Abstract
While the anti-apoptotic effect of paricalcitol has been demonstrated in various animal models, it is not yet clear whether paricalcitol attenuates the apoptosis in gentamicin (GM)-induced kidney injury. We investigated the effect of paricalcitol on apoptotic pathways in rat kidneys damaged by GM. Rats were randomly divided into three groups: 1) Control group (n=8), where only vehicle was delivered, 2) GM group (n=10), where rats were treated with GM (150 mg/kg/day) for 7 days, 3) PARI group (n=10), where rats were co-treated with paricalcitol (0.2 µg/kg/day) and GM for 7 days. Paricalcitol attenuated renal dysfunction by GM administration in biochemical profiles. In terminal deoxynucleotidyl transferase dUTP nick end labeling staining, increased apoptosis was observed in GM group, which was reversed by paricalcitol co-treatment. Immunoblotting using protein samples from rat cortex/outer stripe of outer medulla showed increased Bax/Bcl-2 ratio and cleaved form of caspase-3 in GM group, both of which were reversed by paricalcitol. The phosphorylated Jun-N-terminal kinase (JNK) expression was increase in GM, which was counteracted by paricalcitol. The protein expression of p-Akt and nitro-tyrosine was also enhanced in GM-treated rats compared with control rats, which was reversed by paricalcitol co-treatment. Paricalcitol protects GM-induced renal injury by antiapoptotic mechanisms, including inhibition of intrinsic apoptosis pathway and JNK.
Keywords: Apoptosis, Gentamicin, Kidney, Paricalcitol
INTRODUCTION
Gentamicin (GM) is a potent antimicrobial agent mainly targeting gram negative bacilli, although its use is limited by nephrotoxicity [1]. Despite certain mechanisms have been suggested as GM-induced acute kidney injury (AKI) [2,3], effective prevention of GM nephropathy is still far away from clinical application.
Apoptosis has been emerged as a pivotal mechanism in various experimental models of kidney injury [4-8]. Induction of apoptosis by GM has also been reported in human renal proximal tubular epithelial cells [9]. Inhibition of apoptosis seems to be one of the attractive therapeutic targets, since it has been reported that the blockage of apoptosis suppressed further tissue inflammation and renal injury [10-12], which implies a possibility that apoptosis might be the opening of renal catastrophe.
It has been noted that protective effect of paricalcitol, an active, non-hypercalcemic vitamin D analog, in the kidney against various is mediated by amelioration of apoptosis [13-15]. It is not yet clear, however, whether paricalcitol counteracts GM-induced apoptosis in the kidney.
Considering the previously demonstrated antiapoptotic potential of paricalcitol in various renal injury experiments, it was assumed that renoprotective effect of paricalcitol in GM nephropathy might also be mediated by suppression of apoptosis. The present study, therefore, was designed to investigate the effect of paricalcitol on GM-induced apoptosis in the kidney.
METHODS
Animal model
Animal experiments were performed in accordance with the Ethics Committee of Chonnam National University Medical School. All rats weighed 180~200 g at the start of the experiment. Rats were randomly divided into three groups: 1) Control group (n=8), where only vehicle (phosphate-buffered saline, PBS) was delivered, 2) GM group (n=10), where rats were treated with GM (150 mg/kg/day) intramuscularly for 7 days, 3) PARI group (n=10), where rats were co-treated with paricalcitol (0.2 µg/kg/day) subcutaneously and GM for 7 days. Rats were maintained on a standard rodent diet with pair feeding and allowed free access to drinking water.
On the day of the experiment, the rats were anesthetized with isoflurane. Blood samples were collected from the inferior vena cava and analyzed for creatinine. Plasma creatinine in rats was measured using the Jaffe method (Olympus 5431; Olympus Optical, Tokyo, Japan). The right kidney was rapidly removed, dissected cortex/outer stripe of outer medulla (OSOM) from the whole kidney, and processed for semiquantitative immunoblotting as described below. The left kidney was fixed via retrograde perfusion for preparation of paraffin block.
Protein extraction and semiquantitative immunoblotting
The dissected cortex/outer stripe of the outer medulla (OSOM) was homogenized in ice-cold isolation solution containing 0.3 M sucrose, 25 mM imidazole, 1 mM EDTA, 8.5 µM leupeptin, and 1 mM phenylmethylsulfonyl fluoride (pH 7.2). The homogenates were centrifuged at 1,000×g for 15 min at 4℃ to remove whole cells, nuclei, and mitochondria, and the total protein concentration was measured using the supernatant (Pierce BCA protein assay kit, Pierce, Rockford, IL). All samples were adjusted with isolation solution to normalize the protein concentrations, solubilized at 65℃ for 15 minutes in SDS-containing sample buffer, and the stored at -20℃.
The separated proteins were transferred onto nitrocellulose membranes using Bio-Rad Mini Protean II apparatus (Bio-Rad, Hercules, CA). The blots were blocked with 5% milk in TBST (20 mM Tris-HCl, 140 mM NaCl, 0.1% Tween 20, pH 8.0) for 1 hour and incubated overnight at 4℃ with primary antibodies, followed by incubation with secondary anti-rabbit or anti-mouse horseradish peroxidase-conjugated antibodies. Labeling was visualized with an enhanced chemiluminescence system.
Primary antibodies
The anti-c-Jun N-terminal kinase (JNK), anti-p-JNK, anti-p-Akt, anti-nitro-tyrosine, anti-Bax, anti-Bcl-2, anti-caspase-3, anti-cleaved caspase-3 (Cell Signaling Technology, Danvers, MA),anti-cytochrome C (Santa Cruz Biotechnology, Inc, Dallas, TX) and β-actin (Sigma-Aldrich) antibodies were purchased.
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay
For perfusion fixation, a perfusion needle was inserted in the abdominal aorta and the vena cava was cut to establish an outlet. Blood was flushed from the kidney with cold PBS (pH 7.4) for 15 seconds before a switch to cold 3% paraformaldehyde in PBS for 3 minutes. The kidney was removed and sectioned into 2 to 3 mm transverse sections and immersion fixed for additionally 1 hour, followed by three times of 10-minute washes with PBS. The tissue was dehydrated in graded ethanol and left overnight in xylene. After embedding the tissue in paraffin, 2 µm sections were made on a rotary microtome (Leica Microsystems, Herlev, Denmark).
Apoptosis was determined using the ApopTag Plus Peroxidase in situ apoptosis detection kit (Chemicon International; Temecula, CA, USA) according to the manufacturer's protocol. TUNEL-positive cells were counted in cortical tubular cells in 10 fields per slide (at 200× magnifications).
Statistical analysis
Results are expressed as means±standard error of the mean of 3 independent experiments. Multiple comparisons between groups were made by one-way analysis of variance and post-hoc Tukey's honestly significant difference test. Differences with values of p<0.05 were considered significant.
RESULTS
Effect of GM and paricalcitol on serum creatinine level
The total body weight of the rats were not significantly different among the groups (203.75±9.16 g in control group, 202.50±7.90 g in GM group, 204.50±10.39 g in PARI group), although kidney weight of the rats treated with GM with or without paricalcitol increased compared controls (0.94±0.11 g in control group, 1.41±0.21 g in GM group, 1.27±0.80 g in PARI group, p<0.05 in both groups compared with control group). Serum creatinine levels were elevated in GM rats compared with controls (0.25±0.05 mg/dl in control group, 1.10±0.52 mg/dl in GM group, p<0.05), which was attenuated by paricalcitol co-treatment (0.47±0.12 mg/dl, p<0.05 compared with GM-treated rats).
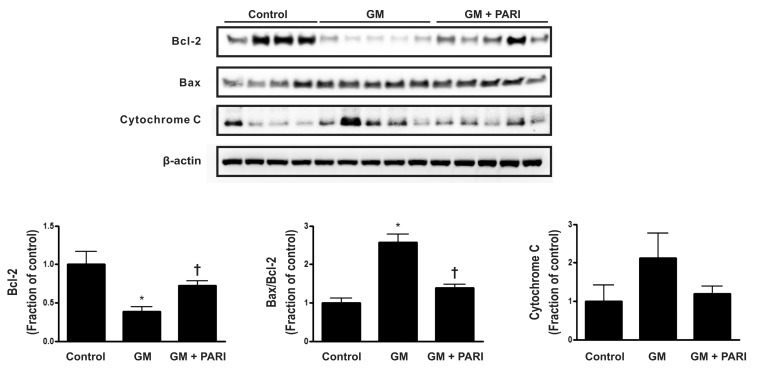
Alteration of Bcl-2, but not Bax, expression by GM and paricalcitol
Immunoblotting of Bax and Bcl-2, which are well known to be modulators of apoptosis, is presented in Fig. 1. GM treatment, compared with vehicle treatment, significantly decreased Bcl-2 expression, while co-administration of paricalcitol restored Bcl-2 expression level. Either of GM or paricalcitol did not affect the expression of Bax protein. The ratio of Bax to Bcl-2 was, therefore, increased in GM group compared to control or PARI groups. The level of cytochrome C expression was not statically significant difference among the three groups.
Fig. 1.
Immunoblotting of Bax and Bcl-2 in renal cortex/OSOM. GM treatment, compared with vehicle treatment, significantly decreased Bcl-2 expression, while co-administration of paricalcitol restored Bcl-2 expression level. Either of GM or paricalcitol did not affect the expression of Bax protein. The ratio of Bax to Bcl-2, accordingly, was increased in GM group, compared to control or PARI groups. The level of cytochrome C expression was not statically significant difference among the three groups. *p<0.05 vs. control. †p<0.05 vs. GM.
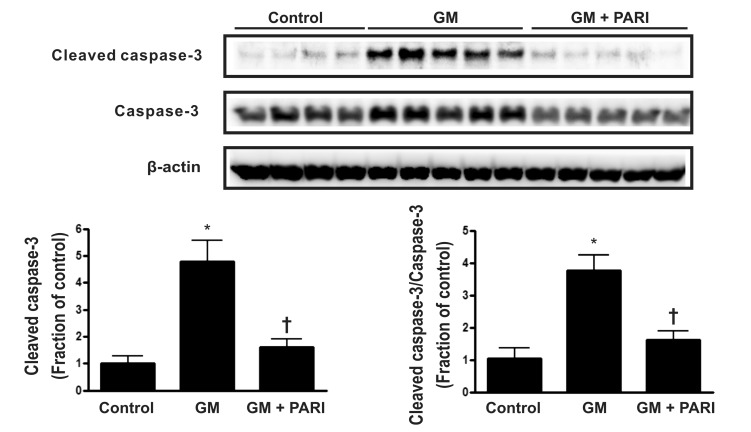
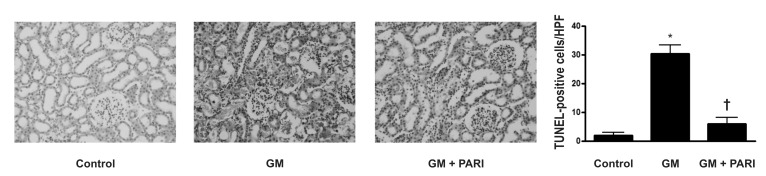
Attenuation of GM-induced apoptosis by paricalcitol in rat kidneys
Immunoblotting analysis demonstrated markedly increased protein expression of cleaved caspase-3 in the kidneys treated GM alone compared in those treated vehicles (Fig. 2), which was counteracted by paricalcitol co-treatment. The ratio of cleaved form to total form caspase-3 expression was also increased in the GM group compared to the other groups. TUNEL staining revealed markedly increased number of TUNEL-positive cells in the kidney of GM group (Fig. 3) compared with in those of control and PARI groups.
Fig. 2.
Immunoblotting of caspase-3 in renal cortex/OSOM. Cleaved caspase-3 was upregulated by GM treatment, compared by vehicle treatment, which was reversed by paricalcitol co-administration. The ratio of cleavage form to total form caspase-3 was also increased in GM group, compared with the other groups. *p<0.05 vs. control. †p<0.05 vs. GM.
Fig. 3.
TUNEL staining in renal cortex (×200 magnifications). Markedly increased numbers of TUNEL-positive cells in the kidney of GM group, compared with in those of control and PARI groups, were observed. *p<0.05 vs. control. †p<0.05 vs. GM.
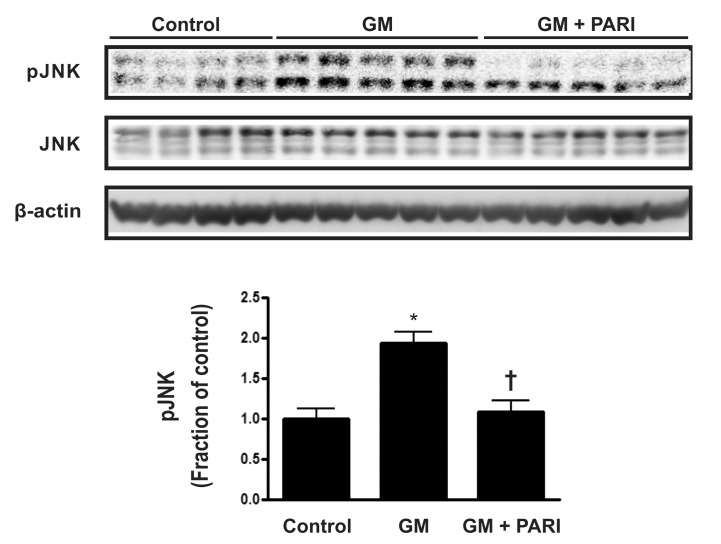
Blockade of GM-induced JNK expression by paricalcitol
To further investigate molecules involved in GM-induced apoptosis in the kidney, protein expression of mitogen- activated protein kinases (MAPKs) was assessed by immunoblotting (Fig. 4). The expression of p-JNK was remarkably increased by GM treatment, compared with vehicle injection, which was reversed by paricalcitol co-treatment, while JNK expression was not affected by either agent. The protein expression of extracellular signal-regulated kinases (ERK) and p38, the other two MAPKs, remained unchanged among the three groups (data not shown).
Fig. 4.
Immnublotting of JNK in renal cortex/OSOM. The expression of p-JNK was remarkably increased by GM treatment, compared with vehicle injection, which was reversed by paricalcitol co-treatment, while JNK expression was not affected by either agent. *p<0.05 vs. control. †p<0.05 vs. GM.
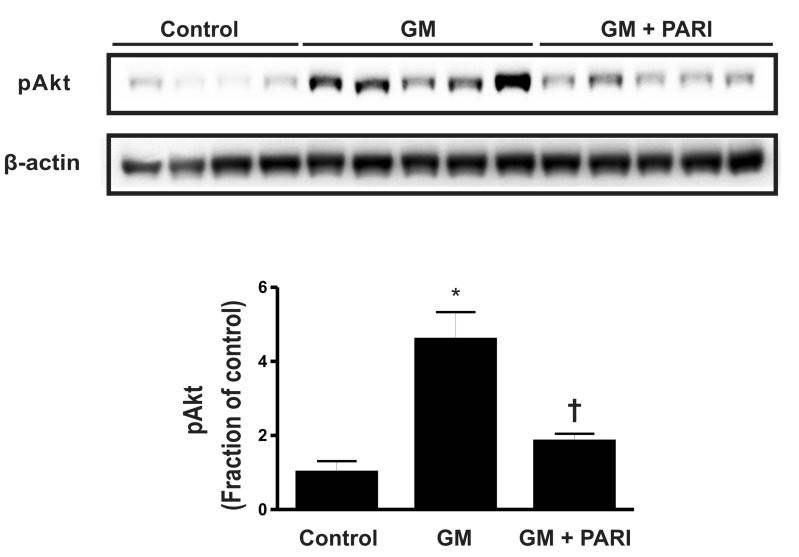
Enhanced phosphorylation of Akt by GM
Akt is one of the molecules involved in the survival signaling pathway. To elucidate the underlying mechanisms of altered apoptosis by GM and paricalcitol, we assessed p-Akt by immunoblotting (Fig. 5). The level of p-Akt was significantly increased in GM-treated rats compared with control rats, which was reversed by paricalcitol co-treatment.
Fig. 5.
Immnublotting of p-Akt in renal cortex/OSOM. The level of p-Akt was significantly increased in GM-treated rats compared with control rats, which was reversed by paricalcitol co-treatment. *p<0.05 vs. control. †p<0.05 vs. GM.
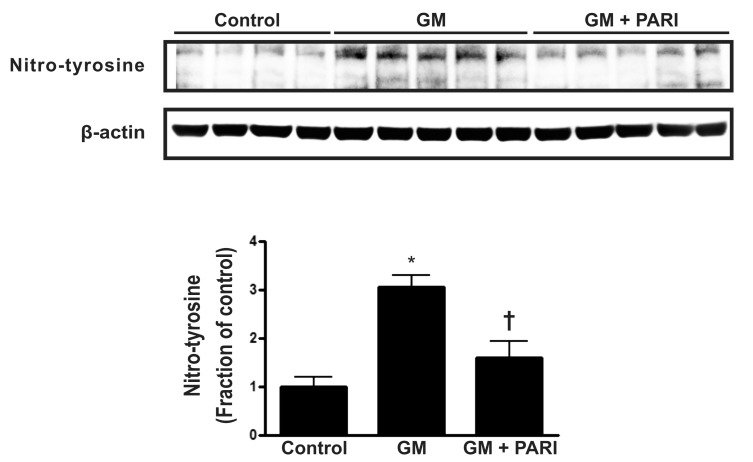
Alterations in nitro-tyrosine level by GM and paricalcitol
To investigate the association of antioxidative effect and antiapoptotic potential of paricalcitol in GM-induced kidney injury, the immunoblotting of nitro-tyrosine, a marker of nitric oxide (NO)-dependent oxidative stress was done (Fig. 6). GM treatment increased the level of nitro-tyrosine in rat kidneys, which alteration was attenuated by paricalcitol co-treatment.
Fig. 6.
the Immunoblotting of nitro-tyrosine in renal cortex/OSOM. GM treatment increased the level of nitro-tyrosine in rat kidneys, which alteration was attenuated by paricalcitol co-treatment. *p<0.05 vs. control. †p<0.05 vs. GM.
DISCUSSION
Overcoming nephrotoxicity following GM administration might be a little, but meaningful advance in the management of gram negative sepsis. It was, therefore, a promising finding that a readily available agent, paricalcitol, attenuated GM-induced AKI.
We have demonstrated the protective potential of paricalcitol against GM-induced AKI [16], where paricalcitol attenuated inflammation and epithelial-to-mesenchymal transition (EMT) process involving such molecules as ERK, nuclear factor-κB, and transforming growth factor-β1. The renoprotective effect of paricalcitol, demonstrated in that study, was strong and effective enough to restore GM-induced dysregulation of water and sodium transporters, which was also observed in other precedent studies [17,18]. It has not been determined, however, whether paricalcitol attenuates apoptosis induced by GM in the kidney. The data presented in this study expand our understating on the way paricalcitol protects kidney against GM and support the action mechanism of paricalcitol is, at least partly, contributed by its anti-apoptotic effect.
Apoptosis is a two-faced phenomenon. While failure or malfunction of apoptotic pathways is relevant with unregulated cell proliferation, offering some chance of cancer growth [19], chronic inflammation [20], and autoimmunity [21], excessive activation of apoptosis in the kidney has been known to result in tubular atrophy and organ failure [22]. The specific pathways of apoptosis consist of two different mechanisms, one of which is the intrinsic pathways, where injuries of intracellular organelles are involved in the following steps, and the other of which is the extrinsic pathway, where specific death receptor with binding its ligand activates the sequential changes [23].
Co-administration of paricalcitol reduced the expression of cleaved caspase-3 and TUNEL-positive cells in the rat kidneys, compared with in those treated GM alone. Although activation of caspase is one of the hallmarks of apoptosis, regardless of its upstream pathways [24], a previous study reported caspase-independent apoptosis in murine proximal tubular cells [25]. Nevertheless, meaningful changes in the numbers of TUNEL-positive cells among the rat group in the present study, despite some caveats of this methodology, such as false positivities in necrotic cells [26], along with results from immunoblotting of caspase-3, support the assumption that paricalcitol attenuates GM-induced AKI.
Bax protein promotes cytochrome c release from mitochondria in reaction to noxious stimuli, and thereby contributes to the activation of caspase-3 [27,28]. Bcl-2 protein, in contrast, by binding to and inactivating Bax protein, suppresses the apoptotic process. GM treatment induced down-regulation of Bcl-2 in the rat kidney, which was reversed by paricalcitol co-treatment. Although Bax expression was not altered by either GM or paricalcitol, it is postulated that recovery of Bcl-2 by paricalcitol suppresses excessive activation in intrinsic pathway of apoptosis, ameliorating GM-induced AKI.
One of MAPKs, JNK, is known to be associated with apoptosis [29] as well as inflammation, and fibrosis [30]. Although it is postulated that suppression of p-JNK expression by paricalcitol, as seen in this study, might, at least partly, contribute to the attenuation of apoptosis, considering data from a previous study [16], which demonstrated marked inflammation induced by GM in the kidney, there some possibilities exist that p-JNK exclusively involved in the tissue inflammation only, instead of apoptosis. Further study should be conducted to elucidate the detailed interaction between paricalcitol and JNK. Additionally, the reason why the expression of the other two MAPKs, ERK and p38, was not affected by GM in this study is uncertain. Specific studies on this topic remain to be done, though there are some suspected causes, such as duration and dose of GM [16], differences in the species [9], and toxic agent used in the experiments [30].
Akt signaling pathway is known to be involved in the survival signaling pathway [31]. Previously reports demonstrated that the activation of Akt signaling pathways is associated with attenuation of apoptosis in the kidney against various noxious stimuli [32,33]. It is postulated that Akt signaling pathway was activated by GM as a defense mechanism, since the level of p-Akt was increased by GM treatment, and was decreased by paricalcitol co-treatment. There are, however, some evidences that the activation of Akt signaling pathway is associated with renal fibrosis [34,35]. Considering the previously reported data that GM caused renal fibrosis in rats [16], the enhanced phosphorylation of Akt in the present study might be a step in the process of epithelial mesenchymal transition. Whether the activation of p-Akt is associated with protection from apoptosis or with acceleration of renal fibrosis or with both remains to be further investigated.
Superoxide anion reacts with excessive NO produced by inducible nitric oxide synthase (iNOS). The resultant product, peroxynitirite, nitrates tyrosine residues in proteins forming nitro-tyrosine [36]. The nitro-tyrosine is, therefore, a marker of NO-dependent oxidative stress. The level of nitro-tyrosine was increased in the rat kidneys treated with GM, compared with those treated with vehicles, while co-treatment with paricalcitol attenuated these alterations in the present study, suggesting the reduced oxidative stress by paricalcitol might be a mechanism explaining its anti-apoptotic effect. This finding is consistent with previously report [16], where increased expression of iNOS by GM was attenuated by paricalcitol. Additional studies, however, are further required, since it is still uncertain whether the reduced oxidative stress by paricalcitol is dependent on the down-regulation of iNOS along with other inflammatory markers, or paricalcitol exerts its own antioxidative potential.
Conclusively, our data support the notion the protective effect of paricalcitol in GM-induced kidney injury is, at least partly, mediated by suppression of apoptosis.
ACKNOWLEDGEMENTS
This study was supported by a grant (CRI11015-1) Chonnam national university hospital research institute of clinical medicine, and by the National Research Foundation of Korea (NRF) grant (MRC for Gene Regulation, 2011-0030132) funded by the Korea government (MSIP).
ABBREVIATIONS
- GM
gentamicin
- JNK
Jun-N-terminal kinase
- PBS
phosphate buffer saline
- OSOM
outer stripe of outer medulla
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling
- MAPK
mitogen-activated protein kinase
- NO
nitric oxide
- EMT
epithelial-to-mesenchymal transition
- ERK
extracellular signal-regulated kinases
- iNOS
inducible nitric oxide synthase
References
- 1.Zappitelli M, Moffett BS, Hyder A, Goldstein SL. Acute kidney injury in non-critically ill children treated with aminoglycoside antibiotics in a tertiary healthcare centre: a retrospective cohort study. Nephrol Dial Transplant. 2011;26:144–150. doi: 10.1093/ndt/gfq375. [DOI] [PubMed] [Google Scholar]
- 2.Martínez-Salgado C, Eleno N, Tavares P, Rodríguez-Barbero A, García-Criado J, Bolaños JP, López-Novoa JM. Involvement of reactive oxygen species on gentamicin-induced mesangial cell activation. Kidney Int. 2002;62:1682–1692. doi: 10.1046/j.1523-1755.2002.00635.x. [DOI] [PubMed] [Google Scholar]
- 3.Peyrou M, Hanna PE, Cribb AE. Cisplatin, gentamicin, and p-aminophenol induce markers of endoplasmic reticulum stress in the rat kidneys. Toxicol Sci. 2007;99:346–353. doi: 10.1093/toxsci/kfm152. [DOI] [PubMed] [Google Scholar]
- 4.Bae EH, Lee J, Ma SK, Kim IJ, Frøkiaer J, Nielsen S, Kim SY, Kim SW. Alpha-Lipoic acid prevents cisplatin-induced acute kidney injury in rats. Nephrol Dial Transplant. 2009;24:2692–2700. doi: 10.1093/ndt/gfp176. [DOI] [PubMed] [Google Scholar]
- 5.Park JW, Park CH, Kim IJ, Bae EH, Ma SK, Lee JU, Kim SW. Rho kinase inhibition by fasudil attenuates cyclosporine-induced kidney injury. J Pharmacol Exp Ther. 2011;338:271–279. doi: 10.1124/jpet.111.179457. [DOI] [PubMed] [Google Scholar]
- 6.Wu X, Guo R, Chen P, Wang Q, Cunningham PN. TNF induces caspase-dependent inflammation in renal endothelial cells through a Rho- and myosin light chain kinase-dependent mechanism. Am J Physiol Renal Physiol. 2009;297:F316–F326. doi: 10.1152/ajprenal.00089.2009. [DOI] [PubMed] [Google Scholar]
- 7.Kelly KJ, Plotkin Z, Dagher PC. Guanosine supplementation reduces apoptosis and protects renal function in the setting of ischemic injury. J Clin Invest. 2001;108:1291–1298. doi: 10.1172/JCI13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bae EH, Cho S, Joo SY, Ma SK, Kim SH, Lee J, Kim SW. 4-Hydroxy-2-hexenal-induced apoptosis in human renal proximal tubular epithelial cells. Nephrol Dial Transplant. 2011;26:3866–3873. doi: 10.1093/ndt/gfr386. [DOI] [PubMed] [Google Scholar]
- 9.Lee KE, Kim EY, Kim CS, Choi JS, Bae EH, Ma SK, Kim KK, Lee JU, Kim SW, Lee KE, Kim EY, Kim CS, Choi JS, Bae EH, Ma SK, Kim KK, Lee JU, Kim SW. Macrophage-stimulating protein attenuates gentamicin-induced inflammation and apoptosis in human renal proximal tubular epithelial cells. Biochem Biophys Res Commun. 2013;434:527–533. doi: 10.1016/j.bbrc.2013.03.108. [DOI] [PubMed] [Google Scholar]
- 10.Homsi E, Janino P, de Faria JB. Role of caspases on cell death, inflammation, and cell cycle in glycerol-induced acute renal failure. Kidney Int. 2006;69:1385–1392. doi: 10.1038/sj.ki.5000315. [DOI] [PubMed] [Google Scholar]
- 11.Daemen MA, van 't Veer C, Denecker G, Heemskerk VH, Wolfs TG, Clauss M, Vandenabeele P, Buurman WA. Inhibition of apoptosis induced by ischemia-reperfusion prevents inflammation. J Clin Invest. 1999;104:541–549. doi: 10.1172/JCI6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo R, Wang Y, Minto AW, Quigg RJ, Cunningham PN. Acute renal failure in endotoxemia is dependent on caspase activation. J Am Soc Nephrol. 2004;15:3093–3102. doi: 10.1097/01.ASN.0000145530.73247.F5. [DOI] [PubMed] [Google Scholar]
- 13.García IM, Altamirano L, Mazzei L, Fornés M, Molina MN, Ferder L, Manucha W. Role of mitochondria in paricalcitol-mediated cytoprotection during obstructive nephropathy. Am J Physiol Renal Physiol. 2012;302:F1595–F1605. doi: 10.1152/ajprenal.00617.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park JW, Cho JW, Joo SY, Kim CS, Choi JS, Bae EH, Ma SK, Kim SH, Lee J, Kim SW. Paricalcitol prevents cisplatin-induced renal injury by suppressing apoptosis and proliferation. Eur J Pharmacol. 2012;683:301–309. doi: 10.1016/j.ejphar.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 15.Park JW, Bae EH, Kim IJ, Ma SK, Choi C, Lee J, Kim SW. Paricalcitol attenuates cyclosporine-induced kidney injury in rats. Kidney Int. 2010;77:1076–1085. doi: 10.1038/ki.2010.69. [DOI] [PubMed] [Google Scholar]
- 16.Park JW, Bae EH, Kim IJ, Ma SK, Choi C, Lee J, Kim SW. Renoprotective effects of paricalcitol on gentamicin-induced kidney injury in rats. Am J Physiol Renal Physiol. 2010;298:F301–F313. doi: 10.1152/ajprenal.00471.2009. [DOI] [PubMed] [Google Scholar]
- 17.Bae WK, Lee JU, Park JW, Bae EH, Ma SK, Kim SH, Kim SW, Bae WK, Lee JU, Park JW, Bae EH, Ma SK, Kim SH, Kim SW. Decreased expression of Na+/KX+-ATPase, NHE3, NBC1, AQP1 and OAT in gentamicin-induced nephropathy. Korean J Physiol Pharmacol. 2008;12:331–336. doi: 10.4196/kjpp.2008.12.6.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J, Yoo KS, Kang DG, Kim SW, Choi KC. Gentamicin decreases the abundance of aquaporin water channels in rat kidney. Jpn J Pharmacol. 2001;85:391–398. doi: 10.1254/jjp.85.391. [DOI] [PubMed] [Google Scholar]
- 19.Lowe SW, Lin AW. Apoptosis in cancer. Carcinogenesis. 2000;21:485–495. doi: 10.1093/carcin/21.3.485. [DOI] [PubMed] [Google Scholar]
- 20.Hung RW, Chow AW. Dissecting the "end game": clinical relevance, molecular mechanisms and laboratory assessment of apoptosis. Clin Invest Med. 2004;27:324–344. [PubMed] [Google Scholar]
- 21.Clemens MJ, van Venrooij WJ, van de Putte LB. Apoptosis and autoimmunity. Cell Death Differ. 2000;7:131–133. doi: 10.1038/sj.cdd.4400633. [DOI] [PubMed] [Google Scholar]
- 22.Mao H, Li Z, Zhou Y, Li Z, Zhuang S, An X, Zhang B, Chen W, Nie J, Wang Z, Borkan SC, Wang Y, Yu X. HSP72 attenuates renal tubular cell apoptosis and interstitial fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol. 2008;295:F202–F214. doi: 10.1152/ajprenal.00468.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Servais H, Ortiz A, Devuyst O, Denamur S, Tulkens PM, Mingeot-Leclercq MP. Renal cell apoptosis induced by nephrotoxic drugs: cellular and molecular mechanisms and potential approaches to modulation. Apoptosis. 2008;13:11–32. doi: 10.1007/s10495-007-0151-z. [DOI] [PubMed] [Google Scholar]
- 24.Oliver L, Vallette FM. The role of caspases in cell death and differentiation. Drug Resist Updat. 2005;8:163–170. doi: 10.1016/j.drup.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Lorz C, Justo P, Sanz AB, Egido J, Ortíz A. Role of Bcl-xL in paracetamol-induced tubular epithelial cell death. Kidney Int. 2005;67:592–601. doi: 10.1111/j.1523-1755.2005.67115.x. [DOI] [PubMed] [Google Scholar]
- 26.Hughes J, Gobe G. Identification and quantification of apoptosis in the kidney using morphology, biochemical and molecular markers. Nephrology (Carlton) 2007;12:452–458. doi: 10.1111/j.1440-1797.2007.00854.x. [DOI] [PubMed] [Google Scholar]
- 27.Scorrano L, Korsmeyer SJ. Mechanisms of cytochrome c release by proapoptotic BCL-2 family members. Biochem Biophys Res Commun. 2003;304:437–444. doi: 10.1016/s0006-291x(03)00615-6. [DOI] [PubMed] [Google Scholar]
- 28.Iverson SL, Orrenius S. The cardiolipin-cytochrome c interaction and the mitochondrial regulation of apoptosis. Arch Biochem Biophys. 2004;423:37–46. doi: 10.1016/j.abb.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Zanke BW, Boudreau K, Rubie E, Winnett E, Tibbles LA, Zon L, Kyriakis J, Liu FF, Woodgett JR. The stress-activated protein kinase pathway mediates cell death following injury induced by cis-platinum, UV irradiation or heat. Curr Biol. 1996;6:606–613. doi: 10.1016/s0960-9822(02)00547-x. [DOI] [PubMed] [Google Scholar]
- 30.Park SJ, Jeong KS. Cell-type-specific activation of mitogen-activated protein kinases in PAN-induced progressive renal disease in rats. Biochem Biophys Res Commun. 2004;323:1–8. doi: 10.1016/j.bbrc.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 31.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 32.Chen YC, Chen CH, Hsu YH, Chen TH, Sue YM, Cheng CY, Chen TW. Leptin reduces gentamicin-induced apoptosis in rat renal tubular cells via the PI3K-Akt signaling pathway. Eur J Pharmacol. 2011;658:213–218. doi: 10.1016/j.ejphar.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 33.Lee KE, Kim EY, Kim CS, Choi JS, Bae EH, Ma SK, Park JS, Jung YD, Kim SH, Lee JU, Kim SW. Macrophage-stimulating protein attenuates hydrogen peroxide-induced apoptosis in human renal HK-2 cells. Eur J Pharmacol. 2013;715:304–311. doi: 10.1016/j.ejphar.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 34.Runyan CE, Schnaper HW, Poncelet AC. The phosphatidylinositol 3-kinase/Akt pathway enhances Smad3-stimulated mesangial cell collagen I expression in response to transforming growth factor-beta1. J Biol Chem. 2004;279:2632–2639. doi: 10.1074/jbc.M310412200. [DOI] [PubMed] [Google Scholar]
- 35.Hubchak SC, Sparks EE, Hayashida T, Schnaper HW. Rac1 promotes TGF-beta-stimulated mesangial cell type I collagen expression through a PI3K/Akt-dependent mechanism. Am J Physiol Renal Physiol. 2009;297:F1316–F1323. doi: 10.1152/ajprenal.00345.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eiserich JP, Patel RP, O'Donnell VB. Pathophysiology of nitric oxide and related species: free radical reactions and modification of biomolecules. Mol Aspects Med. 1998;19:221–357. doi: 10.1016/s0098-2997(99)00002-3. [DOI] [PubMed] [Google Scholar]