Abstract
Background and purpose
In muscle-invasive bladder cancer there is an urgent need to identify relatively non-toxic radiosensitising agents for use in elderly patients. Histone deacetylase inhibitors radiosensitise tumour cells but not normal cells in vitro and variously downregulate DNA damage signalling, homologous recombination (HR) and non-homologous end-joining (NHEJ) repair proteins. We investigated panobinostat (PAN) as a potential radiosensitiser in bladder cancer cells.
Materials and methods
Clonogenic assays were performed in RT112 bladder cancer cells, and RT112 cells stably knocked down for RAD51 or Ku80 by shRNAi. Resolution of γH2AX foci was determined by immunofluorescence confocal microscopy, cell cycle progression by FACS analysis and protein expression by western blotting.
Results
PAN had a greater radiosensitising effect in Ku80KD than RT112 or RAD51KD cells; enhancement ratios 1.35 for Ku80KD at 10 nM (IC20 for Ku80KD) and 1.31 for RT112 and RAD51KD at 25 nM (IC40 for both). PAN downregulated MRE11, NBS1 and RAD51, but not Ku70 and Ku80, increased γH2AX foci formation in a dose-dependent manner and delayed γH2AX foci repair after ionising radiation.
Conclusions
PAN acts as a radiosensitiser in bladder cancer cell lines, and appears to target HR rather than NHEJ. As muscle-invasive bladder tumours have reduced Ku-DNA binding, PAN could be particularly useful as a radiosensitiser in bladder cancer.
Keywords: Bladder cancer, Histone deacetylase inhibitor, Ku80, Panobinostat, Radiosensitisation
Bladder cancer is the 9th commonest cancer worldwide, with over 330,000 people diagnosed and over 130,000 dying from the disease annually [1]. Muscle-invasive bladder cancer can be treated by neo-adjuvant chemotherapy, followed by either surgical removal of the bladder (cystectomy) or radical radiotherapy (RT) with or without concurrent chemotherapy (CRT), with similar results [2]. Older patients tolerate surgery and chemotherapy poorly. As the overall population ages, we are witnessing increasing numbers of patients with bladder cancer who are unable to tolerate toxic treatments. There is therefore an urgent need to test novel, less toxic agents in combination with ionising radiation, as potential clinical radiosensitisers in this disease.
Double strand breaks (DSB) are the lethal DNA lesions caused by ionising radiation. Cells have two major mechanisms to repair DSB: non-homologous end-joining (NHEJ) and homologous recombination (HR). NHEJ predominates in G1/early S phase but is error-prone; Ku70, Ku80 and DNA-PK are its key initiators [3]. HR is active in late S/G2 phase, as it requires a sister chromatid to act as a repair template, and is error free; the MRE11/RAD50/NBS1 complex and RAD51 are key components [4,5].
Histone deacetylase inhibitors (HDACi) have known tumour growth suppressive effects in vitro and in vivo, and these agents act by opening up chromatin thus promoting gene expression, and also by effects on non-histone proteins, including transcription factors, transcription co-regulators, signalling mediators and DNA repair enzymes (reviewed in [6]). High levels of HDACs have been found in bladder tumours, so HDACi’s may be useful in this disease. SAHA and MS275 were well tolerated in phase I studies in bladder cancer [6]. Whilst trichostatin A and belinostat inhibit the growth of bladder cancer cells in nanomolar and micromolar concentrations, respectively [7,8], we are not aware of similar studies using panobinostat (PAN), a potent HDACi.
Several HDACi’s radiosensitise a range of tumour cell types and variously downregulate MRN complex or core NHEJ or HR proteins in tumour but not normal cells, whilst compromising DNA repair post-ionising radiation (reviewed in [9]). In addition, HDAC inhibitors have shown no evidence of toxicity in normal cells and the HDACi H6CAHA even caused an increase in post-irradiation surviving fraction in normal prostate cells [10–12]. Their potential clinical use as radiosensitisers has been reported in anaplastic thyroid carcinoma, glioma, and gastrointestinal carcinoma (reviewed in [9]). However, to date there are no published clinical trials of radiotherapy combined with an HDACi in bladder cancer.
Muscle-invasive bladder tumours have defective NHEJ repair [13,14], so HDACi’s would be particularly useful if they targeted the HR pathway, resulting in ‘synthetic lethality’, and hence an increased therapeutic ratio, in combination with IR [15].
We therefore investigated PAN as a potential radiosensitiser in muscle-invasive bladder cancer. PAN radiosensitised RT112 bladder cancer cells and stably-transfected Ku80 knockdown cells, and appeared to act through the HR pathway, and increased γH2AX foci formation and delayed resolution after ionising radiation.
Materials and methods
Reagents
Panobinostat (LBH589; PAN) was purchased from Selleck Chemicals and dissolved in dimethyl sulfoxide (DMSO; Sigma) to a stock concentration of 1 mmol/L. Single-use aliquots were stored at −80 °C.
Cell culture conditions
TP53 wild-type RT112 bladder transitional cell carcinoma cells were grown in RPMI-1640 (Sigma) supplemented with 10% v/v foetal bovine serum (FBS; Sigma), 2 mmol/L l-glutamine (Sigma). Stably transfected knock-down (KD) 795J (RAD51KD), C13 (Ku80KD) and pSil8 (non-silencing control (NSC)) cells were created from RT112 cells as previously described [15], and cultured in RPMI-1640 medium, 10% v/v FBS, 2 mmol/L l-glutamine (Sigma), 0.04 μg/ml G-418 solution (Roche). All cell lines were grown in a humidified atmosphere containing 5% CO2 at 37 °C and exponentially growing cells were used in all experiments.
Chemosensitivity clonogenic assay
Cells were plated at 700–1000 cells per plate in 10 cm dishes (Greiner Bio-One) for 24 h at appropriate drug concentrations. The next day plates were washed twice with RPMI-1640 medium and 10 ml of fresh medium was added to each plate. Fourteen days later each plate was stained with 0.25% Brilliant blue R (Sigma–Aldrich): 40% methanol: 7% acetic acid for 30 min. Colonies containing more than 50 cells were counted automatically using a Colcounter (Oxford Optronix).
Radiosensitisation clonogenic assay
Cells (7.5 × 105) were plated in 10 cm dishes, and the following day, treated either with DMSO or PAN at appropriate concentrations. After a further 24 h incubation, cells were trypsinised and appropriate numbers plated in 10 cm dishes and irradiated at a dose-rate of 1.7 Gy/min using a caesium-137 source (GSR D1, Gamma-Service Medical GmbH). Cells were then incubated at 37 °C for 14 days, before staining and counting as above. The surviving fraction and radiation survival curves were determined and plotted in GraphPad Prism using the linear-quadratic model as previously described [15].
Western blotting
Western blotting was performed as previously described [15], using the following antibodies: mouse monoclonal anti-Ku80 (Neomarkers), anti-Ku70 (Abcam), anti-Mre11 (Abcam), anti-H3K18 (Cell Signalling) and anti-β-actin (Abcam), and rabbit monoclonal anti-NBS1 (Abcam) and anti-RAD51 (Proteintech Europe).
Cell cycle fluorescence-activated cell sorting (FACS) analysis
Cell-cycle analysis was performed as previously described [15].
Immunofluorescence
Cells (2.5 × 105 per dish) were plated onto sterile glass coverslips, and the following day treated with PAN or DMSO. Twenty-four hours later cells were either fixed or irradiated to 5 Gy prior to fixation, at the time points indicated. Cells were fixed by incubation for 20 min in 4% paraformaldehyde (Thermo Scientific)/0.1% Triton X-100 (Sigma) at room temperature, followed by three PBS rinses, incubation in 0.5% Triton/PBS for 15 min, then three further PBS rinses. Coverslips were then blocked for 30 min at room temperature in 5% bovine serum albumin (VWR International Ltd.) before incubation in mouse monoclonal anti-γH2AX antibody (Millipore) overnight at 4 °C. Following three PBS washes, coverslips were incubated in secondary anti-rabbit (Alexa 488, Invitrogen) and anti-mouse (Alexa 568, Invitrogen) antibodies for 1 h before rinsing in PBS three times. Coverslips were mounted onto slides with Fluoromount G (Sigma) containing 0.1 μg/ml DAPI (Sigma), dried and scanned on a confocal microscope (Zeiss LSM 780).
Statistical analysis
All statistical analyses were conducted using GraphPad Prism software. Clonogenic assays were performed in duplicate at least three times, with the results expressed as mean and standard deviation (SD). The sensitiser enhancement ratio (SER) was calculated at the 0.1 survival fraction (10% survival). In the immunofluorescence experiments, slides were prepared twice for each condition and at least 70 cells counted per slide.
Results
Panobinostat kills bladder cancer cells in the nanomolar range, causes G2/M cell cycle arrest, and causes γH2AX foci increase
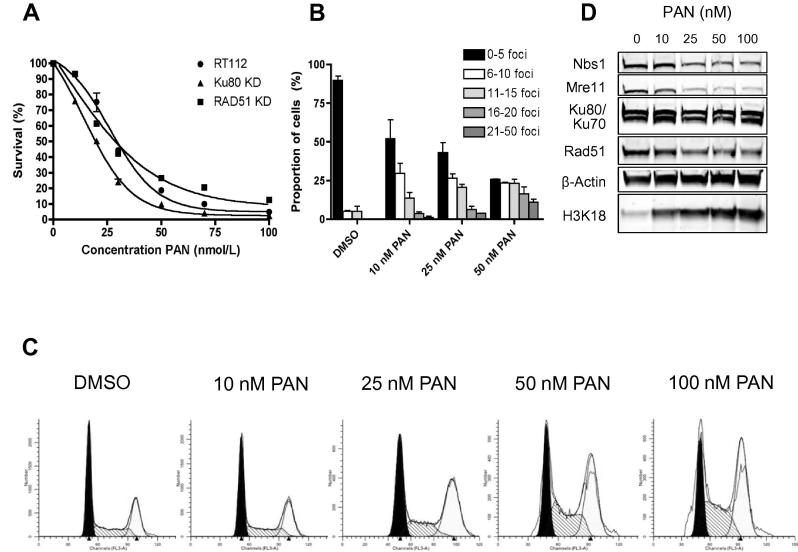
First, we determined the inhibitory effect of panobinostat (PAN) on growth of RT112, RAD51KD, Ku80KD and NSC cells by clonogenic assay. After 24 h treatment, IC50 values for PAN were 27 nM in RT112, 25 nM in RAD51KD, 19 nM in Ku80KD and 27 nM in NSC cells; it was therefore more toxic in Ku knockdown cells (Fig. 1A and Supplementary Fig. 1A).
Fig. 1.
(a) Clonogenic assays following 24 h treatment of RT112, RAD51KD and Ku80KD cells with increasing PAN concentrations. All experiments were performed at least three times. Error bars represent the mean and standard deviation (SD). (b) Quantification of γH2AX foci in DMSO- or PAN-treated cells, 24 h after treatment (see Supplementary Fig. 2 for confocal images). (c) Cell-cycle phase distributions in cells treated with increasing concentrations of PAN for 24 h. (d) Western blots from RT112 cells incubated with PAN for 24 h, showing downregulation of MRE11, NBS1 and RAD51 but not Ku70 or Ku80, with acetylation of H3K18 from 10 nM or greater PAN.
We also studied PAN’s effect on γH2AX foci formation in RT112 cells (Fig. 1B and Supplementary Fig. 2). We detected a significant increase in the number of γH2AX foci per cell (P = 0.022 at 25 nm and P = 0.002 at 50 nM).
We then tested the effects of increasing concentrations of PAN on cell cycle progression (Fig. 1C and Supplementary Fig. 3). PAN caused G2/M phase arrest in a concentration-dependent manner (statistically significant at 25, 50 and 100 nM PAN, P = 0.01, P = 0.02 and P = 0.02, respectively).
Panobinostat downregulates MRE11, NBS1 and RAD51 proteins
Next, we determined the effects of PAN on key DNA damage signalling and repair proteins by western blotting. PAN downregulated protein levels of MRE11 and NBS1 in a dose-dependent manner (Fig. 1D). RAD51 was also downregulated, but PAN did not affect Ku70 or Ku80 levels. Acetylation of histone H3K18 confirmed PAN’s action at 10 nM and above.
Panobinostat radiosensitises bladder cancer cells, particularly Ku-deficient
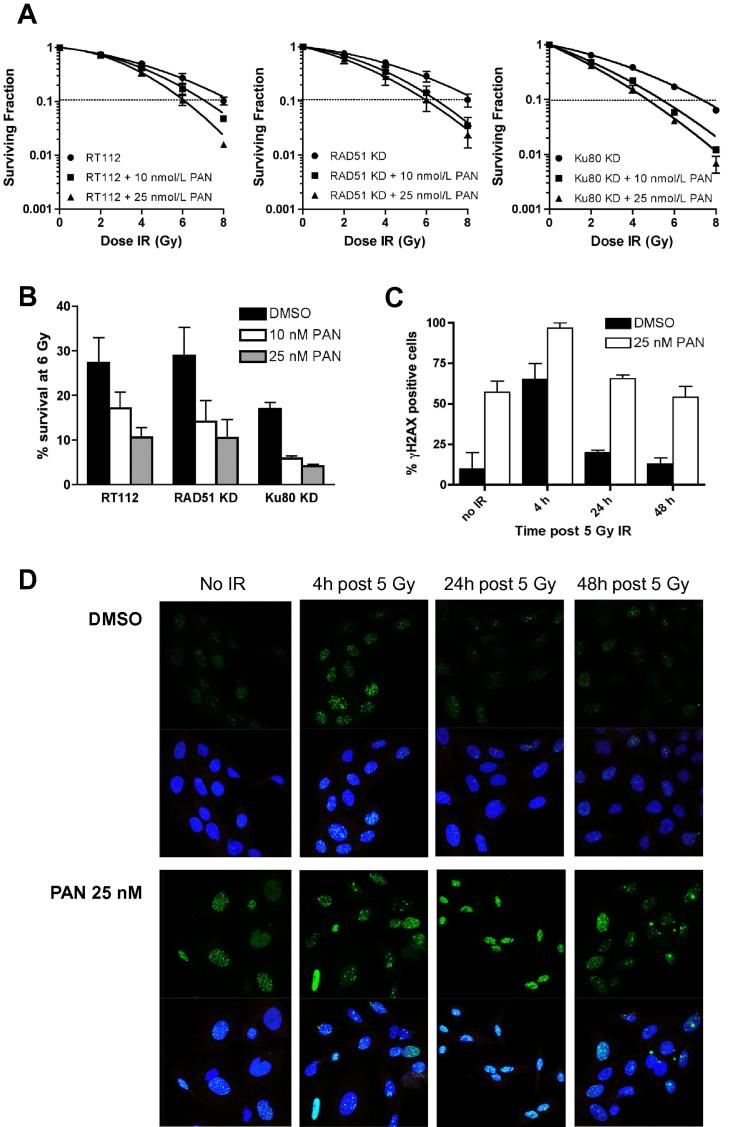
Cells were treated at 10 nM and 25 nM PAN concentrations, approximately equivalent to RT112’s IC10 and IC40. PAN significantly sensitised Ku80KD cells at 10 nM (SER = 1.35, P = 0.04) with less marked effects in parental RT112 cells (SER = 1.16, P = 0.05) and RAD51KD cells (SER = 1.22, P = 0.04). At 25 nM PAN (IC40), the SER for both RT112 and RAD51KD cells was 1.31 (P = 0.01 and P = 0.03, respectively), but this was higher for Ku80KD cells at a Ku80KD IC20 equivalent dose (1.56, P = 0.05; Fig. 2A and B).
Fig. 2.
(a) Clonogenic assays in RT112, RAD51KD and Ku80KD cells treated with increasing ionising radiation doses and 10 nM or 25 nM PAN; (b) Percentages of cells surviving after 6 Gy, quantified from (a); (c) quantification of γH2AX foci from (d); (d) Confocal images of γH2AX foci (top panels) and DAPI stained cells (bottom panels) for cells treated with and without 5 Gy IR and 25 nM PAN.
Panobinostat delays γH2AX foci resolution after 5 Gy irradiation
We examined γH2AX foci resolution at 4, 24 and 48 h post 5 Gy irradiation in PAN and DMSO treated samples (Fig. 2C and D). We detected a significant delay in γH2AX foci resolution in PAN treated samples at 24 and 48 h time points (P = 0.003 and P = 0.03, respectively).
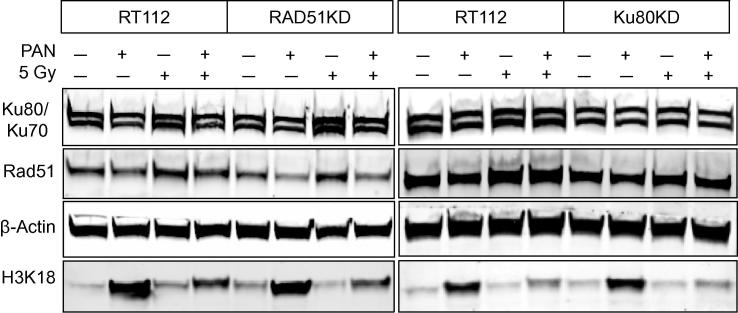
Effects of panobinostat on RAD51 and Ku70/Ku80
RAD51, Ku70/80 and histone H3K18 expression were measured by Western blot after 25 nM PAN ± 5 Gy (Fig. 3). RAD51 and Ku80 levels were reduced as expected in the respective knockdown cells. PAN reduced RAD51 levels in all cell lines and attenuated the RAD51 upregulation seen after 5 Gy. Ku70 and Ku80 protein levels were not markedly affected by PAN treatment.
Fig. 3.
Western blot analyses of RT112, RAD51KD and Ku80KD cells. Cells were incubated with or without 25 nM PAN for 24 h, and then harvested and irradiated to 5 Gy or left untreated. Cells were lysed 4 h later. Experiments were performed at least twice.
Discussion
To our knowledge, this is the first reported study using PAN in bladder cancer cell lines and the first to show its radiosensitising effects in bladder cancer.
We found PAN to have potent cytotoxic effects in the RT112 bladder cancer cell line and its Ku80 and RAD51 stably transfected knockdown counterparts, with downregulation of RAD51, MRE11 and NBS1 but not Ku70 or Ku80 in RT112 cells. Strikingly, PAN was more toxic in the Ku80KD cell line than RT112 or RAD51KD cells. We tested PAN’s effects on cell cycle progression in RT112 cells and, at doses of 25 nM and above, found a significant accumulation of cells in G2/M phase, where HR is the predominant DSB repair mechanism, but this was not seen at 10 nM.
Our RAD51 KD cell lines showed a similar radiosensitivity profile by clonogenic assay to the parental RT112 cell line, whilst the Ku80 KD cell line was more radiosensitive, with surviving fractions after 6 Gy of IR of 27%, 28% and 18%, respectively. HR appears to be less important than NHEJ for repairing IR-induced breaks in higher eukaryotes, especially in G1 phase, although HR contributes in the late S/G2 phase [16]. We found PAN to be an efficient radiosensitiser in the RT112 bladder cancer cells used in this report and also two other high grade bladder cancer cell lines (Supplementary Fig. 1). Here, PAN was a particularly efficient radiosensitiser in Ku80KD cells. PAN had an equal efficacy at Ku80KD IC20 (10 nM) and RT112 IC40 levels (25 nM), with SERs of 1.35 and 1.31, respectively. These data suggest that PAN’s greater radiosensitising effect in KuKD cells is not due solely to the increased toxicity of the drug in these cells. Neither is it likely to be due to cell cycle effects at the lower doses (data not shown). Our results were similar to those we previously found for the tyrosine kinase inhibitor imatinib, which targets RAD51 [15]. However, our PAN results were more striking than with imatinib, and this may reflect the additional effects of PAN on MRE11 and NBS1. Downregulation of the MRN complex by siRNA or NBS1 or MRE11 mutation is known to result in radiosensitisation [17,18] and the additional impact of PAN on these proteins higher in the DNA damage signalling pathway may account for our findings.
We measured γH2AX as a marker of DNA damage and repair and found that PAN upregulated the number of γH2AX foci seen at 24 h. Studies using other HDACi’s also show similar effects [19]. When cells were irradiated with 5 Gy after incubation with 25 nM PAN, we detected a significant delay in γH2AX foci resolution, indicating supressed repair of DNA DSB in PAN treated cells. Geng et al. [20] showed similar effects for PAN with 3 Gy irradiation in lung cancer cells. We are, however, aware of the limitations of the γH2AX assay as a measure of DSB repair, particularly in non-G0/G1 cells, since γH2AX formation can occur at single-stranded DNA regions during replication or repair [21]. Also background, endogenous ‘cryptogenic’ foci can limit the ability to detect residual IR-induced γH2AX foci [22].
In using only the γH2AX assay to measure DNA damage and repair, we have not comprehensively examined the effects of PAN on the NHEJ and HR pathways. To do this requires assays such as the NHEJ assay suitable for use on small scale extracts from cell lines [23] and the widely reported HR I-SceI-based reporter assay [24].
We have found previously that Ku80 expression by western blot and immunohistochemistry is variable and does not correlate with bladder cancer grade and stage [13]. However, muscle-invasive bladder tumours display reduced Ku-DNA binding (as previously seen by Pucci et al. [25]), thus rendering them functionally NHEJ-defective. Therefore agents targeting the HR pathway could result in ‘synthetic lethality’, whereby normal cells, with intact NHEJ, can still repair IR-induced DNA damage via NHEJ despite HR being targeted, whilst tumour cells defective in NHEJ are less able to repair the damage. In terms of biomarker development for the prediction of response to panobinostat, it would be interesting to test whether low Ku-expressing tumours respond better to panobinostat, although the reduced Ku-DNA binding phenotype may over-ride this effect.
Whilst others have variously shown effects of HDACi’s on the MRN complex, RAD51 and Ku70/80, we believe we are the first to study all three aspects together [9]. PAN is a potent HDACi and the plasma levels used in this study can be reached safely in patients [26]. PAN is therefore a promising agent for investigation as an efficient future therapy in combination with radiation in bladder cancer.
Conflicts of interest
None to declare.
Acknowledgements
This study was supported by the Slovene Human Resources Scholarship Fund (B. Groselj) and Cancer Research UK Program Grant C5255/A15935 (M. Kerr and A.E. Kiltie). The study sponsors had no involvement in the study.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.radonc.2013.06.021.
Appendix A. Supplementary data
This file contains Supplementary Figs. 1–3.
References
- 1.Stenzl A., Cowan N.C., De Santis M. Treatment of muscle-invasive and metastatic bladder cancer: update of the EAU guidelines. Eur Urol. 2011;59:1009–1018. doi: 10.1016/j.eururo.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 2.Kotwal S., Choudhury A., Johnston C., Paul A.B., Whelan P., Kiltie A.E. Similar treatment outcomes for radical cystectomy and radical radiotherapy in invasive bladder cancer treated at a United Kingdom specialist treatment center. Int J Radiat Oncol Biol Phys. 2008;70:456–463. doi: 10.1016/j.ijrobp.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 3.Wang C., Lees-Miller S.P. Detection and repair of ionizing radiation-induced DNA double strand breaks: new developments in nonhomologous end joining. Int J Radiat Oncol Biol Phys. 2013 doi: 10.1016/j.ijrobp.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takeda S., Nakamura K., Taniguchi Y., Paull T.T. Ctp1/ctip and the mrn complex collaborate in the initial steps of homologous recombination. Mol Cell. 2007;28:351–352. doi: 10.1016/j.molcel.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 5.Baumann P., West S.C. Role of the human RAD51 protein in homologous recombination and double-stranded-break repair. Trends Biochem Sci. 1998;23:247–251. doi: 10.1016/s0968-0004(98)01232-8. [DOI] [PubMed] [Google Scholar]
- 6.Sharma N.L., Groselj B., Hamdy F.C., Kiltie A.E. The emerging role of histone deacetylase (HDAC) inhibitors in urological cancers. BJU Int. 2013;111:537–542. doi: 10.1111/j.1464-410X.2012.11647.x. [DOI] [PubMed] [Google Scholar]
- 7.Buckley M.T., Yoon J., Yee H. The histone deacetylase inhibitor belinostat (pxd101) suppresses bladder cancer cell growth in vitro and in vivo. J Transl Med. 2007;5:49. doi: 10.1186/1479-5876-5-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li G.C., Zhang X., Pan T.J., Chen Z., Ye Z.Q. Histone deacetylase inhibitor trichostatin a inhibits the growth of bladder cancer cells through induction of p21WAF1 and G1 cell cycle arrest. Int J Urol. 2006;13:581–586. doi: 10.1111/j.1442-2042.2006.01344.x. [DOI] [PubMed] [Google Scholar]
- 9.Groselj B., Sharma N.L., Hamdy F.C., Kerr M., Kiltie A.E. Histone deacetylase inhibitors as radiosensitisers: effects on DNA damage signalling and repair. Br J Cancer. 2013;108:748–754. doi: 10.1038/bjc.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blattmann C., Oertel S., Ehemann V. Enhancement of radiation response in osteosarcoma and rhabdomyosarcoma cell lines by histone deacetylase inhibition. Int J Radiat Oncol Biol Phys. 2010;78:237–245. doi: 10.1016/j.ijrobp.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Konsoula Z., Cao H., Velena A., Jung M. Adamantanyl-histone deacetylase inhibitor h6caha exhibits favorable pharmacokinetics and augments prostate cancer radiation sensitivity. Int J Radiat Oncol Biol Phys. 2011;79:1541–1548. doi: 10.1016/j.ijrobp.2010.11.057. [DOI] [PubMed] [Google Scholar]
- 12.Lee J.H., Choy M.L., Ngo L., Foster S.S., Marks P.A. Histone deacetylase inhibitor induces DNA damage, which normal but not transformed cells can repair. Proc Natl Acad Sci USA. 2010;107:14639–14644. doi: 10.1073/pnas.1008522107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bentley J., L’Hote C., Platt F. Papillary and muscle invasive bladder tumors with distinct genomic stability profiles have different DNA repair fidelity and Ku DNA-binding activities. Genes Chromosomes Cancer. 2009;48:310–321. doi: 10.1002/gcc.20641. [DOI] [PubMed] [Google Scholar]
- 14.Bentley J., Diggle C.P., Harnden P., Knowles M.A., Kiltie A.E. DNA double strand break repair in human bladder cancer is error prone and involves microhomology-associated end-joining. Nucleic Acids Res. 2004;32:5249–5259. doi: 10.1093/nar/gkh842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiao B., Kerr M., Groselj B. Imatinib radiosensitizes bladder cancer by targeting homologous recombination. Cancer Res. 2013;73:1611–1620. doi: 10.1158/0008-5472.CAN-12-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothkamm K., Kruger I., Thompson L.H., Lobrich M. Pathways of DNA double-strand break repair during the mammalian cell cycle. Mol Cell Biol. 2003;23:5706–5715. doi: 10.1128/MCB.23.16.5706-5715.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng R., Tang J., Ma J.G. Pkb/akt promotes dsb repair in cancer cells through upregulating mre11 expression following ionizing radiation. Oncogene. 2011;30:944–955. doi: 10.1038/onc.2010.467. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto Y., Miyamoto T., Sakamoto H. Two unrelated patients with MRE11A mutations and Nijmegen breakage syndrome-like severe microcephaly. DNA Repair (Amst) 2011;10:314–321. doi: 10.1016/j.dnarep.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Munshi A., Kurland J.F., Nishikawa T. Histone deacetylase inhibitors radiosensitize human melanoma cells by suppressing DNA repair activity. Clin Cancer Res. 2005;11:4912–4922. doi: 10.1158/1078-0432.CCR-04-2088. [DOI] [PubMed] [Google Scholar]
- 20.Geng L., Cuneo K.C., Fu A., Tu T., Atadja P.W., Hallahan D.E. Histone deacetylase (hdac) inhibitor lbh589 increases duration of gamma-H2AX foci and confines HDAC4 to the cytoplasm in irradiated non-small cell lung cancer. Cancer Res. 2006;66:11298–11304. doi: 10.1158/0008-5472.CAN-06-0049. [DOI] [PubMed] [Google Scholar]
- 21.Lobrich M., Shibata A., Beucher A. GammaH2AX foci analysis for monitoring DNA double-strand break repair: Strengths, limitations and optimization. Cell Cycle. 2010;9:662–669. doi: 10.4161/cc.9.4.10764. [DOI] [PubMed] [Google Scholar]
- 22.Olive P.L. Retention of gammah2ax foci as an indication of lethal DNA damage. Radiother Oncol. 2011;101:18–23. doi: 10.1016/j.radonc.2011.05.055. [DOI] [PubMed] [Google Scholar]
- 23.Diggle C.P., Bentley J., Kiltie A.E. Development of a rapid, small-scale DNA repair assay for use on clinical samples. Nucleic Acids Res. 2003;31:e83. doi: 10.1093/nar/gng083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao H., Luoto K.R., Meng A.X., Bristow R.G. The receptor tyrosine kinase inhibitor amuvatinib (mp470) sensitizes tumor cells to radio- and chemo-therapies in part by inhibiting homologous recombination. Radiother Oncol. 2011;101:59–65. doi: 10.1016/j.radonc.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 25.Pucci S., Mazzarelli P., Rabitti C. Tumor specific modulation of Ku70/80 DNA binding activity in breast and bladder human tumor biopsies. Oncogene. 2001;20:739–747. doi: 10.1038/sj.onc.1204148. [DOI] [PubMed] [Google Scholar]
- 26.Giles F., Fischer T., Cortes J. A phase I study of intravenous LBH589, a novel cinnamic hydroxamic acid analogue histone deacetylase inhibitor, in patients with refractory hematologic malignancies. Clin Cancer Res. 2006;12:4628–4635. doi: 10.1158/1078-0432.CCR-06-0511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This file contains Supplementary Figs. 1–3.