Abstract
The concept that target tissues determine the survival of neurons has inspired much of the thinking on neuronal development in vertebrates, not least because it is supported by decades of research on nerve growth factor (NGF) in the peripheral nervous system (PNS). Recent discoveries now help to understand why only some developing neurons selectively depend on NGF. They also indicate that the survival of most neurons in the central nervous system (CNS) is not simply regulated by single growth factors like in the PNS. Additionally, components of the cell death machinery have begun to be recognized as regulators of selective axonal degeneration and synaptic function, thus playing a critical role in wiring up the nervous system.
Why do so many neurons die during development?
Programmed cell death occurs throughout life, as cell turnover is part of homeostasis and maintenance in most organs and tissues. The situation in the nervous system is principally different, as the vast majority of neurons undergo their last round of cell division early in development. Soon after exiting the cell cycle, neurons start elongating axons to innervate their targets. It is during this period that they are highly susceptible to undergo programmed cell death: a large percentage, as much as 50% in several ganglia in the peripheral nervous system (PNS) as well as in various central nervous system (CNS) areas, is eliminated around the time that connections are being made with other cells. Later in development, the propensity of neurons to initiate apoptosis progressively decreases. The likelihood for a neuron to undergo apoptosis seems to be determined by a tightly regulated apoptotic machinery (summarized in Fig. 1). Therefore, modulation of the expression levels or the activity of components of this apoptotic balance changes the sensitivity to death-promoting cues, allowing temporal restriction of cell death.
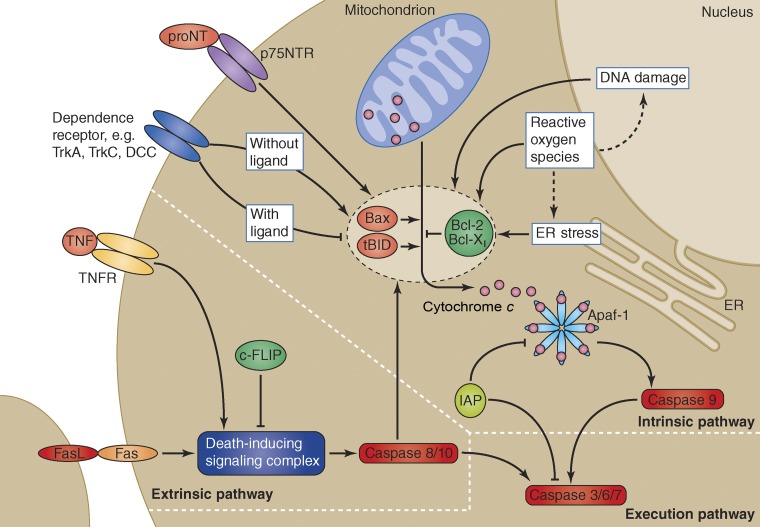
Figure 1.
Core components of the apoptotic machinery. The likelihood that a neuron undergoes apoptosis is determined by the interplay of the tightly interlinked apoptotic machinery, many components of which are highly conserved between species. The critical, and often terminal, step in programmed cell death is the proteolytic activation of the executor caspases (such as caspase 3, 6, 7) by the initiator caspases (i.e., caspase 8, 9, and 10; Riedl and Salvesen, 2007). In mammalian cells, initiation of the executor caspases is regulated by two distinct protein cascades: the intrinsic pathway, also known as the mitochondrial pathway, and the extrinsic pathway. The intrinsic pathway integrates a number of intra- and extracellular signal modalities, such as redox state (for example, the reactive oxygen species; Franklin, 2011), DNA damage (Sperka et al., 2012), ER stress (Puthalakath et al., 2007) and growth factor deprivation (Deckwerth et al., 1998; Putcha et al., 2003; Bredesen et al., 2005), or activation of the p75NTR neurotrophin receptor by pro-neurotrophins (Nykjaer et al., 2005). The stressors converge onto pro- and anti-apoptotic members of the Bcl-2 protein family (for example: BCL-2, BCL-Xl, BAX, and tBID; Youle and Strasser, 2008). These proteins regulate the release of cytochrome c from mitochondria, which activates the initiator caspase 9 through Apaf1 (Riedl and Salvesen, 2007). The extrinsic pathway links activation of ligand-bound death receptors (such as Fas/CD95 and TNFR) to the initiator caspase 8 and 10, through formation of the death-inducing signaling complex (DISC; LeBlanc and Ashkenazi, 2003; Peter and Krammer, 2003). Together with additional regulatory elements (including the Inhibitors of apoptosis proteins [IAP]; Vaux and Silke, 2005) and cFLIP (Scaffidi et al., 1999; Wang et al., 2005), the apoptotic machinery forms a balance that determines the propensity of the neuron to undergo apoptosis.
Programmed cell death eliminates many neurons during development, even in organisms comprised of only few cells, such as Caenorhabditis elegans. As neurons and their targets are initially separated, it is possible that the initial generation of an overabundance of neurons is simply part of a mechanism to ensure that distal targets are adequately innervated (Oppenheim, 1991; Conradt, 2009; Chen et al., 2013). In various tissues other than the nervous system, programmed cell death is used to eliminate cells that are no longer needed, defective, or harmful to the function of the organism. However, there is strong evidence that the elimination of superfluous neurons in the developing nervous system is not essential. For example, early work in C. elegans revealed that preventing programmed cell death does not result in significant behavioral alterations (Ellis and Horvitz, 1986). In the C57BL/6 mouse strain, deletion of the executor caspases 3 and 7 (Fig. 1) has a remarkably limited neuropathological and morphological impact in the CNS (Leonard et al., 2002; Lakhani et al., 2006) compared with the 129X1/SvJ strain, in which deletion of these caspases causes neurodevelopmental defects (Leonard et al., 2002). Similar conclusions were reached by blocking the Bcl-2–associated X protein (BAX)–dependent pathway in many neuronal populations, including motoneurons (Buss et al., 2006a). A recent study in the developing retina showed that in mice lacking the central apoptotic regulator BAX, the normal mosaic distribution of intrinsically photosensitive retinal ganglion cells (ipRGCs) was perturbed (Chen et al., 2013). Although this abnormal distribution is dispensable for the intrinsic photosensitivity of the ipRGCs, it is required for establishing proper connections to other neurons in the retina, which is necessary for rod/cone photo-entrainment (Chen et al., 2013). Even though this finding highlights a physiological role for programmed cell death in the CNS, the functional consequences remain rather underwhelming in the face of a process that eliminates such large numbers of neurons (Purves and Lichtman, 1984; Oppenheim, 1991; Miller, 1995; Gohlke et al., 2004). It thus appears that apoptotic removal of the surplus neurons generated during development mainly serves the purpose to optimize the size of the nervous system to be minimal, but sufficient.
A molecular substrate for the neurotrophic theory
Quantitatively, programmed cell death of neurons in the PNS and CNS is most dramatic when neurons start contacting the cells they innervate. Because experimental manipulations such as target excision typically lead to the death of essentially all innervating neurons (Oppenheim, 1991), the concept emerged that the fate of developing neurons is regulated by their targets. This notion is often referred to as the “neurotrophic theory” (Hamburger et al., 1981; Purves and Lichtman, 1984; Oppenheim, 1991), but it is important to realize that it evolved in the absence of direct mechanistic or molecular support (Purves, 1988). Originally described as a diffusible agent promoting nerve growth, the eponymous NGF later provided a strong and very appealing molecular foundation for this theory (Korsching and Thoenen, 1983; Edwards et al., 1989; Hamburger, 1992). The tyrosine kinase receptor tropomyosin receptor kinase A (TrkA), which was initially identified as an oncogene (Martin-Zanca et al., 1986), was fortuitously discovered to be the critical receptor necessary for the prevention of neuronal cell death by NGF (Klein et al., 1991). Both the remarkable expression pattern of TrkA in NGF-dependent neurons and the onset of its expression during development (Martin-Zanca et al., 1990) provided further additional support for the neurotrophic theory. However, for a surprisingly long time, the question was not asked as to why only specific populations of neurons strictly depend on NGF for survival, while others do not. Indeed, it was only recently shown that TrkA causes cell death of neurons by virtue of its mere expression, and that this death-inducing activity is prevented by addition of NGF (Nikoletopoulou et al., 2010). These findings thus indicate that TrkA acts as a “dependence receptor,” a concept introduced after observations that various cell types die when receptors are expressed in the absence of their cognate ligands (Bredesen et al., 2005; Tauszig-Delamasure et al., 2007). Accordingly, embryonic mouse sympathetic or sensory neurons survive in the absence of NGF when TrkA is deleted (Nikoletopoulou et al., 2010). The closely related neurotrophin receptor TrkC also acts as a dependence receptor (Tauszig-Delamasure et al., 2007; Nikoletopoulou et al., 2010). Here, it is interesting to note a series of older, convergent results indicating that deletion of neurotrophin-3 (NT3), the TrkC ligand, leads to a significantly larger loss of sensory and sympathetic neurons in the PNS than the deletion of TrkC (Tessarollo et al., 1997). This phenotypic discrepancy fits well with the idea that inactivation of the ligand of a dependence receptor is expected to yield a more profound phenotype than inactivation of the receptor itself (Tauszig-Delamasure et al., 2007). How TrkA and TrkC induce apoptosis remains to be fully elucidated. It seems that proteolysis is involved, either of TrkC itself (Tauszig-Delamasure et al., 2007), as was suggested for other dependence receptors (Bredesen et al., 2005), or by the proteolysis of the neurotrophin receptor p75NTR, which associates with both TrkA and TrkC (Fig. 2; Nikoletopoulou et al., 2010). Surprisingly, although TrkA and TrkC cause cell death, the structurally related TrkB receptor does not (Nikoletopoulou et al., 2010), a difference that appears to be accounted for by their differential localization in the cell membrane. TrkA and TrkC colocalize with p75NTR in lipid rafts, whereas TrkB, which also associates with p75NTR (Bibel et al., 1999), is excluded from lipid rafts (Fig. 2; unpublished data). Interestingly, the transmembrane domains of TrkA and TrkC are closely related, and differ clearly from that of TrkB. It turns out that a chimeric protein of TrkB with the transmembrane domain of TrkA causes cell death, which can be prevented by the addition of the TrkB ligand brain-derived neurotrophic factor (BDNF; unpublished data). The suggestion that the lipid raft localization of TrkA and TrkC is important for their death-inducing function is in line with a number of reports indicating that certain apoptotic proteins preferentially localize in lipid rafts in the plasma membrane. After activation of the extrinsic apoptosis pathway, translocation of the activated receptors to lipid rafts in the membrane is required for assembling the death-inducing signaling complex (DISC; Davis et al., 2007; Song et al., 2007). Indeed, regulators of the extrinsic pathway (e.g., cFLIP; Fig. 1) prevent this translocation, explaining how they attenuate cell death induction (Song et al., 2007). Similarly, the localization of the dependence receptor DCC (deleted in colorectal cancer) in lipid rafts is a prerequisite for its pro-apoptotic activity in absence of its ligand, Netrin-1 (Furne et al., 2006).
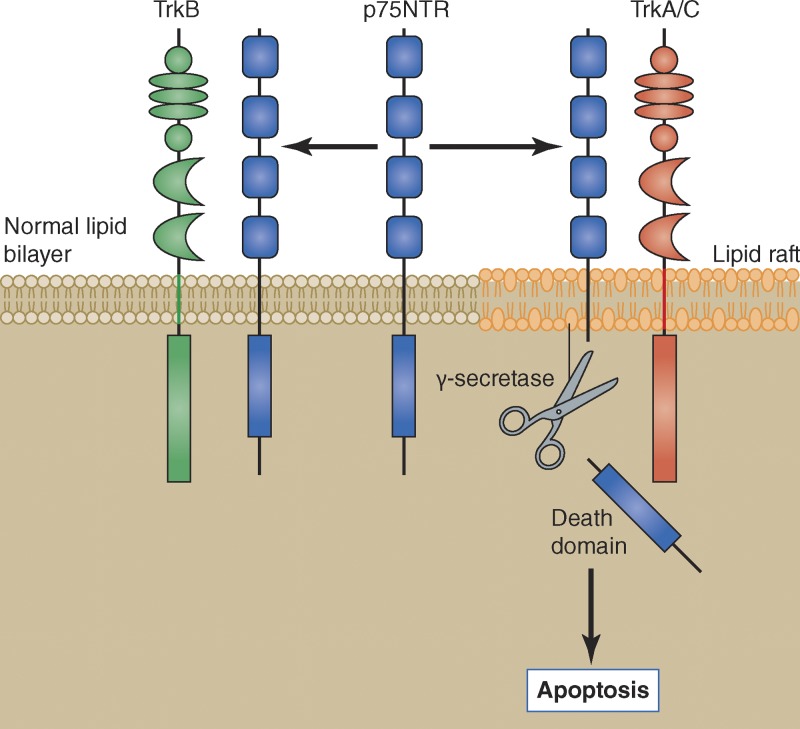
Figure 2.
TrkA and TrkC as dependence receptors: mode of action and contrast with TrkB. All Trk receptors associate with the pan-neurotrophin receptor p75NTR (Bibel et al., 1999). A critical step in the induction of apoptosis by TrkA is the release of the intracellular death domain of p75NTR by the protease γ-secretase (Nikoletopoulou et al., 2010), which is localized in lipid rafts (Urano et al., 2005). Our membrane fractionation studies indicate that while TrkA and TrkC associate with p75NTR in lipid rafts, TrkB associated with p75NTR is excluded from this membrane domain (unpublished data). The 24–amino acid transmembrane domain of the Trk receptors may be responsible for this differential localization (see text).
Despite the fact that TrkB does not act as a dependence receptor, its activation by BDNF is required for the survival of several populations of cranial sensory neurons (Ernfors et al., 1995; Liu et al., 1995). It appears that other death-inducing receptors predispose these neurons to be eliminated, such as p75NTR, which is expressed at high levels in some of these ganglia, or TrkC in vestibular neurons (Stenqvist et al., 2005). This latter case is of special interest, as NT3 is known not to be required for the survival of these neurons (Stenqvist et al., 2005). In addition to inducing apoptosis in the absence of their ligand, TrkA and TrkC have long been recognized to have a pro-survival function similar to TrkB, as can be inferred from the loss of specific populations of peripheral sensory neurons in mutants lacking these receptors (Klein et al., 1994; Smeyne et al., 1994).
Cell death in the CNS
Although TrkA is primarily expressed in peripheral sympathetic and sensory neurons, it is also found in a small population of cholinergic neurons in the basal forebrain (Sobreviela et al., 1994), a proportion of which requires NGF for survival (Hartikka and Hefti, 1988; Crowley et al., 1994; Müller et al., 2012). Selective deletion of TrkA was recently shown not to cause the death of these neurons (Sanchez-Ortiz et al., 2012). This supports the notion that TrkA acts as a dependence receptor for this small population of CNS neurons, like for peripheral sensory and sympathetic neurons. TrkA activation by NGF is essential for the maturation, projections, and function of these neurons (Sanchez-Ortiz et al., 2012), as was previously described for sensory neurons in the PNS as well (Patel et al., 2000).
Whether or not receptors other than TrkA act as dependence receptors in the CNS is an important open question, particularly because TrkB, which is expressed highly by most CNS neurons, does not act as a dependence receptor (Nikoletopoulou et al., 2010). In retrospect, the structural similarities between TrkA and TrkB, just like those between NGF and BDNF (Barde, 1989), have substantially misled the field by suggesting that BDNF would act in the CNS like NGF in the PNS. Adding to the confusion were early findings showing that BDNF supports the growth of spinal cord motoneurons in vitro or in vivo after axotomy (Oppenheim et al., 1992; Sendtner et al., 1992; Yan et al., 1992). However, in the absence of lesion, deletion of BDNF does not lead to significant losses of neurons in the developing or adult CNS (Ernfors et al., 1994a; Jones et al., 1994; Rauskolb et al., 2010), unlike the case in some populations of PNS neurons. The poor correlation of the role of BDNF in CNS development and in axotomy and in vitro experiments is surprising, especially because the role of NGF in vivo could in essence be recapitulated by in vitro experiments. Although the reasons for this discrepancy are not fully understood, the strong up-regulation of death-inducing molecules such as p75NTR after axotomy (Ernfors et al., 1989) may be a part of the explanation. At present, most of the growth factors promoting the survival of PNS neurons fail to show significant survival properties for developing neurons in the CNS, as for example was shown for NT3 (Ernfors et al., 1994b; Fariñas et al., 1994), glial cell line–derived neurotrophic factor (GDNF; Henderson et al., 1994), ciliary neurotrophic factor (CNTF; DeChiara et al., 1995), and several others.
In the developing CNS, neuronal activity and neurotransmitter input seem to play a more significant role than single growth factors in regulating neuronal survival. In particular, it has been known for a long time that blocking synaptic transmission at the neuromuscular junction has a pro-survival effect on spinal cord motoneurons (Pittman and Oppenheim, 1978; Oppenheim et al., 2008). By contrast, surgical denervation of afferent connections leads to increased apoptosis of postsynaptic neurons (Okado and Oppenheim, 1984), whereas inhibiting glycinergic and GABAergic synaptic transmission has both pro- and anti-apoptotic effects on motoneurons (Banks et al., 2005). Throughout the developing brain, blocking glutamate-mediated synaptic transmission involving NMDA receptors markedly increases normally occurring neuronal death (Ikonomidou et al., 1999; Heck et al., 2008). The mechanism involves a reduction of neuronal expression of anti-apoptotic proteins, such as B-cell lymphoma 2 (BCL-2; Hansen et al., 2004). Conversely, a limited increase in neuronal activity leads to down-regulation of the pro-apoptotic genes BAX and caspase 9 (Léveillé et al., 2010), thereby reducing the propensity of these cells to initiate programmed cell death (Hardingham et al., 2002). In addition to directly modulating the expression of apoptotic proteins, neuronal activity affects the expression of several secreted growth factors, such as BDNF (Hardingham et al., 2002; Hansen et al., 2004) and GDNF (Léveillé et al., 2010). So, even though BDNF is not a major survival factor in the developing CNS, it appears to be critical for activity-dependent neuroprotection (Tremblay et al., 1999). A recent publication revealed that certain populations of neurons in the CNS do not follow the predictions of the neurotrophic theory and showed that apoptosis of cortical inhibitory neurons is independent of cues present in the developing cerebral cortex (Southwell et al., 2012). This study indicates that programmed cell death of a large proportion of interneurons in the CNS is regulated by intrinsic mechanisms that are largely resistant to the presence or absence of extrinsic cues (Dekkers and Barde, 2013).
Taken together, even though the extent of naturally occurring cell death in the different regions of the CNS is not nearly as well characterized as in the PNS, let alone quantified, it appears that its regulation may significantly differ. Although single secreted neurotrophic factors seem to be largely dispensable for survival, neuronal activity and other intrinsic mechanisms drive the propensity of the neurons in the CNS to undergo apoptosis. An important open question in this context is a possible involvement of non-neuronal cells, such as glial cells (see Corty and Freeman, in this issue).
The apoptotic machinery as a regulator of connectivity
Activation of the executor caspases has been most studied in cell bodies and typically results in the demise of the entire cell (Williams et al., 2006). However, recent evidence shows that caspases are also activated locally in neuronal processes and branches destined to be eliminated, for example in axons overshooting their targets that are subsequently pruned back to establish the precise adult connectivity (Finn et al., 2000; Raff et al., 2002; Luo and O’Leary, 2005; Buss et al., 2006b). Initially, axonal degeneration and axon pruning were thought to be independent of caspases (Finn et al., 2000; Raff et al., 2002). Later work in Drosophila melanogaster (Kuo et al., 2006; Williams et al., 2006) and in mammalian neurons (Plachta et al., 2007; Nikolaev et al., 2009; Vohra et al., 2010) demonstrated that interfering with the apoptotic balance or the executor caspases can prevent or at least delay axonal degeneration. Simon et al. (2012) have found that a caspase 9 to caspase 3 cascade is crucial for axonal degeneration induced by NGF withdrawal, with caspase 6 activation playing a significant but subsidiary role. Upstream of the caspases, BCL-2 family members such as BAX and BCL-Xl are required (Nikolaev et al., 2009; Vohra et al., 2010). It is conceivable that the failure of ipRGCs in BAX-deficient mice to form appropriate connections to other cells in the retina (Chen et al., 2013) may be in part attributable to defective axonal degeneration. Surprisingly, Apaf1 appears not to be involved in this process (Cusack et al., 2013), suggesting that axon degeneration depends on the concerted activation of the intrinsic initiator complex in a different way from apoptosis.
Strikingly, a series of recent studies showed that several caspases and components of the intrinsic pathway also affect normal synaptic physiology in adulthood (Fig. 3, A–D). Here, pro-apoptotic proteins are predominantly involved in weakening the synapses, whereas the anti-apoptotic proteins have been mainly associated with synaptic strengthening (Fig. 3 B). In particular, caspase 3 promotes long-term depression (LTD), a stimulation paradigm that results in a period of decreased synaptic transmission (Li et al., 2010), and also prevents long-term potentiation (LTP), the converse situation leading to strengthened synaptic transmission (Jo et al., 2011). Likewise, the proapoptotic BCL-2 family members BAX and BAD stimulate LTD (Jiao and Li, 2011). By contrast, the anti-apoptotic protein BCL-Xl increases synapse numbers and strength (H. Li et al., 2008), and the inhibitor of apoptosis protein (IAP) family member survivin was reported to be involved in LTP in the hippocampus (Iscru et al., 2013) and in activity-dependent gene regulation (O’Riordan et al., 2008).
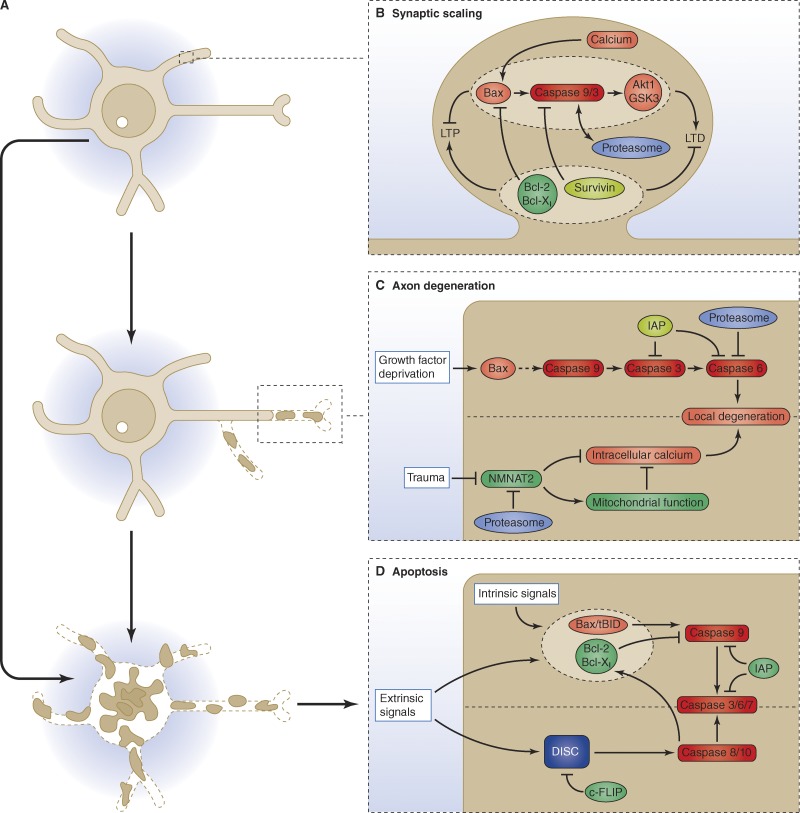
Figure 3.
Canonical and noncanonical functions of the apoptotic machinery. (A) The apoptotic machinery is not only involved in eliminating cells destined to die, but is also a central player in refining neuronal connectivity, by regulating synaptic transmission and by generating the adult connectivity through axon pruning (Luo and O’Leary, 2005; Hyman and Yuan, 2012). But how the canonical and noncanonical roles of the apoptotic machinery are interlinked and spatially restricted is not well understood. (B) In the adult nervous system, the pro-apoptotic proteins BAX, caspase 9, and caspase 3 promote weakening of synapses (long-term depression [LTD]; Li et al., 2010; Jiao and Li, 2011; Jo et al., 2011), while the anti-apoptotic proteins Bcl-Xl and the IAP survivin promote synaptic strengthening (long-term potentiation [LTP]; Li et al., 2008a; Iscru et al., 2013). It is unclear how the activation of these pathways is restricted to a single synapse, but a recent review suggested that the proteasomal degradation of activated caspases may prevent their diffusion (Hyman and Yuan, 2012). (C) Caspase activation is now known to be required for axon pruning during development to generate the adult refined connectivity (Luo and O’Leary, 2005; Simon et al., 2012). Different pathways are activated depending on the stimulus leading to degeneration. Growth factor deprivation during development leads to activation the executor caspases 3 and 6 (Simon et al., 2012) through the intrinsic apoptotic pathway, although its core protein Apaf1 does not seem to be required for this process (Cusack et al., 2013). On the other hand, a traumatic injury leads to reduced influx of NMNAT2 into the axon, which negatively affects the stability and function of mitochondria and leads to an increased calcium concentration (Wang et al., 2012). The effector caspase, caspase 6, is dispensable for this form of axonal degeneration (Vohra et al., 2010; Simon et al., 2012). Regulatory proteins such as the IAPs and also the proteasome seem to play a role in limiting the extent of activation to the degenerating part of the axon (Wang et al., 2012; Cusack et al., 2013; Unsain et al., 2013). (D) Simplified schematic of the main pro- and anti-apoptotic components. DISC, death-induced signaling complex. IAP, inhibitor of apoptosis protein. See Fig. 1 for details.
These findings indicate that the apoptotic machinery acts at different levels in the cell, ranging from driving sub-lethal degradation of a compartment (Fig. 3 C) and attenuating synaptic transmission at the neuronal network level (Fig. 3 B) to destroying the entire cell during development or in disease (Fig. 3 D). How the cell spatially restricts the extent of activation of the apoptotic machinery is yet unclear. For example, elimination of the somata of developing neurons after neurotrophin deprivation is preceded by axonal degeneration, but not all instances of axonal degeneration lead to the death of the neuron (Campenot, 1977; Raff et al., 2002). Local regulation of caspase activation by IAPs is well established as a means for ensuring the elimination of neuronal processes in D. melanogaster (Kuo et al., 2006; Williams et al., 2006). Recent findings suggest a similar role for IAP in mammalian neurons, where it limits caspase activation to the degenerating axon (Fig. 3 C; Cusack et al., 2013; Unsain et al., 2013). The spontaneous mutation Wallerian degeneration slow (WldS; Lunn et al., 1989) has been instrumental to understand that trauma-induced axon degeneration is a regulated process different from, and independent of, cell body degeneration (Wang et al., 2012), but also distinct from axon pruning (Hoopfer et al., 2006). Work on the chimeric protein encoded by the WldS mutation also led to the identification of the protein NMNAT2 (nicotinamide mononucleotide adenylyltransferase 2) as a labile axon survival factor (Gilley and Coleman, 2010). How the WldS chimeric protein and NMNAT2 result in axon protection is unclear, but several lines of evidence seem to converge on local regulation of mitochondrial function and motility (Avery et al., 2012; Fang et al., 2012).
Related to the spatial limiting of apoptotic activity is the question of how a local source of neurotrophins leads to the rescue of a developing peripheral neuron. When neurons encounter a source of neurotrophins, only the receptors close to the target will be activated, whereas the others, located further away, are not. The cell, therefore, needs to integrate a pro-survival signal from the activated receptors, and death-inducing signals from the nonactivated dependence receptors. The continued signaling of activated neurotrophin receptors that are retrogradely transported to the soma (Grimes et al., 1996; Howe et al., 2001; Wu et al., 2001; Harrington et al., 2011) likely play a role in counteracting the pro-apoptotic signaling proximal to the source of neurotrophins. It will be interesting to investigate whether similar mechanisms play a role in axon pruning and traumatic axon degeneration as well.
Programmed cell death in the adult brain
Most of the nervous system becomes post-mitotic early in development. In rodents, two brain areas retain the capacity to generate new neurons in the adult: the sub-ventricular zone, which generates neurons that migrate toward the olfactory bulb, and the sub-granular zone of the dentate gyrus of the hippocampus, where neurons are generated that integrate locally. Similar to what is observed during embryonic development, these adult-generated neurons are produced in excess, and a large fraction undergoes apoptosis when contacting its designated targets (Petreanu and Alvarez-Buylla, 2002; Kempermann et al., 2003; Ninkovic et al., 2007). Preventing apoptosis of adult-generated neurons in the olfactory bulb only has limited functional consequences (Kim et al., 2007), whereas a similar maneuver in the dentate gyrus does lead to impaired performance in memory tasks (Kim et al., 2009). Why superfluous hippocampal neurons would need to be eliminated for proper function is a matter of speculation, but may be linked with the fact that these are excitatory projection neurons, whereas in the olfactory bulb only axon-less inhibitory granule cells are integrated. The extent of survival in both these areas critically depends on the activity of the neuronal network in which these newly born neurons have to integrate (Petreanu and Alvarez-Buylla, 2002; Kempermann et al., 2006; Ninkovic et al., 2007). In this context, BDNF, the expression level of which is well known to be regulated by network activity, supports the survival of young adult–generated neurons and possibly even stimulates the proliferation of neural progenitors (Y. Li et al., 2008; Waterhouse et al., 2012). Interestingly, in young adult mouse mutants that exhibit spontaneous epileptic seizures, significantly higher levels of BDNF have been measured (Lavebratt et al., 2006; Heyden et al., 2011). Concomitantly, the entire hippocampal formation is considerably enlarged by as much as 40% (Lavebratt et al., 2006; Angenstein et al., 2007), which in turn is dependent on the epileptic seizures (Lavebratt et al., 2006). Whether or not there is a causal relationship between increased BDNF levels and hippocampal volume remains to be established.
Conclusion
Now that is has become clear that action of the apoptotic machinery can be limited spatially and temporally, several questions need to be addressed: how do neurons integrate intrinsic and extrinsic pro- and anti-apoptotic signals; and how they are spatially restricted to allow degradation of a dendrite or axon, or modulation of synaptic transmission? Another important issue is the regulation of cell death by intrinsic mechanisms in the central nervous system of vertebrates, not least because programmed cell death is observed in the CNS in a number of neurodegenerative diseases (Vila and Przedborski, 2003). Indeed, several of the central apoptotic components discussed here are also involved in these disorders (Hyman and Yuan, 2012). New insights in the regulation of programmed cell death in the developing nervous system may therefore continue to help to better understand the pathophysiological mechanisms of neurodegenerative disorders.
Acknowledgments
The authors thank Karin Ackema for critically reading the manuscript and Phil Barker for helpful discussions and sharing unpublished results. Illustrations were provided by Neil Smith, www.neilsmithillustration.co.uk.
Footnotes
Abbreviations used in this paper:
- BAX
- Bcl-2–associated X protein
- BCL-2
- B-cell lymphoma 2
- BDNF
- brain-derived neurotrophic factor
- CNS
- central nervous system
- GDNF
- glial cell line–derived neurotrophic factor
- IAP
- inhibitor of apoptosis protein
- ipRGC
- intrinsically photosensitive retinal ganglion cell
- NT3
- neurotrophin-3
- P75NTR
- p75 neurotrophin receptor
- PNS
- peripheral nervous system
- Trk
- tropomyosin receptor kinase
References
- Angenstein F., Niessen H.G., Goldschmidt J., Lison H., Altrock W.D., Gundelfinger E.D., Scheich H. 2007. Manganese-enhanced MRI reveals structural and functional changes in the cortex of Bassoon mutant mice. Cereb. Cortex. 17:28–36 10.1093/cercor/bhj121 [DOI] [PubMed] [Google Scholar]
- Avery M.A., Rooney T.M., Pandya J.D., Wishart T.M., Gillingwater T.H., Geddes J.W., Sullivan P.G., Freeman M.R. 2012. WldS prevents axon degeneration through increased mitochondrial flux and enhanced mitochondrial Ca2+ buffering. Curr. Biol. 22:596–600 10.1016/j.cub.2012.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks G.B., Kanjhan R., Wiese S., Kneussel M., Wong L.M., O’Sullivan G., Sendtner M., Bellingham M.C., Betz H., Noakes P.G. 2005. Glycinergic and GABAergic synaptic activity differentially regulate motoneuron survival and skeletal muscle innervation. J. Neurosci. 25:1249–1259 10.1523/JNEUROSCI.1786-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barde Y.A. 1989. Trophic factors and neuronal survival. Neuron. 2:1525–1534 10.1016/0896-6273(89)90040-8 [DOI] [PubMed] [Google Scholar]
- Bibel M., Hoppe E., Barde Y.A. 1999. Biochemical and functional interactions between the neurotrophin receptors trk and p75NTR. EMBO J. 18:616–622 10.1093/emboj/18.3.616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredesen D.E., Mehlen P., Rabizadeh S. 2005. Receptors that mediate cellular dependence. Cell Death Differ. 12:1031–1043 10.1038/sj.cdd.4401680 [DOI] [PubMed] [Google Scholar]
- Buss R.R., Gould T.W., Ma J., Vinsant S., Prevette D., Winseck A., Toops K.A., Hammarback J.A., Smith T.L., Oppenheim R.W. 2006a. Neuromuscular development in the absence of programmed cell death: phenotypic alteration of motoneurons and muscle. J. Neurosci. 26:13413–13427 10.1523/JNEUROSCI.3528-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss R.R., Sun W., Oppenheim R.W. 2006b. Adaptive roles of programmed cell death during nervous system development. Annu. Rev. Neurosci. 29:1–35 10.1146/annurev.neuro.29.051605.112800 [DOI] [PubMed] [Google Scholar]
- Campenot R.B. 1977. Local control of neurite development by nerve growth factor. Proc. Natl. Acad. Sci. USA. 74:4516–4519 10.1073/pnas.74.10.4516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.K., Chew K.S., McNeill D.S., Keeley P.W., Ecker J.L., Mao B.Q., Pahlberg J., Kim B., Lee S.C., Fox M.A., et al. 2013. Apoptosis regulates ipRGC spacing necessary for rods and cones to drive circadian photoentrainment. Neuron. 77:503–515 10.1016/j.neuron.2012.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradt B. 2009. Genetic control of programmed cell death during animal development. Annu. Rev. Genet. 43:493–523 10.1146/annurev.genet.42.110807.091533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corty M., Freeman M.R. 2013. Architects in neural circuit design: Glia control neuron numbers and connectivity. J. Cell Biol. 203:395–405 10.1083/jcb.201306099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley C., Spencer S.D., Nishimura M.C., Chen K.S., Pitts-Meek S., Armanini M.P., Ling L.H., McMahon S.B., Shelton D.L., Levinson A.D., et al. 1994. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell. 76:1001–1011 10.1016/0092-8674(94)90378-6 [DOI] [PubMed] [Google Scholar]
- Cusack C.L., Swahari V., Hampton Henley W., Michael Ramsey J., Deshmukh M. 2013. Distinct pathways mediate axon degeneration during apoptosis and axon-specific pruning. Nat. Commun. 4:1876 10.1038/ncomms2910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A.R., Lotocki G., Marcillo A.E., Dietrich W.D., Keane R.W. 2007. FasL, Fas, and death-inducing signaling complex (DISC) proteins are recruited to membrane rafts after spinal cord injury. J. Neurotrauma. 24:823–834 10.1089/neu.2006.0227 [DOI] [PubMed] [Google Scholar]
- DeChiara T.M., Vejsada R., Poueymirou W.T., Acheson A., Suri C., Conover J.C., Friedman B., McClain J., Pan L., Stahl N., et al. 1995. Mice lacking the CNTF receptor, unlike mice lacking CNTF, exhibit profound motor neuron deficits at birth. Cell. 83:313–322 10.1016/0092-8674(95)90172-8 [DOI] [PubMed] [Google Scholar]
- Deckwerth T.L., Easton R.M., Knudson C.M., Korsmeyer S.J., Johnson E.M., Jr 1998. Placement of the BCL2 family member BAX in the death pathway of sympathetic neurons activated by trophic factor deprivation. Exp. Neurol. 152:150–162 10.1006/exnr.1998.6846 [DOI] [PubMed] [Google Scholar]
- Dekkers M.P., Barde Y.A. 2013. Developmental biology. Programmed cell death in neuronal development. Science. 340:39–41 10.1126/science.1236152 [DOI] [PubMed] [Google Scholar]
- Edwards R.H., Rutter W.J., Hanahan D. 1989. Directed expression of NGF to pancreatic beta cells in transgenic mice leads to selective hyperinnervation of the islets. Cell. 58:161–170 10.1016/0092-8674(89)90412-1 [DOI] [PubMed] [Google Scholar]
- Ellis H.M., Horvitz H.R. 1986. Genetic control of programmed cell death in the nematode C. elegans. Cell. 44:817–829 10.1016/0092-8674(86)90004-8 [DOI] [PubMed] [Google Scholar]
- Ernfors P., Henschen A., Olson L., Persson H. 1989. Expression of nerve growth factor receptor mRNA is developmentally regulated and increased after axotomy in rat spinal cord motoneurons. Neuron. 2:1605–1613 10.1016/0896-6273(89)90049-4 [DOI] [PubMed] [Google Scholar]
- Ernfors P., Lee K.F., Jaenisch R. 1994a. Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature. 368:147–150 10.1038/368147a0 [DOI] [PubMed] [Google Scholar]
- Ernfors P., Lee K.F., Kucera J., Jaenisch R. 1994b. Lack of neurotrophin-3 leads to deficiencies in the peripheral nervous system and loss of limb proprioceptive afferents. Cell. 77:503–512 10.1016/0092-8674(94)90213-5 [DOI] [PubMed] [Google Scholar]
- Ernfors P., Van De Water T., Loring J., Jaenisch R. 1995. Complementary roles of BDNF and NT-3 in vestibular and auditory development. Neuron. 14:1153–1164 10.1016/0896-6273(95)90263-5 [DOI] [PubMed] [Google Scholar]
- Fang Y., Soares L., Teng X., Geary M., Bonini N.M. 2012. A novel Drosophila model of nerve injury reveals an essential role of Nmnat in maintaining axonal integrity. Curr. Biol. 22:590–595 10.1016/j.cub.2012.01.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fariñas I., Jones K.R., Backus C., Wang X.Y., Reichardt L.F. 1994. Severe sensory and sympathetic deficits in mice lacking neurotrophin-3. Nature. 369:658–661 10.1038/369658a0 [DOI] [PubMed] [Google Scholar]
- Finn J.T., Weil M., Archer F., Siman R., Srinivasan A., Raff M.C. 2000. Evidence that Wallerian degeneration and localized axon degeneration induced by local neurotrophin deprivation do not involve caspases. J. Neurosci. 20:1333–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin J.L. 2011. Redox regulation of the intrinsic pathway in neuronal apoptosis. Antioxid. Redox Signal. 14:1437–1448 10.1089/ars.2010.3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furne C., Corset V., Hérincs Z., Cahuzac N., Hueber A.O., Mehlen P. 2006. The dependence receptor DCC requires lipid raft localization for cell death signaling. Proc. Natl. Acad. Sci. USA. 103:4128–4133 10.1073/pnas.0507864103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilley J., Coleman M.P. 2010. Endogenous Nmnat2 is an essential survival factor for maintenance of healthy axons. PLoS Biol. 8:e1000300 10.1371/journal.pbio.1000300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohlke J.M., Griffith W.C., Faustman E.M. 2004. The role of cell death during neocortical neurogenesis and synaptogenesis: implications from a computational model for the rat and mouse. Brain Res. Dev. Brain Res. 151:43–54 10.1016/j.devbrainres.2004.03.020 [DOI] [PubMed] [Google Scholar]
- Grimes M.L., Zhou J., Beattie E.C., Yuen E.C., Hall D.E., Valletta J.S., Topp K.S., LaVail J.H., Bunnett N.W., Mobley W.C. 1996. Endocytosis of activated TrkA: evidence that nerve growth factor induces formation of signaling endosomes. J. Neurosci. 16:7950–7964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V. 1992. History of the discovery of neuronal death in embryos. J. Neurobiol. 23:1116–1123 10.1002/neu.480230904 [DOI] [PubMed] [Google Scholar]
- Hamburger V., Brunso-Bechtold J.K., Yip J.W. 1981. Neuronal death in the spinal ganglia of the chick embryo and its reduction by nerve growth factor. J. Neurosci. 1:60–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen H.H., Briem T., Dzietko M., Sifringer M., Voss A., Rzeski W., Zdzisinska B., Thor F., Heumann R., Stepulak A., et al. 2004. Mechanisms leading to disseminated apoptosis following NMDA receptor blockade in the developing rat brain. Neurobiol. Dis. 16:440–453 10.1016/j.nbd.2004.03.013 [DOI] [PubMed] [Google Scholar]
- Hardingham G.E., Fukunaga Y., Bading H. 2002. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat. Neurosci. 5:405–414 [DOI] [PubMed] [Google Scholar]
- Harrington A.W., St Hillaire C., Zweifel L.S., Glebova N.O., Philippidou P., Halegoua S., Ginty D.D. 2011. Recruitment of actin modifiers to TrkA endosomes governs retrograde NGF signaling and survival. Cell. 146:421–434 10.1016/j.cell.2011.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartikka J., Hefti F. 1988. Comparison of nerve growth factor’s effects on development of septum, striatum, and nucleus basalis cholinergic neurons in vitro. J. Neurosci. Res. 21:352–364 10.1002/jnr.490210227 [DOI] [PubMed] [Google Scholar]
- Heck N., Golbs A., Riedemann T., Sun J.J., Lessmann V., Luhmann H.J. 2008. Activity-dependent regulation of neuronal apoptosis in neonatal mouse cerebral cortex. Cereb. Cortex. 18:1335–1349 10.1093/cercor/bhm165 [DOI] [PubMed] [Google Scholar]
- Henderson C.E., Phillips H.S., Pollock R.A., Davies A.M., Lemeulle C., Armanini M., Simmons L., Moffet B., Vandlen R.A., Simpson L.C., et al. 1994. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science. 266:1062–1064 10.1126/science.7973664 [DOI] [PubMed] [Google Scholar]
- Heyden A., Ionescu M.C., Romorini S., Kracht B., Ghiglieri V., Calabresi P., Seidenbecher C., Angenstein F., Gundelfinger E.D. 2011. Hippocampal enlargement in Bassoon-mutant mice is associated with enhanced neurogenesis, reduced apoptosis, and abnormal BDNF levels. Cell Tissue Res. 346:11–26 10.1007/s00441-011-1233-3 [DOI] [PubMed] [Google Scholar]
- Hoopfer E.D., McLaughlin T., Watts R.J., Schuldiner O., O’Leary D.D., Luo L. 2006. Wlds protection distinguishes axon degeneration following injury from naturally occurring developmental pruning. Neuron. 50:883–895 10.1016/j.neuron.2006.05.013 [DOI] [PubMed] [Google Scholar]
- Howe C.L., Valletta J.S., Rusnak A.S., Mobley W.C. 2001. NGF signaling from clathrin-coated vesicles: evidence that signaling endosomes serve as a platform for the Ras-MAPK pathway. Neuron. 32:801–814 10.1016/S0896-6273(01)00526-8 [DOI] [PubMed] [Google Scholar]
- Hyman B.T., Yuan J. 2012. Apoptotic and non-apoptotic roles of caspases in neuronal physiology and pathophysiology. Nat. Rev. Neurosci. 13:395–406 10.1038/nrn3228 [DOI] [PubMed] [Google Scholar]
- Ikonomidou C., Bosch F., Miksa M., Bittigau P., Vöckler J., Dikranian K., Tenkova T.I., Stefovska V., Turski L., Olney J.W. 1999. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 283:70–74 10.1126/science.283.5398.70 [DOI] [PubMed] [Google Scholar]
- Iscru E., Ahmed T., Coremans V., Bozzi Y., Caleo M., Conway E.M., D’Hooge R., Balschun D. 2013. Loss of survivin in neural precursor cells results in impaired long-term potentiation in the dentate gyrus and CA1-region. Neuroscience. 231:413–419 10.1016/j.neuroscience.2012.10.049 [DOI] [PubMed] [Google Scholar]
- Jiao S., Li Z. 2011. Nonapoptotic function of BAD and BAX in long-term depression of synaptic transmission. Neuron. 70:758–772 10.1016/j.neuron.2011.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo J., Whitcomb D.J., Olsen K.M., Kerrigan T.L., Lo S.C., Bru-Mercier G., Dickinson B., Scullion S., Sheng M., Collingridge G., Cho K. 2011. Aβ(1-42) inhibition of LTP is mediated by a signaling pathway involving caspase-3, Akt1 and GSK-3β. Nat. Neurosci. 14:545–547 10.1038/nn.2785 [DOI] [PubMed] [Google Scholar]
- Jones K.R., Fariñas I., Backus C., Reichardt L.F. 1994. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 76:989–999 10.1016/0092-8674(94)90377-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G., Gast D., Kronenberg G., Yamaguchi M., Gage F.H. 2003. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 130:391–399 10.1242/dev.00203 [DOI] [PubMed] [Google Scholar]
- Kempermann G., Chesler E.J., Lu L., Williams R.W., Gage F.H. 2006. Natural variation and genetic covariance in adult hippocampal neurogenesis. Proc. Natl. Acad. Sci. USA. 103:780–785 10.1073/pnas.0510291103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W.R., Kim Y., Eun B., Park O.H., Kim H., Kim K., Park C.H., Vinsant S., Oppenheim R.W., Sun W. 2007. Impaired migration in the rostral migratory stream but spared olfactory function after the elimination of programmed cell death in Bax knock-out mice. J. Neurosci. 27:14392–14403 10.1523/JNEUROSCI.3903-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W.R., Park O.H., Choi S., Choi S.Y., Park S.K., Lee K.J., Rhyu I.J., Kim H., Lee Y.K., Kim H.T., et al. 2009. The maintenance of specific aspects of neuronal function and behavior is dependent on programmed cell death of adult-generated neurons in the dentate gyrus. Eur. J. Neurosci. 29:1408–1421 10.1111/j.1460-9568.2009.06693.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R., Jing S.Q., Nanduri V., O’Rourke E., Barbacid M. 1991. The trk proto-oncogene encodes a receptor for nerve growth factor. Cell. 65:189–197 10.1016/0092-8674(91)90419-Y [DOI] [PubMed] [Google Scholar]
- Klein R., Silos-Santiago I., Smeyne R.J., Lira S.A., Brambilla R., Bryant S., Zhang L., Snider W.D., Barbacid M. 1994. Disruption of the neurotrophin-3 receptor gene trkC eliminates la muscle afferents and results in abnormal movements. Nature. 368:249–251 10.1038/368249a0 [DOI] [PubMed] [Google Scholar]
- Korsching S., Thoenen H. 1983. Nerve growth factor in sympathetic ganglia and corresponding target organs of the rat: correlation with density of sympathetic innervation. Proc. Natl. Acad. Sci. USA. 80:3513–3516 10.1073/pnas.80.11.3513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C.T., Zhu S.J., Younger S., Jan L.Y., Jan Y.N. 2006. Identification of E2/E3 ubiquitinating enzymes and caspase activity regulating Drosophila sensory neuron dendrite pruning. Neuron. 51:283–290 10.1016/j.neuron.2006.07.014 [DOI] [PubMed] [Google Scholar]
- Lakhani S.A., Masud A., Kuida K., Porter G.A., Jr, Booth C.J., Mehal W.Z., Inayat I., Flavell R.A. 2006. Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science. 311:847–851 10.1126/science.1115035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavebratt C., Trifunovski A., Persson A.S., Wang F.H., Klason T., Ohman I., Josephsson A., Olson L., Spenger C., Schalling M. 2006. Carbamazepine protects against megencephaly and abnormal expression of BDNF and Nogo signaling components in the mceph/mceph mouse. Neurobiol. Dis. 24:374–383 10.1016/j.nbd.2006.07.018 [DOI] [PubMed] [Google Scholar]
- LeBlanc H.N., Ashkenazi A. 2003. Apo2L/TRAIL and its death and decoy receptors. Cell Death Differ. 10:66–75 10.1038/sj.cdd.4401187 [DOI] [PubMed] [Google Scholar]
- Leonard J.R., Klocke B.J., D’Sa C., Flavell R.A., Roth K.A. 2002. Strain-dependent neurodevelopmental abnormalities in caspase-3-deficient mice. J. Neuropathol. Exp. Neurol. 61:673–677 [DOI] [PubMed] [Google Scholar]
- Léveillé F., Papadia S., Fricker M., Bell K.F., Soriano F.X., Martel M.A., Puddifoot C., Habel M., Wyllie D.J., Ikonomidou C., et al. 2010. Suppression of the intrinsic apoptosis pathway by synaptic activity. J. Neurosci. 30:2623–2635 10.1523/JNEUROSCI.5115-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Chen Y., Jones A.F., Sanger R.H., Collis L.P., Flannery R., McNay E.C., Yu T., Schwarzenbacher R., Bossy B., et al. 2008. Bcl-xL induces Drp1-dependent synapse formation in cultured hippocampal neurons. Proc. Natl. Acad. Sci. USA. 105:2169–2174 10.1073/pnas.0711647105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Luikart B.W., Birnbaum S., Chen J., Kwon C.H., Kernie S.G., Bassel-Duby R., Parada L.F. 2008. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 59:399–412 10.1016/j.neuron.2008.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Jo J., Jia J.M., Lo S.C., Whitcomb D.J., Jiao S., Cho K., Sheng M. 2010. Caspase-3 activation via mitochondria is required for long-term depression and AMPA receptor internalization. Cell. 141:859–871 10.1016/j.cell.2010.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Ernfors P., Wu H., Jaenisch R. 1995. Sensory but not motor neuron deficits in mice lacking NT4 and BDNF. Nature. 375:238–241 10.1038/375238a0 [DOI] [PubMed] [Google Scholar]
- Lunn E.R., Perry V.H., Brown M.C., Rosen H., Gordon S. 1989. Absence of Wallerian Degeneration does not Hinder Regeneration in Peripheral Nerve. Eur. J. Neurosci. 1:27–33 10.1111/j.1460-9568.1989.tb00771.x [DOI] [PubMed] [Google Scholar]
- Luo L., O’Leary D.D. 2005. Axon retraction and degeneration in development and disease. Annu. Rev. Neurosci. 28:127–156 10.1146/annurev.neuro.28.061604.135632 [DOI] [PubMed] [Google Scholar]
- Martin-Zanca D., Hughes S.H., Barbacid M. 1986. A human oncogene formed by the fusion of truncated tropomyosin and protein tyrosine kinase sequences. Nature. 319:743–748 10.1038/319743a0 [DOI] [PubMed] [Google Scholar]
- Martin-Zanca D., Barbacid M., Parada L.F. 1990. Expression of the trk proto-oncogene is restricted to the sensory cranial and spinal ganglia of neural crest origin in mouse development. Genes Dev. 4:683–694 10.1101/gad.4.5.683 [DOI] [PubMed] [Google Scholar]
- Miller M.W. 1995. Relationship of the time of origin and death of neurons in rat somatosensory cortex: barrel versus septal cortex and projection versus local circuit neurons. J. Comp. Neurol. 355:6–14 10.1002/cne.903550104 [DOI] [PubMed] [Google Scholar]
- Müller M., Triaca V., Besusso D., Costanzi M., Horn J.M., Koudelka J., Geibel M., Cestari V., Minichiello L. 2012. Loss of NGF-TrkA signaling from the CNS is not sufficient to induce cognitive impairments in young adult or intermediate-aged mice. J. Neurosci. 32:14885–14898 10.1523/JNEUROSCI.2849-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaev A., McLaughlin T., O’Leary D.D., Tessier-Lavigne M. 2009. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 457:981–989 10.1038/nature07767 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Nikoletopoulou V., Lickert H., Frade J.M., Rencurel C., Giallonardo P., Zhang L., Bibel M., Barde Y.A. 2010. Neurotrophin receptors TrkA and TrkC cause neuronal death whereas TrkB does not. Nature. 467:59–63 10.1038/nature09336 [DOI] [PubMed] [Google Scholar]
- Ninkovic J., Mori T., Götz M. 2007. Distinct modes of neuron addition in adult mouse neurogenesis. J. Neurosci. 27:10906–10911 10.1523/JNEUROSCI.2572-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nykjaer A., Willnow T.E., Petersen C.M. 2005. p75NTR—live or let die. Curr. Opin. Neurobiol. 15:49–57 10.1016/j.conb.2005.01.004 [DOI] [PubMed] [Google Scholar]
- O’Riordan M.X., Bauler L.D., Scott F.L., Duckett C.S. 2008. Inhibitor of apoptosis proteins in eukaryotic evolution and development: a model of thematic conservation. Dev. Cell. 15:497–508 10.1016/j.devcel.2008.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okado N., Oppenheim R.W. 1984. Cell death of motoneurons in the chick embryo spinal cord. IX. The loss of motoneurons following removal of afferent inputs. J. Neurosci. 4:1639–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim R.W. 1991. Cell death during development of the nervous system. Annu. Rev. Neurosci. 14:453–501 10.1146/annurev.ne.14.030191.002321 [DOI] [PubMed] [Google Scholar]
- Oppenheim R.W., Yin Q.W., Prevette D., Yan Q. 1992. Brain-derived neurotrophic factor rescues developing avian motoneurons from cell death. Nature. 360:755–757 10.1038/360755a0 [DOI] [PubMed] [Google Scholar]
- Oppenheim R.W., Calderó J., Cuitat D., Esquerda J., McArdle J.J., Olivera B.M., Prevette D., Teichert R.W. 2008. The rescue of developing avian motoneurons from programmed cell death by a selective inhibitor of the fetal muscle-specific nicotinic acetylcholine receptor. Dev. Neurobiol. 68:972–980 10.1002/dneu.20636 [DOI] [PubMed] [Google Scholar]
- Patel T.D., Jackman A., Rice F.L., Kucera J., Snider W.D. 2000. Development of sensory neurons in the absence of NGF/TrkA signaling in vivo. Neuron. 25:345–357 10.1016/S0896-6273(00)80899-5 [DOI] [PubMed] [Google Scholar]
- Peter M.E., Krammer P.H. 2003. The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ. 10:26–35 10.1038/sj.cdd.4401186 [DOI] [PubMed] [Google Scholar]
- Petreanu L., Alvarez-Buylla A. 2002. Maturation and death of adult-born olfactory bulb granule neurons: role of olfaction. J. Neurosci. 22:6106–6113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman R.H., Oppenheim R.W. 1978. Neuromuscular blockade increases motoneurone survival during normal cell death in the chick embryo. Nature. 271:364–366 10.1038/271364a0 [DOI] [PubMed] [Google Scholar]
- Plachta N., Annaheim C., Bissière S., Lin S., Rüegg M., Hoving S., Müller D., Poirier F., Bibel M., Barde Y.A. 2007. Identification of a lectin causing the degeneration of neuronal processes using engineered embryonic stem cells. Nat. Neurosci. 10:712–719 10.1038/nn1897 [DOI] [PubMed] [Google Scholar]
- Purves D. 1988. A molecular basis for trophic interactions in vertebrates. Body and Brain. Harvard University Press, Cambridge, MA and London, England: 123–143 [Google Scholar]
- Purves D., Lichtman J.W. 1984. Neuronal death during development. Principles of Neural Development. Sinauer Associates, Inc., Sunderland, MA: 131–153 [Google Scholar]
- Putcha G.V., Le S., Frank S., Besirli C.G., Clark K., Chu B., Alix S., Youle R.J., LaMarche A., Maroney A.C., Johnson E.M., Jr 2003. JNK-mediated BIM phosphorylation potentiates BAX-dependent apoptosis. Neuron. 38:899–914 10.1016/S0896-6273(03)00355-6 [DOI] [PubMed] [Google Scholar]
- Puthalakath H., O’Reilly L.A., Gunn P., Lee L., Kelly P.N., Huntington N.D., Hughes P.D., Michalak E.M., McKimm-Breschkin J., Motoyama N., et al. 2007. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 129:1337–1349 10.1016/j.cell.2007.04.027 [DOI] [PubMed] [Google Scholar]
- Raff M.C., Whitmore A.V., Finn J.T. 2002. Axonal self-destruction and neurodegeneration. Science. 296:868–871 10.1126/science.1068613 [DOI] [PubMed] [Google Scholar]
- Rauskolb S., Zagrebelsky M., Dreznjak A., Deogracias R., Matsumoto T., Wiese S., Erne B., Sendtner M., Schaeren-Wiemers N., Korte M., Barde Y.A. 2010. Global deprivation of brain-derived neurotrophic factor in the CNS reveals an area-specific requirement for dendritic growth. J. Neurosci. 30:1739–1749 10.1523/JNEUROSCI.5100-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl S.J., Salvesen G.S. 2007. The apoptosome: signalling platform of cell death. Nat. Rev. Mol. Cell Biol. 8:405–413 10.1038/nrm2153 [DOI] [PubMed] [Google Scholar]
- Sanchez-Ortiz E., Yui D., Song D., Li Y., Rubenstein J.L., Reichardt L.F., Parada L.F. 2012. TrkA gene ablation in basal forebrain results in dysfunction of the cholinergic circuitry. J. Neurosci. 32:4065–4079 10.1523/JNEUROSCI.6314-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi C., Schmitz I., Krammer P.H., Peter M.E. 1999. The role of c-FLIP in modulation of CD95-induced apoptosis. J. Biol. Chem. 274:1541–1548 10.1074/jbc.274.3.1541 [DOI] [PubMed] [Google Scholar]
- Sendtner M., Holtmann B., Kolbeck R., Thoenen H., Barde Y.A. 1992. Brain-derived neurotrophic factor prevents the death of motoneurons in newborn rats after nerve section. Nature. 360:757–759 10.1038/360757a0 [DOI] [PubMed] [Google Scholar]
- Simon D.J., Weimer R.M., McLaughlin T., Kallop D., Stanger K., Yang J., O’Leary D.D., Hannoush R.N., Tessier-Lavigne M. 2012. A caspase cascade regulating developmental axon degeneration. J. Neurosci. 32:17540–17553 10.1523/JNEUROSCI.3012-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeyne R.J., Klein R., Schnapp A., Long L.K., Bryant S., Lewin A., Lira S.A., Barbacid M. 1994. Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature. 368:246–249 10.1038/368246a0 [DOI] [PubMed] [Google Scholar]
- Sobreviela T., Clary D.O., Reichardt L.F., Brandabur M.M., Kordower J.H., Mufson E.J. 1994. TrkA-immunoreactive profiles in the central nervous system: colocalization with neurons containing p75 nerve growth factor receptor, choline acetyltransferase, and serotonin. J. Comp. Neurol. 350:587–611 10.1002/cne.903500407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.H., Tse M.C., Bellail A., Phuphanich S., Khuri F., Kneteman N.M., Hao C. 2007. Lipid rafts and nonrafts mediate tumor necrosis factor related apoptosis-inducing ligand induced apoptotic and nonapoptotic signals in non small cell lung carcinoma cells. Cancer Res. 67:6946–6955 10.1158/0008-5472.CAN-06-3896 [DOI] [PubMed] [Google Scholar]
- Southwell D.G., Paredes M.F., Galvao R.P., Jones D.L., Froemke R.C., Sebe J.Y., Alfaro-Cervello C., Tang Y., Garcia-Verdugo J.M., Rubenstein J.L., et al. 2012. Intrinsically determined cell death of developing cortical interneurons. Nature. 491:109–113 10.1038/nature11523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperka T., Wang J., Rudolph K.L. 2012. DNA damage checkpoints in stem cells, ageing and cancer. Nat. Rev. Mol. Cell Biol. 13:579–590 10.1038/nrm3420 [DOI] [PubMed] [Google Scholar]
- Stenqvist A., Agerman K., Marmigère F., Minichiello L., Ernfors P. 2005. Genetic evidence for selective neurotrophin 3 signalling through TrkC but not TrkB in vivo. EMBO Rep. 6:973–978 10.1038/sj.embor.7400512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauszig-Delamasure S., Yu L.Y., Cabrera J.R., Bouzas-Rodriguez J., Mermet-Bouvier C., Guix C., Bordeaux M.C., Arumäe U., Mehlen P. 2007. The TrkC receptor induces apoptosis when the dependence receptor notion meets the neurotrophin paradigm. Proc. Natl. Acad. Sci. USA. 104:13361–13366 10.1073/pnas.0701243104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessarollo L., Tsoulfas P., Donovan M.J., Palko M.E., Blair-Flynn J., Hempstead B.L., Parada L.F. 1997. Targeted deletion of all isoforms of the trkC gene suggests the use of alternate receptors by its ligand neurotrophin-3 in neuronal development and implicates trkC in normal cardiogenesis. Proc. Natl. Acad. Sci. USA. 94:14776–14781 10.1073/pnas.94.26.14776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay R., Hewitt K., Lesiuk H., Mealing G., Morley P., Durkin J.P. 1999. Evidence that brain-derived neurotrophic factor neuroprotection is linked to its ability to reverse the NMDA-induced inactivation of protein kinase C in cortical neurons. J. Neurochem. 72:102–111 10.1046/j.1471-4159.1999.0720102.x [DOI] [PubMed] [Google Scholar]
- Unsain N., Higgins J.M., Parker K.N., Johnstone A.D., Barker P.A. 2013. XIAP regulates caspase activity in degenerating axons. Cell Rep. 4:751–763 10.1016/j.celrep.2013.07.015 [DOI] [PubMed] [Google Scholar]
- Urano Y., Hayashi I., Isoo N., Reid P.C., Shibasaki Y., Noguchi N., Tomita T., Iwatsubo T., Hamakubo T., Kodama T. 2005. Association of active gamma-secretase complex with lipid rafts. J. Lipid Res. 46:904–912 10.1194/jlr.M400333-JLR200 [DOI] [PubMed] [Google Scholar]
- Vaux D.L., Silke J. 2005. IAPs, RINGs and ubiquitylation. Nat. Rev. Mol. Cell Biol. 6:287–297 10.1038/nrm1621 [DOI] [PubMed] [Google Scholar]
- Vila M., Przedborski S. 2003. Targeting programmed cell death in neurodegenerative diseases. Nat. Rev. Neurosci. 4:365–375 10.1038/nrn1100 [DOI] [PubMed] [Google Scholar]
- Vohra B.P.S., Sasaki Y., Miller B.R., Chang J.F., DiAntonio A., Milbrandt J. 2010. Amyloid precursor protein cleavage-dependent and -independent axonal degeneration programs share a common nicotinamide mononucleotide adenylyltransferase 1-sensitive pathway. J. Neurosci. 30:13729–13738 10.1523/JNEUROSCI.2939-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Wang Y., Zhang J., Kim H.P., Ryter S.W., Choi A.M. 2005. FLIP protects against hypoxia/reoxygenation-induced endothelial cell apoptosis by inhibiting Bax activation. Mol. Cell. Biol. 25:4742–4751 10.1128/MCB.25.11.4742-4751.2005 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wang J.T., Medress Z.A., Barres B.A. 2012. Axon degeneration: molecular mechanisms of a self-destruction pathway. J. Cell Biol. 196:7–18 10.1083/jcb.201108111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse E.G., An J.J., Orefice L.L., Baydyuk M., Liao G.Y., Zheng K., Lu B., Xu B. 2012. BDNF promotes differentiation and maturation of adult-born neurons through GABAergic transmission. J. Neurosci. 32:14318–14330 10.1523/JNEUROSCI.0709-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D.W., Kondo S., Krzyzanowska A., Hiromi Y., Truman J.W. 2006. Local caspase activity directs engulfment of dendrites during pruning. Nat. Neurosci. 9:1234–1236 10.1038/nn1774 [DOI] [PubMed] [Google Scholar]
- Wu C., Lai C.F., Mobley W.C. 2001. Nerve growth factor activates persistent Rap1 signaling in endosomes. J. Neurosci. 21:5406–5416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q., Elliott J., Snider W.D. 1992. Brain-derived neurotrophic factor rescues spinal motor neurons from axotomy-induced cell death. Nature. 360:753–755 10.1038/360753a0 [DOI] [PubMed] [Google Scholar]
- Youle R.J., Strasser A. 2008. The BCL-2 protein family: opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 9:47–59 10.1038/nrm2308 [DOI] [PubMed] [Google Scholar]