Abstract
Cathepsin L (Ctsl) is a proposed therapeutic target to control inflammatory responses in a number of disease states. However, Ctsl is thought to support host defense via its involvement in antigen presentation pathways. Hypothesizing that Ctsl helps combat bacterial infection, we investigated its role in Mycoplasma pulmonis–infected mice as a model of acute and chronic infectious airway inflammation. Responses to the airway inoculation of mycoplasma were compared in Ctsl−/− and Ctsl+/+ mice. After infection, Ctsl−/− mice demonstrated more body weight loss, greater mortality (22% versus 0%, respectively), and heavier lungs than Ctsl+/+ mice, but had smaller bronchial lymph nodes. The burden of live mycoplasma in lungs was 247-fold greater in Ctsl−/− mice than in Ctsl+/+ mice after infection for 3 days. Ctsl−/− mice exhibited more severe pneumonia and neutrophil-rich, airway-occlusive exudates, which developed more rapidly than in Ctsl+/+ mice. Compared with the conspicuous remodeling of lymphatics after infection in Ctsl+/+ mice, little lymphangiogenesis occurred in Ctsl−/− mice, but blood vessel remodeling and tissue inflammation were similarly severe. Titers of mycoplasma-reactive IgM, IgA, and IgG in blood in response to live and heat-killed organisms were similar to those in Ctsl+/+ mice. However, enzyme-linked immunosorbent spot assays revealed profound reductions in the cellular IFN-γ response to mycoplasma antigen. These findings suggest that Ctsl helps contain mycoplasma infection by supporting lymphangiogenesis and cellular immune responses to infection, and our findings predict that the therapeutic inhibition of Ctsl could increase the severity of mycoplasmal infections.
Keywords: cathepsin L, mycoplasma, pneumonia, bronchitis, lymphangiogenesis
Clinical Relevance
Our findings suggest that cathepsin L, a protease expressed in immune cells and that is a target for the therapeutic inhibition of anti-inflammatory and immunosuppressive indications, helps contain lung mycoplasma infection by supporting lymphangiogenesis and cellular immune responses to infection. These findings in mice predict that the therapeutic inhibition of cathepsin L could increase the severity of mycoplasmal infections.
The maintenance of health relies on protein maturation, activation, and breakdown, involving the controlled and coordinated actions of proteases. A subset of these enzymes (the cysteinyl cathepsins) was characterized originally as lysosomal in location and function (1). Later work uncovered additional roles in extracellular spaces as well as in the cytosol and nuclei, revealing that cathepsins are not limited to the unspecific destruction of proteins delivered to lysosomes (2). Rather, some cathepsins process specific substrates important to activities such as antigen presentation, collagen turnover in bone and cartilage, neuropeptide and hormone processing, and leukocyte penetration of the extracellular matrix and cellular monolayers (1, 3–5). The loss of activity among specific cathepsins in mice or humans produces a variety of phenotypes (1, 2, 6). The widely expressed cysteine protease cathepsin L (Ctsl) is implicated in several types of pathology, and is a proposed target for therapeutic inhibition to control inflammatory and immune responses (5, 7, 8). For example, by cleaving fusion proteins, Ctsl helps Ebola, Hendra, and severe acute respiratory syndrome viruses to enter cells, thereby advancing these infections (9–12). However, little is known of Ctsl’s importance in defense against bacterial infections.
Mycoplasmas, which are the smallest free-living, self-replicating bacteria, are distinguished by their minute size and lack of a cell wall (12–14). They possess unusually small genomes, and therefore are models of a “minimal cell” for synthetic biologists (15). Mycoplasma pneumoniae is a leading cause of childhood and adult tracheobronchitis and pneumonia in humans (13, 16). Infection with mycoplasma species can worsen or precipitate noninfectious respiratory diseases, such as asthma and chronic obstructive pulmonary disease in humans (17, 18), as well as in rodent models of these conditions (19–24).
Mycoplasmas can be difficult for host defense systems to defeat and eliminate. Mycoplasma pulmonis causes natural murine respiratory disease with manifestations similar to those in humans infected with M. pneumoniae. In rodents, M. pulmonis can cause life-long infection (19). Previous studies revealed that M. pulmonis induces acute and chronic lung inflammation and airway remodeling with prominent changes in airway blood vessels and lymphatics, which is driven by the immune response, including the production of mycoplasma-specific Ig (24–28).
Because Ctsl is implicated in major histocompatibility complex (MHC) Class II–dependent immune responses and in the degradation of extracellular matrix, we hypothesized that Ctsl is required for protection from lung bacterial pathogens and for the expression of remodeling responses. To test these predictions, we investigated the effects of Ctsl deficiency on the severity of airway mycoplasmal infection and the associated remodeling of blood vessels and lymphatics in the airways of mice. The results revealed severe impairment in defense against M. pulmonis in Ctsl-deficient mice, with defects including blunted cellular immune responses and impaired lymphangiogenesis in infected airways.
Materials and Methods
Infection of Mice with Mycoplasma pulmonis
This study used Ctsl−/− mice described originally by Nakagawa and colleagues (29), and received at the University of California at San Francisco in a C57BL/6/129S background from Sun and colleagues (30), and then backcrossed (≥ 10 generations) into C57BL/6. Ctsl+/+ C57BL/6 mice were used as controls. Mice were inoculated intranasally at 8–12 weeks of age with M. pulmonis strain CT7 (106 colony-forming units [CFUs]) (24). Uninfected control animals received equivalent volumes of sterile broth. The Institutional Review Board of the University of California at San Francisco approved the experiments.
General Observations and Scoring of Pneumonia
After infection, mice were killed at intervals up to 14 days. Endpoints included survival, body weight, and bronchial lymph node and thymus weights. In addition, mice were assessed for severity of pneumonia, as described for grading mycoplasmal infections in hamsters (20), and as adapted to mouse lungs (24, 27). The 26-point score was based on the extent of peribronchiolar and peribronchial infiltrates, luminal exudates, perivascular inflammation, and pneumonia.
Bacterial Burden
The burden of live mycoplasma organisms was measured at intervals after infection, as described elsewhere (24). Briefly, lung homogenates were diluted serially onto plates, and colonies were counted after 10 days.
Lung Fluid Analysis
Cells and proteins in bronchoalveolar lavage (BAL) fluid and lung homogenates were obtained and analyzed as detailed in the online supplement.
Mycoplasma Proliferation
Recombinant Ctsl (R&D Systems, Minneapolis, MN) was activated by incubation for 90 minutes in 25 mM Na acetate (pH 5.0)/5 mM DTT at 37°C. Mycoplasma pulmonis organisms were cocultured with activated Ctsl (1 or 10 μg/ml) for 2 hours, and then frozen at −80°C. For quantification, organisms were diluted in culture medium and plated on agar. Colonies were counted after 13 days at 37°C.
Immunization with Heat-Killed Mycoplasma
Mycoplasma pulmonis organisms (2 × 107), killed by heating to 65°C for 30 minutes, were injected intraperitoneally into Ctsl−/− and Ctsl+/+ mice, as described elsewhere (24). Serum samples were obtained up to 14 days after injection.
Mycoplasma-Specific Igs
Serum Igs reactive to mycoplasma antigens were detected and quantified by immunoassay, as described in the online supplement.
Enzyme-Linked Immunosorbent Spot Assays
To detect T and B lymphocytes responding to mycoplasma antigens, splenocytes from mice infected for 2 weeks were subjected to immunosorbent assays (31), as detailed in the online supplement.
Vessel Morphometry
Tracheal whole mounts were incubated with the antilymphatic vessel endothelial hyaluronan receptor and anti-mouse CD31 to label lymphatic and blood vessels, respectively, for analysis by confocal microscopy (24). Area densities of lymphatic and blood vessels were measured by stereological point counting, as detailed in the online supplement.
Detection of Lung Fibrosis
To determine whether Ctsl−/− mice are prone to develop lung and airway fibrosis after infection, lung and airway tissue sections were stained with Masson trichrome. Additional tissue sections were stained for 1 hour with Sirius red, and staining was quantified in infected lungs, as described in the online supplement.
Statistical Analysis
Survival data were compared according to log-rank tests. Other data were compared using t tests, one-way ANOVA (with the Bonferroni test for pairs of groups), or Mann-Whitney U tests, with P < 0.05 considered significant.
Results
Weight Loss, Organ Weight, and Death after Infection
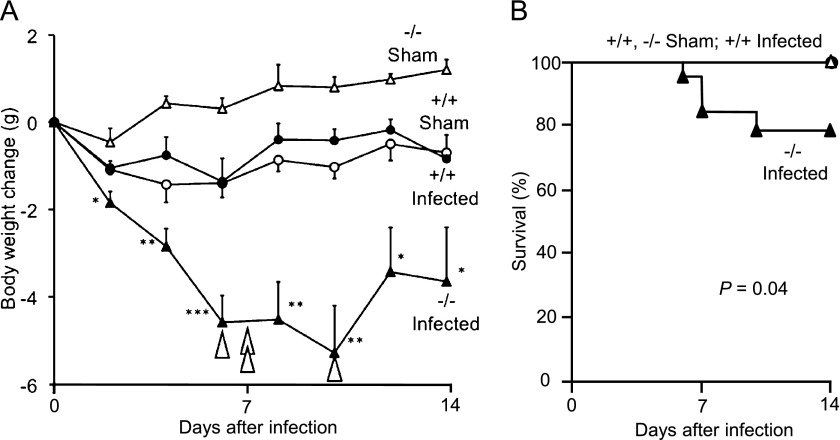
To determine the overall severity of mycoplasma-induced illness, body weight and mortality were compared in age-matched Ctsl−/− and Ctsl+/+ mice. Both types of mice lost weight after infection (Figure 1), but Ctsl−/− mice lost more weight and took longer to recover. By Day 14, the mean change of weight among infected Ctsl+/+ mice was comparable to that of sham-treated Ctsl+/+ mice, whereas the recovery of weight in surviving infected Ctsl−/− mice was minimal. Ctsl−/− mice exhibited heavier lungs but a smaller thymus at all time points after infection, and exhibited smaller bronchial lymph nodes on Days 7 and 14, compared with infected Ctsl+/+ mice (Table 1). Mortality was higher among infected Ctsl−/− mice, four of 18 (22%) among which died during the 14-day study, compared with none of the infected Ctsl+/+ mice.
Figure 1.
Body weight change and death after infection. (A) Mycoplasma-infected Ctsl−/− (−/−) mice lost progressively more weight than infected Ctsl+/+ (+/+) and sham-infected mice during the first 10 days of infection. Four (of 18) infected Ctsl−/− mice died at times indicated by arrowheads, versus zero (of 15) Ctsl+/+ mice. No deaths occurred in sham-infected mice. Values represent means ± SEM (n = 5–18). *P < 0.05, **P < 0.01, and ***P < 0.001, versus infected Ctsl+/+ mice. (B) Kaplan-Meier–type plot of 14-day survival, which was significantly lower (P = 0.04 according to log-rank test for trend) in infected Ctsl−/− mice than in the three other groups of mice. Ctsl, cathepsin L.
TABLE 1.
TISSUE WEIGHTS (IN MILLIGRAMS) AFTER MYCOPLASMA INFECTION IN CTSL-/- AND CTSL+/+ MICE
| Days after Exposure to Mycoplasma or Sterile Broth | Exposure Group | Lung | Bronchial Lymph Node | Thymus |
|---|---|---|---|---|
| 3 |
Ctsl+/+ + medium |
169 ± 4 |
1.4 ± 0.8 |
28 ± 9 |
|
Ctsl−/− + medium |
162 ± 11 |
3.2 ± 0.6 |
19 ± 4 |
|
|
Ctsl+/+ + M.p. |
151 ± 3 |
3.3 ± 1.3 |
29 ± 5 |
|
|
Ctsl−/− + M.p. |
211 ± 17* |
4.9 ± 0.6 |
14 ± 2* |
|
| 7 |
Ctsl+/+ + medium |
139 ± 7 |
2.4 ± 0.7 |
30 ± 9 |
|
Ctsl−/− + medium |
135 ± 1 |
1.7 ± 0.3 |
26 ± 9 |
|
|
Ctsl+/+ + M.p. |
216 ± 21 |
12 ± 2 |
26 ± 6 |
|
|
Ctsl−/− + M.p. |
504 ± 44‡ |
6.8 ± 0.8* |
6 ± 1† |
|
| 14 |
Ctsl+/+ + medium |
127 ± 5 |
5.5 ± 3.5 |
49 ± 5 |
|
Ctsl−/− + medium |
150 ± 2 |
2.4 ± 0.8 |
30 ± 7 |
|
|
Ctsl+/+ + M.p. |
185 ± 34 |
16 ± 4 |
53 ± 5 |
|
| Ctsl−/− + M.p. | 263 ± 56 | 7.3 ± 1.3 | 11 ± 4‡ |
Definition of abbreviations: Ctsl, cathepsin L; M.p., M. pulmonis.
Data represent means ± SEMs (n = 4–6).
P < 0.05, compared with infected Ctsl+/+ mice, according to t tests.
P < 0.01, compared with infected Ctsl+/+ mice, according to t tests.
P < 0.001, compared with infected Ctsl+/+ mice, according to t tests.
Bronchitis and Pneumonia
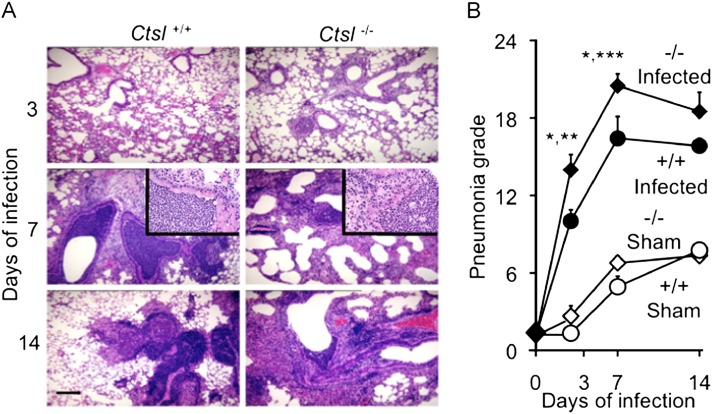
To probe the severity of airway and lung inflammation after infection, lung histopathology and pneumonia scores were compared in Ctsl−/− and Ctsl+/+ mice. Ctsl−/− mice developed neutrophilic pneumonia by Day 3 of infection. Exudates were observed in the airways of Ctsl−/− mice but not Ctsl+/+ mice on Day 3 (Figure 2A). By Day 7, both types of infected mice developed severe lung inflammation. By Day 14, pneumonia and leukocytic infiltrates remained severe, but were stable or decreasing in Ctsl+/+ mice and in survivors among infected Ctsl−/− mice. The results of quantitative scoring, as shown in Figure 2B, reveal significant differences between infected Ctsl−/− and Ctsl+/+ mice in bronchitis/pneumonia severity, 3 and 7 days after infection. Although sham infection after an inoculation of sterile broth for growing M. pulmonis was followed by mild inflammation in Ctsl−/− and Ctsl+/+ mice, the degree was much lower than that produced by mycoplasma, and was similar in the two types of sham-infected animals. These results show that bronchitis and pneumonia appeared earlier and were more severe during the first week of infection in mycoplasma-infected Ctsl−/− mice than in Ctsl+/+ mice.
Figure 2.
Hematoxylin and eosin–stained sections of lung tissue after Mycoplasma pulmonis infection. The photomicrographs in A show representative sections from Ctsl+/+ and Ctsl−/− mouse lungs removed after 3, 7, or 14 days of infection. Scale bar = 100 μm. The insets in the middle row comprise further enlarged images of airway neutrophilic infiltrates. The graph in B shows pneumonia grades in mycoplasma-infected and sham-infected mice. The severity of infiltrates was compared in lungs of Ctsl+/+ (+/+) and Ctsl−/− (−/−) mice after 3, 7, or 14 days of infection. The maximal score on this scale is 26. Values represent means ± SEM (n = 4–5). *P < 0.05, versus pneumonia grade in infected Ctsl+/+ mice. **P < 0.01 and ***P < 0.001, versus grades in sham-infected mice.
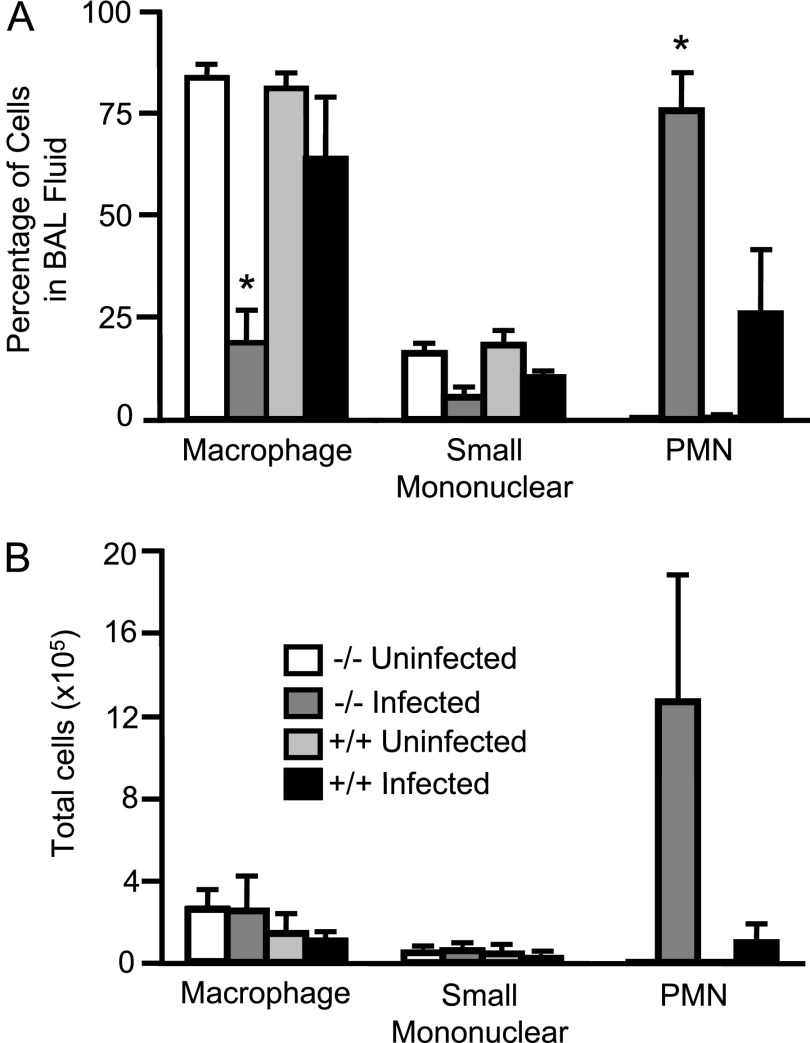
As another means of evaluating lung inflammation before death occurred in infected Ctsl−/− mice, but at a time when leukocyte infiltrates were evident on the histological examination of lungs, BAL fluid cells and protein concentrations were assessed in Ctsl−/− and Ctsl+/+ pathogen-free control mice and in mice 3 days after infection with 106 CFUs of M. pulmonis. Among uninfected mice, total cells in BAL fluid and cellular differential profiles were similar in Ctsl−/− and Ctsl+/+ mice (data not shown), and neutrophils were rare. After infection, BAL fluid from Ctsl−/− mice contained more leukocytes, with a significantly higher percentage of neutrophils and a lower percentage of macrophages than in Ctsl+/+ mice (Figure 3), consistent with the observed histopathology. The total protein in BAL fluid was 2.9-fold higher in infected Ctsl−/− mice than in Ctsl+/+ mice (0.43 ± 0.03 mg/ml in Ctsl−/− mice, versus 0.15 ± 0.03 mg/ml in Ctsl+/+ mice; P < 0.01). Total BAL fluid protein did not differ significantly in pathogen-free mice (0.19 ± 0.02 mg/ml in Ctsl−/− mice, versus 0.16 ± 0.00 mg/ml in Ctsl+/+ mice; P = 0.41).
Figure 3.
Effect of mycoplasmal infection on lung and airway leukocytes. Bronchoalveolar lavage (BAL) fluid was collected from Ctsl+/+ (+/+) and Ctsl−/− (−/−) mice that were either uninfected or had been infected with M. pulmonis for 3 days. (A and B) Differential and total cell counts, respectively. Values represent means ± SEMs (n = 3). *P < 0.05, versus infected Ctsl+/+ mice, according to t test. PMN, polymorphonuclear leukocytes.
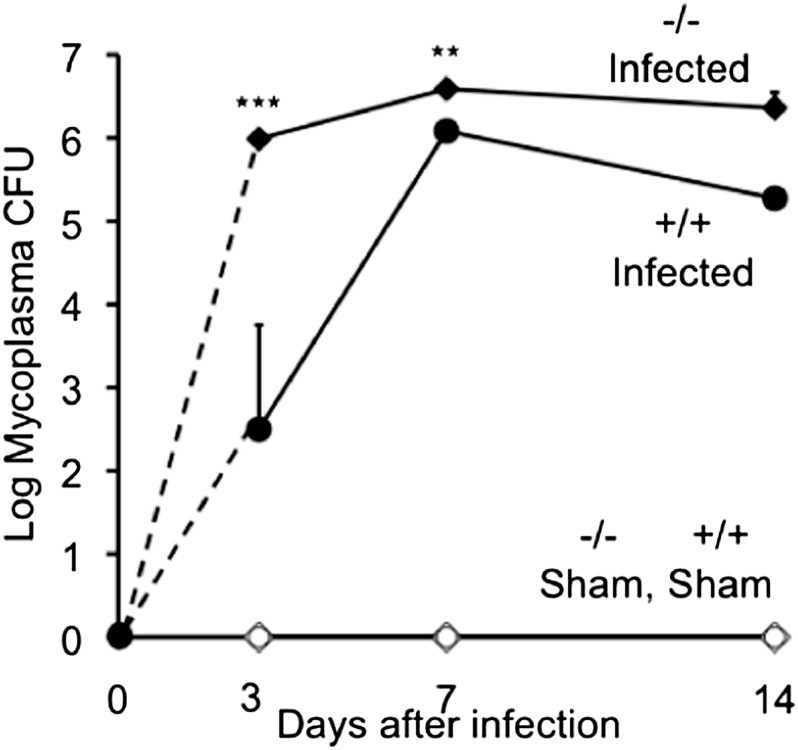
Mycoplasma Burden
To test whether a lack of Ctsl impairs anti-mycoplasma defenses, titers of mycoplasma were measured in lung extracts. This revealed 247-fold more live mycoplasma in Ctsl−/− lungs than in Ctsl+/+ lungs, 3 days after infection (Figure 4). The mycoplasma burden in Ctsl−/− mice was also higher at 7 and 14 days after infection, but the 10-fold difference from Ctsl−/− lungs on Day 14 was not statistically significant. This difference was not likely attributable to a directly toxic effect of Ctsl on mycoplasma, because Ctsl incubated with M. pulmonis in vitro altered neither bacterial viability nor growth. Starting with 10,000 CFUs, the titer increased to 16,700 ± 4,700 CFUs after incubation with buffer alone, 16,000 ± 5,300 CFUs after incubation with 1 μg/ml Ctsl, and 15,300 ± 4,400 CFUs after incubation with 10 μg/ml Ctsl (mean ± SEM, n = 3, P > 0.05).
Figure 4.
Lung burden of live mycoplasma after infection. Mycoplasma colony-forming units (CFUs) were measured in lung homogenates from Ctsl+/+ (+/+) and Ctsl−/− (−/−) mice before infection and 3, 7, and 14 days after intranasal inoculation with live M. pulmonis or medium alone (sham). Bacterial burden is expressed as total CFUs in lung homogenates. Data represent means ± SEMs (n = 3). **P < 0.01 and ***P < 0.001, versus CFUs in infected Ctsl+/+ mice, according to t tests. No CFUs were detected in lungs of uninfected/sham-infected mice.
Production of Mycoplasma-Directed Ig
Anti-mycoplasma IgA appeared earlier in the blood of Ctsl−/− than of Ctsl+/+ mice after infection with live organisms, and the anti-mycoplasma IgG2a titer was slightly lower in Ctsl−/− mice on Day 14 only, with no differences in mycoplasma-directed IgM and IgG1 at any time point (Table 2). IgA, IgG, and IgM responses to heat-killed mycoplasma were no different in Ctsl−/− and Ctsl+/+ mice (Table 3). Similarly, no significant difference was found between Ctsl−/− and Ctsl+/+ mice in an enzyme-linked immunosorbent assay comparing the quantity of M. pulmonis–specific Igs produced by splenocytes 14 days after infection (for IgG1, 0.15 ± 0.04 versus 0.20 ± 0.06 absorbance at 405 nm per 2 × 105 splenocytes, P > 0.05, n = 3–5; for IgG2, no significant difference was evident, but the signal was low). These findings suggest that humoral responses to mycoplasma antigen in Ctsl-deficient mice were largely intact, with the earlier appearance of IgA likely attributable to higher titers of live organisms and associated antigen in the first few days after infection.
TABLE 2.
SERUM MYCOPLASMA-SPECIFIC IMMUNOGLOBULIN TITERS AFTER INFECTION
| Days after Infection | Exposure group | IgM | IgA | IgG1 | IgG2a |
|---|---|---|---|---|---|
| 3 |
Ctsl+/+ + M.p. |
1.05 ± 0.36 |
ND |
ND |
ND |
|
Ctsl−/− + M.p. |
0.65 ± 0.38 |
ND |
ND |
ND |
|
| 7 |
Ctsl+/+ + M.p. |
1.90 ± 0.13 |
ND |
0.80 ± 0.48 |
1.60 ± 0.18 |
|
Ctsl−/− + M.p. |
1.68 ± 0.08 |
0.33 ± 0.33 |
1.90 ± 0.25 |
1.58 ± 0.60 |
|
| 14 |
Ctsl+/+ + M.p. |
1.98 ± 0.08 |
1.73 ± 0.70 |
4.31 ± 0.00 |
4.01 ± 0.17 |
| Ctsl−/− + M.p. | 2.28 ± 0.14 | 1.13 ± 0.40 | 4.01 ± 0.18 | 3.11 ± 0.00* |
Definition of abbreviations: Ctsl, cathepsin L; M.p., M. pulmonis; ND, none detected (IgE is not shown, and was undetectable in any sample).
Values represent means ± SEMs (n = 4–6);
P < 0.01, compared with infected Ctsl+/+ mice, according to t test. No M.p.-specific antibodies were detected in uninfected Ctsl+/+ or Ctsl−/− mice.
TABLE 3.
SERUM IMMUNOGLOBULIN TITERS AFTER IMMUNIZATION WITH HEAT-KILLED MYCOPLASMA
| Days after Infection | Exposure Group | IgM | IgG1 | IgG2a |
|---|---|---|---|---|
| 7 |
Ctsl+/+ + M.p. |
2.00 ± 0.26 |
3.31 ± 0.53 |
2.3 ± 0.53 |
|
Ctsl−/− + M.p. |
0.87 ± 0.43 |
2.3 ± 0.53 |
2.3 ± 0.72 |
|
| 14 |
Ctsl+/+ + M.p. |
1.20 ± 0.42 |
2.48 ± 0.84 |
2.05 ± 0.38 |
| Ctsl−/− + M.p. | 0.65 ± 0.38 | 2.66 ± 0.57 | 2.66 ± 0.96 |
Definition of abbreviations: Ctsl, cathepsin L; M.p., M. pulmonis.
IgA and IgE are not shown, and were undetectable in any sample. Values represent means ± SEMs (n = 3–5). No significant differences were evident between titers in infected Ctsl+/+ and Ctsl−/− serum (according to t tests) at any time point. No M.p.-specific antibodies were detected in PBS-injected control Ctsl+/+ or Ctsl−/− mice.
Mycoplasma-Specific Cellular Immune Responses
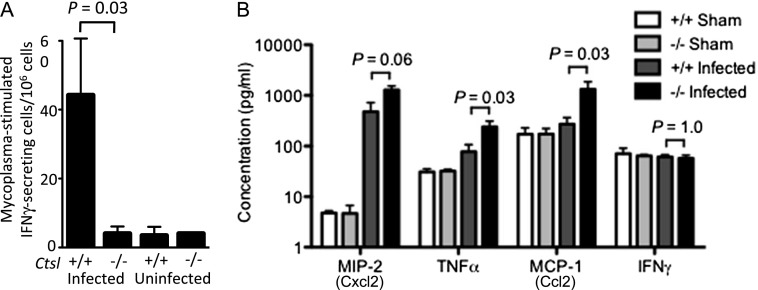
To investigate the role of cellular immune responses in the observed failure to contain M. pulmonis infection, we used an enzyme-linked immunosorbent spot assay to determine the proportion of splenocytes secreting IFN-γ during incubation with mycoplasma antigen. The proportion of splenocytes stimulated to release IFN-γ in response to mycoplasma antigen was similar in uninfected Ctsl−/− and Ctsl+/+ mice (Figure 5A). However, the M. pulmonis–specific cellular immune response in infected Ctsl+/+ mice was 9-fold greater than that in uninfected Ctsl+/+ mice (P = 0.03). This response to mycoplasmal antigen was not found in infected Ctsl−/− mice (Figure 5A), suggesting a Ctsl-related defect in the cellular immune response to mycoplasma.
Figure 5.
Immune responses to mycoplasma. (A) Splenocyte responses to mycoplasma antigen were measured by IFN-γ enzyme-linked immunosorbent spot assay. Cells were harvested 2 weeks after infection. Results are normalized to 106 splenocytes. Data represent means ± SEM (n = 3–5 for infected groups). P < 0.05 for infected Ctsl+/+ mice, compared with each of the other groups of mice, according to t tests. (B) Concentrations of chemokines and IFN-γ determined by immunoassay in lung extracts, 3 days after mycoplasma infection (n = 3–5). Differences between groups were assessed according to Mann-Whitney U tests. MCP (Ccl2), monocyte chemotactic protein–1; MIP (Cxcl2), macrophage inflammatory protein–2.
Local Cytokine and Chemokine Production
To assess whether Ctsl−/− mice possess defects in innate immune signaling as potential explanation for worse outcomes after M. pulmonis infection, we assayed TNF-α, monocyte chemotactic protein (MCP)–1 (Ccl2), macrophage inflammatory protein (MIP)–2 (Cxcl2), and IFN-γ concentrations, 3 days after infection. We found significantly increased concentrations of TNF-α, MCP-1/Ccl2, and MIP-2/Cxcl2 in Ctsl−/− lungs after infection (Figure 5B). The higher concentrations of MCP-1/Ccl2 and TNF-α indicate a potent innate immune response, and suggest a potential basis for the activation and recruitment of monocytic cells and neutrophils into the airways of infected Ctsl−/− mice.
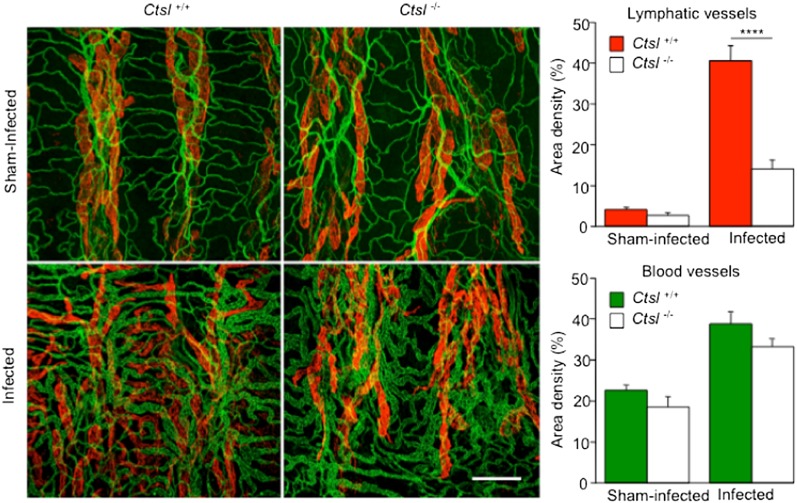
Effects of Mycoplasma on Airway Blood and Lymph Vessel Remodeling
The amount of growth and remodeling of tracheal blood vessels and lymphatics was compared in Ctsl+/+ and Ctsl−/− mice, to assess the importance of Ctsl in changes to the vasculature after infection. At 14 days, blood-vessel caliber was significantly greater in both groups of infected mice than in sham-infected control mice, reflecting vascular enlargement by the conversion of capillaries to venules after infection (Figure 6). The amount of blood-vessel remodeling was similar in Ctsl+/+ and Ctsl−/− mice (P = 0.15). The change in blood vessels is consistent with previous observations of C57BL/6 mice after M. pulmonis infection (23–25). By comparison, changes in tracheal lymphatics after infection were strikingly different in Ctsl+/+ and Ctsl−/− mice (Figure 6). Lymphatics in the mucosa over the cartilaginous rings of Ctsl+/+ mice were 10.2-fold more abundant at 14 days of infection than in pathogen-free control mice, but the difference was only 5-fold in Ctsl−/− mice. A comparison of lymphatic growth in Ctsl+/+ and Ctsl−/− mice at 14 days of infection revealed a 2.9-fold greater lymphangiogenesis in Ctsl+/+ mice (Figure 6). Values for the two groups of corresponding sham-infected mice were not significantly different. These data reflect 69.1% less lymphangiogenesis in Ctsl−/− mice after infection, without a reduction in amount of blood-vessel remodeling.
Figure 6.
Impaired lymphangiogenesis in M. pulmonis–infected Ctsl−/− mice. The confocal photomicrographs are representative of tracheal whole mounts from Ctsl+/+ and Ctsl−/− mice at 14 days of infection. Antilymphatic vessel endothelial hyaluronan receptor (red) and anti-CD31 (green) mark endothelial cells of lymphatics and blood vessels, respectively. Dark vertical bands correspond to cartilage rings. Images show blood-vessel remodeling after infection in the Ctsl+/+ mouse and in the Ctsl−/− mouse, but lymphangiogenesis over a cartilage ring is evident only in the Ctsl+/+ mouse. Graphs show area density measurements of blood vessels and lymphatics. Values represent means ± SEM (n = 5–10). ****P < 0.0001, infected Ctsl+/+ versus infected Ctsl−/−, according to t tests. All differences between area density values of sham-infected and infected mice were significant. Scale bar = 200 μm.
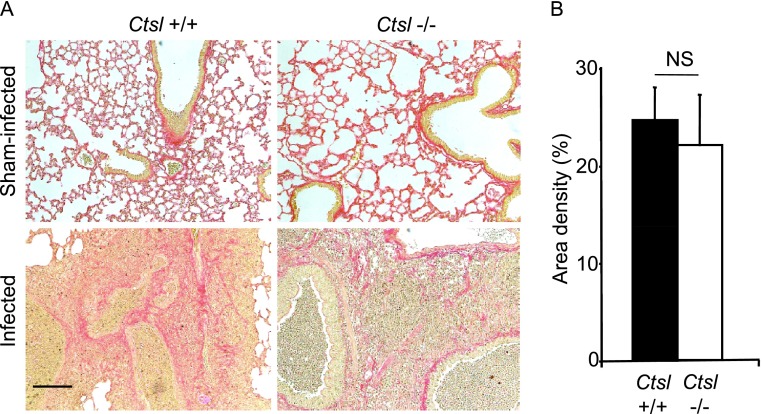
Effects of Mycoplasma on Airway and Lung Fibrosis
No difference in the deposition of collagen in airway walls or alveolar interstitium was visible at 2 weeks between sham-infected Ctsl+/+ and Ctsl−/− mice, or between infected Ctsl+/+ and Ctsl−/− mice in lung sections stained with Masson trichrome (not shown) or Sirius red (Figure 7A). The quantitation of Sirius red staining by stereological area density measurement did not detect significant differences in staining between the two groups of infected mice (Figure 7B). Thus, Ctsl deficiency did not appear to increase the severity of lung fibrosis after mycoplasmal infection for 2 weeks.
Figure 7.
Sirius red staining of lung sections. Lungs were removed 14 days after sham or mycoplasma infection. (A) Regions of matrix and collagen deposition are red. Scale bar = 100 μm. (B) Graphic comparison of Sirius red staining in sections of infected lung was determined using area density measurements. NS, not significant according to t test.
Discussion
The present study suggests critical roles for Ctsl in murine responses to mycoplasma infection. We attribute the higher mortality among infected Ctsl-deficient mice to more severe bronchitis and pneumonia during the early phase of infection. On an absolute scale, the amount of obliteration by inflammatory cells and proteinaceous fluid of lung surface area available for gas transfer was very high in infected Ctsl−/− mice. Although the pneumonia grade seemed to plateau or even diminish in Ctsl−/− mice at 14 days of infection, this may be a survivor effect, because several of the most severely affected mice died before that time point. Nonetheless, pulmonary infection by M. pulmonis may be survivable in Ctsl−/− mice living beyond the initial phase of infection, as it was in all wild-type mice receiving the same initial inoculum of M. pulmonis.
Notably, Ctsl−/− mice also carried higher burdens of mycoplasma organisms than did Ctsl+/+ mice early after infection. This difference is unlikely to be entirely related to a defect in T-lymphocyte responses to mycoplasma antigens, because it occurred before strong adaptive immune responses are expected, whereas early increases in chemokines of the innate immune system MIP-2 and MCP-1 did not support impaired innate responses. The responses to live and heat-killed mycoplasma antigens suggest no major difference between Ctsl+/+ and Ctsl−/− mice in humoral responses, as manifested by the generation of antibodies detected in serum.
The reduced lymphangiogenesis in the airways of infected Ctsl−/− mice was unexpected, given that previous studies reported greater lymphatic growth in the presence of more severe inflammation (24, 25). Defective lymphangiogenesis in Ctsl−/− mice after infection is consistent with the involvement of Ctsl in growing lymphatic endothelial cells. However, in the absence of inflammation, Ctsl is not essential in this regard, because tracheal lymphatics and blood vessels were indistinguishable in pathogen-free Ctsl+/+ and Ctsl−/− mice. Alternately, reduced lymphatic growth could have been a consequence of an ineffective host immune response, which is essential for the full expression of vascular remodeling after mycoplasmal infection (25). Reduced lymphangiogenesis itself could impair host defense later after infection, given that lymphatics are essential conduits for the trafficking of immune cells and proteins. Blunted lymphangiogenesis could increase mucosal edema and other manifestations of inflammation (26).
In the context of infection, Ctsl could coordinate innate and adaptive immune responses. For example, Ctsl activity is increased in mouse corneas after infection with Pseudomonas aeruginosa, and the increase correlates well with the inflammatory response (32). In cancer studies, cathepsin activity localized to areas of tumors, and was possibly attributable to production by immune cells participating in an antitumor response (33). In the present experiments, the inflammatory response was greater in Ctsl−/− mice, as detected by histology and local TNF-α and chemokine production, but this response was less effective, as reflected by an increase in mycoplasmal burden. This disconnection suggests that Ctsl is necessary to initiate a response, but that the response is dysregulated without Ctsl. Given the evidence that Ctsl can facilitate apoptosis in macrophages and neutrophils (34), the finding of increased inflammation and neutrophilic infiltrates could relate in part to dysregulated apoptosis and delayed cell death.
The findings in the present study suggest additional possible bases for the more severe pneumonia, higher mortality, and higher lung bacterial burdens in infected Ctsl−/− mice. Because Ctsl is reported to play a role in MHC Class II–dependent immune responses (29), we hypothesized that Ctsl would be required for normal humoral responses to mycoplasma antigens. We evaluated the contribution of Ctsl to adaptive immune responses by comparing pathogen-specific, Ig class–specific, and Ig subclass–specific responses to live and heat-killed M. pulmonis in Ctsl−/− and Ctsl+/+ mice. Titers of mycoplasma-specific Ig were statistically indistinguishable in the serum of Ctsl−/− and Ctsl+/+ mice after 3 and 7 days of infection, even though measured titers of live mycoplasma in lung extracts were higher in Ctsl−/− mice than in Ctsl+/+ mice at these time points. This might suggest a deficit of antibody production relative to antigen burden. Nonetheless, the similar responses to an injection of heat-killed mycoplasma, which ensured the delivery of equal burdens of antigen, suggest that the capacity of Ctsl−/− mice to mount a humoral response was largely intact for mycoplasma antigens, and extended to all major Ig classes and IgG subclasses. IgE could not be assessed because it was not detected in any of the mice, regardless of Ctsl status.
Although humoral responses were similar, we could not detect M. pulmonis–specific splenic T lymphocytes in Ctsl−/− mice at an interval after infection when Ctsl+/+ mice exhibited robust responses. Primary systemic immune responses typically reflect local responses and often take 7–10 days to develop, so the similar concentrations of IFN-γ at 3 days between Ctsl−/− and wild-type mice with and without infection do not necessarily reflect cellular immune responses. It remains unclear whether this liability in cellular immunity resulted from an MHC Class I–specific defect in antigen processing or an alteration in the peptide repertoire of MHC Class II cells, although Ctsl to date has been implicated more strongly in MHC Class II–restricted antigen presentation (7, 29). Alternately, impaired thymic development in these animals could affect the long-term ability to generate T-lymphocyte responses.
As already noted, we found Ctsl deficiency to be accompanied by impaired lymphangiogenesis, but not blood-vessel remodeling, after mycoplasmal infection. To our knowledge, this is the first time that blunted airway lymphangiogenesis has been found without an associated reduction in blood-vessel remodeling, although the reverse has also been found (26, 35). Similarly, the persistence of remodeled airway lymphatics has been found after remodeled blood vessels regressed, subsequent to treatment of the airway infection (26, 28, 36).
Proteases are important for new vessel formation because sprouting endothelial cells penetrate the extracellular matrix, which consists of laminin, fibronectin, collagens, and other large proteins. In an elastase perfusion model of abdominal aortic aneurysm, Ctsl−/− mice had impaired angiogenesis, with reduced numbers of microvessels and defects of microvessels sprouting from aortic rings (30). Furthermore, Ctsl in cells of bone marrow origin is reported to be necessary for retinal and choroidal vessel development in models of intraocular neovascularization (37). Thus, the finding in the present experiments that a lack of Ctsl neither prevents nor reduces mycoplasma-induced tracheal blood-vessel remodeling was contrary to our initial expectations. However, most of this remodeling is circumferential vessel enlargement with transformation from a capillary to a venule phenotype, rather than angiogenesis. Therefore, Ctsl could be more important for growing new vessels than for the remodeling of existing ones.
Infected Ctsl−/− mice demonstrated much less lymphangiogenesis than Ctsl+/+ mice, even though inflammation was at least as severe as in Ctsl−/− mice. This relative defect in lymphangiogenesis may be attributable to a loss of the effect by Ctsl on lymphatic endothelial cell growth, migration, and matrix penetration, or could result from a dysregulated immune response, upon which lymphatic growth is dependent (25).
In conclusion, these results suggest a critical role for Ctsl in containing airway mycoplasma infection and the resultant lymphangiogenesis. Although Ctsl-null mice mounted robust humoral responses to mycoplasma antigen, the inability of splenocytes from infected Ctsl−/− mice to produce IFN-γ in response to mycoplasma antigen suggests a possible defect in cell-mediated immune responses (38). In the context of M. pulmonis infection, in which activated macrophages are thought to play an important and possibly critical role in controlling infection (13, 16, 39), a deficit of the antigen-stimulated production of IFN-γ, which is a macrophage-activation factor (38), could blunt macrophage responses to mycoplasma. Our experiments predict that Ctsl inhibitors could have the unintended consequence of increasing the severity of respiratory mycoplasmal infection, a possibility that should be assessed in developing and applying Ctsl inhibitors to a clinical context.
Acknowledgments
Acknowledgments
The authors thank Dr. Paul Wolters (University of California at San Francisco) for providing Ctsl−/− mice backcrossed into a C57BL/6 background for the initial experiments.
Footnotes
This work was supported by National Institutes of Health grants P01 HL024136 and R01 HL059157 from the National Heart, Lung, and Blood Institute (G.H.C.).
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2013-0016OC on April 19, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Reiser J, Adair B, Reinheckel T. Specialized roles for cysteine cathepsins in health and disease. J Clin Invest. 2010;120:3421–3431. doi: 10.1172/JCI42918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vasiljeva O, Reinheckel T, Peters C, Turk D, Turk V, Turk B. Emerging roles of cysteine cathepsins in disease and their potential as drug targets. Curr Pharm Des. 2007;13:387–403. doi: 10.2174/138161207780162962. [DOI] [PubMed] [Google Scholar]
- 3.McGrath ME. The lysosomal cysteine proteases. Annu Rev Biophys Biomol Struct. 1999;28:181–204. doi: 10.1146/annurev.biophys.28.1.181. [DOI] [PubMed] [Google Scholar]
- 4.Hsing LC, Rudensky AY. The lysosomal cysteine proteases in MHC Class II antigen presentation. Immunol Rev. 2005;207:229–241. doi: 10.1111/j.0105-2896.2005.00310.x. [DOI] [PubMed] [Google Scholar]
- 5.Kitamoto S, Sukhova GK, Sun J, Yang M, Libby P, Love V, Duramad P, Sun C, Zhang Y, Yang X, et al. Cathepsin L deficiency reduces diet-induced atherosclerosis in low-density lipoprotein receptor-knockout mice. Circulation. 2007;115:2065–2075. doi: 10.1161/CIRCULATIONAHA.107.688523. [DOI] [PubMed] [Google Scholar]
- 6.Turk V, Stoka V, Vasiljeva O, Renko M, Sun T, Turk B, Turk D. Cysteine cathepsins: from structure, function and regulation to new frontiers. Biochim Biophys Acta. 2012;1824:68–88. doi: 10.1016/j.bbapap.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lombardi G, Burzyn D, Mundiñano J, Berguer P, Bekinschtein P, Costa H, Castillo LF, Goldman A, Meiss R, Piazzon I, et al. Cathepsin-L influences the expression of extracellular matrix in lymphoid organs and plays a role in the regulation of thymic output and of peripheral T cell number. J Immunol. 2005;174:7022–7032. doi: 10.4049/jimmunol.174.11.7022. [DOI] [PubMed] [Google Scholar]
- 8.Qin Y, Shi GP. Cysteinyl cathepsins and mast cell proteases in the pathogenesis and therapeutics of cardiovascular diseases. Pharmacol Ther. 2011;131:338–350. doi: 10.1016/j.pharmthera.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chandran K, Sullivan NJ, Felbor U, Whelan SP, Cunningham JM. Endosomal proteolysis of the Ebola virus glycoprotein is necessary for infection. Science. 2005;308:1643–1645. doi: 10.1126/science.1110656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pager CT, Dutch RE. Cathepsin L is involved in proteolytic processing of the Hendra virus fusion protein. J Virol. 2005;79:12714–12720. doi: 10.1128/JVI.79.20.12714-12720.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simmons G, Gosalia DN, Rennekamp AJ, Reeves JD, Diamond SL, Bates P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc Natl Acad Sci USA. 2005;102:11876–11881. doi: 10.1073/pnas.0505577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang IC, Bosch BJ, Li F, Li W, Lee KH, Ghiran S, Vasilieva N, Dermody TS, Harrison SC, Dormitzer PR, et al. SARS coronavirus, but not human coronavirus NL63, utilizes cathepsin L to infect ACE2-expressing cells. J Biol Chem. 2006;281:3198–3203. doi: 10.1074/jbc.M508381200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Razin S, Yogev D, Naot Y. Molecular biology and pathogenicity of mycoplasmas. Microbiol Mol Biol Rev. 1998;62:1094–1156. doi: 10.1128/mmbr.62.4.1094-1156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chambaud I, Heilig R, Ferris S, Barbe V, Samson D, Galisson F, Moszer I, Dybvig K, Wróblewski H, Viari A, et al. The complete genome sequence of the murine respiratory pathogen Mycoplasma pulmonis. Nucleic Acids Res. 2001;29:2145–2153. doi: 10.1093/nar/29.10.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibson DG, Glass JI, Lartigue C, Noskov VN, Chuang RY, Algire MA, Benders GA, Montague MG, Ma L, Moodie MM, et al. Creation of a bacterial cell controlled by a chemically synthesized genome. Science. 2010;329:52–56. doi: 10.1126/science.1190719. [DOI] [PubMed] [Google Scholar]
- 16.Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev. 2004;17:697–728. doi: 10.1128/CMR.17.4.697-728.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yano T, Ichikawa Y, Komatu S, Arai S, Oizumi K. Association of Mycoplasma pneumoniae antigen with initial onset of bronchial asthma. Am J Respir Crit Care Med. 1994;149:1348–1353. doi: 10.1164/ajrccm.149.5.8173777. [DOI] [PubMed] [Google Scholar]
- 18.Martin RJ, Kraft M, Chu HW, Berns EA, Cassell GH. A link between chronic asthma and chronic infection. J Allergy Clin Immunol. 2001;107:595–601. doi: 10.1067/mai.2001.113563. [DOI] [PubMed] [Google Scholar]
- 19.McDonald DM, Schoeb TR, Lindsey JR. Mycoplasma pulmonis infections cause long-lasting potentiation of neurogenic inflammation in the respiratory tract of the rat. J Clin Invest. 1991;87:787–799. doi: 10.1172/JCI115082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cimolai N, Taylor GP, Mah D, Morrison BJ. Definition and application of a histopathological scoring scheme for an animal model of acute Mycoplasma pneumoniae pulmonary infection. Microbiol Immunol. 1992;36:465–478. doi: 10.1111/j.1348-0421.1992.tb02045.x. [DOI] [PubMed] [Google Scholar]
- 21.Martin RJ, Chu HW, Honour JM, Harbeck RJ. Airway inflammation and bronchial hyperresponsiveness after Mycoplasma pneumoniae infection in a murine model. Am J Respir Cell Mol Biol. 2001;24:577–582. doi: 10.1165/ajrcmb.24.5.4315. [DOI] [PubMed] [Google Scholar]
- 22.McDonald DM. Angiogenesis and remodeling of airway vasculature in chronic inflammation. Am J Respir Crit Care Med. 2001;164:S39–S45. doi: 10.1164/ajrccm.164.supplement_2.2106065. [DOI] [PubMed] [Google Scholar]
- 23.Baluk P, Raymond WW, Ator E, Coussens LM, McDonald DM, Caughey GH. Matrix metalloproteinase–2 and –9 expression increases in mycoplasma-infected airways but is not required for microvascular remodeling. Am J Physiol Lung Cell Mol Physiol. 2004;287:L307–L317. doi: 10.1152/ajplung.00404.2003. [DOI] [PubMed] [Google Scholar]
- 24.Xu X, Zhang D, Lyubynska N, Wolters PJ, Killeen NP, Baluk P, McDonald DM, Hawgood S, Caughey GH. Mast cells protect mice from mycoplasma pneumonia. Am J Respir Crit Care Med. 2006;173:219–225. doi: 10.1164/rccm.200507-1034OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aurora AB, Baluk P, Zhang D, Sidhu SS, Dolganov GM, Basbaum C, McDonald DM, Killeen N. Immune complex–dependent remodeling of the airway vasculature in response to a chronic bacterial infection. J Immunol. 2005;175:6319–6326. doi: 10.4049/jimmunol.175.10.6319. [DOI] [PubMed] [Google Scholar]
- 26.Baluk P, Tammela T, Ator E, Lyubynska N, Achen MG, Hicklin DJ, Jeltsch M, Petrova TV, Pytowski B, Stacker SA, et al. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest. 2005;115:247–257. doi: 10.1172/JCI22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu X, Zhang D, Zhang H, Wolters PJ, Killeen NP, Sullivan BM, Locksley RM, Lowell CA, Caughey GH. Neutrophil histamine contributes to inflammation in mycoplasma pneumonia. J Exp Med. 2006;203:2907–2917. doi: 10.1084/jem.20061232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yao LC, Baluk P, Feng J, McDonald DM. Steroid-resistant lymphatic remodeling in chronically inflamed mouse airways. Am J Pathol. 2010;176:1525–1541. doi: 10.2353/ajpath.2010.090909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakagawa T, Roth W, Wong P, Nelson A, Farr A, Deussing J, Villadangos JA, Ploegh H, Peters C, Rudensky AY. Cathepsin L: critical role in Ii degradation and CD4 T cell selection in the thymus. Science. 1998;280:450–453. doi: 10.1126/science.280.5362.450. [DOI] [PubMed] [Google Scholar]
- 30.Sun J, Sukhova GK, Zhang J, Chen H, Sjöberg S, Libby P, Xiang M, Wang J, Peters C, Reinheckel T, et al. Cathepsin L activity is essential to elastase perfusion–induced abdominal aortic aneurysms in mice. Arterioscler Thromb Vasc Biol. 2011;31:2500–2508. doi: 10.1161/ATVBAHA.111.230201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greenland JR, Liu H, Berry D, Anderson DG, Kim WK, Irvine DJ, Langer R, Letvin NL. Beta–amino ester polymers facilitate in vivo DNA transfection and adjuvant plasmid DNA immunization. Mol Ther. 2005;12:164–170. doi: 10.1016/j.ymthe.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 32.Dong Z, Katar M, Linebaugh BE, Sloane BF, Berk RS. Expression of cathepsins B, D and L in mouse corneas infected with Pseudomonas aeruginosa. Eur J Biochem. 2001;268:6408–6416. doi: 10.1046/j.0014-2956.2001.02607.x. [DOI] [PubMed] [Google Scholar]
- 33.Joyce JA, Baruch A, Chehade K, Meyer-Morse N, Giraudo E, Tsai FY, Greenbaum DC, Hager JH, Bogyo M, Hanahan D. Cathepsin cysteine proteases are effectors of invasive growth and angiogenesis during multistage tumorigenesis. Cancer Cell. 2004;5:443–453. doi: 10.1016/s1535-6108(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 34.Mahmood DF, Jguirim-Souissi I, Khadija H, Blondeau N, Diderot V, Amrani S, Slimane MN, Syrovets T, Simmet T, Rouis M. Peroxisome proliferator–activated receptor gamma induces apoptosis and inhibits autophagy of human monocyte–derived macrophages via induction of cathepsin L: potential role in atherosclerosis. J Biol Chem. 2011;286:28858–28866. doi: 10.1074/jbc.M111.273292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baluk P, Hogmalm A, Bry M, Alitalo K, Bry K, McDonald DM. Transgenic overexpression of interleukin-1β induces persistent lymphangiogenesis but not angiogenesis in mouse airways. Am J Pathol. 2013;182:1434–1447. doi: 10.1016/j.ajpath.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yao LC, Baluk P, Srinivasan RS, Oliver G, McDonald DM. Plasticity of button-like junctions in the endothelium of airway lymphatics in development and inflammation. Am J Pathol. 2012;180:2561–2575. doi: 10.1016/j.ajpath.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimada N, Ohno-Matsui K, Iseki S, Koike M, Uchiyama Y, Wang J, Yoshida T, Sato T, Peters C, Mochizuki M, et al. Cathepsin L in bone marrow–derived cells is required for retinal and choroidal neovascularization. Am J Pathol. 2010;176:2571–2580. doi: 10.2353/ajpath.2010.091027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol. 2007;96:41–101. doi: 10.1016/S0065-2776(07)96002-2. [DOI] [PubMed] [Google Scholar]
- 39.Hickman-Davis JM, Michalek SM, Gibbs-Erwin J, Lindsey JR. Depletion of alveolar macrophages exacerbates respiratory mycoplasmosis in mycoplasma-resistant C57BL mice but not mycoplasma-susceptible C3H mice. Infect Immun. 1997;65:2278–2282. doi: 10.1128/iai.65.6.2278-2282.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]