Abstract
Ziziyphus nummularia (family: Rhamnaceae) is a thorny small bush, grows in abundance in the grazing lands of the arid areas of Rajasthan, India. It is an important ethnomedicinal plant of the Thar Desert; local inhabitants use every part of the plant as medicine. Kernels are prescribed in pregnancy as soporific, antiemetic and for relieving abdominal pain. The insect gall is powered and given orally with water to cure bone fracture. Crushed root is applied on the paining shoulder of the bullock. The decoction of leaves is used for the treatment of cough and cold; leaves are also regarded as diaphoretic and prescribed in typhoid. Paste of leaves is used for healing of cuts, boils and cutaneous disease. It is widely used in pain and inflammatory conditions.
Z. nummularia contains a unique group of alkaloids known as cyclopeptide alkaloids, in continuation of our work carried out on the leaves of Z. nummularia, present study was initiated to explore antiinflammatory and analgesic potential of cyclopeptide alkaloids isolated from the leaves of Z. nummularia (IFZN). Anti-inflammatory activity was tested against rat paw oedema, mouse peritonitis and cotton pellet granuloma. For screening of analgesic activity, acetic acid induced writhing, tail flick and hot plate test were performed.
IFZN 30 mg/kg shows the anti-oedematogenic effect against paw oedema induced by carrageenan, dextran, serotonin and histamine; IFZN 20 and 30 mg/kg were found to have highly significant anti-nociceptive effects.
Result of pharmacological studies indicated that IFZN is a potent and efficacious analgesic agent. The analgesic activity of IFZN is mediated by the peripheral as well as central pathways.
Keywords: Ziziyphus nummularia, Analgesic, Anti-inflammatory, Cyclopeptide alkaloids, Cotton pellet granuloma, Acetic acid induced writhing, Tail flick, Hot plate
1. Introduction
Ziziyphus nummularia (family: Rhamnaceae) is a thorny small bush, grows in abundance in the grazing lands of the arid areas of Rajasthan, India (Anonymous, 1989). It is an important ethnomedicinal plant of the Thar Desert; local inhabitants use every part of the plant as medicine.
Kernels are prescribed in pregnancy as a soporific, antiemetic and for relieving abdominal pain (Singh and Pandey, 1998). Filtrate of fruit decoction is used for bathing to cure fever caused due to heat stroke (Kumar et al., 2008; Meena and Yadav, 2010). Extract of fruit is also used for the treatment of infertility (Muhammad and Khan, 2008).
Root is useful in the treatment of diarrhoea and dysentery (Jadhav, 2006). The young branches and roots are used as toothbrushes for preventing decay, pyorrhoea and other diseases of the teeth (Jain et al., 2005; Upadhyay et al., 2011). The insect gall is powered and given orally with water to cure bone fracture. Crushed root is applied on the paining shoulder of the bullock, used in ploughing (Bhattacharjee, 2004; Jadeja et al., 2006).
The decoction of leaves of Z. nummularia is used for the treatment of cough and cold, leaves are regarded as diaphoretic and prescribed in typhoid. Paste of leaves is used for healing of cuts, boils and cutaneous disease (Meena and Yadav, 2010). It is also used in pain and inflammatory conditions (Kumar et al., 2008; Goyal et al., 2011).
We have reported anti-inflammatory and analgesic effects of an ethanolic extract of leaves of Z. nummularia (EZN) against rat paw oedema, mouse peritonitis, acetic acid induced writhing and tail flick test, respectively (Goyal et al., 2012). Similarly, alcoholic extract of leaves has also been reported for topical anti-inflammatory and wound healing effects (Hasan Soliman Yusufoglu, 2011).
Phytochemical analysis of Z. nummularia revealed the presence of number of phytoconstituents such as flavonoids, tannins, sterols, saponins, pectin, glycosides, alkaloids and triterpenoic acids. However, cyclopeptide alkaloids are regarded as active constituents responsible for pharmacological actions of Z. nummularia (Morel Ademir et al., 2009; Hasan Soliman Yusufoglu, 2011; Goyal et al., 2012). Therefore, in continuation of our work carried out on leaves of Z. nummularia, present study was initiated to explore the anti-inflammatory and analgesic potential of cyclopeptide alkaloids isolated from the leaves of Z. nummularia (IFZN). Receptor docking studies were also conducted in addition to in vivo pharmacological studies to understand the possible mechanism of action of IFZN.
2. Materials and methods
2.1. Plant material
The leaves of Z. nummularia were collected from Jodhpur, Rajasthan. Plant was identified by Taxonomists of the Botanical Survey of India (BSI), Jodhpur, and a voucher specimen BSI/AZC/MG-1 was deposited for future reference.
2.2. Extraction of IFZN
IFZN was isolated as per the method described by Ma et al. (2008). One kilogram of dried and powdered leaves of Z. nummularia was extracted in a soxhlet extractor for 24 h with hexane. The residue was extracted with ethanol for 24 h and evaporated to dryness by rotary evaporation. The obtained ethanolic extract was partitioned between 5% hydrochloric acid (60 ml) and ether (50 ml). The aqueous acid solution was extracted further three times with chloroform (60 ml × 3) in the presence of ammonia hydrate (quantity sufficient to produce pH 9.0). The cyclopeptide alkaloid fractions in the chloroform solution were combined, filtered through Whatman No. 1 filter paper, and concentrated using a rotary vacuum evaporator (Ma et al., 2008). The yield of IFZN was found to be 0.02%. The presence of a cyclopeptide alkaloid was confirmed by identification tests, TLC and GCMS analysis.
2.3. Animals
Healthy Wistar rats of both sexes weighing 180–200 g and Swiss mice weighing 20–24 g were used. The animals had free access to a standard commercial diet and water ad libitum and were kept in rooms maintained at 25 °C in a 12-h light/dark cycle. The animals were used after an acclimatization period of 7 days to the laboratory environment. The experiments were performed during the light portion (0800–1600 h). The experimental protocol and procedures used in this study were approved by IAEC of the Lachoo Memorial College of Science and Technology, Pharmacy Wing, Jodhpur vide the approval number LMC/OFFICE/2008/IAEC/17/5/2008/PHD/2.
2.4. Chemicals and drugs
Carrageenan (S.D. Fine Chemicals Limited, Bombay), histamine, serotonin, dextran (Sigma, USA), indomethacin (Recon, Bangalore), aspirin (USV Bombay) and morphine (Bio E) were used in the study.
2.5. Acute toxicity study
Mice were kept on overnight fasting and water was withheld for 3–4 h before the administration of the test compound. Following the period of fasting, the animals were weighed and divided into five groups, three mice in each group. The control group received normal saline 1 ml/kg BW by gavage while the treatment groups received IFZN in a dose of 5 mg/kg BW. Immediately after dosing, the mice were observed continuously for 4 h for symptoms of toxicity like motor activity, tremors, convulsions, tonic extension, muscle spasm, loss of righting reflex, ataxia, sedation, hypnosis, lacrimation, diarrhea, salivation and writhing. Mice were then kept under observation up to 48 h for any mortality, next higher dose 50 and 300 mg/kg. At the dose of 300 mg/kg, two out of three animals died so the dose was lowered to 200 mg/kg and tested on six mice (OECD, 2001).
Body weight and chow consumption of mice were recorded for 14 days. All test animals (including those that die during the test) were subjected to gross necropsy. All gross pathological changes were recorded for each animal.
2.6. Anti-inflammatory activity
2.6.1. Carrageenan-induced rat paw oedema test
Carrageenan-induced rat paw oedema is the most commonly used technique for the screening of anti-inflammatory drugs. The test is based upon the ability of the drug to inhibit the acute edema produced in the hind paw of the rat after injection of a phlogistic agent such as carrageenan, dextran, histamine or serotonin.
Five groups of rats, six in each group were selected for the study. Different groups were treated with saline; indomethacin 10 mg/kg BW and IFZN at doses of 10, 20, 30 mg/kg BW per oral. The animals were treated with the extract 1.0 h before the administration of carrageenan. Acute inflammation was produced by the subplantar administration of 0.1 ml of 1% carrageenan in normal saline in the right hind paw of the rats. The paw volume was measured at 0, 1.0 and 3.0 h after carrageenan injection using plethysmometer (Winter and Porter, 1957). The anti-inflammatory effect was calculated by the following equation:
Anti-inflammatory activity (%) = (1−D/C) × 100.
Where D represents the percentage difference of the paw volume of treated groups and C represents the percentage difference of volume in the control group (Suleyman et al., 1991).
2.6.2. Dextran, histamine and serotonin-induced paw oedema
The rats were treated in a manner similar to that of carrageenan-induced paw oedema models; dextran (0.1 ml, 1% w/v in normal saline), 0.1 ml of freshly prepared histamine (1 mg/kg BW) and serotonin (1 mg/kg BW) were used in the place of carrageenan (Winter and Porter, 1957; Winter et al., 1962).
2.6.3. Cotton pellets-induced granuloma
Cotton pellets induced granuloma are used for the evaluation of steroidal and nonsteroidal anti-inflammatory drugs. The amount of newly formed connective tissue (granuloma) can be measured by this method.
Five groups of mice, six in each group were selected for the study. Mice were anesthetized and fur of their back was removed with an electric razor. Subsequently, an incision was made in the lumbar region and with the help of a blunted forceps; subcutaneous tunnel was made on right side in the scapular region. Sterilized cotton pellet weighing 15 mg was placed in the pouch formed in the scapular region. The different groups of mice were treated with saline; indomethacin 10 mg/kg and IFZN at doses of 10, 20, 30 mg/kg BW per oral for seven consecutive days from the day of cotton pellet implantation. The mice were anaesthetized on the eighth day and cotton pellets were removed surgically (Penn and Ashford, 1963). Pellets were made free from extraneous tissues, moist pellets were weighed and then dried at 60 °C for 24 h, after that dried pellets were weighed again. Increment in the weight of the pellets was taken as a measure of granuloma formation.
2.6.4. Mouse carrageenan peritonitis
Carrageenan induced peritonitis in mice is used for accessing the effect of anti-inflammatory drugs on fluid extravasation and leukocyte migration induced due to inflammation (Griswold et al., 1987).
Five groups of mice, six in each group were selected for the study. Different groups of mice were treated with saline; indomethacin 10 mg/kg BW and IFZN at doses of 10, 20, 30 mg/kg BW per oral. One hour later peritonitis was induced by intraperitoneal injection of carrageenan (0.25 ml, 0.75% w/v in saline). After 4 h, the animals were sacrificed and peritoneal fluid collected for leukocyte count (Griswold et al., 1987).
2.7. Analgesic activity
Pain is frequently associated with inflammation; it is the final product of a complex information processing network involving the central and peripheral pathways. Drugs with anti-inflammatory effect very often possess analgesic properties as well. Analgesic activity of IFZN was evaluated against acetic acid-induced writhing response, which is a method for measuring peripheral analgesic activity. Central analgesic effect was measured by the tail flick test and hot plate.
2.7.1. Acetic acid-induced writhing response in mice
Five groups of mice, six in each group were selected for the study. The different groups were treated with saline; aspirin 100 mg/kg and IFZN at doses of 10, 20, 30 mg/kg BW per oral. Thirty minutes after the drug treatment, 0.1 ml of a 0.6% solution of acetic acid was injected intraperitoneally and the number of writhes during the following 30 min were counted (Koster et al., 1959). Percentage inhibition was calculated by the following equation: Percentage inhibition = (1−D/C) × 100
Where D represents the number of writhes of treated groups and C represents the number of writhes of the control group.
2.7.2. Tail flick test
The tail flick test in mice is used for screening of centrally acting analgesic drugs. Mice are placed into cages leaving the proximal third of the tail exposed to the radiant heat source. Within a few seconds the animal flicks the tail aside or tries to escape. The time until this reaction occurs is measured.
Six groups of mice, six in each group were selected for the study. Mice which had 3.5–4.5 s baseline latency of tail flick were included in study. First four groups were treated with saline and IFZN at doses of 10, 20, 30 mg/kg BW per oral, fifth group was treated with a subcutaneous injection of morphine 5 mg/kg BW and the sixth group was treated with naloxone (1 mg/kg BW i.p.) followed by IFZN 30 mg/kg BW per oral. IFZN, morphine and naloxane were given 60, 30 and 15 min before the test, respectively. Mice were screened by placing their proximal third of the tail to the radiant heat source maintained at 50 ± 2 °C, latency for tail flick was recorded. A cutoff time of 15 s was used to avoid damage to the tail (D’Armour and Smith, 1941).
2.7.3. Hot plate test
Hot plate test is specifically used for the screening of centrally acting analgesics. Administration of centrally acting analgesics causes prolongation of the latency times of response i.e. jumping, withdrawal of the paws and licking of the paws.
Five groups of mice, six in each group were selected for the study. The mice which reacted within 15 s and which did not show a large variation when tested on two separate occasions were included in study. First four groups were treated with saline and IFZN at doses of 10, 20, 30 mg/kg BW per oral, fifth group treated with subcutaneous injection of morphine 5 mg/kg BW. IFZN and morphine were given 60 and 30 min before the test, respectively. Mice were screened by placing them on a hot plate maintained at 55 ± 2 °C and recorded the reaction time in seconds for the licking of paw or jumping (Turner, 1965). A cutoff time of 15 s was used to avoid damage to the paw.
2.8. Docking study
Docking studies were performed against X-ray crystal structure of the μ-opioid receptor (2IQO) using LeadIT FlexX Version: 1.0.1 (BioSolveIT GmbH, Sankt Augustin, Germany).
Structures of ligands (Morphine, Nummularine and Ziziphine) were drawn using Marvin Sketch-5.0.6.1 (ChemAxon Ltd.). The 3D-geometry optimized ligands were saved in .sdf format. Crystal structure of the μ-opioid receptor (2IQO) was downloaded from the Protein data bank (Rscb, 2012).
Docking protocol: Triangle matching base fragment placement and all the poses were used for docking. 200 solutions per iteration and 200 solutions per fragmentation were used.
2.9. Statistical analysis
Results were statistically analysed by ANOVA, followed by the Student’s t-test. The results were expressed as mean ± standard error mean (SEM). P < 0.05 was considered significant.
3. Results
3.1. Acute toxicity study
According to OECD guidelines, IFZN falls in category-3 having LD50 200 mg/kg.
Body weight, RBC and WBC count, and weight of heart and liver of treated mice were found to be well within the limit. However, CNS depression and reduction in locomotor activity were observed in mice treated with IFZN 200 and 300 mg/kg.
Therapeutic range was considered between 1/20 and 1/5 times of LD50. Accordingly, 10, 20 and 30 mg/kg BW of IFZN were selected as doses for pharmacological studies.
3.2. Anti-inflammatory activity
IFZN shows the dose-dependent anti-oedematogenic effects against paw oedema induced by carrageenan, dextran, serotonin and histamine Fig. 1. Results of the paw oedema test are summarized in Table 1.
Figure 1.
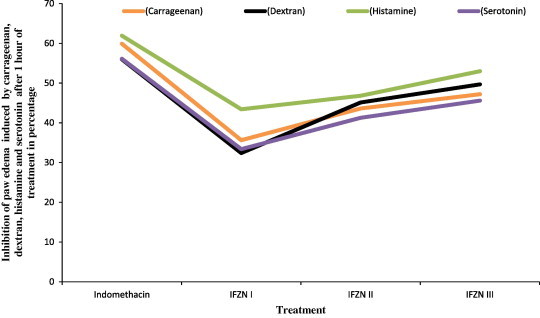
Percentage of inhibition of paw oedema produced by IFZN compared to the control after one hour of treatment; against carrageenan, dextran, histamine and serotonin induced rat paw oedema.
Table 1.
Effect of IFZN on carrageenan, dextran, histamine and serotonin induced rat paw oedema.
| Treatment | Dose mg/kg BW (p.o.) | Increase in paw oedema in milliliters (mean + SEM) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Carrageenan |
Dextran |
Histamine |
Serotonin |
||||||
| 1 h | 3 h | 1 h | 3 h | 1 h | 3 h | 1 h | 3 h | ||
| Control | 5 (ml/kg) | 0.90 ± 0.02 | 0.94 ± 0.03 | 0.92 ± 0.02 | 0.98 ± 0.05 | 0.94 ± 0.01 | 1.02 ± 0.05 | 0.95 ± 0.01 | 0.99 ± 0.03 |
| Indomethacin | 10 | 0.36 ± 0.03⁎ | 0.25 ± 0.03⁎ | 0.41 ± 0.02⁎ | 0.25 ± 0.04⁎ | 0.36 ± 0.01⁎ | 0.25 ± 0.01⁎ | 0.42 ± 0.01⁎ | 0.25 ± 0.01⁎ |
| IFZN I | 10 | 0.57 ± 0.02⁎ | 0.43 ± 0.05⁎ | 0.62 ± 0.01⁎ | 0.46 ± 0.02∗ | 0.53 ± 0.01⁎ | 0.48 ± 0.02⁎ | 0.63 ± 0.01⁎ | 0.48 ± 0.02⁎ |
| IFZN II | 20 | 0.50 ± 0.02⁎ | 0.40 ± 0.04⁎ | 0.51 ± 0.01⁎ | 0.43 ± 0.03⁎ | 0.50 ± 0.01⁎ | 0.48 ± 0.03⁎ | 0.56 ± 0.02⁎ | 0.46 ± 0.03⁎ |
| IFZN III | 30 | 0.47 ± 0.01⁎ | 0.38 ± 0.04⁎ | 0.46 ± 0.01⁎ | 0.41 ± 0.02⁎ | 0.44 ± 0.01⁎ | 0.42 ± 0.02⁎ | 0.52 ± 0.03⁎ | 0.44 ± 0.04⁎ |
Values reported as mean ± SEM (n = 6). The data were analyzed by one way ANOVA followed by Dunnett’s test.
P < 0.05 as compared with the control group. IFZN (cyclopeptide alkaloid fraction of leaves of Ziziphus nummularia).
Carrageenan induced peritonitis in mice was used to access fluid extravasation and leukocyte migration induced due to inflammation. IFZN causes significant dose dependent decreases in the number of leukocytes migrating to the peritoneal cavity (Table 2).
Table 2.
Effect of IFZN on cotton pellet induced granuloma and carrageenan induced peritonitis in mice.
| Treatment | Dose mg/kg (p.o.) | Weight of cotton pellet mg (moist) | Percentage inhibition | Weight of cotton pellet mg (Dried) | Percentage inhibition | Leukocytes (103 cmm) |
|---|---|---|---|---|---|---|
| Control | 5 (ml/kg) | 177.33 ± 5.93 | – | 69.42 ± 2.33 | 4.32 ± 0.21 | |
| Indomethacin | 10 | 76.00 ± 2.88⁎ | 57.06 | 23.32 ± 1.67⁎ | 66.18 | 2.10 ± 0.07⁎ |
| IFZN I | 10 | 132.50 ± 1.61⁎ | 25.14 | 32.50 ± 1.61⁎ | 52.90 | 2.88 ± 0.09⁎ |
| IFZN II | 20 | 120.33 ± 2.35⁎ | 32.02 | 23.50 ± 0.89⁎⁎ | 65.94 | 2.73 ± 0.11⁎ |
| IFZN III | 30 | 96.00 ± 2.07⁎ | 45.76 | 19.00 ± 1.37⁎⁎ | 72.46 | 2.65 ± 0.06⁎ |
Values reported as mean ± SEM (n = 6). The data were analyzed by one way ANOVA followed by the Dunnett’s test.
P < 0.05.
P < 0.01 as compared with the control group. IFZN (cyclopeptide alkaloid fraction of leaves of Ziziphus nummularia).
Effects of IFZN treatment in animals implanted with cotton pellet-induced granuloma are depicted in Table 2. IFZN treatment causes significant (P < 0.05) dose dependent decreases in the weight of moist and dry cotton pellets in comparison to the control group.
3.3. Analgesic activity
The analgesic effects induced by different doses of IFZN and aspirin 100 mg/kg BW on the acetic acid induced writhing in mice are portrayed in Fig. 2. Significant (P < 0.05) dose dependent decrease in the number of writhes was observed in IFZN treated animals. The results obtained for the writhing test are similar to those obtained for the oedematogenic test using carrageenan.
Figure 2.
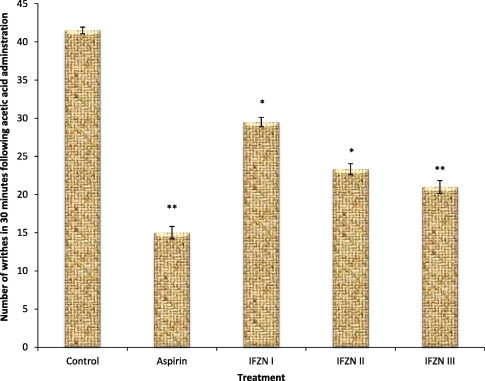
Effect of IFZN on acetic acid induced writhing in mice. Values reported as mean ± SEM (n = 6). The data were analyzed by one way ANOVA followed by the Dunnett’s test. ∗P < 0.05; ∗∗P < 0.01 as compared with the control group. IFZN (cyclopeptide alkaloid fraction of leaves of Ziziphus nummularia).
Results of tail flick and hot plate tests are summarized in Table 3. IFZN and morphine 5 mg/kg causes significant (P < 0.05) dose dependent increases in tail flick latency and hot plate reaction time when compared with the control. Co-administeration of naloxone causes moderate decrease in the tail flick latency of mice when compared with IFZN 30 mg/kg.
Table 3.
Effect of IFZN on hot plate reaction time and tail flick latency of mice.
| Treatment | Dose mg/kg (p.o.)/(s.c.) | Tail flick latency (Second) | Hot plate reaction time (Second) |
|---|---|---|---|
| Control | 5 (ml/kg) | 4.28 ± 0.27 | 4.28 ± 0.27 |
| Morphine | 5 | 12.57 ± 0.41⁎ | 12.57 ± 0.41⁎ |
| IFZN I | 10 | 09.48 ± 0.34⁎ | 09.48 ± 0.34⁎ |
| IFZN II | 20 | 11.07 ± 0.39⁎ | 11.07 ± 0.39⁎⁎ |
| IFZN III | 30 | 11.33 ± 0.45⁎⁎ | 11.33 ± 0.45⁎⁎ |
| IFZN III + Naloxone | 30 + 1 | 09.35 ± 0.42⁎ | – |
Values reported as mean ± SEM (n = 6). The data were analyzed by one way ANOVA followed by the Dunnett’s test.
P < 0.05.
P < 0.01 as compared with the control group. IFZN (cyclopeptide alkaloid fraction of leaves of Ziziphus nummularia).
3.4. Docking studies
The docking scores nummularine on μ-opioid were found to be −14.00. Docking analysis of docked conformers on μ-opioid receptor revealed that nummularine interacts with PHE 237 and TRP 303. Nummularine has also displayed H-bonding interaction with THR 307 (2.02 Ǻ) and LYS 303 (1.87 Ǻ).
4. Discussion
In comparison to our earlier study carried out with alcoholic extracts of leaves of ZN, IFZN was found to be an efficacious and potent analgesic agent.
Anti-inflammatory activity of IFZN is purposed to be mediated by the inhibition of prostaglandin synthesis. Reduction in paw oedema induced by carrageenan and histamine, decreased weight of moist cotton pellet and decreased leukocyte count in carrageenan induced peritonitis are the indicators of decreased vascular permeability and cellular migration to the injured site (Cuman et al., 2001; Linardi et al., 2002). These effects are known to be mediated by the release of histamine, serotonin, cytokines, leukotrienes and prostaglandins (Hwang et al., 1996).
Further, inhibition of the carrageenan induced neutrophil migration to the peritoneal cavity and decreases in acetic acid-induced writhing are indicators of inhibitory action on leukotriene and cytokine synthesis (Canetti et al., 2001; Limet and Lecomte, 1968).
IFZN 30 mg/kg has shown highly significant anti-nociceptive effects against acetic acid-induced writhing, tail flick and hot plate test (Fig. 3).
Figure 3.
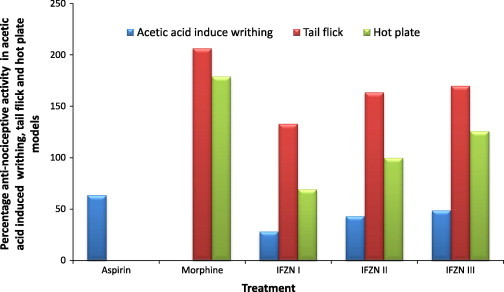
Anti-nociceptive effect induced by IFZN on mice in acetic acid induced writhing, tail flick and hot plate models.
Administration of acetic acid causes increased membrane phospholipase activity and synthesis of pain and inflammation mediators such as cytokines, eicosanoids, leukotrienes and prostaglandins; therefore protection against acetic acid induced writhing is indicative of the inhibition of prostaglandin like mediators (Eun-Mi Choi and Hwang, 2004).
The hot-plate paw-licking as well as the tail-flick responses are used for studying the neuronal mechanisms of opioid and stimulation-induced analgesia. Although thermal stimuli are used in both tests, the tail-flick response is due to spinal reflex, while the hot-plate paw-licking response is a supraspinally integrated response (Suh et al., 1992; Schmauss and Yaksh, 1984). The antinociceptive effect in the tail flick and hot-plate tests indicates that IFZN causes inhibition on both the spinal reflex and supraspinal centers (Dewey et al., 1970). Further, tail flick latency of mice treated with IFZN 30 mg/kg decreases in the presence of naloxone (opioid antagonist), which is an indicative of the involvement of the opioid receptor and the supraspinal centre.
The docking of nummularine with μ-opioid receptor was found to be very encouraging. The docking study confirmed the interaction of cyclopeptide alkaloids with μ-opioid receptor.
5. Conclusion
Based on results obtained in the present pharmacological study, it can be concluded that IFZN is a more potent and efficacious analgesic agent than EZN. IFZN is ten times more potent than EZN. The analgesic activity of IFZN is mediated by the peripheral as well as the central pathways mediated through the μ-opioid receptor. The findings of present studies warrant further research on IFZN for other indications.
Acknowledgments
We are very grateful to the Sophisticated Analytical Instrument Facility, Indian Institute of Technology, Madras for GCMS analysis.
Footnotes
Peer review under responsibility of King Saud University.
References
- Anonymous . Council of Industrial and Scientific Research; New Delhi: 1989. The Wealth of India (Raw Material) p. 590. [Google Scholar]
- Bhattacharjee S.K. Pointer Publication; Jaipur: 2004. Handbook of medicinal plant. pp. 383–385. [Google Scholar]
- Canetti C., Silva J.S., Ferreira S.H., Cunha F.Q. Tumour necrosis factor-alpha and leukotriene B(4) mediate the neutrophil migration in immune inflammation. Bri. J. Pharmacol. 2001;134:1619–1628. doi: 10.1038/sj.bjp.0704403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Eun-Mi, Hwang Jae-Kwan. Antiinflammatory, analgesic and antioxidant activities of the fruit of Foeniculum vulgare. Fitoterapia. 2004;75:557–565. doi: 10.1016/j.fitote.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Cuman R.K.N., Bersani-Amadio C.A., Fortes Z.B. Influence of Type 2 diabetes on the inflammatory response in rats. Inflam. Res. 2001;50:460–465. doi: 10.1007/PL00000271. [DOI] [PubMed] [Google Scholar]
- D’Armour F.E., Smith D.L. A method for determining loss of pain sensation. J. Pharmacol. Exp. Ther. 1941;72:74–79. [Google Scholar]
- Dewey W.I., Harris L.S., Howes J.F., Nuite J.A. The effect of various neurohumoral modulators on the activity of morphine and the narcotic antagonists in the tail-flick and the phenylquinone tests. J. Pharmacol. Exp. Ther. 1970;175:435–442. [PubMed] [Google Scholar]
- Goyal M., Sasmal D., Nagori B.P. Review on medicinal plants used by local community of Jodhpur district of thar desert. Int. J. Pharmacol. 2011;7:333–339. [Google Scholar]
- Goyal M., Nagori B.P., Sasmal D. Analgesic and anti-inflammatory activity of ethanolic extract of Zizyphus nummularia. Res. J. Med. Plant. 2012;6:521–528. [Google Scholar]
- Griswold D.E., Mrarshall P.J., Webb E.F., Godfrey R., Newton J., Diamartina M.J., Sarau H.M., Gleason J.G., Poste G., Hanna N. SK&F, 86002: a structurally novel anti-inflammatory agent that inhibits lipoxygenase- and cyclooxygenase-mediated metabolism of arachidonic acid. Biochem. Pharmacol. 1987;36:3463–3470. doi: 10.1016/0006-2952(87)90327-3. [DOI] [PubMed] [Google Scholar]
- Hasan Soliman Yusufoglu Topical anti-inflammatory and wound healing activities of herbal gel of Ziziphus nummularia L. (F. Rhamnaceae) leaf extract. Int. J. Pharmacol. 2011;7:862–867. [Google Scholar]
- Hwang S., Lam M., Li C., Shen T. Release of platelet activating factor and its involvement in the first phase of carrageenin rat foot oedema. Eur. J. Pharmacol. 1996;120:33–41. doi: 10.1016/0014-2999(86)90636-9. [DOI] [PubMed] [Google Scholar]
- Jadeja B.A., Odedra N.K., Solanki K.M., Baraiya N.M. Indigenous animal healthcare practices in district Porbandar. Gujarat Indian J. Tradit. Knowl. 2006;5:253–258. [Google Scholar]
- Jadhav D. Ethnomedicinal plants used by Bhil tribe of Bibdod. Madhya Pradesh Indian J. Tradit. Knowl. 2006;5:263–267. [Google Scholar]
- Jain A., Katewa S.S., Galav P.K., Sharma P. Medicinal plant diversity of Sitamata wildlife sanctuary, Rajasthan. India J. Ethnopharmacol. 2005;102:143–157. doi: 10.1016/j.jep.2005.05.047. [DOI] [PubMed] [Google Scholar]
- Koster R., Anderson M., de Beer E.J. Acetic acid for analgesic screening. Fed. Proc. 1959;18:412–418. [Google Scholar]
- Kumar S., Parveen F., Goyal S., Chauhan A. Indigenous herbal coolants for combating heat stress in the hot Indian Arid Zone. Indian J. Tradit. Knowl. 2008;7:679–682. [Google Scholar]
- Limet R., Lecomte J. Inhibitory action of indomethacin on generalized oedema induced by dextran in rats. Arch. Int. Pharmacodyn. Ther. 1968;171:109–115. [PubMed] [Google Scholar]
- Linardi A., Costa S.K.P., Da Silva G.R., Antunes E. Involvement of kinins, mast cells and sensory neurons in the plasma exudation and paw oedema induced by staphylococcal entrotoxin-B in the mouse. Eur. J. Pharmacol. 2002;399:235–242. doi: 10.1016/s0014-2999(00)00375-7. [DOI] [PubMed] [Google Scholar]
- Ma Y., Han H., Nam S.Y., Kim Y.B., Hong J.T., Yun Y.P., Oh K.W. Cyclopeptide alkaloid fraction from Zizyphi Spinosi Semen enhances pentobarbital-induced sleeping behaviors. J. Ethnopharmacol. 2008;117:318–324. doi: 10.1016/j.jep.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Meena K.L., Yadav B.L. Some traditional ethnomedicinal plants of southern Rajasthan. Indian Tradit. Knowl. 2010;9:471–474. [Google Scholar]
- Morel Ademir F., Graciela M., Vinicius I. Vol. 67. Academic Press; London: 2009. (The Alkaloids Chemistry and Biology). pp. 79–141. [Google Scholar]
- Muhammad I.C., Khan M.A. An ethnomedicinal inventory of plants used for family planning and sex diseases in Samahni valley. Pakistan. Indian J. Tradit. Knowl. 2008;7:277–283. [Google Scholar]
- OECD . OECD; Paris: 2001. Guidelines for Testing of Chemical, Guideline 423, Acute Oral Toxicity – Acute Toxic Class Method. [Google Scholar]
- Penn G.B., Ashford A. The inflammatory response to implantation of cotton pellets in the rat. J. Pharm. Pharmacol. 1963;15:798–803. doi: 10.1111/j.2042-7158.1963.tb12883.x. [DOI] [PubMed] [Google Scholar]
- Schmauss C., Yaksh T.L. In vivo studies on spinal opiate receptor systems mediating antinociception. II. Pharmacological profiles suggesting a differential association of mu, delta and kappa receptors with visceral, chemical and cutaneous thermal stimuli in the rat. J. Pharmacol. Exp. Ther. 1984;228:1–12. [PubMed] [Google Scholar]
- Singh V., Pandey R.P. Scientific Publishers; Jodhpur: 1998. Ethnobotany of Rajasthan. p. 217. [Google Scholar]
- Suh H.H., Fujimoto J.M., Tseng L.F. Different radiant heat intensities diferentiate intracerebroventricular morphine from b-endorphin-induced inhibition of the tail-lick response in the mouse. Eur. J. Pharmacol. 1992;213:337–341. doi: 10.1016/0014-2999(92)90622-b. [DOI] [PubMed] [Google Scholar]
- Suleyman H., Demirezer L.O., Kuruuzum A., Banoglu Z.N., Gocer F., Ozbakir G., Gepdiremen A. Anti-inflammatory effect of the aqueous extract from Rumex patientia L. roots. J. Ethnopharmacol. 1991;65:141–148. doi: 10.1016/s0378-8741(98)00175-5. [DOI] [PubMed] [Google Scholar]
- Turner R.A. In: Analgesics: Screening Methods in Pharmacology. Turner R., Ebborn P., editors. Academic Press; New York: 1965. p. 100. [Google Scholar]
- Upadhyay B., Singh K.P., Kumar A. Ethno-veterinary uses and informants consensus factor of medicinal plants of Sariska region, Rajasthan. India. J. Ethnopharmacol. 2011;133:14–25. doi: 10.1016/j.jep.2010.08.054. [DOI] [PubMed] [Google Scholar]
- Winter C.A., Porter C.C. Effect of alteration in side chains upon anti-inflammatory and liver glycogen activities in hydrocortisone ester. J. Am. Pharmacol. Soc. 1957;46:515–519. doi: 10.1002/jps.3030460902. [DOI] [PubMed] [Google Scholar]
- Winter C.A., Risley E.A., Nuss G.W. Carregeenan-induced oedema in hind paw of the rats as an assay for anti-inflammatory drugs. Exp. Biol. Med. 1962;111:544–547. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]



