Abstract
MicroRNAs (miRNAs) are increasingly recognized as important posttranscriptional regulators of gene expression, and changes in their actions can contribute to disease states. Little is understood regarding miRNA functions in the airway epithelium under normal or diseased conditions. We profiled miRNA expression in well-differentiated primary cultures of human cystic fibrosis (CF) and non-CF airway epithelia, and discovered that miR-509–3p and miR-494 concentrations were increased in CF epithelia. Human non-CF airway epithelia, transfected with the mimics of miR-509–3p or miR-494, showed decreased cystic fibrosis transmembrane conductance regulator (CFTR) expression, whereas their respective anti-miRs exerted the opposite effect. Interestingly, the two miRNAs acted cooperatively in regulating CFTR expression. Upon infecting non-CF airway epithelial cells with Staphylococcus aureus, or upon stimulating them with the proinflammatory cytokines TNF-α or IL-1β, we observed an increased expression of both miRNAs and a concurrent decrease in CFTR expression and function, suggesting that inflammatory mediators may regulate these miRNAs. Transfecting epithelia with anti-miRs for miR-509–3p and miR-494, or inhibiting NF-κB signaling before stimulating cells with TNFα or IL-1β, suppressed these responses, suggesting that the expression of both miRNAs was responsive to NF-κB signaling. Thus, miR-509–3p and miR-494 are dynamic regulators of CFTR abundance and function in normal, non-CF airway epithelia.
Keywords: ATP binding cassette protein, cystic fibrosis transmembrane conductance regulator, 3′ UTR, epithelial fluid and electrolyte transport
Clinical Relevance
We demonstrate the regulation of cystic fibrosis transmembrane conductance regulator (CFTR) expression and function by microRNAs (miRNAs). The results highlight the ability of miRNAs to influence airway epithelial cell function in response to environmental stimuli. We speculate that changes in CFTR abundance and function might acutely affect airway surface liquid volume and composition, host defense, and mucociliary clearance.
MicroRNAs (miRNAs) comprise an evolutionarily conserved class of small (∼ 21–24 nucleotides), noncoding RNA molecules that regulate post-transcriptional gene expression by binding to the 3′ untranslated region (UTR) of target mRNAs (1, 2). The majority of human genes are under posttranscriptional regulation by miRNAs (1). As such, miRNAs are essential for multiple biological processes such as tissue development, cell-cycle checkpoints, DNA repair, senescence, organogenesis, and innate immunity (3, 4). Interestingly, although a single miRNA can target anywhere from 3–300 genes, distinct miRNAs can act independently or cooperatively, in a tissue-specific manner, to repress the expression of a single gene by binding to their target sequences in the 3′ UTR of the mRNA (2, 4, 5). MiRNAs play an important role in lung development (6, 7) and in several facets of pulmonary biology (7–10). However, the roles of miRNAs in the regulation of fluid and electrolyte transport in the airways are not well understood.
The cystic fibrosis transmembrane conductance regulator (CFTR) gene encodes an anion channel, which when mutated causes the disease cystic fibrosis (CF) (11). Chronic pulmonary infection and the associated inflammation contribute to the decline in respiratory function in patients with CF. The increased expression of proinflammatory cytokines (IL-1, IL-8, IL-6, and TNF-α), along with the decreased expression of anti-inflammatory cytokines and an altered protease/antiprotease balance, has been reported in the airways of patients with CF (12). Similar proinflammatory signals occur in the context of many pulmonary diseases caused by bacteria, viruses, and environmental stimuli. Toll-like receptors on epithelia, macrophages, and other cells recognize pathogen-associated molecular patterns such as bacterial LPS, flagellin, lipoteichoic acid, and additional microbial products. Signal transduction from these receptors, in turn, mediates inflammatory responses, in part by activating the transcription factor NF-κB. NF-κB regulates cellular pathways that induce the production of the inflammatory proteins and cytokines that influence the onset and resolution of numerous pulmonary diseases (13, 14).
Although extensive study of CFTR and its protein product has proceeded since its discovery in 1989 (11), the complex regulation of its expression remains poorly understood (15, 16). CFTR constitutes a low-abundance mRNA in airway epithelia (17), and miRNAs have been suggested to silence low-abundance mRNA targets more efficiently than highly abundant mRNAs (2, 18). Thus, CFTR mRNA concentrations may be tightly controlled post-transcriptionally by miRNAs. Indeed, evidence indicates that miRNAs may influence CFTR expression (19, 20), but the functional effects of these interactions have not been investigated. Furthermore, our understanding of the role miRNAs play in CF and other lung diseases associated with inflammation and infection is nascent. We recently discovered that miR-138 positively influences CFTR expression, post-translational biosynthesis, and function indirectly by inhibiting the transcriptional regulatory protein SIN3A (21). In this study, we hypothesized that miRNAs directly regulate the production of CFTR protein and hence its function in epithelia.
Materials and Methods
RNA Isolation
Total RNA from human primary airway epithelial cells and Calu-3 cells was isolated using the mirVana miRNA Isolation Kit or the TRIzol Reagent (both from Life Technologies, Carlsbad, CA) (22). Total RNA was tested for quality on an Agilent Model 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). Only samples with an RNA integrity number greater than 7.0 were selected for downstream processing.
Primary Human Airway Epithelia
Airway epithelia from human tracheas and primary bronchi removed from organs donated for research were cultured at the air–liquid interface (23).
Detailed materials and methods are available in the online supplement.
Results
MicroRNA Profiling Identifies Candidate Regulators of CFTR
Primary air–liquid interface (ALI) cultures of human airway epithelia replicate many features of in vivo airways (23, 24). We investigated miRNA expression in well-differentiated primary cultures of human non-CF and CF (homozygous for the CFTR-ΔF508 mutation) airway epithelia via quantitative PCR. Using a P-value cutoff of < 0.05, 18 miRNAs were found to be significantly differentially expressed in CF samples (see Table E1 in the online supplement). Of these, miR-509–3p and miR-494 were remarkable for a single predicted binding site each (Targetscan) within the CFTR 3′ UTR (Figure E1), suggesting that these miRNAs might cooperate to regulate CFTR expression posttranscriptionally.
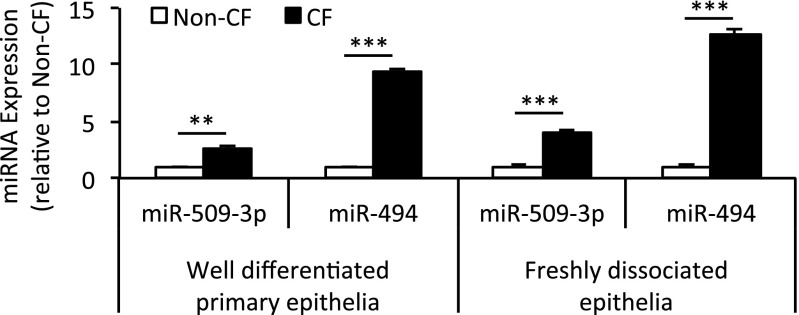
Both miR-509–3p and miR-494 exhibited increased expression in CF epithelia (Table E1). To validate this change in expression, we harvested RNA from well-differentiated primary cultures of human non-CF and CF (homozygous for CFTR-ΔF508) airway epithelia, and from freshly dissociated tracheobronchial airway epithelial cells from human non-CF and CF (homozygous for CFTR-ΔF508) donors. Using miRNA-specific quantitative RT-PCR, we confirmed that both miR-509–3p and miR-494 showed significantly greater expression in CF epithelia (Figure 1).
Figure 1.
Cystic fibrosis (CF) epithelia show increased expression of microRNA (miR)–509–3p and miR-494. RNA harvested from well-differentiated primary epithelia (six donors per genotype) and freshly dissociated tracheobronchial epithelia (five donors per genotype) were profiled for microRNA (miRNA) expression using quantitative RT-PCR. Error bars indicate mean ± SE. Statistical significance was determined by the Student t test. **P < 0.01. ***P < 0.001.
CFTR Is a Target of miR-509–3p and miR-494
The increased expression of miR-509–3p and miR-494 in CF epithelia led us to hypothesize that CFTR is regulated by both miRNAs. To test whether miR-509–3p and miR-494 repress CFTR expression by binding to its 3′ UTR, we performed a dual-luciferase reporter assay. The CFTR 3′ UTR was cloned into the psiCHECK-2 vector and transfected into HEK293T cells with increasing concentrations of either the miR-509–3p mimic (Figure E2A) or the miR-494 mimic (Figure E2B). The results demonstrated a dose-dependent repression of luciferase expression for each mimic. Mutation of the miR-509–3p (Figure E2A) or the miR-494 (Figure E2B) binding site relieved repression by the miRNAs in vitro, indicating that the repression was site-specific.
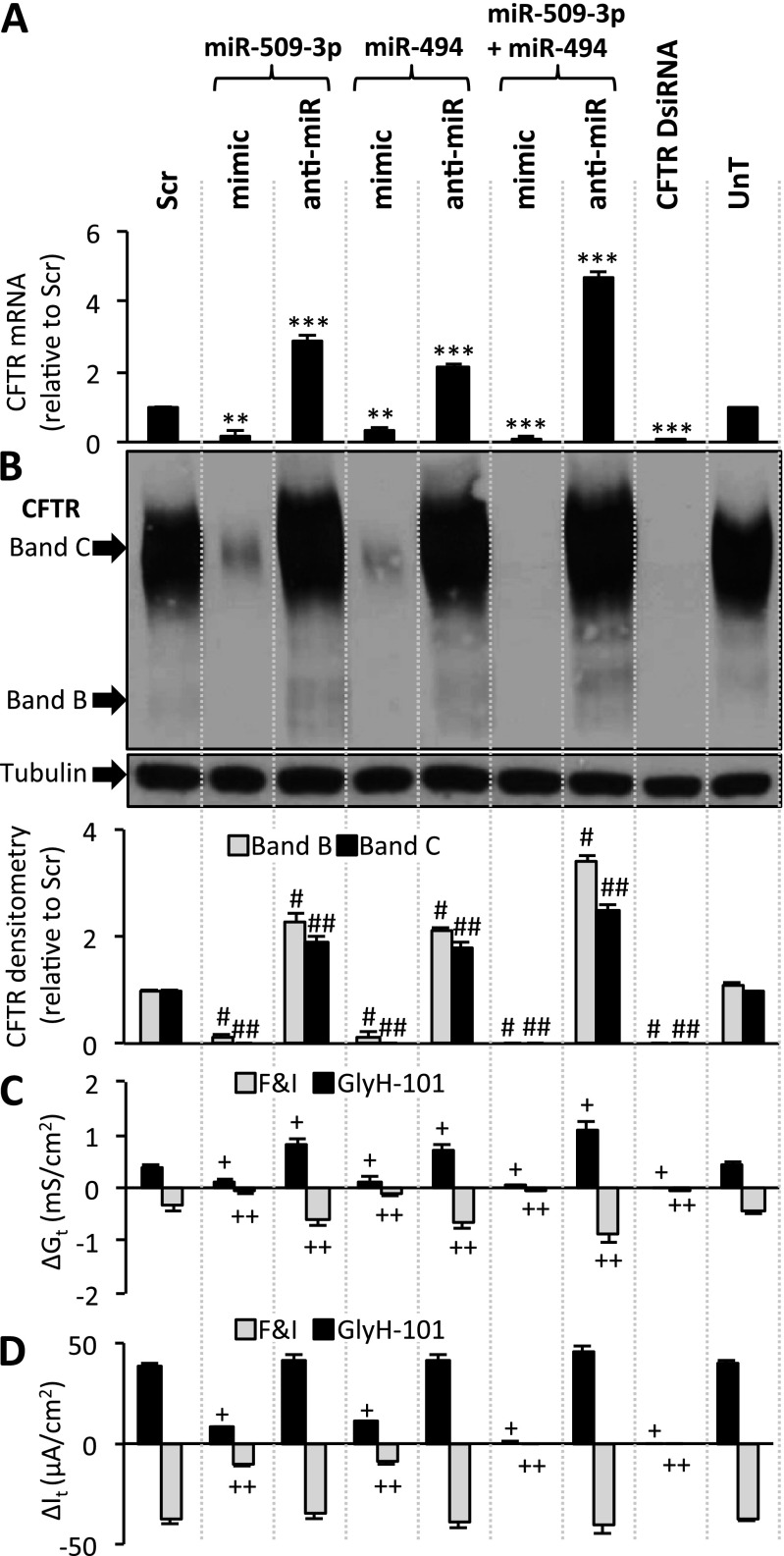
We measured the effects of these miRNAs on endogenous CFTR concentrations in human airway epithelial cells and Calu-3 epithelial cells that express CFTR abundantly (25). We note that in contrast to the expression of recombinant CFTR, endogenous CFTR expression in native tissue and primary airway epithelia produces mainly the band C form of the protein and little band B (26, 27). We have not used radioactive phosphorylation techniques to amplify the signal, and for this reason the band B abundance is often at the lower limits of detection. Transfecting cells with the miR-509–3p mimic caused a significant decrease in CFTR mRNA and protein concentrations in both airway epithelial cells (Figures 2A and 2B) and Calu-3 cells (Figures E3A and E3B). The opposite effects were observed with the miR-509–3p anti-miR, wherein CFTR mRNA and protein expression increased in both airway epithelial cells (Figures 2A and 2B) and Calu-3 cells (Figures E3A and E3B). Similar results were observed with the miR-494 mimic and anti-miR (airway epithelia, Figures 2A and 2B; Calu-3 cells, Figures E3A and E3B), indicating that both miRNAs are potent posttranscriptional repressors of CFTR. Because CFTR creates ion permeability, its function can be assayed by measuring transepithelial electrical conductance in polarized epithelial cells. Transfection with either miR mimic resulted in a significant reduction in CFTR-mediated Cl− conductance (Gt) and current (It) in both polarized ALI primary airway epithelial cell cultures (Figures 2C, 2D, and E4) and polarized ALI Calu-3 epithelial cell cultures (Figures E3C and E3D). The opposite effect was observed after transfecting Calu-3 cells with the anti-miR for each miRNA (Figures E3C and E3D). In polarized ALI primary airway epithelial cell cultures, despite an increase in Gt, the anti-miRs did not increase It (Figures 2D and E4), consistent with the presence of other rate-limiting steps for Cl− secretion in airway epithelia (28). Furthermore, both miRNAs worked cooperatively, because transfecting cells with the mimics of both miRNAs together caused an even greater decrease in CFTR mRNA, protein, Gt, and It in primary airway epithelia (Figures 2A–2D and E4) and in Calu-3 cells (Figures E3A–E3D), compared with either intervention alone. A similar enhanced effect was seen with the combined anti-miR transfection, wherein an increase in CFTR mRNA, protein, Gt, and It was observed compared with either single intervention in primary airway epithelia (Figures 2A–2D) and in Calu-3 cells (Figures E3A–E3D). We note that the transfection of primary cells with anti-miRNAs, miRNA mimics, or Dicer substrate siRNA (DsiRNA) did not appreciably change the morphology of epithelia studied 4 weeks later (Figure E5). These findings provide functional evidence in primary human airway epithelia that CFTR-mediated anion transport is under cooperative posttranscriptional regulation by miRNAs.
Figure 2.
miR-509–3p and miR-494 regulate cystic fibrosis transmembrane conductance regulator (CFTR) gene expression and function in human non-CF airway epithelial cells. (A) CFTR mRNA abundance in primary airway epithelia, 24 hours after indicated transfections (n = 6 donors). (B) CFTR protein abundance in primary airway epithelia, 72 hours after transfection. Densitometry and relative fold change of CFTR protein abundance in primary airway epithelia were determined in six different human donors (eight replicates each). (C and D) Changes in (C) conductance (Gt) and (D) transepithelial current (It) with indicated treatments. Each bar represents data from four different human donors (six replicates each). Basal transepithelial resistance range, 311–383 ohms ⋅ cm2; current (It) range, 24–58 microamperes ⋅ cm2. DsiRNA, Dicer substrate siRNA; UnT, untransfected. Error bars indicate the mean ± SE. Statistical significance was determined by the Student t test. **P < 0.01 and ***P < 0.001, relative to scrambled control (Scr). #P < 0.01, relative to Scr CFTR band B. ##P < 0.01, relative to Scr CFTR band C. +P < 0.01 and ++P < 0.01, relative to ΔGt or ΔIt in Scr-transfected samples upon forskolin and IBMX (F&I) or CFTR inhibitor GlyH-101 treatment, respectively.
Infection and Inflammation Increase miR-509–3p and miR-494 Expression
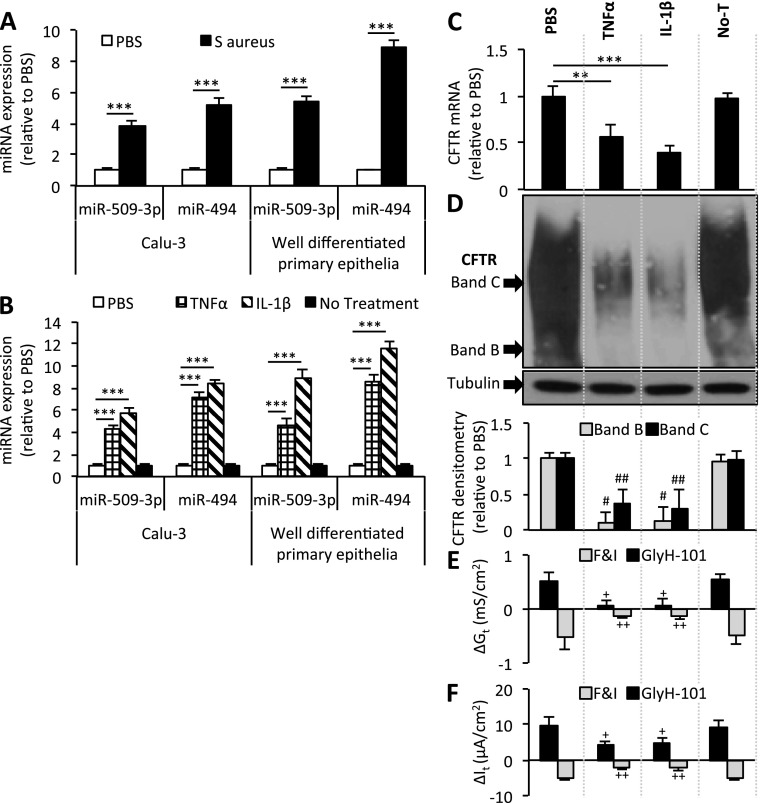
A characteristic feature of CF airway disease involves recurrent and chronic infection and inflammation (12). Exacerbations in other respiratory diseases, such as chronic obstructive pulmonary disease (COPD) and asthma, are also linked to infection and inflammation. We hypothesized that either or both of these stimuli might influence miR-509–3p and miR-494 concentrations, possibly explaining their increased expression in CF epithelia. To test this hypothesis, polarized cultures of Calu-3 epithelia and well-differentiated primary cultures of human non-CF airway epithelia were stimulated with an inoculum of exotoxin A–deficient Staphylococcus aureus. We observed a significant increase in miR-509–3p and miR-494 expression in both cell types (Figure 3A). S. aureus is a frequent pathogen in patients with CF, particularly during the first decade of life (29). Whereas S. aureus may elicit innate immune responses through multiple mechanisms, the increased expression of miR-509–3p and miR-494 was notable and raises questions about how complex infectious stimuli may alter CFTR expression in the airways.
Figure 3.
Bacteria stimuli and TNF-α and IL-1β regulate CFTR expression. (A) Calu-3–polarized cultures (n = 10) and well-differentiated primary epithelia (eight donors) were apically stimulated with an inoculum of Staphylococcus aureus (USA300Δhla mutant) suspended in PBS (1.0 × 107 colony-forming units/millicell). RNA was harvested 48 hours after stimulation, and profiled for miRNA expression by quantitative RT-PCR. (B) Calu-3–polarized cultures (n = 10) and well-differentiated primary epithelia (eight donors) were basolaterally stimulated with 100 ng/ml of the indicated cytokines. RNA was harvested 8 hours after stimulation and profiled for miRNA expression by quantitative RT-PCR. (C) CFTR mRNA concentrations were determined according to quantitative RT-PCR in well-differentiated primary epithelia. (n = 8). (D) CFTR protein abundance 8 hours after stimulation in well-differentiated primary epithelia. Densitometry and relative fold change of CFTR protein abundance (n = 3, three replicates each) were ascertained. Changes in (E) conductance (Gt) and (F) transepithelial current (It) were measured in well-differentiated primary epithelia, 8 hours after the indicated treatments (n = 6, two replicates each). Basal transepithelial resistance range, 191–264 ohms · cm2; It range, 9–28 microamperes · cm2. No-T, no treatment. Error bars indicate the mean ± SE. Statistical significance was determined by the Student t test. **P < 0.01, ***P < 0.001, and #P < 0.01, relative to PBS CFTR band B. ##P < 0.01, relative to PBS CFTR band C. +P < 0.01 and ++P < 0.01, relative to ΔGt or ΔIt in PBS-treated samples upon forskolin and IBMX (F&I) or CFTR inhibitor GlyH-101 treatment, respectively.
We next asked whether a proinflammatory cytokine stimulus might influence the expression of miR-509–3p and miR-494. Treatment with either TNF-α or IL-1β caused a significant increase in miR-509–3p and miR-494 expression in both cell types (Figure 3B). These results suggest that miR-509–3p and miR-494 are regulated in part by a common pathway that responds to bacterial and proinflammatory stimuli.
TNF-α and IL-1β Regulate CFTR Expression
Previous studies suggested a role for proinflammatory cytokines in the regulation of CFTR expression (30–37). To test the possibility that the TNF-α–mediated or IL-1β–mediated increases in miR-509–3p and miR-494 expression influence CFTR abundance, we measured CFTR expression in well-differentiated primary cultures of human non-CF airway epithelia and polarized ALI cultures of Calu-3 airway epithelia after TNF-α or IL-1β treatment. The increased expression of miR-509–3p and miR-494 (Figure 3B) was associated with concurrent decreases in CFTR mRNA and protein concentrations in primary airway epithelia (Figures 3C and 3D) and Calu-3 cells (Figures E6A and E6B). Furthermore, TNF-α and IL-1β significantly diminished cyclic adenosine monophosphate (cAMP)–stimulated Gt, and It in primary airway epithelia (Figures 3E, 3F, and E7) and in polarized Calu-3 cultures (Figures E6C and E6D).
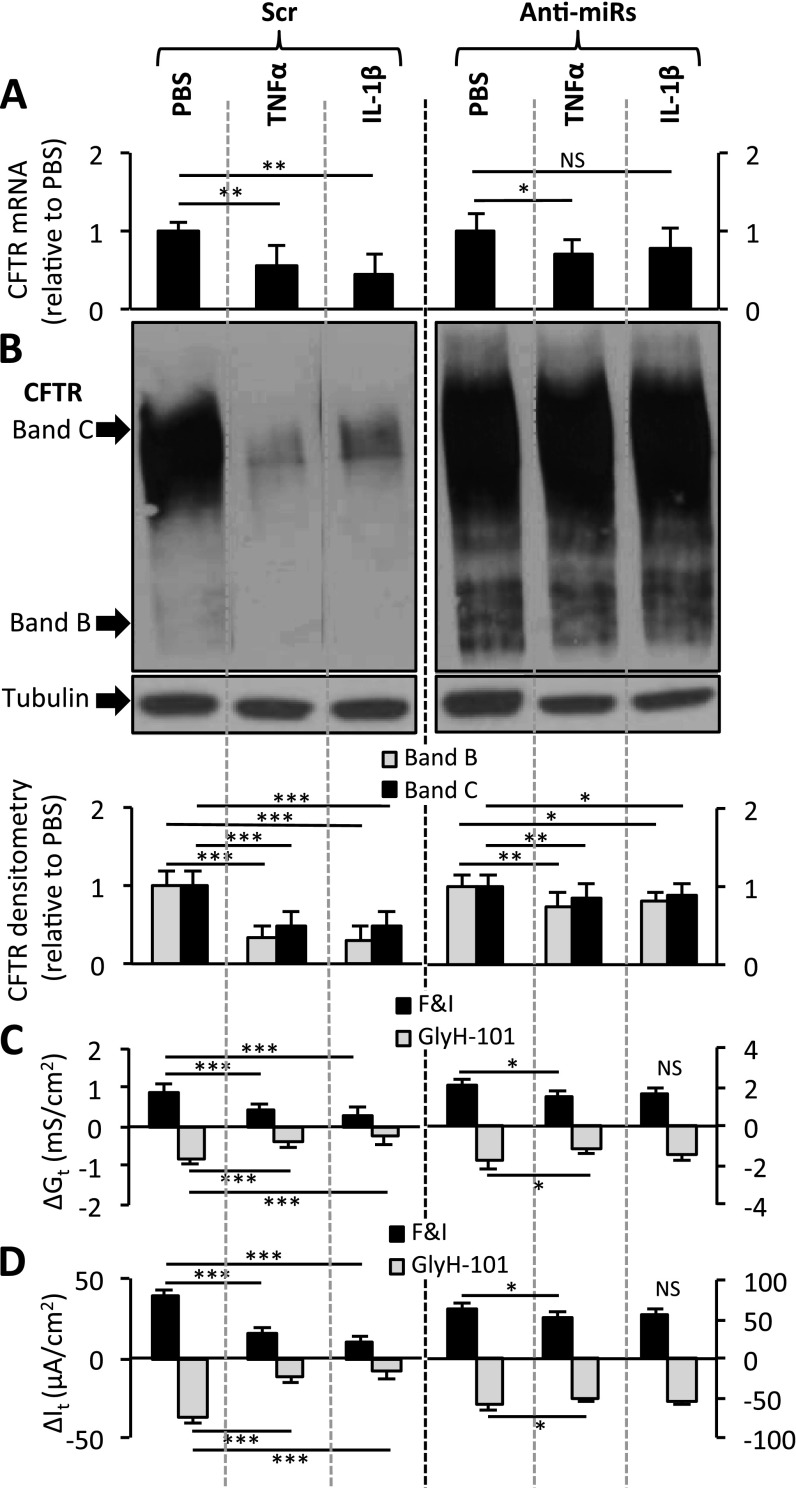
These results suggest the possibility that the repression of CFTR expression by TNF-α or IL-1β occurs via the action of miR-509–3p and miR-494. To test this hypothesis, we transfected Calu-3 and primary human non-CF airway epithelial cells with a scrambled control oligonucleotide (Scr oligo), or with the anti-miRs for both miR-509–3p and miR-494. Notably, anti-miR transfections did not interfere with the induction of miRNA expression in Calu-3 cells (Figure E8) or in primary airway epithelia (Figures E9A and E9B). After these procedures, we treated the cells with TNF-α or IL-1β. TNF-α or IL-1β stimulation alone (pretransfected with Scr oligo) decreased CFTR expression and function in Calu-3 cells (Figures 4A–4D), compared with the PBS control. However, the TNF-α–treated or IL-1β–treated Calu-3 cultures that were pretransfected with the anti-miRs showed a significantly reduced effect on CFTR mRNA, protein, Gt, and It (Figures 4A–4D). Similar results were observed in primary cultures of human non-CF airway epithelia, where the absence of miRNA activity significantly reduced the effects of TNF-α or IL-1β on CFTR mRNA concentrations (Figure E9C).
Figure 4.
TNF-α and IL-1β regulate CFTR expression via the actions of miR-509–3p and miR-494. (A) CFTR mRNA concentrations were determined by quantitative RT-PCR in polarized Calu-3 cells, 8 hours after cytokine stimulation. Cultures were transfected with oligonucleotides 10 days before cytokine stimulation (n = 4, four replicates each). (B) CFTR immunoblot in pretransfected Calu-3 cells, 8 hours after PBS or TNF-α or IL-1β stimulation. Densitometry and relative fold change of CFTR protein abundance (n = 4, four replicates each) were determined. Immunoblotting was rearranged to suit the presentation order. Changes in (C) conductance (Gt) and (D) transepithelial current (It) were measured in polarized Calu-3 cells with the indicated treatments (n = 4). Basal transepithelial resistance range, 294–415 ohms · cm2; It range, 39–63 microamperes · cm2. All error bars indicate the mean ± SE. Statistical significance was determined by the Student t test. *P < 0.05. **P < 0.01. ***P < 0.001. NS, not statistically significant.
To examine the influence of miRNA activity on cytokine-mediated CFTR repression, we performed two-way ANOVA. The results indicate that the repression of CFTR expression by TNF-α and IL-1β is greater when miR-509–3p and miR-494 bind to the CFTR 3′ UTR (Calu-3 cells, Table E2A; primary airway epithelia, Table E2B). Interestingly, the effect of IL-1β on CFTR was almost entirely abrogated by blocking both miRNAs (Figures 4 and E9C), suggesting that the IL-1β–stimulated repression of CFTR is more dependent on miR-509–3p and miR-494 than is that of TNF-α. These results indicate that the binding of miR-509–3p and miR-494 to the 3′ UTR of CFTR influences TNF-α–mediated and IL-1β–mediated regulation of CFTR expression in airway epithelia.
Expression of miR-509–3p and miR-494 Is Regulated by NF-κB Signaling
The increase in miRNAs in response to S. aureus or proinflammatory cytokines suggests the possibility that common transcriptional machinery is activated in response to these stimuli. A number of bacterial products are known to activate NF-κB signaling (38). Similarly, both TNF-α and IL-1β signal in part via NF-κB (39, 40). Because the promoter regions of miR-509–3p and miR-494 have not been characterized, we looked for NF-κB binding sites matching the general consensus binding sequence GGGRNNYYCC (R-purine and Y-pyrimidine) (41). We identified three predicted binding sites in the 5′ flanking sequence of each miRNA (Figure E10), suggesting that NF-κB may interact with the promoters of miR-509–3p and miR-494. Therefore, we hypothesized that NF-κB might contribute to the transcriptional activation of miR-509–3p and miR-494 in response to these cytokines.
To test this hypothesis, we first transduced polarized ALI cultures of Calu-3 cells and well-differentiated primary cultures of human non-CF airway epithelia with either an empty adenovirus (Ad-e) or an adenovirus expressing a dominant-negative IκB-α (Ad.IkBaS32/36A or Ad-dnIκB-α) (42) under the control of a cytomegalovirus (CMV) promoter. Three days later, we treated the cells with cytokines. Cells expressing the dominant-negative IκB-α failed to increase miR-509–3p and miR-494 concentrations significantly after stimulation with TNF-α or IL-1β, in contrast to control samples (Ad-e–transduced) (Calu-3 cells, Figures E11A and E11B; airway epithelia, Figures E12A and E12B). Hence, an activated NF-κB complex is needed for miR-509–3p and miR-494 induction by TNF-α or IL-1β, suggesting that the NF-κB transcription factor complex regulates the expression of miR-509–3p and miR-494.
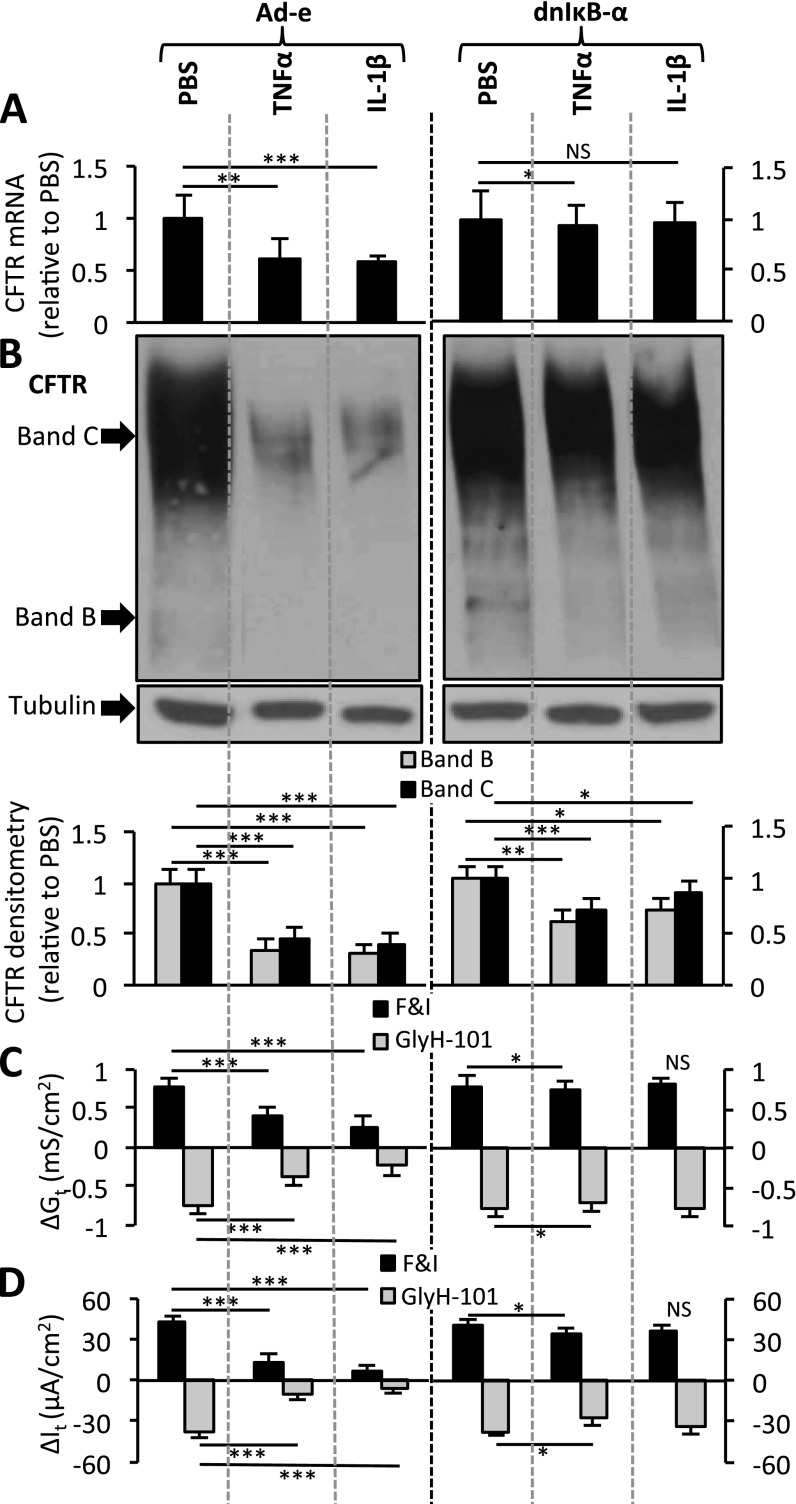
To address whether blocking NF-κB activity diminishes the effect of cytokines on CFTR, we compared the effect of cytokines on CFTR in the presence or absence of a dominant-negative inhibitor of NF-κB in Calu-3 cells (Figure 5). TNF-α or IL-1β stimulation alone (transduced with Ad-e) decreased CFTR expression and function (Figures 5A–5D), and increased miRNA concentrations (Figures E11A and E11B), compared with the PBS control samples. However, Calu-3 epithelia pretreated with the dominant-negative inhibitor of NF-κB and then treated with TNF-α or IL-1β showed almost no induction of miR-509–3p and miR-494 concentrations (Figures E11A and E11B), concurrent with a significantly diminished effect on CFTR mRNA, protein, Gt, and It (Figures 5A–5D). Similar results were observed in well-differentiated primary cultures of human non-CF airway epithelia, where the inhibition of NF-κB led to a reduced induction of miR-509–3p and miR-494 expression in response to TNF-α or IL-1β (Figures E12A and E12B), concurrent with significantly diminished effects on CFTR mRNA expression (Figure E12C).
Figure 5.
TNF-α and IL-1β require a functional NF-κB complex to regulate CFTR. (A) CFTR mRNA concentrations were ascertained according to quantitative RT-PCR in Calu-3–polarized cultures, 8 hours after cytokine stimulation (n = 4, four replicates each). Cultures were transduced 3 days before cytokine stimulation. (B) CFTR immunoblot in pretransduced Calu-3 cells, 8 hours after PBS or TNF-α or IL-1β stimulation. Densitometry and relative fold change of CFTR protein abundance (n = 4, three replicates each) were determined. Immunoblotting was rearranged to suit the presentation order. Changes in (C) conductance (Gt) and (D) transepithelial current (It) were measured 8 hours after the indicated treatments in Calu-3–polarized cultures (n = 4). Basal transepithelial resistance range, 318–479 ohms · cm2; and It range, 32–58 microamperes · cm2. All error bars indicate the mean ± SE. Statistical significance was determined by the Student t test. *P < 0.05, **P < 0.01, and ***P < 0.001. Ad-e, empty adenovirus; dnIκB-α, dominant-negative IκB-α; NS, not statistically significant.
To examine the influence of NF-κB activity on cytokine-mediated CFTR repression, we performed two-way ANOVA. The results indicate that the repression of CFTR expression by TNF-α and IL-1β is greater in the presence of a functional NF-κB complex (Calu-3 cells, Table E3A; primary airway epithelia, Table E3B). Thus, a dominant-negative IκB-α suppressed both the induction of miR-509–3p and miR-494, and the repression of CFTR in response to a proinflammatory stimulus. These data provide evidence that the cytokine-mediated repression of CFTR is regulated in part via the NF-κB–dependent induction of miR-509–3p and miR-494 and their actions on the CFTR 3′ UTR.
Discussion
Here we show that miR-509–3p and miR-494 influence CFTR expression in human airway epithelia. Both miRNAs repress CFTR mRNA, protein abundance, and function in a site-specific manner. Interestingly, the binding sites of both miRNAs lie within 100 base pairs of each other in the CFTR 3′ UTR. This finding, along with the enhanced effect seen when the mimic or anti-miR of both miRNAs is cotransfected, strongly suggests that miR-509–3p and miR-494 may act cooperatively to regulate CFTR expression and function in the airway epithelium. The expression of both miRNAs was increased in epithelia isolated from patients with CF. In addition, bacterial infection and first-response cytokines increased miR-509–3p and miR-494 concentrations. We show for the first time that TNF-α and IL-1β increase miR-509–3p and miR-494 concentrations, in part via the action of the NF-κB transcriptional activator complex, and that the increased abundance of miR-509–3p and miR-494 results in a correlative decrease of CFTR expression and function. These findings are significant because they implicate inflammatory responses, and in particular TNF-α or IL-1β, in miRNA-mediated CFTR repression.
Our findings are consistent with previous results indicating that the abundance of CFTR mRNA (17) and protein is similar in CF and non-CF airway epithelia (43). Although we did not detect a reduced abundance of CFTR mRNA in CF epithelia (Figure 6A), we note that the regulation of CFTR expression is complex, and that counterregulatory effectors may exert an impact (21, 35, 44–46). For example, NF-κB has been reported to interact directly with the CFTR promoter, positively regulating its transcription (35). Moreover, we previously showed that miR-138 positively regulates CFTR transcription and expression (21), increasing CFTR mRNA concentrations by de-repressing transcription. When we profiled for miRNA expression in well-differentiated primary cultures of human non-CF and CF (homozygous for CFTR-ΔF508) airway epithelia, we found miR-138 to be increased in CF airway epithelia (Table E1). These results suggest that counterregulatory effectors in CF airway epithelia could increase CFTR mRNA abundance.
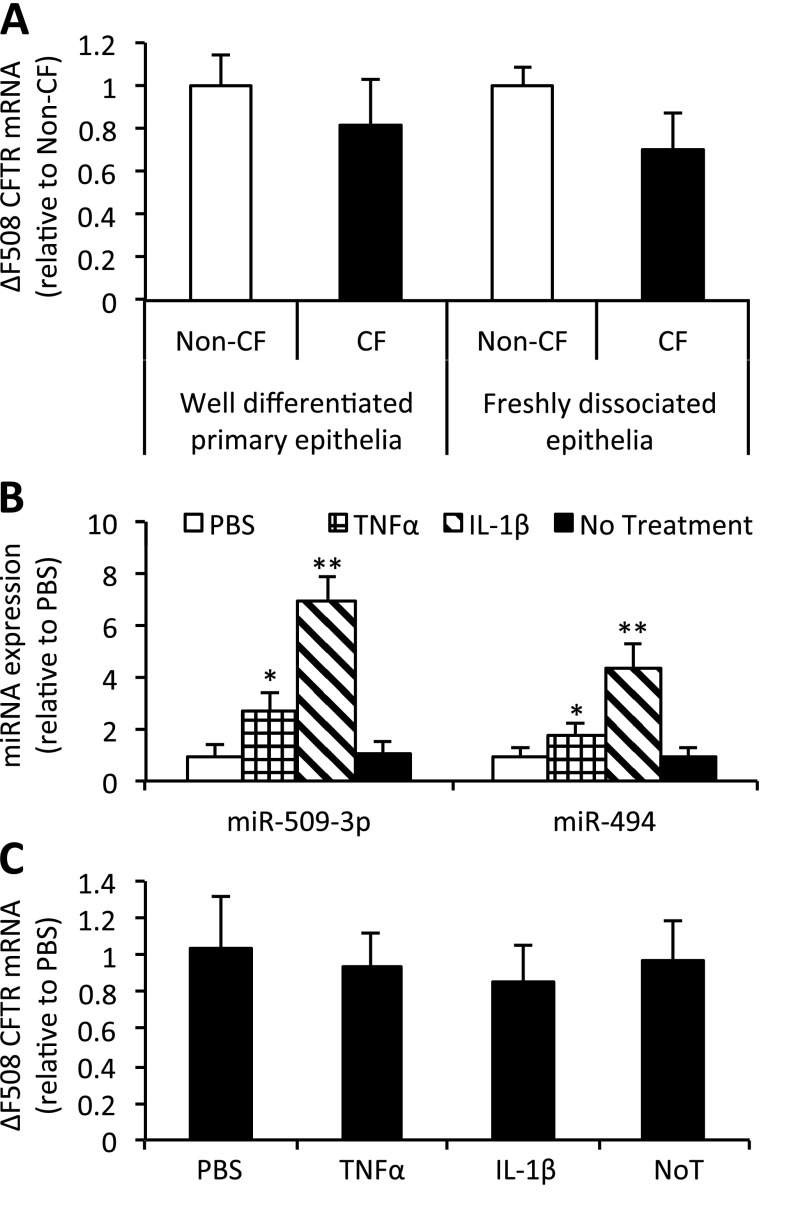
Figure 6.
CFTR mRNA expression is not significantly different in CF airway epithelia. (A) RNA harvested from well-differentiated primary epithelia (six donors per genotype) and from freshly dissociated tracheobronchial airway epithelia from donors (five donors per genotype) were profiled for CFTR mRNA concentrations by quantitative RT-PCR. CF donors were homozygous for CFTR-ΔF508. (B and C) Well-differentiated primary epithelia (three donors, with three cultures each) were basolaterally stimulated with 100 ng/ml of the indicated cytokines. RNA was harvested 8 hours after stimulation, and profiled for (B) miRNA expression and (C) CFTR mRNA concentrations according to quantitative RT-PCR. All error bars indicate the mean ± SE. Statistical significance was determined by the Student t test. *P < 0.05. **P < 0.01. NoT, no treatment.
Such counterregulatory mechanisms may also vary between genotypes. To test this, well-differentiated primary airway epithelia from three CF donors (homozygous for CFTR-ΔF508) were treated with PBS, TNF-α, or IL-1β. The abundance of both miR-509–3p and miR-494 increased (Figure 6B), although the magnitude of increase was lower compared with non-CF primary epithelia treated with cytokines (Figure 3B). This genotype-dependent difference could be attributable to the unavailability of the NF-κB transcription factor complex or a saturation of miRNA expression. CFTR mRNA concentrations remained unchanged after treatment (Figure 6C). This result suggests that CFTR mRNA is subject to regulatory pathways that may differ between CF and non-CF airway epithelia. In addition, the effects of chronic inflammatory stimuli may modify responses in CF epithelia.
The miRNA binding sites on the mutant CFTR 3′ UTR may possess an altered secondary structure, thereby preventing or reducing miRNA binding. The ΔF508 mutation was reported to change the secondary structure of CFTR mRNA (47). This might influence the site availability or its thermodynamics, factors known to influence miRNA binding (48–50). CFTR expression and biosynthesis are subject to complex regulatory mechanisms involving multiple pathways (16, 21, 46, 51), some interacting with the CFTR 3′ UTR (19, 30). These scenarios point to the possibility that the miRNA-mediated regulation of CFTR expression may be transient, dynamic, or subject to counterregulatory effectors that can increase CFTR transcription in CF airway epithelia (21, 35).
Oglesby and colleagues previously reported an increase in miR-494 concentrations in bronchial epithelial brushings from human CF airways (52). The repression of CFTR by miR-494 has also been reported previously (19, 20), without investigation of the functional effects on anion transport. Our results confirm an increase in miR-494 expression in human CF airway epithelia, and provide new evidence that miR-494 and miR-509–3p regulate both CFTR expression and anion transport. MiR-509–3p is a primate-specific small RNA transcript expressed from three distinct genomic loci within a 2-kb region on chromosome Xq, and has not previously been reported to play a functional role in airway epithelia.
The TNF-α–mediated and IL-1β–mediated regulation of CFTR expression was addressed in previous studies (30–37). TNF-α was reported to decrease CFTR expression (33) and reduce CFTR mRNA stability via the influence of mitogen-activated protein kinase cascades (30, 33), and may be more effective in reducing CFTR mRNA concentrations in combination with IFN-γ (35). IL-1β, on the other hand, exhibits a biphasic effect on CFTR expression (31, 37). Concentrations greater than 1 to 10 ng/ml decrease CFTR expression, whereas concentrations below 1 ng/ml exert the opposite effect. In experiments performed using human bronchial epithelial cells, low concentrations of IL-1β (with prolonged exposure) increased CFTR expression and function (37), whereas TNF-α exerted no effect on cAMP-dependent current (36). These studies emphasize the dose-dependent and time-dependent effects of cytokines on CFTR expression and function (30–37). Here we provide evidence that at higher concentrations and with acute exposure, TNF-α and IL-1β both repress CFTR via the induction of miRNAs that target the CFTR 3′ UTR, and coincidently alter CFTR function. Under the experimental conditions used in primary human airway epithelia and Calu-3 cells, we observed the TNF-α–mediated and IL-1β–mediated repression of CFTR function in airway epithelia. Although the genomic elements controlling the expression of miR-494 and miR-509–3p remain poorly understood, our findings indicate that inflammatory stimuli and NF-κB signaling play a role in regulating their abundance. The findings that both miRNAs increase in response to inflammatory stimuli suggest the possibility of a cooperative partnership between miR-509–3p and miR-494 in regulating ion and liquid transport.
miRNAs regulate multiple biological processes through their action on the 3′ UTR of target mRNA. Our discovery that miR-494 and miR-509–3p regulate both CFTR expression and function has implications for airway biology in health and disease. Because the overexpression of ΔF508-CFTR exerts little or no effect on rescuing Cl− transport in native epithelia (21, 53), increasing ΔF508-CFTR concentrations with anti-miRs will be unlikely to pose therapeutic benefit for CF (caused by ΔF508-CFTR). However, in other settings, influencing CFTR abundance via miRNAs may have therapeutic implications. One example involves the overexpression of miR-138, which we demonstrated can rescue ΔF508-CFTR maturation and function in CF airway epithelia (21). Our findings highlight the potential for miRNAs to regulate airway epithelial cell function acutely in response to environmental stimuli. The dynamic modulation of airway surface liquid (ASL) volume and composition is an important homeostatic function in the respiratory tract. We speculate that changes in CFTR abundance might acutely affect ASL volume and composition, host defense, and mucociliary clearance (54). Further studies are needed to better define the role of miRNAs in airway epithelial cell biology and pulmonary disease pathogenesis.
Acknowledgments
Acknowledgments
The authors thank Omar Itani, Patrick Sinn, and Colleen Stein for critically reviewing the manuscript. The authors acknowledge Dr. Kathryn Chaloner for assistance with biostatistical analyses. The authors also thank Thomas Moninger for preparing hematoxylin-and-eosin–stained paraffin sections for histochemistry.
Footnotes
This work was supported by National Institutes of Health grant R21 HL-91808 (P.B.M. and Y.X.), the Roy J. Carver Charitable Trust, and the Cystic Fibrosis Foundation Research Development Program (M.J.W.). M.J.W. is an Investigator at the Howard Hughes Medical Institute. The authors also acknowledge the support of the In Vitro Models and Cell Culture Core and the Gene Transfer Vector Core, partly supported by National Institutes of Health grants P01 HL-51670 (P.B.M.), P01 HL-091842 (M.J.W.), and P30 DK-54759, and by the Cystic Fibrosis Foundation.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2012-0430OC on May 6, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466:835–840. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bushati N, Cohen SM. MicroRNA functions. Annu Rev Cell Dev Biol. 2007;23:175–205. doi: 10.1146/annurev.cellbio.23.090506.123406. [DOI] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shkumatava A, Stark A, Sive H, Bartel DP. Coherent but overlapping expression of microRNAs and their targets during vertebrate development. Genes Dev. 2009;23:466–481. doi: 10.1101/gad.1745709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marcet B, Chevalier B, Luxardi G, Coraux C, Zaragosi LE, Cibois M, Robbe-Sermesant K, Jolly T, Cardinaud B, Moreilhon C, et al. Control of vertebrate multiciliogenesis by miR-449 through direct repression of the Delta/Notch pathway. Nat Cell Biol. 2011;13:693–699. doi: 10.1038/ncb2241. [DOI] [PubMed] [Google Scholar]
- 7.Harris KS, Zhang Z, McManus MT, Harfe BD, Sun X. Dicer function is essential for lung epithelium morphogenesis. Proc Natl Acad Sci USA. 2006;103:2208–2213. doi: 10.1073/pnas.0510839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams AE, Larner-Svensson H, Perry MM, Campbell GA, Herrick SE, Adcock IM, Erjefalt JS, Chung KF, Lindsay MA. MicroRNA expression profiling in mild asthmatic human airways and effect of corticosteroid therapy. PLoS ONE. 2009;4:e5889. doi: 10.1371/journal.pone.0005889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moschos SA, Williams AE, Perry MM, Birrell MA, Belvisi MG, Lindsay MA. Expression profiling in vivo demonstrates rapid changes in lung microRNA levels following lipopolysaccharide-induced inflammation but not in the anti-inflammatory action of glucocorticoids. BMC Genomics. 2007;8:240. doi: 10.1186/1471-2164-8-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams AE, Perry MM, Moschos SA, Lindsay MA. MicroRNA expression in the aging mouse lung. BMC Genomics. 2007;8:172. doi: 10.1186/1471-2164-8-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 12.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 13.Koehler DR, Downey GP, Sweezey NB, Tanswell AK, Hu J. Lung inflammation as a therapeutic target in cystic fibrosis. Am J Respir Cell Mol Biol. 2004;31:377–381. doi: 10.1165/rcmb.2004-0124TR. [DOI] [PubMed] [Google Scholar]
- 14.Muir A, Soong G, Sokol S, Reddy B, Gomez MI, Van Heeckeren A, Prince A. Toll-like receptors in normal and cystic fibrosis airway epithelial cells. Am J Respir Cell Mol Biol. 2004;30:777–783. doi: 10.1165/rcmb.2003-0329OC. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Z, Ott CJ, Lewandowsa MA, Leir SH, Harris A. Molecular mechanisms controlling CFTR gene expression in the airway. J Cell Mol Med. 2012;16:1321–1330. doi: 10.1111/j.1582-4934.2011.01439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewandowska MA, Costa FF, Bischof JM, Williams SH, Soares MB, Harris A. Multiple mechanisms influence regulation of the cystic fibrosis transmembrane conductance regulator gene promoter. Am J Respir Cell Mol Biol. 2010;43:334–341. doi: 10.1165/rcmb.2009-0149OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trapnell BC, Chu C-S, Paakko PK, Banks TC, Yoshimura K, Ferrans VJ, Chernick MS, Crystal RG. Expression of the cystic fibrosis transmembrane conductance regulator gene in the respiratory tract of normal individuals and individuals with cystic fibrosis. Proc Natl Acad Sci USA. 1991;88:6565–6569. doi: 10.1073/pnas.88.15.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukherji S, Ebert MS, Zheng GX, Tsang JS, Sharp PA, van Oudenaarden A. MicroRNAs can generate thresholds in target gene expression. Nat Genet. 2011;43:854–859. doi: 10.1038/ng.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gillen AE, Gosalia N, Leir SH, Harris A. MicroRNA regulation of expression of the cystic fibrosis transmembrane conductance regulator gene. Biochem J. 2011;438:25–32. doi: 10.1042/BJ20110672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Megiorni F, Cialfi S, Dominici C, Quattrucci S, Pizzuti A. Synergistic post-transcriptional regulation of the cystic fibrosis transmembrane conductance regulator (CFTR) by miR-101 and miR-494 specific binding. PLoS ONE. 2011;6:e26601. doi: 10.1371/journal.pone.0026601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramachandran S, Karp PH, Jiang P, Ostedgaard LS, Walz AE, Fisher JT, Keshavjee S, Lennox KA, Jacobi AM, Rose SD, et al. A microRNA network regulates expression and biosynthesis of wild-type and DeltaF508 mutant cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci USA. 2012;109:13362–13367. doi: 10.1073/pnas.1210906109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramachandran S, Clarke LA, Scheetz TE, Amaral MD, McCray PB., Jr Microarray mRNA expression profiling to study cystic fibrosis. Methods Mol Biol. 2011;742:193–212. doi: 10.1007/978-1-61779-120-8_12. [DOI] [PubMed] [Google Scholar]
- 23.Karp PH, Moninger TO, Weber SP, Nesselhauf TS, Launspach JL, Zabner J, Welsh MJ. An in vitro model of differentiated human airway epithelia: methods for establishing primary cultures. Methods Mol Biol. 2002;188:115–137. doi: 10.1385/1-59259-185-X:115. [DOI] [PubMed] [Google Scholar]
- 24.Yamaya M, Finkbeiner WE, Chun SY, Widdicombe JH. Differentiated structure and function of cultures from human tracheal epithelium. Am J Physiol. 1992;262:L713–L724. doi: 10.1152/ajplung.1992.262.6.L713. [DOI] [PubMed] [Google Scholar]
- 25.Haws C, Finkbeiner WE, Widdicombe JH, Wine JJ. CFTR in Calu-3 human airway cells: channel properties and role in cAMP-activated Cl− conductance. Am J Physiol. 1994;266:L502–L512. doi: 10.1152/ajplung.1994.266.5.L502. [DOI] [PubMed] [Google Scholar]
- 26.Varga K, Jurkuvenaite A, Wakefield J, Hong JS, Guimbellot JS, Venglarik CJ, Niraj A, Mazur M, Sorscher EJ, Collawn JF, et al. Efficient intracellular processing of the endogenous cystic fibrosis transmembrane conductance regulator in epithelial cell lines. J Biol Chem. 2004;279:22578–22584. doi: 10.1074/jbc.M401522200. [DOI] [PubMed] [Google Scholar]
- 27.Ostedgaard LS, Meyerholz DK, Chen JH, Pezzulo AA, Karp PH, Rokhlina T, Ernst SE, Hanfland RA, Reznikov LR, Ludwig PS, et al. The ΔF508 mutation causes CFTR misprocessing and cystic fibrosis–like disease in pigs. Sci Transl Med. 2011;3:74ra24. doi: 10.1126/scitranslmed.3001868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farmen SL, Karp PH, Ng P, Palmer DJ, Koehler DR, Hu J, Beaudet AL, Zabner J, Welsh MJ. Gene transfer of CFTR to airway epithelia: low levels of expression are sufficient to correct Cl− transport and overexpression can generate basolateral CFTR. Am J Physiol Lung Cell Mol Physiol. 2005;289:L1123–L1130. doi: 10.1152/ajplung.00049.2005. [DOI] [PubMed] [Google Scholar]
- 29.Szaff M, Høiby N. Antibiotic treatment of Staphylococcus aureus infection in cystic fibrosis. Acta Paediatr Scand. 1982;71:821–826. doi: 10.1111/j.1651-2227.1982.tb09526.x. [DOI] [PubMed] [Google Scholar]
- 30.Baudouin-Legros M, Hinzpeter A, Jaulmes A, Brouillard F, Costes B, Fanen P, Edelman A. Cell-specific posttranscriptional regulation of CFTR gene expression via influence of MAPK cascades on 3′UTR part of transcripts. Am J Physiol Cell Physiol. 2005;289:C1240–C1250. doi: 10.1152/ajpcell.00595.2004. [DOI] [PubMed] [Google Scholar]
- 31.Cafferata EG, González-Guerrico AM, Giordano L, Pivetta OH, Santa-Coloma TA. Interleukin-1beta regulates CFTR expression in human intestinal T84 cells. Biochim Biophys Acta. 2000;1500:241–248. doi: 10.1016/s0925-4439(99)00105-2. [DOI] [PubMed] [Google Scholar]
- 32.Cafferata EG, Guerrico AM, Pivetta OH, Santa-Coloma TA. NF-kappaB activation is involved in regulation of cystic fibrosis transmembrane conductance regulator (CFTR) by interleukin-1beta. J Biol Chem. 2001;276:15441–15444. doi: 10.1074/jbc.M010061200. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura H, Yoshimura K, Bajocchi G, Trapnell BC, Pavirani A, Crystal RG. Tumor necrosis factor modulation of expression of the cystic fibrosis transmembrane conductance regulator gene. FEBS Lett. 1992;314:366–370. doi: 10.1016/0014-5793(92)81507-i. [DOI] [PubMed] [Google Scholar]
- 34.Besançon F, Przewlocki G, Baró I, Hongre AS, Escande D, Edelman A. Interferon-gamma downregulates CFTR gene expression in epithelial cells. Am J Physiol. 1994;267:C1398–C1404. doi: 10.1152/ajpcell.1994.267.5.C1398. [DOI] [PubMed] [Google Scholar]
- 35.Brouillard F, Bouthier M, Leclerc T, Clement A, Baudouin-Legros M, Edelman A. NF-kappa B mediates up-regulation of CFTR gene expression in Calu-3 cells by interleukin-1beta. J Biol Chem. 2001;276:9486–9491. doi: 10.1074/jbc.M006636200. [DOI] [PubMed] [Google Scholar]
- 36.Galietta LJ, Folli C, Marchetti C, Romano L, Carpani D, Conese M, Zegarra-Moran O. Modification of transepithelial ion transport in human cultured bronchial epithelial cells by interferon-gamma. Am J Physiol Lung Cell Mol Physiol. 2000;278:L1186–L1194. doi: 10.1152/ajplung.2000.278.6.L1186. [DOI] [PubMed] [Google Scholar]
- 37.Gray T, Coakley R, Hirsh A, Thornton D, Kirkham S, Koo JS, Burch L, Boucher R, Nettesheim P. Regulation of MUC5AC mucin secretion and airway surface liquid metabolism by IL-1beta in human bronchial epithelia. Am J Physiol Lung Cell Mol Physiol. 2004;286:L320–L330. doi: 10.1152/ajplung.00440.2002. [DOI] [PubMed] [Google Scholar]
- 38.Müller-Anstett MA, Müller P, Albrecht T, Nega M, Wagener J, Gao Q, Kaesler S, Schaller M, Biedermann T, Götz F. Staphylococcal peptidoglycan co-localizes with NOD2 and TLR2 and activates innate immune response via both receptors in primary murine keratinocytes. PLoS ONE. 2010;5:e13153. doi: 10.1371/journal.pone.0013153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Viemann D, Goebeler M, Schmid S, Klimmek K, Sorg C, Ludwig S, Roth J. Transcriptional profiling of IKK2/NF-kappa B– and p38 MAP kinase–dependent gene expression in TNF-alpha–stimulated primary human endothelial cells. Blood. 2004;103:3365–3373. doi: 10.1182/blood-2003-09-3296. [DOI] [PubMed] [Google Scholar]
- 40.Lin FS, Lin CC, Chien CS, Luo SF, Yang CM. Involvement of p42/p44 MAPK, JNK, and NF-kappaB in IL-1beta–induced ICAM-1 expression in human pulmonary epithelial cells. J Cell Physiol. 2005;202:464–473. doi: 10.1002/jcp.20142. [DOI] [PubMed] [Google Scholar]
- 41.Udalova IA, Mott R, Field D, Kwiatkowski D. Quantitative prediction of NF-kappa B DNA–protein interactions. Proc Natl Acad Sci USA. 2002;99:8167–8172. doi: 10.1073/pnas.102674699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fan C, Yang J, Engelhardt JF. Temporal pattern of NFkappaB activation influences apoptotic cell fate in a stimuli-dependent fashion. J Cell Sci. 2002;115:4843–4853. doi: 10.1242/jcs.00151. [DOI] [PubMed] [Google Scholar]
- 43.Kälin N, Claass A, Sommer M, Puchelle E, Tümmler B. DeltaF508 CFTR protein expression in tissues from patients with cystic fibrosis. J Clin Invest. 1999;103:1379–1389. doi: 10.1172/JCI5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blackledge NP, Ott CJ, Gillen AE, Harris A. An insulator element 3′ to the CFTR gene binds CTCF and reveals an active chromatin hub in primary cells. Nucleic Acids Res. 2009;37:1086–1094. doi: 10.1093/nar/gkn1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ott CJ, Bischof JM, Unti KM, Gillen AE, Leir SH, Harris A. Nucleosome occupancy reveals regulatory elements of the CFTR promoter. Nucleic Acids Res. 2012;40:625–637. doi: 10.1093/nar/gkr754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ott CJ, Blackledge NP, Kerschner JL, Leir SH, Crawford GE, Cotton CU, Harris A. Intronic enhancers coordinate epithelial-specific looping of the active CFTR locus. Proc Natl Acad Sci USA. 2009;106:19934–19939. doi: 10.1073/pnas.0900946106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bartoszewski RA, Jablonsky M, Bartoszewska S, Stevenson L, Dai Q, Kappes J, Collawn JF, Bebok Z. A synonymous single nucleotide polymorphism in DeltaF508 CFTR alters the secondary structure of the mRNA and the expression of the mutant protein. J Biol Chem. 2010;285:28741–28748. doi: 10.1074/jbc.M110.154575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baek D, Villén J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature. 2008;455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Long D, Lee R, Williams P, Chan CY, Ambros V, Ding Y. Potent effect of target structure on microRNA function. Nat Struct Mol Biol. 2007;14:287–294. doi: 10.1038/nsmb1226. [DOI] [PubMed] [Google Scholar]
- 50.Kertesz M, Iovino N, Unnerstall U, Gaul U, Segal E. The role of site accessibility in microRNA target recognition. Nat Genet. 2007;39:1278–1284. doi: 10.1038/ng2135. [DOI] [PubMed] [Google Scholar]
- 51.Ott CJ, Blackledge NP, Leir SH, Harris A. Novel regulatory mechanisms for the CFTR gene. Biochem Soc Trans. 2009;37:843–848. doi: 10.1042/BST0370843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oglesby IK, Bray IM, Chotirmall SH, Stallings RL, O’Neill SJ, McElvaney NG, Greene CM. MiR-126 is downregulated in cystic fibrosis airway epithelial cells and regulates TOM1 expression. J Immunol. 2010;184:1702–1709. doi: 10.4049/jimmunol.0902669. [DOI] [PubMed] [Google Scholar]
- 53.Granio O, Norez C, Ashbourne Excoffon KJ, Karp PH, Lusky M, Becq F, Boulanger P, Zabner J, Hong SS. Cellular localization and activity of Ad-delivered GFP-CFTR in airway epithelial and tracheal cells. Am J Respir Cell Mol Biol. 2007;37:631–639. doi: 10.1165/rcmb.2007-0026TE. [DOI] [PubMed] [Google Scholar]
- 54.Pezzulo AA, Tang XX, Hoegger MJ, Alaiwa MH, Ramachandran S, Moninger TO, Karp PH, Wohlford-Lenane CL, Haagsman HP, van Eijk M, et al. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature. 2012;487:109–113. doi: 10.1038/nature11130. [DOI] [PMC free article] [PubMed] [Google Scholar]