101/gad.1973010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelemen O, Convertini P, Zhang Z, Wen Y, Shen M, Falaleeva M, et al. Function of alternative splicing. Gene. 2013;514:1–30. doi: 10.1016/j.gene.2012.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nilsen TW, Graveley BR. Expansion of the eukaryotic proteome by alternative splicing. Nature. 2010;463:457–63. doi: 10.1038/nature08909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bland CS, Wang ET, Vu A, David MP, Castle JC, Johnson JM, et al. Global regulation of alternative splicing during myogenic differentiation. Nucleic Acids Res. 2010;38:7651–7664. doi: 10.1093/nar/gkq614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson MB, Kawasawa YI, Mason CE, Krsnik Z, Coppola G, Bogdanovic D, et al. Functional and evolutionary insights into human brain development through global transcriptome analysis. Neuron. 2009;62:494–509. doi: 10.1016/j.neuron.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He C, Zhou F, Zuo Z, Cheng H, Zhou R. A global view of cancer-specific transcript variants by subtractive transcriptome-wide analysis. PloS One. 2009;4:e4732. doi: 10.1371/journal.pone.0004732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venables JP, Klinck R, Bramard A, Inkel L, Dufresne-Martin G, Koh C, et al. Identification of alternative splicing markers for breast cancer. Cancer Res. 2008;68:9525–9531. doi: 10.1158/0008-5472.CAN-08-1769. [DOI] [PubMed] [Google Scholar]
- 11.Shapiro IM, Cheng AW, Flytzanis NC, Balsamo M, Condeelis JS, Oktay MH, et al. An EMT-driven alternative splicing program occurs in human breast cancer and modulates cellular phenotype. PLoS Genet. 2011;7:e1002218. doi: 10.1371/journal.pgen.1002218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prinos P, Garneau D, Lucier JF, Gendron D, Couture S, Boivin M, et al. Alternative splicing of SYK regulates mitosis and cell survival. Nat Struct Mol Biol. 2011;18:673–679. doi: 10.1038/nsmb.2040. [DOI] [PubMed] [Google Scholar]
- 13.Grosso AR, Martins S, Carmo-Fonseca M. The emerging role of splicing factors in cancer. EMBO Rep. 2008;9:1087–1093. doi: 10.1038/embor.2008.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper TA, Wan L, Dreyfuss G. RNA and disease. Cell. 2009;136:777–793. doi: 10.1016/j.cell.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen M, Manley JL. Mechanisms of alternative splicing regulation: insights from molecular and genomics approaches. Nat Rev Mol Cell Biol. 2009;10:741–754. doi: 10.1038/nrm2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barbosa-Morais NL, Irimia M, Pan Q, Xiong HY, Gueroussov S, Lee LJ, et al. The evolutionary landscape of alternative splicing in vertebrate species. Science. 2012;338:1587–1593. doi: 10.1126/science.1230612. [DOI] [PubMed] [Google Scholar]
- 17.Merkin J, Russell C, Chen P, Burge CB. Evolutionary dynamics of gene and isoform regulation in Mammalian tissues. Science. 2012;338:1593–1599. doi: 10.1126/science.1228186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaudino G, Follenzi A, Naldini L, Collesi C, Santoro M, Gallo KA, et al. RON is a heterodimeric tyrosine kinase receptor activated by the HGF homologue MSP. EMBO J. 1994;13:3524–3532. doi: 10.1002/j.1460-2075.1994.tb06659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagh PK, Peace BE, Waltz SE. Met-related receptor tyrosine kinase Ron in tumor growth and metastasis. Adv Cancer Res. 2008;100:1–33. doi: 10.1016/S0065-230X(08)00001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghigna C, Giordano S, Shen H, Benvenuto F, Castiglioni F, Comoglio PM, et al. Cell motility is controlled by SF2/ASF through alternative splicing of the Ron protooncogene. Mol Cell. 2005;20:881–890. doi: 10.1016/j.molcel.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 21.Collesi C, Santoro MM, Gaudino G, Comoglio PM. A splicing variant of the RON transcript induces constitutive tyrosine kinase activity and an invasive phenotype. Mol Cell Biol. 1996;16:5518–5526. doi: 10.1128/mcb.16.10.5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 23.Poulikakos PI, Persaud Y, Janakiraman M, Kong X, Ng C, Moriceau G, et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E) Nature. 2011;480:387–390. doi: 10.1038/nature10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coopman PJ, Do MT, Barth M, Bowden ET, Hayes AJ, Basyuk E, et al. The Syk tyrosine kinase Suppresses malignant growth of human breast cancer cells. Nature. 2000;406:742–747. doi: 10.1038/35021086. [DOI] [PubMed] [Google Scholar]
- 25.Feldman AL, Sun DX, Law ME, Novak AJ, Attygalle AD, Thorland EC, et al. Overexpression of Syk tyrosine kinase in peripheral T-cell lymphomas. Leukemia. 2008;22:1139–1143. doi: 10.1038/leu.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buchner M, Fuchs S, Prinz G, Pfeifer D, Bartholome K, Burger M, et al. Spleen tyrosine kinase is overexpressed and represents a potential therapeutic target in chronic lymphocytic leukemia. Cancer Res. 2009;69:5424–5432. doi: 10.1158/0008-5472.CAN-08-4252. [DOI] [PubMed] [Google Scholar]
- 27.Luangdilok S, Box C, Patterson L, Court W, Harrington K, Pitkin L, et al. Syk tyrosine kinase is linked to cell motility and progression in squamous cell carcinomas of the head and neck. Cancer Res. 2007;67:7907–7916. doi: 10.1158/0008-5472.CAN-07-0331. [DOI] [PubMed] [Google Scholar]
- 28.Chao DT, Korsmeyer SJ. BCL-2 family: regulators of cell death. Annu Rev Immunol. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- 29.Boise LH, Gonzalez-Garcia M, Postema CE, Ding L, Lindsten T, Turka LA, et al. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- 30.Xerri L, Parc P, Brousset P, Schlaifer D, Hassoun J, Reed JC, et al. Predominant expression of the long isoform of Bcl-x (Bcl-xL) in human lymphomas. Br J Haematol. 1996;92:900–906. doi: 10.1046/j.1365-2141.1996.423958.x. [DOI] [PubMed] [Google Scholar]
- 31.Olopade OI, Adeyanju MO, Safa AR, Hagos F, Mick R, Thompson CB, et al. Overexpression of BCL-x protein in primary breast cancer is associated with high tumor grade and nodal metastases. Cancer J Sci Am. 1997;3:230–237. [PubMed] [Google Scholar]
- 32.Takehara T, Liu X, Fujimoto J, Friedman SL, Takahashi H. Expression and role of Bcl-xL in human hepatocellular carcinomas. Hepatology. 2001;34:55–61. doi: 10.1053/jhep.2001.25387. [DOI] [PubMed] [Google Scholar]
- 33.Ma X, Zhao Y, Li Y, Lu H, He Y. Relevance of Bcl-x expression in different types of endometrial tissues. J Exp Clin Cancer Res. 2010;29:14. doi: 10.1186/1756-9966-29-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keck PJ, Hauser SD, Krivi G, Sanzo K, Warren T, Feder J, et al. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989;246:1309–1312. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- 35.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 36.Biselli-Chicote PM, Oliveira AR, Pavarino EC, Goloni-Bertollo EM. VEGF gene alternative splicing: pro- and anti-angiogenic isoforms in cancer. J Cancer Res Clin Oncol. 2012;138:363–370. doi: 10.1007/s00432-011-1073-2. [DOI] [PubMed] [Google Scholar]
- 37.Kawamura H, Li X, Harper SJ, Bates DO, Claesson-Welsh L. Vascular endothelial growth factor (VEGF)-A165b is a weak in vitro agonist for VEGF receptor-2 due to lack of coreceptor binding and deficient regulation of kinase activity. Cancer Res. 2008;68:4683–4692. doi: 10.1158/0008-5472.CAN-07-6577. [DOI] [PubMed] [Google Scholar]
- 38.Gough DJ, Levy DE, Johnstone RW, Clarke CJ. IFNgamma signaling-does it mean JAK-STAT? Cytokine Growth Factor Rev. 2008;19:383–394. doi: 10.1016/j.cytogfr.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 39.Goldstein D, Laszlo J. The role of interferon in cancer therapy: a current perspective. CA Cancer J Clin. 1988;38:258–277. doi: 10.3322/canjclin.38.5.258. [DOI] [PubMed] [Google Scholar]
- 40.Du Z, Fan M, Kim JG, Eckerle D, Lothstein L, Wei L, et al. Interferon-resistant Daudi cell line with a Stat2 defect is resistant to apoptosis induced by chemotherapeutic agents. J Biol Chem. 2009;284:27808–27815. doi: 10.1074/jbc.M109.028324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 42.David CJ, Chen M, Assanah M, Canoll P, Manley JL. HnRNP proteins controlled by c-Myc deregulate pyruvate kinase mRNA splicing in cancer. Nature. 2010;463:364–368. doi: 10.1038/nature08697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clower CV, Chatterjee D, Wang Z, Cantley LC, Vander Heiden MG, Krainer AR. The alternative splicing repressors hnRNP A1/A2 and PTB influence pyruvate kinase isoform expression and cell metabolism. Proc Natl Acad Sci U S A. 2010;107:1894–1899. doi: 10.1073/pnas.0914845107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 45.Momand J, Zambetti GP, Olson DC, George D, Levine AJ. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69:1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- 46.Bartel F, Taubert H, Harris LC. Alternative and aberrant splicing of MDM2 mRNA in human cancer. Cancer cell. 2002;2:9–15. doi: 10.1016/s1535-6108(02)00091-0. [DOI] [PubMed] [Google Scholar]
- 47.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 48.Kubbutat MH, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature. 1997;387:299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- 49.Sigalas I, Calvert AH, Anderson JJ, Neal DE, Lunec J. Alternatively spliced mdm2 transcripts with loss of p53 binding domain sequences: transforming ability and frequent detection in human cancer. Nat Med. 1996;2:912–917. doi: 10.1038/nm0896-912. [DOI] [PubMed] [Google Scholar]
- 50.Manfredi JJ. The Mdm2-p53 relationship evolves: Mdm2 swings both ways as an oncogene and a tumor suppressor. Genes & Dev. 2010;24:1580–1589. doi: 10.1101/gad.1941710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fu XD. The superfamily of arginine/serine-rich splicing factors. RNA. 1995;1:663–680. [PMC free article] [PubMed] [Google Scholar]
- 52.Graveley BR. Sorting out the complexity of SR protein functions. RNA. 2000;6:1197–1211. doi: 10.1017/s1355838200000960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Long JC, Caceres JF. The SR protein family of splicing factors: master regulators of gene expression. Biochem J. 2009;417:15–27. doi: 10.1042/BJ20081501. [DOI] [PubMed] [Google Scholar]
- 54.Martinez-Contreras R, Cloutier P, Shkreta L, Fisette JF, Revil T, Chabot B. hnRNP proteins and splicing control. Adv Exp Med Biol. 2007;623:123–147. doi: 10.1007/978-0-387-77374-2_8. [DOI] [PubMed] [Google Scholar]
- 55.Manley JL, Tacke R. SR proteins and splicing control. Genes & Dev. 1996;10:1569–1579. doi: 10.1101/gad.10.13.1569. [DOI] [PubMed] [Google Scholar]
- 56.Karni R, de Stanchina E, Lowe SW, Sinha R, Mu D, Krainer AR. The gene encoding the splicing factor SF2/ASF is a proto-oncogene. Nat Struct Mol Biol. 2007;14:185–193. doi: 10.1038/nsmb1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anczukow O, Rosenberg AZ, Akerman M, Das S, Zhan L, Karni R, et al. The splicing factor SRSF1 regulates apoptosis and proliferation to promote mammary epithelial cell transformation. Nat Struct Mol Biol. 2012;19:220–228. doi: 10.1038/nsmb.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Michlewski G, Sanford JR, Caceres JF. The splicing factor SF2/ASF regulates translation initiation by enhancing phosphorylation of 4E-BP1. Mol Cell. 2008;30:179–189. doi: 10.1016/j.molcel.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 59.Das S, Anczukow O, Akerman M, Krainer AR. Oncogenic splicing factor SRSF1 is a critical transcriptional target of MYC. Cell Rep. 2012;1:110–117. doi: 10.1016/j.celrep.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jia R, Li C, McCoy JP, Deng CX, Zheng ZM. SRp20 is a proto-oncogene critical for cell proliferation and tumor induction and maintenance. Int J Biol Sci. 2010;6:806–826. doi: 10.7150/ijbs.6.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kurokawa K, Akaike Y, Masuda K, Kuwano Y, Nishida K, Yamagishi N, et al. Downregulation of serine/arginine-rich splicing factor 3 induces G1 cell cycle arrest and apoptosis in colon cancer cells. Oncogene. 2013 doi: 10.1038/onc.2013.86. [DOI] [PubMed] [Google Scholar]
- 62.Tang Y, Horikawa I, Ajiro M, Robles AI, Fujita K, Mondal AM, et al. Downregulation of splicing factor SRSF3 induces p53beta, an alternatively spliced isoform of p53 that promotes cellular senescence. Oncogene. 2012 doi: 10.1038/onc.2012.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Z, Chatterjee D, Jeon HY, Akerman M, Vander Heiden MG, Cantley LC, et al. Exon-centric regulation of pyruvate kinase M alternative splicing via mutually exclusive exons. J Mol Cell Biol. 2012;4:79–87. doi: 10.1093/jmcb/mjr030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Patry C, Bouchard L, Labrecque P, Gendron D, Lemieux B, Toutant J, et al. Small interfering RNA-mediated reduction in heterogeneous nuclear ribonucleoparticule A1/A2 proteins induces apoptosis in human cancer cells but not in normal mortal cell lines. Cancer Res. 2003;63:7679–7688. [PubMed] [Google Scholar]
- 65.Golan-Gerstl R, Cohen M, Shilo A, Suh SS, Bakacs A, Coppola L, et al. Splicing factor hnRNP A2/B1 regulates tumor suppressor gene splicing and is an oncogenic driver in glioblastoma. Cancer Res. 2011;71:4464–4472. doi: 10.1158/0008-5472.CAN-10-4410. [DOI] [PubMed] [Google Scholar]
- 66.Huelga SC, Vu AQ, Arnold JD, Liang TY, Liu PP, Yan BY, et al. Integrative genome-wide analysis reveals cooperative regulation of alternative splicing by hnRNP proteins. Cell Rep. 2012;1:167–178. doi: 10.1016/j.celrep.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen M, David CJ, Manley JL. Concentration-dependent control of pyruvate kinase M mutually exclusive splicing by hnRNP proteins. Nat Struct Mol Biol. 2012;19:346–354. doi: 10.1038/nsmb.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xue Y, Zhou Y, Wu T, Zhu T, Ji X, Kwon YS, et al. Genome-wide analysis of PTB-RNA interactions reveals a strategy used by the general splicing repressor to modulate exon inclusion or skipping. Mol Cell. 2009;36:996–1006. doi: 10.1016/j.molcel.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Izaguirre DI, Zhu W, Hai T, Cheung HC, Krahe R, Cote GJ. PTBP1-dependent regulation of USP5 alternative RNA splicing plays a role in glioblastoma tumorigenesis. Mol Carcinog. 2012;51:895–906. doi: 10.1002/mc.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.He X, Pool M, Darcy KM, Lim SB, Auersperg N, Coon JS, et al. Knockdown of polypyrimidine tract-binding protein suppresses ovarian tumor cell growth and invasiveness in vitro. Oncogene. 2007;26:4961–4968. doi: 10.1038/sj.onc.1210307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang C, Norton JT, Ghosh S, Kim J, Fushimi K, Wu JY, et al. Polypyrimidine tract-binding protein (PTB) differentially affects malignancy in a cell line-dependent manner. J Biol Chem. 2008;283:20277–20287. doi: 10.1074/jbc.M803682200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jin W, Bruno IG, Xie TX, Sanger LJ, Cote GJ. Polypyrimidine tract-binding protein down-regulates fibroblast growth factor receptor 1 alpha-exon inclusion. Cancer Res. 2003;63:6154–6157. [PubMed] [Google Scholar]
- 73.Wang F, Kan M, Yan G, Xu J, McKeehan WL. Alternately spliced NH2-terminal immunoglobulin-like Loop I in the ectodomain of the fibroblast growth factor (FGF) receptor 1 lowers affinity for both heparin and FGF-1. J Biol Chem. 1995;270:10231–10235. doi: 10.1074/jbc.270.17.10231. [DOI] [PubMed] [Google Scholar]
- 74.Yamaguchi F, Saya H, Bruner JM, Morrison RS. Differential expression of two fibroblast growth factor-receptor genes is associated with malignant progression in human astrocytomas. Proc Natl Acad Sci U S A. 1994;91:484–488. doi: 10.1073/pnas.91.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dayal S, Sparks A, Jacob J, Allende-Vega N, Lane DP, Saville MK. Suppression of the deubiquitinating enzyme USP5 causes the accumulation of unanchored polyubiquitin and the activation of p53. J Biol Chem. 2009;284:5030–5041. doi: 10.1074/jbc.M805871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Llorian M, Schwartz S, Clark TA, Hollander D, Tan LY, Spellman R, et al. Position-dependent alternative splicing activity revealed by global profiling of alternative splicing events regulated by PTB. Nat Struct Mol Biol. 2010;17:1114–1123. doi: 10.1038/nsmb.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lefave CV, Squatrito M, Vorlova S, Rocco GL, Brennan CW, Holland EC, et al. Splicing factor hnRNPH drives an oncogenic splicing switch in gliomas. EMBO J. 2011;30:4084–4097. doi: 10.1038/emboj.2011.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mulherkar N, Prasad KV, Prabhakar BS. MADD/DENN splice variant of the IG20 gene is a negative regulator of caspase-8 activation Knockdown enhances TRAIL-induced apoptosis of cancer cells. J Biol Chem. 2007;282:11715–11721. doi: 10.1074/jbc.M701085200. [DOI] [PubMed] [Google Scholar]
- 79.Castello A, Fischer B, Eichelbaum K, Horos R, Beckmann BM, Strein C, et al. Insights into RNA biology from an atlas of mammalian mRNA-binding proteins. Cell. 2012;149:1393–1406. doi: 10.1016/j.cell.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 80.Barash Y, Calarco JA, Gao W, Pan Q, Wang X, Shai O, et al. Deciphering the splicing code. Nature. 2010;465:53–59. doi: 10.1038/nature09000. [DOI] [PubMed] [Google Scholar]
- 81.Yoshida K, Sanada M, Shiraishi Y, Nowak D, Nagata Y, Yamamoto R, et al. Frequent pathway mutations of splicing machinery in myelodysplasia. Nature. 2011;478:64–69. doi: 10.1038/nature10496. [DOI] [PubMed] [Google Scholar]
- 82.Visconte V, Makishima H, Jankowska A, Szpurka H, Traina F, Jerez A, et al. SF3B1, a splicing factor is frequently mutated in refractory anemia with ring sideroblasts. Leukemia. 2012;26:542–545. doi: 10.1038/leu.2011.232. [DOI] [PubMed] [Google Scholar]
- 83.Papaemmanuil E, Cazzola M, Boultwood J, Malcovati L, Vyas P, Bowen D, et al. Somatic SF3B1 mutation in myelodysplasia with ring sideroblasts. N Engl J Med. 2011;365:1384–1395. doi: 10.1056/NEJMoa1103283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang L, Lawrence MS, Wan Y, Stojanov P, Sougnez C, Stevenson K, et al. SF3B1 and other novel cancer genes in chronic lymphocytic leukemia. N Engl J Med. 2011;365:2497–2506. doi: 10.1056/NEJMoa1109016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rossi D, Bruscaggin A, Spina V, Rasi S, Khiabanian H, Messina M, et al. Mutations of the SF3B1 splicing factor in chronic lymphocytic leukemia: association with progression and fludarabine-refractoriness. Blood. 2011;118:6904–6908. doi: 10.1182/blood-2011-08-373159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Quesada V, Conde L, Villamor N, Ordonez GR, Jares P, Bassaganyas L, et al. Exome sequencing identifies recurrent mutations of the splicing factor SF3B1 gene in chronic lymphocytic leukemia. Nat Genet. 2012;44:47–52. doi: 10.1038/ng.1032. [DOI] [PubMed] [Google Scholar]
- 87.Harbour JW, Roberson ED, Anbunathan H, Onken MD, Worley LA, Bowcock AM. Recurrent mutations at codon 625 of the splicing factor SF3B1 in uveal melanoma. Nat Genet. 2013;45:133–135. doi: 10.1038/ng.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Martin M, Masshofer L, Temming P, Rahmann S, Metz C, Bornfeld N, et al. Exome sequencing identifies recurrent somatic mutations in EIF1AX and SF3B1 in uveal melanoma with disomy 3. Nat Genet. 2013 doi: 10.1038/ng.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hicks MJ, Mueller WF, Shepard PJ, Hertel KJ. Competing upstream 5′ splice sites enhance the rate of proximal splicing. Mol Cell Biol. 2010;30:1878–1886. doi: 10.1128/MCB.01071-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Visconte V, Rogers HJ, Singh J, Barnard J, Bupathi M, Traina F, et al. SF3B1 haploinsufficiency leads to formation of ring sideroblasts in myelodysplastic syndromes. Blood. 2012;120:3173–3186. doi: 10.1182/blood-2012-05-430876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Boultwood J, Pellagatti A, Nikpour M, Pushkaran B, Fidler C, Cattan H, et al. The role of the iron transporter ABCB7 in refractory anemia with ring sideroblasts. PloS One. 2008;3:e1970. doi: 10.1371/journal.pone.0001970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nikpour M, Scharenberg C, Liu A, Conte S, Karimi M, Mortera-Blanco T, et al. The transporter ABCB7 is a mediator of the phenotype of acquired refractory anemia with ring sideroblasts. Leukemia. 2013;27:889–896. doi: 10.1038/leu.2012.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Malcovati L, Papaemmanuil E, Bowen DT, Boultwood J, Della Porta MG, Pascutto C, et al. Clinical significance of SF3B1 mutations in myelodysplastic syndromes and myelodysplastic/myeloproliferative neoplasms. Blood. 2011;118:6239–6246. doi: 10.1182/blood-2011-09-377275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Patnaik MM, Lasho TL, Hodnefield JM, Knudson RA, Ketterling RP, Garcia-Manero G, et al. SF3B1 mutations are prevalent in myelodysplastic syndromes with ring sideroblasts but do not hold independent prognostic value. Blood. 2012;119:569–572. doi: 10.1182/blood-2011-09-377994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Makishima H, Visconte V, Sakaguchi H, Jankowska AM, Abu Kar S, Jerez A, et al. Mutations in the spliceosome machinery, a novel and ubiquitous pathway in leukemogenesis. Blood. 2012;119:3203–3210. doi: 10.1182/blood-2011-12-399774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hirabayashi S, Flotho C, Moetter J, Heuser M, Hasle H, Gruhn B, et al. Spliceosomal gene aberrations are rare, coexist with oncogenic mutations, and are unlikely to exert a driver effect in childhood MDS and JMML. Blood. 2012;119:e96–e99. doi: 10.1182/blood-2011-12-395087. [DOI] [PubMed] [Google Scholar]
- 97.Schilling B, Bielefeld N, Sucker A, Hillen U, Zimmer L, Schadendorf D, et al. Lack of SF3B1 R625 mutations in cutaneous melanoma. Diagn Pathol. 2013;8:87. doi: 10.1186/1746-1596-8-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kaida D, Schneider-Poetsch T, Yoshida M. Splicing in oncogenesis and tumor suppression. Cancer Sci. 2012;103:1611–1616. doi: 10.1111/j.1349-7006.2012.02356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bonnal S, Vigevani L, Valcarcel J. The spliceosome as a target of novel antitumour drugs. Nat Rev Drug Discov. 2012;11:847–859. doi: 10.1038/nrd3823. [DOI] [PubMed] [Google Scholar]
- 100.Webb TR, Joyner AS, Potter PM. The development and application of small molecule modulators of SF3b as therapeutic agents for cancer. Drug Discov Today. 2013;18:43–49. doi: 10.1016/j.drudis.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kaida D, Motoyoshi H, Tashiro E, Nojima T, Hagiwara M, Ishigami K, et al. Spliceostatin A targets SF3b and inhibits both splicing and nuclear retention of pre-mRNA. Nat Chem Biol. 2007;3:576–583. doi: 10.1038/nchembio.2007.18. [DOI] [PubMed] [Google Scholar]
- 102.Furumai R, Uchida K, Komi Y, Yoneyama M, Ishigami K, Watanabe H, et al. Spliceostatin A blocks angiogenesis by inhibiting global gene expression including VEGF. Cancer Sci. 2010;101:2483–2489. doi: 10.1111/j.1349-7006.2010.01686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]