Abstract
Glia serve many important functions in the mature nervous system. In addition, these diverse cells have emerged as essential participants in nearly all aspects of neural development. Improved techniques to study neurons in the absence of glia, and to visualize and manipulate glia in vivo, have greatly expanded our knowledge of glial biology and neuron–glia interactions during development. Exciting studies in the last decade have begun to identify the cellular and molecular mechanisms by which glia exert control over neuronal circuit formation. Recent findings illustrate the importance of glial cells in shaping the nervous system by controlling the number and connectivity of neurons.
Introduction
The nervous system is comprised of two main cell types: neurons and glia. Glia represent a heterogeneous population of nervous system cells that associate with neurons and have diverse roles in nervous system development, maintenance, and function. Major glial cell types in vertebrates include astrocytes, microglia, oligodendrocytes, and NG2+ oligodendrocyte precursor cells (OPCs) in the central nervous system, and Schwann cells in the peripheral nervous system. Active roles for glia in all major steps of neural development have been described in a variety of model organisms from worms to mammals. Among the first recognized (and perhaps the most well-known) developmental functions of glia is in helping to guide axons to their correct targets. Glia serve as contact-dependent guideposts and sources of numerous attractive and repulsive cues in many circuits (Chotard and Salecker, 2004). It is also now understood that glia serve as stem cells in both embryonic and adult vertebrate nervous systems (Doetsch et al., 1999; Noctor et al., 2001; Seri et al., 2001), in addition to providing important substrates for neuronal migration (Rakic, 1971). In recent years, new functions for glia in synaptogenesis and plasticity have revealed a very active role for glia in neural circuit formation. In this review, we highlight recent insights into active roles for glia during nervous system development, focusing primarily on glial shaping of circuit formation via control of neuron and synapse numbers.
Glia regulate neuron production
A key step in neural development is generating the appropriate number of neurons and glia at the correct time and in the right place. In both invertebrate and vertebrate systems, glial cells have been identified as crucial constituents of neural stem cell niches that modulate neurogenesis by dynamically regulating stem cell proliferation and precursor differentiation in response to a variety of changing developmental time points and functional needs.
In Drosophila, neural stem cells called neuroblasts undergo a stereotyped period of quiescence during early larval stages before resuming divisions to give rise to the adult nervous system (Truman and Bate, 1988), and proper transition between these states is crucial for normal nervous system formation (Ebens et al., 1993). As with many other stem cell types, neuroblast exit from quiescence is tied to overall animal development through nutrient signaling. Signals from the larval fat body, which monitors nutrient status in the periphery, are required to activate neuroblasts to reenter the cell cycle (Britton and Edgar, 1998). Two recent studies have shown that glia are required to act as an intermediate to transduce this information to neuroblasts. In response to nutrient-dependent fat body signaling, glia secrete insulin/IGF-like peptides (Drosophila insulin-like peptides [dILPs]). These dILPs activate insulin receptor–PI3K/Akt signaling in neuroblasts, triggering reentry into the cell cycle (Chell and Brand, 2010; Sousa-Nunes et al., 2011). Neuroblast proliferation is significantly delayed in animals lacking dILPs, or when glia are prevented from signaling by inhibiting vesicle trafficking with a mutant form of the fly dynamin gene, shibire. Moreover, glial expression of dILPs is sufficient to induce precocious activation of neuroblasts under normal conditions or bypass the requirement for dietary amino acids in starvation conditions (Chell and Brand, 2010; Sousa-Nunes et al., 2011). Glia, therefore, play a specific role in receiving systemic signals and transmitting them to neural stem cells to modulate their growth. Using glia as an intermediary may allow for more stable and precisely timed regulation of stem cell activation by allowing glia to serve as an integration site for multiple cues, which clearly include nutritional status. Auto-regulation of their own activity also appears to modulate glial control of stem cell activity: there is evidence glia may cell-autonomously modulate their own dILP production through expression of the RNA-binding protein FMRP, and that this regulation is critical for proper timing of neuroblast reactivation (Callan et al., 2012). Thus, glia may not simply relay information, but serve as important integration or filtering sites for multiple signals that impinge on neuroblast biology.
Glial cells also play important roles in regulating progenitor cell division and specification in the proliferative zones of embryonic and adult mammalian brains. Microglia derived from myeloid precursors in the yolk sac migrate into the brain as early as E10.5 (Hirasawa et al., 2005; Ginhoux et al., 2010), where they can modulate neural precursor proliferation and neural cell fate specification during embryonic cortical neurogenesis. Evidence from in vitro studies comparing isolated progenitors to those cultured with young microglia suggest that the presence of microglia enhances proliferation and biases progeny toward an astroglial fate (Antony et al., 2011). A novel role for microglia in the negative regulation of neurogenesis has also recently been described. Data from immunostaining of fixed tissue and imaging of microglia in cultured brain slices suggest that young microglia phagocytose cortical precursor cells as cortical neurogenesis nears completion, which may serve as a key mechanism to curb neuron production or reduce neural populations at this time (Cunningham et al., 2013). One intriguing feature of this finding is that microglia appear to engulf cells that lack several standard cell death markers and may even engulf actively dividing progenitors (Cunningham et al., 2013). These observations suggest that microglia may not simply be acting as scavengers, but might be initiating cell death via engulfment. There is evidence that strongly suggests that engulfment by microglia is required to fully execute partially activated developmental cell death programs in post-mitotic Purkinje neurons (Marín-Teva et al., 2004; see next section), and roles for engulfing cells in promoting final execution of apoptosis in target cells have been described in Caenorhabditis elegans (Hoeppner et al., 2001; Reddien et al., 2001). However, the above study goes further, suggesting that microglia somehow decide to engulf and destroy seemingly healthy neural precursors. Defining the molecular mechanisms that govern this choice will be an important focus for future work.
Radial glia are a key feature of the developing cortex, long understood to serve as important scaffolds for neuronal migration in the developing embryo. Studies in the last decade have now demonstrated that radial glia cells are in fact neuronal progenitors in the developing brain (Noctor et al., 2001; Tamamaki et al., 2001). This important finding came on the heels of the identification of astrocytes as adult neural stem cells in the mammalian brain (Doetsch et al., 1999). In addition to serving as stem cells, mammalian astrocytes in adult proliferative zones can modulate progenitor division and differentiation through the release of secreted molecules (including FGF2) and contact-dependent mechanisms (such as Eph/ephrin signaling; Morrens et al., 2012). Only astrocytes from proliferative zones are capable of promoting neurogenesis; thus, astrocytes constrain where new neurons are generated in the adult (Song et al., 2002). In addition, astrocytes can also couple neurogenesis to physiological status or injury. For example, IGF-I, normally expressed by neurons, is strongly expressed in astrocytes only after brain injury, and may contribute to injury-induced increases in adult neurogenesis (Yan et al., 2006).
These exciting new studies of neural stem cells and neurogenesis in different species and at different developmental stages demonstrate conserved and wide-ranging roles for glia as regulators of neuron production. Through mechanisms that remain to be defined, glia appear to serve as sites of integration that allow dynamic modulation of neurogenesis in response to changing physiological needs during development and after injury or disease in the adult. Understanding the cellular and molecular mechanisms by which glia monitor animal physiology and modulate neurogenesis should have important implications for the understanding and treatment of many neurodevelopmental disorders and neurodegenerative diseases.
Glia in developmental programmed cell death
Overproduction, followed by programmed cell death (PCD) of excess neurons, is a well-described and conserved feature of nervous system development across phyla that is designed to ensure a sufficient number of neurons are initially formed to accomplish neural circuit construction (see Dekkers et al., in this issue). Competition between neurons for limited environmental and target-derived trophic factors results in cell death of extraneous or weakly connected neurons (Levi-Montalcini, 1987). PCD most often occurs via apoptosis followed by phagocytosis of the cellular debris by microglia, the resident immune cells and phagocytes of the central nervous system. A number of recent studies in the rodent have challenged the view that microglia act as passive scavengers of cellular debris. Rather, accumulating evidence now demonstrates an active role for microglia and innate immune mechanisms in promoting developmental PCD (Fig. 1).
Figure 1.
Microglia promote cell death and clearance of neuronal debris in the developing brain. (A) Model of the role of microglia in promoting neuronal programmed cell death in the developing cerebellum and hippocampus. Caspase-3+ neurons that have initiated apoptosis express as-yet-unknown “kill me/eat me” signals that recruit microglia to engulf them. Contact between dying neurons and microglia expressing the integrin CD11b and the immunoreceptor DAP12 triggers production of reactive oxygen species (including O2.−) in microglia, which is locally released to promote completion of apoptosis in the dying neuron. CD11b and DAP12 are required in microglia for efficient ROS production and for normal levels of PCD, but it remains unclear whether CD11b/DAP12 act as a receptor for one of the elusive neuronal signal(s), or if other receptors indirectly activate this pathway. (B) Microglia are also required for the clearance of cellular debris generated by PCD. The neuronal signals and specific microglial receptors that mediate corpse recognition and phagocytosis also remain unknown. Proper clearance of corpses is required to prevent inflammation.
Close association or partial engulfment of intact but caspase-3–expressing Purkinje cells and microglia is seen in fixed tissue and in cultured cerebellar slices from postembryonic day 3 (P3) mice, a time corresponding to high levels of Purkinje cell PCD in vivo (Marín-Teva et al., 2004). At this stage, microglia were observed to be amoeboid in shape, which is characteristic of phagocytic function. Despite the fact that many of the Purkinje cells at this stage are already positive for activated caspases, depletion of microglia from the slices using clodronate containing liposomes (which target phagocytic cells) dramatically increases the number of surviving Purkinje cells after 3 days in vitro (Marín-Teva et al., 2004). These data fit a model proposed by PCD studies in C. elegans that shows engulfment by phagocytes is not simply required for debris clearance, but is itself required for the completion of cell death, even in cells already expressing activated caspases (Hoeppner et al., 2001; Reddien et al., 2001).
In the innate immune response, engulfing cells can release reactive oxygen species (ROS) in a process called “respiratory burst” to aid in killing pathogens (Chanock et al., 1994). Using pharmacology, Marín-Teva et al. (2004) found evidence that microglia produce such respiratory bursts upon engulfment of Purkinje cells that contributed to their death. Subsequent studies demonstrated similar requirements for microglia in mediating PCD in the developing hippocampus and identified two immune molecules expressed in microglia that have been co-opted from the innate immune system for use in developmental PCD (Wakselman et al., 2008). In peripheral immune cells, the cell surface molecules CD11b and DAP12 work together to target pathogens for destruction using ROS (Mócsai et al., 2006). In the developing hippocampus, CD11b and DAP12 are expressed exclusively by microglia, and examination of DAP12 or CD11b mutant mice showed reduced microglial superoxide production along with significantly reduced PCD in the hippocampus (Fig. 1 A; Wakselman et al., 2008). In both the cerebellum and hippocampus, caspase activation is still assumed to be cell-autonomously induced, and although greatly reduced, some PCD is still observed in the absence of microglial signaling. These data suggest that cell-autonomous mechanisms are capable of both inducing PCD and carrying it to completion in some cases, but that microglia play an important role in a majority of cases. Thus, microglia appear to have two important roles in developmental PCD. First, they can act to promote cell death via production of reactive oxygen species (and potentially additional mechanisms) to ensure the appropriately high levels of cell death that are required for normal development (Fig. 1 A). In addition, microglia act as phagocytes to clear the debris PCD generates (Fig. 1 B). It is assumed that dying neurons produce “kill me” and/or “eat me” signals that help recruit microglia and initiate CD11b/DAP12-dependent ROS production and engulfment, but these putative signals and their microglial receptors remain unknown.
Whether microglia play such an active role in cell death outside of development is unclear. In uninjured adult hippocampus, for example, microglia lack DAP12 (Wakselman et al., 2008) and have very low levels of CD11b (Sierra et al., 2010), yet there are high levels of cell death among newly born neurons in the subgranular zone of the hippocampus (a site of adult neurogenesis; Sierra et al., 2010). This suggests that microglia may not play the same active role in driving apoptosis of the newly born neurons, but this remains to be tested directly. Interestingly, engulfment of these cells in the mature hippocampus is performed by the processes of ramified (resting) microglia that efficiently phagocytose dying neurons without any morphological signs of activation (Sierra et al., 2010). Engulfment earlier in development is generally performed by amoeboid-like microglia, so this morphological difference may suggest the molecular mechanisms engaged may also be distinct at different stages. On the other hand, microglial activity during PCD at all stages is clearly distinguished from microglial response to injury or infection, which causes inflammation. Rapid engulfment of neurons undergoing PCD is considered immunologically silent—actually preventing inflammation that might be caused by unattended cell corpses.
Glia control connectivity by mediating developmental axon pruning
During development neurons often make inappropriate, excessive, or transient connections that must be eliminated as circuits mature. Though this process sometimes results in developmental cell death, selective pruning of axonal and dendritic branches is an important alternative method of neural circuit refinement and remodeling. Glia play key roles in two of the most well-characterized examples of developmental axon pruning—mammalian neuromuscular junction (NMJ) refinement and fly mushroom body γ-neuron remodeling.
In early development, mammalian NMJs are innervated by multiple motor neuron axons. Activity-dependent competition between the axonal branches innervating the same NMJ eventually results in exactly one “winning” axon that maintains connectivity, while all the other “losing” branches are eliminated (Colman et al., 1997; Walsh and Lichtman, 2003). Detailed analysis of branch elimination using confocal and electron microscopy has revealed that losing axons retract from the NMJ by shedding pieces of themselves (termed axosomes) in a distal-to-proximal manner (Bishop et al., 2004; Song et al., 2008). Individual axon branches are ensheathed by perisynaptic Schwann cells—a specialized subtype of glia cell in the peripheral nervous system—before and during retraction. Shed axosomes are engulfed into the ensheathing perisynaptic Schwann cell where they undergo lysosomal degradation in the Schwann cell cytoplasm (Song et al., 2008). Thus, Schwann cells appear to play an essential role in at least the clearance of axonal debris, a feature that appears to be conserved at the fly NMJ (Fuentes-Medel et al., 2009).
An unresolved question is whether axosomes are shed cell-autonomously by the retracting axon, or whether the ensheathing Schwann cell may contribute to the formation or “pinching off” of these structures in the losing axon branch. Some imaging evidence reveals thin Schwann cell processes around budding axosomes, suggesting glia might play an active role in axosome formation and/or shedding as they are engulfed (Bishop et al., 2004). Demonstrating such an active role is technically challenging due to the requirement of Schwann cell ensheathment for axon survival (Koirala et al., 2003). Schwann cell ablation does result in loss of nerve terminals, suggesting that retraction can take place in the absence of glia, but whether retraction in this situation is similar to activity-induced pruning is unclear (Reddy et al., 2003)—perhaps in the absence of Schwann cells axon terminals simply degenerate due to lack of support. Experiments in which Schwann cell endocytosis or membrane dynamics can be acutely disrupted during dual-color live imaging will likely be required to definitively resolve this question. Moreover, such an active role in eliminating weak synapses should require that Schwann cells are capable of discriminating and evaluating the relative synaptic strength of multiple inputs so that they target the correct input for elimination. A recent study using simultaneous glial calcium imaging and neuronal electrophysiological recording demonstrates that perisynaptic Schwann cells are capable of “sensing” the relative activity levels of each synapse at a multiply innervated NMJ. An individual PSC contacting two NMJs displays stronger calcium responses to “stronger” than to “weaker” synapses, an effect mediated by purinergic receptors on the glial cells (Darabid et al., 2013). If and how this responsiveness might affect synaptic competition or branch retraction awaits future studies in which glial responses are perturbed and the effects on innervation determined.
In Drosophila, axon pruning during metamorphosis is important for the remodeling required to form mature adult circuits. Mushroom body γ-neurons prune their larval axons during early metamorphosis, followed by formation of new adult-specific connections as metamorphosis continues (Fig. 2; Lee et al., 1999). In addition to many cell-autonomous requirements for proper axon pruning, there are multiple essential roles for neighboring glia in mediating axon removal in this circuit. Initiation of pruning is triggered by a pulse of the molting hormone ecdysone just before metamorphosis (Lee et al., 2000). γ-Neuron responsiveness to this critical signal is controlled by surrounding cortex and astrocyte-like glia, which express the TGF-β ligand Myoglianin (Myo) during late larval stages (Awasaki et al., 2011). Glial-derived Myo must activate TGF-β signaling within γ-neurons to up-regulate expression of the ecdysone receptor B1 isoform (EcR-B1) so that γ-neurons can receive the hormonal signal that will trigger pruning (Zheng et al., 2003; Awasaki et al., 2011). Myo therefore represents perhaps the first glial-derived factor required for neurons to become competent to prune their axons (Fig. 2 A).
Figure 2.
Developmental pruning of mushroom body γ-neuron axons requires glia. (A) Before metamorphosis, cortex and astrocyte-like glia secrete the TGF-β ligand Myoglianin, which acts through γ-neuron baboon receptors to up-regulate expression of the ecdysone receptor EcR-B1. This makes γ-neurons competent to respond to the ecdysone pulse that will initiate pruning. (B) As metamorphosis begins, glia infiltrate the mushroom body neuropil. This infiltration is dependent on the glial cell surface receptor Draper. Arrival of glia coincides with axon blebbing and fragmentation, but it remains uncertain whether glia actively promote this fragmentation. (C) Glia are required for clearance of axonal debris. Recognition and phagocytosis of the debris is also mediated by the glial cell surface receptor Draper.
γ-Neuron axons are pruned by local degeneration in which specific axonal branches fragment in place and are subsequently cleared by glia cells in a process primarily mediated by the glial cell surface receptor Draper (Fig. 2, B and C; Watts et al., 2003, 2004; Awasaki and Ito, 2004; Awasaki et al., 2006). Although glial clearance and degradation of axonal debris is required for complete pruning, it remains unclear if engulfing glia play active roles in promoting degeneration and fragmentation of pruned axons, or whether they simply respond to and clear cell-autonomously fragmented axons. Preventing glial infiltration by blocking membrane dynamics strongly suppresses axon pruning, providing support for an active role for glia in driving pruning (Awasaki and Ito, 2004). However, these results are inconclusive because the experiments lacked sufficient resolution to distinguish whether axons had been broken down but not cleared, or remained completely intact. Additional evidence in support of an active role is that glia infiltrate the mushroom body lobes before any observable fragmentation and can even infiltrate if fragmentation is suppressed via genetic mutations (Watts et al., 2004). Subsequent work showed that glial infiltration is dependent on the engulfment receptor Draper (Awasaki et al., 2006). Draper is believed to recruit engulfing cells to dying cells by responding to an unidentified “eat me” signal (Zhou et al., 2001; MacDonald et al., 2006). The requirement of Draper for infiltration, therefore, suggests glia are responding to axon-derived signals that may be generated independently of, or before, fragmentation. Microtubule disorganization occurs before fragmentation and can occur even in the absence of glial infiltration (Awasaki et al., 2006), suggesting that axons are already committed to pruning before visible fragmentation. Together, these findings suggest that initiation of axon degeneration (as defined by microtubule breakdown) may be cell-autonomous to neurons, but does not rule out a role for glia in driving fragmentation in addition to engulfment of debris. Live imaging experiments taking advantage of new techniques to independently label and genetically manipulate engulfing glia and mushroom body γ-neurons with single-cell resolution (Lai and Lee, 2006; Potter et al., 2010) will likely be necessary to fully resolve the role of glia in this process. If glia are indeed promoting axonal degeneration, the next exciting question would be—how do they choose which axons to destroy?
Glia promote synapse formation
A key step in the formation of a functional nervous system is the establishment of appropriate synaptic connections between neurons. Unraveling how neurons make appropriate synaptic connections has been a major focus of neurodevelopment research for decades, and we now understand that synaptogenesis is a multistep process including recognition and selection of appropriate targets, assembly and localization of synaptic components, and stabilization of appropriate connections. Research in the last decade has identified important roles for glia in many of these steps.
An important breakthrough was the development of methods to isolate and maintain purified neurons in culture in the absence of glia. Pfrieger and Barres (1997) developed a technique to purify retinal ganglion cell (RGC) neurons and keep them healthy in culture without glia. Intriguingly, they found that purified RGCs cultured alone formed sevenfold fewer functional excitatory synapses than RGCs cultured with astrocyte feeder layers as assayed by electrophysiological recordings, immunostaining for synaptic protein localization, and EM analysis (Pfrieger and Barres, 1997; Nägler et al., 2001; Ullian et al., 2001). Subsequent in vitro studies with other neuronal subtypes demonstrated that these findings were not just specific to rat RGCs (Peng et al., 2003; Ullian et al., 2004; Cao and Ko, 2007). Hints that astrocytes might play similar synaptogenic roles in vivo first came from observations that timing of synaptogenesis between RGCs and their in vivo targets correlated with glial infiltration of the area, even though RGC axons had reached their target region several days prior (Ullian et al., 2001).
In culture, the synapse-promoting effects of glia did not require physical contact between the glia and neurons, implicating secreted factors as the primary mediators of glia-induced synaptogenesis (Fig. 3). Fractionation of astrocyte-conditioned media (ACM) was used to identify thrombospondins (TSPs)—extracellular matrix proteins with known roles in cell adhesion—as key glial-derived synaptogenic factors (Fig. 3 A; Christopherson et al., 2005). Purified TSP-1 or TSP-2 mimicked ACM to promote structural synapse formation, whereas depleting TSP-2 from ACM abrogated its synaptogenic effects on cultured neurons. With a specific molecule in hand, researchers could now ask whether a glial-derived molecule was required for synaptogenesis in vivo. Christopherson et al. (2005) showed that TSPs are expressed in astrocytes in vivo when synapses are forming and colocalize with synapse-associated astrocyte processes. Importantly, mice mutant for both TSP-1 and TSP-2 had significantly fewer synapses than wild-type animals (∼40% reduction at P8), demonstrating a requirement for an astrocyte-derived factor for normal synapse development in vivo (Christopherson et al., 2005).
Figure 3.
Model of astrocyte–neuron interactions during synapse formation and maturation. (A) Astrocytes secrete several factors to promote structural synapse formation. Astrocytes secrete thrombospondins (TSPs), which act through α2–δ1 calcium channel subunit/gabapentin receptors to drive formation of structurally intact glutamatergic synapses. The exact mechanism by which TSP binding to α2–δ1 promotes adhesion and structural synapse formation is unknown. Astrocytes also secrete hevin and SPARC. Hevin also promotes structural synapse formation, whereas SPARC antagonizes this function. Competition between hevin and SPARC for a common unknown binding partner on neurons may explain the antagonism. The synapses formed in response to TSPs or hevin are structurally intact with docked vesicles and PSD-95 correctly localized; however, they lack surface expression of AMPARs at the postsynapse and are therefore not fully functional. (B) Other astrocyte-secreted factors influence surface expression and clustering of glutamatergic AMPARs. Glypicans secreted from astrocytes act through an unidentified receptor to promote surface expression and clustering of AMPARs during development. Activity can also induce astrocytes to secrete SPARC, which can perturb integrin interactions with surface AMPARs, leading to decreased surface expression of AMPARs in response to excess activity.
In an elegant follow-up study, Eroglu et al. (2009) identified the α2–δ1 calcium channel subunit/gabapentin receptor as the neuronal receptor for glial TSPs, which bind the receptor through their EGF-like domains. α2–δ1 colocalizes with both pre- and postsynaptic puncta, and in vitro overexpression experiments suggest it functions postsynaptically. The intracellular mechanisms by which TSP/α2–δ1 signaling promotes synapse assembly remains an open question, though α2–δ1 modulation of calcium channel function is not likely to be involved because perturbation of calcium channel expression or function is unable to interfere with TSP- or astrocyte-induced synaptogenesis (Eroglu et al., 2009).
The extracellular matrix protein hevin is a second astrocyte-derived factor capable of inducing structural synapse formation in vitro (Fig. 3 A). Although purified hevin works just as well as TSPs at inducing glutamatergic synapses in culture, depletion of TSPs from ACM completely abolishes the synaptogenic activity of ACM despite the presence of hevin (Christopherson et al., 2005; Kucukdereli et al., 2011), implying that there might be ACM components that can antagonize hevin function. This inhibitory factor was identified as SPARC (secreted protein acidic and rich in cysteine), a hevin homologue that is also highly expressed in ACM. SPARC selectively interferes with hevin’s synapse-promoting ability, likely by competing with hevin for an as-yet-unidentified common binding partner on neurons. Hevin and SPARC are coexpressed in vivo, with peak expression in the superior colliculus at a time when RGCs are actively forming synapses. hevin mutant mice have reduced, and SPARC mutant mice have increased, RGC-collicular synapses, supporting antagonistic roles for these proteins in vivo. The overall positive effect of synaptogenesis in this circuit during development is likely due to a high hevin/SPARC ratio. (This ratio is low in ACM.) Expression of hevin and SPARC is maintained into adulthood, unlike TSPs (Christopherson et al., 2005), indicating that hevin and SPARC might also play changing roles in synapse maintenance and plasticity throughout life, potentially through dynamic regulation of their relative levels.
Although TSPs and hevin play critical roles in promoting synapse formation, these molecules are only capable of promoting structural assembly but not functional maturation of synapses (Christopherson et al., 2005; Eroglu et al., 2009; Kucukdereli et al., 2011). Synapses formed in response to TSPs and hevin are “silent” synapses: synapses that look ultrastructurally normal, but lack postsynaptic AMPA glutamate receptors (AMPARs), rendering them incapable of neurotransmission. Astrocytes or ACM are sufficient to induce the formation of functional synapses, as well as strengthen newly formed synapses by increasing the amplitude and frequency of miniature excitatory postsynaptic potentials (mEPSPs; Ullian et al., 2001). Thus, there must be additional glia-derived factors that work in conjunction with TSPs and hevin to specifically instruct the addition of AMPARs at the postsynapse. This was an outstanding question in the field until the glypicans Gpc-4 and Gpc-6, which are part of the heparan sulfate proteoglycan family, were identified as ACM factors that fulfill this role (Fig. 3 B; Allen et al., 2012). When supplied to isolated neurons in conjunction with TSPs, Gpc-4 and Gpc-6 were each sufficient to increase synaptic activity in cultured RGCs, primarily by increasing surface expression and clustering of postsynaptic AMPAR GluA1 subunits. Synapse function is impaired in gpc-4 knockout mice, as evidenced by reductions in the amplitude of mEPSPs in hippocampal slices, supporting an in vivo role for glypicans in promoting functional synapse formation (Allen et al., 2012). The mechanism by which glypicans induce surface expression and clustering of GluA1 AMPARs is still unknown. In other contexts, glypicans have roles in concentrating morphogens to enhance signaling pathways, but examination of several glypican interactors (including FGFs, Sonic hedgehog, and Wnts) failed to show roles for these molecules in AMPAR regulation. This suggests that Gpc-4 and Gpc-6 might act directly on a postsynaptic receptor to mediate their effects. Identification of the synaptic glypican receptor or binding partner, and of additional molecules that regulate the surface expression and clustering of AMPAR subunits, will be important for determining how astrocytes contribute to synaptic maturation and the plasticity of neural circuits in vivo.
It is unlikely the above-described molecules represent the sum of pro-synaptogenic factors supplied by glia. For example, recent work on inhibitory GABAergic synapse formation indicates the effect of TSPs is specific to glutamatergic synapses, having virtually no effect on inhibitory synapse development. Nonetheless, ACM is required for normal inhibitory synapse development (Hughes et al., 2010); thus, ACM must contain additional synaptogenic factor(s) that can affect inhibitory synapses that have yet to be identified. In addition, astrocytes are not the only glial cell type to support synapse formation—peripheral glia also promote NMJ synapse formation and growth in vertebrates and Drosophila through secretion of TGF-β ligands. Schwann cell–derived TGF-β1 can drive synapse formation in Xenopus motor neuron/muscle co-cultures by acting on presynaptic neurons to increase expression of a protein called agrin, which is required to cluster acetylcholine receptors at neuron/muscle contact sites (Feng and Ko, 2008). In Drosophila, the role of glial TGF-β signaling on synapse growth is distinct. In this system, glia express the TGF-β ligand Maverick (Mav), which acts on postsynaptic muscle to modulate muscle release of a distinct TGF-β ligand (Glass bottom boat) that acts directly on neurons to drive synaptic growth (Fuentes-Medel et al., 2012). Thus, although a synaptogenic role for glia cells is highly conserved, diverse mechanisms exist to drive this function that may be specific to species, glial cell type, or synapse type.
Several important questions emerge from these studies. First, are the signals discussed above nonspecific permissive signals that allow synapses to form with specificity being driven by entirely distinct factors, or are they capable of selectively driving the formation of specific, appropriate synapses in vivo? Implication of mainly secreted factors as mediators of synapse promotion from in vitro studies suggests the former. There are some studies, however, that have demonstrated close association and dynamic interactions of glial processes and developing synaptic spines that suggest that glia can and do act in synapse-specific ways as well. Whether similar or different molecular mechanisms are involved in these interactions remains to be determined.
A second question is how glial synapse promotion is regulated and modulated during and after development. The effects of TSPs and hevin are robust in vitro, and aside from controlling the general timing of expression or the coexpression SPARC and hevin (mechanisms that seem relatively imprecise and slow), it remains unclear how synapse formation is regulated, particularly in vivo both during development and throughout life. Most glial cells are capable of sensing and responding to neural activity, but how activity affects the production or secretion of synaptogenic factors (particularly before synapse formation) has not been fully resolved. It has been recently reported that SPARC is up-regulated in astrocytes in response to neural activity. In this context though, SPARC seems to have a distinct primary function from inhibiting hevin—negative regulation of AMPAR levels at synapses via inhibition of neuronal β-integrin function (Fig. 3 B; Jones et al., 2011). Thus, SPARC appears to play at least two distinct roles in synapse development and plasticity at different times. At early stages, SPARC limits the number of synapses by abrogating hevin function before synapse assembly. After synapses have formed, SPARC can refine synaptic strength by interfering with integrin localization and function that normally serves to stabilize AMPARs at the membrane. Thus, production of SPARC in response to activity can indirectly limit AMPAR clustering at highly active synapses (Jones et al., 2011). Glial regulation of synapse formation, function, and plasticity throughout life is likely to be a rich and fruitful area of future study.
Finally, we need to understand how these astrocyte-derived pro-synaptogenic factors function in vivo to coordinate synapse formation and plasticity in different brain regions. This is clearly a complex issue—for example, TSP knockout mice have an ∼40% reduction in cortical synapses and hevin knockout mice show an ∼35% reduction in collicular synapses. These observations indicate that there are likely additional partially redundant glial-derived factors yet to be discovered. Determining how these glial signals interact or synergize with the molecular cues required between pre- and postsynaptic neurons in vivo will be an important future goal.
Glia prune exuberant synaptic connections
Activity-dependent mechanisms ensure that appropriate connections are strengthened while inappropriate or weak connections are eliminated, but the cellular and molecular mechanisms that underlie experience-dependent synapse elimination are largely unknown. A flurry of research over the last several years has identified microglia as key mediators of developmental synaptic pruning by phagocytosing excessive presynaptic inputs.
Microglia were once assumed to be relatively quiescent in healthy brains, but advances in live imaging techniques have revealed “resting” microglia to be highly active cells that constantly extend and retract their processes to make contact with neighboring neurons, glia, and synapses (Davalos et al., 2005; Nimmerjahn et al., 2005; Wake et al., 2009). Moreover, microglia activity changes in response to perturbations in neurotransmission, indicating that microglia might be actively monitoring local neural activity, perhaps playing a role in activity-dependent plasticity. High-resolution imaging studies combining live imaging with electron microscopy soon revealed that microglia made frequent contact with dendritic spines during periods of plasticity and remodeling. This contact has consequences on spine morphology and stability with microglia contact frequently predicting the loss of individual spines (Tremblay et al., 2010).
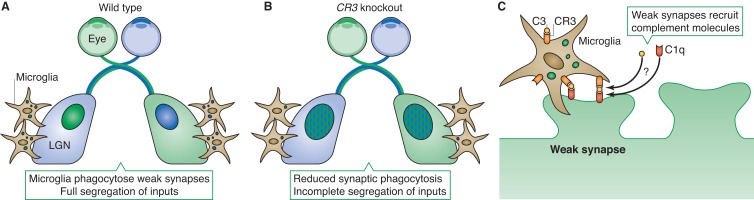
Evidence of synaptic elements contained within microglia cytoplasm combined with the known role of microglia as phagocytes, and a report implicating the classical complement cascade with synaptic pruning provided highly suggestive evidence that microglia were involved in activity-dependent synapse elimination via phagocytosis (Stevens et al., 2007; Tremblay et al., 2010; Paolicelli et al., 2011; Schafer et al., 2012). In an elegant study, Schafer et al. (2012) used the mammalian retinogeniculate system to clearly demonstrate the in vivo role for microglia in mediating activity-dependent synaptic pruning that relies on signaling from the classical complement system. This circuit undergoes a well-characterized period of robust activity-dependent synaptic elimination during postnatal development with a clear readout. RGC axons from both eyes form synapses in the lateral geniculate nucleus (LGN) of the thalamus. Due to exuberant connectivity, distinct ipsilateral and contralateral eye domains are poorly defined until approximately postnatal day 5, at which point activity-dependent synaptic pruning results in clearly segregated eye-specific domains (Fig. 4 A). Injecting unique fluorescent anterograde tracers in each eye labels RGC axons and synapses independently, allowing a clear readout of the success of activity-dependent synaptic pruning (Huberman et al., 2008). GFP-expressing microglia in the LGN were closely associated with synapses, and were often found with tracer-labeled presynaptic membrane within their processes and soma during the period (∼P5) of robust synaptic pruning, similar to previous findings in the developing cortex and hippocampus (Tremblay et al., 2010; Paolicelli et al., 2011).
Figure 4.
Microglia refine circuits by complement-mediated phagocytosis of weak synapses. Schematic of retinogeniculate connectivity in wild-type (A) and complement receptor CR3 knockout (B) animals. Inputs from each eye are completely segregated in the LGNs of wild-type animals (A, separate blue and green regions). Synaptic debris from inappropriate connections is observed within local microglia (brown cells) during the pruning process. In CR3 knockout animals (B), segregation of inputs in the LGN is incomplete, resulting in regions with overlapping inputs from both eyes (blue/green regions). Significantly less synaptic debris is observed in microglia in these animals. (C) Proposed model for complement-dependent synaptic pruning by microglia. Weak synapses are tagged for elimination by recruitment of the classical complement molecule C1q. C1q can recruit and activate C3, which then serves as a ligand for the CR3 receptor (expressed exclusively by microglia) to recruit microglia to clear the synapse. How C1q and C3 are localized to and activated at weak synapses is still unknown.
To test whether microglia were indeed ingesting synapses destined for elimination, the authors injected one eye with tetrodotoxin (TTX) to create a strong imbalance of activity between the RGCs from each eye. As expected, the authors found significantly more engulfed synapses that originated from the TTX-injected eye as compared with the control-injected eye, indicating that microglia were pruning synapses in an activity-dependent manner. A wealth of anatomical data supported the idea that microglia engulf weak synapses for elimination, but what were the molecular mechanisms that controlled this engulfment? A good candidate came from an earlier study that identified the classical complement components C1q and C3 as synaptically localized molecules required for activity-dependent pruning of retinogeniculate synapses (Stevens et al., 2007). As microglia are the only cell type in the brain that express the complement receptor CR3, it was hypothesized that binding between synaptic C3 and microglial CR3 might mediate synapse–microglia interactions required for engulfment (Fig. 4 C). To test this hypothesis, Schafer et al. (2012) examined CR3 knockout mice and found that CR3-deficient microglia showed an ∼50% reduction in phagocytosed presynaptic material. This in turn resulted in incomplete pruning, as evidenced by incomplete segregation of eye-specific domains in the LGN, and demonstrated a clear role for complement signaling in microglia pruning of synapses (Fig. 4 B).
Among the key questions this work raises is how weak synapses are tagged for removal by the complement system. How the diffusible molecules C1q and activated C3 are localized to or activated at specific synapses is unclear, so the identification of complement system binding partners, inhibitors, and activators at synapses and determining how they are regulated by neuronal activity will be an important focus of future studies (Stevens et al., 2007). In addition, although complement signaling plays a major role in synaptic pruning, loss of complement signaling does not completely prevent pruning, causing only an ∼50% reduction. Thus, it is likely that other molecules and signaling pathways contribute to microglial synaptic pruning. One candidate pathway suggested by studies in fly is that of Draper (Megf10 in mouse), which has been shown to mediate developmental axon pruning (Awasaki et al., 2006) and can function in peripheral glia and muscle to mediate engulfment of shed synapses at the NMJ (Fuentes-Medel et al., 2009). This study from Drosophila also elegantly demonstrates the importance of synaptic clearance by glia. Failure to clear synaptic debris leads to severe defects in synapse growth at the NMJ, indicating that the elimination of synaptic debris by glia is an important event required for synaptic plasticity (Fuentes-Medel et al., 2009).
In addition to synaptic pruning by microglia, other glial subtypes play important roles in synaptic plasticity. For example, as discussed above, astrocyte-secreted factors including SPARC can influence synaptic plasticity. SPARC expression in the postnatal hippocampus is regulated by activity. SPARC affects clustering and stabilization of glutamate receptor subunits at hippocampal synapses during postnatal development, and is required for normal synaptic plasticity during this time (Jones et al., 2011). Visual cortex plasticity is also strongly affected by glia, for example implantation of immature astrocytes into the brains of older animals can restore ocular dominance plasticity (Müller and Best, 1989). Maturation of oligodendrocytes might also affect the duration of ocular dominance plasticity via releasing factors that bind to neuronal Nogo receptors, as knockout of these receptors can lead to an extension of the plastic period in mice (McGee et al., 2005). These are just a few of the contributions of different glial subtypes to activity-dependent plasticity throughout development (and into adulthood) that have been described thus far. Continued work in this field should reveal many more unexpected roles for glia in modulating plasticity and activity in neural circuits.
Conclusions and future directions
There has been remarkable progress in our understanding of how glia function in the developing nervous system to shape neuronal connectivity. In particular, a number of cellular and molecular mechanisms by which glia regulate neural stem cell biology, and neural fate, survival, and connectivity have begun to be elucidated, but there is undoubtedly much more for us to learn about these remarkable cells. Exciting and fundamental questions about neuron–glia interactions await exploration and molecular or functional insight. For example, determining whether synaptogenic signals from astrocytes are perceived as permissive or instructive will be important, and if they are instructive for specific connections, how is specificity embedded in the message? In the context of neural circuit refinement we need to understand cell–cell communication events and how they are integrated with circuit function. How are “loser” synapses that need to be eliminated molecularly tagged? How does neuronal activity regulate glial pruning function or synaptic tagging during synapse elimination? Are microglia the only glia pruning synapses in mammals? What are the functional consequences of failed pruning in different brain regions? More broadly, we must explore how neural activity shapes glial developmental events, or neuron–glia interactions. It is well known that many types of glia express neurotransmitter receptors that could allow them to “listen in” on neuronal activity if they are in close contact with synapses. What are these used for? Is this a primary way that neurons signal to glia? If so, how does neurotransmitter signaling to glia shape their biology?
In contrast to the growing list of astrocyte and microglial functions during embryonic and postnatal development, relatively little is known about specific roles for oligodendrocytes or their precursors (NG2+ cells) in the developing nervous system. Oligodendrocytes mediate myelination during the latest stages of development, though the cellular interactions between axons and glia that mediate myelination are still largely unknown. In vivo ablation studies indicate that loss of oligodendrocytes from the developing cerebellum near the onset of myelination disrupts overall cellular architecture and connectivity and alters the gene expression patterns of neurons, suggesting there may be a number of as-yet-identified functions for these cells during development (Mathis et al., 2003; Doretto et al., 2011). Intriguingly, NG2+ OPCs receive synapses from neurons during development (Bergles et al., 2000). Why these cells receive direct synaptic input is unclear, as is the exact function of NG2+ OPCs, but they are seemingly poised to respond rapidly to neural activity via their direct synaptic connections and generate new oligodendrocytes (De Biase et al., 2010; Mangin et al., 2012). Determining what these cells do in the developing and mature brain is a major question for the field in the future.
Finally, although most communication between neurons and glia is likely bi-directional, many of our current models have elucidated only one half of the conversation. In several examples discussed above we have some knowledge of a glial-derived molecule, but many receptors and downstream signaling pathways remain to be identified. It is also notable that the expression of many glial-derived signals often changes over developmental time (e.g., TSPs and Gpcs). Is this the result of intrinsic changes in glial pro-synaptic potential, or is it the result of feedback signaling from neurons? Misregulation of several of the processes and/or molecules discussed above that modulate neural circuit construction are associated with disease. Understanding these functions more deeply and how they are altered in disease may be crucial for generating new therapeutic strategies to program appropriate levels of neural circuit plasticity.
Acknowledgments
M.R. Freeman is an Investigator of the Howard Hughes Medical Institute. Work in the Freeman laboratory is supported by National Institutes of Health grant RO1 NS053538.
Illustrations were provided by Neil Smith, www.neilsmithillustration.co.uk.
Footnotes
Abbreviations used in this paper:
- ACM
- astrocyte-conditioned media
- AMPAR
- AMPA receptor
- dILP
- Drosophila insulin-like peptide
- LGN
- lateral geniculate nucleus
- NMJ
- neuromuscular junction
- OPC
- oligodendrocyte precursor cell
- PCD
- programmed cell death
- RGC
- retinal ganglion cell
- ROS
- reactive oxygen species
- SPARC
- secreted protein acidic and rich in cysteine
- TSP
- thrombospondin
References
- Allen N.J., Bennett M.L., Foo L.C., Wang G.X., Chakraborty C., Smith S.J., Barres B.A. 2012. Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature. 486:410–414 10.1038/486473e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony J.M., Paquin A., Nutt S.L., Kaplan D.R., Miller F.D. 2011. Endogenous microglia regulate development of embryonic cortical precursor cells. J. Neurosci. Res. 89:286–298 10.1002/jnr.22533 [DOI] [PubMed] [Google Scholar]
- Awasaki T., Ito K. 2004. Engulfing action of glial cells is required for programmed axon pruning during Drosophila metamorphosis. Curr. Biol. 14:668–677 10.1016/j.cub.2004.04.001 [DOI] [PubMed] [Google Scholar]
- Awasaki T., Tatsumi R., Takahashi K., Arai K., Nakanishi Y., Ueda R., Ito K. 2006. Essential role of the apoptotic cell engulfment genes draper and ced-6 in programmed axon pruning during Drosophila metamorphosis. Neuron. 50:855–867 10.1016/j.neuron.2006.04.027 [DOI] [PubMed] [Google Scholar]
- Awasaki T., Huang Y., O’Connor M.B., Lee T. 2011. Glia instruct developmental neuronal remodeling through TGF-β signaling. Nat. Neurosci. 14:821–823 10.1038/nn.2833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles D.E., Roberts J.D., Somogyi P., Jahr C.E. 2000. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 405:187–191 10.1038/35012083 [DOI] [PubMed] [Google Scholar]
- Bishop D.L., Misgeld T., Walsh M.K., Gan W.B., Lichtman J.W. 2004. Axon branch removal at developing synapses by axosome shedding. Neuron. 44:651–661 10.1016/j.neuron.2004.10.026 [DOI] [PubMed] [Google Scholar]
- Britton J.S., Edgar B.A. 1998. Environmental control of the cell cycle in Drosophila: nutrition activates mitotic and endoreplicative cells by distinct mechanisms. Development. 125:2149–2158 [DOI] [PubMed] [Google Scholar]
- Callan M.A., Clements N., Ahrendt N., Zarnescu D.C. 2012. Fragile X Protein is required for inhibition of insulin signaling and regulates glial-dependent neuroblast reactivation in the developing brain. Brain Res. 1462:151–161 10.1016/j.brainres.2012.03.042 [DOI] [PubMed] [Google Scholar]
- Cao G., Ko C.P. 2007. Schwann cell-derived factors modulate synaptic activities at developing neuromuscular synapses. J. Neurosci. 27:6712–6722 10.1523/JNEUROSCI.1329-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanock S.J., el Benna J., Smith R.M., Babior B.M. 1994. The respiratory burst oxidase. J. Biol. Chem. 269:24519–24522 [PubMed] [Google Scholar]
- Chell J.M., Brand A.H. 2010. Nutrition-responsive glia control exit of neural stem cells from quiescence. Cell. 143:1161–1173 10.1016/j.cell.2010.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotard C., Salecker I. 2004. Neurons and glia: team players in axon guidance. Trends Neurosci. 27:655–661 10.1016/j.tins.2004.09.001 [DOI] [PubMed] [Google Scholar]
- Christopherson K.S., Ullian E.M., Stokes C.C., Mullowney C.E., Hell J.W., Agah A., Lawler J., Mosher D.F., Bornstein P., Barres B.A. 2005. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 120:421–433 10.1016/j.cell.2004.12.020 [DOI] [PubMed] [Google Scholar]
- Colman H., Nabekura J., Lichtman J.W. 1997. Alterations in synaptic strength preceding axon withdrawal. Science. 275:356–361 10.1126/science.275.5298.356 [DOI] [PubMed] [Google Scholar]
- Cunningham C.L., Martínez-Cerdeño V., Noctor S.C. 2013. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J. Neurosci. 33:4216–4233 10.1523/JNEUROSCI.3441-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darabid H., Arbour D., Robitaille R. 2013. Glial cells decipher synaptic competition at the mammalian neuromuscular junction. J. Neurosci. 33:1297–1313 10.1523/JNEUROSCI.2935-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D., Grutzendler J., Yang G., Kim J.V., Zuo Y., Jung S., Littman D.R., Dustin M.L., Gan W.B. 2005. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 8:752–758 10.1038/nn1472 [DOI] [PubMed] [Google Scholar]
- De Biase L.M., Nishiyama A., Bergles D.E. 2010. Excitability and synaptic communication within the oligodendrocyte lineage. J. Neurosci. 30:3600–3611 10.1523/JNEUROSCI.6000-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekkers M.P.J., Nikoletopoulou V., Barde Y.A. 2013. Death of developing neurons: New insights and implications for connectivity. J. Cell Biol. 203:385–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F., Caillé I., Lim D.A., García-Verdugo J.M., Alvarez-Buylla A. 1999. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 97:703–716 10.1016/S0092-8674(00)80783-7 [DOI] [PubMed] [Google Scholar]
- Doretto S., Malerba M., Ramos M., Ikrar T., Kinoshita C., De Mei C., Tirotta E., Xu X., Borrelli E. 2011. Oligodendrocytes as regulators of neuronal networks during early postnatal development. PLoS ONE. 6:e19849 10.1371/journal.pone.0019849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebens A.J., Garren H., Cheyette B.N., Zipursky S.L. 1993. The Drosophila anachronism locus: a glycoprotein secreted by glia inhibits neuroblast proliferation. Cell. 74:15–27 10.1016/0092-8674(93)90291-W [DOI] [PubMed] [Google Scholar]
- Eroglu Ç., Allen N.J., Susman M.W., O’Rourke N.A., Park C.Y., Özkan E., Chakraborty C., Mulinyawe S.B., Annis D.S., Huberman A.D., et al. 2009. Gabapentin receptor α2δ-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 139:380–392 10.1016/j.cell.2009.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z., Ko C.P. 2008. Schwann cells promote synaptogenesis at the neuromuscular junction via transforming growth factor-beta1. J. Neurosci. 28:9599–9609 10.1523/JNEUROSCI.2589-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes-Medel Y., Logan M.A., Ashley J., Ataman B., Budnik V., Freeman M.R. 2009. Glia and muscle sculpt neuromuscular arbors by engulfing destabilized synaptic boutons and shed presynaptic debris. PLoS Biol. 7:e1000184 10.1371/journal.pbio.1000184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes-Medel Y., Ashley J., Barria R., Maloney R., Freeman M., Budnik V. 2012. Integration of a retrograde signal during synapse formation by glia-secreted TGF-β ligand. Curr. Biol. 22:1831–1838 10.1016/j.cub.2012.07.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F., Greter M., Leboeuf M., Nandi S., See P., Gokhan S., Mehler M.F., Conway S.J., Ng L.G., Stanley E.R., et al. 2010. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 330:841–845 10.1126/science.1194637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa T., Ohsawa K., Imai Y., Ondo Y., Akazawa C., Uchino S., Kohsaka S. 2005. Visualization of microglia in living tissues using Iba1-EGFP transgenic mice. J. Neurosci. Res. 81:357–362 10.1002/jnr.20480 [DOI] [PubMed] [Google Scholar]
- Hoeppner D.J., Hengartner M.O., Schnabel R. 2001. Engulfment genes cooperate with ced-3 to promote cell death in Caenorhabditis elegans. Nature. 412:202–206 10.1038/35084103 [DOI] [PubMed] [Google Scholar]
- Huberman A.D., Feller M.B., Chapman B. 2008. Mechanisms underlying development of visual maps and receptive fields. Annu. Rev. Neurosci. 31:479–509 10.1146/annurev.neuro.31.060407.125533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes E.G., Elmariah S.B., Balice-Gordon R.J. 2010. Astrocyte secreted proteins selectively increase hippocampal GABAergic axon length, branching, and synaptogenesis. Mol. Cell. Neurosci. 43:136–145 10.1016/j.mcn.2009.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E.V., Bernardinelli Y., Tse Y.C., Chierzi S., Wong T.P., Murai K.K. 2011. Astrocytes control glutamate receptor levels at developing synapses through SPARC-beta-integrin interactions. J. Neurosci. 31:4154–4165 10.1523/JNEUROSCI.4757-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koirala S., Reddy L.V., Ko C.P. 2003. Roles of glial cells in the formation, function, and maintenance of the neuromuscular junction. J. Neurocytol. 32:987–1002 10.1023/B:NEUR.0000020637.71452.3c [DOI] [PubMed] [Google Scholar]
- Kucukdereli H., Allen N.J., Lee A.T., Feng A., Ozlu M.I., Conatser L.M., Chakraborty C., Workman G., Weaver M., Sage E.H., et al. 2011. Control of excitatory CNS synaptogenesis by astrocyte-secreted proteins Hevin and SPARC. Proc. Natl. Acad. Sci. USA. 108:E440–E449 10.1073/pnas.1104977108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai S.L., Lee T. 2006. Genetic mosaic with dual binary transcriptional systems in Drosophila. Nat. Neurosci. 9:703–709 10.1038/nn1681 [DOI] [PubMed] [Google Scholar]
- Lee T., Lee A., Luo L. 1999. Development of the Drosophila mushroom bodies: sequential generation of three distinct types of neurons from a neuroblast. Development. 126:4065–4076 [DOI] [PubMed] [Google Scholar]
- Lee T., Marticke S., Sung C., Robinow S., Luo L. 2000. Cell-autonomous requirement of the USP/EcR-B ecdysone receptor for mushroom body neuronal remodeling in Drosophila. Neuron. 28:807–818 10.1016/S0896-6273(00)00155-0 [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. 1987. The nerve growth factor: thirty-five years later. EMBO J. 6:1145–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald J.M., Beach M.G., Porpiglia E., Sheehan A.E., Watts R.J., Freeman M.R. 2006. The Drosophila cell corpse engulfment receptor Draper mediates glial clearance of severed axons. Neuron. 50:869–881 10.1016/j.neuron.2006.04.028 [DOI] [PubMed] [Google Scholar]
- Mangin J.M., Li P., Scafidi J., Gallo V. 2012. Experience-dependent regulation of NG2 progenitors in the developing barrel cortex. Nat. Neurosci. 15:1192–1194 10.1038/nn.3190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín-Teva J.L., Dusart I., Colin C., Gervais A., van Rooijen N., Mallat M. 2004. Microglia promote the death of developing Purkinje cells. Neuron. 41:535–547 10.1016/S0896-6273(04)00069-8 [DOI] [PubMed] [Google Scholar]
- Mathis C., Collin L., Borrelli E. 2003. Oligodendrocyte ablation impairs cerebellum development. Development. 130:4709–4718 10.1242/dev.00675 [DOI] [PubMed] [Google Scholar]
- McGee A.W., Yang Y., Fischer Q.S., Daw N.W., Strittmatter S.M. 2005. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 309:2222–2226 10.1126/science.1114362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mócsai A., Abram C.L., Jakus Z., Hu Y., Lanier L.L., Lowell C.A. 2006. Integrin signaling in neutrophils and macrophages uses adaptors containing immunoreceptor tyrosine-based activation motifs. Nat. Immunol. 7:1326–1333 10.1038/ni1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrens J., Van Den Broeck W., Kempermann G. 2012. Glial cells in adult neurogenesis. Glia. 60:159–174 10.1002/glia.21247 [DOI] [PubMed] [Google Scholar]
- Müller C.M., Best J. 1989. Ocular dominance plasticity in adult cat visual cortex after transplantation of cultured astrocytes. Nature. 342:427–430 10.1038/342427a0 [DOI] [PubMed] [Google Scholar]
- Nägler K., Mauch D.H., Pfrieger F.W. 2001. Glia-derived signals induce synapse formation in neurones of the rat central nervous system. J. Physiol. 533:665–679 10.1111/j.1469-7793.2001.00665.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A., Kirchhoff F., Helmchen F. 2005. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 308:1314–1318 10.1126/science.1110647 [DOI] [PubMed] [Google Scholar]
- Noctor S.C., Flint A.C., Weissman T.A., Dammerman R.S., Kriegstein A.R. 2001. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 409:714–720 10.1038/35055553 [DOI] [PubMed] [Google Scholar]
- Paolicelli R.C., Bolasco G., Pagani F., Maggi L., Scianni M., Panzanelli P., Giustetto M., Ferreira T.A., Guiducci E., Dumas L., et al. 2011. Synaptic pruning by microglia is necessary for normal brain development. Science. 333:1456–1458 10.1126/science.1202529 [DOI] [PubMed] [Google Scholar]
- Peng H.B., Yang J.F., Dai Z., Lee C.W., Hung H.W., Feng Z.H., Ko C.P. 2003. Differential effects of neurotrophins and schwann cell-derived signals on neuronal survival/growth and synaptogenesis. J. Neurosci. 23:5050–5060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfrieger F.W., Barres B.A. 1997. Synaptic efficacy enhanced by glial cells in vitro. Science. 277:1684–1687 10.1126/science.277.5332.1684 [DOI] [PubMed] [Google Scholar]
- Potter C.J., Tasic B., Russler E.V., Liang L., Luo L. 2010. The Q system: a repressible binary system for transgene expression, lineage tracing, and mosaic analysis. Cell. 141:536–548 10.1016/j.cell.2010.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. 1971. Neuron-glia relationship during granule cell migration in developing cerebellar cortex. A Golgi and electronmicroscopic study in Macacus Rhesus. J. Comp. Neurol. 141:283–312 10.1002/cne.901410303 [DOI] [PubMed] [Google Scholar]
- Reddien P.W., Cameron S., Horvitz H.R. 2001. Phagocytosis promotes programmed cell death in C. elegans. Nature. 412:198–202 10.1038/35084096 [DOI] [PubMed] [Google Scholar]
- Reddy L.V., Koirala S., Sugiura Y., Herrera A.A., Ko C.P. 2003. Glial cells maintain synaptic structure and function and promote development of the neuromuscular junction in vivo. Neuron. 40:563–580 10.1016/S0896-6273(03)00682-2 [DOI] [PubMed] [Google Scholar]
- Schafer D.P., Lehrman E.K., Kautzman A.G., Koyama R., Mardinly A.R., Yamasaki R., Ransohoff R.M., Greenberg M.E., Barres B.A., Stevens B. 2012. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 74:691–705 10.1016/j.neuron.2012.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seri B., García-Verdugo J.M., McEwen B.S., Alvarez-Buylla A. 2001. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J. Neurosci. 21:7153–7160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A., Encinas J.M., Deudero J.J., Chancey J.H., Enikolopov G., Overstreet-Wadiche L.S., Tsirka S.E., Maletic-Savatic M. 2010. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 7:483–495 10.1016/j.stem.2010.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H., Stevens C.F., Gage F.H. 2002. Astroglia induce neurogenesis from adult neural stem cells. Nature. 417:39–44 10.1038/417039a [DOI] [PubMed] [Google Scholar]
- Song J.W., Misgeld T., Kang H., Knecht S., Lu J., Cao Y., Cotman S.L., Bishop D.L., Lichtman J.W. 2008. Lysosomal activity associated with developmental axon pruning. J. Neurosci. 28:8993–9001 10.1523/JNEUROSCI.0720-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa-Nunes R., Yee L.L., Gould A.P. 2011. Fat cells reactivate quiescent neuroblasts via TOR and glial insulin relays in Drosophila. Nature. 471:508–512 10.1038/nature09867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B., Allen N.J., Vazquez L.E., Howell G.R., Christopherson K.S., Nouri N., Micheva K.D., Mehalow A.K., Huberman A.D., Stafford B., et al. 2007. The classical complement cascade mediates CNS synapse elimination. Cell. 131:1164–1178 10.1016/j.cell.2007.10.036 [DOI] [PubMed] [Google Scholar]
- Tamamaki N., Nakamura K., Okamoto K., Kaneko T. 2001. Radial glia is a progenitor of neocortical neurons in the developing cerebral cortex. Neurosci. Res. 41:51–60 10.1016/S0168-0102(01)00259-0 [DOI] [PubMed] [Google Scholar]
- Tremblay M.È., Lowery R.L., Majewska A.K. 2010. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 8:e1000527 10.1371/journal.pbio.1000527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truman J.W., Bate M. 1988. Spatial and temporal patterns of neurogenesis in the central nervous system of Drosophila melanogaster. Dev. Biol. 125:145–157 10.1016/0012-1606(88)90067-X [DOI] [PubMed] [Google Scholar]
- Ullian E.M., Sapperstein S.K., Christopherson K.S., Barres B.A. 2001. Control of synapse number by glia. Science. 291:657–661 10.1126/science.291.5504.657 [DOI] [PubMed] [Google Scholar]
- Ullian E.M., Harris B.T., Wu A., Chan J.R., Barres B.A. 2004. Schwann cells and astrocytes induce synapse formation by spinal motor neurons in culture. Mol. Cell. Neurosci. 25:241–251 10.1016/j.mcn.2003.10.011 [DOI] [PubMed] [Google Scholar]
- Wake H., Moorhouse A.J., Jinno S., Kohsaka S., Nabekura J. 2009. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J. Neurosci. 29:3974–3980 10.1523/JNEUROSCI.4363-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakselman S., Béchade C., Roumier A., Bernard D., Triller A., Bessis A. 2008. Developmental neuronal death in hippocampus requires the microglial CD11b integrin and DAP12 immunoreceptor. J. Neurosci. 28:8138–8143 10.1523/JNEUROSCI.1006-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh M.K., Lichtman J.W. 2003. In vivo time-lapse imaging of synaptic takeover associated with naturally occurring synapse elimination. Neuron. 37:67–73 10.1016/S0896-6273(02)01142-X [DOI] [PubMed] [Google Scholar]
- Watts R.J., Hoopfer E.D., Luo L. 2003. Axon pruning during Drosophila metamorphosis: evidence for local degeneration and requirement of the ubiquitin-proteasome system. Neuron. 38:871–885 10.1016/S0896-6273(03)00295-2 [DOI] [PubMed] [Google Scholar]
- Watts R.J., Schuldiner O., Perrino J., Larsen C., Luo L. 2004. Glia engulf degenerating axons during developmental axon pruning. Curr. Biol. 14:678–684 10.1016/j.cub.2004.03.035 [DOI] [PubMed] [Google Scholar]
- Yan Y.P., Sailor K.A., Vemuganti R., Dempsey R.J. 2006. Insulin-like growth factor-1 is an endogenous mediator of focal ischemia-induced neural progenitor proliferation. Eur. J. Neurosci. 24:45–54 10.1111/j.1460-9568.2006.04872.x [DOI] [PubMed] [Google Scholar]
- Zheng X., Wang J., Haerry T.E., Wu A.Y., Martin J., O’Connor M.B., Lee C.H., Lee T. 2003. TGF-beta signaling activates steroid hormone receptor expression during neuronal remodeling in the Drosophila brain. Cell. 112:303–315 10.1016/S0092-8674(03)00072-2 [DOI] [PubMed] [Google Scholar]
- Zhou Z., Hartwieg E., Horvitz H.R. 2001. CED-1 is a transmembrane receptor that mediates cell corpse engulfment in C. elegans. Cell. 104:43–56 10.1016/S0092-8674(01)00190-8 [DOI] [PubMed] [Google Scholar]