Abstract
Baicalein (BA), a plant-derived active flavonoid present in the root of Scutellaria baicalensis, has been widely used for the treatment of stress-related neuropsychiatric disorders including depression. Previous studies have demonstrated that repeated restraint stress disrupts the activity of the hypothalamic-pituitary-adrenal (HPA) axis, resulting in depression. The behavioral and neurochemical basis of the BA effect on depression remain unclear. The present study used the forced swimming test (FST) and changes in brain neurotransmitter levels to confirm the impact of BA on repeated restraint stress-induced behavioral and neurochemical changes in rats. Male rats received 10, 20, or 40 mg/kg BA (i.p.) 30 min prior to daily exposure to repeated restraint stress (2 h/day) for 14 days. Activation of the HPA axis in response to repeated restraint stress was confirmed by measuring serum corticosterone levels and the expression of corticotrophin-releasing factor in the hypothalamus. Daily BA administration significantly decreased the duration of immobility in the FST, increased sucrose consumption, and restored the stress-related decreases in dopamine concentrations in the hippocampus to near normal levels. BA significantly inhibited the stress-induced decrease in neuronal tyrosine hydroxylase immunoreactivity in the ventral tegmental area and the expression of brain-derived neurotrophic factor (BDNF) mRNA in the hippocampus. Taken together, these findings indicate that administration of BA prior to the repeated restraint stress significantly improves helpless behaviors and depressive symptoms, possibly by preventing the decrease in dopamine and BDNF expression. Thus, BA may be a useful agent for the treatment or alleviation of the complex symptoms associated with depression.
Keywords: Baicalein, Chronic stress, Depression, Dopamine, Hypothalamus-pituitary-adrenal axis
INTRODUCTION
Baicalein (BA; 5,6,7,-trihydroxyflavone), one of the most active natural plant flavonoids, is found in the dry roots of Scutellaria baicalensis Georgi [1]. This compound exhibits to improve multiple physiological actions, and produce a variety of biological effects in the central nervous and immune systems, and several studies have investigated the anti-inflammatory, antioxidant, anti-proliferative, anti-apoptotic, and anti-tumor, properties of BA [2-4]. BA has been shown to cross the blood-brain barrier and may act directly in brain nuclei to produce pharmacological effects [5]. Several studies in experimental animal models have shown that BA has antidepressant-like effects and the underlying mechanisms may be related to modulation of the extracellular signal-regulated kinase (ERK) signaling pathway in the hippocampus [1]. In addition, BA has been reported to attenuate irradiation-induced impairment of hippocampal neurogenesis by modulating oxidative stress and elevating brain-derived neurotrophic factor (BDNF) signaling [6], and to attenuate memory impairment in beta-amyloid peptide-(25-35)-induced amnesia in rats [7]. BA may improve cognitive deficits and reduce apoptosis following transient global cerebral ischemia/reperfusion injury-induced hippocampal neuronal damage in mice via phosphorylation of ERK (pERK) and stimulation of BDNF expression in vivo [8,9]. A limited amount of information on the clinical effects of BA on depression and morbid forgetfulness is available [10], and the effect of BA treatment on depression-like symptoms induced by repeated restraint stress in rats is not known.
Chronic exposure to stressful life events is a well-established and significant risk factor for the development and maintenance of several neuropsychological conditions and helplessness including major depression [11,12]. Chronic stress can trigger or exacerbate a disruption in the activity of the hypothalamic-pituitary-adrenal (HPA) axis, as evidenced by observations that the elevated circulating corticosterone (CORT) levels disrupt the circadian regulation of CORT secretion as well as the glucocorticoid (GC) receptor-negative feedback circuit [13,14]. Elevated GC levels cause changes in brain function that impair the regulation of physiological and behavioral responses to stressors and are closely associated with psychosomatic disorders and affective behaviors that are indicative of or consistent with depressive-like symptoms [15,16]. Furthermore, several animal studies have shown that chronic stress disrupts HPA axis activity, leading to morphological changes in the hypothalamus, hippocampus, and prefrontal cortex [17,18] as well as in a variety of neurotransmitters [19,20], reductions in body weight, and altered behaviors [21-24]. A reduction in brain dopamine (DA) and serotonin (5-HT) levels has been reported to disrupt HPA axis activity and cause depressive-like symptoms in rats [25], mimicking the symptoms of human depression [19]. Several antidepressant medicines currently in use were developed several decades ago based on evidence from basic and clinical studies suggesting that low levels of monoamines cause depression [26]. Thus, current treatment for depression primarily relies on traditional therapeutic strategies that modulate the serotonergic and noradrenergic systems with the goal of restoring 5-HT and DA levels in the brain [24].
Therefore, the present study used repeated restraint-induced stress to investigate the effect of BA on the symptoms of chronic stress-induced depression in an animal model. We used the forced swimming test (FST) as a behavioral measure and brain concentration of DA and 5-HT and BDNF mRNA expression in the hippocampus as a neurobiological measure of BA action and potential underlying mechanisms.
METHODS
Animals
Adult male Sprague-Dawley (SD) rats weighing 260~280 g were obtained from Samtako Animal Co. (Seoul, Korea). Animals were maintained on a 12-hour light/dark cycle (lights on at 7:00 a.m., lights off at 7:00 p.m.) under controlled temperature (22±2℃) and humidity (55±15%), and they were given standard diet and water during the experiments. The rats were housed in a limited access rodent facility with up to five rats per polycarbonate cage. The animal experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23), revised in 1996, and were approved by the Kyung Hee University Institutional Animal Care and Use Committee. All animal experiments began at least 7 days after the animals arrived. The effects were made to minimize the number and suffering of animals.
Experimental groups
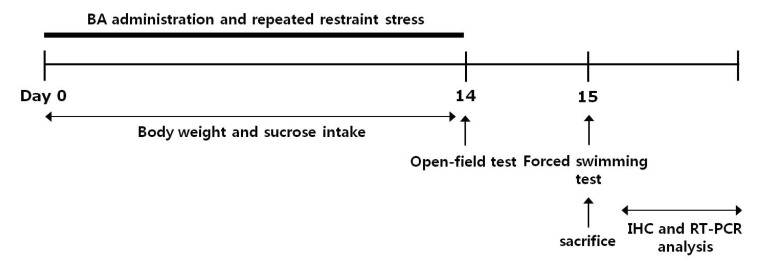
This study was designed to explore the efficacy of BA administration for healing repeated restraint stress-induced depression-like behavior in an animal model using behavioral and neurobiological methodologies. The rats were randomly divided into six groups of six to seven individuals each as follows: unstressed group daily treated with saline instead of BA (0.9% NaCl, i.p., SAL group, n=6), restraint-stressed group daily treated with saline instead of BA (STR group, as a negative control, n=7), restraint-stressed plus 10 mg/kg BA-treated group (STR+BA10 group, n=6), restraint-stressed plus 20 mg/kg BA-treated group (STR+BA20 group, n=6), restraint-stressed plus 40 mg/kg BA-treated group (STR+BA40 group, n=6), and restraint-stressed plus 10 mg/kg fluoxetine-treated group (STR+FLX group, as a positive control, n=6). BA and fluoxetine (FLX) were purchased from Sigma-Aldrich Chemical Co. (St. Louise, MO, USA). The rats were administrated by intraperitoneally (i.p.) with BA and FLX 30 min prior to a daily restraint stress for 14 days, and BA and FLX were dissolved in 0.9% physiological saline solution before use. All drugs were freshly prepared right before every experiment. The restraint stress procedure was carried out once daily for 2 h from 10:00 a.m. to 12:00 p.m., and 14 consecutive days in rodent immobilization bags. In brief, rats were forced to be placed in a transparent plastic tubes (20×7 cm), of which one end is conical shaped and has several 3 mm-holes for breathing, and the other end is open, as described previous our study [27]. The animals have ample air but were unable to move within the tubes. The following parameters were measured to monitor the effects of the development of psychosomatic disorders by repeated restraint stress: changes of body weight gains (at the beginning step of restraint stress), and serum CORT levels (after repeated restraint stress-induced depression-like symptoms). Behavioral testing for depression-like behavior was done 24 h after the end of the chronic physiological stress protocol. All rats sequentially performed to take the FST on the 15th day after repeated restraint stress. After the behavioral testing and body weighting, rats were sacrificed and brain tissues were immediately collected for experiments or stored at -70℃ for later use. All rat groups except SAL group were received same restraint stress. The entire experimental schedule of all drug administration and behavioral examinations are shown in Fig. 1.
Fig. 1.
Experimental schedule of developing repeated restraint stress-induced depression-like behaviors and administration of BA in the rats. IHC, immunohistochemistry.
Measurement of sucrose intake
The sucrose intake test was performed as described previously with minor modifications [28]. For this test, rats were trained to consume 1% sucrose solution prior to the start of the experiment. Briefly, 48 hours before the test, the rats were trained to adapt to 1% sucrose solution (w/v): two bottle of 1% sucrose solution were placed in each cage, and 24 hours later 1% sucrose solution in one bottle was replaced with tap water for 24 hours. After the adaption, rats were deprived of water and food for 10 hours. Sucrose preference test was conducted at 9:00 a.m. in which rats were housed in individual cages and were free to access to two bottles containing 100 ml of sucrose solution (1%, w/v) and 100 ml of water, respectively. After 3 hours, the volumes of consumed sucrose solution and water were measured, and the sucrose preference was calculated by the following formula: sucrose preference=sucrose consumption/(water consumption+sucrose consumption)×100% [24,28].
CORT, DA and 5-HT analysis
After restraint stress for 14 days, CORT concentration in blood, and DA and 5-HT concentration in brain tissue were determined. Animals were killed by decapitation one day after behavioral measurement. For this, the unanesthetized rats were rapidly decapitated, and blood was quickly collected via the abdominal aorta. The hippocampus or medial prefrontal cortex were rapidly removed from the rat brains in randomized order. Special care was taken to avoid pre-decapitation stress; while rats were rapidly decapitated, the other animals were left outside the room and handled for a few minutes prior to sampling. The blood samples were centrifuged at 4,000 g for 10 min, and serum was collected and stored at -20℃ until use. The CORT concentration was measured by a competitive enzyme-linked immunoassay (ELISA) using a rabbit polyclonal CORT antibody (Novus Biologicals Corticosterone kit; Novus Biologicals, LLC., Littleton, CO, USA) according to the manufacturer's protocol. The brain tissue samples were stored at -80℃ until use. Hippocampus or medial prefrontal cortex were homogenized in a lysis buffer containing 137 mM NaCl, 20 mM Tris (pH 8.0), 1% NP40, 10% glycerol, 1 mM PMSF, 10 mg/ml aprotinin, 1 mg/ml leupeptin and 0.5 mM sodium vanadate. Homogenization was carried out on ice using a tissue homogenizer and incubated for 1 min at 4℃ with shaking. Homogenates were centrifuged and supernatants were collected. Protein concentrations were estimated by the procedure of Gmeiner and Seelos [29] with BSA as the standard. The DA and 5-HT concentration was measured by a competitive enzyme-linked immunoassay (ELISA) using a mouse monoclonal DA and 5-HT antibody (Novus biologicals DA and 5-HT kit; Novus Biologicals, LLC., Littleton, CO, USA) according to the manufacturer's protocol. Samples (or standard) and conjugate were added to each well, and the plate was incubated for 1 h at room temperature without blocking. After wells were washed several times with buffers and proper color developed, the optical density was measured at 450 nm using an ELISA reader (MutiRead 400; Authos Co., Vienna, Austria).
Forced swimming test (FST)
Forced swimming test, a representative behavioral test for depression, is frequently used to evaluate the activities of potential antidepressant drugs in rodent models. Forced immersion of rats in water for an extended period produces a characteristic behaviors of immobility. The antidepressant treatments decrease the immobility behavior accompanying with an increase in the escape responses such as climbing and swimming behaviors. A transparent Plexiglas cylinder (20 cm diameter×50 cm height) was filled up to a depth of 30 cm with water at 25℃. At this depth, rats could not touch the bottom of the cylinder with their tails or hind limbs. On day 14, the rats in all groups were trained for 5 min by placing them in the water-filled cylinder. On day 15, animals were subjected to 5 min of forced swim, and escape behaviors (climbing and swimming behaviors) were determined. The duration of immobility was scored during the 5 min test period. Climbing behavior was defined as upward-directed movements of the forepaws alone the side of the swim chamber and swimming behavior was considered as movements throughout the swim chamber including crossing into another quadrant. Immobility behavior was calculated as the length of time in which the animal did not show escape responses (e.g., total time of the test minus time spent in climbing and swimming behaviors). The animals' behavior was continuously recorded throughout the testing session with an overhead video camera. After the test, the rat was removed from the tank, dried with a towel and placed back in its home cage. The water in the swim tank was changed between rats.
Open field test
Prior to forced swimming test, the rats were individually housed in a rectangular container that was made of dark polyethylene (60×60×30 cm) to provide best contrast to the white rats in a dimly lit room equipped with a video camera above the center of the room, and their locomotor activities (animal's movements) were then measured. The locomotor activity indicated by the speed and the distance of movements was monitored by a computerized video-tracking system using S-MART program (Panlab Co., Barcelona, Spain). After 5 min adaptation, the distance they traveled in the container was recorded for another 5 min. The locomotor activity was measured in centimeters. The floor surface of each chamber was thoroughly cleaned with 70% ethanol between tests.
Immunohistochemistry of corticotrophin-releasing factor (CRF) and tyrosine hydroxylase (TH)
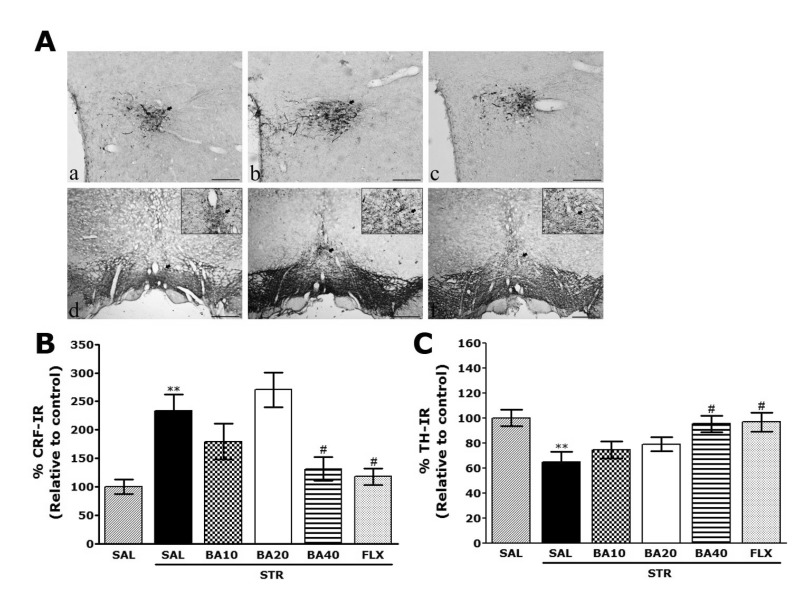
For immunohistochemical studies, the three rats in each groups were deeply anesthetized with sodium pentobarbital (80 mg/kg, by intraperitoneal injection) and perfused through the ascending aorta with normal saline (0.9%) followed by 300 ml (per rat) of 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS). The brains were removed in a randomized order, post-fixed over-night, and cryoprotected with 20% sucrose in 0.1 M PBS at 4℃. Coronal sections 30 µm thick were cut through hypothalamus and ventral tegmental area (VTA) using a cryostat (Leica CM1850; Leica Microsystems Ltd., Nussloch, Germany). The sections were obtained according to the rat atlas of Paxinos and Watson (Paxinos and Watson, 1986). The sections were immunostained for CRF and TH expression using the avidin-biotin-peroxidase complex (ABC) method. Briefly, the sections were incubated with primary goat anti-CRF antibody (1:500 dilution; Santa Cruz Biotechnology Inc., California, CA, USA) and sheep anti-TH antibody (1:2,000 dilution; Chemicon International Inc., Temecular, CA, USA) in PBST (PBS plus 0.3% Triton X-100) for 72 h at 4℃. The sections were incubated for 120 min at room temperature with secondary antibody. The secondary antibodies were obtained from Vector Laboratories Co. (Burlingame, CA, USA) and diluted 1:200 in PBST containing 2% normal serum. To visualize immunoreactivity, the sections were incubated for 90 min in ABC reagent (Vectastain Elite ABC kit; Vector Labs. Co., Burlingame, CA, USA), and incubated in a solution containing 3,3'-diaminobenzidine (DAB; Sigma-Aldrich Chemical Co., St. Louis, MO, USA) and 0.01% H2O2 for 1 min. Finally, the tissues were washed in PBS, followed by a brief rinse in distilled water, and mounted individually onto slides. Images were captured using the AxioVision 3.0 imaging system (Carl Zeiss, Inc., Oberkochen, Germany) and processed using Adobe Photoshop (Adobe Systems, Inc., San Jose, CA, USA). The sections were viewed at 200× magnification, and the numbers of CRF and TH labeled cells was quantified in the hypothalamus and VTA. CRF- and TH-labeled cells were counted by an observer blinded to the experimental groups. Counting the immunepositive cells were performed within the square (100×100 µm2), anatomically localized in at least three different hypothalamus and VTA sections per rat brain according to the stereotactic rat brain atlas of Paxinos and Watson [30]. The counted sections were randomly chosen from equal levels of serial sections along the rostral-caudal axis. The stained cells of which intensities were reached to a defined value above the background were only considered as immunopositive cells. Distinct brown spots indicating CRF- and TH-immunopositive cells were observed in the hypothalamus and VTA. The differences of brightness and contrast among raw images were not adjusted to exclude any possibility of subjective selection of the immunereactive cells.
Total RNA preparation and RT-PCR analysis
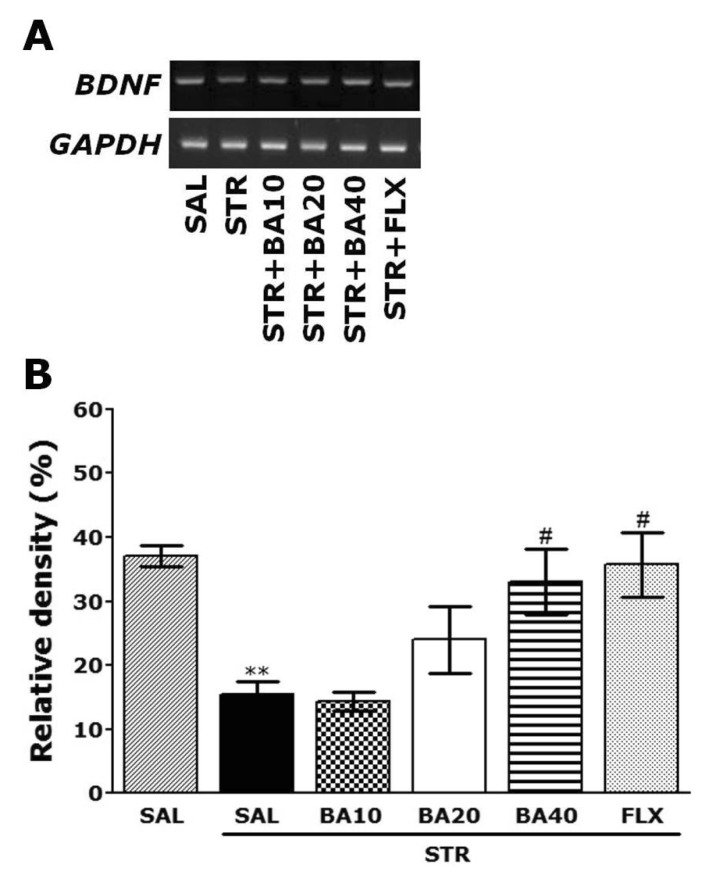
The expression levels of BDNF mRNA were determined by the reverse transcription-polymerase chain reaction (RT-PCR). The brain hippocampus was isolated from four rats per group. After decapitation, the brain was quickly removed and stored at -80℃ until use. The total RNA was prepared from the brain tissues using TRIzol® reagent (Invitrogen Co., Carlsbad, CA, USA) according to the supplier's instruction. Complementary DNA was first synthesized from total RNA using a reverse transcriptase (Takara Co., Shiga, Japan). PCR was performed using a PTC-100 programmable thermal controller (MJ Research, Inc., Watertown, MA, USA). The operating conditions were as follows: for glyceraldehydes-3-phosphate dehydrogenase (GAPDH), 30 cycles of denaturation at 95℃ for 30 sec, annealing at 58℃ for 30 sec, and extension at 72℃ for 30 sec; for BDNF, 27 cycles of denaturation at 95℃ for 30 sec, annealing at 57℃ for 30 sec, and extension at 72℃ for 30 sec. All primers were designed using published mRNA sequences and a primer designing software, Primer 3, offered by the Whitehead Institute for Biomedical Research (Cambridge, MA, USA; www.genome.wi.mit.edu) on the website. The following sequences were used: for GAPDH (409 bp), (forward) 5'-ATC CCA TCA CCA TCT TCC AG-3' and (reverse) 5'-CCT GCT TCA CCA CCT TCT TG-3'; for BDNF (153 bp), (forward) 5'-CAG GGG CAT AGA CAA AAG-3' and (reverse) 5'-CTT CCC CTT TTA ATG GTC-3'. The PCR products were separated on 1.2% agarose gels and stained with ethidium bromide. The density of each band was quantified using an image-analyzing system (i-Max™, CoreBio System Co., Seoul, Korea). The expression levels were compared each other by calculating the relative density of target band, such as BDNF, to that of GAPDH.
Statistical analysis
All measurements were performed by an independent investigator blinded to the experimental conditions. Results in figures are expressed as mean±standard error of means (SE). Differences within or between normally distributed data were analyzed by analysis of variance (ANOVA) using SPSS (Version 13.0; SPSS, Inc., Chicago, IL, USA) followed by Tukey's post-hoc test. Statistical significance was set at p<0.05.
RESULTS
Effect of BA on repeated restraint stress-induced body weight loss, increase in serum CORT levels and reduction in consumed sucrose intake
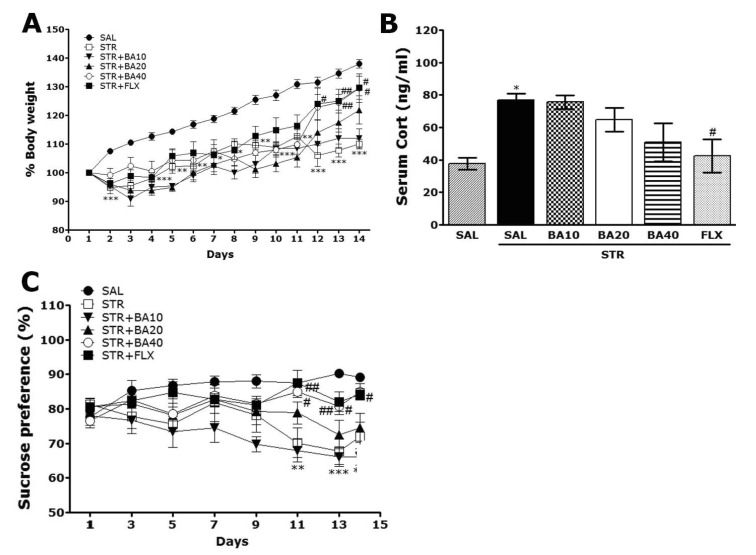
Rats exposed to repeated restraint stress begin to lose their body weights on the first day of restraint stress and this initial reduction of body weight is sustained for a while without restoring or even exacerbated in some cases [27]. In the present study, we also examined body weights daily for 14 days to identify whether repeated restraint stress (STR group) caused body weight loss (difference between daily weights and starting weight) (Fig. 2A). Analysis of the body weight values revealed a significant gradually reduction in body weight gain for 14 day in the STR group, as compared to the normal control rats (SAL) group. During this period, 40 mg/kg BA-treated rats showed significant inhibitions of reductions in body weight gains, as compared to STR group (p<0.05 on day 12 and 14).
Fig. 2.
Effect of BA administration on body weight (A), serum corticosterone levels (B), and sucrose intake (C) of rats subjected to repeated restraint stress for 14 consecutive days. *p<0.05, **p<0.01, ***p<0.001 vs. the SAL group; #p<0.05, ##p <0.01 vs. the STR group.
Acute restraint stress induces a large increase in serum CORT level, which gradually decreased as the restraint stress was repeatedly applied to the rats, probably due to adrenal habituation [31]. The serum CORT levels were measured in each group after exposure to repeated restraint stress for 14 days (Fig. 2B). The ELISA analysis demonstrated that repeated restraint stress for 14 days significantly increased the serum CORT concentration in the rats by 203.88%, as compared to SAL group (p<0.05). It indicated that the repeated restraint stress was sufficiently stressful despite the evoked CORT responses (physiological responses) to repeated restraint stress was significantly more than the response to single restraint stress (data not shown). Daily administration of BA slightly inhibited the repeated restraint stress-induced increase in serum CORT level as compared to STR group in spite of little statistical significance (p=0.199).
In present study, we examined sucrose intake once two days for 14 days to indentify whether repeated restraint stress (STR group) caused consumed sucrose solution much less than the SAL group, as seen in Fig. 2C. Analysis of the sucrose intake values revealed a significant gradually reduction in consumed sucrose intake gain for 14 day in the STR group, as compared to the normal control rats (SAL) group (p<0.01 on day 11 and 14; p<0.001 on day 13). During this period, 40 mg/kg BA-treated rats showed significant inhibitions of reductions in consumed sucrose intake, as compared to STR group (p<0.05 on day 11, 13 and 14). The results also showed that the recovery of consumed sucrose intake in the STR+BA40 group was almost comparable to that in the STR+FLX group.
Effect of BA on repeated restraint stress-induced depression-like behavior
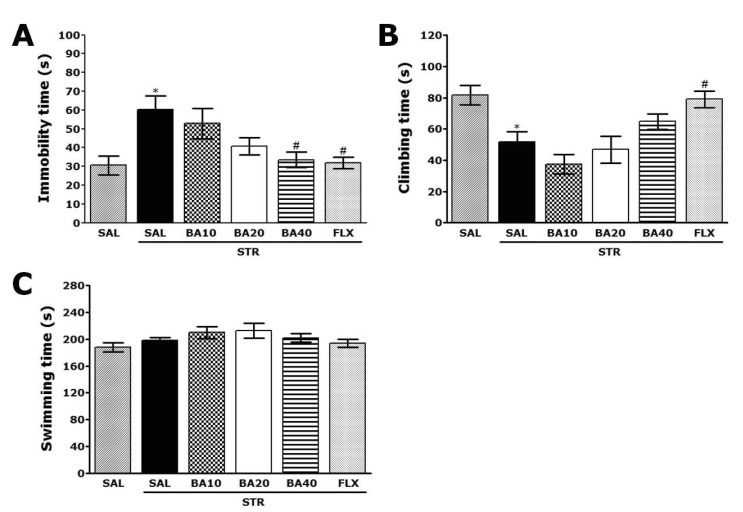
Rats subjected to repeated restraint stress for 14 days exhibited a significant depression phenotype, characterized by increased immobility time during the FST, as compared to saline-treated controls (SAL group) (Fig. 3A; p<0.05). However, the rats in STR+BA40 group showed significant decrease in immobility time during 5 min in the FST, as compared to those in STR group (p<0.05), indicating that administration of 40 mg/kg BA decreased depression-like behavior. Similarly, we next focused on another key behavior manifested as "climbing behavior". The rats in STR group showed significant decrease in climbing behavior during the FST, as compared to SAL group (Fig. 3B; p<0.05). However, it was shown that the rats in STR+BA40 group showed slightly restoration in climbing behavior time during 5 min in the FST, as compared to those of the STR group in spite of little statistical significance (p=0.697). Also, repeated restraint stress for 14 days did not induce significant differences of swimming behavior among all groups during the FST (Fig. 3C; p=0.853). This results also showed that the reduction of immobility on depression-like behavior in the STR+BA40 group was almost comparable to that in the STR+FLX group.
Fig. 3.
Effect of BA administration on immobility time (A), climbing behavior (B), and swimming behavior (C) in the forced swimming test during repeated restraint stress for 14 consecutive days. *p<0.05 vs. the SAL group; #p<0.05 vs. the STR group.
Effect of BA on repeated restraint stress-induced motor functions
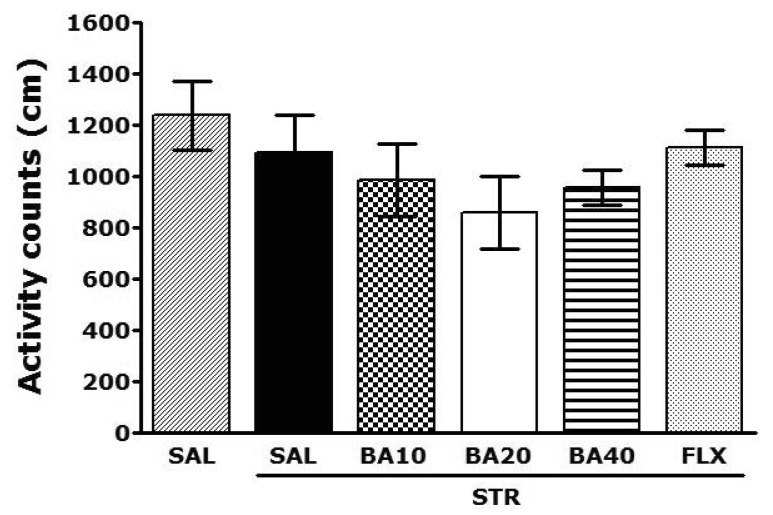
Open-field activity was used to evaluate locomotor activity among the rats receiving repeated restraint stress for 14 days (Fig. 4). No significant individual differences in locomotor activity were observed between groups (p=0.432). It shows that administration of BA did not affect the psychomotor of the rat's performance.
Fig. 4.
Effect of BA administration on activity counts of locomotor activity in the open-field test during repeated restraint stress.
Effect of BA on repeated restraint stress-induced CRF- and TH-like immunoreactivity
Following the behavioral tasks, CRF-like immunoreactivity was analyzed in the cell bodies of various hypothalamic regions including the paraventricular nucleus (PVN; Fig. 5A). In the rat brains in the STR group, the numbers of CRF immunoreactive neurons in the PVN were increased by 233.47%. Analysis of the numbers of CRF-immunoreactive neurons values revealed that the rats receiving repeated restraint stress exhibited a significant increase in CRF expression compared to the SAL group (p<0.01; Fig. 5B). The number of CRF-immunoreactive neurons was significantly decreased in hypothalamic PVN regions of the STR+BA40 group compared to the STR group (p<0.05). It also indicated that the increased CRF-immunoreactivity induced by the repeated restraint stress was significantly restored by BA administration and that the numbers of CRF-immunopositive neurons in the STR+BA40 group was similar to that in the STR+FLX group. TH-like immunoreactivity was analyzed in the cell bodies of VTA (Fig. 5A). In the rat brains in the STR group, the numbers of TH immunoreactive neurons in the VTA were decreased by 64.84%. Analysis of the numbers of TH-immunoreactive neurons values revealed that the rats receiving repeated restraint stress exhibited a significant decrease in TH expression compared to the SAL group (p<0.01; Fig. 5C). The number of TH-immunoreactive neurons was significantly increased in VTA regions of the STR+BA40 group compared to the STR group (p<0.05). It also indicated that the decreased TH-immunoreactivity induced by the repeated restraint stress was significantly restored by BA administration and the numbers of TH-immunopositive neurons in the STR+BA40 group was similar to that in the STR+ FLX group.
Fig. 5.
Effect of BA administration on the mean number of corticotrophin-releasing factor (CRF) expression in the paraventricular nucleus (PVN) of the hypothalamus and tyrosine hydroxylase (TH) expression in the ventral tegmental area (VTA). Representative photographs and the relative percentage values are indicated in (A~C), respectively. (a): CRF expression of hypothalamus in the SAL group, (b): CRF expression of hypothalamus in the STR group, (c): CRF expression of hypothalamus in the STR+BA40 group, (d): TH expression of VTA in the SAL group, (e): TH expression of VTA in the STR group, and (f): TH expression of VTA in the STR+BA40 group. The scale bar indicates 50 µm. **p<0.01 vs. the SAL group; #p<0.05 vs. the STR group.
Effect of BA on repeated restraint stress-induced decrease of dopamine and serotonin concentrations in the hippocampus and medial prefrontal cortex
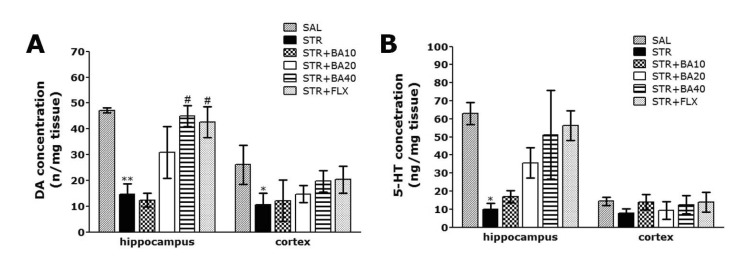
The ELISA analysis demonstrated that repeated restraint stress for 14 days significantly decreased the DA concentration in the hippocampus and medial prefrontal cortex by 31.12% and 40.85%, respectively, compared with rats in the non-treated SAL group. The concentration of DA in the hippocampus was markedly decreased in the STR group, as compared to the SAL group (p<0.01; Fig. 6A). Daily administration of BA showed significantly increased the repeated restraint stress-induced decrease of DA concentration in the hippocampus, as compared to STR group (p<0.05). It also indicated that the concentration of DA in the hippocampus in rats receiving 40 mg/kg BA administration was almost compatible with the rats receiving 10 mg/kg FLX administration. However, there was no significant difference in DA concentration among the six groups in the medial prefrontal cortex. The concentration of DA in the STR+BA40 group was significantly higher than that in the STR group (p=0.868), while the STR group had significantly lower DA concentration compared to the SAL group (p=0.430).
Fig. 6.
Effect of BA administration on dopamine (A) and serotonin (B) concentration in the hippocampus and medical prefrontal cortex of rats subjected to repeated restraint stress for 14 consecutive days. *p<0.05, **p<0.01 vs. the SAL group; #p<0.05 vs. the STR group.
The ELISA analysis demonstrated that repeated restraint stress for 14 days significantly decreased the 5-HT concentration in the hippocampus and medial prefrontal cortex by 15.78% and 55.04%, respectively, compared with rats in the non-treated SAL group (p<0.05; Fig. 6B). Daily administration of BA showed slightly increased the repeated restraint stress-induced decrease of 5-HT concentration in the hippocampus, as compared to STR group in spite of little statistical significance (p=0.169). However, there was no significant difference in 5-HT concentration among the six groups in the medial prefrontal cortex. The concentration of 5-HT in the STR+BA40 group was significantly higher than that in the STR group (p=0.973), while the STR group had significantly lower 5-HT concentration compared to the SAL group (p=0.883).
Effect of BA on repeated restraint stress-induced expression of BDNF mRNA in the hippocampus
The effect of BA administration on the expression level of BDNF mRNA in rats with repeated restraint stress-induced hippocampus lesions were investigated using RT-PCR analysis (Fig. 7). The BDNF mRNA expression levels were normalized against glyceraldehydes-3-phophate dehydrogenase (GAPDH) mRNA, an internal control. BDNF mRNA expression in the hippocampus in the STR group was significantly decreased compared with that in the SAL group (p<0.01). The decreased expression of BDNF mRNA in the STR group was significantly restored in the STR+BA40 group (p<0.05), and the restored level was similar to that of normal rats in the SAL group. This also indicated that the expression of BDNF mRNA in the hippocampus in rats receiving 40 mg/kg BA administration was similar to that in rats receiving 10 mg/kg FLX administration.
Fig. 7.
Effect of BA administration on the expression of (BDNF) mRNA in rats subjected to repeated restraint stress for 14 consecutive days. PCR bands on agarose gel and their relative intensities are indicated in (A) and (B), respectively. The expression levels of BDNF mRNA were normalized to that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA as an internal control. **p<0.01 vs. the SAL group; #p<0.05 vs. the STR group.
DISCUSSION
We found that the plant-derived flavonoid BA has antidepressant-like effects in a rat model of depression, and identified potential mechanisms underlying the effect. BA decreased the duration of immobility at doses of 10, 20, and 40 mg/kg in the FST following repeated restraint stress; however, the effect was significant only at 40 mg/kg. This behavioral effect was likely the result of BA-induced modulation of hypothalamic CRF activity, preventing the reduction in brain DA that underlies the development of major depressive disorder. BA reduced stress-related depression-like behaviors in the FST in a dose-dependent manner; however, 40 mg/kg was the most effective dose, which is consistent with the findings of a previous study [32].
Our findings are consistent with those of previous studies that have shown that repeated restraint stress disrupts HPA axis activity, increasing the probability of depression-like behavior [27,33]. Furthermore, the gradual decrease in body weight and increase in serum CORT levels found immediately before behavioral testing indicate that the repeated restraint stress procedure was valid [27,34]. The restraint stress model used in our study is a well-established method of inducing stress that has several advantages. Animals placed in a restraint stress chamber for a specified period of time over 14 days undergo physiological changes in body weight and serum CORT levels. BA restored body weight and serum CORT levels to near normal levels toward the end of the 2-week treatment period.
Furthermore, disruption of HPA axis activity induced by restraint stress was the likely cause of increased immobility duration during FST and reduced sucrose preference, both common symptoms of depression [35,36]. This hypothesis is supported by several studies in which elevated levels of CORT altered HPA axis activity and affected behavioral activity and sucrose preference [35,36]. By the end of the treatment period, animals administered BA prior to restraint stress increased their preference for sucrose compared to rats in the restraint stress control group, suggesting that BA counteracts chronic stress-induced depressive symptoms or psychological disorders [37]. These results are consistent with previous studies in which repeated restraint stress produced dramatic effects during the FST, indicating that BA has a powerful effect on systems disrupted during the FST [27]. It may take several weeks before the mood elevating effects of antidepressant are felt in humans and several restraint stress trials in rats; thus we administered BA for 14 consecutive days to investigate the antidepressant-like effects of BA in the FST of rats [38]. The decrease in immobility duration in the STR+BA40 group was similar to that of STR+FLX group, further supporting the antidepressant activity of BA.
The present data suggest that CRF circuits in the PVN of the hypothalamus are activated by repeated restraint stress, which causes HPA axis hyperactivity resulting in depressive-like activity in behavioral tests [39]. This animal model of stress focuses on PVN region of the hypothalamus because PVN sends projections to the limbic system and several points in the hypothalamus [40,41]. Our results show that BA significantly blocked the stress-induced increase in CRF immunoreactivity in the PVN, suggesting that the anti-depressive effects of BA are closely associated with CRF modulation in the PVN. Also, the enzyme TH is involved in stress-induced activation of the central nervous system and in stress-related psychopathological conditions such as depression [42,43]. These results are consistent with previous studies indicating that depression-like behavior induced by chronic stress is the result of changes in the dopaminergic system [44]. Moreover, we demonstrated that administration of BA significantly increased TH-like immunoreactivity in the VTA of rats subjected to repeated restraint stress. Together, these findings indicate that BA attenuates behaviors and neurochemical responses associated with depression by modulating the HPA axis and the dopaminergic system, suggesting that administration of BA, like FLX, may indirectly alter catecholamine synthesis in the brain to produce physiological effects. Thus, our results indicate that BA acts by stimulating dopamine synthesis in the rat brain, suggesting that an overactive dopaminergic system may contribute to depressive symptomology, and that the therapeutic action of antidepressants reverses this activity by decreasing TH expression in the VTA [45].
Several studies have focused on the role of monoamines specifically DA and 5-HT overflow in the hippocampus, and have shown that changes in monoamines are strongly correlated with depressive-like behaviors in the FST [46]. Repeated exposure to restraint stress decreases the release and turnover of DA and 5-HT in areas of the brain implicated in behavioral and physiological responses to stress, such as the medical prefrontal cortex and hippocampus [47]. We suggested that the repeated restraint stress-induced impairment of FST performance is caused by a reduction in DA and 5-HT in the brain. In the present study, BA inhibited the decrease in hippocampal DA attributed to repeated exposure to restraint stress, but did not alter release in the medial prefrontal cortex. The elevated levels were restored to near control values. However, our values differ from those reported by previous studies [25]. This disparity may be attributed to differences in protocol, immobilization schedule and the brain regions analyzed. DA producing neurons in the medical prefrontal cortex and hippocampus, which directly innervate CRF secreting neurons in the hypothalamus, constitute a major stimulatory pathway in the stress-induced activation of the HPA axis [48]. Moreover, the finding that the secretion of CRF, which plays a pivotal role in basal and stress-induced CORT secretion, is controlled by a variety of brain monoamines and peptides, such as DA and neuropeptide Y, is consistent with the findings of our previous studies [16]. Thus, CRF may play an important role in the neurobiological and behavioral mechanisms medicated by the dopaminergic system and HPA axis.
A recent study has shown that chronic social defeat stress and chronic restraint stress are associated with a long-lasting downregulation of BDNF in the brain which can be reversed by treatment with an antidepressant [49,50]. Thus, the increase in BDNF expression may have a role in the treatment of depression. These results suggest that a decrease in the expression of BDNF in the hippocampus may be related to the pathogenesis of depression-like symptoms [51,52]. In the present study, repeated restraint stress was associated with decreased expression of BDNF mRNA in the rat hippocampus and depression-like behavior. However, administration of BA restored the level of BDNF mRNA in the hippocampus of rats subjected to repeated restraint stress, suggesting that BDNF may play a role in the antidepressant effect of BA [53,54]. Our data provide further support for the hypothesis that the antidepressant effect of BA is at least in part correlated with the CREB or ERK signaling pathways.
Administration of BA normalized the stress-induced decrease in DA concentrations and reduced hypersecretion of CORT and, thus should be considered a potential therapeutic agent for reducing stress. Furthermore, ample experimental and clinical evidence suggests that BA has no adverse effects following long-term use for 2 weeks. Patients suffering from stress need special care and protection against the risk of iatrogenic stress, and using a safe natural product is the obvious choice in such cases [55]. Our results showing an association between depression-like behavior and disruption of HPA axis activity and neurochemical interactions between CRF in hypothalamus and dopaminergic pathway suggest a novel hypothesis for the mechanisms mediating the antidepressant effect of BA.
In summary, the present study demonstrates that repeated restraint stress significantly increases the duration of immobility in the FST compared to unstressed normal controls. Furthermore, the administration of BA significantly alleviates depression-like symptoms following repeated restraint stress, possibly by modulating the hypothalamic CRF and dopaminergic systems. Together, these findings indicate that BA is capable of ameliorating the complex behaviors and neurochemical responses involved in depression by modulating BDNF mRNA expression. Accordingly, BA may be a useful alternative therapeutic agent for stress-related disorders such as depression.
ACKNOWLEDGEMENTS
This work was supported by a grant from the National Research Foundation of Korea Grant funded by the Korean Government (MEST)(2010-0003678) and by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (No. 2005-0049404).
ABBREVIATIONS
- BA
Baicalein
- HPA
hypothalamic-pituitary-adrenal
- FST
forced swimming test
- CRF
corticotrophin-releasing factor
- BDNF
brain-derived neurotrophic factor
- ERK
extracellular signal-regulated kinase
- CORT
corticosterone
- GC
glucocorticoid
- DA
dopamine
- 5-HT
serotonin
- FLX
fluoxetine
- ELISA
enzyme-linked immunoassay
- TH
tyrosine hydroxylase
- PBS
phosphate-buffered saline
- VTA
ventral tegmental area
- ABC
avidin-biotin-peroxidase complex
- DAB
diaminobenzidine
- RT-PCR
reverse transcription-polymerase chain reaction
- GAPDH
glyceraldehydes-3-phosphate dehydrogenase
References
- 1.Xiong Z, Jiang B, Wu PF, Tian J, Shi LL, Gu J, Hu ZL, Fu H, Wang F, Chen JG. Antidepressant effects of a plant-derived flavonoid baicalein involving extracellular signal-regulated kinases cascade. Biol Pharm Bull. 2011;34:253–259. doi: 10.1248/bpb.34.253. [DOI] [PubMed] [Google Scholar]
- 2.Chandrashekar N, Selvamani A, Subramanian R, Pandi A, Thiruvengadam D. Baicalein inhibits pulmonary carcinogenesis-associated inflammation and interferes with COX-2, MMP-2 and MMP-9 expressions in-vivo. Toxicol Appl Pharmacol. 2012;261:10–21. doi: 10.1016/j.taap.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Park SJ, Kim DH, Kim JM, Shin CY, Cheong JH, Ko KH, Ryu JH. Mismatch between changes in baicalein-induced memory-related biochemical parameters and behavioral consequences in mouse. Brain Res. 2010;1355:141–150. doi: 10.1016/j.brainres.2010.07.098. [DOI] [PubMed] [Google Scholar]
- 4.Wang W, Wang F, Yang YJ, Hu ZL, Long LH, Fu H, Xie N, Chen JG. The flavonoid baicalein promotes NMDA receptor-dependent long-term potentiation and enhances memory. Br J Pharmacol. 2011;162:1364–1379. doi: 10.1111/j.1476-5381.2010.01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsai TH, Liu SC, Tsai PL, Ho LK, Shum AY, Chen CF. The effects of the cyclosporin A, a P-glycoprotein inhibitor, on the pharmacokinetics of baicalein in the rat: a microdialysis study. Br J Pharmacol. 2002;137:1314–1320. doi: 10.1038/sj.bjp.0704959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oh SB, Park HR, Jang YJ, Choi SY, Son TG, Lee J. Baicalein attenuates impaired hippocampal neurogenesis and the neurocognitive deficits induced by γ-ray radiation. Br J Pharmacol. 2013;168:421–431. doi: 10.1111/j.1476-5381.2012.02142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang SY, Wang HH, Chi CW, Chen CF, Liao JF. Effects of baicalein on beta-amyloid peptide-(25-35)-induced amnesia in mice. Eur J Pharmacol. 2004;506:55–61. doi: 10.1016/j.ejphar.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 8.Liu C, Wu J, Gu J, Xiong Z, Wang F, Wang J, Wang W, Chen J. Baicalein improves cognitive deficits induced by chronic cerebral hypoperfusion in rats. Pharmacol Biochem Behav. 2007;86:423–430. doi: 10.1016/j.pbb.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Liu C, Wu J, Xu K, Cai F, Gu J, Ma L, Chen J. Neuroprotection by baicalein in ischemic brain injury involves PTEN/AKT pathway. J Neurochem. 2010;112:1500–1512. doi: 10.1111/j.1471-4159.2009.06561.x. [DOI] [PubMed] [Google Scholar]
- 10.Guo QL, Ding QL, Wu ZQ. Effect of baicalein on experimental prostatic hyperplasia in rats and mice. Biol Pharm Bull. 2004;27:333–337. doi: 10.1248/bpb.27.333. [DOI] [PubMed] [Google Scholar]
- 11.Colman I, Ataullahjan A. Life course perspectives on the epidemiology of depression. Can J Psychiatry. 2010;55:622–632. doi: 10.1177/070674371005501002. [DOI] [PubMed] [Google Scholar]
- 12.Uliaszek AA, Zinbarg RE, Mineka S, Craske MG, Sutton JM, Griffith JW, Rose R, Waters A, Hammen C. The role of neuroticism and extraversion in the stress-anxiety and stress-depression relationships. Anxiety Stress Coping. 2010;23:363–381. doi: 10.1080/10615800903377264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skórzewska A, Bidziński A, Lehner M, Turzyńska D, Wisłowska-Stanek A, Sobolewska A, Szyndler J, Maciejak P, Taracha E, Płaźnik A. The effects of acute and chronic administration of corticosterone on rat behavior in two models of fear responses, plasma corticosterone concentration, and c-Fos expression in the brain structures. Pharmacol Biochem Behav. 2006;85:522–534. doi: 10.1016/j.pbb.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Steiner MA, Marsicano G, Nestler EJ, Holsboer F, Lutz B, Wotjak CT. Antidepressant-like behavioral effects of impaired cannabinoid receptor type 1 signaling coincide with exaggerated corticosterone secretion in mice. Psychoneuroendocrinology. 2008;33:54–67. doi: 10.1016/j.psyneuen.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Z, Zhong XM, Li ZY, Feng CR, Pan AJ, Mao QQ. Curcumin reverses corticosterone-induced depressive-like behavior and decrease in brain BDNF levels in rats. Neurosci Lett. 2011;493:145–148. doi: 10.1016/j.neulet.2011.02.030. [DOI] [PubMed] [Google Scholar]
- 16.Lee B, Shim I, Lee HJ, Yang Y, Hahm DH. Effects of acupuncture on chronic corticosterone-induced depression-like behavior and expression of neuropeptide Y in the rats. Neurosci Lett. 2009;453:151–156. doi: 10.1016/j.neulet.2009.01.076. [DOI] [PubMed] [Google Scholar]
- 17.Mokler DJ, Torres OI, Galler JR, Morgane PJ. Stress-induced changes in extracellular dopamine and serotonin in the medial prefrontal cortex and dorsal hippocampus of prenatally malnourished rats. Brain Res. 2007;1148:226–233. doi: 10.1016/j.brainres.2007.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizoguchi K, Yuzurihara M, Ishige A, Aburada M, Tabira T. Saiko-ka-ryukotsu-borei-to, a herbal medicine, ameliorates chronic stress-induced depressive state in rotarod performance. Pharmacol Biochem Behav. 2003;75:419–425. doi: 10.1016/s0091-3057(03)00131-x. [DOI] [PubMed] [Google Scholar]
- 20.Torres IL, Gamaro GD, Vasconcellos AP, Silveira R, Dalmaz C. Effects of chronic restraint stress on feeding behavior and on monoamine levels in different brain structures in rats. Neurochem Res. 2002;27:519–525. doi: 10.1023/a:1019856821430. [DOI] [PubMed] [Google Scholar]
- 21.Anisman H, Matheson K. Stress, depression, and anhedonia: caveats concerning animal models. Neurosci Biobehav Rev. 2005;29:525–546. doi: 10.1016/j.neubiorev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Bravo JA, Díaz-Veliz G, Mora S, Ulloa JL, Berthoud VM, Morales P, Arancibia S, Fiedler JL. Desipramine prevents stress-induced changes in depressive-like behavior and hippocampal markers of neuroprotection. Behav Pharmacol. 2009;20:273–285. doi: 10.1097/FBP.0b013e32832c70d9. [DOI] [PubMed] [Google Scholar]
- 23.O'Mahony CM, Clarke G, Gibney S, Dinan TG, Cryan JF. Strain differences in the neurochemical response to chronic restraint stress in the rat: relevance to depression. Pharmacol Biochem Behav. 2011;97:690–699. doi: 10.1016/j.pbb.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 24.Zhu KY, Mao QQ, Ip SP, Choi RC, Dong TT, Lau DT, Tsim KW. A standardized chinese herbal decoction, kai-xin-san, restores decreased levels of neurotransmitters and neurotrophic factors in the brain of chronic stress-induced depressive rats. Evid Based Complement Alternat Med. 2012;2012:149256. doi: 10.1155/2012/149256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Y, Ku BS, Yao HY, Lin YH, Ma X, Zhang YH, Li XJ. Antidepressant effects of curcumin in the forced swim test and olfactory bulbectomy models of depression in rats. Pharmacol Biochem Behav. 2005;82:200–206. doi: 10.1016/j.pbb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Molina-Hernández M, Téllez-Alcántara NP, Olivera-López JI, Jaramillo MT. Intra-lateral septal infusions of folic acid alone or combined with various antidepressant drugs produce antidepressant-like actions in male Wistar rats forced to swim. Prog Neuropsychopharmacol Biol Psychiatry. 2012;36:78–84. doi: 10.1016/j.pnpbp.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 27.Lee B, Shim I, Lee H, Hahm DH. Effect of ginsenoside Re on depression- and anxiety-like behaviors and cognition memory deficit induced by repeated immobilization in rats. J Microbiol Biotechnol. 2012;22:708–720. doi: 10.4014/jmb.1112.12046. [DOI] [PubMed] [Google Scholar]
- 28.Mao QQ, Ip SP, Ko KM, Tsai SH, Che CT. Peony glycosides produce antidepressant-like action in mice exposed to chronic unpredictable mild stress: effects on hypothalamic-pituitary-adrenal function and brain-derived neurotrophic factor. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1211–1216. doi: 10.1016/j.pnpbp.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Gmeiner BM, Seelos CC. Measurement of phosphotyrosine phosphatase activity using the Folin-Ciocalteu phenol reaction. Biochem Mol Biol Int. 1995;36:943–948. [PubMed] [Google Scholar]
- 30.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3th ed. New York, USA: Academic Press; 1986. pp. 54–85. [Google Scholar]
- 31.Ling S, Jamali F. Effect of cannulation surgery and restraint stress on the plasma corticosterone concentration in the rat: application of an improved corticosterone HPLC assay. J Pharm Pharm Sci. 2003;6:246–251. [PubMed] [Google Scholar]
- 32.Sun H, Che QM, Zhao X, Pu XP. Antifibrotic effects of chronic baicalein administration in a CCl4 liver fibrosis model in rats. Eur J Pharmacol. 2010;631:53–60. doi: 10.1016/j.ejphar.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Chiba S, Numakawa T, Ninomiya M, Richards MC, Wakabayashi C, Kunugi H. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:112–119. doi: 10.1016/j.pnpbp.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 34.Nacher J, Pham K, Gil-Fernandez V, McEwen BS. Chronic restraint stress and chronic corticosterone treatment modulate differentially the expression of molecules related to structural plasticity in the adult rat piriform cortex. Neuroscience. 2004;126:503–509. doi: 10.1016/j.neuroscience.2004.03.038. [DOI] [PubMed] [Google Scholar]
- 35.Sigwalt AR, Budde H, Helmich I, Glaser V, Ghisoni K, Lanza S, Cadore EL, Lhullier FL, de Bem AF, Hohl A, de Matos FJ, de Oliveira PA, Prediger RD, Guglielmo LG, Latini A. Molecular aspects involved in swimming exercise training reducing anhedonia in a rat model of depression. Neuroscience. 2011;192:661–674. doi: 10.1016/j.neuroscience.2011.05.075. [DOI] [PubMed] [Google Scholar]
- 36.Strekalova T, Gorenkova N, Schunk E, Dolgov O, Bartsch D. Selective effects of citalopram in a mouse model of stress-induced anhedonia with a control for chronic stress. Behav Pharmacol. 2006;17:271–287. doi: 10.1097/00008877-200605000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Ulloa JL, Castañeda P, Berríos C, Díaz-Veliz G, Mora S, Bravo JA, Araneda K, Menares C, Morales P, Fiedler JL. Comparison of the antidepressant sertraline on differential depression-like behaviors elicited by restraint stress and repeated corticosterone administration. Pharmacol Biochem Behav. 2010;97:213–221. doi: 10.1016/j.pbb.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Zhang W, Rosenkranz JA. Repeated restraint stress increases basolateral amygdala neuronal activity in an age-dependent manner. Neuroscience. 2012;226:459–474. doi: 10.1016/j.neuroscience.2012.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng J, Babygirija R, Bülbül M, Cerjak D, Ludwig K, Takahashi T. Hypothalamic oxytocin mediates adaptation mechanism against chronic stress in rats. Am J Physiol Gastrointest Liver Physiol. 2010;299:G946–G953. doi: 10.1152/ajpgi.00483.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cook CJ. Stress induces CRF release in the paraventricular nucleus, and both CRF and GABA release in the amygdala. Physiol Behav. 2004;82:751–762. doi: 10.1016/j.physbeh.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 41.Raone A, Cassanelli A, Scheggi S, Rauggi R, Danielli B, De Montis MG. Hypothalamus-pituitary-adrenal modifications consequent to chronic stress exposure in an experimental model of depression in rats. Neuroscience. 2007;146:1734–1742. doi: 10.1016/j.neuroscience.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 42.Park HJ, Shim HS, Kim H, Kim KS, Lee H, Hahm DH, Shim I. Effects of glycyrrhizae radix on repeated restraint stress-induced neurochemical and behavioral responses. Korean J Physiol Pharmacol. 2010;14:371–376. doi: 10.4196/kjpp.2010.14.6.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang YC, Ho UC, Ko MC, Liao CC, Lee LJ. Differential neuronal changes in medial prefrontal cortex, basolateral amygdala and nucleus accumbens after postweaning social isolation. Brain Struct Funct. 2012;217:337–351. doi: 10.1007/s00429-011-0355-4. [DOI] [PubMed] [Google Scholar]
- 44.Sevgi S, Ozek M, Eroglu L. L-NAME prevents anxiety-like and depression-like behavior in rats exposed to restraint stress. Methods Find Exp Clin Pharmacol. 2006;28:95–99. doi: 10.1358/mf.2006.28.2.977840. [DOI] [PubMed] [Google Scholar]
- 45.Spasojevic N, Gavrilovic L, Dronjak S. Effects of repeated maprotiline and fluoxetine treatment on gene expression of catecholamine synthesizing enzymes in adrenal medulla of unstressed and stressed rats. Auton Autacoid Pharmacol. 2010;30:213–217. doi: 10.1111/j.1474-8673.2010.00458.x. [DOI] [PubMed] [Google Scholar]
- 46.Shishkina GT, Kalinina TS, Dygalo NN. Effects of swim stress and fluoxetine on 5-HT1A receptor gene expression and monoamine metabolism in the rat brain regions. Cell Mol Neurobiol. 2012;32:787–794. doi: 10.1007/s10571-012-9828-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cui J, Jiang L, Xiang H. Ginsenoside Rb3 exerts antidepressant-like effects in several animal models. J Psychopharmacol. 2012;26:697–713. doi: 10.1177/0269881111415735. [DOI] [PubMed] [Google Scholar]
- 48.Dallman MF, Akana SF, Strack AM, Scribner KS, Pecoraro N, La Fleur SE, Houshyar H, Gomez F. Chronic stress-induced effects of corticosterone on brain: direct and indirect. Ann N Y Acad Sci. 2004;1018:141–150. doi: 10.1196/annals.1296.017. [DOI] [PubMed] [Google Scholar]
- 49.Bergström A, Jayatissa MN, Mørk A, Wiborg O. Stress sensitivity and resilience in the chronic mild stress rat model of depression; an in situ hybridization study. Brain Res. 2008;1196:41–52. doi: 10.1016/j.brainres.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 50.Cirulli F, Berry A, Bonsignore LT, Capone F, D'Andrea I, Aloe L, Branchi I, Alleva E. Early life influences on emotional reactivity: evidence that social enrichment has greater effects than handling on anxiety-like behaviors, neuroendocrine responses to stress and central BDNF levels. Neurosci Biobehav Rev. 2010;34:808–820. doi: 10.1016/j.neubiorev.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 51.Dagnino-Subiabre A, Zepeda-Carreño R, Díaz-Véliz G, Mora S, Aboitiz F. Chronic stress induces upregulation of brain-derived neurotrophic factor (BDNF) mRNA and integrin alpha5 expression in the rat pineal gland. Brain Res. 2006;1086:27–34. doi: 10.1016/j.brainres.2006.02.118. [DOI] [PubMed] [Google Scholar]
- 52.Gatt JM, Nemeroff CB, Dobson-Stone C, Paul RH, Bryant RA, Schofield PR, Gordon E, Kemp AH, Williams LM. Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety. Mol Psychiatry. 2009;14:681–695. doi: 10.1038/mp.2008.143. [DOI] [PubMed] [Google Scholar]
- 53.Franklin TB, Perrot-Sinal TS. Sex and ovarian steroids modulate brain-derived neurotrophic factor (BDNF) protein levels in rat hippocampus under stressful and non-stressful conditions. Psychoneuroendocrinology. 2006;31:38–48. doi: 10.1016/j.psyneuen.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 54.Murakami S, Imbe H, Morikawa Y, Kubo C, Senba E. Chronic stress, as well as acute stress, reduces BDNF mRNA expression in the rat hippocampus but less robustly. Neurosci Res. 2005;53:129–139. doi: 10.1016/j.neures.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 55.Zhang W, Li J, Zhu J, Shi Z, Wang Y, Kong L. Chinese medicine Banxia-houpu decoction regulates c-fos expression in the brain regions in chronic mild stress model in rats. Phytother Res. 2004;18:200–203. doi: 10.1002/ptr.1391. [DOI] [PubMed] [Google Scholar]