Abstract
Human pulmonary artery smooth muscle cells (HPASMCs) express both adenosine monophosphate–activated protein kinase (AMPK) α1 and α2. We investigated the distinct roles of AMPK α1 and α2 in the survival of HPASMCs during hypoxia and hypoxia-induced pulmonary hypertension (PH). The exposure of HPASMCs to hypoxia (3% O2) increased AMPK activation and phosphorylation, and the inhibition of AMPK with Compound C during hypoxia decreased their viability and increased lactate dehydrogenase activity and apoptosis. Although the suppression of either AMPK α1 or α2 expression led to increased cell death, the suppression of AMPK α2 alone increased caspase-3 activity and apoptosis in HPASMCs exposed to hypoxia. It also resulted in the decreased expression of myeloid cell leukemia sequence 1 (MCL-1). The knockdown of MCL-1 or MCL-1 inhibitors increased caspase-3 activity and apoptosis in HPASMCs exposed to hypoxia. On the other hand, the suppression of AMPK α1 expression alone prevented hypoxia-mediated autophagy. The inhibition of autophagy induced cell death in HPASMCs. Our results suggest that AMPK α1 and AMPK α2 play differential roles in the survival of HPASMCs during hypoxia. The activation of AMPK α2 maintains the expression of MCL-1 and prevents apoptosis, whereas the activation of AMPK α1 stimulates autophagy, promoting HPASMC survival. Moreover, treatment with Compound C, which inhibits both isoforms of AMPK, prevented and partly reversed hypoxia-induced PH in mice. Taking these results together, our study suggests that AMPK plays a key role in the pathogenesis of pulmonary arterial hypertension, and AMPK may represent a novel therapeutic target for the treatment of pulmonary arterial hypertension.
Keywords: hypoxia, adenosine monophosphate–activated protein kinase, pulmonary artery smooth muscle cells, apoptosis, autophagy
Clinical Relevance
Our results suggest that adenosine monophosphate–activated protein kinase (AMPK) α1 and AMPK α2 play differential roles in the survival of human pulmonary artery smooth muscle cells (HPASMCs) during hypoxia. The activation of AMPK α2 maintains the expression of MCL-1 and prevents apoptosis, whereas the activation of AMPK α1 stimulates autophagy, promoting HPASMC survival. Treatment with an AMPK inhibitor prevented and partly reversed hypoxia-induced pulmonary hypertension in mice. Thus, our study suggests that AMPK plays a key role in the pathogenesis of pulmonary arterial hypertension (PAH), and AMPK may represent a novel therapeutic target for the treatment of PAH.
Pulmonary arterial hypertension (PAH) is a devastating disease characterized by persistent increases in pulmonary arterial pressure, ultimately leading to right ventricular failure and death (1, 2). Despite recent improvements in treatment, the annual mortality rate of patients with PAH remains at around 20% (3). Both active vasoconstriction and vascular remodeling with smooth muscle cell proliferation contribute to PAH (4). Hypoxia is a well-established stimulus for the induction of a modest and variable magnitude of pulmonary hypertension (PH) in several animal models in which hypoxia induces the survival and proliferation of pulmonary artery smooth muscle cells (PASMCs) (5). However, the mechanisms by which hypoxia induces PASMC survival remain unclear.
Adenosine monophosphate–activated protein kinase (AMPK) is a serine–threonine kinase composed of three subunits, α, β, and γ (6–8). The α subunit contains the kinase domain, where threonine-172 can be phosphorylated by upstream kinases, whereas the β and γ subunits have a regulatory function. Two α, two β, and three γ subunits have been identified, and 12 possible holoenzymes are expressed in a tissue-specific and subcellular location–specific pattern (9). In the lung, both isoforms of the catalytic α subunit are expressed (6, 10, 11). During hypoxia, AMPK serves as a metabolic master switch to maintain cellular energy homeostasis (12, 13). Hypoxia increases the ratio of adenosine monophosphate (AMP)/ATP, and facilitates the association of AMP with the γ subunit, leading to the activation of AMPK (10, 14). Recent reports suggest that during acute or moderate hypoxia, Ca2+/calmodulin-dependent protein kinase kinase activates AMPK in several cell lines, independent of the AMP/ATP ratio (15, 16).
AMPK is known to regulate cell survival and proliferation (17). AMPK is activated in rapidly proliferating cells such as cardiac fibroblasts and cancer cells (18, 19). Furthermore, in some cell types, AMPK is critical in cell-cycle regulation, the decision to enter autophagy or apoptosis, and other cell-fate decisions during development (17). In systemic vascular smooth muscle cells, a few studies suggest that the activation of AMPK inhibits cell proliferation (20, 21). However, it remains unknown whether AMPK plays a role in the survival of PASMCs during hypoxia.
In this study, we show that hypoxia activates AMPK in human PASMCs (HPASMCs), and that the inhibition of either isoform of the catalytic subunit of AMPK leads to the death of HPASMCs when exposed to hypoxia. The inhibition of AMPK α1 prevented hypoxia-induced autophagy, causing HPASMC death. The inhibition of AMPK α2 decreased the expression of myeloid cell leukemia sequence 1 (MCL-1), leading to cell apoptosis. Moreover, we found that an AMPK inhibitor, Compound C, that inhibited both isoforms prevented and partly reversed hypoxia-induced PH. Therefore, our study provides evidence for the first time that AMPK plays a key role in the survival of PASMCs during hypoxia, and that the differential mechanisms by which AMPK α1 and α2 promote cell survival may represent potential therapeutic targets for the treatment of PAH.
Materials and Methods
Cell Culture and Western Blot Analysis
HPASMCs were obtained from Lonza (Walkersville, MD) (22). HPASMCs isolated from patients and donors with idiopathic PAH (IPAH) were used in this study, and were provided by the Pulmonary Hypertension Breakthrough Initiative. Wild-type (WT), AMPK α1–null, and AMPK α2–null mouse embryo fibroblasts (MEFs) were described in a previous study (23). Cells at Passages 6–9 were used for these experiments. Hypoxia (3% O2) was achieved using INVIVO2 300 (Ruskinn Technology Limited, Bridgend, UK) with an O2 sensor. The antibodies used are described in the online supplement. The density of protein bands was determined using ImageJ (National Institutes of Health, Bethesda, MD).
Inhibition of AMPK, MCL-1, and Autophagy
Compound C (EMD Chemicals, Gibbstown, NJ), an inhibitor of AMPK, was dissolved in dimethylsulfoxide (DMSO) and diluted to a 10-μM concentration, using smooth muscle cell basal medium (SmBM) media. Obatoclax mesylate (GX 15-070) and TW-37 (Selleck, Houston, TX), which are both MCL-1/B-cell lymphoma 2 (BCL-2) inhibitors, were used at various concentrations. 3-methyladenine (3-MA) (Sigma-Aldrich, St. Louis, MO) was used as a specific inhibitor of autophagy (24). HPASMCs were pretreated with these chemicals for 1 hour before exposure to normoxia or hypoxia. Small interfering RNAs (siRNAs) were used to suppress AMPK α1 and AMPK α2 expression (Santa Cruz Biotechnology, Santa Cruz, CA) and MCL-1 (Cell Signaling Danvers, MA), with scrambled siRNA (Ambion/Applied Biosystems, Foster City, CA) as control.
Cell Viability Analysis, Lactate Dehydrogenase Assay, and Cell Apoptosis
Cell viability was determined using the Cell Titer 96 AQueous One Solution Cell Proliferation Assay (Promega Corporation, Madison, WI). Cell death was measured with the Cytotoxicity Detection (using lactate dehydrogenase; LDH) Kit (Roche, Mannheim, Germany). Cell apoptosis was determined by three methods, namely, the detection of cleavage of caspase-3 by Western blot analysis, a colorimetric caspase-3 activity assay (Sigma-Aldrich), and a terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling (TUNEL) assay (Trevigen, Inc., Gaithersburg, MD). The experiments were performed in media containing 0.1% FBS.
Hypoxic Pulmonary Hypertension Model in Mice
Male C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME) were used throughout this study. All animals were handled according to National Institutes of Health guidelines, and the experimental protocols were approved by our Institutional Animal Care and Use Committee. Compound C (CC) was dissolved in DMSO and administered intraperitoneally once a week at a volume of 100 μl and a dose of 20 mg/kg body weight (25). We injected the first dose of CC 1 day before the exposure to normoxia or hypoxia. Mice were exposed to room air (normoxia) or 10% oxygen (hypoxia) for 3 weeks in a BioSpherix A chamber (BioSpherix, Lacona, NY), and the oxygen concentration (10%) was monitored with a Proox Model P110 oxygen controller (BioSpherix). Mice were weighed before the experiment and once a week during the experiment. Right-ventricular (RV) pressure was measured with a 1.4-French pressure transducer catheter (Millar Instruments, Houston, TX) and AcqKnowledge software (Biopac Systems, Inc., Goleta, CA), as previously described (26). Right-ventricular systolic pressure (RVSP) was recorded and used as a surrogate for pulmonary artery pressure. The blood was drawn to determine a complete blood count, and hearts were excised and dissected to determine the right ventricle/(left ventricle + septum) (RV/[LV + S]) ratio as a parameter of RV hypertrophy. Lung tissues were fixed, embedded, and sectioned. Slides were stained with hematoxylin and eosin for morphometric analysis to quantitate pulmonary arterial wall thickness (27), and were immunolabeled with phospho-AMPK (pAMPK) and α–smooth muscle actin (α–SMA) antibodies for immunofluorescence microscopy analysis. To test whether AMPK inhibition would reverse the development of PAH, we exposed C57BL/6 mice to hypoxia or normoxia for 2 weeks, followed by the injection of CC or DMSO once weekly for another 2 weeks.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism, version 4 (GraphPad, La Jolla, CA), and P < 0.05 was considered statistically significant.
Results
AMPK Is Essential for HPASMC Survival during Hypoxia
To investigate whether hypoxia increases AMPK phosphorylation in HPASMCs, we exposed the cells to normoxia (21% O2) or hypoxia (3% O2) for 15 minutes, 30 minutes, and up to 24 hours, followed by the measurement of phosphorylated and total AMPK according to Western blot analysis. As shown in Figure 1A, hypoxia increased AMPK phosphorylation at Thr-172, which was maximal after a 15-minute exposure to hypoxia. After a longer exposure to hypoxia (up to 24 h), AMPK phosphorylation levels were back to basal concentrations (Figure 1B). The total concentrations of AMPK remained unchanged. These results suggest that hypoxia transiently activates AMPK in HPASMCs.
Figure 1.
The activation of adenosine monophosphate–activated protein kinase (AMPK) is essential for the survival of human pulmonary artery smooth muscle cells (HPASMCs) during hypoxia. (A and B) HPASMCs were exposed to normoxia (N) (21% O2) or hypoxia (H) (3% O2) for 15 and 30 minutes (A) and 24 hours (B). The amounts of phospho-AMPK (pAMPK) at Thr-172 (T172) and total AMPK were determined, and the pAMPK/AMPK ratios were calculated. (C-F) HPASMCs were pretreated with dimethylsulfoxide (DMSO) or 10 μM Compound C (CC) for 1 hour, and then incubated under normoxia (N) or hypoxia (H) for 24 hours for the cell viability assay (C), 8 hours for the lactate dehydrogenase (LDH) assay (D), and for up to 5 hours to detect the cleavage of caspase-3 (E) and for a terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling (TUNEL) assay (F). Data are expressed as means ± SEMs (n ≥ 3). *P < 0.05. **P < 0.01. Tubulin was used as loading control. CTL, control.
To determine whether AMPK is essential for the survival of HPASMCs during hypoxia, we pretreated HPASMCs with 10 μM CC, a reversible AMPK inhibitor for 1 hour, and then exposed the cells to normoxia or hypoxia for 24 hours, followed by the cell viability assay. As shown in Figure 1C, the inhibition of AMPK decreased the viability of HPASMCs exposed to hypoxia, whereas the inhibition of AMPK exerted little effect on the viability of HPASMCs exposed to normoxia. To determine whether the decreased viability was attributable to increased cell death, and to determine the time course of cell death, we pretreated HPASMCs with CC, and then exposed HPASMCs to normoxia or hypoxia for up to 8 hours, followed by the measurement of cell death with an LDH assay. We found that the inhibition of AMPK significantly increased cell death during hypoxia, whereas the inhibition of AMPK did not increase cell death during normoxia (Figure 1D). These results suggest that AMPK is necessary for the survival of HPASMCs during hypoxia.
To determine whether AMPK contributes to cell survival during hypoxia by preventing apoptosis, HPASMCs were pretreated with CC before exposure to normoxia or hypoxia for 4 or 5 hours. As shown in Figure 1E, HPASMCs treated with CC and exposed to hypoxia exhibited an increase in the amount of cleaved caspase-3 as early as 4 hours after exposure, suggesting that the inhibition of AMPK induces apoptosis during hypoxia. The inhibition of AMPK did not cause the cleavage of caspase-3 in HPASMCs exposed to normoxia. We confirmed these results by performing a TUNEL assay. As shown in Figure 1F, CC also increased TUNEL activity in HPASMCs exposed to hypoxia. These results suggest that the activation of AMPK prevents cell apoptosis during hypoxia.
Inhibition of AMPK α2 Induces Apoptosis of HPASMCs during Hypoxia
Because HPASMCs express both the α1 and α2 isoforms (6, 10, 11), we investigated which isoforms plays a role in cell survival during hypoxia. We transfected HPASMCs with siRNA against the AMPK α1 or α2 isoform, exposed them to normoxia or hypoxia for 6 hours, and determined the LDH activity to measure cell death. We found that siRNAs against AMPK α1 or α2 isoform specifically suppressed the expression of the AMPK α1 or α2 isoform, respectively (Figure 2A). The suppression of either AMPK α1 or α2 resulted in elevated LDH activity in HPASMCs exposed to hypoxia, suggesting that both AMPK α1 and α2 are essential for cell survival during hypoxia (Figure 2A). To confirm these results, we exposed WT, AMPK α1–null, and AMPK α2–null MEFs to normoxia or hypoxia for 8 hours, and measured the LDH activity. As shown in Figure 2B, hypoxia induced LDH activity in AMPK α1–null or AMPK α2–null MEFs.
Figure 2.
The inhibition of AMPK α2 induces HPASMC apoptosis during hypoxia. (A) HPASMCs were transfected with small interfering RNA (siRNA) against AMPK α1 or α2 before exposure to normoxia (N) or hypoxia (H) for 6 hours, and LDH activity was determined as already described. Scrambled siRNA (si-Neg) was used as control. The amounts of AMPK α1 and α2 in the cell lysates were determined by Western blot analysis. β-actin was used as loading control. (B) Wild-type (WT), AMPK α1–null, and AMPK α2–null mouse embryo fibroblasts (MEFs) were exposed to normoxia or hypoxia for 8 hours, and then LDH activity was measured. (C) HPASMCs transfected with siRNA against AMPK α1 or α2 were incubated during normoxia or hypoxia for 8 hours, and then caspase-3 activity was measured. (D) MEFs were exposed to normoxia or hypoxia for 8 hours, and cell lysates were collected to measure caspase-3 activity. Data are expressed as means ± SEMs (n ≥ 3). *P < 0.05.
To determine which specific isoforms of the catalytic α subunit are responsible for apoptosis during hypoxia, we transfected HPASMCs with siRNA against AMPK α1 or α2, exposed the cells to normoxia or hypoxia, and measured the caspase-3 activity. Our results showed that the suppression of AMPK α2 but not AMPK α1 increased caspase-3 activity in HPASMCs exposed to hypoxia (Figure 2C). The suppression of either AMPK α1 or α2 exerted little effect on caspase-3 activity in HPASMCs exposed to normoxia (Figure 2C), suggesting that AMPK α2 is critical for HPASMC survival during hypoxia by preventing apoptosis. We also exposed WT, AMPK α1–null, and AMPK α2–null MEFs to normoxia or hypoxia, and measured the caspase-3 activity. We found that caspase-3 activity was significantly increased in AMPK α2–null MEFs exposed to hypoxia (Figure 2D), confirming that AMPK α2 plays a role in the survival of HPASMCs during hypoxia via the prevention of apoptosis.
Suppression of AMPK α2 Reduces Expression of Prosurvival Protein MCL-1
Cell apoptosis is regulated by the balance between the amount of prosurvival proteins (e.g., MCL-1, B-cell lymphoma-extra large [BCL-XL], and BCL-2) and proapoptotic proteins (e.g., Bcl-2–associated death promoter [BAD] and BH3 interacting-domain death agonist [BID]) (28). To investigate the mechanism underlying AMPK α2–mediated HPASMC survival during hypoxia, we pretreated cells with 10 μM CC for 1 hour, and then exposed the cells to normoxia or hypoxia for 8 hours. We determined the amounts of prosurvival proteins MCL-1 and BCL-XL and proapoptotic BAD and BID by Western blot analysis. As shown in Figure 3A, treatment with CC resulted in a decrease of the concentration of MCL-1 during normoxia and hypoxia. However, treatment with CC did not alter the expression levels of BCL-XL, BAD, and BID. To determine which specific α isoform is responsible for MCL-1 expression, we transfected HPASMCs with siRNA against AMPK α1 or α2, exposed the cells to normoxia or hypoxia, and measured MCL-1 concentrations. We found that the suppression of AMPK α2 but not α1 significantly decreased MCL-1 in HPASMCs exposed to normoxia and hypoxia (Figure 3B), suggesting that AMPK α2 plays a role in the regulation of MCL-1 expression.
Figure 3.
Inhibition of AMPK α2 decreases the expression of prosurvival protein MCL-1, leading to HPASMC cell apoptosis during hypoxia. (A) HPASMCs were pretreated with dimethylsulfoxide (DMSO) or 10 μM Compound C (CC) for 1 hour, and then incubated under normoxia (N) or hypoxia (H) for 8 hours. The amount of prosurvival myeloid cell leukemia sequence 1 (MCL-1) and B-cell lymphoma-extra large (BCL-XL) and proapoptotic Bcl-2–associated death promoter (BAD) and BH3 interacting-domain death agonist (BID) in the cell lysates were determined by Western blot analysis. (B) Cells were transfected with siRNA against AMPK α1 or α2 (si-α1 or si-α2) and then exposed to normoxia (N) or hypoxia (H) for 6 hours. The amount of MCL-1 in the cell lysates was determined as described previously. Tubulin was used as loading control. The MCL-1/tubulin ratios are shown at the top. (C–E) HPASMCs were transfected with si–MCL-1 and exposed to normoxia (N) or hypoxia (H) for 6 hours. (C) The amount of MCL-1 in the cell lysates was determined by Western blot analysis, and the MCL-1/tubulin ratios are shown at the top. LDH activity (D) and caspase-3 activity (E) were determined as described previously. (F and G) HPASMCs were pretreated with various doses of obatoclax mesylate (GX 15-070) (F) and TW-37 (G) for 1 hour, and then incubated under normoxia (NMX) or hypoxia (HPX) for 24 hours. The HPASMC viability was measured as described previously. Data are expressed as means ± SEMs (n ≥ 3). *P < 0.05. **P < 0.01.
MCL-1 Is Required for the Survival of HPASMCs during Hypoxia
To investigate whether AMPK α2, by maintaining MCL-1 expression, prevents the apoptosis of HPASMCs during hypoxia, we transfected HPASMCs with siRNA against MCL-1 to suppress MCL-1 expression (Figure 3C), exposed the cells to normoxia or hypoxia for 6 hours, and measured cell death according to an LDH assay (Figure 3D) and caspase-3 activity (Figure 3E). We found that siRNA against MCL-1 specifically and efficiently suppressed the expression of MCL-1 (Figure 3C), and the suppression of MCL-1 induced LDH and caspase-3 activity in HPASMCs exposed to hypoxia (Figures 3D and 3E). The suppression of MCL-1 did not affect LDH and caspase-3 activity in HPASMCs exposed to normoxia (Figures 3D and 3E). These results suggest that the AMPK α2/MCL-1 pathway participates in preventing HPASMC apoptosis during hypoxia.
A few small molecules have been developed to inhibit the MCL-1/BCL-2 family for therapeutic purposes, two of which are GX 15-070 and TW-37 (29, 30). To explore whether the chemical inhibition of the MCL-1/BCL-2 family induces HPASMC death during hypoxia, we treated cells with various concentrations of GX 15-070 and TW-37 and exposed them to normoxia or hypoxia for 24 hours, followed by the measurement of cell viability. As shown in Figure 3F, during normoxia, 50% of HPASMCs were viable at 86.9 μM of GX 15-070, whereas during hypoxia, 50% of HPASMCs were viable at 3.39 μM of GX 15-070. Similarly, treatment with 9.73 μM TW-37 caused 50% HPASMC cell death in normoxia, and 6.76 μM TW-37 caused 50% cell death in hypoxia (Figure 3G). Together, these results suggest that MCL-1 may be a potential target to induce HPASMC apoptosis during hypoxia.
Inhibition of AMPK α1 Prevents Autophagy
We found that both AMPK α1 and α2 are required for the survival of HPASMCs during hypoxia (Figure 2A), whereas AMPK α2 promotes HPASMC survival by the prevention of apoptosis (Figure 3). However, the underlying mechanism of AMPK α1–mediated HPASMC survival remains unknown. Previously, hypoxia was reported to induce autophagy, a process that can either induce cell death or promote cell survival, and AMPK is a key regulator of autophagy (31–37). Thus we investigated the role of AMPK α1 in hypoxia-mediated autophagy and the survival of HPASMCs during hypoxia. We transfected HPASMCs with siRNAs against AMPK α1 or α2 to suppress the expression of AMPK α1 or α2, respectively, and then exposed cells to normoxia or hypoxia. We then determined the amount of microtubule-associated protein 1 light chain 3B (LC3B) according to Western blot analysis. The presence of fast-migrating LC3B-II is an indicator of autophagy. As shown in Figure 4A, hypoxia induced the expression levels of LC3B-II, and the suppression of AMPK α1 diminished hypoxia-induced LC3B-II. However, the suppression of AMPK α2 exerted little effect on hypoxia-induced LC3B-II, suggesting that AMPK α1 but not AMPK α2 is the mediator of hypoxia-induced autophagy.
Figure 4.
AMPK α1 facilitates HPASMC survival during hypoxia by promoting autophagy. HPASMCs were either transfected with siRNA for AMPK α1 or α2 (A) or pretreated with dimethylsulfoxide (DMSO) or 1 mM 3-methyladenine (3-MA) for 1 hour (B), and then exposed to normoxia or hypoxia for 6 hours. The cleavage of microtubule-associated protein 1 light chain 3B (LC3B) was measured by Western blot analysis. β-actin (A) and tubulin (B) were used as loading controls. The LC3B-II/I ratios are shown at the top (B). After pretreatment with 3-MA and exposure to normoxia or hypoxia, LDH activity (C) and caspase-3 activity (D) were determined. Data are expressed as means ± SEMs (n ≥ 3). *P < 0.05. **P < 0.01.
To investigate the role of autophagy in the survival of HPASMCs during hypoxia, we pretreated HPASMCs with the autophagy inhibitor 3-MA for 1 hour, and then exposed them to normoxia or hypoxia, followed by the measurement of LC3B-II, LDH activity, and caspase-3 activity. As shown in Figure 4B, treatment with 3-MA prevented hypoxia-induced LC3B-II, suggesting that 3-MA inhibited hypoxia-induced autophagy in HPASMCs. Furthermore, treatment with 3-MA increased LDH activity in HPASMCs exposed to either normoxia or hypoxia (Figure 4C). However, 3-MA did not affect caspase-3 activity in HPASMCs exposed to either normoxia or hypoxia (Figure 4D), suggesting that the inhibition of autophagy induces HPASMC death, independent of apoptosis.
Pulmonary Arterial Hypertensive Human and Mouse PASMCs Express Elevated Levels of AMPK Phosphorylation
To investigate the biological function of AMPK phosphorylation in PAH, we compared the concentrations of phosphorylated and total AMPK in normal HPASMCs and in HPASMCs isolated from patients with PAH. We found that PAH HPASMCs contained elevated pAMPK concentrations, whereas total AMPK concentrations remained unchanged (Figure 5A). In addition, we also measured the phosphorylated and total AMPK concentrations in whole-lung homogenates from a model of hypoxia-induced PH, in which mice were exposed to normoxia or hypoxia (10% O2) for 3 weeks. We found that hypoxic lung tissues also demonstrated increased pAMPK, whereas AMPK concentrations were unchanged (Figure 5B). Furthermore, we determined the amounts of pAMPK in mouse lungs with immunofluorescence staining, and found that in hypoxic mouse PASMCs (labeled with the anti–α-SMA antibody), pAMPK was elevated (Figure 5C). Moreover, pAMPK concentrations appeared to be elevated in other cell types in the lung (Figure 5C). Taken together, our results suggest that AMPK is hyperphosphorylated in PASMCs during PAH.
Figure 5.
Hypertensive human and mouse PASMCs express elevated levels of AMPK phosphorylation. (A) We compared the concentrations of phosphorylated and total AMPK in normal HPASMCs and in HPASMCs isolated from patients with pulmonary arterial hypertension (PAH). Representative blots are shown at the bottom, and the amounts of pAMPK and total AMPK are shown as the pAMPK/AMPK and AMPK/tubulin ratios at the top and middle, respectively. (B) Mice were exposed to normoxia (ambient air, N) or hypoxia (10% O2, H) for 3 weeks. Whole-lung homogenates were used to determine the concentrations of phosphorylated and total AMPK by Western blot analysis. Data are expressed as means ± SEMs (n ≥ 3). *P < 0.05. Tubulin was used as loading control. (C) The lung sections of mice described in B were immunolabeled with pAMPK and α–smooth muscle actin (SMA) antibodies and 4′6-diamidino-2-phenylindole (DAPI) for study, using fluorescence microscopy.
Inhibition of AMPK by Compound C Prevents Hypoxia-Induced PAH In Vivo
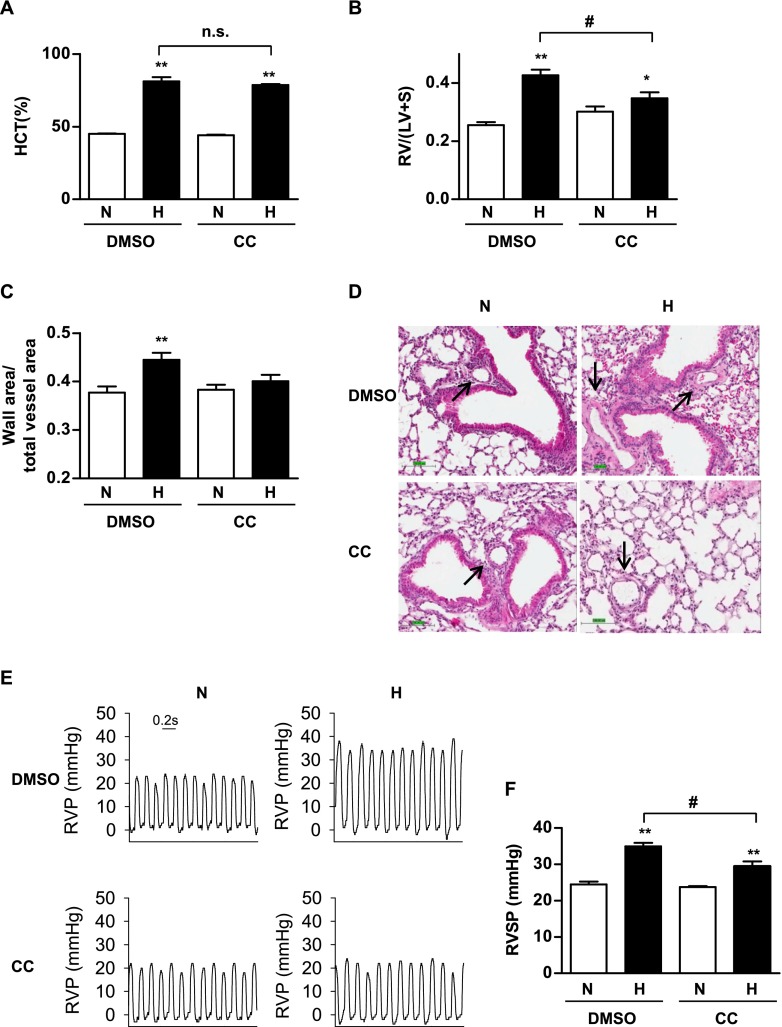
To investigate whether the inhibition of AMPK prevents PAH in vivo, we adopted a mouse model of hypoxia-induced PH. We injected CC to C57BL/6 mice once weekly, and exposed mice to normoxia or hypoxia (10% O2) for 3 weeks. The mice were weighed every week. After exposure, the mice were killed, their blood was drawn to determine a complete blood count, and their hearts were excised to measure the heart weight, RV weight, and RV/(LV + S) ratio. As shown in Figure E1A in the online supplement, although exposure to hypoxia caused weight loss in mice, CC did not affect the weight of mice exposed to normoxia or hypoxia, suggesting that CC may exert minimal side effects. Exposure to hypoxia is known to raise hematocrit (HCT), red blood cell counts (RBC), and hemoglobin content (HGB). However, CC exerted little effect on the hypoxia-induced increase in HCT (Figure 6A), RBC count, and HGB (Figures E1B and E1C). Furthermore, CC did not affect the heart weight and heart weight/body weight ratio (Figures E1D and E1E). Interestingly, CC prevented hypoxia-induced elevation of RV/(LV + S) and RV/heart weight, suggesting that CC prevents hypoxia-induced right ventricle hypertrophy (Figures 6B and E1F). We measured the pulmonary arterial wall thickness of these mice, and found that CC prevented hypoxia-induced increases in pulmonary arterial wall thickness (Figures 6C and 6D). We also determined the RVSP in these mice, and found that CC prevented hypoxia-induced elevations in RVSP (Figures 6E and 6F). Taken together, these results suggest that the inhibition of AMPK may prevent PAH.
Figure 6.
The inhibition of AMPK by Compound C prevents hypoxia-induced pulmonary hypertension in mice. Mice were weighed and injected with DMSO or Compound C (CC), 1 day before exposure to normoxia (ambient air, N) or hypoxia (10% O2, H) for 3 weeks. During the experimental period, mice were injected with DMSO or Compound C once a week, and were weighed weekly. (A) After the completion of exposure, mice were anesthetized, and blood was drawn from the heart and used to measure hematocrit (HCT). (B) Tight ventricular (RV) hypertrophy, expressed as the weight ratio of right ventricle/(left ventricle + septum) (RV/(LV + S)), was determined as described in Materials and Methods. (C) Images of hematoxylin-and-eosin–stained sections of mouse lung were used to calculate the thickness of pulmonary arterial wall. (D) Representative hematoxylin-and-eosin–stained images of mouse lung sections are shown, and the arrows indicate pulmonary arteries. Scale bar at the lower left corner = 100 μm. (E and F) We measured the right ventricular pressure (RVP) in these mice. Representative diagrams of RVP are shown in E, and the quantification of right ventricular systolic pressure (RVSP) is shown in F. Data are presented as means ± SEMs, and are compared with those mice injected with DMSO and exposed to normoxia. The insert in E indicates a duration of 0.2 s. *P < 0.05 and **P < 0.01 (n ≥ 5 for each group). #P < 0.05, significant difference between DMSO + H and CC + H groups. n.s., no significance.
Inhibition of AMPK by Compound C Partly Reverses Existing Hypoxia-Induced PAH In Vivo
To investigate whether the inhibition of AMPK may represent a novel therapeutic approach to the treatment of PAH, we tested whether CC reverses existing PAH in hypoxic mice. We exposed C57BL/6 mice to hypoxia or normoxia for 2 weeks, followed by an injection of CC or DMSO once weekly for another 2 weeks. After exposure, the mice were killed, and we measured HCT, RV/(LV + S) ratio, arterial wall thickness, and RVSP. Although CC was unable to reverse the elevated RVSP in hypoxic mice (Figures 7A and 7B), CC decreased the RV/(LV + S) ratio (Figure 7C) and arterial wall thickening (Figure 7D) in hypoxic mice. Moreover, CC exerted little effect on the hypoxia-induced increase in HCT (Figure 7E), suggesting that CC partly reverses existing PAH.
Figure 7.
Inhibition of AMPK by Compound C partly reverses hypoxia-induced pulmonary hypertension in mice. We exposed C57BL/6 mice to hypoxia or normoxia for 2 weeks, followed by an injection of CC or DMSO once weekly for another 2 weeks. After the completion of exposure, mice were anesthetized, and RVPs were measured. Representative diagrams of RVP are shown in A, and the quantification of RVSP is shown in B. RV/(LV + S), arterial wall thickness, and HCT values are shown in C, D, and E, respectively. Data are presented as means ± SEMs, and are compared with data for mice injected with DMSO and exposed to normoxia (DMSO + N). *P < 0.05 and **P < 0.01 (n ≥ 5 for each group). ##P < 0.01, significant difference between DMSO + H and CC + H groups. n.s., no significance.
Discussion
Although the role of AMPK in metabolism and in the maintenance of cellular energy homeostasis has been well studied, the role of AMPK in cell survival remains elusive. In our study, we provide evidence for the first time that the activation of AMPK is essential for HPASMC survival during hypoxia, and that AMPK α1 and α2 differentially regulate HPASMC survival via distinct mechanisms. AMPK α1 promotes HPASMC survival via the induction of autophagy, whereas AMPK α2 facilitates HPASMC survival by maintaining the expression of MCL-1 and the prevention of apoptosis. Interestingly, we also found that AMPK is phosphorylated and activated in the HPASMCs of patients with PAH and of hypertensive mice. These results suggest that AMPK may participate in the pathogenesis of PAH, and may represent a novel therapeutic target for the treatment of PAH.
The role of AMPK in cell survival appears to be cell-specific. Some studies show that the inhibition of AMPK induces growth arrest and reduces viability (19, 38), whereas others report that the activation of AMPK inhibits the growth and/or survival of cells (39). Although HPASMCs express AMPK (40), it remains unknown whether AMPK promotes or inhibits HPASMC survival during hypoxia. Our results show that hypoxia activates AMPK, and the inhibition of AMPK induces cell death during hypoxia in a time-dependent manner (Figure 1), suggesting that the activation of AMPK is required for HPASMC survival. These results are in line with a previous report in which the inhibition of AMPK significantly decreased the viability of cells exposed to hypoxia (41). In our study, we show that the exposure of HPASMCs to 3% O2 induces transient AMPK phosphorylation, which reaches a maximum in 15 minutes, and returns to baseline in 30 minutes (Figure 1). These results are consistent with previous reports in which the exposure of Type II alveolar epithelial cells to hypoxia induced the activation of AMPK (42, 43). Krymskaya and colleagues reported that AMPK is activated in hypoxic PASMCs, and that the activation of AMPK is not required for the hypoxia-induced proliferation of PASMCs (40), which is consistent with our finding that AMPK plays a role in hypoxia-induced PH via the regulation of PASMC survival.
In cells that express only AMPK α1, the inhibition of AMPK α1 induces apoptosis (19, 44, 45). PASMCs express both AMPK α1 and AMPK α2 (6, 10, 11), and our results show that both the α1 and α2 isoforms are required for the survival of HPASMCs during hypoxia (Figure 2). However, the inhibition of AMPK α2 induces HPASMC apoptosis during hypoxia, whereas the inhibition of AMPK α1 causes HPASMC death independent of apoptosis, suggesting a differential role of AMPK isoforms in the survival of HPASMCs and a PASMC-specific role of AMPK α2 in the prevention of apoptosis. Similarly, other studies suggest that the differential sensitivity to hypoxia and different intracellular localizations determine the biological function of AMPK α1 and AMPK α2 (23, 46, 47).
The balance between the expressions of proapoptotic and antiapoptotic proteins regulates cell apoptosis and survival (28). We show that the suppression of AMPK α2 down-regulates a prosurvival protein MCL-1 (Figure 3), resulting in a shift toward apoptosis. The knockdown of MCL-1 significantly increases HPASMC apoptosis during hypoxia (Figure 3). Furthermore, small molecule inhibitors of the MCL-1/BCL-2 family also increase the cell death of HPASMCs during hypoxia (Figure 3), suggesting that MCL-1 may be a novel target for the treatment of PAH. Our results support a previous study in which AMPK was required for the maintenance of MCL-1 and BCL-XL in myeloma cells (44). However, other studies suggest that the activation of AMPK increases BAD phosphorylation and reduces the interaction between BAD and BCL-XL, leading to the prevention of apoptosis (48), suggesting that AMPK may prevent apoptosis by different mechanisms in specific cell types. Interestingly, the suppression of MCL-1 is not sufficient to induce apoptosis in normoxic HPASMCs, which is consistent with our result that the suppression of AMPK α2 decreases MCL-1 expression during normoxia, but does not induce apoptosis (Figures 2 and 3). These results suggest that in addition to MCL-1 down-regulation, other mechanisms may contribute to HPASMC apoptosis during hypoxia. Accordingly, two MCL-1 inhibitors, GX 15-070 and TW-37, which also inhibit other BCL-2 proteins, induce cell death under normoxic conditions (Figures 3F and 3G). A previous study suggested that a second proapoptotic signal was required to facilitate MCL-1 degradation–mediated apoptosis (49). MCL-1 is extremely unstable and has a short half-life, and MCL-1 is known to undergo ubiquitination and proteolysis (50, 51). The underlying mechanism by which AMPK α2 regulates MCL-1 expression warrants further investigation.
Autophagy is a catabolic process in eukaryotic cells that degrades and recycles damaged cytoplasmic constituents (31, 32). Although excessive autophagy may lead to cell death, autophagy can protect cells against metabolic stress (31). Hypoxia is known to induce autophagy, and AMPK activates autophagy (33–37). Because AMPK α1 is important for HPASMC survival in an apoptosis-independent manner (Figures 2 and 3), we investigated its role in hypoxia-induced autophagy and survival. We show that AMPK α1 is required for hypoxia-mediated autophagy, and the inhibition of autophagy inhibits HPASMC survival (Figure 4), suggesting that AMPK α1 mediates HPASMC survival during hypoxia by inducing autophagy. Our results are consistent with those of a previous study in which autophagy prevented ischemia–reperfusion injury in cardiomyocytes (52). It is noteworthy that previous reports indicate crosstalk between autophagic and apoptotic pathways in which MCL-1, BCL-2, and BCL-XL regulate autophagy (31, 49). In our study, although the knockdown of AMPK α2 decreased MCL-1, it did not affect autophagy (Figure 4). These results suggest that in HPASMCs, AMPK α1–mediated autophagy and the AMPK α2–mediated prevention of apoptosis are distinct from each other. In our in vitro experiments, we used HPASMCs from Lonza, which are obtained from large-diameter arteries. These cells have limitations because they may not completely recapitulate the behavior of HPASMCs in small vessels, where most of the remodeling occurs in PAH.
Our finding that AMPK plays a key role in the survival of HPASMCs during hypoxia implicates an important role for AMPK activation in the pathogenesis of PAH. Indeed, we found that AMPK is activated in PASMCs isolated from patients with PAH, and pulmonary hypertensive mice contain hyperphosphorylated AMPK (Figure 5), suggesting that the inhibition of AMPK may represent a novel therapeutic strategy for the treatment of PAH. Treatment with the AMPK inhibitor CC prevented hypoxia-induced increases in RV/(LV + S), arterial wall thickness, and RVSP (Figure 6). Furthermore, we found that CC reverses RV hypertrophy and arterial wall remodeling without affecting RVSP in existing hypoxia-induced PH (Figure 7), suggesting that CC may partly reverse PAH. The dissociation between pulmonary vascular remodeling and right ventricular pressure has been reported previously (53). We suggest, as one possible explanation for this dissociation in our PAH treatment experiment, that to achieve a complete reversal of PAH, we may have to consider the contributions of structural changes in other pulmonary vessels and hypoxia-induced vasoconstriction (53, 54). Emerling and colleagues previously reported that CC inhibits hypoxia-inducible factor (HIF) activation during hypoxia-independent AMPK activation (55), whereas we show that CC exerts little effect on hypoxia-induced increases in HCT, RBC count, and HGB (Figures 6, 7, and E1), which are known to be induced by HIF activation. These results suggest that in our model, CC may act in an AMPK-dependent but HIF-independent manner, which is consistent with the results from our siRNA experiments. Interestingly, the antidiabetic drug metformin, which is an indirect AMPK activator, protects rodents from hypoxia-induced or monocrotaline-induced PH (56). These seemingly contradictory results emphasize the importance of a delicate balance in AMPK-mediated signaling during the treatment of PAH.
In conclusion, our results show for the first time that AMPK activation during hypoxia is essential for HPASMC survival, and that AMPK α1 and α2 contribute to HPASMC survival via different mechanisms. Furthermore, we provide evidence that small molecule inhibitors targeting AMPK α1/autophagy and the AMPK α2/MCL-1 pathway induce PASMC death. Importantly, we have discovered that treatment with an AMPK inhibitor prevents and partly reverses hypoxia-induced PH in vivo, suggesting that it may be a novel therapeutic agent in the treatment of PAH. Because CC is not a specific AMPK inhibitor, further studies with smooth muscle cell–specific AMPK α1 and α2 knockout mice are warranted.
Acknowledgments
Acknowledgments
The authors thank Dr. Ramaswamy Ramchandran and Dr. Aarti Raghavan for helpful discussions, Dr. Sekhar Reddy for critical reading of the manuscript, Dr. Benoit Viollet for providing the AMPK knockout mouse embryo fibroblasts, and the Pulmonary Hypertension Breakthrough Initiative for HPASMCs isolated from patients and donors with IPAH.
Footnotes
This study was supported by an Advancing Newborn Medicine Grant from Ikaria (J.C.F.I.), National Institutes of Health grants HL075187 and HL110829 (J.U.R.), and a Pulmonary Hypertension Association/Pfizer Proof-of-Concept Award (G.Z.), for which the American Thoracic Society provides administrative support.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2012-0446OC on May 13, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med. 2004;351:1425–1436. doi: 10.1056/NEJMra040291. [DOI] [PubMed] [Google Scholar]
- 2.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation. 2009;119:2250–2294. doi: 10.1161/CIRCULATIONAHA.109.192230. [DOI] [PubMed] [Google Scholar]
- 3.Walsh-Sukys MC, Tyson JE, Wright LL, Bauer CR, Korones SB, Stevenson DK, Verter J, Stoll BJ, Lemons JA, Papile LA, et al. Persistent pulmonary hypertension of the newborn in the era before nitric oxide: practice variation and outcomes. Pediatrics. 2000;105:14–20. doi: 10.1542/peds.105.1.14. [DOI] [PubMed] [Google Scholar]
- 4.Durmowicz AG, Stenmark KR. Mechanisms of structural remodeling in chronic pulmonary hypertension. Pediatr Rev. 1999;20:e91–e102. [PubMed] [Google Scholar]
- 5.Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res. 2006;99:675–691. doi: 10.1161/01.RES.0000243584.45145.3f. [DOI] [PubMed] [Google Scholar]
- 6.Evans AM, Hardie DG, Peers C, Wyatt CN, Viollet B, Kumar P, Dallas ML, Ross F, Ikematsu N, Jordan HL, et al. Ion channel regulation by AMPK: the route of hypoxia-response coupling in the carotid body and pulmonary artery. Ann N Y Acad Sci. 2009;1177:89–100. doi: 10.1111/j.1749-6632.2009.05041.x. [DOI] [PubMed] [Google Scholar]
- 7.Nagata D, Hirata Y. The role of AMP-activated protein kinase in the cardiovascular system. Hypertens Res. 2010;33:22–28. doi: 10.1038/hr.2009.187. [DOI] [PubMed] [Google Scholar]
- 8.Viollet B, Athea Y, Mounier R, Guigas B, Zarrinpashneh E, Horman S, Lantier L, Hebrard S, Devin-Leclerc J, Beauloye C, et al. AMPK: lessons from transgenic and knockout animals. Front Biosci. 2009;14:19–44. doi: 10.2741/3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horman S, Morel N, Vertommen D, Hussain N, Neumann D, Beauloye C, El Najjar N, Forcet C, Viollet B, Walsh MP, et al. AMP-activated protein kinase phosphorylates and desensitizes smooth muscle myosin light chain kinase. J Biol Chem. 2008;283:18505–18512. doi: 10.1074/jbc.M802053200. [DOI] [PubMed] [Google Scholar]
- 10.Evans AM, Mustard KJW, Wyatt CN, Dipp M, Kinnear NP, Hardie DG. Does AMP-activated protein kinase couple inhibition of mitochondrial oxidative phosphorylation by hypoxia to pulmonary artery constriction? Adv Exp Med Biol. 2006;580:147–154. doi: 10.1007/0-387-31311-7_22. [DOI] [PubMed] [Google Scholar]
- 11.Creighton J, Jian M, Sayner S, Alexeyev M, Insel PA. Adenosine monophosphate–activated kinase alpha1 promotes endothelial barrier repair. FASEB J. 2011;25:3356–3365. doi: 10.1096/fj.10-179218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heidrich F, Schotola H, Popov AF, Sohns C, Schuenemann J, Friedrich M, Coskun KO, von Lewinski D, Hinz J, Bauer M, et al. AMPK: activated protein kinase and its role in energy metabolism of the heart. Curr Cardiol Rev. 2010;6:337–342. doi: 10.2174/157340310793566073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardie DG. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25:1895–1908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans AM. AMP-activated protein kinase and the regulation of Ca2+ signalling in O2-sensing cells. J Physiol. 2006;574:113–123. doi: 10.1113/jphysiol.2006.108381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gusarova GA, Trejo HE, Dada LA, Briva A, Welch LC, Hamanaka RB, Mutlu GM, Chandel NS, Prakriya M, Sznajder JI. Hypoxia leads to Na,K-ATPase downregulation via Ca(2+) release–activated Ca(2+) channels and AMPK activation. Mol Cell Biol. 2011;31:3546–3556. doi: 10.1128/MCB.05114-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mungai PT, Waypa GB, Jairaman A, Prakriya M, Dokic D, Ball MK, Schumacker PT. Hypoxia triggers AMPK activation through reactive oxygen species–mediated activation of calcium release–activated calcium channels. Mol Cell Biol. 2011;31:3531–3545. doi: 10.1128/MCB.05124-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mishra R, Cool BL, Laderoute KR, Foretz M, Viollet B, Simonson MS. AMP-activated protein kinase inhibits transforming growth factor–beta–induced Smad3-dependent transcription and myofibroblast transdifferentiation. J Biol Chem. 2008;283:10461–10469. doi: 10.1074/jbc.M800902200. [DOI] [PubMed] [Google Scholar]
- 18.Hattori Y, Akimoto K, Nishikimi T, Matsuoka H, Kasai K. Activation of AMP-activated protein kinase enhances angiotensin II–induced proliferation in cardiac fibroblasts. Hypertension. 2006;47:265–270. doi: 10.1161/01.HYP.0000198425.21604.aa. [DOI] [PubMed] [Google Scholar]
- 19.Park HU, Suy S, Danner M, Dailey V, Zhang Y, Li H, Hyduke DR, Collins BT, Gagnon G, Kallakury B, et al. AMP-activated protein kinase promotes human prostate cancer cell growth and survival. Mol Cancer Ther. 2009;8:733–741. doi: 10.1158/1535-7163.MCT-08-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Igata M, Motoshima H, Tsuruzoe K, Kojima K, Matsumura T, Kondo T, Taguchi T, Nakamaru K, Yano M, Kukidome D, et al. Adenosine monophosphate–activated protein kinase suppresses vascular smooth muscle cell proliferation through the inhibition of cell cycle progression. Circ Res. 2005;97:837–844. doi: 10.1161/01.RES.0000185823.73556.06. [DOI] [PubMed] [Google Scholar]
- 21.Nagata D, Takeda R, Sata M, Satonaka H, Suzuki E, Nagano T, Hirata Y. AMP-activated protein kinase inhibits angiotensin II–stimulated vascular smooth muscle cell proliferation. Circulation. 2004;110:444–451. doi: 10.1161/01.CIR.0000136025.96811.76. [DOI] [PubMed] [Google Scholar]
- 22.Raghavan A, Zhou G, Zhou Q, Ibe JCF, Ramchandran R, Yang Q, Racherla H, Raychaudhuri P, Raj JU. Hypoxia-induced pulmonary arterial smooth muscle cell proliferation is controlled by Forkhead box M1. Am J Respir Cell Mol Biol. 2012;46:431–436. doi: 10.1165/rcmb.2011-0128OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laderoute KR, Amin K, Calaoagan JM, Knapp M, Le T, Orduna J, Foretz M, Viollet B. 5′-AMP–activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments. Mol Cell Biol. 2006;26:5336–5347. doi: 10.1128/MCB.00166-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seglen PO, Gordon PB. 3-methyladenine: specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc Natl Acad Sci USA. 1982;79:1889–1892. doi: 10.1073/pnas.79.6.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Zeng Z, Viollet B, Ronnett GV, McCullough LD. Neuroprotective effects of adenosine monophosphate–activated protein kinase inhibition and gene deletion in stroke. Stroke. 2007;38:2992–2999. doi: 10.1161/STROKEAHA.107.490904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao Y-Y, Zhao YD, Mirza MK, Huang JH, Potula H-HSK, Vogel SM, Brovkovych V, Yuan JXJ, Wharton J, Malik AB. Persistent ENOS activation secondary to caveolin-1 deficiency induces pulmonary hypertension in mice and humans through PKG nitration. J Clin Invest. 2009;119:2009–2018. doi: 10.1172/JCI33338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma W, Han W, Greer PA, Tuder RM, Toque HA, Wang KKW, Caldwell RW, Su Y. Calpain mediates pulmonary vascular remodeling in rodent models of pulmonary hypertension, and its inhibition attenuates pathologic features of disease. J Clin Invest. 2011;121:4548–4566. doi: 10.1172/JCI57734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 29.Heidari N, Hicks MA, Harada H. GX15-070 (obatoclax) overcomes glucocorticoid resistance in acute lymphoblastic leukemia through induction of apoptosis and autophagy. Cell Death Dis. 2010;1:e76. doi: 10.1038/cddis.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohammad RM, Goustin AS, Aboukameel A, Chen B, Banerjee S, Wang G, Nikolovska-Coleska Z, Wang S, Al-Katib A. Preclinical studies of TW-37, a new nonpeptidic small-molecule inhibitor of BCL-2, in diffuse large cell lymphoma xenograft model reveal drug action on both BCL-2 and MCL-1. Clin Cancer Res. 2007;13:2226–2235. doi: 10.1158/1078-0432.CCR-06-1574. [DOI] [PubMed] [Google Scholar]
- 31.Hsieh Y-C, Athar M, Chaudry IH. When apoptosis meets autophagy: deciding cell fate after trauma and sepsis. Trends Mol Med. 2009;15:129–138. doi: 10.1016/j.molmed.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yen W-L, Klionsky DJ. How to live long and prosper: autophagy, mitochondria, and aging. Physiology (Bethesda) 2008;23:248–262. doi: 10.1152/physiol.00013.2008. [DOI] [PubMed] [Google Scholar]
- 33.Liang J, Shao SH, Xu Z-X, Hennessy B, Ding Z, Larrea M, Kondo S, Dumont DJ, Gutterman JU, Walker CL, et al. The energy sensing LKB1–AMPK pathway regulates p27(KIP1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol. 2007;9:218–224. doi: 10.1038/ncb1537. [DOI] [PubMed] [Google Scholar]
- 34.Grotemeier A, Alers S, Pfisterer SG, Paasch F, Daubrawa M, Dieterle A, Viollet B, Wesselborg S, Proikas-Cezanne T, Stork B. AMPK-independent induction of autophagy by cytosolic Ca2+ increase. Cell Signal. 2010;22:914–925. doi: 10.1016/j.cellsig.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 35.Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, et al. Phosphorylation of ULK1 (HATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hardie DG. AMPK and autophagy get connected. EMBO J. 2011;30:634–635. doi: 10.1038/emboj.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim J, Kundu M, Viollet B, Guan K-L. AMPK and mTor regulate autophagy through direct phosphorylation of ULK1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chhipa RR, Wu Y, Mohler JL, Ip C. Survival advantage of AMPK activation to androgen-independent prostate cancer cells during energy stress. Cell Signal. 2010;22:1554–1561. doi: 10.1016/j.cellsig.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vucicevic L, Misirkic M, Janjetovic K, Harhaji-Trajkovic L, Prica M, Stevanovic D, Isenovic E, Sudar E, Sumarac-Dumanovic M, Micic D, et al. AMP-activated protein kinase–dependent and –independent mechanisms underlying in vitro antiglioma action of Compound C. Biochem Pharmacol. 2009;77:1684–1693. doi: 10.1016/j.bcp.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 40.Krymskaya VP, Snow J, Cesarone G, Khavin I, Goncharov DA, Lim PN, Veasey SC, Ihida-Stansbury K, Jones PL, Goncharova EA. mTor is required for pulmonary arterial vascular smooth muscle cell proliferation under chronic hypoxia. FASEB J. 2011;25:1922–1933. doi: 10.1096/fj.10-175018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang X, Gao R, Li J, Qi Y, Song X, Zhao L, Wang H, Pu Y, Xu K, Li J. A pharmacological activator of AMP-activated protein kinase protects hypoxic neurons in a concentration-dependent manner. Neurochem Res. 2010;35:1281–1289. doi: 10.1007/s11064-010-0186-3. [DOI] [PubMed] [Google Scholar]
- 42.Gusarova GA, Dada LA, Kelly AM, Brodie C, Witters LA, Chandel NS, Sznajder JI. Alpha1-AMP–activated protein kinase regulates hypoxia-induced Na,K-ATPase endocytosis via direct phosphorylation of protein kinase C zeta. Mol Cell Biol. 2009;29:3455–3464. doi: 10.1128/MCB.00054-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Emerling BM, Weinberg F, Snyder C, Burgess Z, Mutlu GM, Viollet B, Budinger GRS, Chandel NS. Hypoxic activation of AMPK is dependent on mitochondrial ROS but independent of an increase in AMP/ATP ratio. Free Radic Biol Med. 2009;46:1386–1391. doi: 10.1016/j.freeradbiomed.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baumann P, Mandl-Weber S, Emmerich B, Straka C, Schmidmaier R. Inhibition of adenosine monophosphate–activated protein kinase induces apoptosis in multiple myeloma cells. Anticancer Drugs. 2007;18:405–410. doi: 10.1097/CAD.0b013e32801416b6. [DOI] [PubMed] [Google Scholar]
- 45.Liu C, Liang B, Wang Q, Wu J, Zou M-H. Activation of AMP-activated protein kinase alpha1 alleviates endothelial cell apoptosis by increasing the expression of anti-apoptotic proteins BCL-2 and survivin. J Biol Chem. 2010;285:15346–15355. doi: 10.1074/jbc.M110.102491. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Kodiha M, Rassi JG, Brown CM, Stochaj U. Localization of AMP kinase is regulated by stress, cell density, and signaling through the MEK→ERK1/2 pathway. Am J Physiol Cell Physiol. 2007;293:C1427–C1436. doi: 10.1152/ajpcell.00176.2007. [DOI] [PubMed] [Google Scholar]
- 47.Witczak CA, Sharoff CG, Goodyear LJ. AMP-activated protein kinase in skeletal muscle: from structure and localization to its role as a master regulator of cellular metabolism. Cell Mol Life Sci. 2008;65:3737–3755. doi: 10.1007/s00018-008-8244-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kewalramani G, Puthanveetil P, Wang F, Kim MS, Deppe S, Abrahani A, Luciani DS, Johnson JD, Rodrigues B. AMP-activated protein kinase confers protection against TNF-{alpha}–induced cardiac cell death. Cardiovasc Res. 2009;84:42–53. doi: 10.1093/cvr/cvp166. [DOI] [PubMed] [Google Scholar]
- 49.Germain M, Nguyen AP, Le Grand JN, Arbour N, Vanderluit JL, Park DS, Opferman JT, Slack RS. MCL-1 is a stress sensor that regulates autophagy in a developmentally regulated manner. EMBO J. 2011;30:395–407. doi: 10.1038/emboj.2010.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase–3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21:749–760. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 51.Millman SE, Pagano M. MCL1 meets its end during mitotic arrest. EMBO Rep. 2011;12:384–385. doi: 10.1038/embor.2011.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hamacher-Brady A, Brady NR, Gottlieb RA. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem. 2006;281:29776–29787. doi: 10.1074/jbc.M603783200. [DOI] [PubMed] [Google Scholar]
- 53.van Suylen RJ, Aartsen WM, Smits JF, Daemen MJ. Dissociation of pulmonary vascular remodeling and right ventricular pressure in tissue angiotensin–converting enzyme–deficient mice under conditions of chronic alveolar hypoxia. Am J Respir Crit Care Med. 2001;163:1241–1245. doi: 10.1164/ajrccm.163.5.2003144. [DOI] [PubMed] [Google Scholar]
- 54.Stenmark KR, McMurtry IF. Vascular remodeling versus vasoconstriction in chronic hypoxic pulmonary hypertension: a time for reappraisal? Circ Res. 2005;97:95–98. doi: 10.1161/01.RES.00000175934.68087.29. [DOI] [PubMed] [Google Scholar]
- 55.Emerling BM, Viollet B, Tormos KV, Chandel NS. Compound C inhibits hypoxic activation of HIF-1 independent of AMPK. FEBS Lett. 2007;581:5727–5731. doi: 10.1016/j.febslet.2007.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Agard C, Rolli-Derkinderen M, Dumas-de-La-Roque E, Rio M, Sagan C, Savineau JP, Loirand G, Pacaud P. Protective role of the antidiabetic drug metformin against chronic experimental pulmonary hypertension. Br J Pharmacol. 2009;158:1285–1294. doi: 10.1111/j.1476-5381.2009.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]