Abstract
A membrane barrier important for assembly of the nodes of Ranvier is found at the paranodal junction. This junction is comprised of axonal and glial adhesion molecules linked to the axonal actin–spectrin membrane cytoskeleton through specific adaptors. In this issue, Zhang et al. (2013. J. Cell Biol. http://dx.doi.org/10.1083/jcb.201308116) show that axonal βII spectrin maintains the diffusion barrier at the paranodal junction. Thus, βII spectrin serves to compartmentalize the membrane of myelinated axons at specific locations that are determined either intrinsically (i.e., at the axonal initial segment), or by axoglial contacts (i.e., at the paranodal junction).
Cell polarization is an essential feature that allows many cell types to fulfill their unique functions. Upon differentiation, polarized cells establish specialized membrane domains with distinct protein composition. In myelinated axons, such membrane compartmentalization is essential for fast and efficient propagation of action potentials in a saltatory manner. The membrane of these axons is divided into several distinct domains that include (1) the nodes of Ranvier, which are gaps between myelin segments where sodium channels are clustered; (2) the paranodal axoglial junction, where the terminal loops of the myelin attach to the axon; (3) the juxtaparanodal region, where Kv1 potassium channels are concentrated; and (4) the internode, which are covered by compact myelin (Fig. 1). In the peripheral nervous system (PNS), this intricate axonal organization requires specific intercellular contact sites between the axon and myelinating Schwann cells (Poliak and Peles, 2003; Eshed-Eisenbach and Peles, 2013), as well as the formation of membrane diffusion barriers that restrict the movement of proteins and lipids in the plasma membrane across different domains (Lasiecka et al., 2009; Katsuki et al., 2011).
Figure 1.
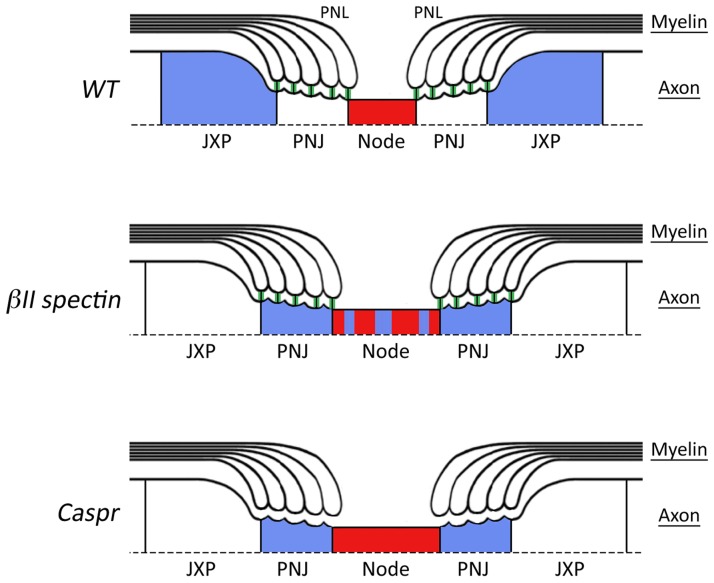
βII spectrin helps organize membrane domains in myelinated axons. A schematic view depicting the organization of myelinated peripheral nerves around the nodes of Ranvier of wild type (WT, top), and mice mutants lacking axonal βII spectrin (middle) or the adhesion molecule Caspr (bottom). The presence of intact paranodal junction (PNJ) is marked by green vertical lines between the paranodal loops (PNL) and the axon. In wild-type nerves (top), both the paranodal junction and the cytoskeletal barrier are intact, resulting in the sequestering of Kv1 channels (blue) in the juxtaparanodal region (JXP) away from nodal sodium channels (red). In contrast to the paranodes in Caspr knockout that lack both the junction and the barrier function (bottom), in the βII spectrin mutant (middle) the barrier is compromised while the junction is intact. Note that the nodes in both mutants are wider compared to the wild type.
The main membrane barrier that plays an important role in the assembly of the nodes of Ranvier is present at the paranodal junction (Feinberg et al., 2010; Susuki et al., 2013). These septate-like junctions are composed of axonal (Caspr and contactin) and glial (neurofascin 155-kD isoform) adhesion molecules, and are linked through specific adaptor proteins to the actin–spectrin membrane cytoskeleton (Ogawa et al., 2006; Perkins et al., 2008; Nans et al., 2011). Cytoskeletal components of the paranodal junction include the scaffold protein 4.1B, which is required for the organization of myelinated axons (Horresh et al., 2010; Buttermore et al., 2011; Cifuentes-Diaz et al., 2011; Einheber et al., 2013), as well as ankyrin B and αII and βII spectrin (Ogawa et al., 2006). A paranodal membrane barrier has long been described as the boundary separating nodal and juxtaparanodal ion channels. The barrier function has been attributed to the axoglial contact and formation of the septate-like junctions (Bhat et al., 2001; Boyle et al., 2001). Nonetheless, the molecular mechanism forming the barrier itself has never been resolved. In general, membrane barriers can form by several mechanisms (Lasiecka et al., 2009). For example, a barrier at the axonal initial segment (AIS), which maintains axo-dendritic polarity, is formed by anchoring various transmembrane proteins to the actin-based membrane skeleton (Nakada et al., 2003; Galiano et al., 2012). In the base of the cilium, yeast bud and dendritic spines septins, proteins that are absent from AIS and tight junctions (Caudron and Barral, 2009), form high order ring-like structure that immobilize lipids in the inner membrane leaflet. In erythrocytes, direct binding of spectrin to membrane lipids forms a diffusion barrier for both proteins and lipids in the absence of actin (Sheetz et al., 2006). Interestingly, at the epithelial tight junction, the diffusion barriers for lipids and proteins are probably achieved by separate mechanisms, as targeting some junctional components results in loss of lipid but not of protein polarity (Jou et al., 1998).
In the current issue, Zhang et al. succeeded to uncouple the assembly of the paranodal membrane domain from its barrier function. This was accomplished by specifically ablating βII spectrin in peripheral sensory neurons and analyzing the axonal organization of these nerves. The unique domain organization of myelinated axons allows for a simple and highly reproducible examination of the barrier function at the paranode. That is, impairment of the barrier will result in the displacement of juxtaparanodal components (i.e., Caspr2, Kv1.2, and TAG-1) into the paranodes and nodes, as observed in mutants that lack an intact paranodal junction (Bhat et al., 2001; Boyle et al., 2001). In the affected nerves of the βII spectrin mutant, the authors made the surprising observation that although the axoglial paranodal junction remained completely intact, juxtaparanodal complexes were no longer excluded from paranodes and nodes (Fig. 1). Developmental analysis of the mutant revealed a dramatic increase in the number of paranodes and nodes containing juxtaparanodal components with age, an observation suggesting that a βII spectrin–based diffusion barrier mainly contributes to the maintenance of a paranodal membrane barrier. Interestingly, these results are in line with a previous study showing that the linkage between Caspr and the adaptor protein 4.1B is crucial for the paranodal barrier (Horresh et al., 2010). Zhang et al. (2013) also observed that the absence of βII spectrin results in a significant widening of the nodes of Ranvier (Fig. 1), further supporting a role for the paranodal junction barrier in the maintenance of nodal sodium channels (Rios et al., 2003). The assembly of the nodes of Ranvier in the PNS is achieved by initial clustering of Na+ channels at heminodes, a process that requires binding of glial gliomedin and NrCAM to their axonal receptor Neurofascin 186, as well as by restricting the distribution of these channels to the nodal gap by the paranodal junction barrier (Feinberg et al., 2010). To examine whether the βII spectrin–based membrane barrier at the paranodal junction also participates in node formation would require additional analysis of mice lacking both βII spectrin and the glial clustering signal (i.e., gliomedin or NrCAM). Surprisingly, despite the abnormal presence of Kv1 channels at the paranodes and nodes, and in contrast to all known mutants lacking the paranodal junction, βII mutant mice exhibit normal nerve conduction. These results may indicate that the paranodal junctions that provide an intercellular sealing, similarly to epithelial tight junctions, are critical for proper nerve conduction. In contrast, an intact paranodal membrane barrier is not necessary for normal conduction.
The similarity between mice lacking βII spectrin in sensory neurons and paranodal mutants lacking Caspr, NF155, and contactin uncovers a hierarchy in axonal domain organization: adhesion molecules that form the axon–glial junction independently of cytoskeletal interactions induce the formation of a βII spectrin–based membrane barrier, which in turn is responsible for maintaining axonal domain organization. Furthermore, the exact location of a barrier on the membrane can be determined by cell-intrinsic or -extrinsic factors (Katsuki et al., 2011). AISs are formed by intrinsic factors, whereas the paranodal junction is determined by axon–glia interactions. Strikingly, a previous paper from Rasband and colleagues has shown that an axonal barrier controlling the formation of the AIS is composed of the same cytoskeletal proteins as the paranodal barrier, namely ankB, βII spetrin, and αII spectrin (Galiano et al., 2012). Thus, the same membrane barrier can be localized by either external or internal cues and participate in either the formation (AIS and nodes of Ranvier) or maintenance (nodes of Ranvier and juxtaparanodal region) of axonal domains.
Acknowledgments
Work in the authors’ laboratory is supported by the National Institutes of Health (NS50220), the Israel Science Foundation, and the Adelson Medical Research Foundation. E. Peles is the Incumbent of the Hanna Hertz Professorial Chair for Multiple Sclerosis and Neuroscience.
Footnotes
Abbreviations used in this paper:
- AIS
- axonal initial segment
- PNS
- peripheral nervous system
References
- Bhat M.A., Rios J.C., Lu Y., Garcia-Fresco G.P., Ching W., St Martin M., Li J., Einheber S., Chesler M., Rosenbluth J., et al. 2001. Axon-glia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/Paranodin. Neuron. 30:369–383 10.1016/S0896-6273(01)00294-X [DOI] [PubMed] [Google Scholar]
- Boyle M.E., Berglund E.O., Murai K.K., Weber L., Peles E., Ranscht B. 2001. Contactin orchestrates assembly of the septate-like junctions at the paranode in myelinated peripheral nerve. Neuron. 30:385–397 10.1016/S0896-6273(01)00296-3 [DOI] [PubMed] [Google Scholar]
- Buttermore E.D., Dupree J.L., Cheng J., An X., Tessarollo L., Bhat M.A. 2011. The cytoskeletal adaptor protein band 4.1B is required for the maintenance of paranodal axoglial septate junctions in myelinated axons. J. Neurosci. 31:8013–8024 10.1523/JNEUROSCI.1015-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudron F., Barral Y. 2009. Septins and the lateral compartmentalization of eukaryotic membranes. Dev. Cell. 16:493–506 10.1016/j.devcel.2009.04.003 [DOI] [PubMed] [Google Scholar]
- Cifuentes-Diaz C., Chareyre F., Garcia M., Devaux J., Carnaud M., Levasseur G., Niwa-Kawakita M., Harroch S., Girault J.A., Giovannini M., Goutebroze L. 2011. Protein 4.1B contributes to the organization of peripheral myelinated axons. PLoS ONE. 6:e25043 10.1371/journal.pone.0025043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einheber S., Meng X., Rubin M., Lam I., Mohandas N., An X., Shrager P., Kissil J., Maurel P., Salzer J.L. 2013. The 4.1B cytoskeletal protein regulates the domain organization and sheath thickness of myelinated axons. Glia. 61:240–253 10.1002/glia.22430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshed-Eisenbach Y., Peles E. 2013. The making of a node: a co-production of neurons and glia. Curr. Opin. Neurobiol. 23:1–8 10.1016/j.conb.2012.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg K., Eshed-Eisenbach Y., Frechter S., Amor V., Salomon D., Sabanay H., Dupree J.L., Grumet M., Brophy P.J., Shrager P., Peles E. 2010. A glial signal consisting of gliomedin and NrCAM clusters axonal Na+ channels during the formation of nodes of Ranvier. Neuron. 65:490–502 10.1016/j.neuron.2010.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galiano M.R., Jha S., Ho T.S., Zhang C., Ogawa Y., Chang K.J., Stankewich M.C., Mohler P.J., Rasband M.N. 2012. A distal axonal cytoskeleton forms an intra-axonal boundary that controls axon initial segment assembly. Cell. 149:1125–1139 10.1016/j.cell.2012.03.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horresh I., Bar V., Kissil J.L., Peles E. 2010. Organization of myelinated axons by Caspr and Caspr2 requires the cytoskeletal adapter protein 4.1B. J. Neurosci. 30:2480–2489 10.1523/JNEUROSCI.5225-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou T.S., Schneeberger E.E., Nelson W.J. 1998. Structural and functional regulation of tight junctions by RhoA and Rac1 small GTPases. J. Cell Biol. 142:101–115 10.1083/jcb.142.1.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki T., Joshi R., Ailani D., Hiromi Y. 2011. Compartmentalization within neurites: its mechanisms and implications. Dev. Neurobiol. 71:458–473 10.1002/dneu.20859 [DOI] [PubMed] [Google Scholar]
- Lasiecka Z.M., Yap C.C., Vakulenko M., Winckler B. 2009. Compartmentalizing the neuronal plasma membrane from axon initial segments to synapses. Int Rev Cell Mol Biol. 272:303–389 10.1016/S1937-6448(08)01607-9 [DOI] [PubMed] [Google Scholar]
- Nakada C., Ritchie K., Oba Y., Nakamura M., Hotta Y., Iino R., Kasai R.S., Yamaguchi K., Fujiwara T., Kusumi A. 2003. Accumulation of anchored proteins forms membrane diffusion barriers during neuronal polarization. Nat. Cell Biol. 5:626–632 10.1038/ncb1009 [DOI] [PubMed] [Google Scholar]
- Nans A., Einheber S., Salzer J.L., Stokes D.L. 2011. Electron tomography of paranodal septate-like junctions and the associated axonal and glial cytoskeletons in the central nervous system. J. Neurosci. Res. 89:310–319 10.1002/jnr.22561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y., Schafer D.P., Horresh I., Bar V., Hales K., Yang Y., Susuki K., Peles E., Stankewich M.C., Rasband M.N. 2006. Spectrins and ankyrinB constitute a specialized paranodal cytoskeleton. J. Neurosci. 26:5230–5239 10.1523/JNEUROSCI.0425-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins G.A., Sosinsky G.E., Ghassemzadeh S., Perez A., Jones Y., Ellisman M.H. 2008. Electron tomographic analysis of cytoskeletal cross-bridges in the paranodal region of the node of Ranvier in peripheral nerves. J. Struct. Biol. 161:469–480 10.1016/j.jsb.2007.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliak S., Peles E. 2003. The local differentiation of myelinated axons at nodes of Ranvier. Nat. Rev. Neurosci. 4:968–980 10.1038/nrn1253 [DOI] [PubMed] [Google Scholar]
- Rios J.C., Rubin M., St Martin M., Downey R.T., Einheber S., Rosenbluth J., Levinson S.R., Bhat M., Salzer J.L. 2003. Paranodal interactions regulate expression of sodium channel subtypes and provide a diffusion barrier for the node of Ranvier. J. Neurosci. 23:7001–7011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz M.P., Sable J.E., Döbereiner H.G. 2006. Continuous membrane-cytoskeleton adhesion requires continuous accommodation to lipid and cytoskeleton dynamics. Annu. Rev. Biophys. Biomol. Struct. 35:417–434 10.1146/annurev.biophys.35.040405.102017 [DOI] [PubMed] [Google Scholar]
- Susuki K., Chang K.J., Zollinger D.R., Liu Y., Ogawa Y., Eshed-Eisenbach Y., Dours-Zimmermann M.T., Oses-Prieto J.A., Burlingame A.L., Seidenbecher C.I., et al. 2013. Three mechanisms assemble central nervous system nodes of Ranvier. Neuron. 78:469–482 10.1016/j.neuron.2013.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Susuki K., Zollinger D.R., Dupree J.L., Rasband M.N. 2013. Membrane domain organization of myelinated axons requires βII spectrin. J. Cell Biol. 203:437–443 10.1083/jcb.201308116 [DOI] [PMC free article] [PubMed] [Google Scholar]