Abstract
Tracheal brush cells (BCs) are specialized epithelial chemosensors that use the canonical taste transduction cascade to detect irritants. To test whether BCs are replaced at the same rate as other cells in the surrounding epithelium of adult mice, we used 5-bromo-2′-deoxyuridine (BrdU) to label dividing cells. Although scattered BrdU-labeled epithelial cells are present 5–20 days after BrdU, no BCs are labeled. These data indicate that BCs comprise a relatively static population. To determine how BCs are generated during development, we injected 5-day-old mice with BrdU and found labeled BCs and non-BC epithelial cells 5 days after BrdU. During the next 60 days, the percentage of labeled BCs increased, whereas the percentage of other labeled cell types decreased. These data suggest that BCs are generated from non-BC progenitor cells during postnatal tracheal growth. To test whether the adult epithelium retains the capacity to generate BCs, tracheal epithelial cells were recovered from adult mice and grown in an air–liquid interface (ALI) culture. After transition to differentiation conditions, BCs are detected, and comprise 1% of the total cell population by Day 14. BrdU added to cultures before the differentiation of BCs was chased into BCs, indicating that the increase in BC density is attributable to the proliferation of a non-BC progenitor. We conclude that: (1) BCs are normally a static population in adult mice; (2) BC progenitors proliferate and differentiate during neonatal development; and (3) BCs can be regenerated from a proliferative population resident in adult epithelium.
Keywords: trachea, brush cell, chemosensory, air–liquid interface, 5-bromo-2′-deoxyuridine
Clinical Relevance
In 2005, a working group of the National Heart, Lung, and Blood Institute on the brush cell concluded that an understanding of basic brush-cell biology could be important to developing new therapeutic strategies. However, this group also concluded that the major obstacle to brush-cell research was the absence of reliable cellular markers (Reid and colleagues, Am J Respir Crit Care Med 2005;172:136–139). Recent reports have finally established markers for the mysterious brush cell (Krasteva and colleagues, Proc Natl Acad Sci USA 2011;108:9478–9483; Tizzano and colleagues, BMC Pulm Med 2011;11:3). Our experiments are the first to take advantage of this development to determine the lifespan of the brush cell.
The tracheal epithelium is a complex tissue containing diverse cell types, which include ciliated cells, club (Clara)–like cells, and basal cells. These cells are slowly replaced in the adult, but can be regenerated rapidly in the case of cell death or overt epithelial injury (1–6). Relatively less studied are brush cells (BCs), which are specialized epithelial chemosensors. BCs are relatively rare compared with other epithelial cell types, and are distributed throughout the tracheal epithelium (7). Whether these specialized chemosensors are replaced at the same rate as other cells in the adult trachea remains unknown (8).
Although BCs were long speculated to be sensory elements (9), only recently has this chemosensory function been confirmed (7). In responding to many noxious substances, BCs use the canonical taste transduction pathway of Type 2 taste receptors (T2Rs), Gα-gustducin, phospholipase Cβ2 (PLCβ2), inositol 1,4,5-trisphosphate receptor, type 3 (IP3R3), and transient receptor potential melastatin 5 (TRPM5) (10–13). TRPM5 is a nonspecific cation channel, which has been used as a marker for several taste cell–like airway chemosensors (11, 12, 14, 15). BCs respond to “bitter-tasting” irritants by releasing acetylcholine, which activates nearby vagal nerve fibers to elicit protective respiratory reflexes (7). The tracheal BCs are molecularly similar to nasal solitary chemosensory cells (SCCs), which also use the canonical taste transduction cascade (12, 16).
In other chemosensory systems (e.g., main and accessory olfactory epithelia, taste buds, and nasal solitary chemosensory cells), the sensory cells are replaced at a rate similar to that of surrounding epithelium (17–19) (e.g., during the span of a few weeks). The lifespan of ciliated and club (Clara)–like cells in the tracheal epithelium is longer than in nasal and lingual epithelia (1, 3, 20, 21), but the lifespan of BCs has yet to be determined. We predicted that BC renewal and generation would occur at the same rate as that of the surrounding epithelium, and we tested this with 5-bromo-2′-deoxyuridine (BrdU) labeling. Surprisingly, BCs show no evidence of turnover in the adult epithelium. This finding led us to examine the initial generation of BCs during development and the capacity to generate BCs in an in vitro injury model of the adult epithelium.
Materials and Methods
Mice
The mice used in these experiments included C57Bl/6, TRPM5-GFP (22), and choline acetyltransferase (ChAT)–green fluorescent protein (GFP) (23) lines for in vivo experiments, and A/J and TRPM5-GFP mice for trachea epithelial cultures (see the online supplement for details). All experimental procedures were approved by the Institutional Animal Care and Use Committees at the University of Colorado Anschutz Medical Campus and National Jewish Health.
Immunohistochemistry
Immunohistochemistry was performed using standard methods, as described in the online supplement. For the adult BrdU experiments, 3-month-old mice received three intraperitoneal injections of 150 mg/kg BrdU evenly spaced during 12 hours. Because tracheal epithelial cells proliferate infrequently (1), a multiple injection protocol was used to increase the likelihood that BrdU would be bioavailable when BC progenitors were replicating DNA. For the perinatal BrdU experiments, 5-day-old mice received a single intraperitoneal injection of 100 mg/kg BrdU. Slides were evaluated with an epifluorescence microscope, and imaged with either a scanning laser or spinning disk confocal microscope.
Cell Culture
The production and maintenance of mouse trachea epithelial air–liquid interface (ALI) cultures are fully described in the online supplement. For BrdU labeling experiments, 10 μmol/L of BrdU were added to the culture medium for a 12-hour period.
Results
Identification of Chemosensory Brush Cells
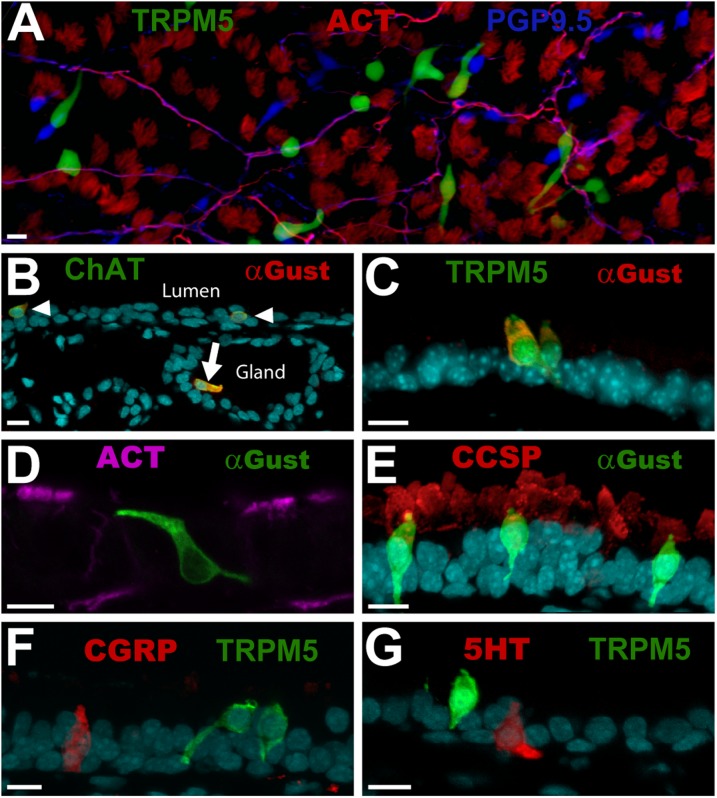
BCs are readily identifiable as a unique cell type within the tracheal epithelium. They are elongated, and typically have an apical process that reaches the lumen, as well as one or more basal processes (Figures 1A–1G). Consistent with previous reports (7, 11), BCs are present at every level of the trachea from the larynx to the bronchi, and coexpress TRPM5, ChAT, and Gα-gustducin (Figures 1B and 1C, and Figure E2 in the online supplement). BCs are not, however, immunoreactive for the ciliated cell marker, acetylated tubulin (ACT; Figure 1D) or the club (Clara)–like cell marker, club (Clara) cell secretory protein (CSSP; Figure 1E). Although morphologically similar to neuroendocrine cells of the trachea, BCs are not immunoreactive for protein gene product 9.5 (PGP9.5, also known as ubiquitin carboxyl terminal hydrolase-1; Figures 1A and E2C), calcitonin gene related peptide (CGRP; Figure 1F), or 5-hydroxytryptamine (5HT; Figure 1G), three markers of neuroendocrine cells. BCs are present throughout the tracheal epithelium, and occur both as solitary cells (Figures 1A, 1B, 1D, 1E, and 1G) and in clusters (Figures 1C and 1F). Cells that are similar to BCs occasionally occur in submucosal glands, as indicated by Gα-gustducin immunoreactivity (Figure 1B, arrow). A similar distribution of BCs is demonstrated by GFP positivity in transgenic mice that harbor transgenes regulated by the ChAT or TRPM5 promoters (Figures 1A–1C).
Figure 1.
Brush cells (BCs) comprise a distinct cell type in the tracheal epithelium. (A) Triple-labeled, whole-mount tracheal epithelium shows transient receptor potential melastatin 5 (TRPM5)–green fluorescent protein (GFP; green), protein gene product 9.5 (PGP9.5; blue), and acetylated tubulin (ACT; red). BCs appear green. Nerve fibers (magenta) are immunoreactive for both PGP9.5 and acetylated tubulin. Neuroendocrine cells are immunoreactive for PGP9.5 (blue), whereas cilia appear red, and are immunoreactive for ACT. (B) BCs coexpress Gα-gustducin (Gust; red) and choline acetyltransferase (ChAT; green), and occur within the tracheal epithelium (arrowheads) and in submucosal glands (arrow). (C) Most BCs are immunoreactive for both Gα-gustducin (red) and TRPM5 (green), and have multiple processes. (D) Gα-gustducin (green) immunoreactive BCs are not immunoreactive for ACT (magenta), a marker for ciliated cells. (E) Gα-gustducin (green) immunoreactive BCs are not immunoreactive for club (Clara)–cell secretory protein (CCSP) (red), a marker for club (Clara)–like cells. (F and G) TRPM5 (green)–expressing BCs appear morphologically similar to neuroendocrine cells, but are not immunoreactive for the known neuroendocrine cell markers (E) calcitonin gene related peptide (CGRP; red) and (F) 5-hydroxytryptamine (5HT; red). (B–G) Counterstains with DRAQ5 are shown in cyan. Photographs are of tissues from mice that ranged in age from 90–180 days. Scale bars = 10 μm.
Chemosensory Brush Cells Do Not Turn over in Adult Mice
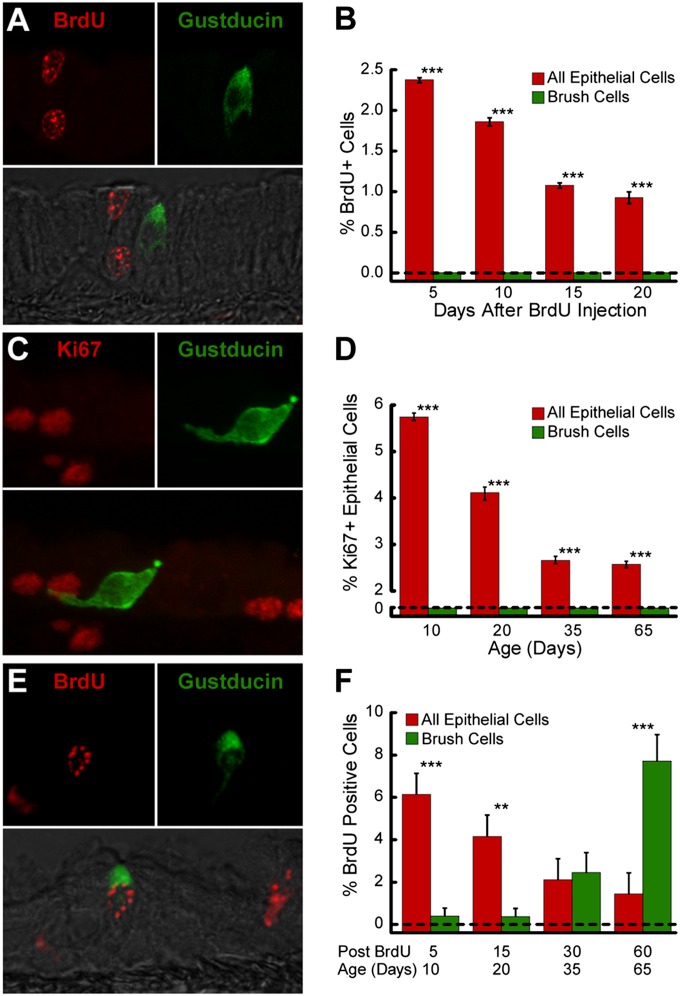
In adult mice, BrdU immunoreactive nuclei were present throughout the tracheal epithelium, 5, 10, 15, and 20 days after BrdU injection (Figure 2A and Figure E1A in the online supplement). BrdU+ nuclei were more common near the basement membrane at shorter time intervals. Five days after BrdU injection, 2.37% ± 0.03% of tracheal epithelial cells were labeled (Figure 2B). The frequency of BrdU+ cells decreased to 1.86% ± 0.05% on Day 10, 1.07% ± 0.03% on Day 15, and 0.92% ± 0.07% on Day 20. The extrapolation of these values predicts that the epithelial-cell lifespan is greater than 211 days, consistent with findings reported by others (1, 21). In the 16 mice used in this experiment, 2,849 BCs were examined. No BrdU-positive BCs were detected at any time (Figure 2B). Thus, BrdU labeling revealed no evidence for the replacement of BCs in adult epithelium. We conclude that BC replacement, if it occurs at all in healthy adult mice, is significantly slower than that in the surrounding epithelium (Figure 2B; P < 0.001, according to χ2 test).
Figure 2.
BCs comprise a static population in the adult trachea, but are generated by a population of proliferative progenitor cells during perinatal development. (A) Double-labeled image with Nomarski overlay shows 5-bromo-2′-deoxyuridine (BrdU; red)–labeled epithelial cell nuclei near an unlabeled Gα-gustducin (green) immunoreactive BC in an adult mouse, 20 days after BrdU treatment. (B) Percentages of tracheal epithelial cells and BCs labeled with BrdU at 5–20 days after BrdU treatment of adult mice. Bars depict the mean ± SEM (n = 4) for each point. (C) Double-labeled image shows Ki67 (red)–labeled epithelial cell nuclei near a Gα-gustducin (green) immunoreactive BC in a trachea from a 35-day-old mouse. (D) Percentages of perinatal tracheal epithelial cells and BCs immunoreactive for the mitotic marker Ki67. Bars depict the mean ± SEM (n = 3) for each time. (E) Double-labeled image with Nomarski overlay shows a BrdU (red)–labeled, Gα-gustducin (green) immunoreactive BC in a 35-day-old mouse, 30 days after BrdU treatment. (F) Percentages of perinatal tracheal epithelial cells and BCs labeled with BrdU at different times after the injection of 5-day-old mice. Bars depict the mean ± SEM (n = 4) for each time point. **P < 0.01 and ***P < 0.001, according to χ2 test. Scale bars = 10 μm.
Perinatal Generation of Chemosensory Brush Cells
Because BCs are a relatively stable population in adults, we investigated the origin of BCs during development. The trachea lengthens and enlarges during postnatal development (24), resulting in a substantial increase of epithelial surface area (Figures E2A and E2B). If BCs comprise a static population established before birth, the density of BCs in the trachea should decrease as the trachea enlarges. Conversely, if the density of BCs is constant during this period of expansion, then new BCs must be added over time. To evaluate this question, we imaged whole-mounted tracheas from TRPM5-GFP mice. The density of BCs did not change significantly from postnatal Day (p) 5 to p30, implying that significant cell addition occurs during this period (Figure E2C; 43.20 ± 4.92 BCs/mm2 on p5, 38.40 ± 4.26 BCs/mm2 on p10, 42.80 ± 6.95 BCs/mm2 on p15, and 57.20 ± 6.09 BCs/mm2 on p30, one-way ANOVA, F(3,16) = 2.09, P = 0.14).
To test whether BCs themselves are mitotic during postnatal growth, we stained the tracheas for the proliferation marker Ki67, which is present during all phases of the cell cycle except G0 (25). Numerous epithelial cells, but no Gα-gustducin immunoreactive BCs, were immunoreactive for Ki67. The mitotic index (percent cells immunoreactive for Ki67) within the tracheal epithelium decreased significantly as mice aged from p10 to p65 (Figure 2D; one-way ANOVA, F(3,8) = 23.80, P < 0.001; p10, 5.75% ± 0.06%; p20, 4.11% ± 0.11%; p35, 2.66% ± 0.05%; p65, 2.57% ± 0.05%). Among the 12 mice used in this experiment, 415 BCs and 3,767 Ki67-positive nuclei were examined. At no time were Ki67 and Gα-gustducin double-positive cells detected (Figures 2C and 2D).
To test whether BCs are added from a proliferative population during early development, we injected mice with BrdU on p5, and examined their tracheas for labeled BCs at 5 hours, and at 5, 15, 30, and 60 days after injection (Figure E1B). In tracheas examined 5 hours after BrdU injection, the majority of BrdU-labeled cells expressed cytokeratin 14, or were adjacent to cytokeratin 14–expressing basal cells (Figure E2D). No BCs were BrdU-labeled at this time. This finding is consistent with previous reports that the main tracheal progenitor is a basal cell that expresses cytokeratin 14 (3, 26, 27). As early as 5 days after injection (chase Day 5 = p10), BCs with BrdU-labeled nuclei (Figure 2E) were detected along with BrdU-labeled non-BC epithelial cells. Apparently, these labeled BCs arise from the proliferative basal cells labeled shortly after the BrdU injection.
The fraction of BCs labeled with BrdU increased with time after injection, suggesting a slow addition of new BCs from the proliferative basal cell pool. In contrast, the fraction of BrdU-labeled non-BC epithelial cells decreased (Figure 2F). On chase Day 5, the proportion of BrdU-labeled to total BCs was significantly lower than the proportion of labeled to total non-BC epithelial cells (0.38% ± 0.38% of BCs compared with 4.16% ± 0.29% of non-BC epithelial cells, n = 4, χ2 analysis, P < 0.01). By chase Day 30, the proportion of BrdU-labeled to total BCs (2.46% ± 0.93%) was not significantly different from the proportion of BrdU-labeled to total non-BC epithelial cells (2.12% ± 0.32%). On chase Day 60, the proportion of BrdU-labeled BCs was significantly higher than the proportion of non-BC epithelial cells labeled by BrdU (7.71% ± 0.12% of BCs compared with 1.44% ± 0.03% of other epithelial cells, n = 4, χ2 analysis, P < 0.001). The BC progenitor was labeled by BrdU on p5, but BC progeny were not detected for 5 days. Taken together, these findings indicate that the BC progenitor is proliferative on p5, but that the differentiation of BCs occurs over a period of weeks.
New Chemosensory Brush Cells Can Be Generated from Adult Tracheal Epithelial Progenitors
To test whether the adult tracheal epithelium retains the capacity to generate new BCs, we used ALI cultures (Figure E1C). ALI cultures are produced by seeding dissociated tracheal epithelial cells at low density to model epithelial regeneration after injury (26, 28–30). These epithelial cells are grown to confluence on a porous polycarbonate membrane, and differentiate into an epithelial layer that recapitulates the anatomy and gene expression of the native mouse trachea (26, 28, 30). This model can be used to determine the cell types generated by adult tracheal facultative progenitor cells (26, 28), and was adapted to evaluate tissue stem-cell differentiation (27).
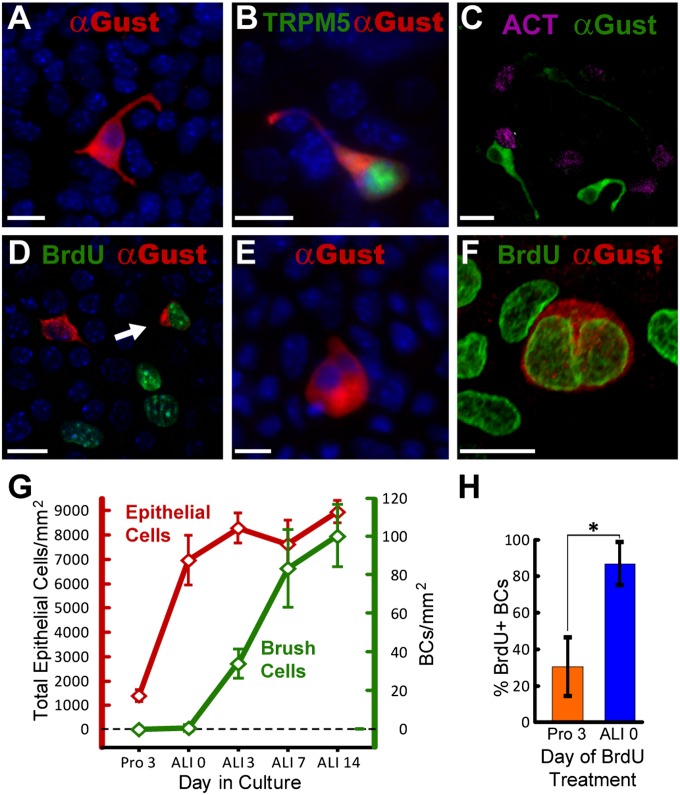
Immediately after the transition to differentiation conditions, rare Gα-gustducin immunoreactive cells were evident (Figure 3A), but constituted only 0.01% ± 0.01% of the total population (Figure 3G). In culture, as in vivo, BCs express both Gα-gustducin and TRPM5 (Figure 3B), and are not immunoreactive for acetylated tubulin (Figure 3C). In vitro, BCs were multipolar, with two to three processes of various lengths (Figures 3A–3C), similar to their appearance in vivo in adult trachea. Doublet BCs (i.e., pairs of adjacent Gα-gustducin–positive BCs) were common (Figures 3E and 3F). Closely spaced BCs are also present in vivo, but are not as intimately associated as those in culture (Figures 1C, 3E, and 3F).
Figure 3.
New BCs can be generated from adult tracheal epithelium in vitro. (A) Gα-gustducin (red) immunoreactive BCs in tracheal epithelial cell culture on air–liquid interface (ALI) Day 7. Cultured BCs have multiple processes like their in vivo counterparts. (B) BCs in cultures produced from TRPM5-GFP (green) transgenic mice were immunoreactive for Gα-gustducin (red) and expressed GFP on ALI Day 7. (C) Gα-gustducin (green) immunoreactive BCs were negative for the ciliated cell marker acetylated tubulin (ACT, magenta) on ALI Day 7. (D) A double-labeled cell with a BrdU (green) and Gα-gustducin (red) double-immunoreactive BC (arrow). BrdU also labeled other cells in culture (ALI Day 14). (E) Several doublet Gα-gustducin (red) immunoreactive BCs, suggestive of daughter cells, were observed culture on ALI Day 1. (F) Double-labeled image shows a Gα-gustducin (red) immunoreactive doublet, where both nuclei are labeled with BrdU (green), on ALI Day 14. Counterstains with 4′6-diamidino-2-phenylindole are shown in blue. Scale bars = 10 μm. (G) Tracheal epithelial cell density (red line) and the fraction of BCs (green line) over time in ALI cultures. Points depict the mean ± SEM (n = 3) for each time. (H) Fraction of cultured BCs labeled when dosed with BrdU, either during the proliferation period (Pro 3) or on ALI Day 0. *P < 0.001, according to t test. Bars depict the mean ± SEM (n = 3) for each point.
The overall cell density within the cultures is stable after the switch to differentiation conditions (Figure 3G), but the proportion of BCs increases significantly (one-way ANOVA, F(4,85) = 62.71, P < 0.001) to 0.38% ± 0.09% on ALI Day 3, 0.93% ± 0.22% at ALI Day 7, and 1.12% ± 0.18% by ALI Day 14 (Figure 3G). To test the possibility that this increase in BC density was attributable to cell division, BrdU was added to the cultures on proliferation Day 3 (2 d before the switch to ALI) or on ALI Day 0. No BrdU-labeled BCs were present in cultures fixed immediately after the 12-hour BrdU pulse, indicating that differentiated BCs are not proliferative. BrdU-labeled BCs are, however, present on ALI Day 14 (chase Day 16 or 14; Figure 3D), indicating that BCs differentiate from a proliferative population labeled in culture some days prior during cell division. Proliferative cells may undergo asymmetric divisions, so that one daughter cell remains proliferative, whereas the other assumes a differentiated cell fate, potentially becoming one of a variety of cell types, including ciliated, club (Clara)–like, or BC. The determination of exact lineage relationships within the epithelial population will require further study.
Significantly more BCs were labeled when BrdU was added on ALI Day 0 (Figure 3H; t test, P < 0.001) than when BrdU was added on proliferation Day 3, suggesting that progenitor cells that proliferate on proliferation Day 3 are biased toward population expansion. In contrast, progenitors that proliferate after the transition to ALI have the potential to generate differentiated BCs. Continued cell divisions between proliferation Day 3 and the onset of ALI would likely dilute the BrdU label in the proliferative population, resulting in no detectable label in later-forming BCs. Thus, the present study faithfully evaluates early BC generation, but may not detect BCs that are generated as a consequence of multiple rounds of progenitor proliferation. Taken together, the in vitro results indicate that the adult tracheal epithelium has the capacity to generate new BCs, although few are generated under normal healthy conditions.
Discussion
Respiratory epithelia, including the tracheal epithelium, are at risk of damage by inhaled particulates, toxins, and pathogens. In the normal healthy trachea, ciliated cells and club (Clara)–like cells receive the most exposure to the airway lumen, and in mice are replaced approximately every 6–9 months. In contrast, the tracheal BC population is essentially static after being produced during the perinatal period of tracheal growth. Moreover, the lifespan of BCs and consequently their turnover rate are distinct from those of other tracheal epithelial cell types in healthy adult mice. However, our in vitro experiments demonstrate that BCs can be regenerated if they are lost because of tracheal damage. These data raise interesting questions regarding the mechanisms that determine BC longevity and replacement.
Reliable Markers for Chemosensory Brush Cells
The lack of reliable markers for BCs has presented a significant impediment to elucidating details of their biology (8). Initial speculations that BCs were chemosensory were based solely on ultrastructural analyses of tracheal epithelia (9). These observations were not confirmed until 40 years later (7, 10), largely because BCs could not be identified among the more common cell types in the epithelium (8).
In the present study, we used Gα-gustducin, a component of the bitter-taste transduction cascade, as a marker for BCs because this protein was detectable even after applying the harsh antigen-retrieval methods required to detect BrdU. Gα-gustducin has been used extensively as a marker for bitter-sensitive, sweet-sensitive, and umami-sensitive Type II taste receptor cells, including several BrdU dating studies (31, 32). Similarly, Gα-gustducin is a reliable marker for nasal solitary chemosensory cells (19).
The majority of BCs are immunoreactive for Gα-gustducin, but a small fraction can lack this or other taste-related markers (11, 33). In the auditory tube, 3% of ChAT-expressing BCs are not immunoreactive for Gα-gustducin (33). Likewise, the occasional tracheal BC that does not express all of the phenotypic markers has also been observed (7). In the taste system, subpopulations of receptor cells use different downstream transduction elements (34). Thus, in BCs as in taste receptor cells, different second messengers could be used by different subpopulations. Alternately, these “atypical” non–Gα-gustducin BCs could be immature cells that have yet to express the full complement of chemosensory transduction proteins.
A previous study reported that ciliated cells of cultured human respiratory epithelia were immunoreactive for Gα-gustducin (35), although others noted the absence of Gα-gustducin in ciliated cells from the respiratory epithelia of rats (36). Likewise, we never observed cilia or ciliated cells immunoreactive for Gα-gustducin in our in vivo (Figure 1D) or in vitro (Figure 3C) preparations of mouse tissue. This disparity may represent differences in species-specific expression patterns. The distinct morphology of ciliated cells and BCs is sufficient to ensure that no ciliated cells were counted as BCs in the present study (Figures 1D and 3C).
Tracheal Brush Cells Are Generated Perinatally but Comprise a Stable Population in Adults
The lack of BC turnover in the trachea is unusual for chemosensory epithelia. Most chemosensory cells (e.g., taste buds, olfactory receptor cells, and even solitary chemosensory cells of the nasal epithelium) are replaced every 2–4 weeks (17–19, 37–39). However, other chemosensors, including those of the carotid body, are stable (40). Carotid body cells are internal chemosensors (41), and their lack of turnover may reflect their protected intravascular position. Similarly, other types of sensory cells in protected end organs, such as photoreceptors (42), auditory hair cells (43), and Merkel cells (44), are not replaced in a normal healthy individual.
The stability of murine tracheal BCs may also reflect the relatively protected environment in which they reside. The rodent nose provides an efficient filtering mechanism, leaving the trachea relatively free of inhaled toxins. In contrast, the human nose is not as effective at this protective function (45). Thus, the human tracheobronchial BC population is likely to be more susceptible to damage, and therefore less stable than its murine counterpart. Following a similar line of logic, SCCs in the nasal cavity of adult mice are likely to be exposed to a higher concentration of inhaled toxins than are tracheal BCs. Thus, the finding that nasal SCCs turn over every few weeks (19), whereas BCs are stable, may reflect cell locations rather than cell-intrinsic differences in maximal lifespan.
During the perinatal growth period, the surface area of the trachea increases, and new epithelial cells are added. The density of ciliated and club (Clara)–like cells remains constant even as new cells are generated (24). Likewise, new BCs form during this period (Figures 2E and 2F), resulting in a constant proportion of BCs (Figure E3). The constant density of BCs, ciliated cells, and club (Clara)–like cells suggests a regulated generation of different cell types, likely reflecting intraepithelial signaling to maintain the proportionate generation of different cell types.
Does Injury Result in the Production of New Brush Cells?
The airway epithelium is a remarkably resilient tissue, capable of reconstituting diverse cell types from a small reservoir of progenitor cells (21, 46). Although the mitotic index for adult epithelium is low, the rate at which new epithelial cells are produced after injury increases substantially (5, 6, 47). This new cell addition results in the regeneration of all major tracheal cell types.
We used the ALI culture system to test the ability of tracheal facultative progenitor cells to reconstitute BCs along with other elements of the tracheal epithelium after disruption (26, 28). Our results demonstrate that BCs, like ciliated and club (Clara)–like cells, can be regenerated after damage (Figures 3D, 3G, and 3H). Although ALI cultures mimic many aspects of the naphthalene injury model (26), in vitro models may not always faithfully recapitulate in vivo circumstances, and thus these data must be viewed with that caveat in mind. However, the idea that tracheal progenitor cells are capable of regenerating BCs is completely consistent with current models of airway plasticity.
BC activity may itself play a role in regulating the plasticity of the surrounding epithelium. BCs respond to irritants, including bacteria metabolites, by releasing the neurotransmitter acetylcholine (7, 10) (Figures 1B and E2B). However, the acetylcholine released by BCs lying close to mitotic basal cells (Figure 2C) may act in a paracrine fashion on basal-cell nicotinic acetylcholine receptors to modulate the proliferation and differentiation of the mitotic population (48). This model provides a mechanism by which BC activation could result in changes in the proliferation and differentiation of tracheal progenitor cells.
What Is the Progenitor of the Chemosensory Brush Cell?
The main progenitor of neonatal and adult tracheal epithelial cells is a cytokeratin (K) 5–expressing and K14–expressing basal cell (3, 26, 27, 49). In the adult tracheal epithelium, basal cells self-renew (3) while also producing CCSP+ club (Clara)–like secretory cells (27). Club (Clara)–like cells, under normal conditions, are capable of producing ciliated cells (27), or can undergo injury-induced metaplasia to become mucus-secreting cells (3). Ciliated cells and mucus-secreting cells appear to be terminally differentiated, whereas club (Clara)–like cells are capable of both self-renewal and transdifferentiation (3).
Our data indicate that BCs, like ciliated cells, are terminally differentiated cells, likely generated from a K14+ basal cell. In both adult and perinatal animals, no cells expressing BC markers underwent cell division, as assessed by the mitotic marker Ki67 (Figures 2C and 2D). Furthermore, in our perinatal BrdU experiments, we found that nearly all BrdU-labeled cells expressed K14 or were adjacent to K14+ basal cells within 5 hours of injection (Figure E2D), suggesting that K14+ cells represent the proliferative pool at this time. Although BrdU+ BCs appeared with longer chase times, BC progenitors are likely a subset of the K14+ basal cells labeled by BrdU in these short-chase experiments. Moreover, in our ALI experiments, adult epithelial progenitor cells retained the ability to generate new BCs in vitro (Figures 3D and 3F–3H). Other experiments have implicated the K5-expressing and K14-expressing basal cells (26, 27) as the progenitors responsible for the recovery of the epithelium in ALI cultures. Taken together with these previous results, our data suggests that BCs are generated by K5-expressing and K14-expressing basal cells as part of the epithelial repair response.
These results represent the first attempt to place BCs within the tracheal epithelial cell linage. The lack of reliable BC markers and their scarcity (1% of total epithelial cells) have hindered previous examinations of lineage relationships (8). Although our results suggest that the K14-expressing and K5-expressing basal cell gives rise to BCs, lineage-tracing in vivo will be needed to establish a lineage relationship. Likewise, our observation of doublets BCs in vitro is suggestive of daughter cells (Figures 3E and 3F), and may indicate that a symmetric terminal division is the final step in the generation of BCs.
Conclusions
Our results indicate that BCs comprise a static population in the healthy adult trachea. These cells show no evidence of the turnover typical of surrounding epithelial cells, a trait unusual for epithelial chemoreceptor cells. The majority of BCs present in the healthy adult mouse are generated perinatally while the trachea is expanding. Despite the lack of turnover in adult epithelium, BCs can be regenerated from proliferative epithelial cells after tracheal damage, allowing for a full reconstitution of the tracheal epithelium.
Acknowledgments
Acknowledgments
The authors thank Robert Margolskee (Monell Chemical Senses Center, Philadelphia, PA) for providing the TRPM5-GFP mice, and Sukumar Vijayaraghavan (University of Colorado School of Medicine) for providing the ChAT-tauGFP mice. In addition, the authors thank Russell Smith (National Jewish Health) for his assistance with BrdU–cytokeratin 14 immunohistochemistry, and Jennifer Strafford (University of Colorado School of Medicine) for statistical advice. Parts of this manuscript were presented at the 2010, 2011, and 2012 Annual Meetings of the Association of Chemoreceptive Sciences and at the 2011 American Thoracic Society International Conference.
Footnotes
This work was supported by National Institutes of Health grants R01 DC009820 (T.E.F.), P30 DC004657 (D. Restrepo), and RO1 HL075585, and by National Institutes of Health supplement HL075585-S1 (S.D.R.).
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2012-0485OC on March 22, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Rawlins EL, Hogan BL. Ciliated epithelial cell lifespan in the mouse trachea and lung. Am J Physiol Lung Cell Mol Physiol. 2008;295:L231–L234. doi: 10.1152/ajplung.90209.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, Wang F, Hogan BL. The role of SCGB1A1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009;4:525–534. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reynolds SD, Malkinson AM. Clara cell: progenitor for the bronchiolar epithelium. Int J Biochem Cell Biol. 2010;42:1–4. doi: 10.1016/j.biocel.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rock JR, Randell SH, Hogan BLM. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis Model Mech. 2010;3:545–556. doi: 10.1242/dmm.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. Basal cells are a multipotent progenitor capable of renewing the bronchial epithelium. Am J Pathol. 2004;164:577–588. doi: 10.1016/S0002-9440(10)63147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole BB, Smith RW, Jenkins KM, Graham BB, Reynolds PR, Reynolds SD. Tracheal basal cells: a facultative progenitor cell pool. Am J Pathol. 2010;177:362–376. doi: 10.2353/ajpath.2010.090870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krasteva G, Canning BJ, Hartmann P, Veres TZ, Papadakis T, Mühlfeld C, Schliecker K, Tallini YN, Braun A, Hackstein H, et al. Cholinergic chemosensory cells in the trachea regulate breathing. Proc Natl Acad Sci USA. 2011;108:9478–9483. doi: 10.1073/pnas.1019418108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reid L, Meyrick B, Antony VB, Chang LY, Crapo JD, Reynolds HY. The mysterious pulmonary brush cell: a cell in search of a function. Am J Respir Crit Care Med. 2005;172:136–139. doi: 10.1164/rccm.200502-203WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luciano L, Reale E, Ruska H. Brush cells in the alveolar epithelium of the rat lung. Z Zellforsch Mikrosk Anat. 1969;95:198–201. [PubMed] [Google Scholar]
- 10.Krasteva G, Canning BJ, Papadakis T, Kummer W. Cholinergic brush cells in the trachea mediate respiratory responses to quorum sensing molecules. Life Sci. 2012;91:992–996. doi: 10.1016/j.lfs.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Tizzano M, Cristofoletti M, Sbarbati A, Finger TE. Expression of taste receptors in solitary chemosensory cells of rodent airways. BMC Pulm Med. 2011;11:3. doi: 10.1186/1471-2466-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finger TE, Böttger B, Hansen A, Anderson KT, Alimohammadi H, Silver WL. Solitary chemoreceptor cells in the nasal cavity serve as sentinels of respiration. Proc Natl Acad Sci USA. 2003;100:8981–8986. doi: 10.1073/pnas.1531172100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merigo F, Benati D, Tizzano M, Osculati F, Sbarbati A. Alpha-gustducin immunoreactivity in the airways. Cell Tissue Res. 2005;319:211–219. doi: 10.1007/s00441-004-1007-2. [DOI] [PubMed] [Google Scholar]
- 14.Kaske S, Krasteva G, König P, Kummer W, Hofmann T, Gudermann T, Chubanov V. TRPM5, a taste-signaling transient receptor potential ion-channel, is a ubiquitous signaling component in chemosensory cells. BMC Neurosci. 2007;8:49. doi: 10.1186/1471-2202-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansen A, Finger TE. Is TRPM5 a reliable marker for chemosensory cells? Multiple types of microvillous cells in the main olfactory epithelium of mice. BMC Neurosci. 2008;9:115. doi: 10.1186/1471-2202-9-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silver W, Roe P, Saunders CJ. Functional neuroanatomy of the upper airway in experimental animals. Toxicology of the nose and upper airways. In: Morris JB, Shusterman D, editors. New York: Informa Healthcare; 2010. pp. 45–64. [Google Scholar]
- 17.Moulton DG. Dynamics of cell populations in the olfactory epithelium. Ann N Y Acad Sci. 1974;237:52–61. doi: 10.1111/j.1749-6632.1974.tb49843.x. [DOI] [PubMed] [Google Scholar]
- 18.Beidler LM, Smallman RL. Renewal of cells within taste buds. J Cell Biol. 1965;27:263–272. doi: 10.1083/jcb.27.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gulbransen BD, Finger TE. Solitary chemoreceptor cell proliferation in adult nasal epithelium. J Neurocytol. 2005;34:117–122. doi: 10.1007/s11068-005-5051-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rawlins EL, Okubo T, Que J, Xue Y, Clark C, Luo X, Hogan BL. Epithelial stem/progenitor cells in lung postnatal growth, maintenance, and repair. Cold Spring Harb Symp Quant Biol. 2008;73:291–295. doi: 10.1101/sqb.2008.73.037. [DOI] [PubMed] [Google Scholar]
- 21.Basbaum C, Jany B. Plasticity in the airway epithelium. Am J Physiol. 1990;259:L38–L46. doi: 10.1152/ajplung.1990.259.2.L38. [DOI] [PubMed] [Google Scholar]
- 22.Clapp TR, Medler KF, Damak S, Margolskee RF, Kinnamon SC. Mouse taste cells with G protein–coupled taste receptors lack voltage-gated calcium channels and SNAP-25. BMC Biol. 2006;4:7. doi: 10.1186/1741-7007-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grybko MJ, Hahm ET, Perrine W, Parnes JA, Chick WS, Sharma G, Finger TE, Vijayaraghavan S. A transgenic mouse model reveals fast nicotinic transmission in hippocampal pyramidal neurons. Eur J Neurosci. 2011;33:1786–1798. doi: 10.1111/j.1460-9568.2011.07671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Winkle LS, Fanucchi MV, Miller LA, Baker GL, Gershwin LJ, Schelegle ES, Hyde DM, Evans MJ, Plopper CG.Epithelial cell dis-tribution and abundance in rhesus monkey airways during postnatal lung growth and development J Appl Physiol 2004972355–2363.discussion 2354. [DOI] [PubMed] [Google Scholar]
- 25.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 26.Brechbuhl HM, Ghosh M, Smith MK, Smith RW, Li B, Hicks DA, Cole BB, Reynolds PR, Reynolds SD. β-catenin dosage is a critical determinant of tracheal basal cell fate determination. Am J Pathol. 2011;179:367–379. doi: 10.1016/j.ajpath.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghosh M, Brechbuhl HM, Smith RW, Li B, Hicks DA, Titchner T, Runkle CM, Reynolds SD. Context-dependent differentiation of multipotential keratin 14–expressing tracheal basal cells. Am J Respir Cell Mol Biol. 2011;45:403–410. doi: 10.1165/rcmb.2010-0283OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.You Y, Richer EJ, Huang T, Brody SL. Growth and differentiation of mouse tracheal epithelial cells: selection of a proliferative population. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1315–L1321. doi: 10.1152/ajplung.00169.2002. [DOI] [PubMed] [Google Scholar]
- 29.Ghosh M, Helm KM, Smith RW, Giordanengo MS, Li B, Shen H, Reynolds SD. A single cell functions as a tissue-specific stem cell and the in vitro niche-forming cell. Am J Respir Cell Mol Biol. 2011;45:459–469. doi: 10.1165/rcmb.2010-0314OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith MK, Koch PJ, Reynolds SD. Direct and indirect roles for β-catenin in facultative basal progenitor cell differentiation. Am J Physiol Lung Cell Mol Physiol. 2012;302:L580–L594. doi: 10.1152/ajplung.00095.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho YK, Farbman AI, Smith DV. The timing of alpha-gustducin expression during cell renewal in rat vallate taste buds. Chem Senses. 1998;23:735–742. doi: 10.1093/chemse/23.6.735. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen HM, Reyland ME, Barlow LA. Mechanisms of taste bud cell loss after head and neck irradiation. J Neurosci. 2012;32:3474–3484. doi: 10.1523/JNEUROSCI.4167-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krasteva G, Hartmann P, Papadakis T, Bodenbenner M, Wessels L, Weihe E, Schütz B, Langheinrich AC, Chubanov V, Gudermann T, et al. Cholinergic chemosensory cells in the auditory tube. Histochem Cell Biol. 2012;137:483–497. doi: 10.1007/s00418-012-0911-x. [DOI] [PubMed] [Google Scholar]
- 34.Tizzano M, Dvoryanchikov G, Barrows JK, Kim S, Chaudhari N, Finger TE. Expression of Galpha14 in sweet-transducing taste cells of the posterior tongue. BMC Neurosci. 2008;9:110. doi: 10.1186/1471-2202-9-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science. 2009;325:1131–1134. doi: 10.1126/science.1173869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Merigo F, Benati D, Cristofoletti M, Amarù F, Osculati F, Sbarbati A. Glucose transporter/T1R3–expressing cells in rat tracheal epithelium. J Anat. 2012;221:138–150. doi: 10.1111/j.1469-7580.2012.01522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farbman AI. Renewal of taste bud cells in rat circumvallate papillae. Cell Tissue Kinet. 1980;13:349–357. doi: 10.1111/j.1365-2184.1980.tb00474.x. [DOI] [PubMed] [Google Scholar]
- 38.Hamamichi R, Asano-Miyoshi M, Emori Y. Taste bud contains both short-lived and long-lived cell populations. Neuroscience. 2006;141:2129–2138. doi: 10.1016/j.neuroscience.2006.05.061. [DOI] [PubMed] [Google Scholar]
- 39.Mackay-Sim A, Kittel P. Cell dynamics in the adult mouse olfactory epithelium: a quantitative autoradiographic study. J Neurosci. 1991;11:979–984. doi: 10.1523/JNEUROSCI.11-04-00979.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang ZY, Olson EB, Jr, Bjorling DE, Mitchell GS, Bisgard GE. Sustained hypoxia-induced proliferation of carotid body Type I cells in rats. J Appl Physiol. 2008;104:803–808. doi: 10.1152/japplphysiol.00393.2007. [DOI] [PubMed] [Google Scholar]
- 41.Milsom WK, Burleson ML. Peripheral arterial chemoreceptors and the evolution of the carotid body. Respir Physiol Neurobiol. 2007;157:4–11. doi: 10.1016/j.resp.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 42.Zeiss CJ, Johnson EA. Proliferation of microglia, but not photoreceptors, in the outer nuclear layer of the RD-1 mouse. Invest Ophthalmol Vis Sci. 2004;45:971–976. doi: 10.1167/iovs.03-0301. [DOI] [PubMed] [Google Scholar]
- 43.López-Schier H. Regeneration: did you hear the news? Curr Biol. 2004;14:R127–R128. [PubMed] [Google Scholar]
- 44.Vaigot P, Pisani A, Darmon YM, Ortonne JP. The majority of epidermal Merkel cells are non-proliferative: a quantitative immunofluorescence analysis. Acta Derm Venereol. 1987;67:517–520. [PubMed] [Google Scholar]
- 45.Harkema JR, Carey SA, Wagner JG. The nose revisited: a brief review of the comparative structure, function, and toxicologic pathology of the nasal epithelium. Toxicol Pathol. 2006;34:252–269. doi: 10.1080/01926230600713475. [DOI] [PubMed] [Google Scholar]
- 46.Reynolds SD, Giangreco A, Power JH, Stripp BR. Neuroepithelial bodies of pulmonary airways serve as a reservoir of progenitor cells capable of epithelial regeneration. Am J Pathol. 2000;156:269–278. doi: 10.1016/S0002-9440(10)64727-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. In vivo differentiation potential of tracheal basal cells: evidence for multipotent and unipotent subpopulations. Am J Physiol Lung Cell Mol Physiol. 2004;286:L643–L649. doi: 10.1152/ajplung.00155.2003. [DOI] [PubMed] [Google Scholar]
- 48.Maouche K, Polette M, Jolly T, Medjber K, Cloëz-Tayarani I, Changeux JP, Burlet H, Terryn C, Coraux C, Zahm JM, et al. Alpha7 nicotinic acetylcholine receptor regulates airway epithelium differentiation by controlling basal cell proliferation. Am J Pathol. 2009;175:1868–1882. doi: 10.2353/ajpath.2009.090212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daniely Y, Liao G, Dixon D, Linnoila RI, Lori A, Randell SH, Oren M, Jetten AM. Critical role of p63 in the development of a normal esophageal and tracheobronchial epithelium. Am J Physiol Cell Physiol. 2004;287:C171–C181. doi: 10.1152/ajpcell.00226.2003. [DOI] [PubMed] [Google Scholar]