Abstract
A bacterial strain SSZ01 isolated from a eutrophic lake in Saudi Arabia dominated by cyanobacterial blooms, showed an antialgal activity against cyanobacteria species. Based on the analysis of the 16S rDNA gene sequence, the isolated strain (SSZ01) most likely belonged to the genus Bacillus with a 99% similarity to Bacillus flexus strain EMGA5. The thin layer chromatography (TLC) analysis of the ethyl acetate extract of this bacterium revealed that this strain can produce harmine and norharmane compared to different β-carboline analog standards. Harmine and norharmane were also detected in considerable amounts in bacterial growth medium, indicating a potential excretion of these compounds into the aquatic environment. The crude extract of Bacillus flexus as well as pure materials of harmine and norharmane inhibited the growth of tested species of cyanobacteria. However, the bacterial crude extract has a higher toxicity against tested species of cyanobacteria than harmine and norharmane. In addition, harmine was more toxic to cyanobacteria than norharmane. On the other hand, neither pure compounds of harmine and norharmane nor crude bacterial extract showed any antialgal activity against tested species of green algae. The results of the present study suggest that B. flexus SSZ01 or its crude extract containing harmine and norharmane could be a candidate for the selective control of cyanobacterial blooms without affecting other algal species.
Keywords: Antialgal bacteria, Bacillus flexus, Cyanobacteria, Harmine, Norharmane
1. Introduction
Cyanobacteria usually dominate in eutrophic lakes and cause environmental problems due to the production of malodorous compounds and toxins (Oliver and Ganf, 2000). Cyanobacteria blooms are also responsible for wildlife and human health hazards, causing economic losses on fisheries, aquaculture and recreational activities (Codd, 1999; Anderson et al., 2002; Hallegraeff, 2003). Cyanobacteria may produce hepatotoxins (microcystins, nodularins and cylindrospermopsins), neurotoxins (anatoxins and saxitoxins), cytotoxins and irritant compounds (lipopolysaccharides (LPS)) (Codd, 1999; Long and Carmichael, 2003; Apeldoorn et al., 2007). Some of these toxins can act as cancer promoters (Falconer et al., 1999; Kuiper-Goodman et al., 1999). In addition, cyanobacterial toxins significantly affect the growth of plants and accumulate in their edible tissues (Mohamed and Al-Shehri, 2009) providing an additional route of human and animal exposure to these toxins.
Much attention has been given in recent decades to strategies for cyanobacteria bloom control and management. Various chemical or synthetic agents (e.g., copper, chlorine, aluminum, calcium, and potassium permanganate) are used to control nuisance phytoplankton in aquatic ecosystems (Lam et al., 1995; Falconer et al., 1999). However, these algaecides often induce the release of phytotoxins, which threaten drinking water supplies, accumulate and persist in the environment, and are toxic to fish (Lam et al., 1995; Karan et al., 1998; Boyd and Massaut, 1999; Meepagala et al., 2005). Therefore, biological control agents such as viruses (Garry et al., 1998), bacteria (Imai et al., 1995; Park et al., 2006a), and protozoa (Kim et al., 2009) are of particular interest.
Research into the relationship between bacteria and algae has resulted in the isolation of several strains of bacteria capable of inhibiting or killing harmful algal bloom species (Lovejoy et al., 1998; Yoshinaga et al., 1998; Amaro et al., 2005; Su et al., 2007). This is because these bacteria may contain natural compounds acting as algaecides against algae and cyanobacteria (Kodani et al., 2002). Such algaecides are likely to be specific and biodegradable, and may therefore offer an environmentally friendly method for control of algal blooms (Park et al., 2006a,b). Among antialgal substances isolated from bacteria are β-carbolines (e.g. harmane, harmine norharmane and norharmalane) (Kodani et al., 2002; Volk, 2005, 2006; Volk and Furkert, 2006; Volk and Mundt, 2007).
In this study, different strains of bacteria, isolated from phytoplankton samples collected from a eutrophic lake in Saudi Arabia, were screened for their antialgal activity. The strongest antialgal bacterium was checked for the presence of β-carbolines. In addition, the current study investigates the effect of β-carboline compounds, isolated from such an antialgal bacterium, on the selective inhibition of cyanobacterial growth.
2. Materials and methods
2.1. Tested algae
The tested cyanobacteria (Merismopedia tennuisssima, Oscillatoria limnetica) and green algae (Chorella vulgaris, Ankistrodesmus falcatus) used in the antialgal activity experiments, were isolated from the same place (Tendaha Lake), where the antialgal bacteria were isolated. All algal species were grown and maintained in BG-11 medium (Stanier et al., 1971) under the conditions of 25 °C and illumination of 30 μmol photon m−2 s−1.
2.2. Isolation and screening of antialgal bacteria
Surface water samples were collected during May 2011 from Tendaha Lake, southwest of Saudi Arabia. Samples were serially diluted with sterile distilled water. One hundred μl aliquots of each dilution were spread onto Nutrient Broth (NB) Agar (Difco) medium plates, containing 0.3% Beef Extract, 0.5% Peptone, 0.5% NaCl and 1.5% Agar, followed by incubation for 3 d at 30 °C. Colonies with different colony color and morphological shape were chosen for isolation. As a result, three different strains were isolated in this study. To test the antialgal activity of bacterial strains, a small sample of each colony was inoculated on plates of BG-11 medium containing a tested algal species. The plates were incubated for 7 d at 25 °C under illumination of 30 μmol photon m−2 s−1. A bacterial strain exhibiting antialgal activity against either species of cyanobacteria or eukaryotic algae was selected for further study.
Antialgal bacteria were inoculated and precultured into three sterilized tissue culture tubes containing 10 ml of liquid NB at 30 °C, at 200 rpm for 48 h. These 10 ml cultures were then transferred into three 250 ml Erlenmeyer flasks containing10 ml of liquid NB. Flasks were incubated for 3 d at 30 °C and 200 rpm. Bacterial cells were then removed by centrifugation (5,000g for 20 min at 4 °C) and filtration (0.2 μm pore-size membrane filter). Both the pellets and supernatants were ultrasonicated and extracted separately with ethyl acetate. Subsequently, the ethyl acetate layer was evaporated and stored at −20 °C until the next procedure.
The analysis of β-carboline compounds in bacterial extract was performed according to Kodani et al. (2002) using thin layer chromatography (TLC). Briefly, the ethyl acetate extracts were subjected to silica gel chromatography TLC (Kieselgel 60F254, 20 × 20 cm, Merck) and eluted with chloroform:methanol (9:1) according to Kodani et al. (2002). Ten μl of test solutions (cell extract and medium extract) and references solutions (harmane, harmine and norharmane) with concentration of 200 μg ml−1 methanol were applied to the plates. β-carboline compounds (harmane, harmine and norharmane) were noticed in UV light (UV 254 nm). TLC plates were scanned, digitized and analyzed by UN-scan-it gel scanning software (Silk Scientific, Orem, Utah).
2.3. Growth inhibition experiments
As the yield of harmine and norharmane isolated from Bacillus flexus SSZ01 during the present study was very low, it was decided to use harmine and norharmane obtained from Fluka and Sigma companies, respectively. Different concentrations of chemical harmine and norharmane (1, 10, 20, 30 μg ml−1), as well as crude bacillus extract containing the same concentrations of harmine and norharmane were added to each tested algal suspension in BG-11 medium (25 ml) in 50 ml Erlenmeyer flasks. The flasks were incubated at the same above conditions of algal growth for 7 d. The numbers of surviving cells were counted directly on a hemacytometer at a magnification of ×400. The antialgal activity of antialgal bacterium was calculated using the following equation (Kim et al., 2009).
2.4. Antialgal activity (%) = (1−Tt/Ct) × 100
where Tt and Ct are the cell numbers of treated and control cultures, respectively, of tested species. Algal cultures with no treatments were used as control. All experiments were carried out in triplicate, and results are given as the mean and standard deviation of raw data. The IC50 values (i.e. the concentrations of tested compounds that inhibited growth (cell numbers) by 50% relative to the controls) were calculated by those obtained from probity regression analysis.
2.5. Identification and sequence analysis of 16S rRNA gene
The antialgal bacterium was identified on the basis of an analysis of 16S rRNA gene sequences as reported in the previous study (Alamri, 2012).
2.6. Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences for Windows (SPSS, version 10.0, Chicago, IL, USA). Data are presented as means with their standard deviation. Statistical evaluation of the data was performed using a one-way ANOVA test. Values were considered statistically significant when p ⩽ 0.05.
3. Results
3.1. Screening of antialgal bacteria
The preliminary screening based on agar diffusion assay for antialgal activity of three morphologically different strains of bacteria isolated from phytoplankton samples collected from a eutrophic lake in Saudi Arabia, showed that only one strain (SSZ01) had antialgal activity against tested species of cyanobacteria. The bacterial 16S rRNA gene sequences showed close relationships between the strain SSZ01 and the genus Bacillus. The highest sequence similarity value (99%) was obtained between strain SSZ0l and B. flexus strain EMGA5 (Genbank accession number, EU602312). Thus, this strain was designated to be B. flexus and deposited in the Genbank with an accession number of GU112451.
The TLC analysis of ethyl acetate extract of antialgal bacterium (B. flexus), revealed that both bacterial cells and NB medium contained the β-carboline derivatives, harmine and norharmane with different concentrations compared to standard β-carbolins (Fig. 1). By digitizing the scanned TLC plates, the results showed that the extract of antialgal bacterim cells contained 10 and 15 μg harmine and norharmane ml−1, respectively while growth medium of this bacterium contained 5 and 8 μg ml−1 harmine and norharmane, respectively.
Figure 1.

TLC of standards of harmane (1), harmine (2), and norharmane (3), and of ethyl acetate extracts of Bacillus flexus cells (4) and growth medium (5).
3.2. Growth inhibition of algae β-carboline compounds produced by bacteria
The results of 7 d exposure of cyanobacteria and green algae to harmine and/or norharmane in a liquid medium showed that the crude extract of B. flexus cells containing harmine and norharmane inhibited the growth of cyanobacteria (M. tennuisssima and O. limnetica) as determined by cell number (Fig. 2). Although there was no significant variation in the growth as affected by this extract between these two species, Merismopedia was more sensitive than Oscillatoria. IC50 values of bacterial crude extract varied substantially between the two species (p < 0.05), i.e. IC50 value for Merismopedia was less than that of Oscillatoria (Table 1).
Figure 2.
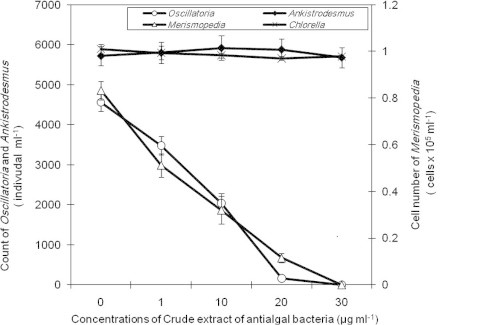
Effect of ethyl acetate extract of Bacillus flexus on the cell number of tested species of cyanobacteria and green algae.
Table 1.
IC50 values (μg ml−1) obtained for crude extract of antialgal Bacillus sp., pure harmine and norharmane toward cyanobacteria and green algae species screened in this study.
| Tested algae | IC50 (μg ml−1) |
||
|---|---|---|---|
| Crude extract | Harmine | Norharmane | |
| M. tennuisssima | 5.3 ± 0.9 | 11.62 ± 1.7 | 13.2 ± 1.2 |
| O. limnetica | 9.6 ± 1.1 | 11.25 ± 1.3 | 12.5 ± 1.4 |
| A falcatus | – | – | – |
| C. vulgaris | – | – | – |
The pure materials of either harmine or norharmane were less toxic to tested cyanobacteria species than crude bacterial extract containing harmine and norharmane. These β-carboline analogs decreased the cell number and chl.a contents in treated cultures of tested cyanobacteria compared to controls (Figs. 3 and 4). In contrast to crude extract, IC50 values of either harmine or norharmane did not differ significantly between the two cyanobacteria species (M. tennuisssima and O. limnetica) (Table 1). However, harmine was more toxic to cyanobacteria than norharmane (as shown by higher IC50 values). Furthermore, the antialgal activities of either crude bacterial extract or pure harmine/norharmane toward tested cyanobacteria increased with increasing the concentration of antialgal substance. The strongest antialgal activity for both tested species of cyanobacteria was obtained at a concentration of 30 μg ml−1 (Table 2). However, the antialgal activities differed significantly among crude extract, harmine and norharmane for both species of cyanobacteria at the lowest concentrations only (1, 10 μg ml−1) (p < 0.05), but no significant variation in these activities was observed at the highest concentrations (20, 30 μg ml−1) used during the present study. In contrast to cyanobacteria, green algae (A. falcatus and C. vulgaris) had not been affected by either bacterial crude extract or pure β-carboline compounds (harmine, norharmane). The cell number of tested species of green algae did not differ significantly between treated and control cultures (Figs. 3 and 4).
Figure 3.
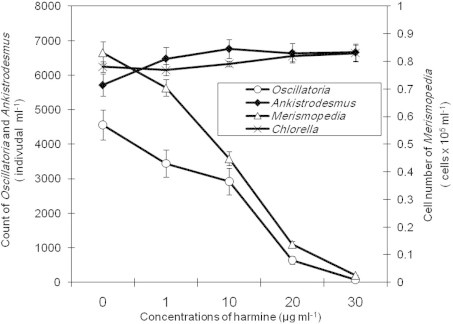
Effect of a pure harmine of Bacillus flexus on the cell number of tested species of cyanobacteria and green algae.
Figure 4.
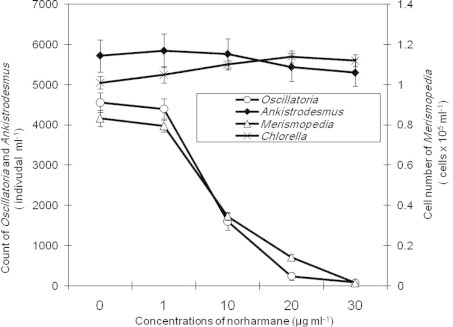
Effect of a pure norharmane of Bacillus flexus on the cell number of tested species of cyanobacteria and green algae.
Table 2.
Antialgal activity (%) obtained for different concentrations (μg ml−1) of crude extract of antialgal Bacillus flexus, pure harmine and norharmane toward cyanobacteria species screened in this study.
| Tested species/antialgal concentrations | Antialgal activity (%) |
||
|---|---|---|---|
| Crude extract | Harmine | Norharmane | |
| M. tennuisssima | |||
| 0 | 0 | 0 | 0 |
| 1 | 38.5 | 15.4 | 4.6 |
| 10 | 61.5 | 46.2 | 41.6 |
| 20 | 86.2 | 83.4 | 83.1 |
| 30 | 100 | 96.9 | 98.4 |
| O. limnetica | |||
| 0 | 0 | 0 | 0 |
| 1 | 45.6 | 24.6 | 3.5 |
| 10 | 77.2 | 59.6 | 64.9 |
| 20 | 96.5 | 86 | 94.7 |
| 30 | 100 | 98.2 | 98.2 |
4. Discussion
Bacteria with antialgal activities were reported to belong to Cytophaga-Flavobacteria-Bacteroides CFB group (50%) and γ-Proteobacteria (45%), while the remaining strains represent the Gram-positive genera Micrococcus, Bacillus and Planomicrobium (Fukuyo et al., 2002; Mayali and Azam, 2004; Hare et al., 2005). Some Bacillus strains were reported to completely inhibit the growth of bloom-forming cyanobacteria and green algae in highly eutrophic lakes (Ahn et al., 2003; Mu et al., 2007). In the present study, B. flexus was isolated as bacterial strain with an antialgal activity against cyanobacteria, from a Saudi eutrophic pond containing toxic cyanobacterial blooms.
Antialgal bacteria may inhibit the algal growth effectively through direct attack that is required for cell-to-cell contact (Imai et al., 1993) or indirect attack by the production of extracellular compounds (Fukami et al., 1992; Imai et al., 1993; Wang et al., 2005). In this study, B. flexus strain might inhibit the growth of cyanobacteria indirectly by the production of two β-carboline derivatives (harmine and norharmane). β-carboline derivatives have been described to occur naturally in some higher plants (Allen and Holmstedt, 1980; Bourke et al., 1992; Cheng and Mitchelson, 1997), and in Indonesian sponge (Rao et al., 2003). Recently, these compounds were isolated and identified in some species of fresh and marine cyanobacteria (Volk, 2005, 2006, 2008; Volk and Furkert, 2006; Volk and Mundt, 2007; Mohamed, 2013), but their production in bacteria was confined to a few species including the gliding bacterium Myxobacter (Böhlendorf et al., 1996), Pseudomonas (Kodani et al., 2002) Pseudoalteromonas piscicida (Zheng et al., 2005) and Enterococcus faecium (Aassila et al., 2003).
The bacterial crude extract containing the β-carbolines, harmine and norharmane, showed antagonistic activity against cyanobacteria, affecting the cell division in these species. These results confirmed the results of previous studies reporting the inhibition of many species of cyanobacteria by norharmane and other related β-carbolines. Kodani et al. (2002) reported the antialgal activity of β-carbolines, harmane and norharmane, produced by Pseudomonas sp. against the cyanobacteria Anabaena cylindrica, A. variabilis, Anacystis marina, Microcystis aeruginosa, M. viridis and Oscillatoria agardhii at a concentration of 30 μg disk−1. Volk (2006) also investigated the activity of three β-carbolines harmane, norharmane and norharmalane toward some species of cyanobacteria (Arthrospira laxissim, Chroococcus minutus, Nostoc carneum, Nostoc insulare, Synechocystis aquatilis, Synechococcus species), and he found that all these compounds were cytotoxic against the cyanobacterial test organisms in low quantities (0.5–18.0 μg).
The results of the present study showed that both crude bacterial extracts containing harmine, norharmane as well as synthetic harmine and norharmane exhibited an antialgal activity against cyanobacteria species at different IC50s. The crude extract was more toxic to cyanobacteria (IC50 = 5.3–9.6 μg ml−1) than harmine and norharmane (IC50 = 11.25–13.2 μg ml−1). The high toxicity of crude extract may be due to the synergistic effect of both harmine and norharmane present together in the extract. Previously, it has been stated that the inhibitory effect of algicides is likely to be a synergistic effect of various compounds (Ball et al., 2001; Ferrier et al., 2005). On the other hand, the results of this study revealed differences in the antialgal activity between harmine and norharmane toward tested species of cyanobacteria; where harmine was of a significant higher toxicity than norharmane. These results are in conformity with those obtained by Volk (2006) reporting differences in the activity of norharmane and harmane (the 1-methyl-derivate of norharmane) toward the cyanobacteria, Arthrospira laxissima and Synechococcus sp., harmane was of a significant higher toxicity than norharmane. Based on the hypothesis of Volk (2006) that the hydrophobic alkaloid bases could easily penetrate the external plasma membrane of cells than the hydrophilic ones, a higher penetration rate of the more hydrophobic harmine could also explain the higher activity of this β-carboline in comparison to the less hydrophobic and unmethylated norharmane.
In contrast to cyanobacteria, the tested species of green algae for antialgal activity of harmine and norharmane during the present study did not show any growth inhibition toward these compounds. Accordingly, previous studies showed that β-carbolines have antialgal activity toward cyanobacteria with no remarkable effect on green algae (Kodani et al., 2002). This finding indicates that these β-carboline compounds have selective inhibition of cyanobacterial growth rather than green algae. The potential for selectively controlling cyanobacteria without affecting other algal species could be useful for the preservation of ecosystems with the least impact on the environment.
The results of the present study also showed that B. flexus not only contains the β-carbolines, harmine and norharmane, within the cells, but also can release considerable amounts of these compounds into culture medium. This study is not the first to demonstrate that bacteria and cyanobacteria can excrete such antialgal metabolites into the culture media. Previous studies detected β-carboline derivatives in culture media of bacteria (Kodani et al., 2002) and cyanobacteria (Volk, 2005, 2006, 2008; Volk and Furkert, 2006). These results indicate the potential excretion of these antialgal compounds into the aquatic environment and thus function naturally as allelopathic substances.
5. Conclusion
This study is the first to report that B. flexus can produce antialgal β-carbolines (harmine and norharmane). This bacterium and its β-carbolines inhibited the growth of cyanobacteria only, but not green algae, which is an advantage of bacterial agents in removing undesirable algal species but providing niches for other beneficial species to colonize and thrive.
Acknowledgment
The author would like to acknowledge and thank King Khalid University for supporting this study.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aassila H., Bourguet-Kondracki M.L., Rifai S., Fassouane A., Guyot M. Identification of harman as the antibiotic compound produced by a tunicate-associated bacterium. Marine Biotechnol. 2003;5:163–166. doi: 10.1007/s10126-002-0060-7. [DOI] [PubMed] [Google Scholar]
- Ahn C.Y., Joung S.H., Jeon J.W., Kim H.S., Yoon B.D., Oh H.M. Selective control of cyanobacteria by surfactin-containing culture broth of Bacillus subtilis C1. Biotechnol. Lett. 2003;25:1137–1142. doi: 10.1023/a:1024508927361. [DOI] [PubMed] [Google Scholar]
- Alamri S. Biodegradation of microcystin-RR by Bacillus flexus isolated from a Saudi freshwater lake. Saudi J. Biol. Sci. 2012;19:435–440. doi: 10.1016/j.sjbs.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J.R., Holmstedt B.R. The simple-carboline alkaloids. Phytochem. 1980;19:1573–1582. [Google Scholar]
- Amaro A.M., Fuentes M.S., Ogalde S.R., Venegas J.A., Suarez-Isla B.A. Identification and characterization of potentially algal-lytic marine bacteria strongly associated with the toxic dinoflagellate Alexandrium catenella. J. Eukaryot. Microbiol. 2005;52:191–200. doi: 10.1111/j.1550-7408.2005.00031.x. [DOI] [PubMed] [Google Scholar]
- Anderson D., Glibert P., Burkholder J. Harmful algal blooms and eutrophication: nutrient sources, composition, and consequences. Estuaries. 2002;25:704–726. [Google Scholar]
- Apeldoorn M., Egmond H., Speijers G., Bakker G. Toxins of cyanobacteria. Mol. Nutr. Food Res. 2007;51:7–60. doi: 10.1002/mnfr.200600185. [DOI] [PubMed] [Google Scholar]
- Ball A., Williams M., Vincent D., Robinson J. Algal growth control by a barley straw extract. Bioresour. Technol. 2001;77:177–181. doi: 10.1016/s0960-8524(00)00148-6. [DOI] [PubMed] [Google Scholar]
- Böhlendorf B., Bedorf N., Jansen R., Trowitzsch-Kienast W., Höfle G., Forche E., Gerth K., Irschik H., Kunze B., Reichenbach H. Antibiotics from gliding bacteria, LXXIII indole and quinoline derivatives as metabolites of tryptophan in myxobacteria. Liebigs Ann. 1996;1:49–53. [Google Scholar]
- Bourke C.A., Stevens G.R., Carrigan M.J. Locomotor effects in sheep of alkaloids identified in Australien Tribulus terrestris. Aust. Vet. J. 1992;69:163–165. doi: 10.1111/j.1751-0813.1992.tb07502.x. [DOI] [PubMed] [Google Scholar]
- Boyd C., Massaut L. Risks associated with the use of chemicals in pond aquaculture. Aquaculture Eng. 1999;20:113–132. [Google Scholar]
- Cheng J., Mitchelson K.R. Improved separation of six harmane alkaloids by high-performance capillary electrophoresis. J. Chromatogr. 1997;761:297–305. [Google Scholar]
- Codd G.A. Cyanobacterial toxins: their occurrence in aquatic environments and significance to health. Bull. Inst. Oceanogr. Monaco. 1999;19:483–500. [Google Scholar]
- Falconer I., Bartram J., Chorus I., Kuiper-Goodman T., Utkilen H., Burch M., Codd G. Safe levels and safe practices. In: Chorus I., Bartram J., editors. Toxic Cyanobacteria in Water: a Guide to their Public Health Consequences, Monitoring and Management. WHO/ Spon; London: 1999. pp. 155–178. [Google Scholar]
- Ferrier M., Butler B., Terlizzi D., Lacouture R. The effects of barley straw (Hordeum vulgare) on the growth of freshwater algae. Bioresour. Technol. 2005;96:1788–1795. doi: 10.1016/j.biortech.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Fukami K., Yuzawa A., Nishijima T., Hata Y. Isolation and properties of a bacterium inhibiting the growth of Gymnodinium nagasakiense. Nippon Suisan Gakkai-shi. 1992;58:1073–1077. [Google Scholar]
- Fukuyo Y., Imai I., Kodama M., Tamai K. Red tides and other harmful algal blooms in Japan. In: Taylor F.J., Trainer V.L., editors. Harmful Algal Blooms in the PICES Region of the North Pacific. North Pacific Marine Science Organization (PICES); Sidney, Canada: 2002. pp. 7–20. [Google Scholar]
- Garry R.T., Hearing P., Cosper E.M. Characterization of a lytic virus infectious to the bloom-forming microalga Aureococcus anophagefferens (Pelagophyceae) J. Phycol. 1998;34:616–621. [Google Scholar]
- Hallegraeff G.M. Harmful algal blooms: a global overview. In: Hallegraeff G.M., Anderson D.M., Cembella A.D., editors. Manual on Harmful Marine Microalgae. UNESCO Publishing; Paris: 2003. pp. 25–49. [Google Scholar]
- Hare C.E., Demir E., Coyne K.J., Cary S.C., Kirchman D.L., Hutchins D.A. A bacterium that inhibits the growth of Pfiesteria piscicida and other dinoflagellates. Harmful Algae. 2005;4:221–234. [Google Scholar]
- Imai I., Ishida Y., Hata Y. Killing of marine phytoplankton by a gliding bacterium Cytophaga sp., isolated from the coastal sea of Japan. Marine Biol. 1993;116:527–532. [Google Scholar]
- Imai I., Ishida Y., Sakaguchi K., Hata Y. Antialgal marine-bacteria isolated from northern Hiroshima Bay. Jpn. Fish. Sci. 1995;61:628–636. [Google Scholar]
- Karan V., Vitorovic S., Tutundzjic V., Poleksic V. Functional enzymes activity and gill histology of carp after copper sulfate exposure and recovery. Ecotoxicol. Environ. Saf. 1998;40:49–55. doi: 10.1006/eesa.1998.1641. [DOI] [PubMed] [Google Scholar]
- Kim Y.S., Lee D.S., Jeong S.Y., Lee W.J., Lee M.S. Isolation and characterization of a marine antialgal bacterium against the harmful raphidophyceae Chattonella marina. J. Microbiol. 2009;47:9–18. doi: 10.1007/s12275-008-0141-z. [DOI] [PubMed] [Google Scholar]
- Kodani S., Imoto A., Mitsutani A., Murakami M. Isolation and identification of the antialgal compound, harmane (1-methyl-β-carboline), produced by the antialgal bacterium, Pseudomonas sp. K44-1. J. Appl. Phycol. 2002;14:109–114. [Google Scholar]
- Kuiper-Goodman T., Falconer I., Fitzgerald J. Human health aspects. In: Chorus I., Bartram J., editors. Toxic Cyanobacteria in Water: Aguide to their Public Health Consequences, Monitoring and Management. WHO/Spon; London: 1999. pp. 113–153. [Google Scholar]
- Lam A.K., Prepas E.E., Spink D., Hrudey S.E. Chemical control of hepatotoxic phytoplankton blooms: implications for human health. Water Res. 1995;29:1845–1854. [Google Scholar]
- Long B.M., Carmichael W. Marine cyanobacterial toxins. In: Hallegraeff G.M., Anderson D.M., Cembella A.D., editors. Manual on Harmful Marine Microalgae. UNESCO; Venice: 2003. pp. 279–296. [Google Scholar]
- Lovejoy C., Bowman J.P., Hallegraeff G.M. Antialgal effects of a novel marine Pseudoalteromonas isolate (class Proteobacteria, gamma subdivision) on harmful algal bloom species of the genera Chattonella, Gymnodinium, and Heterosigma. Appl. Environ. Microbiol. 1998;64:2806–2813. doi: 10.1128/aem.64.8.2806-2813.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayali X., Azam F. Antialgal bacteria in the sea and their impact on algal blooms. J. Eukaryot. Microbiol. 2004;51:139–144. doi: 10.1111/j.1550-7408.2004.tb00538.x. [DOI] [PubMed] [Google Scholar]
- Meepagala K., Schader K., Wedge D., Duke S. Antialgal and antifungal compounds from the roots of Ruta graveolens and synthesis of their analogs. Phytochemical. 2005;66:2689–2695. doi: 10.1016/j.phytochem.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Mohamed Z.A. Allelopathic activity of the norharmane-producing cyanobacterium Synechocystis aquatilis against cyanobacteria and microalgae. Oceanol. Hydrobiol. Stud. 2013;42:1–7. [Google Scholar]
- Mohamed Z.A., Al-Shehri A.M. Microcystins in groundwater wells and their accumulation in vegetable plants irrigated with contaminated waters in Saudi Arabia. J. Hazard. Mater. 2009;172:310–315. doi: 10.1016/j.jhazmat.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Mu R.M., Fan Z.Q., Pei H.Y., Yuan X.L., Liu S.X., Wang X.R. Isolation and algae-lysing characteristics of the antialgal bacterium B5. J. Environ. Sci. 2007;19:1336–1340. doi: 10.1016/s1001-0742(07)60218-6. [DOI] [PubMed] [Google Scholar]
- Oliver R.L., Ganf G. Freshwater blooms. In: Whitton B.A., Potts M., editors. The Ecology of Cyanobacteria: their Diversity in Time and Space. Kluwer Academic Publishers; Dordrecht: 2000. pp. 149–194. [Google Scholar]
- Park M.H., Han M.S., Ahn C.Y., Kim H.S., Yoon B.D., Oh H.M. Growth inhibition of bloom-forming cyanobacterium Microcystis aeruginosa by rice straw extract. Lett. Appl. Microbiol. 2006;43:307–312. doi: 10.1111/j.1472-765X.2006.01951.x. [DOI] [PubMed] [Google Scholar]
- Park M.H., Hwang S.J., Ahn C.Y., Kim B.H., Oh H.M. Screening of seventeen oak extracts for the growth inhibition of the cyanobacterium Microcystis aeruginosa Kutz. em. Elenkin. Bull. Environ. Contam. Toxicol. 2006;77:9–14. doi: 10.1007/s00128-006-1025-8. [DOI] [PubMed] [Google Scholar]
- Rao K.V., Santarsiero B.D., Mesecar A.D., Schinazi R.F., Tekwani B.L., Hamann M.T. New manzamine alkaloids with activity against infectious and tropical parasitic diseases from an Indonesian sponge. J. Nat. Prod. 2003;66:823–828. doi: 10.1021/np020592u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R.Y., Kunisawa R., Mandel M., Cohen-Baziere G. Purification and properties of unicellular blue-green algae (order Chroococcales) Bacteriol. Rev. 1971;35:171–205. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J., Yang X., Zheng T., Tian Y., Jiao N., Cai L., Hong H. Isolation and characterization of marine antialgal bacterium against the toxic dinoflagellate Alexandrium tamarense. Harmful Algae. 2007;6:799–810. [Google Scholar]
- Volk R. Screening of microalgal culture media for the presence of antialgal compounds and isolation and identification of two bioactive metabolites excreted by the cyanobacteria Nostoc insulare and Nodularia harveyana. J. Appl. Phycol. 2005;17:339–347. [Google Scholar]
- Volk R. Antialgal activity of several cyanobacterial exometabolites. J. Appl. Phycol. 2006;18:145–151. [Google Scholar]
- Volk R. Screening of microalgae for species excreting norharmane a manifold biologically active indole alkaloid. Microbiol. Res. 2008;163:307–313. doi: 10.1016/j.micres.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Volk R., Furkert F. Antialgal, antibacterial and antifungal activity of two metabolites produced and excreted by cyanobacteria during growth. Microbiol. Res. 2006;161:180–186. doi: 10.1016/j.micres.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Volk R., Mundt S. Cytotoxic and non-cytotoxic exometabolites of the cyanobacterium Nostoc insulare. J. Appl. Phycol. 2007;19:55–62. [Google Scholar]
- Wang X.L., Gong L.Y., Liang S.K., Han X.R., Zhu C.J., Li Y.B. Antialgal activity of rhamnolipid biosurfactants produced by Pseudomonas aeruginosa. Harmful Algae. 2005;4:433–443. [Google Scholar]
- Yoshinaga I., Kim M.C., Katanozaka N., Imai I., Uchida A., Ishida Y. Population structure of antialgal marine bacteria targeting the red tide forming alga Heterosigma akashiwo (Raphidophyceae), determined by restriction fragment length polymorphism analysis of the bacterial 16S ribosomal RNA genes. Mar. Ecol. Prog. Ser. 1998;170:33–44. [Google Scholar]
- Zheng L., Chen H., Han X., Lin W., Yan X. Antimicrobial screening and active compound isolation from marine bacterium NJ6-3-1 associated with the sponge Hymeniacidon perleve. World J. Microbiol. Biotechnol. 2005;21:201–206. [Google Scholar]



