Abstract
Background
Transgenic Bt rice line T2A-1 expresses a synthesized cry2A gene that shows high resistance to Lepidoptera pests, including Cnaphalocrocis medinalis (Guenée) (Lepidoptera: Pyralidae). Plant volatile orientation cues and the physical characteristics of the leaf surface play key roles in host location or host-plant acceptance of phytophagous insects. These volatile compounds and physical traits may become altered in Bt rice and it is not known whether this influences the behavior of C. medinalis when searching for oviposition sites.
Results
The results of electronic nose analysis showed that the Radar map of Bt rice cultivars was analogous to the non- Bt rice cultivars at each growing stage. PCA analysis was able to partly discriminate between some of the Bt vs. non-Bt rice sensors, but could not to separate Bt cultivars from non-Bt cultivars. The total ion chromatogram between Bt and non-Bt rice cultivars at the seedling, booting and tillering stages were similar and 25 main compounds were identified by GC-MS. For most compounds, there was no significant difference in compound quantities between Bt and non-Bt rice cultivars at equivalent growth stages. The densities of the tubercle papicles and the trichomes on the upper and lower surfaces were statistically equal in Bt and non-Bt rice. The target pest, C. medinalis, was attracted to host rice plants, but it could not distinguish between the transgenic and the isogenic rice lines.
Conclusions
There were no significant differences between the Bt rice line, T2A-1 and the non-Bt rice for volatiles produced or in its physical characteristics and there were no negative impacts on C. medinalis oviposition behavior. These results add to the mounting evidence that Bt rice has no negative impact on the target insect oviposition behavior.
Introduction
Transgenic crops that are resistant to insects, due to the expression of Bacillus thuringiensis Berliner (Bt) genes, have been introduced worldwide and remarkable progress has been achieved [1]–[4]. To date, numerous genotypes of transgenic rice expressing different Bt genes have been successfully developed. Laboratory and field investigations have confirmed that Bt rice can effectively control the infestation of target Lepidopteran insect pests, such as stem-boring and leaf-folding species [5]–[9]. And a long-term large-scale survey have showed Bt crops could enhance biocontrol services in agricultural landscapes [10], however, because there is still a significant ongoing public debate over the social, economic and ecological implications of genetically modified (GM) agriculture [11], [12], the commercial release of Bt rice has not been permitted.
Many phytophagous insects use plant volatiles as orientation cues for food plant resources, which are used for nutritional purposes, for mate-location or for depositing their offspring [13]–[18]. The rice leaffolder, Cnaphalocrocis medinalis (Guenée) (Lepidoptera: Pyralidae), has been shown to have broad, strong electroantennogram (EAG) responses to individual rice odors [19], [20]. However, it is not known whether these volatile compounds are altered in GM crops. If they have changed, then this may influence the behavior of herbivores searching for oviposition sites natural enemies when foraging [21].
Studies on Heliothis virescens (Fabricius) and Helicoverpa zea (Boddie) in the Africa suggested that there were no changes in the oviposition behavior of female moths between cotton plant structures after almost 1 decade of widespread Bt cotton planting [22], [23]. Van den Berg & van Wyk reported that Sesamia calamistis adults did not differentiate between Bt and non-Bt maize plants during oviposition choice experiments [23]. Chilo partellus and Sesamia calamistis moths could not discriminate between Bt and non-Bt maize plants with regards to egg laying [24] and uninfested Bt and non-Bt maize were similarly attractive to females of the larval parasitoids: Cotesia flavipes and C. sesamiae [25]. However, it has been reported that there were significant quantitative differences in volatile emissions between Bt and non-Bt plants, although C. marginiventris and Microplitis rufiventris could not distinguish between the odors of a Bt maize cultivar and its near-isogenic line [26]. H. armigera hatching larvae have been shown to preferentially seek structures with lower Cry protein levels when selecting plants to feed on [27]. In addition, the olfactory responses of the parasitoid Cotesia marginiventris were shown to be weaker toward host (Spodoptera frugiperda) frass derived from Bt maize compared to frass derived from conventional maize [28].
Most insect-plant interactions occur on the leaf surface. The physical and/or chemical characteristics of the leaf surface play key roles in host-plant acceptance by phytophagous insects. Any changes to the physical and/or chemical characteristics of the leaf surfaces of transgenic crops, due to the insertion of exotic genes, may influence the search for or the acceptance of host plants by herbivore insects [21]. Significant phenotypic changes induced by genetic modification are not without precedent. Xue showed that three cotton lines were quite different from each other in the densities of certain kinds of covering trichomes [29] and the production of lignin, a major structural component of plants, increased in vascular tissues by 33–97% in Bt maize compared to non-Bt isolines [30].
The transgenic Bt rice line, T2A-1, expresses a synthesized cry2A gene and has a high resistance to Lepidoptera pests [7], [8], including Cnaphalocrocis medinalis [31]. It has been reported that T2A-1 has no significant effects on the occurrence of four predatory arthropod natural enemies (Ummeliata insecticeps, Paegerus fuscipes, Hylyphantes graminicola, Pardosa pseudoannulata) of C. medinalis [31] and previous studies have reported no significant adverse effects on the population dynamics of three plant hoppers (Nilaparvata lugens, Sogatella furcifera and Laodelphax striatellus) and their predators (Cyrtorhinus lividipennis, Pirata subpiraticus and Theridium octomaculatum) [32]. But it is not clear whether Bt genetic engineering in rice may have an effect on phenotypic changes (e.g. densities coverings and volatile emissions) which could cause unintended pleiotropic effects in the environment. The aim of the study was to investigate whether Bt genetic engineering in rice may affect volatile emissions of rice plants as well as olfactory- & physically-mediated host location behavior of C. medinalis.
Materials and Methods
Plant Growth
The rice varieties used in this study were the transgenic Bt rice line, T2A-1 and the non-transgenic parental indica rice line, Minghui 63 (MH63). T2A-1 expresses a synthesized cry2A gene which was driven by the maize ubiquitin promoter and introduced into the elite indica rice restorer line Minghui 63 by Agrobacterium-mediated transformation. All the rice lines were gifted by Lin Yong-Jun, National Key Laboratory of Crop Genetic Improvement, Wuhan, China. The rice seeds were sown in a greenhouse under 26±4°C, a 16 h light:8 h dark cycle and 60–90% relative humidity. After 20–25 d, the seedlings were transplanted into clay pots (20 cm (diam)×30 cm (height)) and each pot contained one plant. The plants were watered daily, and each pot was supplied with 10 ml of nutrient solution (Ca(NO3)2·4H2O, 0.5 g/l; K(NO3)2·4H2O, 0.125 g/l; MgSO4·7H2O, 0.125 g/l; K2HPO4, 0.125 g/l and FeCl2, 0.005 g/l) every 3 d. The pots were divided into three groups: plants representing the seedling stage, 25–30 d after potting for the tillering stage and 20–25 d after potting for the booting stage. Planting continued at regular intervals so that there were enough plants of a suitable age available for all the experiments.
Electronic Nose Measurement
The electronic nose, FOX 4000 (Alpha MOS, France), was used in this study. It was equipped with 18 different thermo-regulated metal oxide semiconductor sensors that were sensitive to different classes of chemical compounds (Table S1). The equipment used clean air (through an activated charcoal filter) as a reference gas. For each sample, three seedlings or three flag leaves at the tillering/booting stage were placed in 100 ml Pyrex vials equipped with a pierceable silicon/Teflon cap. Measurements were performed in dynamic headspace mode by extracting the headspace through the 0.45 µm syringe filter outlet in order to prevent environmental contamination. Each measurement cycle consisted of: exposure of the sensors to clean filtered ambient air, a sensor measurement period of 120 s, exposure of the sensors to the sample headspace for 120 s and finally, sensor exposure to filtered ambient air for 360 s for baseline recovery before the next analysis. The experimental conditions adapted from Camurati et al were used [33], and measurement was repeated six times for each sample. In order to perform data statistical correlation, e-nose measurements were performed at the same time when volatile samples were collected for Gas chromatography/mass spectrometry (GC-MS) analyses.
Collection, Isolation and Identification of Volatile Compounds
The seedlings and potted plants (1 or 3 per pot) were washed with running water to remove soil. The treated plants were placed in water-filled conical flasks, which were wrapped with tinfoil. The plants were then placed separately into four glass containers (20 cm (diam)×50 cm (height)) and connected to Super Q (200 mg each, Alltech Associates, Inc. Deerfield, Illinois) traps (15 cm L×0.6 cm OD) [34]. Air was filtered using three adsorbent traps (20 cm×3 cm OD): 1) charcoal (Activated Carbon, 6–14 mesh, Fisher Scientific), 2) 5A molecular sieves (beads, 8–12 mesh, Sigma-Fluka), and 3) silica gel Rubin (Drying agent free of metal salts* silica gel, Sigma-Fluka) before being pulled through the apparatus with a vacuum pump. The flow rate was controlled at 0.4 L/min, and volatile collection was conducted at 25±1°C for 24 hours (Figure S1). The airborne volatiles were eluted by percolating each Super Q trap with glass-distilled dichlormethane (0.5 ml/container), and the resultant solutions were concentrated using nitrogen to a volume of about 100 µl. Exactly 200 ng of nonyl acetate (Sigma, Buchs, Switzerland) in 10 µl of dichlormethane was added to the samples as internal standards and the solutions were then stored at –40°C. Collections were replicated five times for each cultivar.
GC/MS analysis was performed on DSQ II instrument (Thermo Fisher Scientific, USA) with an automated on-column injection system. A 1 µl aliquot from each sample was injected onto a HP-5 capillary column (30 m, 0.25 mm i.d. and with a 0.25 mm film thickness, Alltech, Deerfield, IL, USA) in splitless mode. Helium (24 cm/s) was used as the carrier gas. After injection, the oven temperature was maintained at 40°C for 2 min, increased to 250°C at 6°C/min and then held at 250°C for 2 min. The detector signal was processed using Hewlett Packard (HP) GC Chemstation software.
Initial identification of volatile compounds was based on the Wiley MS library data base matching and comparisons of retention times with those from previous studies [35]. The identities of potential candidate compounds and were confirmed by GC retention times and MS spectra of the standard samples. For the quantitative purpose, 1 µl aliquot was injected in pulsed splitless mode into a HP GC equipped with a flame ionization detector, using the same column and temperature program as above. Total volatile contents were calculated based on their peak areas when compared to those of the internal standards.
Trichome and Tubercle Papicle Density
Flag leaves from plants at the booting stage were collected. Measurements were undertaken using a scanning electron microscope (SEM) (Tokyo, Japan) depending on the number of trichomes and tubercle papicles on the upper and lower surfaces [29]. Thirty samples were counted per treatment and the subsequent data is presented in terms of trichome and tubercle papicle density per 0.1 mm2.
Bioassays
Cnaphalocrocis medinalis pupae were collected from an experimental plot in Wu’xue county of Hubei province in China (115°33′E; 29°51′N) in 2012. The locations sampled were not privately owned or protected in any way, and this field study did not involve endangered or protected species. Sexed pupae were kept inside culture dishes (20 cm diameter) in an environmental chamber (25±1°C, 75±5% RH and 16 L:8 D photoperiod) until the moths emerged. In order to assess oviposition preference, ten doubles of newly emerged females and male C. medinalis were released in a cage covered with a cotton mesh (70×70×100 cm) that contained four potted plants (two MH-63 and two T2A-1 plants) at the booting stage and placed in a diagonal formation (Figure S2).
The assay consisted of 30 replicates and was carried out in an outdoor environment under high humidity conditions (Figure S2). The position of the Bt and non-Bt rice in the cages was alternately changed in the different replicates. The plants were exposed for oviposition for 6 d and then the number of eggs laid was recorded.
Data Analysis
The complex data sets created by e-nose analysis were submitted to Principal Component Analysis (PCA) using a projection method that allowed an easy visualization of the information contained in the data sets and also permitted dimensional reduction [36]. Two-way ANOVA was used to analyze the differences between cultivars and stages, together with their interactions, on the measured indexes of relative abundance (P-value). A paired-sample t-test was used to analyze the differences in the quantity of volatiles, trichome/tubercle papicle density and C. medinalis oviposition performance between MH63 and T2A-1. The data were analyzed statistically using SPSS 20 for Windows software.
Results
Electronic Nose Analysis
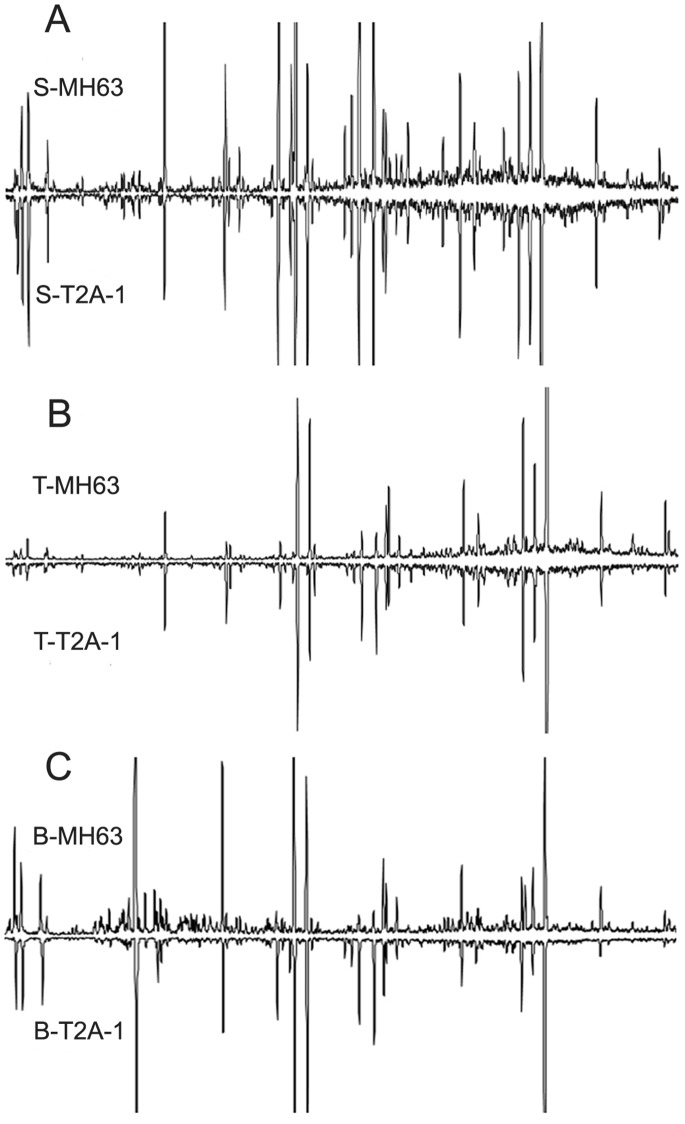
The response values for each sensor are shown in Figure 1. These graphs were constructed using the changes in relative resistance and represented the peak height of each sensor as a radial vector. The sensor responses were highly reproducible and the outline of Bt rice cultivars was analogous to the non-Bt rice cultivars. Two-way ANOVA analysis showed that there were no obvious changes in relative abundance for each sensor when comparing the two cultivars (P>0.05) and cultivar×stage did not affect relative abundance either (P>0.05), but growth stage did significantly affect sensor relative abundance (P<0.05) (Table 1).
Figure 1. Radar map, created using an electronic nose, of rice leaves from Bt and non-Bt rice cultivars: (A) seedling stage(S); (B) tillering stage (T); (C) booting stage (B).
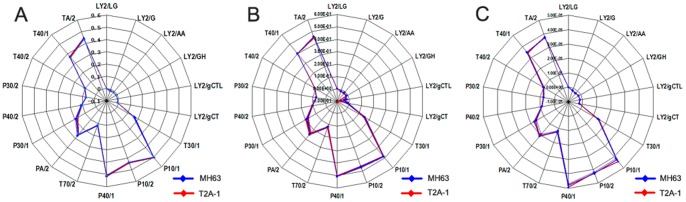
Table 1. Two-way ANOVAs of the effects of cultivar, stage and their interactions on the measured indexes of relative abundance (P-value).
| Alpha MOS Sensor Type | Cultivar | Stage | Cultivar×Stage |
| LY2/LG | 0.481 | 0.534 | 0.281 |
| LY2/G | 0.900 | <0.0001 | 0.425 |
| LY2/AA | 0.633 | <0.0001 | 0.352 |
| LY2/GH | 0.825 | <0.0001 | 0.071 |
| LY2/gCTL | 0.817 | <0.0001 | 0.974 |
| LY2/gCT | 0.236 | <0.0001 | 0.594 |
| T30/1 | 0.917 | <0.0001 | 0.261 |
| P10/1 | 0.529 | <0.0001 | 0.713 |
| P10/2 | 0.433 | <0.0001 | 0.730 |
| P40/1 | 0.486 | <0.0001 | 0.671 |
| T70/2 | 0.547 | <0.0001 | 0.794 |
| PA/2 | 0.928 | <0.0001 | 0.911 |
| P30/1 | 0.851 | <0.0001 | 0.322 |
| P40/2 | 0.813 | <0.0001 | 0.524 |
| P30/2 | 0.466 | <0.0001 | 0.723 |
| T40/2 | 0.766 | <0.0001 | 0.671 |
| T40/1 | 0.506 | <0.0001 | 0.468 |
| TA/2 | 0.409 | <0.0001 | 0.573 |
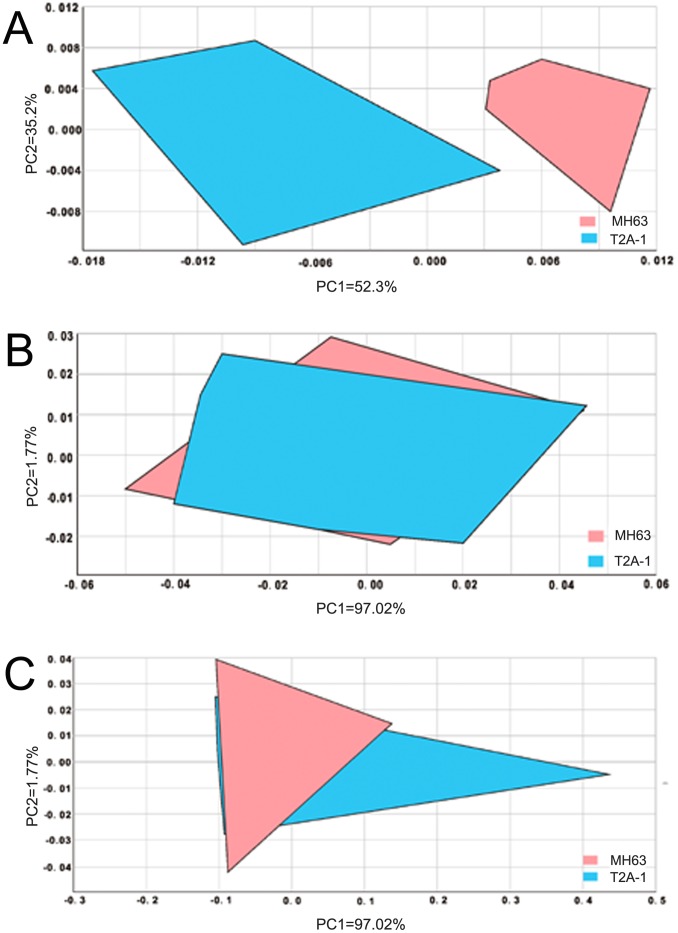
The PCA analysis results are shown in Figure 2, which exhibited the results for principal component1 (PC1) and principal component2 (PC2) on a two-dimensional plane. PCA is viewed as a linear combinatorial method and enabled the complexity of the data set to be reduced. PCA was performed in order to describe the volatile changes seen during the different stages for Bt and non-Bt rice cultivars. At the seedling stage, the first principal component, PC1, explained 52.30% of the total variation, while 35.21% of the total variance was explained by PC2, with a distance of 0.01 (Figure 2A). At the tillering stage, PC1 explained 66.33% of the variation, while PC2 explained 20.89%, with a distance of 0.02 (Figure 2B). At the booting stage, the values were 97.02% for PC1 and 1.77% for PC2, with a distance of 0.04 (Figure 2C). The distances indicated that there was a low discrimination power between the components of the non-Bt and Bt samples at all three growth stages. This method was able to partially discriminate between the Bt and non-Bt rice cultivars for some of the sensors but was not able to separate out the Bt cultivar from the non-Bt cultivar.
Figure 2. Score plots of PC1 vs. PC2 for the PCAs of leaves from Bt and non-Bt rice cultivars analyzed using an electronic nose: (A) seedling stage (S); (B) tillering stage (T); (C) booting stage (B).
Volatile Compounds Emitted from Rice
The total ion chromatogram between Bt and non- Bt rice cultivars at the seedling, booting and tillering stages were similar (Figure 3) and 25 main compounds, belonging to the following chemical classes: alkanes, terpenoids, carbonyls, hydroxyl and carbonyl compounds, benzenes, aromatic compounds and heterocyclil compounds, were identified by GC-MS (Table 2). The alkanes, such as: dodecane, tridecane, tetradecane, pentadecane, hexadecane, eicosane, heneicosane and tetracosane, were the most abundant class of compounds, whereas the major components identified were alkanes. Naphthalene and dodecane were the predominant components identified at all three growth stages.
Figure 3. Gas chromatography-mass spectrometry (GC-MS) profiles of representative solvent extracts of Bt and non-Bt rice cultivars: (A) seedling stage (S); (B) tillering stage (T); (C) booting stage (B).
Table 2. Volatiles released at the seedling (S), tillering (T) and booting (B) stages by Bt and non-Bt rice.
| Volatile | S-T2A-1 | S-MH63 | T-T2A-1 | T-MH63 | B-T2A-1 | B-MH63 |
| Alkanes | ||||||
| Dodecane | 1.95±0.27 | 1.51±0.07ns | 3.50±0.54 | 4.12±0.26ns | 3.82±0.96 | 5.06±1.66ns |
| Tridecane | 1.94±0.20 | 1.82±0.15ns | 1.10±0.44 | 1.12±0.25ns | 0.99±0.17 | 1.00±0.73ns |
| Heptylcyclohexane | 0.25±0.69 | 0.24±0.37ns | 0.19±0.26 | 0.17±0.20ns | 0.15±0.12 | 0.13±0.13ns |
| Tetradecane | 1.96±0.14 | 1.63±0.93ns | 1.97±0.20 | 1.82±0.15ns | 1.43±0.49 | 1.31±0.25ns |
| Pentadecane | 2.16±0.18 | 1.87±0.04ns | 2.76±0.97 | 2.83±0.19ns | 1.31±0.64 | 1.29±0.31ns |
| Hexadecane | 1.73±0.23 | 1.77±0.13ns | 1.66±0.11 | 1.90±0.18ns | 0.95±0.87 | 1.02±0.19ns |
| Eicosane | 1.53±0.15 | 1.71±0.10ns | 1.29±0.18 | 1.68±0.19ns | 0.80±0.26 | 0.72±0.18ns |
| Heneicosane | 0.66±0.25 | 0.66±0.11ns | 1.40±0.18 | 1.67±0.19ns | 0.37±0.64 | 0.56±0.28ns |
| Tetracosane | 0.24±0.36 | 0.21±0.23ns | 0.39±0.12 | 0.44±0.91ns | 0.17±0.15 | 0.16±0.34ns |
| Terpenoids | ||||||
| Longifolene-(V4) | 0.53±0.08 | 0.49±0.07ns | 0.40±0.10 | 0.41±0.07ns | 0.23±0.10 | 0.30±0.05ns |
| Cedrene | 1.26±0.12 | 0.95±0.12ns | 1.42±0.94 | 1.35±0.11ns | 0.72±0.27 | 0.31±0.54ns |
| 4-Carene | 0.17±0.31 | 0.17±0.03ns | 0.16±0.12 | 0.17±0.21ns | 0.15±0.09 | 0.15±0.02ns |
| à-Pinene | 0.20±0.15 | 0.17±0.26ns | 0.22±0.41 | 0.19±0.19ns | 0.17±0.16 | 0.16±0.16ns |
| D-Limonene | 1.75±0.15 | 1.99±0.37ns | 1.74±0.32 | 1.88±0.30ns | 0.47±0.25 | 0.44±0.44ns |
| Carbonyls | ||||||
| Tetradecanal | 0.48±0.67 | 0.49±0.68ns | 0.18±0.14 | 0.15±0.13ns | 0.09±0.31 | 0.17±0.12ns |
| Nonanal | 0.56±0.35 | 0.54±0.24ns | 0.68±0.067 | 0.65±0.68ns | 0.28±0.30 | 0.30±0.36ns |
| 2-Hexanone | 0.20±0.02 | 0.22±0.47ns | 0.18±0.58 | 0.13±0.13ns | 0.07±0.01 | 0.07±0.02ns |
| Hydroxyl and carbonyl compound | ||||||
| Cedrol | 0.33±0.12 | 0.35±0.23ns | 0.34±0.47 | 0.35±0.45ns | 0.18±0.06 | 0.20±0.35ns |
| 3-Hexanol | 0.81±0.18 | 0.95±0.18ns | 0.25±0.18 | 0.26±0.43ns | 0.28±0.41 | 0.31±0.78ns |
| (Z)3-Hexen-1-ol | 1.11±0.64 | 1.17±0.22ns | 1.20±0.14 | 1.54±0.20ns | 0.10±0.01 | 0.14±0.24ns |
| Benzene | ||||||
| Ethylbenzene | 1.17±0.10 | 0.80±0.07* | 0.35±0.04 | 0.36±0.23ns | 1.89±0.31 | 1.87±0.47ns |
| o-Xylene | 1.10±0.22 | 0.75±0.04ns | 0.30±0.06 | 0.45±0.06ns | 1.27±0.11 | 1.34±0.25ns |
| Biphenyl | 0.39±0.08 | 0.41±0.06ns | 0.40±0.92 | 0.50±0.95ns | 0.32±0.60 | 0.27±0.06ns |
| Aromatic compounds | ||||||
| Indane | 0.10±0.01 | 0.08±0.01ns | 0.06±0.02 | 0.09±0.02ns | 0.13±0.02 | 0.14±0.04ns |
| Heterocyclil compound | ||||||
| Naphthalene | 7.40±0.30 | 6.47±0.64ns | 5.03±0.35 | 5.20±0.61ns | 5.24±1.12 | 6.12±0.89ns |
Note: paired values with an asterisk indicate that there is an significant difference between Bt (T2A-1) and non-Bt (MH63) rice (P<0.05) and ns indicates there are no significant differences between Bt (T2A-1) and non-Bt (MH63) rice at the seedling stage (S), tillering stage (T) and booting stage (B), respectively.
For each compound, with the exception of the ethylbenzene content in Bt rice, which was higher than that in non-Bt rice (P>0.05), there were no significant quantitative differences between Bt and non-Bt rice cultivars at the same growth stage (P>0.05). However, within each rice cultivar, the quantity of some volatiles changed as the rice grew and, both rice cultivars showed the same trend, which had three distinct patterns: first there was an uptrend for some compounds, such as dodecane, then there was a downtrend for some compounds, such as 2-hexanone, 3-hexanol, D-limonene, tridecane, tetradecanal, heptylcyclohexane, longifolene-(V4) and eicosane, and finally the trend was flat for some compounds, such as 4-carene, à-pinene, and naphthalene (Table 2).
Trichome and Tubercle Papicle Density
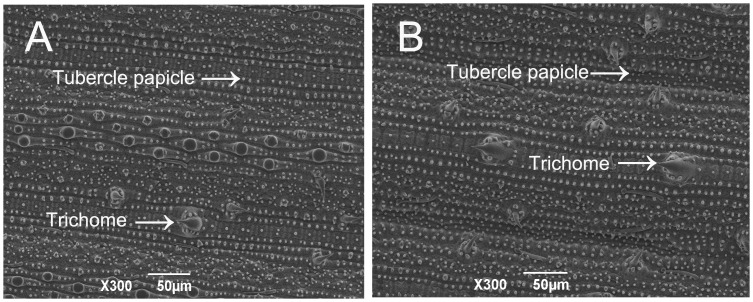
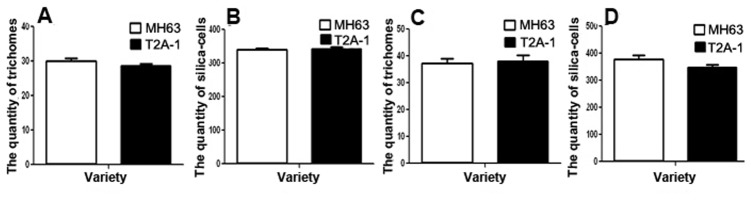
Under the SEM, tubercle papicles were found to be widespread across the leaf surfaces of Bt rice and non-Bt rice lines and the trichomes were located along the veins on both surfaces of the leaf (Figure 4). There were no significant differences in the total density of the different types of trichomes covering the upper surface (t = 1.5105; P = 0.1363), the lower surface (t = 0.2569; P = 0.7981) or between the two rice lines. There were also no significant differences between the two rice lines for tubercle papicles on the upper surface (t = 0.3271; P = 0.7448)) or on the lower leaf surface (t = 1.7119; P = 0.0931) (Figure 5).
Figure 4. The morphology of trichomes and tubercle papicles on the surfaces of rice leaves: (A) upper surfaces; (B) lower surfaces.
Figure 5. Density of trichomes and tubercle papicles on the surfaces of rice leaves: (A) trichomes on the upper surfaces; (B) tubercle papicles on the upper surfaces; (C): trichomes on the lower surfaces; (D) tubercle papicles on the lower surfaces.
Target Pest Performance
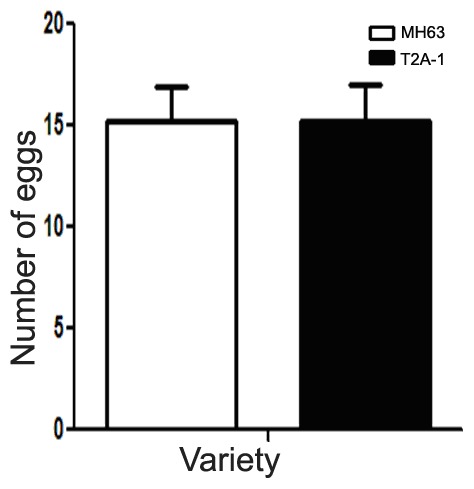
The average number of eggs (mean ± SE) per Bt and non-Bt rice plant was 15.2 and 15.1, respectively, and there were no significant differences between the two rice lines (t = 0.0219; P = 0.9826) (Figure 6).
Figure 6. The number of eggs (mean ± SE) on Bt and non-Bt rice.
Discussion
Concern over the environmental safety of Bt crops has led to a large number of studies that have investigated the potential effect of these crops on non-target phytophagous insects and their natural enemies. In general, significant negative effects or not on natural enemies due to Bt-transgenic rice have been observed, as measured by fitness indicators, population density and dynamics and biodiversity indices [37]–[42]. However, little attention has been paid to the potential changes in rice plant volatiles due to the genetic modification of rice plants, which could affect the insect host location process.
The electronic nose was based on the principal of gas chromatography and an array of solid-state sensors that are non-selectively sensitive to the relevant chemicals and the responses of which reflect the chemical information contained in the sample. This detection scheme is, in many respects, similar to natural olfaction where hundreds of different receptors can distinguish between tens of thousands of different odors [43]. FOX 4000 electronic noses have been applied in a number of different fields and have identified and classified many different samples [43]–[46]. In this study, for the first time, the FOX 4000 has been used to compare Bt and non-Bt rice. Both the Radar map analysis and the PCA results showed that there were no significant differences in volatiles between the Bt and non-Bt rice cultivars, whereas growth stage could affect volatile contents in the two cultivars. However, it was hard to discriminate between the Bt and non-Bt rice varieties on the Radar maps and by PCA after using the electronic nose.
Overall, the types of volatiles present in Bt and non-Bt rice identified by GC-MS were similar and in good agreement with those previously reported in non-Bt rice cultivars using different extraction methods [47]–[50]. There were no significant differences for almost compounds between the two rice lines at all three growth stages. This suggested that genetic modification did not appear to alter the volatile chemical profile of rice. The same results have been found in Bt maize, where genetic modification did not alter the volatile chemical profile of undamaged maize [51]. Furthermore, Bt maize did not alter the aphid–parasitoid associations and had no effect on the aphid parasitism and hyperparasitism rates [52]. However, ethylbenzene at the seeding stage where the results showed a significant difference between Bt and non-Bt rice. Ethylbenzene is in a range of plants such as green coffee [53], Olea europaea [54] and Coffea Arabica [55]. Ethylbenzene emitted by the leaves and half-ripe olives was significantly attractive to Dacus oleae, while as an oviposition weak activants of D. oleae [54]. In the olfactometer bioassay, a pest of plants Hypothenemus hampei showed a significant response to ethylbenzene emitted by the Coffea Arabica [55]. While the correlation of ethylbenzene emitted by rice to the rice pests or natural enemies are not clear. Differences between Bt and non-Bt plants, including changes in the blend of plant volatiles emitted, have been found and undamaged Bt cotton plants have been shown to emit unique compounds and different proportions of typical compounds when compared to non-Bt cotton [56]. The HD-non Bt cultivar has been found to release a higher quantity of volatile compounds compared to HD-Bt maize [26] and transgenic Bt (expressing the cry1Ac endotoxin gene) oilseed rape plants affect the emissions of DMNT and (E,E)-alpha-farnesene after herbivore damage [57]. The headspace volatiles of two representative cultivars of transgenic scab resistant apple plants differed quantitatively for four terpenes and an aromatic compound [58]. The data available to date, relating to Bt transgenes and induced volatile release by crop plants implied that the diversity of effects by genetic modification could be obtained in different plant species.
In addition, leaf surface physical characteristics can influence the searching behaviors of insects [59]. Physical factors on the leaf surface are mainly exhibited as the density of trichomes and tubercle papicles. There are two primary types of epidermal outgrowths, i.e. covering trichomes and capitate or glandular trichomes [60], [61]. The results of this study showed that the covering trichomes and tubercle papicle densities were similar in Bt and non-Bt rice lines. Studies have also shown that there were no significant differences in the physical profiles of Bt and non-Bt arabidopsis plants [62] and cotton plants [56].
The bioassay results indicated that C. medinalis oviposition was similar on the Bt rice lines and the regular rice line. The target pest, C. medinalis, was attracted to the host rice plants, but couldn’t distinguish between the transgenic and the isogenic rice lines. As far as can be ascertained, this is the first study that describes the changes in volatile and physical characteristics introduced by the Bt gene in rice and this study has been the one of the very few that has focused on the target pest behavioral response to Bt plants. These results add to the mounting evidence that Bt rice has no negative impact on target insect behavior. Plant volatile emissions are likely to be affected by herbivore damage, which could have an effect of on plant-herbivore-parasitoid tritrophic relationships. The herbivore-induced volatile emissions from Bt rice plants compared to those from a non-transformed isoline will be studied further in the future.
Supporting Information
The headspace collection of both Bt and non-Bt rice.
(TIF)
Oviposition preference bioassays of Cnaphalocrocis medinalis : (A) Rice plants and target pests were covered with net in order to prevent the pests from flying away; (B) Experiments were conducted in humid and warm conditions.
(TIF)
Sensor sensitivity of eighteen individual sensors within the sensor array of the FOX 4000 e-nose.
(TIF)
Acknowledgments
The authors thank Zhang Hong-Yan (Key Laboratory of Horticultural Plant Biology (Huazhong Agricultural University), Ministry of Education), Yan Miao-Jun and Feng Ying-Ying for their technical assistance during the GC-MS analysis. We thank Wang Ru-Feng and Mi Feng for their technical assistance during the E-nose analysis and we thank Qin Li-Hong for his technical assistance when using the SEM.
Funding Statement
This study was supported and funded by the National Genetically Modified Organisms Breeding Major Projects of China (No: 2011ZZX08001-001), Huazhong Agricultural University Scientific & Technological Self-innovation Foundation and The Industry Project of the Ministry of Agriculture of China (200903051). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Clive J (2009) Global status of commercialized biotech/GM crops. ISAAA brief 41.
- 2. Wu K-M, Lu Y-H, Feng H-Q, Jiang Y-Y, Zhao J-Z (2008) Suppression of cotton bollworm in multiple crops in China in areas with Bt toxin–containing cotton. Science 321: 1676–1678. [DOI] [PubMed] [Google Scholar]
- 3.Jaffe G (2009) Complacency on the farm. Center for Insect Science in the Public Interest.
- 4. Kruger M, Van Rensburg J, Van den Berg J (2009) Perspective on the development of stem borer resistance to Bt maize and refuge compliance at the Vaalharts irrigation scheme in South Africa. Crop Protection 28: 684–689. [Google Scholar]
- 5. Tu J, Zhang G, Datta K, Xu C, He Y, et al. (2000) Field performance of transgenic elite commercial hybrid rice expressing Bacillus thuringiensis δ-endotoxin. Nature Biotechnology 18: 1101–1104. [DOI] [PubMed] [Google Scholar]
- 6. Ye G, Shu Q, Cui H, Hu C, Gao M, et al. (2000) A leaf-section bioassay for evaluating rice stem borer resistance in transgenic rice containing a synthetic cry1Ab gene from Bacillus thuringiensis Berliner. Bulletin of Entomological Research 90: 179–182. [DOI] [PubMed] [Google Scholar]
- 7. Chen H, Tang W, Xu C, Li X, Lin Y, et al. (2005) Transgenic indica rice plants harboring a synthetic cry2A* gene of Bacillus thuringiensis exhibit enhanced resistance against lepidopteran rice pests. TAG Theoretical and Applied Genetics 111: 1330–1337. [DOI] [PubMed] [Google Scholar]
- 8. Tang W, Chen H, Xu C, Li X, Lin Y, et al. (2006) Development of insect-resistant transgenic indica rice with a synthetic cry1C* gene. Molecular Breeding 18: 1–10. [Google Scholar]
- 9. Wang Y, Zhang G, Du J, Liu B, Wang M (2010) Influence of transgenic hybrid rice expressing a fused gene derived from cry1Ab and cry1Ac on primary insect pests and rice yield. Crop Protection 29: 128–133. [Google Scholar]
- 10. Lu Y, Wu K, Jiang Y, Guo Y, Desneux N (2012) Widespread adoption of Bt cotton and insecticide decrease promotes biocontrol services. Nature 487: 362–365. [DOI] [PubMed] [Google Scholar]
- 11. Romeis J, Bartsch D, Bigler F, Candolfi MP, Gielkens MM, et al. (2008) Assessment of risk of insect-resistant transgenic crops to nontarget arthropods. Nature Biotechnology 26: 203–208. [DOI] [PubMed] [Google Scholar]
- 12. Desneux N, Bernal JS (2010) Genetically modified crops deserve greater ecotoxicological scrutiny. Ecotoxicology 19: 1642–1644. [DOI] [PubMed] [Google Scholar]
- 13. Jyothi K, Prasuna A, Sighamony S, Kumari BK, Prasad A, et al. (2002) Electroantennogram responses of Apanteles obliquae (Hym., Braconidae) to various infochemicals. Journal of Applied Entomology 126: 175–181. [Google Scholar]
- 14. van Der Goes vN, Carlson JR (2006) Insects as chemosensors of human sand crops. Nature 444: 302–307. [DOI] [PubMed] [Google Scholar]
- 15. Gallego D, Galián J, Diez J, Pajares J (2008) Kairomonal responses of Tomicus destruens (Col., Scolytidae) to host volatiles α-pinene and ethanol. Journal of Applied Entomology 132: 654–662. [Google Scholar]
- 16. Fettig C, McKelvey S, Dabney C, Borys R, Huber D (2009) Response of Dendroctonus brevicomis to different release rates of nonhost angiosperm volatiles and verbenone in trapping and tree protection studies. Journal of Applied Entomology 133: 143–154. [Google Scholar]
- 17. Hu J, Angeli S, Schuetz S, Luo Y, Hajek AE (2009) Ecology and management of exotic and endemic Asian longhorned beetle Anoplophora glabripennis . Agricultural and Forest Entomology 11: 359–375. [Google Scholar]
- 18. Zhuge PP, Luo SL, Wang MQ, Zhang G (2010) Electrophysiological responses of Batocera horsfieldi (Hope) adults to plant volatiles. Journal of Applied Entomology 134: 600–607. [Google Scholar]
- 19. Ramachandran R, Khan Z, Caballero P, Juliano B (1990) Olfactory sensitivity of two sympatric species of rice leaf folders (Lepidoptera: Pyralidae) to plant volatiles. Journal of Chemical Ecology 16: 2647–2666. [DOI] [PubMed] [Google Scholar]
- 20.Sun X, Liu Z, Zhang A, Dong H-B, Zeng F-F, et al. (2013) Electrophysiological Responses of the Rice Leaffolder, Cnaphalocrocis medinalis (Lepidoptera: Pyralidae), to Rice Plant Volatiles(Accpeted). Journal of Insect Science. [DOI] [PMC free article] [PubMed]
- 21. Schuler TH, Poppy GM, Kerry BR, Denholm I (1999) Potential side effects of insect-resistant transgenic plants on arthropod natural enemies. Trends in Biotechnology 17: 210–216. [DOI] [PubMed] [Google Scholar]
- 22. Torres JB, Ruberson JR (2006) Spatial and temporal dynamics of oviposition behavior of bollworm and three of its predators in Bt and non-Bt cotton fields. Entomologia Experimentalis et Applicata 120: 11–22. [Google Scholar]
- 23. Van den Berg J, Van Wyk A (2007) The effect of Bt maize on Sesamia calamistis in South Africa. Entomologia Experimentalis et Applicata 122: 45–51. [Google Scholar]
- 24. Obonyo D, Songa J, Oyieke F, Nyamasyo G, Mugo S (2008) Bt-transgenic maize does not deter oviposition by two important African cereal stem borers, Chilo partellus Swinhoe (Lepidoptera: Crambidae) and Sesamia calamistis Hampson (Lepidoptera: Noctuidae). Journal of Applied Biosciences 10: 424–433. [Google Scholar]
- 25.Obonyo DN (2009) Tritrophic Interactions Between Parasitoids, Lepidopteran Stem Borers and Bt Maize, PhD Thesis.
- 26. Turlings TC, Jeanbourquin PM, Held M, Degen T (2005) Evaluating the induced-odour emission of a Bt maize and its attractiveness to parasitic wasps. Transgenic Research 14: 807–816. [DOI] [PubMed] [Google Scholar]
- 27. Lu B, Downes S, Wilson L, Gregg P, Knight K, et al. (2011) Preferences of field bollworm larvae for cotton plant structures: impact of Bt and history of survival on Bt crops. Entomologia Experimentalis et Applicata 140: 17–27. [Google Scholar]
- 28. Desneux N, Ramírez-Romero R, Bokonon-Ganta AH, Bernal JS (2010) Attraction of the parasitoid Cotesia marginiventris to host (Spodoptera frugiperda) frass is affected by transgenic maize. Ecotoxicology 19: 1183–1192. [DOI] [PubMed] [Google Scholar]
- 29. Xue K, Deng S, Wang R, Yan F, Xu C (2008) Leaf surface factors of transgenic Bt cotton associated with the feeding behaviors of cotton aphids: A case study on non-target effects. Science in China Series C: Life Sciences 51: 145–156. [DOI] [PubMed] [Google Scholar]
- 30. Saxena D, Stotzky G (2001) Bt corn has a higher lignin content than non-Bt corn. American Journal of Botany 88: 1704–1706. [PubMed] [Google Scholar]
- 31. Xu X, Han Y, Wu G, Cai W, Yuan B, et al. (2011) Field evaluation of effects of transgenic cry1Ab/cry1Ac, cry1C and cry2A rice on Cnaphalocrocis medinalis and its arthropod predators. Science China Life Sciences 54: 1019–1028. [DOI] [PubMed] [Google Scholar]
- 32. Han Y, Xu X, Ma W, Yuan B, Wang H, et al. (2011) The Influence of Transgenic cry1Ab/cry1Ac, cry1C and cry2A Rice on Non-Target Planthoppers and Their Main Predators Under Field Conditions. Agricultural Sciences in China 10: 1739–1747. [Google Scholar]
- 33. Camurati F, Tagliabue S, Bresciani A, Sberveglieri G, Zaganelli P (2006) Sensory analysis of virgin olive oil by means of organoleptic evaluation and electronic olfactory system. Rivista Italiana Delle Sostanze Grasse 83: 205–211. [Google Scholar]
- 34. Heath RR, Manukian A (1994) An automated system for use in collecting volatile chemicals released from plants. Journal of Chemical Ecology 20: 593–608. [DOI] [PubMed] [Google Scholar]
- 35. Turlings TC, Benrey B (1998) Effects of plant metabolites on the behavior and development of parasitic wasps. Ecoscience 5: 321–333. [Google Scholar]
- 36. Yu H, Wang J (2007) Discrimination of LongJing green-tea grade by electronic nose. Sensors and Actuators B: Chemical 122: 134–140. [Google Scholar]
- 37. Bai Y, Jiang M, Cheng J (2005) Effects of transgenic cry1Ab rice pollen on the oviposition and adult longevity of Chrysoperla sinica Tjeder. Acta Phytophylacica Sinica 32: 225. [Google Scholar]
- 38. Bai Y, Jiang M, Cheng J (2005) Effects of transgenic cry1Ab rice pollen on fitness of Propylea japonica (Thunberg). Journal of Pest Science 78: 123–128. [Google Scholar]
- 39. Bai Y, Jiang M, Cheng J, Wang D (2006) Effects of Cry1Ab toxin on Propylea japonica (Thunberg) (Coleoptera: Coccinellidae) through its prey, Nilaparvata lugens Stål (Homoptera: Delphacidae), feeding on transgenic Bt rice. Environmental Entomology 35: 1130–1136. [Google Scholar]
- 40. Chen M, Shelton A, Ye G-y (2011) Insect-resistant genetically modified rice in China: from research to commercialization. Annual Review of Entomology 56: 81–101. [DOI] [PubMed] [Google Scholar]
- 41. Chen M, Ye G, Liu Z, Yao H, Chen X, et al. (2006) Field assessment of the effects of transgenic rice expressing a fused gene of cry1Ab and cry1Ac from Bacillus thuringiensis Berliner on nontarget planthopper and leafhopper populations. Environmental Entomology 35: 127–134. [Google Scholar]
- 42. Peterson JA, Lundgren JG, Harwood JD (2011) Interactions of transgenic Bacillus thuringiensis insecticidal crops with spiders (Araneae). Journal of Arachnology 39: 1–21. [Google Scholar]
- 43. Eifler J, Martinelli E, Santonico M, Capuano R, Schild D, et al. (2011) Differential Detection of Potentially Hazardous Fusarium Species in Wheat Grains by an Electronic Nose. PloS one 6: e21026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Korsching S (2002) Olfactory maps and odor images. Current Opinion in Neurobiology 12: 387–392. [DOI] [PubMed] [Google Scholar]
- 45. Perkowski J, Bu<$>\vskip -0.3 \raster(50%)="rg2"<$>ko M, Chmielewski J, Góral T, Tyrakowska B (2008) Content of trichodiene and analysis of fungal volatiles (electronic nose) in wheat and triticale grain naturally infected and inoculated with Fusarium culmorum . International Journal of Food Microbiology 126: 127–134. [DOI] [PubMed] [Google Scholar]
- 46. Lopez de Lerma N, Bellincontro A, Mencarelli F, Moreno J, Peinado RA (2012) Use of electronic nose, validated by GC–MS, to establish the optimum off-vine dehydration time of wine grapes. Food Chemistry 130: 447–452. [Google Scholar]
- 47. Lou Y-G, Ma B, Cheng J-A (2005) Attraction of the Parasitoid Anagrus nilaparvatae to Rice Volatiles Induced by the Rice Brown Planthopper Nilaparvata lugens . Journal of Chemical Ecology 31: 2357–2372. [DOI] [PubMed] [Google Scholar]
- 48. Wechgama K, Laopaiboon L, Laopaiboon P (2008) Quantitative analysis of main volatile and other compounds in traditional distilled spirits from Thai rice. Biotechnology 7: 718–724. [Google Scholar]
- 49. Lu K, Li X, Zhou JL, Jie XJ, Qi S, et al. (2010) Influence of the herbivore-induced rice volatiles on fungal disease (in Chinese). Chinese Science Bulletin 55: 2925–2930. [Google Scholar]
- 50. Yan F, Wang X, Lv J, Pang B-P, Lou Y-G (2010) Comparison of the volatiles from rice plants infested by rice striped stem borer, Chilo suppressalis and rice leaf folder, Cnaphalocrocis medinali(In Chinese with English Abstract). Entomological Knowledge 47: 96–101. [Google Scholar]
- 51. Dean JM, De Moraes CM (2006) Effects of genetic modification on herbivore-induced volatiles from maize. Journal of Chemical Ecology 32: 713–724. [DOI] [PubMed] [Google Scholar]
- 52. Lumbierres B, Starý P, Pons X (2011) Effect of Bt maize on the plant-aphid–parasitoid tritrophic relationships. BioControl 56: 133–143. [Google Scholar]
- 53. Spadone JC, Takeoka G, Liardon R (1990) Analytical investigation of Rio off-flavor in green coffee. Journal of Agricultural and Food Chemistry 38: 226–233. [Google Scholar]
- 54. Scarpati ML, Scalzo RL, Vita G (1993) Olea europaea volatiles attractive and repellent to the olive fruit fly (Dacus oleae, Gmelin). Journal of Chemical Ecology 19: 881–891. [DOI] [PubMed] [Google Scholar]
- 55. Mendesil E, Bruce TJ, Woodcock CM, Caulfield JC, Seyoum E, et al. (2009) Semiochemicals used in host location by the coffee berry borer, Hypothenemus hampei . Journal of Chemical Ecology 35: 944–950. [DOI] [PubMed] [Google Scholar]
- 56. Yan F, Bengtsson M, Anderson P, Ansebo L, Xu C, et al. (2004) Antennal response of cotton bollworm (Heliocoverpa armigera) to volatiles in transgenic Bt cotton. Journal of Applied Entomology 128: 354–357. [Google Scholar]
- 57. Ibrahim M, Stewart-Jones A, Pulkkinen J, Poppy G, Holopainen J (2008) The influence of different nutrient levels on insect-induced plant volatiles in Bt and conventional oilseed rape plants. Plant Biology 10: 97–107. [DOI] [PubMed] [Google Scholar]
- 58. Vogler U, Rott AS, Gessler C, Dorn S (2010) Comparison between volatile emissions from transgenic apples and from two representative classically bred apple cultivars. Transgenic Research 19: 77–89. [DOI] [PubMed] [Google Scholar]
- 59. KAMEL SA, ELKASSABY FY (1965) Relative resistance of cotton varieties in Egypt to spider mites, leafhoppers, and aphids. Journal of Economic Entomology 58: 209–212. [Google Scholar]
- 60. Bryson CT, McCarty JC, Jenkins JN, Parrott W (1983) Frequency of pigment glands and capitate and covering trichomes in nascent leaves of selected cottons. Crop Science 23: 369–371. [Google Scholar]
- 61. Bondada BR, Oosterhuis DM (2000) Comparative epidermal ultrastructure of cotton (Gossypium hirsutum L.) leaf, bract and capsule wall. Annals of Botany 86: 1143–1152. [Google Scholar]
- 62. Aharoni A, Giri AP, Deuerlein S, Griepink F, de Kogel W-J, et al. (2003) Terpenoid metabolism in wild-type and transgenic Arabidopsis plants. The Plant Cell Online 15: 2866–2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The headspace collection of both Bt and non-Bt rice.
(TIF)
Oviposition preference bioassays of Cnaphalocrocis medinalis : (A) Rice plants and target pests were covered with net in order to prevent the pests from flying away; (B) Experiments were conducted in humid and warm conditions.
(TIF)
Sensor sensitivity of eighteen individual sensors within the sensor array of the FOX 4000 e-nose.
(TIF)