Abstract
The emergence of nanotechnology has produced a multitude of engineered nanomaterials such as carbon nanotubes (CNTs), and concerns have been raised about their effects on human health, especially for susceptible populations such as individuals with asthma. Multiwalled CNTs (MWCNTs) have been shown to exacerbate ovalbumin (OVA)–induced airway remodeling in mice. Moreover, cyclooxygenase-2 (COX-2) has been described as a protective factor in asthma. We postulated that COX-2–deficient (COX-2−/−) mice would be susceptible to MWCNT-induced exacerbations of allergen-induced airway remodeling, including airway inflammation, fibrosis, and mucus-cell metaplasia (i.e., the formation of goblet cells). Wild-type (WT) or COX-2−/− mice were sensitized to OVA to induce allergic airway inflammation before a single dose of MWCNTs (4 mg/kg) delivered to the lungs by oropharyngeal aspiration. MWCNTs significantly increased OVA-induced lung inflammation and mucus-cell metaplasia in COX-2−/− mice compared with WT mice. However, airway fibrosis after exposure to allergen and MWCNTs was no different between WT and COX-2−/− mice. Concentrations of certain prostanoids (prostaglandin D2 and thromboxane B2) were enhanced by OVA or MWCNTs in COX-2−/− mice. No differences in COX-1 mRNA concentrations were evident between WT and COX-2−/− mice treated with OVA and MWCNTs. Interestingly, MWCNTs significantly enhanced allergen-induced cytokines involved in Th2 (IL-13 and IL-5), Th1 (CXCL10), and Th17 (IL-17A) inflammatory responses in COX-2−/− mice, but not in WT mice. We conclude that exacerbations of allergen-induced airway inflammation and mucus-cell metaplasia by MWCNTs are enhanced by deficiencies in COX-2, and are associated with the activation of a mixed Th1/Th2/Th17 immune response.
Keywords: carbon nanotubes, nanoparticles, asthma, inflammation, COX-2
Clinical Relevance
Our results demonstrate that cyclooxygenase-2 (COX-2) deficiency constitutes a susceptibility factor to occupational, consumer, or environmental exposure carbon nanotubes in allergic airway disease. Our findings suggest that individuals with asthma who manifest reduced concentrations of COX-2, or those who demonstrate reduced COX-2 activity as a consequence of treatment with COX-2 inhibitors, are at greatest risk for exposure to certain engineered nanomaterials.
Asthma is a chronic airway disease affecting over 22 million people in the United States, and is characterized by periodic acute bronchospasm, accompanied by chronic airway inflammation and remodeling (1, 2). Airway remodeling involves alterations to the airway epithelium such as mucus-cell hyperplasia and the recruitment and/or activation of fibroblasts with accompanying subepithelial fibrosis, as well as airway smooth muscle cell hypertrophy and hyperplasia (3). The pathogenesis of asthma is exacerbated by a variety of agents such as allergen exposure in sensitized individuals, viral infections, and inhaled irritants (4). Concentrations of airborne particulates have been epidemiologically linked to the incidence and severity of asthma attacks (5). Ultrafine air-pollution particles (i.e., nanoparticles) have been suggested to be the most important in asthma exacerbations, because these particles are capable of reaching the distal regions of the lung, and have a greater surface area per unit mass for the generation of reactive oxygen species (6, 7).
The rapid emergence of nanotechnology has led to the development of a multitude of engineered nanoparticles, namely, those defined as a particle intentionally designed or manufactured with any dimension less than 100 nm. Carbon nanotubes (CNTs) represent an important family of emerging nanotechnologies because of their many potential uses in engineering, electronics, and medicine, based on their small size, ease of functionalization, unusual strength, and electrical conductivity. However, these novel engineered nanostructures also represent a potential risk for the respiratory tract because of the potential for inhalation exposure and evidence that the lung is a key target organ for hazardous effects (8, 9). We and others have previously demonstrated that the inhalation of multiwalled CNTs (MWCNTs) exacerbates airway remodeling in mice prechallenged with ovalbumin (OVA) (10, 11). In addition, MWCNTs delivered to mice by oropharyngeal aspiration (OPA) impair pulmonary function (12). These studies suggested that CNTs could pose a potential health concern for individuals with asthma.
In addition to environmental factors such as inhaled nanoparticles, the aberrant expression of multiple genes is thought to contribute to the severity of asthma pathogenesis, including IL-13, IL-5, and cyclooxygenase-2 (COX-2) (13–16). The overabundance or increased biological activity of certain gene products (e.g., IL-13) mediates the pathogenesis of asthma (17–19). However, deficiencies in protective genes could be equally important. For example, mRNA concentrations encoding the COX-2 enzyme are reduced in airway epithelial cells isolated from patients with aspirin-sensitive asthma (20). Moreover, IL-13 has been shown to reduce concentrations of COX-2 in human bronchial epithelial cells from patients with asthma, and decreased COX-2 concentrations were associated with decreased concentrations of prostaglandin E2 (PGE2), a principal arachidonic acid metabolite generated by the action of COX enzymes (21). Other research has demonstrated that airway remodeling in murine asthma is correlated with diminished COX-2 inducibility and reduced PGE2 synthesis after repeated allergen challenges (22). Furthermore, genetic deficiencies, as well as the pharmacological inhibition of COX-2, have been shown to exacerbate lung inflammation in mice after OVA allergen challenges (23–26). These observations suggest a protective role for COX-2 in the asthmatic lung during chronic airway remodeling.
Because COX-2 has been implicated in the pathogenesis of asthma and fibrosis, we investigated whether COX-2 plays a role in allergic airway inflammation, mucus-cell hyperplasia, and fibrosis in response to MWCNT exposure in OVA allergen–challenged mice. We report that MWCNTs significantly increased OVA-induced lung inflammation and mucus-cell metaplasia in COX-2−/− mice compared with wild-type (WT) mice, but that airway fibrosis after exposure to allergen and MWCNTs was not affected by the loss of COX-2. Our findings also indicate that MWCNTs, coupled with allergen exposure, produce a mixed Th1/Th2/Th17 immune response. We conclude that COX-2 deficiency is a susceptibility factor in the exacerbation of allergen-induced airway inflammation and mucus-cell metaplasia caused by exposure to MWCNTs.
Materials and Methods
Multiwalled Carbon Nanotubes
MWCNTs (Helix Material Solutions, Inc., Richardson, TX) were synthesized by carbon vapor deposition with nickel and lanthanum catalysts. Characterization of the size, purity, surface area, and elemental composition provided by the manufacturer was verified independently (Millennium Research Laboratories, Inc., Woburn, MA), and was previously reported by our laboratory (10, 27, 28). MWCNTs were suspended in 0.1% pluronic surfactant in PBS, and sonicated before the delivery of OPA (see the online supplement for details).
Animals
Pathogen-free adult male and female WT C57BL/6J × 129/Ola mice or homozygous knockout (COX-2−/−) mice, 6 to 9 weeks of age, were obtained from Taconic Laboratories (Germantown, NY). Numbers of animals per experimental groups are indicated within the figures and the online supplement. Mice were housed in a temperature-controlled and humidity-controlled animal facility, and received food and water ad libitum. The Institutional Animal Care and Use Committee at North Carolina State University approved all procedures involving animal use.
Experimental Design
Details of the experimental design are provided in the text of the online supplement and in Figure E1 of the online supplement.
Bronchoalveolar Lavage, Lactate Dehydrogenase, and Cytology
Lungs were serially lavaged three times with 0.5 ml Dulbecco's phosphate-buffered saline. All three lavages were combined, and two 0.1-ml samples were taken for lactate dehydrogenase (CytoTox 96 Non-Radioactive Cytotoxicity Assay; Promega, Madison, WI) and differential cell counts (see the online supplement for details).
ELISA
Cytokines (IL-13 and IL-5) and IgE were measured in bronchoalveolar lavage fluid (BALF) and serum, respectively, using commercial kits (see the online supplement for details).
TaqMan Real-Time RT-PCR
One-step, TaqMan quantitative RT-PCR was used to quantify the expression of genes of interest in lung tissue collected 1 day after MWCNT aspiration (see the online supplement for details).
Semiquantitative Airway Morphometry
Airway inflammation.
The scoring of lung inflammation was performed 1 day after MWCNT exposure. Digitized photomicrographs of hematoxylin and eosin–stained lung sections were evaluated in a blinded fashion, using a semiquantitative scoring system (10).
Mucus goblet-cell hyperplasia.
The quantification of airway mucin production was performed as described previously (29).
Airway fibrosis.
The morphometry of airway collagen thickness was performed using an airway-intersect method (10, 30) and an area/perimeter ratio method (31) (see the online supplement for further details on morphometry).
Transmission Electron Microscopy
Lung tissues were postfixed in 1% osmium tetroxide in 0.1 M sodium phosphate buffer (pH 7.2), dehydrated through graded ethanol solutions, cleared in acetone, and then infiltrated and embedded in Spurr resin (North Carolina State University). Unstained thin sections (800–1,000 mm) were mounted on copper grids and then examined on a Philips EM208S transmission electron microscope.
Sircol Collagen Assay
Soluble collagen was measured by Sircol assay (Biocolor Ltd, Carrickfergus, UK), as previously described (10).
Eicosanoid Measurement in BALF
Concentrations of eicosanoids were measured in BALF by liquid chromatography–tandem mass spectrometry (LC-MS/MS), as previously described (32, 33).
Statistical Analysis
All graphs were constructed and statistical analysis was performed using GraphPad Prism software, version 5.0 (GraphPad Software, Inc., San Diego, CA). One-way ANOVA with a post hoc Bonferroni test was used to identify significant differences among treatment groups. Two-way ANOVA with the Bonferroni post hoc test was used to identify significant differences between genotypes. Significance was set at P < 0.05, unless otherwise stated.
Results
MWCNTs Accumulate within Macrophages Adjacent to Inflamed Airways
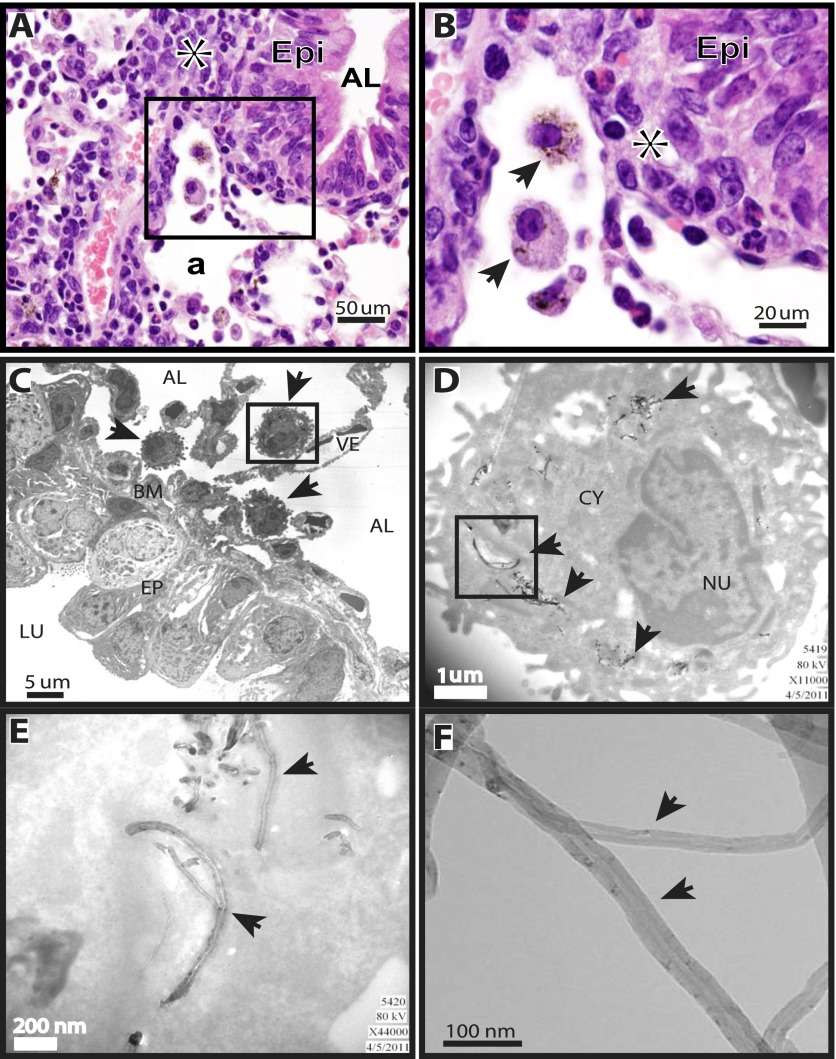
After a 34-day allergen sensitization protocol, WT or COX-2−/− mice were exposed to MWCNTs by OPA, and lung tissue was harvested 1 day or 14 days after exposure. We first sought to determine the cellular localization of MWCNTs according to light microscopy, and to confirm the nanotube structure within cells by transmission electron microscopy (TEM). MWCNTs delivered to the lungs of mice by OPA were found primarily within alveolar macrophages, as determined by the oil immersion light microscopy of lung sections from WT mice stained with hematoxylin and eosin (Figures 1A and 1B). Similar MWCNT localizations in lung tissue and macrophages were observed in COX-2−/− mice (data not shown). TEM was performed in lung-tissue sections from samples taken 1 day after MWCNT exposure. MWCNT aggregates were clearly visible by TEM within the cytoplasm of alveolar macrophages that accumulated beneath the airway epithelium (Figure 1C). Individual and aggregated MWCNTs within the alveolar macrophages were visible at higher magnifications (Figures 1D and 1E). MWCNTs were also observed in macrophages of both genotypes at 14 days (data not shown). TEM of the MWCNT sample before suspension in pluronic/saline delivery OPA in mice showed the classic hollow-tube structures, with widths varying from 30–50 nm (Figure 1F).
Figure 1.
Localization of multiwalled carbon nanotubes (MWCNTs) in mouse lungs, 1 day after oropharyngeal aspiration. (A) Photomicrograph at ×40 magnification of hematoxylin and eosin–stained lung section from a wild-type (WT) mouse shows alveolar macrophages containing MWCNTs. Asterisk indicates inflammation. Epi, epithelium; AL, airway lumen; a, alveolar region. (B) Higher magnification (×100) of inset from ×40 photomicrograph in A. Asterisk indicates inflammation. Arrows in ×100 photomicrograph indicate alveolar macrophages containing MWCNTs. Epi, epithelium. (C) Transmission electron microscopy (TEM) shows localization of alveolar macrophages containing MWCNTs (arrows) adjacent to the airway basement membrane (BM). The macrophage enclosed by the inset box frame is shown at a higher magnification in D. AL, alveolus; LU, airway lumen; VE, blood vessel; EP, airway epithelium. (D) MWCNTs within a macrophage are indicated by arrows. Inset box frame is shown at a higher magnification in E. CY, cytoplasm; NU, nucleus. (E) MWCNTs within the cytoplasm of a macrophage (arrows). (F) Arrows indicate MWCNTs visualized by high-resolution TEM before delivery to mice.
MWCNTs Exacerbate OVA-Induced Airway Inflammation in COX-2−/− Mice
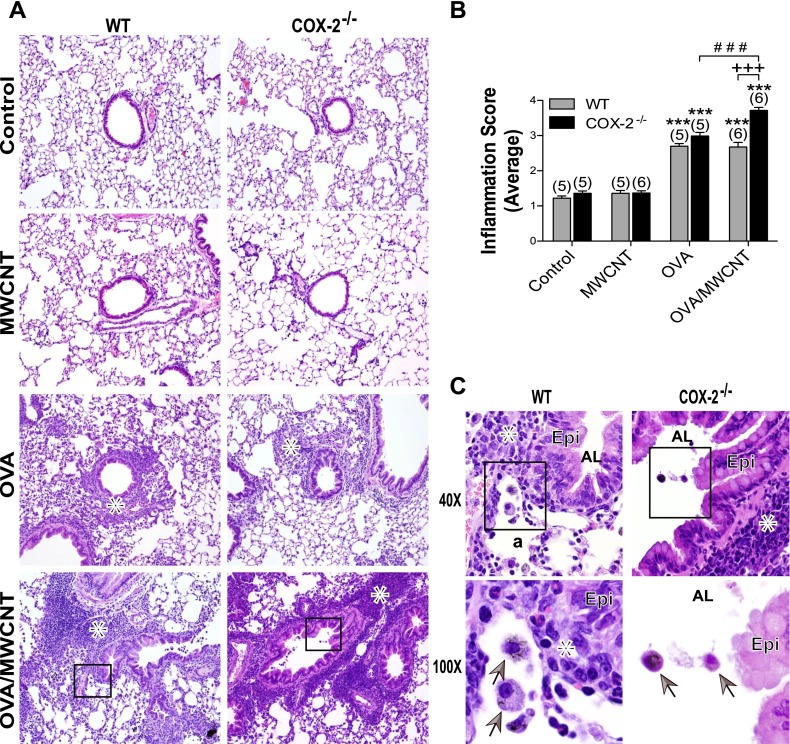
Lung inflammation was not evident in mice exposed to MWCNTs compared with control animals among either WT or COX-2−/− mice (Figure 2A). However, inflammation was present in OVA-sensitized mice, with or without MWCNT exposure, in both genotypes (Figure 2A). The semiquantitative scoring of hematoxylin and eosin–stained lung sections (as described in Materials and Methods) revealed significant inflammation in both genotypes after OVA sensitization with or without MWCNT exposure, compared with respective control mice on Day 1. In WT mice, the addition of MWCNTs did not increase airway inflammation caused by OVA sensitization alone (Figure 2B). However, in the COX-2−/− mice, MWCNTs 1 day after exposure significantly increased the inflammation associated with OVA sensitization (Figure 2B). The effect of MWCNTs on enhancing OVA-induced inflammation was not observed 14 days after exposure. Structural changes typical of allergic inflammation, such as immune cell infiltration and alveolar wall thickening, were evident at higher magnification in both WT and COX-2−/− mice receiving combination OVA/MWCNT treatment. Alveolar macrophages containing engulfed MWCNTs were evident at higher magnification (Figure 2C). Cell differential counting showed that MWCNTs after OVA sensitization caused a significant increase in the numbers of macrophages and eosinophils recovered from the BALF of COX-2−/− mice compared with WT mice (Table 1). However, OVA sensitization alone resulted in significantly more macrophages and eosinophils in the BALF of WT mice compared with COX-2−/− mice.
Figure 2.
Lung inflammation, 1 day after MWCNT exposure. (A) Photomicrographs of representative hematoxylin and eosin–stained sections at low magnification (×10). Inset boxes are shown in higher magnification in C. Asterisks denote areas of inflammation. COX-2, cyclooxygenase-2. (B) Lung pathology scoring in mice, 1 day after MWCNT exposure. Lungs were scored for the number of inflammatory cells (polymorphonuclear cells and alveolar macrophages) and the thickness of the alveolar walls. Data are presented as the means ± SEMs of scores determined by three independent observers. ***P < 0.001, compared with control groups of the respective genotypes. +++P < 0.001, compared with wild-type (WT) ovalbumin (OVA)/MWCNT. ###P < 0.001, compared with COX-2−/− (COX-2 null) OVA. Numbers in parentheses indicate the number of animals in each dosage group. (C) Higher magnification (×40) of inset from micrograph of OVA-sensitized, WT, and COX-2−/− mice exposed to MWCNTs, as well as higher magnification (×100) of inset from ×40 micrographs of OVA-sensitized, WT, and COX-2 null mice exposed to MWCNTs. Asterisk indicates inflammation. Arrows in ×100 photomicrographs indicate alveolar macrophages containing MWCNTs. Epi, epithelium; AL, airway lumen; a, alveolar region.
TABLE 1.
NUMBERS AND CELL TYPES RECOVERED IN BRONCHOALVEOLAR LAVAGE FLUID FROM OVA-CHALLENGED, WILD-TYPE, AND COX-2−/− MICE, 1 DAY AFTER MWCNT EXPOSURE
| Genotype | Pretreatment | OPA Treatment | Macrophages | Neutrophils | Eosinophils | Lymphocytes |
|---|---|---|---|---|---|---|
| WT |
Saline |
Vehicle |
19.26 ± 8.83 |
3.20 ± 1.47 |
0.32 ± 0.15 |
0.21 ± 0.10 |
| |
|
|
(84) |
(14) |
(1) |
(1) |
| |
Saline |
MWCNT |
38.45 ± 2.21 |
6.25 ± 0.36 |
1.84 ± 0.11 |
0.12 ± 0.01 |
| |
|
|
(82) |
(13) |
(4) |
(0.3) |
| |
OVA |
Vehicle |
108.65 ± 35.24†,‡,§ |
29.97 ± 9.72 |
102.13 ± 33.12†,§ |
3.58 ± 1.16 |
| |
|
|
(45) |
(12) |
(42) |
(1) |
| |
OVA |
MWCNT |
41.62 ± 14.35 |
13.92 ± 4.80 |
36.83 ± 12.70 |
2.79 ± 0.96 |
| |
|
|
(44) |
(14) |
(39) |
(3) |
| COX-2−/− |
Saline |
Vehicle |
26.17 ± 5.75 |
1.38 ± 0.30 |
0.91 ± 0.20 |
0.53 ± 0.12 |
| |
|
|
(90) |
(5) |
(3) |
(2) |
| |
Saline |
MWCNT |
12.61 ± 3.70 |
4.10 ± 1.20 |
2.89 ± 0.85 |
0.59 ± 0.17 |
| |
|
|
(62) |
(20) |
(14) |
(3) |
| |
OVA |
Vehicle |
29.67 ± 12.49 |
7.04 ± 2.96 |
58.67 ± 24.69* |
4.18 ± 1.76 |
| |
|
|
(30) |
(7) |
(59) |
(4) |
| |
OVA |
MWCNT |
81.28 ± 16.75*,¶ |
13.71 ± 2.82 |
98.04 ± 20.20†,§ |
6.53 ± 1.34 |
| (41) | (7) | (49) | (3) |
Definition of abbreviations: COX-2, cyclooxygenase-2; MWCNT, multiwalled carbon nanotubes; OPA; oropharyngeal aspiration; OVA, ovalbumin.
Data represent the mean ± SEM for each dosage group of the total number of cells counted on three nonoverlapping bronchoalveolar lavage fluid cytospin images, taken at ×20 magnification for each animal. Numbers in parentheses refer to the average percentage of total cells. The vehicle consisted of saline/0.1% pluronic solution.
P < 0.01, compared with same cell type in the saline/0.9% pluronic group of the respective genotype.
P < 0.001, compared with same cell type in the saline/0.9% pluronic group of the respective genotype.
P < 0.001, compared with the COX-2−/− OVA/0.9% pluronic group.
P < 0.001, compared with the wild-type OVA/MWCNT group.
P < 0.05, compared with the wild-type OVA/MWCNT group.
MWCNTs Enhance OVA-Induced Mucus-Cell Metaplasia in COX-2−/− Mice
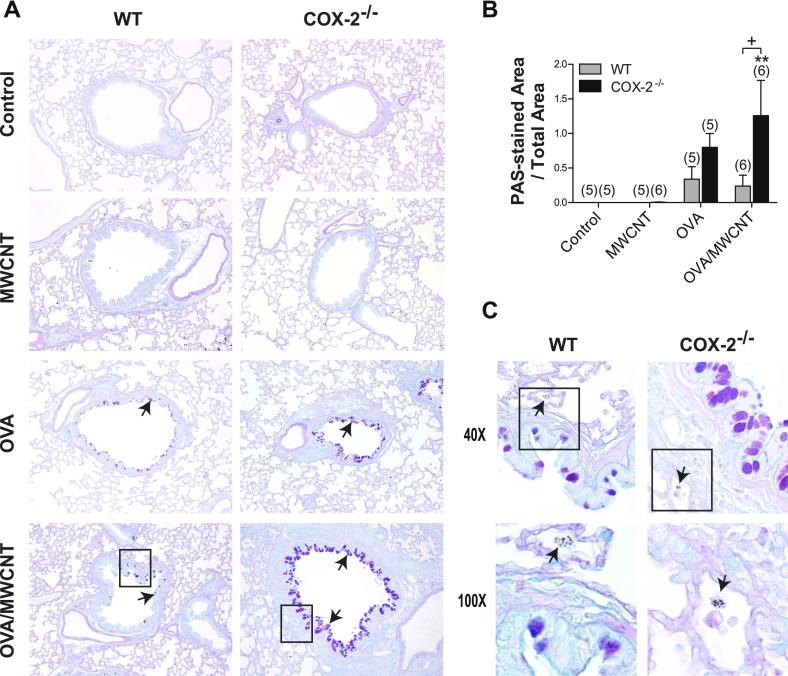
Mucus-cell metaplasia was defined as the appearance of alcian blue–periodic acid–Schiff (AB-PAS)–positive goblet cells within the airway epithelium after exposure to allergen and/or MWCNTs. Mucus-cell metaplasia was not evident in mice exposed to MWCNTs, compared with control animals among either WT or COX-2−/− mice (Figure 3A). Goblet cells were evident at 1 day in OVA-sensitized mice, with or without MWCNT exposure, in both genotypes (Figure 3A). The semiquantitative scoring of AB-PAS–stained lung sections (as described in Materials and Methods) revealed significant increases in mucus-cell metaplasia (i.e., areas of AB-PAS–positive airway epithelium) in both genotypes after OVA sensitization, with or without MWCNT exposure, compared with their respective control mice. In WT mice, the addition of MWCNTs did not increase the mucus-cell metaplasia caused by OVA sensitization alone (Figure 3B). However, in COX-2−/− mice, MWCNT exposure at 1 day significantly increased this effect was not observed in WT mice (Figure 3B). Moreover, MWCNTs did not cause an enhancement of OVA-induced goblet-cell metaplasia in COX-2−/− mice at 14 days, indicating that the observed effect of a single exposure to MWCNTs at 1 day was transient and resolved by 14 days (data not shown). Structural changes typical of allergic inflammation, such as immune cell infiltration and alveolar wall thickening, were evident at higher magnification in both WT and COX-2−/− mice that received combination OVA/MWCNT treatment. Alveolar macrophages containing engulfed MWCNTs were observed adjacent to the airways in close association with goblet cells in both WT and COX-2−/− mice (Figure 3C).
Figure 3.
Mucus-cell hyperplasia after allergen and multiwalled carbon nanotube (MWCNT) exposure. (A) Photomicrographs of alcian blue–periodic acid–Schiff (AB-PAS)–stained sections of lung tissue taken 1 day after MWCNT exposure, low magnification (×10). Inset boxes are shown in higher magnification in C. Arrows indicate AB-PAS-positive mucus-producing cells in airways. (B) Semiquantitative scoring of AB-PAS–stained lung sections from mice, 1 day after exposure, shows that airway goblet cells (arrows) are significantly increased in the lungs of COX-2−/− mice after combined allergen challenge and MWCNT exposure, compared with WT mice. **P < 0.01, compared with COX-2−/− control mice. +P < 0.05, compared with wild-type (WT) ovalbumin (OVA)/MWCNT mice. Numbers in parentheses indicate number of animals in dosage group. (C) Higher magnification (×40) of inset from ×10 micrographs of OVA-sensitized, WT, and COX-2−/− mice exposed to MWCNTs, as well as higher magnification (×100) of inset from ×40 micrographs of OVA-sensitized, WT, and COX-2−/− mice exposed to MWCNTs. Arrows indicating alveolar macrophages containing MWCNTs are depicted in the photomicrographs at ×100.
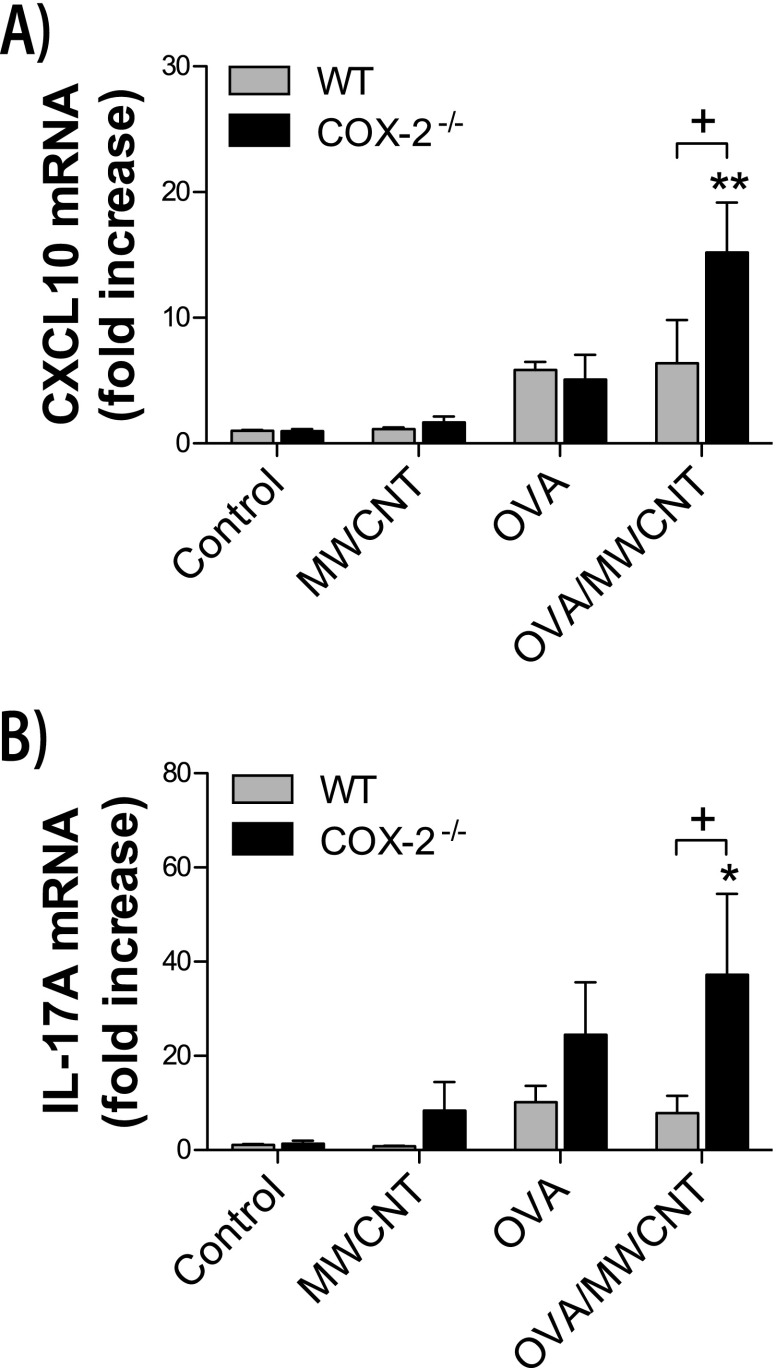
MWCNTs Amplify OVA-Induced Th2, Th1, and Th17 Mediators in COX-2−/− Mice
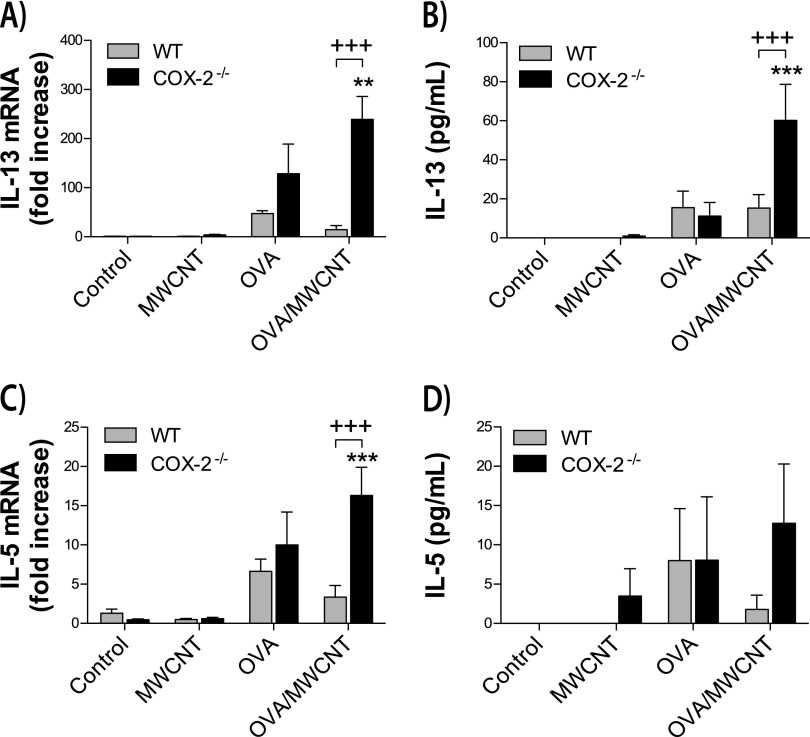
Cytokine protein concentrations were measured in lung BALF, and mRNA concentrations of cytokines were measured in whole lung extracts. Allergen challenges increased mRNA and protein concentrations of the Th2 cytokines, IL-13 and IL-5 (Figure 4). In addition, a systemic allergic response was induced by OVA allergen challenge, as determined by increased concentrations of serum IgE (Figure 5). MWCNTs significantly amplified concentrations of allergen-induced IL-13, IL-5, and IgE at 1 day after exposure in COX-2−/− mice but not in WT mice. However, MWCNTs alone did not cause a significant increase in these Th2 mediators. OVA challenge also increased mRNA concentrations of interferon-inducible CXCL10, a mediator of the Th1 immune response, and increased mRNA concentrations of IL-17A, a mediator of Th17 inflammation (Figure 6). MWCNTs significantly enhanced OVA-induced mRNA concentrations of CXCL10 and IL-17A in COX-2−/− mice, but not in WT mice, on Day 1. However, MWCNTs alone did not significantly increase concentrations of CXCL10 or IL-17A. Moreover, the enhancement of OVA-induced CXCL10 and IL-17A by MWCNTs was not observed at 14 days after exposure.
Figure 4.
Th2 cytokines in the lungs of mice after ovalbumin (OVA) allergen and multiwalled carbon nanotube (MWCNT) exposure. (A) IL-13 mRNA concentrations were measured in whole lung tissue by TaqMan quantitative real-time RT-PCR, 1 day after exposure to MWCNTs. **P < 0.01, compared with COX-2−/− control mice. +++P < 0.001, compared with wild-type (WT) OVA/MWCNT mice. (B) IL-13 protein concentrations were measured in bronchoalveolar lavage fluid (BALF), using ELISA. ***P < 0.001, compared with COX-2−/− control mice. +++P < 0.001 compared with WT OVA/MWCNT mice. (C) IL-5 mRNA concentrations were measured in whole lung tissue by TaqMan quantitative real-time RT-PCR. ***P < 0.001, compared with COX-2−/− control mice. +++P < 0.001, compared with WT OVA/MWCNT mice. (D) IL-5 protein concentrations were measured in BALF using ELISA.
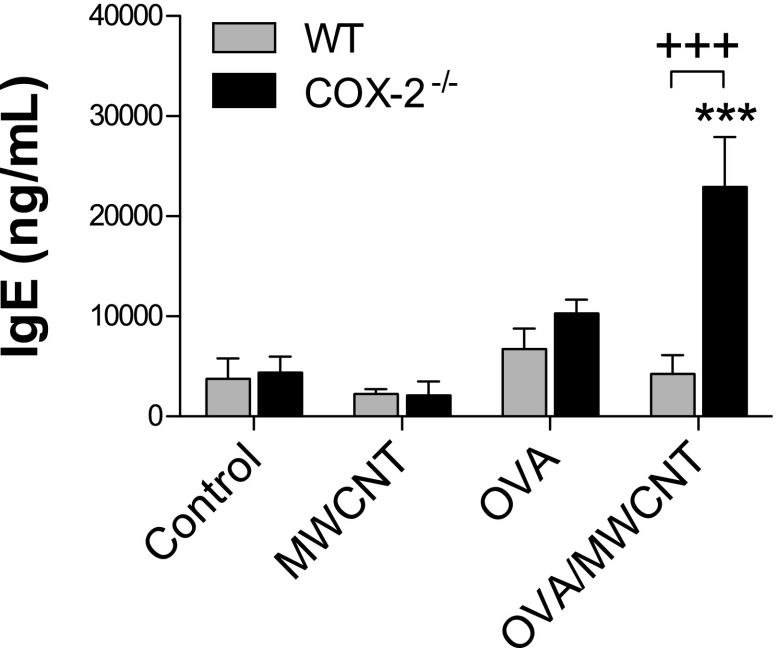
Figure 5.
Serum IgE protein concentrations after ovalbumin (OVA) allergen and multiwalled carbon nanotube (MWCNT) exposure. IgE concentrations were measured in serum, using ELISA. ***P < 0.001, compared with COX-2−/− control mice. +++P < 0.001, compared with wild-type (WT) OVA/MWCNT mice. One-way ANOVA with a post hoc Bonferroni test was used to identify significant differences among treatment groups. Two-way ANOVA with the Bonferroni post hoc test was used to identify significant differences between genotypes.
Figure 6.
Lung Th1 and Th17 cytokines after allergen and MWCNT exposure. (A) Interferon-inducible chemokine (CXCL10) mRNA concentrations were measured in whole lung tissue by TaqMan quantitative real-time RT-PCR, 1 day after exposure to MWCNTs. **P < 0.01, compared with COX-2−/− control mice. +P < 0.05, compared with wild-type (WT) OVA/MWCNT mice. (B) IL-17A mRNA concentrations were measured in whole lung tissue by TaqMan quantitative real-time RT-PCR, 1 day after exposure to MWCNTs. *P < 0.05, compared with COX-2−/− control mice. +P < 0.05, compared with WT OVA/MWCNT mice.
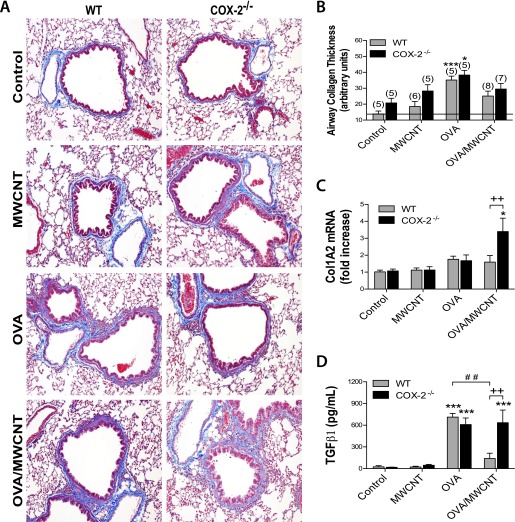
Effects of MWCNT on Allergen-Induced Airway Fibrosis in WT and COX-2−/− Mice
The histopathology of trichrome-stained lung sections at 14 days after MWCNT exposure suggested differences in airway-associated collagen between the dosage groups (Figure 7A). Therefore, we performed semiquantitative morphometry, as described previously (10, 30). An approximately 2-fold increase in airway-associated collagen deposition was observed after OVA sensitization alone, compared with control mice, in both genotypes (Figure 7B). The combination of exposure to OVA and MWCNTs resulted in less airway-associated collagen compared with OVA exposure alone in either WT or COX-2−/− mice, although not to a significant extent. A similar pattern of collagen deposition was also found in the area/perimeter ratio scoring method (Figure E2). To assess total lung collagen, we measured collagen mRNA and soluble collagen protein in whole lung tissue at 1 day and 14 days after MWCNT dosing, respectively. Collagen 1A2 (Col1A2) mRNA, as measured by quantitative RT-PCR, was slightly elevated in both genotypes after OVA sensitization. The combination of OVA sensitization and MWCNTs increased lung Col1A2 mRNA concentrations approximately 2-fold in COX-2−/− mice compared with OVA sensitization alone, but not in WT mice (Figure 7C). Soluble collagen protein in whole lung tissue measured by Sircol assay was not significantly different between genotypes after the combination of OVA sensitization and MWCNT exposure, 14 days after MWCNT treatment (Figure E3). Transforming growth factor (TGF)–β1 protein measured in BALF by ELISA at 1 day was significantly increased in both genotypes after OVA sensitization, compared with the respective control mice (Figure 7D). After combined OVA sensitization and MWCNT exposure, TGF-β1 total protein concentrations in WT mice were decreased, compared with WT mice that received only OVA sensitization. In contrast, combined OVA sensitization and MWCNT exposure in COX-2−/− mice resulted in TGF-β1 total protein concentrations similar to concentrations measured in the COX-2−/− mice that received OVA sensitization alone. Furthermore, after combined OVA sensitization and MWCNT exposure, TGF-β1 total protein concentrations were significantly increased in COX-2−/− mice compared with WT mice.
Figure 7.
Airway fibrogenic response after allergen and MWCNT exposure. (A) Photomicrographs of trichrome-stained sections depict representative airways (with collagen staining in blue) at low magnification (×10). (B) Quantitative morphometry was used to measure collagen deposition around the airways between 100 and 200 μm at 14 days (see the online supplement for details). *P < 0.05, compared with COX-2−/− control mice. ***P < 0.001, compared with wild-type (WT) control mice. Numbers in parentheses indicate the number of animals in dosage groups. (C) Collagen 1A2 (Col1A2) mRNA concentrations, 1 day after MWCNT exposure. Concentrations of mRNA were measured in whole lung tissue, using Taqman quantitative real-time RT-PCR. *P < 0.05, compared with COX-2−/− control mice. ++P < 0.01 compared to WT. (D) Transforming growth factor–β1 (TGF-β1) protein concentrations were measured in BALF, using ELISA, 1 day after MWCNT exposure. ***P < 0.001, compared with control mice of the respective genotype. ++P < 0.01, compared with WT OVA/MWCNT mice. ##P < 0.01, compared with WT OVA/MWCNT mice.
MWCNTs Modulate OVA-Stimulated Production of Prostanoids via COX-2
Prostanoid concentrations were measured according to LC-MS/MS in BALF collected from mice, to evaluate the effects of OVA and MWCNT exposure (Table 2). OVA-stimulated PGE2 was increased by approximately 2-fold, 1 day after MWCNT exposure in WT or COX-2−/− mice, and MWCNT exposure enhanced OVA-induced PGE2 concentrations another approximate twofold in both genotypes. The concentration of OVA-induced 6-keto prostaglandin F1α (6-keto PGF1α; the stable metabolite of prostacyclin) was increased more than twofold, 1 day after exposure to MWCNT in WT mice, but not in COX-2−/− mice. OVA-induced prostaglandin F2α was slightly elevated in both WT and COX-2−/− mice at 1 day after exposure to MWCNTs. Thromboxane B2 (TXB2) was significantly increased by OVA sensitization in WT, but not in COX-2−/− mice. Moreover, MWCNTs reduced OVA-stimulated TXB2 at 1 day after exposure in WT mice, and yet MWCNTs increased OVA-induced TXB2 in COX-2−/− mice at 1 day after exposure. MWCNTs amplified OVA-induced concentrations of Prostaglandin D2 (PGD2), a major mediator of asthma, at 1 day after exposure in COX-2−/− mice but not WT mice (Table 2). Furthermore, MWCNTs alone significantly increased PGD2 at 14 days after exposure in COX-2−/− mice. To address whether altered prostanoid concentrations could be attributed to a change in COX-1 expression between WT and COX-2−/− mice, TaqMan real-time RT-PCR was performed to measure total lung concentrations of COX-1 mRNA after combined OVA/MWCNT exposure. Although a combined exposure to OVA and MWCNTs reduced lung COX-1 mRNA concentrations by approximately 25%, compared with control samples (saline/pluronic treatment), no differences in COX-1 mRNA concentrations were observed between WT and COX-2−/− mice (Figure E4).
TABLE 2.
EFFECTS OF MWCNT EXPOSURE AND OVA SENSITIZATION ON PROSTANOID CONCENTRATIONS DETECTED BY LIQUID CHROMATOGRAPHY–TANDEM MASS SPECTROSCOPY IN BRONCHOALVEOLAR LAVAGE FLUID FROM WILD-TYPE AND COX-2−/− MICE
| Genotype | Pretreatment | OPA | PGE2 |
6-keto PGF1α |
PGF2α |
TXB2 |
PGD2 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 d | 14 d | 1 d | 14 d | 1 d | 14 d | 1 d | 14 d | 1 d | 14 d | |||
| WT |
Saline |
Vehicle |
47 ± 16 |
38 ± 6 |
11 ± 5 |
4 ± 2 |
17 ± 1 |
10 ± 1 |
21 ± 7 |
7 ± 1 |
107 ± 26 |
72 ± 15 |
| |
Saline |
MWCNT |
56 ± 14 |
59 ± 4 |
11 ± 7 |
7 ± 2 |
12 ± 2 |
13 ± 1 |
14 ± 3 |
11 ± 1 |
72 ± 11 |
85 ± 6 |
| |
OVA |
Vehicle |
88 ± 13 |
91 ± 7 |
16 ± 5 |
6 ± 1 |
39 ± 8 |
26 ± 5 |
102 ± 28* |
42 ± 13 |
106 ± 32 |
118 ± 23 |
| |
OVA |
MWCNT |
161 ± 68 |
85 ± 12 |
39 ± 16* |
6 ± 1 |
49 ± 16 |
15 ± 1 |
63 ± 20 |
25 ± 7 |
96 ± 32 |
131 ± 19 |
| COX-2−/− |
Saline |
Vehicle |
76 ± 9 |
60 ± 8 |
12 ± 5 |
10 ± 4 |
20 ± 4 |
13 ± 2 |
26 ± 6 |
17 ± 5 |
106 ± 17 |
120 ± 25 |
| |
Saline |
MWCNT |
98 ± 12 |
91 ± 32 |
14 ± 5 |
11 ± 8 |
19 ± 2 |
18 ± 3 |
24 ± 4 |
23 ± 3 |
137 ± 20 |
170 ± 12* |
| |
OVA |
Vehicle |
94 ± 43 |
63 ± 10 |
5 ± 1 |
3 ± 1 |
30 ± 8 |
13 ± 1 |
38 ± 12 |
21 ± 9 |
169 ± 104 |
112 ± 10 |
| OVA | MWCNT | 175 ± 42 | 62 ± 7 | 9 ± 3 | 7 ± 3 | 66 ± 13 | 14 ± 1 | 120 ± 36* | 20 ± 1 | 227 ± 97 | 87 ± 11 | |
Definition of abbreviations: COX-2, cyclooxygenase-2; d, days; MWCNT, multiwalled carbon nanotubes; OPA; oropharyngeal aspiration; OVA, ovalbumin; PGD2, prostaglandin D2; PGE2, prostaglandin E2; PGF2α, prostaglandin F2α; 6-keto PGF1α, 6-keto prostaglandin F1α; TXB2, thromboxane B2; WT, wild-type.
Data are expressed as pg/ml (mean ± SEM). The vehicle consisted of saline/0.1% pluronic solution.
P < 0.05, compared with the corresponding genotype group.
Discussion
The emergence of nanotechnology has generated a wide variety of engineered nanoparticles that could pose potential health risks for individuals with allergic asthma. We investigated the effects of CNT exposure on OVA-induced airway remodeling in WT and COX-2−/− mice. The rationale for determining the effects of MWCNT exposure after allergen sensitization in mice involved modeling the response to nanoparticles in individuals with preexisting asthma. Furthermore, we sought to investigate the role of COX-2, an enzyme that has been reported to exert protective effects in asthma. We observed that MWCNTs significantly enhanced OVA-induced airway inflammation and mucus-cell metaplasia in COX-2−/− mice but not WT mice. Moreover, these enhanced airway remodeling effects in COX-2−/− mice caused by MWCNTs and allergen coexposure were associated with a mixed Th1/Th2/Th17 immune response and enhanced concentrations of PGD2 and TXB2.
The most significant airway remodeling endpoint observed in this study comprised mucus-cell metaplasia, which resulted in increased numbers of goblet cells that were readily detectable by AB-PAS staining, and that were quantifiable by morphometric analysis. Mucus-cell metaplasia and subsequent mucus hypersecretion by goblet cells comprise a hallmark feature of asthma and a major problem in individuals with asthma, causing occlusion of the airways with mucus plugs. IL-13, a key Th2 cytokine, has been shown to mediate mucus-cell metaplasia and mucus hypersecretion by goblet cells after allergen challenge in mice (18, 34, 35). In the present study, the pattern of IL-13 mRNA and protein expression correlated with increased mucus-cell metaplasia at 1 day after exposure to MWCNTs in COX-2−/− mice that were prechallenged with OVA. A systemic endpoint of allergic Th2 inflammation involves increased serum IgE. We observed that OVA sensitization increased serum IgE concentrations in WT and COX-2−/− mice by approximately 2-fold. MWCNTs have been previously demonstrated to exacerbate OVA challenges and increase serum IgE concentrations (11). In the present study, MWCNT exposure significantly enhanced OVA-induced serum IgE only in COX-2−/− mice, but not in WT mice, and elevated serum IgE concentrations in COX-2−/− mice exposed to MWCNTs after OVA sensitization closely mirrored the increased concentrations of the Th2 cytokines, IL-5 and IL-13.
A Th2 immune response is typical of allergic airway inflammation in asthma. However, more recent findings suggest that a Th17 inflammatory response is more typical of severe experimental allergic asthma (36). Moreover, inflammatory cytokine profiles from patients with mild and severe asthma support the hypothesis that a Th2 inflammatory response is typical of mild asthma, whereas a mixed Th2/Th17 inflammatory response is more typical of severe asthma (37). Our data showed that MWCNTs exacerbate allergen-induced airway inflammation and mucus-cell metaplasia in COX-2−/− mice and increase the Th17 cytokine IL-17A as well as the Th2 cytokines, IL-5 and IL-13, thereby supporting the hypothesis that a more severe allergic airway remodeling event is mediated by a mixed Th2/Th17 response. Significant differences in Th2 cytokines (IL-13 and IL-5) were not observed between WT and COX-2−/− mice that received OVA sensitization without MWCNT exposure. This finding is in agreement with an earlier study showing that COX-2−/− mice do not have different concentrations of IL-13 in BALF after OVA sensitization (38). Therefore, MWCNT exposure after OVA sensitization is required to augment a Th2 response in COX-2−/− mice. COX-2 regulates Th17 cell differentiation during allergic lung inflammation, and lower concentrations of IL-17A have been reported in the BALF recovered from the lungs of COX-2−/− mice compared with WT mice after OVA challenge (32). We observed a slight increase of IL-17A in the BALF of COX-2−/− mice compared with WT mice after OVA exposure. The difference in IL-17A regulation observed in the present study could be attributed to a much longer OVA sensitization protocol (see Figure E1 compared with the findings of Li and colleagues) (32). Significant increases in IL-17A measured in BALF were only seen in COX-2−/− mice treated with MWCNTs after OVA sensitization.
We showed that the expression of the Th1 cytokine CXCL10 was enhanced by MWCNTs and OVA sensitization in COX-2−/− mice, but not WT mice. CXCL10 is an interferon-inducible cytokine that is increased in patients with asthma, but is most dramatically increased in individuals with sarcoidosis, a Th1 inflammatory disease (39). In addition, CXCL10 has been identified as a biomarker of virus-induced asthma exacerbation (40). In the present study, MWCNTs alone did not induce CXCL10, and OVA alone marginally increased CXCL10 to a similar extent in either WT or COX-2−/− mice. However, the combination of OVA and MWCNTs significantly induced CXCL10 in COX-2−/− mice, suggesting that COX-2 plays a role in suppressing Th1 inflammation in the exacerbation of allergic airway inflammation. In general, our findings show that the convergent effects of MWCNTs, which induce a Th1 response, and OVA, which induces a Th2 response, result in a mixed Th1/Th2 response that also features the activation of the Th17 immune response.
The role of COX in the pathogenesis of asthma is complex, in that some of the prostaglandin (PG) metabolites produced during COX activity are beneficial in asthma, whereas others are detrimental. Both PGE2 and prostacyclin are bronchoprotective, whereas PGD2 and TXB2 are bronchoconstrictors (41, 42), and have been shown to mediate enhanced eosinophilic lung inflammation after OVA challenge in mice (43). In the present study, lung concentrations of OVA-induced PGD2 and TXB2 in BALF were enhanced by MWCNTs in COX-2−/− mice but not in WT mice, which supports a pathogenic role for these mediators. PGD2 in particular has been linked to airway inflammation and mucus-cell metaplasia in asthma (44, 45). PGD2, a product of mast cells, is produced in large amounts by individuals with asthma, and antagonists of PGD2 receptors have been proposed for the treatment of allergic airway disease (46). Importantly, mice deficient in PGD2 receptor type 2 (DP2), the receptor for PGD2, demonstrate reduced eosinophilia and do not develop airway mucus-cell metaplasia (45). Furthermore, the pharmacologic blockade of DP2 inhibits cigarette smoke–induced mucus-cell metaplasia in mice (44). In the present study, MWCNTs enhanced OVA-induced concentrations of PGD2 on Day 1 in COX-2−/− but not WT mice, and lung PGD2 concentrations closely corresponded to the enhanced mucus-cell metaplasia observed in COX-2−/− mice after MWCNT exposure. However, the possible link between PGD2 and enhanced mucus-cell metaplasia after MWCNT exposure in OVA-challenged mice is associative and not causative. Future investigations should address this putative link by crossing COX-2−/− mice with DP2 mice to determine whether the high level of mucus-cell metaplasia seen in COX-2−/− mice after coexposure to MWCNT and allergen is ablated or reduced in mice lacking both COX-2 and DP2.
Airway fibrosis constitutes an important component of the chronic remodeling process in asthma. TGF-β1 is a central mediator of fibrosis that stimulates collagen production by fibroblasts, and IL-13 has been shown to induce tissue fibrosis by stimulating the production and activation of TGF-β1 (47). In the present study, OVA challenge increased IL-13 and TGF-β1 in both WT and COX-2−/− mice, whereas MWCNTs caused a significant decrease in IL-13 and TGF-β1 in WT mice, but not COX-2−/− mice. Similarly, MWCNTs caused a significant increase in OVA-induced collagen (Col1A2) mRNA concentrations in COX-2−/− mice but not WT mice, suggesting that COX-2 plays a role in reducing collagen mRNA concentrations in this model system. However, a morphometric analysis showed that OVA challenge increased airway collagen thickness similarly (2- to 3-fold) in both WT and COX-2−/− mice, and that MWCNTs suppressed OVA-induced airway collagen thickness in both WT and COX-2−/− mice, although this effect was not statistically significant. Why increased collagen mRNA expression in COX-2−/− mice treated with OVA and MWCNTs did not translate into increased airway fibrosis remains unknown. The collagen mRNAs that we measured were obtained from whole lung tissue, whereas morphometric measurements were performed only in airway collagen. However, total lung collagen, as measured by Sircol assay, indicated no significant increase in overall lung fibrosis in any of the treatment groups. Collagen-degrading proteinases may also be increased in mice exposed to OVA and MWCNTs, resulting in a net decrease in airway collagen. We and others previously reported that airway fibrosis is increased by combined OVA and MWCNT exposure (10, 11). However, these previous studies used different mouse strains (C57BL/6 and ICR), compared with the present study (a C57BL/6 × 129/Ola strain). Genetic strain variation has been reported for lung physiologic and pathologic outcomes in mice, including fibrosis (48). Therefore, future studies should focus on genetic variation as a factor in predicting the pathologic response to CNTs.
COX-2−/− and COX-1−/− cells were previously reported to exhibit substantially enhanced concentrations of the remaining functional COX gene, and cells with the genetic ablation of either COX isozyme have the ability to overcome defects in PG biosynthesis (49, 50). Moreover, others have reported that PG concentrations in BALF are not significantly altered in COX-2−/− mice during allergic lung inflammation, relative to WT mice (32). In addition, our data suggest that the compensatory production of certain PGs is regulated differently. For example, PGE2 concentrations were similarly increased by MWCNTs and OVA in WT and COX-2−/− mice, and yet PGD2 concentrations were increased by MWCNTs and OVA in COX-2−/− mice but not in WT mice. To determine whether the increase in PGs produced by COX-2−/− mice after coexposure to MWCNTs and/or OVA in the present study was attributable to a compensatory increase in COX-1, we measured concentrations of COX-1 mRNA in lung tissue from WT and COX-2−/− mice after exposure to OVA and MWCNTs. Exposure to OVA and MWCNTs mildly suppressed COX-1 mRNA concentrations in lung tissue at 1 day after exposure, and no differences were observed between genotypes. This finding suggests that enzymes downstream of COX-1, such as PGD synthase and thromboxane A (TXA) synthase, might be regulated differently in WT versus COX-2−/− mice.
The findings in our study are consistent with the hypothesis that COX-2 is protective during asthma. Several lines of experimental evidence support this hypothesis. First, a deficiency of cyclooxygenase transcripts has been reported in cultured primary bronchial epithelial cells of aspirin-sensitive patients with asthma (20). Second, allergic airway inflammation is increased in COX-2−/− mice (24, 26). Third, selective COX-2 inhibitors increase allergic airway inflammation in mice (23). Collectively, these studies provide strong evidence that COX-2 deficiency is an important susceptibility factor in the pathogenesis of allergic asthma.
The exacerbation of allergen-induced airway inflammation and mucus-cell metaplasia that we observed in the present study is likely not specific to MWCNTs. Ultrafine particulate matter (i.e., air pollution particles in the nanoscale range) is well-known to exacerbate asthma in humans and experimental animals (5, 51). However, our work is important in that we used a well-characterized source of a specific type of engineered nanomaterial that could pose a human health risk during occupational or consumer exposure scenarios. Moreover, we demonstrated COX-2 deficiency as a susceptibility factor in the exacerbation of allergen-induced airway inflammation and mucus-cell metaplasia by MWCNTs. A wide variety of other types of engineered nanomaterials could also present a possible health risk by exacerbating preexisting asthma or by acting as allergens themselves. Therefore, addressing the vast array of emerging engineered nanomaterials as potential health risks for individuals with asthma poses an important future challenge.
Finally, our findings may have some clinical implications, and suggest that individuals with asthma who also have reduced concentrations of COX-2, or individuals with reduced COX-2 activity as a consequence of treatment with COX-2 inhibitors, are at greater risk for exposure to MWCNTs. COX inhibitors are very commonly used medications. Therefore, individuals with underlying allergic airway inflammation who are taking a COX inhibitor would likely demonstrate a more severe airway inflammatory response and enhanced mucus production if exposed to an inhalation dose of CNTs.
Acknowledgments
Acknowledgments
The authors thank the staff of the Toxicology Department Vivarium at North Carolina State University for the exceptional quality of care provided in regard to animal welfare and husbandry.
Footnotes
This work was funded by National Institute of Environmental Health Sciences grants RC2-ES018772 (J.C.B.) and R01-ES020897 (J.C.B.), and the Intramural Research Program of the National Institutes of Health and the National Institutes of Environmental Health Sciences (D.C.Z., R.L., R.T.D., M.L.E., F.B.L., and K.B.T.). E.E.G.-B. and B.C.S. are supported by National Institute of Environmental Health Sciences training grant T32-ES007046-31.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2013-0019OC on May 3, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and management of asthma—summary report. J Allergy Clin Immunol. 2007;120:S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 2.Davies DE, Wicks J, Powell RM, Puddicombe SM, Holgate ST. Airway remodeling in asthma: new insights. J Allergy Clin Immunol. 2003;111:215–225. doi: 10.1067/mai.2003.128. [DOI] [PubMed] [Google Scholar]
- 3.Al-Muhsen S, Johnson JR, Hamid Q. Remodeling in asthma. J Allergy Clin Immunol. 2011;128:451–462. doi: 10.1016/j.jaci.2011.04.047. [DOI] [PubMed] [Google Scholar]
- 4.Lemanske RF, Jr, Busse WW. Asthma: clinical expression and molecular mechanisms. J Allergy Clin Immunol. 2010;125:S95–S102. doi: 10.1016/j.jaci.2009.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gavett SH, Koren HS. The role of particulate matter in exacerbation of atopic asthma. Int Arch Allergy Immunol. 2001;124:109–112. doi: 10.1159/000053685. [DOI] [PubMed] [Google Scholar]
- 6.Peters A, Wichmann H, Tuch T, Heinrich J, Heyder J. Respiratory effects are associated with the number of ultrafine particles. Am J Respir Crit Care Med. 1997;155:1376–1383. doi: 10.1164/ajrccm.155.4.9105082. [DOI] [PubMed] [Google Scholar]
- 7.Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonner JC. Nanoparticles as a potential cause of pleural and interstitial lung disease. Proc Am Thorac Soc. 2010;7:138–141. doi: 10.1513/pats.200907-061RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Card JW, Zeldin DC, Bonner JC, Nestmann ER. Pulmonary applications and toxicity of engineered nanoparticles. Am J Physiol Lung Cell Mol Physiol. 2008;295:L400–L411. doi: 10.1152/ajplung.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryman-Rasmussen JP, Tewksbury EW, Moss OR, Cesta MF, Wong BA, Bonner JC. Inhaled multiwalled carbon nanotubes potentiate airway fibrosis in murine allergic asthma. Am J Respir Cell Mol Biol. 2009;40:349–358. doi: 10.1165/rcmb.2008-0276OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inoue K-I, Koike E, Yanagisawa R, Hirano S, Nishikawa M, Takano H. Effects of multi-walled carbon nanotubes on a murine allergic airway inflammation model. Toxicol Appl Pharmacol. 2009;237:306–316. doi: 10.1016/j.taap.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Katwa P, Podila R, Chen P, Ke PC, Rao AM, Walters DM, Wingard CJ, Brown JM. Multi-walled carbon nanotube instillation impairs pulmonary function in C57BL/6 mice. Part Fibre Toxicol. 2011;8:24. doi: 10.1186/1743-8977-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kabesch M, Schedel M, Carr D, Woitsch B, Fritzsch C, Weiland SK, von Mutius E. IL-4/IL-13 pathway genetics strongly influence serum IgE levels and childhood asthma. J Allergy Clin Immunol. 2006;117:269–274. doi: 10.1016/j.jaci.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 14.Kim H-B, Kang M-J, Lee S-Y, Jin H-S, Kim J-H, Kim B-S, Jang S-O, Lee Y-C, Sohn M-H, Kim K-E, et al. Combined effect of tumour necrosis factor–alpha and interleukin-13 polymorphisms on bronchial hyperresponsiveness in Korean children with asthma. Clin Exp Allergy. 2008;38:774–780. doi: 10.1111/j.1365-2222.2008.02965.x. [DOI] [PubMed] [Google Scholar]
- 15.London SJ. Gene–air pollution interactions in asthma. Proc Am Thorac Soc. 2007;4:217–220. doi: 10.1513/pats.200701-031AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vercelli D. Discovering susceptibility genes for asthma and allergy. Nat Rev Immunol. 2008;8:169–182. doi: 10.1038/nri2257. [DOI] [PubMed] [Google Scholar]
- 17.Arima K, Umeshita-Suyama R, Sakata Y, Akaiwa M, Mao X-Q, Enomoto T, Dake Y, Shimazu S-I, Yamashita T, Sugawara N, et al. Upregulation of IL-13 concentration in vivo by the IL13 variant associated with bronchial asthma. J Allergy Clin Immunol. 2002;109:980–987. doi: 10.1067/mai.2002.124656. [DOI] [PubMed] [Google Scholar]
- 18.Kuperman D, Huang X, Koth L, Chang G, Dolganov G, Zhu Z, Elias J, Sheppard D, Erle D. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med. 2002;8:885–889. doi: 10.1038/nm734. [DOI] [PubMed] [Google Scholar]
- 19.Vladich FD, Brazille SM, Stern D, Peck ML, Ghittoni R, Vercelli D. IL-13 R130Q, a common variant associated with allergy and asthma, enhances effector mechanisms essential for human allergic inflammation. J Clin Invest. 2005;115:747–754. doi: 10.1172/JCI22818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pierzchalska M, Soja J, Woś M, Szabó Z, Nizankowska-Mogielnicka E, Sanak M, Szczeklik A. Deficiency of cyclooxygenases transcripts in cultured primary bronchial epithelial cells of aspirin-sensitive asthmatics. J Physiol Pharmacol. 2007;58:207–218. [PubMed] [Google Scholar]
- 21.Trudeau J, Hu H, Chibana K, Chu HW, Westcott JY, Wenzel SE. Selective downregulation of prostaglandin E2–related pathways by the Th2 cytokine IL-13. J Allergy Clin Immunol. 2006;117:1446–1454. doi: 10.1016/j.jaci.2006.01.049. [DOI] [PubMed] [Google Scholar]
- 22.Stumm CL, Wettlaufer SH, Jancar S, Peters-Golden M. Airway remodeling in murine asthma correlates with a defect in PGE2 synthesis by lung fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2011;301:L636–L644. doi: 10.1152/ajplung.00158.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peebles RS, Hashimoto K, Morrow JD, Dworski R, Collins RD, Hashimoto Y, Christman JW, Kang K-H, Jarzecka K, Furlong J, et al. Selective cyclooxygenase-1 and -2 inhibitors each increase allergic inflammation and airway hyperresponsiveness in mice. Am J Respir Crit Care Med. 2002;165:1154–1160. doi: 10.1164/ajrccm.165.8.2106025. [DOI] [PubMed] [Google Scholar]
- 24.Gavett SH, Madison SL, Chulada PC, Scarborough PE, Qu W, Boyle JE, Tiano HF, Lee CA, Langenbach R, Roggli VL, et al. Allergic lung responses are increased in prostaglandin H synthase–deficient mice. J Clin Invest. 1999;104:721–732. doi: 10.1172/JCI6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stokes Peebles RJ, Dworski R, Collins RD, Jarzecka K, Mitchell DB, Graham BS, Sheller JR. Cyclooxygenase inhibition increases interleukin 5 and interleukin 13 production and airway hyperresponsiveness in allergic mice. Am J Respir Crit Care Med. 2000;162:676. doi: 10.1164/ajrccm.162.2.9911063. [DOI] [PubMed] [Google Scholar]
- 26.Nakata J, Kondo M, Tamaoki J, Takemiya T, Nohara M, Yamagata K, Nagai A. Augmentation of allergic inflammation in the airways of cyclooxygenase-2–deficient mice. Respirology. 2005;10:149–156. doi: 10.1111/j.1440-1843.2005.00687.x. [DOI] [PubMed] [Google Scholar]
- 27.Ryman-Rasmussen JP, Cesta MF, Brody AR, Shipley-Phillips JK, Everitt JI, Tewksbury EW, Moss OR, Wong BA, Dodd DE, Andersen ME, et al. Inhaled carbon nanotubes reach the subpleural tissue in mice. Nat Nanotechnol. 2009;4:747–751. doi: 10.1038/nnano.2009.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JK, Sayers BC, Chun K-S, Lao H-C, Shipley-Phillips JK, Bonner JC, Langenbach R. Multi-walled carbon nanotubes induce COX-2 and iNOS expression via MAP kinase–dependent and –independent mechanisms in mouse RAW264.7 macrophages. Part Fibre Toxicol. 2012;9:14. doi: 10.1186/1743-8977-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ledford JG, Goto H, Potts EN, Degan S, Chu HW, Voelker DR, Sunday ME, Cianciolo GJ, Foster WM, Kraft M, et al. SP-A preserves airway homeostasis during Mycoplasma pneumoniae infection in mice. J Immunol. 2009;182:7818–7827. doi: 10.4049/jimmunol.0900452. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 30.Card JW, Voltz JW, Carey MA, Bradbury JA, Degraff LM, Lih FB, Bonner JC, Morgan DL, Flake GP, Zeldin DC. Cyclooxygenase-2 deficiency exacerbates bleomycin-induced lung dysfunction but not fibrosis. Am J Respir Cell Mol Biol. 2007;37:300–308. doi: 10.1165/rcmb.2007-0057OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brass DM, Savov JD, Gavett SH, Haykal-Coates N, Schwartz DA. Subchronic endotoxin inhalation causes persistent airway disease. Am J Physiol Lung Cell Mol Physiol. 2003;285:L755–L761. doi: 10.1152/ajplung.00001.2003. [DOI] [PubMed] [Google Scholar]
- 32.Li H, Bradbury JA, Dackor RT, Edin ML, Graves JP, DeGraff LM, Wang PM, Bortner CD, Maruoka S, Lih FB, et al. Cyclooxygenase-2 regulates Th17 cell differentiation during allergic lung inflammation. Am J Respir Crit Care Med. 2011;184:37–49. doi: 10.1164/rccm.201010-1637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newman JW, Watanabe T, Hammock BD. The simultaneous quantification of cytochrome P450 dependent linoleate and arachidonate metabolites in urine by HPLC-MS/MS. J Lipid Res. 2002;43:1563–1578. doi: 10.1194/jlr.d200018-jlr200. [DOI] [PubMed] [Google Scholar]
- 34.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 35.Ingram JL, Kraft M. IL-13 in asthma and allergic disease: asthma phenotypes and targeted therapies. J Allergy Clin Immunol. 2012;130:829–842. doi: 10.1016/j.jaci.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 36.Lajoie S, Lewkowich IP, Suzuki Y, Clark JR, Sproles AA, Dienger K, Budelsky AL, Wills-Karp M. Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nature Publishing Group. 2010;11:928–935. doi: 10.1038/ni.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y-H, Wills-Karp M. The potential role of interleukin-17 in severe asthma. Curr Allergy Asthma Rep. 2011;11:388–394. doi: 10.1007/s11882-011-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carey MA, Germolec DR, Bradbury JA, Gooch RA, Moorman MP, Flake GP, Langenbach R, Zeldin DC. Accentuated T helper Type 2 airway response after allergen challenge in cyclooxygenase-1−/− but not cyclooxygenase-2−/− mice. Am J Respir Crit Care Med. 2003;167:1509–1515. doi: 10.1164/rccm.200211-1383OC. [DOI] [PubMed] [Google Scholar]
- 39.Miotto D, Christodoulopoulos P, Olivenstein R, Taha R, Cameron L, Tsicopoulos A, Tonnel AB, Fahy O, Lafitte JJ, Luster AD, et al. Expression of IFN-gamma–inducible protein; monocyte chemotactic proteins 1, 3, and 4; and eotaxin in Th1- and Th2-mediated lung diseases. J Allergy Clin Immunol. 2001;107:664–670. doi: 10.1067/mai.2001.113524. [DOI] [PubMed] [Google Scholar]
- 40.Wark PA, Bucchieri F, Johnston SL, Gibson PG, Hamilton L, Mimica J, Zummo G, Holgate ST, Attia J, Thakkinstian A, et al. IFN-gamma–induced protein 10 is a novel biomarker of rhinovirus-induced asthma exacerbations. J Allergy Clin Immunol. 2007;120:586–593. doi: 10.1016/j.jaci.2007.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wenzel SE. Arachidonic acid metabolites: mediators of inflammation in asthma. Pharmacotherapy: J of Human Pharmacol Drug Therapy. 1997;17:3S–12S. [PubMed] [Google Scholar]
- 42.Park GY, Christman JW. Involvement of cyclooxygenase-2 and prostaglandins in the molecular pathogenesis of inflammatory lung diseases. Am J Physiol Lung Cell Mol Physiol. 2006;290:L797–L805. doi: 10.1152/ajplung.00513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shiraishi Y, Asano K, Niimi K, Fukunaga K, Wakaki M, Kagyo J, Takihara T, Ueda S, Nakajima T, Oguma T, et al. Cyclooxygenase-2/prostaglandin D2/CRTH2 pathway mediates double-stranded RNA–induced enhancement of allergic airway inflammation. J Immunol. 2008;180:541–549. doi: 10.4049/jimmunol.180.1.541. [DOI] [PubMed] [Google Scholar]
- 44.Stebbins KJ, Broadhead AR, Correa LD, Scott JM, Truong YP, Stearns BA, Hutchinson JH, Prasit P, Evans JF, Lorrain DS. Therapeutic efficacy of AM156, a novel prostanoid DP2 receptor antagonist, in murine models of allergic rhinitis and house dust mite–induced pulmonary inflammation. Eur J Pharmacol. 2010;638:142–149. doi: 10.1016/j.ejphar.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 45.Matsuoka T, Hirata M, Tanaka H, Takahashi Y, Murata T, Kabashima K, Sugimoto Y, Kobayashi T, Ushikubi F, Aze Y, et al. Prostaglandin D2 as a mediator of allergic asthma. Science. 2000;287:2013–2017. doi: 10.1126/science.287.5460.2013. [DOI] [PubMed] [Google Scholar]
- 46.Pettipher R, Hansel TT, Armer R. Antagonism of the prostaglandin D2 receptors DP1 and CRTH2 as an approach to treat allergic diseases. Nat Rev Drug Discov. 2007;6:313–325. doi: 10.1038/nrd2266. [DOI] [PubMed] [Google Scholar]
- 47.Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, Koteliansky V, Shipley JM, Gotwals P, Noble P, Chen Q, et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor 1. J Exp Med. 2001;194:809–822. doi: 10.1084/jem.194.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brewer JP, Kisselgof AB, Martin TR. Genetic variability in pulmonary physiological, cellular, and antibody responses to antigen in mice. Am J Respir Crit Care Med. 1999;160:1150–1156. doi: 10.1164/ajrccm.160.4.9806034. [DOI] [PubMed] [Google Scholar]
- 49.Kirtikara K, Morham SG, Raghow R, Laulederkind SJ, Kanekura T, Goorha S, Ballou LR. Compensatory prostaglandin E2 biosynthesis in cyclooxygenase 1 or 2 null cells. J Exp Med. 1998;187:517–523. doi: 10.1084/jem.187.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sandee D, Sivanuntakorn S, Vichai V, Kramyu J, Kirtikara K. Up-regulation of microsomal prostaglandin E synthase–1 in COX-1 and COX-2 knock-out mouse fibroblast cell lines. Prostaglandins Other Lipid Mediat. 2009;88:111–116. doi: 10.1016/j.prostaglandins.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 51.Matsumoto A, Hiramatsu K, Li Y, Azuma A, Kudoh S, Takizawa H, Sugawara I. Repeated exposure to low-dose diesel exhaust after allergen challenge exaggerates asthmatic responses in mice. Clin Immunol. 2006;121:227–235. doi: 10.1016/j.clim.2006.08.003. [DOI] [PubMed] [Google Scholar]