Abstract
The lung hosts multiple populations of macrophages and dendritic cells, which play a crucial role in lung pathology. The accurate identification and enumeration of these subsets are essential for understanding their role in lung pathology. Flow cytometry is a mainstream tool for studying the immune system. However, a systematic flow cytometric approach to identify subsets of macrophages and dendritic cells (DCs) accurately and consistently in the normal mouse lung has not been described. Here we developed a panel of surface markers and an analysis strategy that accurately identify all known populations of macrophages and DCs, and their precursors in the lung during steady-state conditions and bleomycin-induced injury. Using this panel, we assessed the polarization of lung macrophages during the course of bleomycin-induced lung injury. Alveolar macrophages expressed markers of alternatively activated macrophages during both acute and fibrotic phases of bleomycin-induced lung injury, whereas markers of classically activated macrophages were expressed only during the acute phase. Taken together, these data suggest that this flow cytometric panel is very helpful in identifying macrophage and DC populations and their state of activation in normal, injured, and fibrotic lungs.
Keywords: pulmonary macrophages, alveolar macrophages, interstitial macrophages, macrophage polarization, lung fibrosis
Clinical Relevance
Flow cytometry is a mainstream tool for studying the immune system. However, a systematic flow cytometric approach to identify subsets of macrophages and dendritic cells (DCs) accurately and consistently in the normal mouse lung has not been described. Here we developed a panel of surface markers and an analysis strategy that accurately identifies all known populations of macrophages and DCs, and their precursors in the lung, during steady-state conditions and bleomycin-induced injury.
Cells of the innate immune system, and especially myeloid cells such as neutrophils, eosinophils, monocytes, macrophages (alveolar and interstitial), and dendritic cells (DCs, i.e., plasmacytoid DCs, CD103+ DCs, and CD11b+ DCs), play an important role in lung development and physiology, and contribute to important lung diseases, including pulmonary infection, cancer, asthma, chronic obstructive pulmonary disease, and pulmonary fibrosis (1–5). Alveolar and interstitial lung macrophages exhibit different origins and life spans in lungs, and have been identified as key regulators of pathological and reparative processes. Alveolar macrophages, which are considered tissue-resident macrophages, populate lung tissue during early embryogenesis and remain viable for prolonged periods, with minimal replenishment from bone marrow–derived monocytes (6). In contrast, interstitial macrophages originate from bone marrow–derived monocytes and have a shorter half-life (7, 8). In recent studies, several groups of investigators suggested that these two populations of lung macrophages play opposing roles in lung injury. Alveolar macrophages appear to limit neutrophil influx into the lung during acute lung injury (9) or chronic exposure to organic dust (10), whereas interstitial macrophages promote neutrophil extravasation (11, 12). An additional layer of complexity is added by the phenotypic plasticity of macrophages. Classically activated macrophages (sometimes referred to as M1-polarized) have been suggested to promote the development of acute lung injury, whereas alternatively activated macrophages (M2) may play a role in limiting or resolving lung inflammation (13) and/or potentially promoting the development of fibrosis (14–17).
Understanding the roles played by these different macrophage populations and macrophage phenotypes in the pathophysiology of lung injury, repair, and fibrosis requires proper identification, enumeration, and phenotypic characterization. Flow cytometry has become an essential tool for analyses of the immune system, because it offers a short turnaround time between sample preparation, acquisition, and analysis, allows for the accurate enumeration of individual cell subsets (including very rare subsets), and provides an opportunity for detailed molecular phenotyping. However, flow cytometric analyses of innate immune cells are challenging even in the normal lung, and these problems are magnified in the presence of lung inflammation or fibrosis.
Recently, Gautier and colleagues used gene-expression profiling to identify differentially expressed genes in tissue macrophages, compared with other tissue-resident myeloid cells (18). We combined some of these newly identified markers with those described elsewhere in the literature to develop a panel of antibodies for use in flow cytometry that meets three a priori criteria, namely, the panel (1) clearly distinguishes between different myeloid populations in the mouse lung, (2) relies exclusively on surface markers to allow for live cell sorting, and (3) performs well in the injured or fibrotic lung. We used this panel to identify a minimal set of surface markers that can be used by investigators without access to eight or more parameter instruments. We validated the usefulness of both full and minimal panels during early acute lung injury and the late fibrosis after intratracheal administrations of bleomycin, and we evaluated the utility of these markers for differentiating between what are currently understood as M1 and M2 macrophage subsets. This systematic approach to flow cytometric analyses of the innate immune system in the mouse lung should prove helpful in improving our understanding of the role that individual subsets of macrophages and DCs play in the development of lung disease.
Materials and Methods
Mice
Eight-week-old male C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME) and housed at a barrier-free and specific pathogen–free facility at the Center for Comparative Medicine at Northwestern University (Chicago, IL). All procedures were approved by the Institutional Animal Care and Use Committee at Northwestern University.
Bleomycin-Induced Lung Injury and Fibrosis
Mice were anesthetized with isoflurane, their lungs were intubated orally with a 20-gauge Angiocath (Franklin Lanes, NJ), and two 50-μl sterile aliquots of PBS (control) or 0.025 IU bleomycin (AAP Pharmaceuticals LLC, Shamburg, IL) were instilled through the catheter, 3 minutes apart. After each aliquot, the mice were placed on their right side and then left side for 10–15 seconds. Mice were killed 5 and 21 days after the instillation of bleomycin.
Flow Cytometry and Cell Sorting
After the mice wee killed, their lungs were perfused through the right ventricle with 5 ml of PBS. The lungs were removed, and the large airways were dissected from the peripheral lung tissue. The peripheral lung tissue was cut into small pieces with scissors, transferred into C-tubes (Miltenyi, Auburn, CA), and processed in digestion buffer (1 mg/ml of Collagenase D and 0.1 mg/ml DNase I, both from Roche, Indianapolis, IN, in Hanks’ balanced salt solution) and a GentleMACS dissociator (Miltenyi), according to the manufacturer’s instructions. Homogenized lungs were passed through 40-μm nylon mesh to obtain a single-cell suspension. The remaining red blood cells were lysed using BD Pharm Lyse (BD Biosciences, San Jose, CA). The resultant cells were counted using a Countess cell counter (Invitrogen, Carlsbad, CA). Dead cells were excluded, using trypan blue.
Cells were stained with viability dye Aqua (Invitrogen) or eFluor 506 (eBioscience, San Diego, CA), incubated with FcBlock (BD Biosciences), and stained with a mixture of fluorochrome-conjugated antibodies (Table for a list of antibodies, clones, fluorochromes, and manufacturers). Data were acquired on a BD LSR II flow cytometer using BD FACSDiva software (BD Biosciences; see Table E2 for instrument configuration), and compensation and data analyses were performed “offline” using FlowJo software (TreeStar, Ashland, OR). Cell sorting was performed on a FACSAria II instrument (BD Biosciences) with the same configuration as the LSR II. Cytospins were prepared from sorted cells and stained with a Diff-Quik Stain Set (Siemens Healthcare, Malvern. PA). “Fluorescence minus one” controls were used when necessary. Cell populations were identified using sequential gating strategy (see Results), and the percentage of cells in the live/singlets gate was multiplied by the number of live cells (after trypan blue exclusion) to obtain an absolute live-cell count. The expression of activation markers is presented as median fluorescence intensity (MFI).
Statistical Analysis
Differences between groups were determined according to ANOVA. When ANOVA indicated a significant difference, individual differences were examined using t tests with a Tukey correction for multiple comparisons, as indicated. All analyses were performed using GraphPad Prism, version 5.00 (GraphPad Software, San Diego CA). Data are shown as means ± SEMs. P < 0.05 was considered statistically significant in all tests.
Results
Characterization of Myeloid Cell Subsets in the Normal Mouse Lung
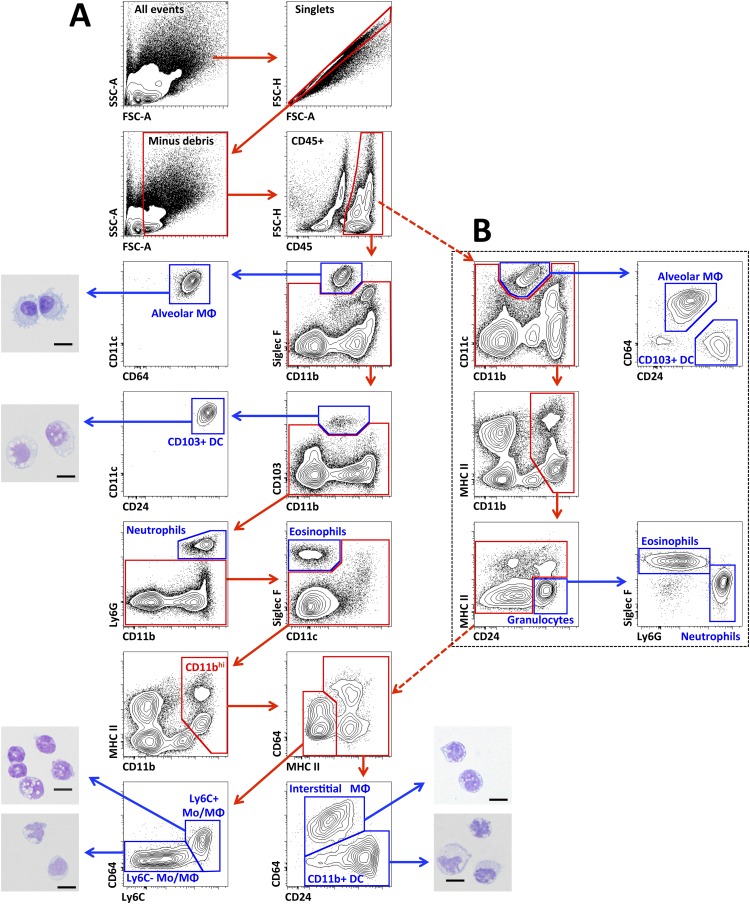
To identify myeloid populations in lungs accurately under steady-state conditions, we performed 10-color flow cytometry and sequential gating analysis (Figure 1). After the exclusion of doublets and debris, immune cells were identified using the pan-hematopoietic marker CD45. Dead cells may also be excluded at this step, using live/dead staining (Figure E1). In normal mouse lungs, alveolar macrophages were readily identified, based on the expression of Siglec F, CD11c, CD64, F4/80, the absence of CD11b, high side scatter, and high autofluorescence (Table 1, Figure 1A, and Figure E2). CD103+ DCs were enumerated based on their high expression of CD11c, CD24, CD103, and major histocompatibility class II (MHC II), and their absence of CD11b. Neutrophils express Ly6G, which is not detected in any other cell types, thus allowing for their clear and unambiguous identification. Eosinophils were identified based on their expression of Siglec F and F4/80, and after gating out alveolar macrophages (which also express high concentrations of Siglec F). High concentrations of CD11b and CD24, as well as high side scatter and the absence of CD11c and MHC II molecules, provided additional help in the identification of neutrophils and eosinophils. Moreover, the high expression of CD11b permitted the separation of monocytes, interstitial macrophages, and CD11b+ DCs from natural killer cells, which expressed intermediate concentrations of CD11b. Interstitial macrophages and CD11b+ DCs expressed MHC II and high-to-intermediate concentrations of CD11c, and were distinguished based on their expression of CD64 and CD24, correspondingly. CD11bhi cells do not express MHC II and express only low concentrations of CD64, and were separated into Ly6C+ and Ly6C− subsets. The expression of CD64, MHC II, and activation markers such as CD40, CD80, and CD86 was low in Ly6C+ and Ly6C− subsets (data not shown). Plasmacytoid DCs were identified as mPDCA-1+, as well as CD11cintB220+ (Figure E3).
Figure 1.
Gating strategy used to identify myeloid-cell subsets in the normal mouse lung. Cells were isolated from enzymatically digested mouse lungs, and after the exclusion of doublets and debris, immune cells were identified by CD45 staining. (A) A sequential gating strategy was first used to identify populations expressing specific markers: alveolar macrophages (MΦ) (Siglec F+ CD11b− CD11c+ CD64+), CD103+ dendritic cells (DCs) (CD11c+ CD103+ CD24+), neutrophils (CD11b+ Ly6G+), and eosinophils (Siglec F+ CD11b+ CD11c−), followed by the identification of populations with overlapping expression patterns: interstitial macrophages (CD11b+ MHC II+ CD11c+ CD64+ CD24−), CD11b+ DCs (CD11b+ MHC II+ CD11c+ CD24+ CD64−), and monocytes (Mo)/undifferentiated macrophages (CD11b+ MHC II− CD64+/− Ly6Clo). Scale bar in microphotographs = 5 μm. (B) Identification of macrophages and DCs using the minimal panel of surface markers. Both alveolar macrophages and CD103+ DCs are identified as CD11b−CD11c+ cells, and are further separated using CD64 and CD24, correspondingly. If necessary, MHC II can be used to confirm gating in CD103+ DCs (not shown). Gating on CD11bhi cells allows for the separation of myeloid cells from lymphoid cells that either do not express this marker (T and B cells), or express it at intermediate level (natural killer cells). Granulocytes (neutrophils and eosinophils) can be gated out as CD24+CD11c−, and the identification of CD11b+ DCs (CD11b+ MHC II+ CD11c+ CD24− CD64−), interstitial macrophages (CD11b+ MHC II+ CD11c+ CD64+ CD24−), and monocytes/undifferentiated macrophages can be continued as in the full panel (CD11b+ MHC II− CD64+/− Ly6Clo). FSC, forward scatter; MHC II, major histocompatibility complex class II; SSC, side scatter.
TABLE 1.
PHENOTYPES OF MYELOID CELLS IN THE NORMAL MOUSE LUNG
| Marker | Eosinophils | Neutrophils | Plasmacytoid DCs | CD103+ DCs | CD11b+ DCs | AM | IM | Ly6C+ Mo/MΦ | Ly6C− Mo/MΦ | |
|---|---|---|---|---|---|---|---|---|---|---|
|
1 |
CD45 |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
2 |
CD11b |
+ |
+ |
– |
– |
+ |
– |
+ |
+ |
+ |
|
3 |
CD11c |
− |
– |
± |
+ |
+ |
+ |
+ |
– |
± |
|
4 |
CD24 |
+ |
+ |
+ |
+ |
+ |
– |
– |
– |
– |
|
5 |
CD64 |
– |
– |
– |
– |
– |
+ |
+ |
± |
± |
|
6 |
Ly6C |
– |
± |
+ |
– |
± |
– |
– |
+ |
– |
|
7 |
MHC II |
– |
– |
± |
+ |
+ |
± |
+ |
– |
– |
| 8 |
CD14 |
– |
– |
NT |
– |
± |
± |
+ |
– |
– |
| 9 |
CD36 |
– |
– |
NT |
+ |
± |
+ |
+ |
± |
– |
| 10 |
CD103 |
– |
– |
– |
+ |
– |
– |
– |
– |
– |
| 11 |
CD206 |
– |
– |
– |
– |
± |
+ |
± |
– |
– |
| 12 |
Siglec F |
+ |
– |
– |
– |
– |
+ |
– |
– |
– |
| 13 |
Ly6G |
– |
+ |
– |
– |
– |
– |
– |
– |
– |
| 14 |
F4/80 |
+ |
– |
– |
– |
± |
+ |
+ |
+ |
+ |
| 15 | PDCA-1 | – | – | + | – | – | – | – | – | – |
Definition of abbreviations: AM, alveolar macrophages; DCs, dendritic cells; IM, interstitial macrophages; Mo, monocytes; MΦ, alveolar macrophages; MHC II, major histocompatibility complex class II; NT, not tested.
Symbols indicate the expression of a given marker. +, high expression; ±, low or intermediate expression; −, absence of expression. For a more differentiated assessment of expression, see Figure E2 in the online supplement. The minimal set of surface markers required for the accurate identification of macrophages and DCs in the mouse lung is shown in boldface.
To validate the results of flow cytometric experiments, we sorted individual populations from normal mouse lungs and examined their morphology (Figure 1A). CD103+ and CD11b+ DCs and interstitial macrophages exhibited irregularly shaped nuclei and numerous vacuoles in the cytoplasm, consistent with their role as phagocytes. Alveolar macrophages exhibited a similar morphology, but with more prominent pseudopodia. Ly6C+ and Ly6C− populations contained both monocyte-like and macrophage-like cells. The monocyte-like cells contained bean-shaped nuclei without vacuoles, and the macrophage-like cells contained irregularly shaped nuclei with numerous vacuoles.
Although our approach allows for the accurate identification of all myeloid subsets in the mouse lung, it requires instruments capable of analyzing at least 10 fluorescent parameters, and these instruments may not be available to all investigators. Therefore, we developed a minimal set of surface markers and corresponding antibodies that allowed for a clear identification of all populations of myeloid cells in normal mouse lungs (Figure 1B). This approach identified well-defined populations such as alveolar macrophages and CD103+ DCs, while allowing for the separation of monocyte-derived populations such as interstitial macrophages (CD11b+CD11c+CD64highMHC II+CD24−), CD11b+ DCs (CD11b+CD11c+CD64−MHC II+CD24+), and less mature monocytes and macrophages (CD11b+CD11c+/−CD64lowMHC II−CD24low).
The Phenotype of Myeloid Cells Changes during the Course of Bleomycin-Induced Lung Injury
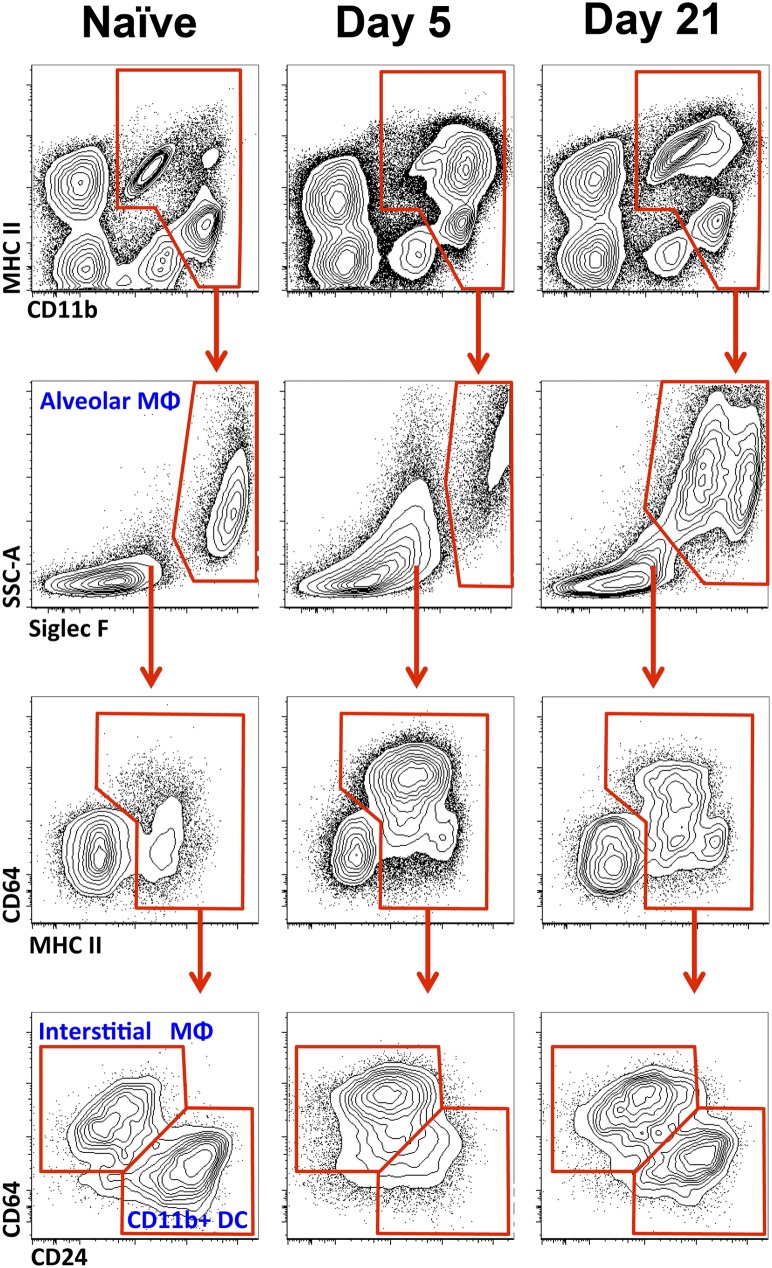
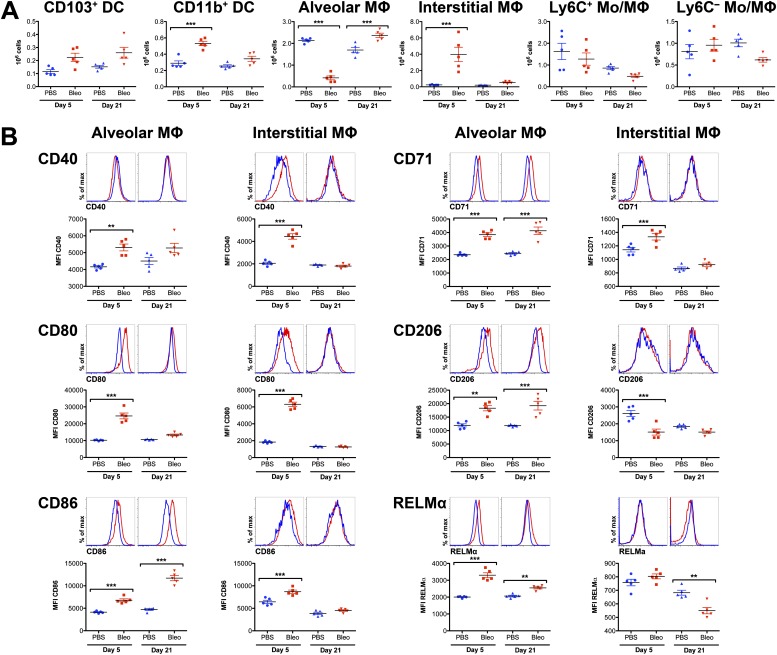
We examined our panel of markers for the identification of myeloid populations in the lung in the bleomycin model of lung injury followed by fibrosis. Previous studies have shown that an intratracheal administration of bleomycin results in acute lung injury, which becomes maximal 3 to 5 days after instillation and subsequently resolves. This is followed by the transforming growth factor–β–mediated development of lung fibrosis, which peaks between Days 21 and 28 after injury, and resolves slowly thereafter (16). In the acute (Day 5) and fibrotic (Day 21) phases of bleomycin-induced lung injury, the full and minimal panels of surface markers readily identified all myeloid subsets in homogenized lung tissue (Figure 2). During the acute phase (Day 5), the number of alveolar macrophages significantly decreased, whereas the number of interstitial macrophages and CD11b+ DCs increased (Figure 3A). In contrast, during the fibrotic phase (Day 21), the number of alveolar macrophages in bleomycin-treated animals was higher than in control animals, whereas the number of interstitial macrophages returned to control concentrations (Figure 3A). An increase in the number of alveolar macrophages during the fibrotic phase coincided with the emergence of a new subpopulation of Siglec Flow alveolar macrophages. These Siglec Flow alveolar macrophages expressed CD11b and elevated concentrations of CD11c, CD14, CD36, and CD64 (Figure E4). Siglec F was required for their identification, because neither CD11b nor CD11c allowed for the clear separation of these two subsets of alveolar macrophages. Importantly, even 38 days after bleomycin-induced lung injury, the Siglec Flow population was still present, and the ratio of Siglec Fhigh to Siglec Flow macrophages was unchanged (data not shown).
Figure 2.
The phenotype of myeloid cells in mouse lungs changes during the course of bleomycin-induced lung injury. Left to right: Normal lung, 5 and 21 days after instillation of bleomycin. Top images were gated on CD45+ cells, with neutrophils and eosinophils gated out.
Figure 3.
Changes of myeloid-cell subsets in mouse lungs during bleomycin-induced lung injury (Days 5 and 21). (A) Numerical changes of myeloid-cell subsets were identified as described in Figure 1. Values represent means ± SEMs. Differences between groups were compared using one-way ANOVA. ***P < 0.001. (B) Expression of markers associated with classically (CD40, CD80, and CD86) and alternatively (CD71, CD206, and RELMα) activated macrophages on alveolar and interstitial macrophages during bleomycin-induced lung injury. Values represent means ± SEMs for median fluorescence intensity (MFI) for the given marker. Differences between groups were compared using one-way ANOVA. **P < 0.01. ***P < 0.001.
Polarization of Lung Macrophages during the Course of Bleomycin-Induced Lung Injury
During the acute phase of bleomycin-induced lung injury, the expression of CD64 and markers of “classically activated” or “M1-like” macrophages (CD40, CD80, and CD86) (19) were increased in both alveolar and interstitial macrophages (Figures 3B and E5). However, during the fibrotic phase (Day 21), the expression of CD40 and CD80 returned to control concentrations (Figure 3B), whereas CD86 expression in alveolar macrophages remained elevated. In contrast to activation markers, the expression of CD71 (transferrin receptor), CD206 (mannose receptor), and resistin-like molecule alpha (RELMα), which are all associated with “alternatively activated,” “regulatory,” or “M2-like” macrophages (13, 19, 20), was elevated in alveolar macrophages during both the acute and fibrotic phases (Figure 3B). Importantly, no difference was evident in the expression of CD71, CD206, RELMα, and CD86 between Siglec Fhigh and Siglec Flow alveolar macrophages on Day 21.
Discussion
Flow cytometric analyses of innate immune cells in the lung are complicated for several reasons. First, many myeloid cells, and particularly alveolar macrophages, exhibit high autofluorescence, which often decreases the resolution between “positive” and “negative” populations, leading to false positivity for a given antigen/fluorochrome (21–23). Moreover, the autofluorescence of myeloid cells in the lung may further increase after exposure to environmental particulate matter. Second, many populations of myeloid cells, and especially macrophages and DCs, express similar surface markers, which makes an accurate identification of individual cellular subsets using just one or two surface markers almost impossible (18, 24–26). We describe a panel of surface markers that can be used to identify different myeloid populations unambiguously in the mouse lung, using flow cytometry. In a well-described model of bleomycin-induced lung injury followed by fibrosis, we found that this panel was able to distinguish different myeloid populations and assess their level of activation. Unlike other approaches (26), this panel relies exclusively on surface markers, and therefore can be used to sort live cells for use in subsequent studies.
The normal mouse lung contains multiple populations of macrophages and DCs. Lung macrophages consist of two distinct populations, namely, alveolar macrophages that represent long-lived tissue-resident macrophages, and short-lived monocyte-derived interstitial macrophages. Alveolar macrophages play an important role in maintaining lung homeostasis by removing pathogens and noxious particles without inducing inflammation or recruiting monocytes and neutrophils (3, 4, 27). In contrast, monocyte-derived interstitial macrophages are recruited to the lung from the circulation in response to acute lung injury, and are major contributors to the inflammatory response (3, 4, 27). DCs in the lung are represented by monocyte-derived CD11b+ DCs, plasmacytoid DCs, and CD103+ DCs, which originate from distinct precursors and play a crucial role in the induction and suppression of innate immune responses (2, 28). A precise identification of the relative and absolute compositions, as well as the activation state, of inflammatory populations in the lung is required if we are to understand their role in disease pathogenesis. This identification has been complicated by the absence of a specific macrophage or DC marker (18, 24) and the expression of similar surface markers in macrophages and DCs in lungs. Although plasmacytoid DCs and CD103+ DCs are easily identified using mPDCA-1 and CD103 antibodies, respectively (24, 29), discriminating between interstitial macrophages and CD11b+ DCs in the lung is less straightforward (28). Historically, CD11b+ DCs in the lungs were identified as CD11b+CD11c+MHC II+. However, this population was recently found to be heterogeneous (30). CD64, also known as Fc-gamma-Receptor 1 (FcγR1), has been shown to be useful in discriminating macrophages from CD11b+ DCs in the mouse gut and muscle (31, 32), and together with proto-oncogene tyrosine-protein kinase MER (MerTK) and CD14, is one of the most specific macrophage markers (18). We found that a combination of CD64 with CD24 and MHC II allowed not only for the separation of CD11b+ DCs from interstitial macrophages, but also for their discrimination from monocytes and undifferentiated macrophages.
Alveolar macrophages are long-lived tissue-resident macrophages. They populate the lung during early embryogenesis, and are able to maintain themselves for months with minimal replenishment from bone marrow–derived cells (6, 33). Moreover, resident alveolar macrophages have been shown to persist after LPS-induced or influenza A–induced acute lung injury, when they participate in the resolution of inflammation by phagocytosing apoptotic neutrophils and recruiting monocyte-derived macrophages (33). However, if alveolar macrophages are depleted using clodronate-loaded liposomes or the administration of diphtheria toxin to CD11c–diphtheria toxin receptor mice, they can be restored by monocyte-derived interstitial macrophages (7). The instillation of bleomycin induces the apoptosis of alveolar macrophages (34–36), which are then reconstituted from bone marrow–derived cells. Siglec F, which is typically considered an eosinophil marker (37), is highly expressed in murine alveolar macrophages, and when used in combination with CD11c or CD64, provides the most accurate identification of alveolar macrophages in the mouse lung. We found that in the normal mouse lung and during the acute phase of bleomycin-induced lung injury, alveolar macrophages maintain their distinct phenotype (Siglec FhighCD11c+CD64+CD11b−) and can be easily separated from interstitial macrophages and CD11b+ DCs, using flow cytometry. However, during the fibrotic phase, a new subpopulation of Siglec Flow alveolar macrophages appears. In comparison to Siglec Fhigh alveolar macrophages, these Silgec Flow alveolar macrophages express higher concentrations of CD11b, CD11c, CD64, CD14, and CD36, and likely represent monocyte-derived interstitial macrophages taking on intermediate phenotypes as they differentiate into alveolar macrophages. Therefore, although an accurate identification of macrophage and DC subsets in the mouse lung is possible without using Siglec F, we found this marker very useful in providing additional information on the origins of alveolar macrophages. In contrast, F4/80, which is often considered a “classic” macrophage marker, did not separate interstitial macrophages, monocytes/undifferentiated macrophages, eosinophils, and CD11b+ DCs. The addition of F4/80 to the panel provided no additional discriminatory power. Another “classic” macrophage marker, CD68, was recently proposed for the identification of myeloid cell subsets in the mouse lung (26). However, because CD68 is an intracellular marker, its use requires cellular fixation and permeabilization, precluding use of the cells in subsequent experiments. Our approach relies exclusively on surface markers, and therefore can be used to sort live cells by means of FACS.
In the past, many groups reported the results of two-parameter approaches to identify myeloid cell subsets in the murine lung. For example, investigators have used CD11b versus CD11c plots to identify alveolar and interstitial macrophages and even DCs (7, 23, 38). Our data suggest that although this approach allows for a fairly accurate identification of alveolar macrophages, it does not permit discrimination between CD11b+ DCs, interstitial macrophages, and immature monocytes/macrophages. Furthermore, unless neutrophils and eosinophils are explicitly gated out before examinations of CD11b versus CD11c staining, they would fall into the CD11b+CD11c− region and possibly be incorrectly identified as monocytes and macrophages. Other multiparameter panels for analyzing the myeloid compartment of the mouse lung failed to discriminate between interstitial macrophages, CD11b+ DCs, and immature macrophages/monocytes, and often failed to identify eosinophils (25, 26, 38).
Although flow cytometry provides a wealth of information about cell phenotypes, information about anatomical localization is essentially lost during sample preparation. One valid approach to overcome this problem involves the in vivo labeling of cells in the intravascular compartment with an anti-CD45 antibody (38). Another commonly used approach involves comparing the populations present in bronchoalveolar lavage with those present after enzymatic digestion of the lung. The origin of cells recovered from lavage fluid is attributed to the alveolar space, whereas cells recovered from the digested lung are attributed to the interstitium. However, results using this approach should be cautiously interpreted, because even after multiple lavages, only a fraction of cells can be recovered from the alveolar space (26, 39). Future studies might use immunohistochemical or immunofluorescence techniques to provide better correlations between cell surface markers and anatomic localizations in the intact lung.
During the past several years, the importance of macrophage polarization during inflammation and fibrosis has been increasingly recognized. Markers associated with “classically activated” “M1-like” macrophages are up-regulated only during the acute phase of bleomycin-induced lung injury, whereas markers associated with “alternatively” or “regulatory” “M2-like” macrophages are increased in alveolar macrophages during both the acute and fibrotic phases. Our data suggest that in the model of bleomycin-induced lung injury followed by fibrosis and repair, an “M2-like” macrophage response begins very early, in parallel with an initial “M1-like” response, rather than after its cessation. Unlike Listeria monocytogenes–infected peritoneum (40) or infarcted myocardium (41), the acute-phase “M2-type” response in bleomycin-treated mouse lungs is driven not by recruited monocytes, but by resident tissue macrophages. Of interest, similar findings have been reported in the mouse gut, where tissue-resident macrophages exhibited an anti-inflammatory profile, both in the native state and during acute inflammation (42). However, macrophage polarization is not limited to the M1 and M2 states, but also includes regulatory and resolution-phase macrophages (43, 44). Moreover, overlapping phenotypes and populations may exist simultaneously within the same tissue. Therefore, the proper assignment of macrophage polarization cannot be performed using only this limited number of surface markers. Perhaps analyses of gene expression in individually sorted populations of pulmonary myeloid-cell subsets during different stages of disease will allow for a better understanding of macrophage polarization status, and potentially help identify new markers and their associated functions.
In conclusion, we provide a flow cytometric approach to identify subsets of macrophages and DCs in the normal and inflamed mouse lung. Our panel can be used by investigators as a starting point to examine the role of resident and recruited macrophages and DCs in murine models of lung disease. When experimentally indicated, other markers, such as markers of neutrophils, plasmacytoid DCs, markers of macrophage activation, or viability dyes, can be included with this panel. This panel and its future refinements will provide a useful tool for investigators to examine the complex immune responses of the lung to its changing environment during health and disease.
Footnotes
This work was supported by grants from the Veterans Administration and by National Institutes of Health grants BX000201, ES013995, HL071643, and HL071643 (G.R.S.B.), ES015024 (G.M.M.), and AR050250, AR054796, AI092490, and HL108795 (H.P.). Solovy/Arthritis Research Society Professor provided funds to H.P.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2013-0086MA on May 14, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Gill MA. The role of dendritic cells in asthma. J Allergy Clin Immunol. 2012;129:889–901. doi: 10.1016/j.jaci.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 2.Lambrecht BN, Hammad H. Lung dendritic cells in respiratory viral infection and asthma: from protection to immunopathology. Annu Rev Immunol. 2012;30:243–270. doi: 10.1146/annurev-immunol-020711-075021. [DOI] [PubMed] [Google Scholar]
- 3.Balhara J, Gounni AS. The alveolar macrophages in asthma: a double-edged sword. Mucosal Immunol. 2012;5:605–609. doi: 10.1038/mi.2012.74. [DOI] [PubMed] [Google Scholar]
- 4.Lee JS. Heterogeneity of lung mononuclear phagocytes in chronic obstructive pulmonary disease. J Innate Immun. 2012;4:489–497. doi: 10.1159/000337434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byers DE, Holtzman MJ. Alternatively activated macrophages and airway disease. Chest. 2011;140:768–774. doi: 10.1378/chest.10-2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landsman L, Jung S. Lung macrophages serve as obligatory intermediate between blood monocytes and alveolar macrophages. J Immunol. 2007;179:3488–3494. doi: 10.4049/jimmunol.179.6.3488. [DOI] [PubMed] [Google Scholar]
- 8.Landsman L, Varol C, Jung S. Distinct differentiation potential of blood monocyte subsets in the lung. J Immunol. 2007;178:2000–2007. doi: 10.4049/jimmunol.178.4.2000. [DOI] [PubMed] [Google Scholar]
- 9.Beck-Schimmer B, Schwendener R, Pasch T, Reyes L, Booy C, Schimmer RC. Alveolar macrophages regulate neutrophil recruitment in endotoxin-induced lung injury. Respir Res. 2005;6:61. doi: 10.1186/1465-9921-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poole JA, Gleason AM, Bauer C, West WW, Alexis N, van Rooijen N, Reynolds SJ, Romberger DJ, Kielian TL. CD11c(+)/CD11b(+) cells are critical for organic dust-elicited murine lung inflammation. Am J Respir Cell Mol Biol. 2012;47:652–659. doi: 10.1165/rcmb.2012-0095OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhaliwal K, Scholefield E, Ferenbach D, Gibbons M, Duffin R, Dorward DA, Morris AC, Humphries D, MacKinnon A, Wilkinson TS, et al. Monocytes control second-phase neutrophil emigration in established lipopolysaccharide-induced murine lung injury. Am J Respir Crit Care Med. 2012;186:514–524. doi: 10.1164/rccm.201112-2132OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maus UA, Waelsch K, Kuziel WA, Delbeck T, Mack M, Blackwell TS, Christman JW, Schlöndorff D, Seeger W, Lohmeyer J. Monocytes are potent facilitators of alveolar neutrophil emigration during lung inflammation: role of the CCL2–CCR2 axis. J Immunol. 2003;170:3273–3278. doi: 10.4049/jimmunol.170.6.3273. [DOI] [PubMed] [Google Scholar]
- 13.Nair MG, Du Y, Perrigoue JG, Zaph C, Taylor JJ, Goldschmidt M, Swain GP, Yancopoulos GD, Valenzuela DM, Murphy A, et al. Alternatively activated macrophage-derived RELM-alpha is a negative regulator of Type 2 inflammation in the lung. J Exp Med. 2009;206:937–952. doi: 10.1084/jem.20082048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pechkovsky DV, Prasse A, Kollert F, Engel KM, Dentler J, Luttmann W, Friedrich K, Müller-Quernheim J, Zissel G. Alternatively activated alveolar macrophages in pulmonary fibrosis–mediator production and intracellular signal transduction. Clin Immunol. 2010;137:89–101. doi: 10.1016/j.clim.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 15.Gibbons MA, MacKinnon AC, Ramachandran P, Dhaliwal K, Duffin R, Phythian-Adams AT, van Rooijen N, Haslett C, Howie SE, Simpson AJ, et al. Ly6Chi monocytes direct alternatively activated profibrotic macrophage regulation of lung fibrosis. Am J Respir Crit Care Med. 2011;184:569–581. doi: 10.1164/rccm.201010-1719OC. [DOI] [PubMed] [Google Scholar]
- 16.Mouratis MA, Aidinis V. Modeling pulmonary fibrosis with bleomycin. Curr Opin Pulm Med. 2011;17:355–361. doi: 10.1097/MCP.0b013e328349ac2b. [DOI] [PubMed] [Google Scholar]
- 17.Osterholzer JJ, Olszewski MA, Murdock BJ, Chen GH, Erb-Downward JR, Subbotina N, Browning K, Lin Y, Morey RE, Dayrit JK, et al. Implicating exudate macrophages and Ly-6C(high) monocytes in CCR2-dependent lung fibrosis following gene-targeted alveolar injury. J Immunol. 2013;190:3447–3457. doi: 10.4049/jimmunol.1200604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, et al. Immunological Genome Consortium. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13:1118–1128. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lech M, Anders HJ. Macrophages and fibrosis: how resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochim Biophys Acta. 2012;1832:989–997. doi: 10.1016/j.bbadis.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Johnston LK, Rims CR, Gill SE, McGuire JK, Manicone AM. Pulmonary macrophage subpopulations in the induction and resolution of acute lung injury. Am J Respir Cell Mol Biol. 2012;47:417–426. doi: 10.1165/rcmb.2012-0090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garn H. Specific aspects of flow cytometric analysis of cells from the lung. Exp Toxicol Pathol. 2006;57:21–24. doi: 10.1016/j.etp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell AJ, Pradel LC, Chasson L, Van Rooijen N, Grau GE, Hunt NH, Chimini G. Technical advance: autofluorescence as a tool for myeloid cell analysis. J Leukoc Biol. 2010;88:597–603. doi: 10.1189/jlb.0310184. [DOI] [PubMed] [Google Scholar]
- 23.Duan M, Li WC, Vlahos R, Maxwell MJ, Anderson GP, Hibbs ML. Distinct macrophage subpopulations characterize acute infection and chronic inflammatory lung disease. J Immunol. 2012;189:946–955. doi: 10.4049/jimmunol.1200660. [DOI] [PubMed] [Google Scholar]
- 24.Miller JC, Brown BD, Shay T, Gautier EL, Jojic V, Cohain A, Pandey G, Leboeuf M, Elpek KG, Helft J, et al. Immunological Genome Consortium. deciphering the transcriptional network of the dendritic cell lineage. Nat Immunol. 2012;13:888–899. doi: 10.1038/ni.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hackstein H, Wachtendorf A, Kranz S, Lohmeyer J, Bein G, Baal N. Heterogeneity of respiratory dendritic cell subsets and lymphocyte populations in inbred mouse strains. Respir Res. 2012;13:94. doi: 10.1186/1465-9921-13-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zaynagetdinov R, Sherrill TP, Kendall PL, Segal BH, Weller KP, Tighe RM, Blackwell TS. Identification of myeloid cell subsets in murine lungs using flow cytometry. Am J Respir Cell Mol Biol. doi: 10.1165/rcmb.2012-0366MA. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gwyer Findlay E, Hussell T. Macrophage-mediated inflammation and disease: a focus on the lung. Mediators Inflamm. 2012;2012:140937. doi: 10.1155/2012/140937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.GeurtsvanKessel CH, Lambrecht BN. Division of labor between dendritic cell subsets of the lung. Mucosal Immunol. 2008;1:442–450. doi: 10.1038/mi.2008.39. [DOI] [PubMed] [Google Scholar]
- 29.Sung SS, Fu SM, Rose CE, Jr, Gaskin F, Ju ST, Beaty SR. A major lung CD103 (alphaE)–beta7 integrin–positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J Immunol. 2006;176:2161–2172. doi: 10.4049/jimmunol.176.4.2161. [DOI] [PubMed] [Google Scholar]
- 30.Satpathy AT, Kc W, Albring JC, Edelson BT, Kretzer NM, Bhattacharya D, Murphy TL, Murphy KM. ZBTB46 expression distinguishes classical dendritic cells and their committed progenitors from other immune lineages. J Exp Med. 2012;209:1135–1152. doi: 10.1084/jem.20120030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langlet C, Tamoutounour S, Henri S, Luche H, Ardouin L, Grégoire C, Malissen B, Guilliams M. CD64 expression distinguishes monocyte-derived and conventional dendritic cells and reveals their distinct role during intramuscular immunization. J Immunol. 2012;188:1751–1760. doi: 10.4049/jimmunol.1102744. [DOI] [PubMed] [Google Scholar]
- 32.Tamoutounour S, Henri S, Lelouard H, de Bovis B, de Haar C, van der Woude CJ, Woltman AM, Reyal Y, Bonnet D, Sichien D, et al. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. Eur J Immunol. 2012;42:3150–3166. doi: 10.1002/eji.201242847. [DOI] [PubMed] [Google Scholar]
- 33.Janssen WJ, Barthel L, Muldrow A, Oberley-Deegan RE, Kearns MT, Jakubzick C, Henson PM. Fas determines differential fates of resident and recruited macrophages during resolution of acute lung injury. Am J Respir Crit Care Med. 2011;184:547–560. doi: 10.1164/rccm.201011-1891OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamilton RF, Jr, Li L, Felder TB, Holian A. Bleomycin induces apoptosis in human alveolar macrophages. Am J Physiol. 1995;269:L318–L325. doi: 10.1152/ajplung.1995.269.3.L318. [DOI] [PubMed] [Google Scholar]
- 35.Ortiz LA, Moroz K, Liu JY, Hoyle GW, Hammond T, Hamilton RF, Holian A, Banks W, Brody AR, Friedman M. Alveolar macrophage apoptosis and TNF-alpha, but not p53, expression correlate with murine response to bleomycin. Am J Physiol. 1998;275:L1208–L1218. doi: 10.1152/ajplung.1998.275.6.L1208. [DOI] [PubMed] [Google Scholar]
- 36.Zhao HW, Hu SY, Barger MW, Ma JK, Castranova V, Ma JY. Time-dependent apoptosis of alveolar macrophages from rats exposed to bleomycin: involvement of TNF receptor 2. J Toxicol Environ Health A. 2004;67:1391–1406. doi: 10.1080/15287390490471569. [DOI] [PubMed] [Google Scholar]
- 37.Zhang JQ, Biedermann B, Nitschke L, Crocker PR. The murine inhibitory receptor mSiglec-E is expressed broadly on cells of the innate immune system whereas mSiglec-F is restricted to eosinophils. Eur J Immunol. 2004;34:1175–1184. doi: 10.1002/eji.200324723. [DOI] [PubMed] [Google Scholar]
- 38.Barletta KE, Cagnina RE, Wallace KL, Ramos SI, Mehrad B, Linden J. Leukocyte compartments in the mouse lung: distinguishing between marginated, interstitial, and alveolar cells in response to injury. J Immunol Methods. 2012;375:100–110. doi: 10.1016/j.jim.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soroosh P, Doherty TA, Duan W, Mehta AK, Choi H, Adams YF, Mikulski Z, Khorram N, Rosenthal P, Broide DH, et al. Lung-resident tissue macrophages generate Foxp3+ regulatory T cells and promote airway tolerance. J Exp Med. 2013;210:775–788. doi: 10.1084/jem.20121849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 41.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zigmond E, Varol C, Farache J, Elmaliah E, Satpathy AT, Friedlander G, Mack M, Shpigel N, Boneca IG, Murphy KM, et al. Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity. 2012;37:1076–1090. doi: 10.1016/j.immuni.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 43.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Novak ML, Koh TJ. Macrophage phenotypes during tissue repair. J Leukoc Biol. doi: 10.1189/jlb.1012512. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]