Abstract
The developmental origins of health and disease hypothesis holds that inappropriate environmental cues in utero, a period marked by tremendous developmental sensitivity, facilitate cellular reprogramming to ultimately predispose disease in adulthood. In this review, we analyze if stress during early stages of development can affect future health. This has wide clinical importance, given that 5 million children have been conceived with assisted reproductive technologies (ART). Because the primary outcome of assisted reproduction procedures is delivery at term of a live, healthy baby, the postnatal effects occurring outside of the neonatal period are often overlooked. To this end, the long-term outcome of ART is appropriately the most relevant concern of the field today. Evidence of adverse consequences is controversial. The majority of studies have concluded no obvious problems in IVF-conceived children, although a number of isolated cases of imprinted diseases, cancers, or malformations have been reported. Given that animal studies suggest alteration of metabolic pathways following preimplantation stress, it will be of great importance to follow-up ART individuals as they enter later stages of adult life.
Keywords: assisted reproductive technologies, in vitro culture, developmental-origin-of-health-and-disease hypothesis, preimplantation stress
INTRODUCTION
Mammalian preimplantation development is a period of elegantly orchestrated molecular events, extending from fertilization of the oocyte to invasion of the uterine epithelium by the blastocyst [reviewed in (Cockburn and Rossant, 2010)]. These early stages are remarkably sensitive to environmental states, which stimulate signaling events and changes in gene regulatory networks (Fernandez-Gonzalez et al., 2004). As will be described in detail, gametes and embryos are exposed to a myriad of conditions throughout the fertilization and preimplantation periods. For example, the progression of the embryo from the fallopian tube to the uterus consists of successive changes in nutrient composition and growth factor availability, as well as serial decreases in pH from alkaline to mildly acidic, all of which have measurable influences on development and viability. Alterations to or disruptions within the circumstances of preimplantation development have profound implications for an organism’s health and predisposition to disease. Given that the number of individuals conceived by assisted reproductive technologies (ART), such as in vitro fertilization, has now reached 5 million (ICMART, 2012), the impact of unusual or suboptimal fertilization and preimplantation environments is particularly relevant. As will be described, many of the conditions associated with ART have the potential to be perceived as stressful.
This review evaluates our current understanding of preimplantation stress and its consequences for disease susceptibility. We analyze the physiologic events that occur before implantation in mammals, and describe how maternal disease or culture in vitro can have long-term health consequences. Finally we will focus on the possible different conditions in vitro that can modify metabolism and growth trajectories.
Importantly, we define stress broadly, as anything that jeopardizes homeostasis. In addition to conditions that threaten macromolecular damage or the integrity of various cell functions, stress may also occur as inappropriate physiological signals. These forms of stress do not necessarily evoke a stress response, but have a measurable effect on cell viability and/or alter typical development on the order of growth, gene expression, and overall competence. Stress therefore encompasses stimuli that perturb normal physiological functions with the potential to induce adverse pathological outcomes.
THE PREIMPLANTATION EMBRYO
Preimplantation development encompasses fertilization through the invasion of the hatched blastocyst into the uterus (Fig. 1). Soon after the oocyte is fertilized by a spermatozoan to form a single-celled zygote, the cellular constituents are replicated and partitioned over several rounds of cleavage without increasing whole embryo volume (Cockburn and Rossant, 2010). At eight cells, embryo polarization allows compaction, providing a foundation for establishing distinct cell lineages: the trophectoderm, precursor to extraembryonic tissues like the placenta, and the inner cell mass, which will give rise to the embryo proper as well as part of its extraembryonic membrane. These lineages evolve over subsequent asymmetric cleavage divisions, continuing through the formation of the blastocoel cavity. Cavitation permits full expansion of the blastocyst, which hatches from the protective zona pellucida before implanting into the uterus. The first attachment of the human blastocyst to the uterus (apposition stage) occurs on day 8 postfertilization (Dey et al., 2004).
Figure 1.
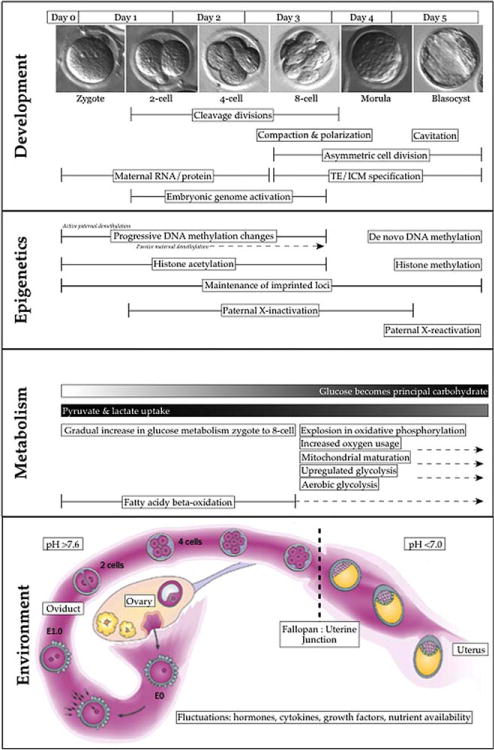
Summary of developmental milestones occurring during preimplantation development.
Analysis of metabolic physiology in preimplantation embryos indicates diverse nutritional requirements during development from zygote to blastocyst. Progression from the zygote to two-cell stage has an absolute requirement for pyruvate, and further cleavage stages are synergistically supported by lactate (Brinster, 1965; Biggers et al., 1967). Glucose cannot exclusively sustain development until the late four-cell/early eight-cell stage, but becomes the principal carbohydrate metabolized beginning around compaction (Brinster and Thomson, 1966). These requirements are relatively conserved across different mammalian species, with slight variations in time of increase in glucose oxidation. Effectively, aerobic and anaerobic respiration are not mutually exclusive but together function to meet the changing energy demands of early embryos (Wilding et al., 2009). This transition in energy substrate preference is influenced by the availability of carbohydrates, amino acids, and extracellular pH, which affect glycolytic activity, oxidative phosphorylation, and membrane transport, with potential consequences for proliferation and growth (Lane and Gardner, 2000, 2003). For example, the absense of glucose in culture prevents the characteristic decline of pyruvate uptake after compaction (Brinster, 1965). Consequently, preimplantation development occurs over a dynamic range of conditions, demonstrating strong metabolic plasticity and the ability of embryos to compensate for nutrient fluctuations at this time (Gardner and Leese, 1988; Edwards et al., 1998; Horsthemke and Ludwig, 2005).
Mammalian preimplantation development is additionally characterized by coordinated reprogramming of the genome and establishment of epigenetic marks that are maintained after birth (Bermejo-Alvarez et al., 2011). Epigenetic regulation occurs at the DNA level via methylation of cytosine bases residing in CpG dinucleotides (Ooi et al., 2009), or by modification of histone proteins through methylation, acetylation, ubiquitination, sumoylation, or phosphorylation (Zhang and Reinberg, 2001). These covalent moieties combine into cooperative epigenetic signatures that affect gene expression through a variety of mechanisms, including (but not limited to) control of gene or promoter accessibility by relaxation or condensation of chromatin, the recruitment of chromatin remodeling enzymes, and occlusion of transcriptional machinery (Fischer et al., 2008; Wang et al., 2008; Burdge and Lillycrop, 2010; Khorasanizadeh, 2011). Shortly after fertilization, the complementary parental genomes undergo dramatic changes in DNA methylation status via the TET family of dioxygenases, allowing for subsequent re-methylation to establish somatic differentiation patterns after implantation (Reik, 2007). Because preimplantation development is typified by epigenetic rearrangement, the embryo may be particularly vulnerable to disturbances. Environmental perturbations could cause errors or epimutations, or affect the programming of cell states and response pathways.
THE DEVELOPMENTAL ORIGINS OF HEALTH AND DISEASE HYPOTHESIS
Emerging research over the past several decades has supported a clear biological basis for health and disease. More specifically, there is now appreciable evidence that exposure to stressful environments during critical periods of development can increase an organism’s susceptibility to a variety of diseases later in life. The administration of thalidomide to alleviate morning sickness in the late 1950s and early 1960s—resulting in an epidemic of phocomelia and other related birth defects—was paramount to demonstrating that events in utero could affect developmental outcomes (Brent and Holmes, 1988; McBride, 1961). Although previous researchers had suggested that developmental experiences could inform postnatal health outcomes (Kermack et al., 1934; McCance and Widdowson, 1974; Wadsworth et al., 1985), Barker et al. crystallized this theory in the 1980s by correlating nutritional stress in utero, manifested by low birth weights, with heightened risk of adult cardiovascular disease in particular regions of England and Wales (Barker and Osmond, 1986). Their fetal origins of adult disease hypothesis posited that fetal undernutrition could reprogram gene pathways to permanently affect body composition and metabolism. Today, the Developmental Origins of Health and Disease (DOHaD) hypothesis states that different environmental conditions occurring at critical points during development have the potential to shape developmental trajectories.
One of the most frequently cited examples in support of the DOHaD hypothesis is the Dutch Hunger Winter study, and deserves recognition here. In 1944, during the German occupation of specific regions of the Netherlands, rations were reduced to as few as 400 to 800 calories per day for a period of 5 months (Schulz, 2010). As with Barker’s study, the results are undeniable: nutrient deprivation in utero is associated with glucose intolerance, obesity, and cardiac dysfunction in adulthood (de Rooij et al., 2006a, b, c, d, 2010).
It is now apparent that fertilization and preimplantation development are additional periods of vulnerability to stressful environments. This becomes particularly relevant for individuals conceived by ART, given that many facets of fertilization and culture in vitro may be perceived as stressful (Fig. 2). Unfortunately, there is little data available for long-term epidemiological analysis due to the relatively brief history of ART. IVF children are at most 34 years old and too young to exhibit signs of chronic middle-aged syndromes, such that the impact of ART on adult health and age-related diseases remains obscure. Although the majority of children are apparently healthy, the increased prevalence of birth defects, epigenetic disorders, and metabolic discrepancies indicates a potential danger associated with assisted reproductive technologies.
Figure 2.
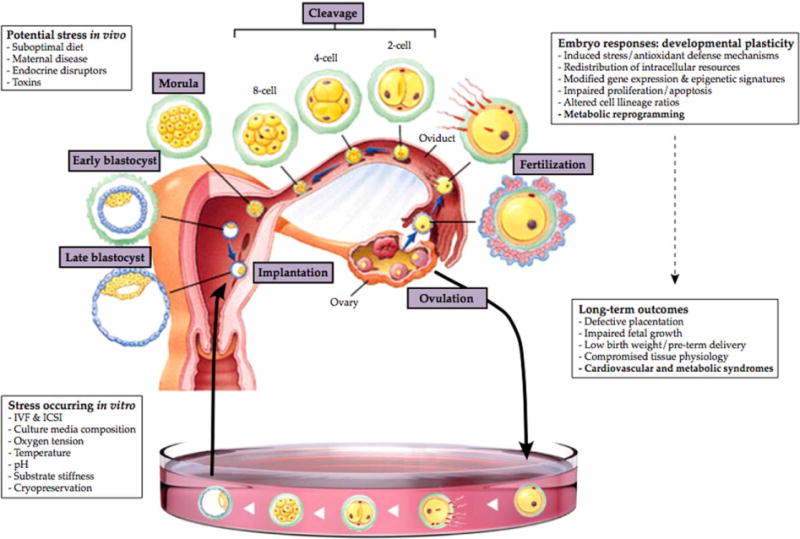
Different types of in vivo or in vitro stress may affect preimplantation embryo development.
CONSEQUENCES OF STRESS IN VIVO DURING THE PREIMPLANTATION PERIOD
With respect to human development, it is challenging to differentiate a plausible stress limited to the preimplantation period that does not persist for the remainder of pregnancy. However, there is evidence from animal studies suggesting that stress limited to this period can have both short and long-term consequences. In particular, low protein diet administered exclusively during the preimplantation period in rats is associated with cardiovascular pathologies, as well as perturbations to renin-angiotensin homeostasis in adulthood (Watkins et al., 2008, 2010). Specifically, the authors of this study observed significantly elevated systolic blood pressure, impaired arterial vasodilation, and increased expression of angiotensin-converting enzyme, an important mediator of vasoconstriction, in the lung. Within the oviduct and uterus, maternal diet affects nutrient availability and hormone composition (Leese et al., 2008). It is therefore possible to speculate that because embryo utilization of energy substrates is based primarily upon nutrient accessibility, altered concentrations of key metabolites and growth signals during the preimplantation period will affect embryo development and competence. Given that the worldwide prevalence of obesity and diabetes are at all-time highs, the effects of unhealthy maternal nutrition during preimplantation development are of serious concern.
An excellent study by Moley et al. evaluated the impact of high glucose during early gestation in mice (Wyman et al., 2008). Either zygotes or blastocysts obtained from streptozotocin-induced diabetic or control mothers were transferred into nondiabetic surrogate dams. Fetuses derived from zygotes and blastocysts of the diabetic mice displayed significantly higher neural tube closure problems and abdominal wall or limb deformities at midgestation. In addition, both groups of fetuses were significantly growth retarded, demonstrating a significant effect of maternal environment as early as the one-cell stage.
In addition to dietary or metabolic conditions, toxicologic agents can impact early pregnancy. Developmental exposure to xenoestrogens, such as diethylstilbestrol or bisphenol A, have been documented activating estrogen-responsive genes, affecting proliferation, and increasing adult cancer susceptibility (Nadal et al., 2000; Markey et al., 2001). This could result in reprogramming of hormone-response pathways, disruption of normal endocrine function, and altered establishment of hypothalamic-pituitary homeostasis (Maffini et al., 2006). Exposure of mouse embryos to environmentally relevant quantities of bisphenol A advanced blastocyst development and resulted in higher weight at weaning (Takai et al., 2001), whereas incubation with higher doses impaired embryo development (Takai et al., 2000).
Several interesting studies investigating the effects of tobacco on preimplantation development have shown that smoking before pregnancy can affect early embryonic growth. Tobacco exposure is correlated with ovulation of fewer oocytes, altered tubal function, (Knoll et al., 1995; Magers et al., 1995; DiCarlantonio and Talbot, 1999; Gieseke and Talbot, 2005), and impaired embryo development in female mice (Hassa et al., 2007). In rats, smoke reduces both preimplantation embryo development and sperm count (Polyzos et al., 2009). Similarly, maternal exposure to tobacco smoke in the immediate postconception period is associated with a dose-related retardation in embryo growth, increased pregnancy wastage (Seller and Bnait, 1995; Bnait and Seller, 1995), and delayed migration of embryos from the fallopian tubes into the uterus (Tachi and Aoyama, 1989). Yoshinaga et al. (1979) obtained similar results after daily maternal nicotine injections, observing a 12-hr delay in cleavage at the two-cell stage, and subsequent retardations at each step thereafter, including implantation.
CONSEQUENCES OF IN VITRO STRESS DURING THE PREIMPLANTATION PERIOD: ART
Human in vitro fertilization (IVF) as a treatment for infertility is regarded as one of the most outstanding accomplishments of the 20th century, leading to the awarding of a Nobel Prize to its visionary Sir Robert Edwards in 2010. However, IVF has been viewed with some skepticism since it deviates rather severely from natural conception. The dynamic conditions existent in the genital tract are not only lost with IVF, but the procedure introduces many additional stressors. It is therefore not entirely surprising that numerous health complications are present following manipulation of embryos in vitro (Table 1).
TABLE 1.
Complications Reported in Humans After ART, With Selected References
| Complications | Reference | |
|---|---|---|
| Obstetric | Multiple pregnancy | (Vitthala et al., 2009; Prevention CfDCa, 2011) |
| Pre-eclampsia | (Jackson et al., 2004) | |
| Placenta abnormalities | ||
| Placenta previa | (Jackson et al., 2004; Romundstad et al. 2006) | |
| Placenta abruption | (Shevell et al., 2005) | |
| Abnormal cord insertion | ||
| Cesarean delivery | ||
| Pre-term delivery | (Helmerhorst et al., 2004; Jackson et al., 2004; de Rooij et al., 2011; McDonald et al., 2010) | |
| Perinatal | Low or very low birth weight | (Ceelen et al., 2008a) |
| Birth defects & malformations | (Hansen et al., 2002) | |
| Anotia, microtia | (Reefhuis et al., 2009) | |
| Septal heart defects | ||
| Cleft lip and/or palate | ||
| Esophageal atresia | ||
| Anorectal atresia | ||
| Hypospadia | ||
| Craniosynostosis | ||
| Congenital malformations | (Davies MJ et al., 2012) | |
| Cardiovascular malformations | ||
| Musculoskeletal malformations | ||
| Urogenital malformations | ||
| Gastrointestinal malformations | ||
| Respiratory defects | ||
| Metabolic defects | ||
| Pediatric | Cerebral palsy and neurological sequelae | (Stromberg et al., 2002; Hvidtjorn et al., 2009) |
| Chromosomal anomalies | ||
| Imprinting disorders | (Manipalviratn et al., 2009) | |
| Angelman syndrome | ||
| Beckwith-Wiedemann syndrome | ||
| Cancer | (Kallen et al., 2010) | |
| Hepatoblastoma | (McLaughlin et al., 2006) | |
| Retinoblastoma | (Moll et al., 2003) | |
| Leukemia | (Petridou et al., 2012) | |
| Metabolic discrepancies | (Ceelen et al., 2007; Ceelen et al., 2008a; Ceelen et al., 2009) | |
| Higher systolic & diastolic blood pressure | ||
| Elevated fasting glucose | ||
| Increased peripheral fat deposition | ||
| Trending altered bone mineral density | ||
| Trending increases in total body fat | ||
However, it is important to emphasize the limitations of ART epidemiological studies: because ART patients represent a rather exclusive population, reliable data is often confounded by variables such as increased maternal age, infertility, or lack of appropriate control groups. In fact, subfertility itself is a risk factor for several health complications, including cardiovascular disease, depression, and certain reproductive cancers (Brinton et al., 2005; Wilkins et al., 2010; Parikh et al., 2012). It is therefore difficult to parse an effect of preimplantation stress attributable to ART procedures from outcomes secondary to the infertility status of the patients themselves. Nevertheless, important conclusions about embryo stress, adaptation, and reprogramming may be drawn from human ART analyses.
The major risk associated with ART in humans is the problem of multiple gestations, which are linked with numerous health complications. In 2010, the American Society of Reproductive Technologies (SART) reported that the risk of a woman under the age of 35 bearing twins after ART was 32.9% (Center for Disease Control and Prevention ASfRM, 2012). This relative risk is increased 25- to 30fold in contrast to twinning in spontaneous conception, which occurs in 1/80 pregnancies (1.25%) (Jaddoe and Witteman, 2006). Monozygotic twinning is twice as common in ART pregnancies, and the incidence of higher order multiples (triplets or more) is 2 orders of magnitude greater (1.5%) than in natural pregnancy (1/6400 or 0.01%) (Vitthala et al., 2009).
There is an overall increased risk of preterm delivery (PTD; defined as <37 wks gestation), low birth weight (LBW < 2500 g), and very low birth weight (VLBW < 1500 g) for ART pregnancies, even in singleton gestations (Helmerhorst et al., 2004; Jackson et al., 2004; Ombelet et al., 2005). Both PTD and LBW are correlated with several unfavorable neonatal outcomes, including neuromotor, behavioral, and cognitive dysfunctions, necrotizing enterocolitis, and cerebral palsy (Saigal et al., 1991; Lems et al., 1993; Holman et al., 1997; O’Shea et al., 1998; Buck et al., 2000; Saigal, 2000; Hille et al., 2001; Jackson et al., 2004). Compared with infants weighing 2500 to 2999 g and 3000 to 3499 g at birth, low birth weight alone increases the risk of neonatal death by 4- and 10-fold, respectively (Ashworth, 1998). ART is also associated with placenta abnormalities, including placenta previa, placental abruption, and abnormal cord insertion (Jackson et al., 2004; Shevell et al., 2005; Romundstad et al., 2006), which may influence fetal nutrition and growth, gestational age, and size at birth. These conditions are exacerbated by twin or higher order multiple gestations (McDonald et al., 2010). Additional obstetric risks correlated with ART include pregnancy-induced hypertension, and labor complications such as neonatal asphyxia and increased delivery by Cesarean section, although the latter may reflect a more cautious management of ART pregnancies (Gillet et al., 2011).
It has become increasingly clear that different forms of stress correlate with unique results. One interesting assessment—albeit with limited evidence—explored whether certain infertility etiologies are associated with particular outcomes. In a Finnish study, the risk of PTD was highest in women diagnosed with anovulation or endometriosis, whereas admission into neonatal intensive care was higher in pregnancies associated with male factor infertility (Kuivasaari-Pirinen et al., 2012). More complete analyses are required, but may inform the use of different ART procedures or pregnancy management options within ART populations.
Several groups have reported an increased incidence of epigenetic diseases in association with ART, implying that preimplantation stress can alter the epigenome. This includes the imprinting disorders Beckwith-Wiedemann Syndrome (BWS) and Angelman Syndrome (AS), which are associated with impaired development, growth defects, cognitive dysfunction, and cancer (Williams et al., 1995; Maher et al., 2003). Imprinted genes are genes in which a particular allele is inactivated in a parent-of-origin-dependent manner, and imprinting disorders arise when a maternal or paternal allele is inappropriately expressed because of abnormal DNA methylation [reviewed in (Manipalviratn et al., 2009; Batcheller et al., 2011)]. ART has previously been linked with altered methylation at several imprinting control regions (Doherty et al., 2000; Tremblay et al., 2000; Mann et al., 2004; Li et al., 2005; Rivera et al., 2008; Giritharan et al., 2010, 2012; Bloise et al., 2012). Although imprinted genes encompass 0.1 to 0.5% of the genome, they play a pivotal role in early development by controlling processes such as nutrient consumption and the cell cycle (Fowden et al., 2006). Inappropriate regulation of imprinted genes—inherited, sporadic, or environmentally induced—has been associated by some with diseases of growth and development (Miozzo and Simoni, 2002), but not by others (Rancourt et al., 2012; Puumala et al., 2012). While rare, the increased observation of BWS and AS may reflect more widespread, as-of-yet unidentified epigenetic alterations occurring after preimplantation stress. For example, assisted conception has been connected to widespread methylation changes in several mammalian species, including humans (Shi and Haaf, 2002; Zaitseva et al. 2007; Katari et al., 2009; Deshmukh et al., 2011). Mouse embryos cultured in vitro exhibit global hypermethylation (Wright et al., 2011), which could influence higher-order organization of chromatin structure. Interestingly, in the same study, IVF embryos were overall hypomethylated at 1500 selected foci. In another report, extensive DNA methylation analyses in placenta and cord blood cells revealed no obvious changes in global methylation between IVF- and in vivo-conceived newborns, indicating that IVF individuals may not have a noticeable epigenetic fingerprint (Katari et al., 2009). Yet, there were locus-specific methylation and gene expression discrepancies, suggesting there may be epigenetic differences in children born after IVF. As many imprinted genes are involved in growth and development, stress-induced epigenetic aberrancies could have a significant effect on postnatal health (Miozzo and Simoni, 2002). Furthermore, epigenetic changes may also account for previously described transgenerational effects (Holemans et al., 1991; Harrison et al., 2009). These investigations have revealed that culture-derived mice can produce offspring exhibiting organomegaly of the brain, pituitary, and kidney, with lower body weights at weaning (Mahsoudi et al., 2007).
Because the primary objective of assisted reproduction procedures is delivery at term of a live, healthy baby, postnatal effects occurring outside of the neonatal period are often overlooked. To this end, the long-term outcome of ART is appropriately the most relevant concern of the field today. Evidence of adverse consequences is controversial. The majority of studies have concluded no obvious problems in IVF-conceived children, although a number of isolated cases of rare diseases, cancers, or malformations have been reported (Table 1; see these excellent reviews and meta-analyses: Finnstrom et al., 2011; Fortunato and Tosti, 2011; Kallen et al., 2005; Labouesse, 2011; Steel and Sutcliffe, 2009; Tosti et al., 2006; Wennerholm et al., 2009). However, the relatively short history of IVF has restricted long-term analyses, preventing a more complete understanding of middle-age health and susceptibility to age-related diseases following preimplantation stress.
Arguably the most compelling evaluation of growth and metabolic outcome of IVF treatments compared approximately 225 IVF-conceived children with 225 children conceived naturally by subfertile parents (Wagenaar, 2008; Ceelen et al., 2007, 2008a, b, 2009). The subfertility control is an ideal way to remove a potential infertility effect, hence it is more reasonable to conclude that observed differences were induced by IVF procedures. The authors discovered that IVF babies were significantly smaller regardless of gestational age, and more likely to be born prematurely. Comparisons of weight, height, and BMI between the two cohorts during the first four years of life demonstrated that the growth velocity of IVF infants was considerably faster, such that their physiological characteristics were statistically indistinguishable from controls by age 3 (Ceelen et al., 2009). Of note, rapid weight gain in early postnatal life is an independent risk factor for metabolic syndrome (Khuc et al., 2012), adult hypertension (Eriksson et al., 2000, 2007), and coronary heart disease (Eriksson et al., 1999; Forsen et al., 1999). In the same study, children (average age 12.3 yrs) born through IVF had significantly higher systolic and diastolic blood pressure, peripheral fat deposition, and fasting glucose levels, with trending increases in bone mineral density and total fat mass (Ceelen et al., 2007; Ceelen et al., 2008a). IVF did not appear to affect the onset of puberty, although pubertal girls had significantly increased circulating levels of LH and DHEAS (Ceelen et al., 2008b). These data indicate that ART might alter long-term metabolic homeostasis, and it will be of great importance to continue follow up analyses of IVF individuals as they enter later stages of adult life.
Animal Models
The use of animal models to investigate the impact of preimplantation stress on subsequent development and health has been vital to our understanding of the mechanisms underlying the embryo stress response (Table 2) and potential consequences for adult fitness (Table 3). Animal models are valuable in corroborating human ART findings because they remove infertility as a potential confounding variable, permit investigation of a particular controlled stress, and extend analyses with available molecular tools (Jansson and Lambert, 1999; Walters and Edwards, 2003; Neitzke et al., 2008). However, studies in animals are not immune to bias and errors (Jansson and Lambert 1999; Walters and Edwards, 2003; Neitzke et al., 2008). An important caveat is that the conditions required for successful fertilization and preimplantation development can vary between different mammalian species, such that normal physiological signals for one species may be perceived as stressful by another. As a result, animal models may imprecisely or incompletely depict the needs of the human embryo (He et al., 2010).
TABLE 2.
Effects of Preimplantation Stress on Embryo Development in Animals, with Selected References
| Stressor | Model | Impact | Reference |
|---|---|---|---|
| Culture medium | Mouse | Impaired development, altered ICM:TE number and ratio, modified metabolism, changes to gene expression/imprinted loci | (Gardner and Leese, 1988; Lane and Gardner, 2000; Rinaudo and Schultz, 2004; Giritharan et al., 2012; Schwarzer et al., 2012) |
| pH | Mouse | Impaired development, modified metabolism, changes to gene expression | (Edwards et al., 1998a, b; Lane and Gardner, 2003) |
| IVF | Mouse | Impaired development, altered ICM:TE number and ratio, changes to gene expression/imprinted loci | (Giritharan et al., 2007) |
| ICSI | Mouse | Impaired development, altered ICM:TE number and ratio, changes to gene expression/imprinted loci | (Giritharan et al., 2010) |
| Oxygen tension | Mouse | Impaired development, altered ICM:TE number and ratio, changes to gene expression/imprinted loci | (Rinaudo et al., 2006) |
| Culture dish elasticity | Mouse | Different developmental velocity, altered ICM:TE number and ratio, changes to gene expression | (Rinaudo et al., 2006) |
| Endocrine disruption | Mouse | Different developmental velocity | (Takai et al., 2001) |
| Maternal diet | Rat | Altered ICM:TE number and ratio | (Kwong et al., 2000) |
| Tobacco | Rat | Developmental delay | (Tachi and Aoyama, 1989) |
TABLE 3.
Selected Evidence from Animal Models of Long-Term Effects Observed after Preimplantation Stress
| Impact | Stressor | Reference |
|---|---|---|
| Impaired placentation and fetal growth | Embryo culture | (Bloise et al., 2012) |
| IVF | (Delle Piane et al., 2010) | |
| Reduced Birthweight | Maternal diet | (Kwong et al., 2000) |
| Altered Behavior (anxiety) | Embryo culture | (Fernandez-Gonzalez et al., 2004; Ecker et al., 2004) |
| Alteration of imprinted/epigenetic loci | Embryo culture | (Fernandez-Gonzalez et al., 2004) |
| Altered growth curves | Maternal diet | (Kwong et al., 2000; Watkins et al., 2008) |
| Hypertension | Maternal diet | (Kwong et al., 2000; Watkins et al., 2007) |
| Glucose intolerance | IVF and in vitro culture | (Rinaudo et al., 2009; Scott et al., 2010) |
It is well described that culture in vitro delays embryo development by 18 to 24 hr (Bowman and McLaren, 1970; Harlow and Quinn, 1982). Our laboratory has explored the impact of various forms of pre-implantation stress in mice, and has reported that factors, such as culture media composition, oxygen tension, method of fertilization, and culture dish rigidity will impact the percentage of embryos developing to blastocyst, ICM and TE lineage ratio and cell number, embryo morphology, and gene expression (Rinaudo and Schultz, 2004; Rinaudo et al., 2006; Giritharan et al., 2007, 2010, 2012; Kolahi et al., 2012). A recent study by Schwarzer et al. (2012) compared 13 ART culture protocols for mouse embryo development and observed distinct effects on developmental competence, lineage composition, and global gene expression with different conditions (Schwarzer et al., 2012). Each of these disparities could have a significant impact on fetal development after implantation, and thus influence organismal growth and health. In fact, there is evidence that placentation may be compromised following in vitro stress. TE cell number is reduced in blastocysts after IVF, ICSI, or various forms of culture (Rinaudo et al., 2006; Giritharan et al., 2007, 2010), with downregulated expression of genes involved in solute transport and placentation, as well as altered expression of several imprinted genes (Giritharan et al., 2012). This is particularly relevant since placental growth, efficiency, and nutrient transport capacity are frequently regulated by imprinted genes (Angiolini et al., 2006).
Mouse embryos fertilized and cultured under either optimal or substandard conditions in vitro exhibit similar implantation rates to in vivo embryos or to blastocysts flushed from oviducts and transferred into pseudo pregnant mothers (Delle et al., 2010). This suggests that despite the severity and type of stress, as well as variation in TE cell number, initial invasion of the uterine epithelium is a stable process. However, in these same embryos, abortion rates were significantly increased in the IVF groups and highest after suboptimal culture, possibly reflecting defective placentation or an inability to satisfy fetal needs. Impaired embryo viability following IVF or embryo culture has been confirmed by other groups (Holm et al., 1996; Khosla et al., 2001; Fernandez-Gonzalez et al., 2004; Block et al., 2010; Block et al., 2011; Bermejo-Alvarez et al., 2012).
Our laboratory has shown that IVF fetal and placental growth trajectories are significantly different throughout mouse fetal development. IVF placentae originate from fewer TE cells, are of normal weight by midgestation, and are significantly larger toward the end of pregnancy. Comparably, IVF fetuses are smaller throughout the majority of gestation, but display rapid catch-up growth to controls during the second half of gestation, possibly due to enlarged placentae (Bloise et al., 2012). At birth, weights are statistically indistinguishable between IVF and control animals, demonstrating that birth weight may be a weak indicator of fetal growth and nutrition.
The first record that preimplantation-specific stress can produce clear definable postnatal phenotypes came from Ecker et al. in 2004, who observed decreased anxiety and impaired spatial memory in adult mice after in vitro culture from the two-cell to blastocyst stage, without a significant effect of culture or embryo transfer on development to term (Ecker et al., 2004). Similar behavioral changes have been observed in mice transferred at the two-cell stage following ICSI (Fernandez-Gonzalez et al., 2008).
Animal models have additionally demonstrated that preimplantation conditions may affect long-term growth and metabolic health. Several groups have described increased body weights in mice after IVF, ICSI, or preimplantation culture (Fernandez-Gonzalez et al., 2004, 2008; Scott et al., 2010). Importantly, these differences may be present throughout postnatal growth, or appear later in adulthood, demonstrating latent effects of stress on physiology. Forms of in vitro stress reportedly affect organ size and integrity: Fernandez-Gonzalez et al. (2004) observed cases of pneumonia, kidney inflammation, and testicular atrophy in adult mice after embryo culture, as well as liver steatosis, possibly reflecting defective glycogen metabolism and resulting lipid mobilization as a compensatory energy source. Mice derived by IVF or ICSI exhibit signs of glucose intolerance and insulin resistance (Scott et al., 2010), and our group has noted similar detriment to long-term glucose homeostasis after IVF (Rinaudo et al., 2009). Embryo culture stress is also associated with increased systolic blood pressure in 21-wk mice, and alters the expression of several metabolic and cardiovascular genes (Watkins et al., 2007). These findings not only substantiate similar observations in humans, but suggest that metabolic reprogramming after assisted conception may not be an infertility effect or an outcome exclusive to ART populations.
POTENTIAL SOURCES OF IN VITRO STRESS OCCURRING DURING THE PREIMPLANTATION PERIOD
Studies of embryo development under controlled conditions have identified a number of different sources of stress influencing preimplantation development. Because the environment in vitro can be exactly manipulated, we have included a review of specific conditions that can play an important role in affecting embryo growth.
Intracytoplasmic Sperm Injection (ICSI)
Since the introduction of intracytoplasmic sperm injection (ICSI) as a method of fertilization in 1992, ICSI has become the most extensively used fertilization technique, accounting for approximately two-thirds of all fertilization treatments worldwide (Palermo et al., 1993; Ferraretti et al., 2012). Briefly, a micromanipulation device is used to pierce the zona pellucida of a denuded oocyte and inject a single spermatozoon into the ooplasm. ICSI has been confronted with certain suspicion, however, as the circumstances of the procedure itself employ many stressful techniques. Both sets of gametes must be handled to assess maturity, and in oocytes this is accomplished after enzymatic and mechanical removal of the protective surrounding cumulous and corona cells (Kimura and Yanagimachi, 1995). Moreover, direct penetration of the zona pellucida is physically destructive. Breach of the intra/extracellular barrier may result in cytoplasmic leakage or introduce culture media into the oocyte, thereby contaminating the cytoplasm. Animal studies show that ICSI-generated zygotes cleave at a slower rate, have a reduced hatching rate and reduced cell number in both trophectoderm and inner cell mass (Giritharan et al., 2010). ICSI zygotes also exhibit shorter calcium oscillations with an altered pattern (Kurokawa and Fissore, 2003). Blastocysts resulting from ICSI fertilization show approximately 1000 genes differentially expressed, compared with in vivo blastocysts (Giritharan et al., 2010). Importantly, as described earlier, ICSI-generated mice using fresh spermatozoa develop long-term consequences, such as obesity and organomegaly (Fernandez-Gonzalez et al., 2008).
Culture Media
The energy demands of the mammalian preimplantation embryo are perhaps the most vital aspects to consider when examining the various facets of preimplantation stress. Embryos exhibit diverse nutritional requirements, and the oviduct functions to fulfill those needs by providing energy substrates suitable for optimal development and metabolism [reviewed in (Leese et al., 2008; Leese, 2012)]. Tubal fluid is composed of nutrients, macromolecules, and electrolytes, the concentrations of which are remarkably conserved throughout different mammalian species (Gardner and Leese, 1990). Oviductal secretions are receptive to embryos and their evolving needs, providing different hormones, growth factors, and antioxidant defenses as embryos mature from zygote to blastocyst (Aviles et al., 2010). Surprisingly, the concentrations of many components found in culture media differ markedly from their physiological quantities (Gardner and Leese, 1990). This is important because the metabolism of preimplantation embryos relies heavily on feedback mechanisms (Summers and Biggers, 2003). For example, the concentration of lactate in culture media influences the degree of pyruvate metabolism (Lane and Gardner, 2000). In vitro, the build up of metabolic derivatives, including carbohydrate substrates or toxic byproducts, may contaminate culture media and affect energy usage by embryos (Lane and Gardner, 2003; Gardner and Lane, 2003). Although the use of sequential media is combative against this, in vitro culture is a closed system compared with the metabolic waste dissipation capacities provided by the oviduct (which to an embryo are essentially infinite) (Gardner and Lane, 1998). Variations in nutrient availability alter developmental competence (Edwards et al., 1998a, b), implying that culture in vitro is a source of great stress. Yet embryos can adapt to poor conditions, although embryo recovery rates after nutritional disturbances correlate with the severity of stress (Zhao and Baltz, 1996).
Independently, embryos culture is conducted under paraffin oil. Oil prevents evaporation of the medium to preserve a constant osmolarity, as well as minimizes fluctuations of pH and temperature (Tae et al., 2006). Oil also, however, can be toxic to gametes and embryos (Van Langendonckt et al., 1996).
pH
pH is a powerful modulator of metabolic activity, and manipulations of intracellular pH are associated with changes to cellular proliferation, transcriptional activity, protein localization and synthesis, as well as glycolytic function [reviewed in (Shrode et al., 1997; Webb et al., 2011)]. In the mammalian reproductive tract, gametes and cleavage stage embryos are exposed to markedly alkaline conditions in the oviduct (pH ≥7.5), before implanting into a more acidic uterine environment (pH 6.96) (Zhao and Baltz, 1996). The stratified reproductive environment is suggestive of stage-specific pH requirements during fertilization and preimplantation development, a theory validated by a recent study demonstrating enhanced development of cultured embryos allowed to progress through cleavage stages at higher pH before transition into a more acidic pH after compaction (Hentemann et al., 2011).
Fluctuations in pH are common in reproductive biology. pH oscillations are related to menstrual periodicity: parallel increases and decreases of pH in oviductal and uterine fluid (respectively) at the time of ovulation signify a role for pH regulation in optimizing fertilization and implantation events (Pommerenke and Breckenridge, 1952; Macdonald and Lumley, 1970; Maas et al., 1976). In humans, intercourse increases the pH of uterine fluid by 0.2 to 0.95 units for approximately 30 min, presumably to enhance capacitation and protect sperm from harsh acidic vaginal secretions, cervical mucus, and the uterine environment (Fox et al., 1973).
Importantly, the evolving pH environment from oviduct to uterus is lost with in vitro fertilization and culture systems. Culture media are routinely provided at pH 7.2 to 7.4, and most embryo culture protocols utilize a bicarbonate and CO2 buffering system. Incubation chambers maintaining CO2 concentrations of 5 to 7% under pressure alter the pH of culture media. In addition, the pH of culture conditions is unstable. Accumulation of pH-altering byproducts of embryo metabolism, such as lactate or ammonium, will pollute culture media over time (Edwards et al., 1998b; Lane and Gardner, 2003). Changes to pH (or lack thereof) could perturb metabolic homeostasis, stimulate or prevent induction of specific molecular pathways, and facilitate potentially irreversible metabolic changes.
Several groups have reported that extracellular pH manipulation during preimplantation development can initiate metabolic changes. For example, acid and alkaline loads acutely alter embryo intracellular pH, and are correlated with adjusted glycolytic activity to restore homeostasis (Gardner and Leese, 1988; Edwards et al., 1998a, b; Lane and Gardner, 2000, 2003). This implies the induction of an adaptive response to ensure optimal physiological activity. Separately, PFK1, a rate-limiting glycolytic enzyme, is released from allosteric regulation and increases its activity >100-fold between pH 7.0 and 7.5 (Bock and Frieden 1976a, b; Frieden et al., 1976). In embryo culture, the loss of normal pH shift from greater than 7.6 to below 7.0 during the progression from zygote to blastocyst would undoubtedly have an effect on metabolic activity, possibly through a PFK1-related mechanism. We have observed a 1.63-fold increase in PFK1 transcription in suboptimally cultured blastocysts after IVF, which due to the sensitivity of the enzyme, may have an enormous impact on glycolytic activity (Giritharan et al., 2007). Additionally, preimplantation stress modifies the expression of several genes encoding membrane-bound transporters that buffer pH within a narrow range (Zhao et al., 1995; Phillips et al., 2000), leading to changes in intracellular pH and affected transcriptional activity (Putney et al., 2004; Chen et al., 2008).
Stiffness of the Environment
A cell’s location within its physical surroundings is tremendously important for different cellular behaviors. Mechanotransduction is the process by which cells interpret mechanical information obtained from the environment, providing a means of transducing biophysical stimuli into an appropriate biochemical response [reviewed in (Ingber, 2006)]. Variations in tensile force have been linked to several developmental processes, including proliferative capacity, morphogenesis, and feedback mechanisms of growth control (Ingber, 2006; Labouesse, 2011). Not only can the relative elasticity of an extracellular environment affect cell differentiation (Wang et al., 2001), but mechanical force is a key factor in the ability of tumor cells to invade and metastasize (Butcher et al., 2009). This depends in part upon the distribution of intercellular junctions, as well as extracellular matrix composition (Levental et al., 2009). Still, the relevance of tension and stiffness during pre-implantation development has been largely overlooked. Embryo blastomeres, oviducts, and uterine epithelia are relatively soft, with elasticities ranging from 100 to 1000 Pascals (Pa; the unit of pressure or tensile strength incorporating forces of stress and strain, used to describe force per unit area) (Kolahi et al., 2012). A polystyerene petri dish, routinely used to culture preimplantation embryos, is roughly six orders of magnitude stiffer at 1 GPa (Discher et al., 2005). As a result, development in an in vitro culture system, in conjunction with the physical manipulation of embryos or gametes, may be a source of mechanical stress influencing cell fate specification and developmental potential. Our laboratory has confirmed this hypothesis in a recent study demonstrating that fertilization and blastulation rates, as well as TE cell numbers, are higher for embryos developing on a collagen matrix versus a standard polystyrene petri dish (Kolahi et al., 2012).
Temperature
Even small fluctuations in temperature are relevant to gamete and embryo viability. Sperm development occurs 1 to 2°C below core body temperature, and is impaired by heat stress. Ovarian follicles also maintain a temperature approximately 1.5 to 2.5°C lower than deep body temperature [reviewed in (Leese et al., 2008)], which has implications for in vitro maturation technology. Temperature gradients have been described along the Fallopian tube (David et al., 1971; Hunter and Nichol, 1986), with correlation to menstrual periodicity (Bahat et al., 2005). In human oocytes, microtubule depolymerization initiates after temperature decreases as small as 5°C, and is irreversible after a 10°C decrease to 27°C (Almeida and Bolton, 1995). Ten minutes at 33°C causes absolute microtubule disassembly, leaving spindles permanently disorganized (Wang et al., 2001). Unfortunately, exposure to room temperature conditions is unavoidable during in vitro culture of embryos. While this is minimized by culture under a protective coat of paraffin oil, longer intervals outside of 37°C incubators will result in a temperature change in culture.
Cryopreservation of Gametes and Embryos
Thermal forces as a source of stress have become increasingly significant to preimplantation development after the introduction of cryopreservation techniques as a method of storing and preserving gametes and embryos. Human IVF laboratories conduct embryo freezing from 37°C to −196°C in two fashions, either by a controlled, slow-rate cooling, or by ultra-rapid (<1 s) vitrification; warming occurs over 1 to 2 min at room temperature. Freezing and thawing procedures can be damaging to intracellular structures and membrane integrity, to which delicate oocytes and embryos are particularly sensitive (Mandelbaum et al., 2004). Cryoinjury occurs in many forms, including ice crystal formation, structural damage to water-bound enzymes, separation of membrane proteins from lipids, altered membrane permeability resulting from phase transitions of phospholipids, and osmotic stress due to changes in cell volume (Carrell and Peterson, 2010). Indeed, cryodamage frequently results in embryo loss, although there are reports of healthy pregnancy in cases with up to 50% cell injury after freezing (Hartshorne et al., 1990). Freezing may additionally induce hardening of the zona pellucida (Matson et al., 1997), necessitating assisted blastocyst hatching (Tucker et al., 1991). Cryopreservation has been associated with zona cracking and resulting cytoplasmic leakage (Fabbri et al., 2001), microtubule disarray leading to meiotic spindle disruption, and chromosome dispersal (Boiso et al., 2002), as well as other cytoskeletal rearrangements and cytoplasmic disorder (Saunders and Parks, 1999).
Oxygen
The role of oxygen concentration in early development has become a subject of great interest. Culture of early embryos has historically been conducted similarly to somatic cells, under atmospheric conditions (~20% oxygen) (Gomes Sobrinho et al., 2011). However, several studies of oxygen concentration in the oviduct and uterus have demonstrated that oxygen tension fluctuates within 2–8% across different mammalian species (Mastroianni and Jones 1965; Mitchell and Yochim, 1968; Maas et al., 1976; Fischer and Bavister, 1993; Kaufman and Mitchell, 1994; Bavister, 2004). Despite the variation, this indicates that the female reproductive tract is absolutely capable of supporting aerobic metabolism in gametes and embryos (Leese, 1988). The generation of harmful reactive oxygen species (ROS) such as superoxide, hydrogen peroxide, and hydroxyl radicals is one major feature of aerobic respiration, although these may also originate from embryo surroundings (Guerin et al., 2001). Moreover, rises in oxygen concentration during culture are directly related to the increased production of oxygen free radicals in embryos (Goto et al., 1993). The accumulation of ROS threatens cellular viability, as they are damaging to all major cellular macromolecules, membrane and organelle integrity, as well as transcription. In fact, oxidative stress is considered to be a principal cause of the two-cell developmental block (Johnson and Nasr-Esfahani, 1994; Favetta et al., 2007). Although embryos are equipped with several endogenous means of countering oxidative stress, many antioxidant genes are not expressed until later stages of preimplantation development (Harvey et al., 1995). Production and accumulation of ROS from exposure to atmospheric oxygen concentrations during in vitro culture may therefore overwhelm embryo defense mechanisms.
In contrast, ROS at low concentrations function as critical physiological signals in many important cellular pathways, such as signal transduction, apoptosis, and transcriptional activity (Hancock et al., 2001). As a result, high oxygen tensions may induce unconventional downstream pathways. Oxidative stress can modulate gene expression, cause aberrant intracellular signaling, or affect the redox ratios of several key metabolites, and these changes are associated with supplementary rippling effects throughout metabolism (Guerin et al., 2001).
CONCLUSIONS
The embryo response to preimplantation stress, manifested by changes in cell number, lineage ratio, growth velocity, or gene expression patterns, highlights its remarkable plasticity and capacity for survival. Analysis of the literature reveals that many different sources of stress during the preimplantation period will have widespread effects on growth and metabolism. Stress can divert resources away from networks involved in coordinating optimal development, down alternative metabolic branches. The quiet embryo hypothesis postulates that stressed embryos will require more resources and energy in an attempt to alleviate molecular damage, leading to greater metabolic activity (Leese et al., 2008). An increase in mitochondrial oxidative phosphorylation to fuel cell repair machinery would be associated with an increase in free oxygen radicals, leading to aberrant signaling or cell damage. If this occurs during epigenetic reorganization, cellular defense strategies against transient stress exposure may be inappropriately programmed as homeostasis, leading to atypical responses to normal physiological signals, exhaustion of cell machinery, and long-term phenotypes. Alternatively, subtle metabolite fluctuations or atypical nutrient availability might not redirect resources towards damage responses, but rather be sensed as a ‘normal’ environment, eliciting an ‘appropriate’ programming reaction. However, these reprogramming events might be strategic for early survival, but inadequate for optimal postnatal life. This proves that long-term follow-up studies on the health of ART children are imperative, because although the great majority of children conceived by ART appear healthy, the long-term health impacts of these procedures are unknown.
Acknowledgments
Grant support: NICHD grant R01 HD062803-02 to PR.
Contributor Information
Sky Feuer, Department of Obstetrics, Gynecology and Reproductive Sciences, Division of Reproductive Endocrinology and Infertility, University of California, San Francisco, California 94115.
Paolo Rinaudo, Eli and Edythe Broad Center for Regeneration Medicine and Stem Cell Research, University of California, San Francisco, California 94143.
References
- Almeida PA, Bolton VN. The effect of temperature fluctuations on the cytoskeletal organisation and chromosomal constitution of the human oocyte. Zygote. 1995;3:357–365. doi: 10.1017/s0967199400002793. [DOI] [PubMed] [Google Scholar]
- Angiolini E, Fowden A, Coan P, et al. Regulation of placental efficiency for nutrient transport by imprinted genes. Placenta. 2006;27(Suppl A):S98–S102. doi: 10.1016/j.placenta.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Ashworth A. Effects of intrauterine growth retardation on mortality and morbidity in infants and young children. Eur J Clin Nutr. 1998;52(Suppl 1):S34–S41. discussion S41–S32. [PubMed] [Google Scholar]
- Aviles M, Gutierrez-Adan A, Coy P. Oviductal secretions: will they be key factors for the future ARTs? Mol Hum Reprod. 2010;16:896–906. doi: 10.1093/molehr/gaq056. [DOI] [PubMed] [Google Scholar]
- Bahat A, Eisenbach M, Tur-Kaspa I. Periovulatory increase in temperature difference within the rabbit oviduct. Hum Reprod. 2005;20:2118–2121. doi: 10.1093/humrep/dei006. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–1081. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- Batcheller A, Cardozo E, Maguire M, et al. Are there subtle genome-wide epigenetic alterations in normal offspring conceived by assisted reproductive technologies? Fertil Steril. 2011;96:1306–1311. doi: 10.1016/j.fertnstert.2011.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavister B. Oxygen concentration and preimplantation development. Reprod Biomed Online. 2004;9:484–486. doi: 10.1016/s1472-6483(10)61630-6. [DOI] [PubMed] [Google Scholar]
- Bermejo-Alvarez P, Rizos D, Lonergan P, et al. Transcriptional sexual dimorphism during preimplantation embryo development and its consequences for developmental competence and adult health and disease. Reproduction. 2011;141:563–570. doi: 10.1530/REP-10-0482. [DOI] [PubMed] [Google Scholar]
- Bermejo-Alvarez P, Roberts RM, Rosenfeld CS. Effect of glucose concentration during in vitro culture of mouse embryos on development to blastocyst, success of embryo transfer, and litter sex ratio. Mol Reprod Dev. 2012;79:329–336. doi: 10.1002/mrd.22028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggers JD, Whittingham DG, Donahue RP. The pattern of energy metabolism in the mouse oocyte and zygote. Proc Natl Acad Sci USA. 1967;58:560–567. doi: 10.1073/pnas.58.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block J, Bonilla L, Hansen PJ. Efficacy of in vitro embryo transfer in lactating dairy cows using fresh or vitrified embryos produced in a novel embryo culture medium. J Dairy Sci. 2010;93:5234–5242. doi: 10.3168/jds.2010-3443. [DOI] [PubMed] [Google Scholar]
- Block J, Hansen PJ, Loureiro B, et al. Improving post-transfer survival of bovine embryos produced in vitro: actions of insulin-like growth factor-1, colony stimulating factor-2 and hyaluronan. Theriogenology. 2011;76:1602–1609. doi: 10.1016/j.theriogenology.2011.07.025. [DOI] [PubMed] [Google Scholar]
- Bloise E, Lin W, Liu X, et al. Impaired placental nutrient transport in mice generated by in vitro fertilization. Endocrinology. 2012;153:3457–3467. doi: 10.1210/en.2011-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bnait KS, Seller MJ. Ultrastructural changes in 9-day old mouse embryos following maternal tobacco smoke inhalation. Exp Toxicol Pathol. 1995;47:453–461. doi: 10.1016/S0940-2993(11)80327-1. [DOI] [PubMed] [Google Scholar]
- Bock PE, Frieden C. Phosphofructokinase. II. Role of ligands in pH-dependent structural changes of the rabbit muscle enzyme. J Biol Chem. 1976a;251:5637–5643. [PubMed] [Google Scholar]
- Bock PE, Frieden C. Phosphofructokinase. I. Mechanism of the pH-dependent inactivation and reactivation of the rabbit muscle enzyme. J Biol Chem. 1976b;251:5630–5636. [PubMed] [Google Scholar]
- Boiso I, Marti M, Santalo J, et al. A confocal microscopy analysis of the spindle and chromosome configurations of human oocytes cryopreserved at the germinal vesicle and metaphase II stage. Hum Reprod. 2002;17:1885–1891. doi: 10.1093/humrep/17.7.1885. [DOI] [PubMed] [Google Scholar]
- Bowman P, McLaren A. Viability and growth of mouse embryos after in vitro culture and fusion. J Embryol Exp Morphol. 1970;23:693–704. [PubMed] [Google Scholar]
- Brent RL, Holmes LB. Clinical and basic science lessons from the thalidomide tragedy: what have we learned about the causes of limb defects? Teratology. 1988;38:241–251. doi: 10.1002/tera.1420380308. [DOI] [PubMed] [Google Scholar]
- Brinster RL, Thomson JL. Development of eight-cell mouse embryos in vitro. Exp Cell Res. 1966;42:308–315. doi: 10.1016/0014-4827(66)90295-3. [DOI] [PubMed] [Google Scholar]
- Brinster RL. Studies on the development of mouse embryos in vitro. IV. Interaction of energy sources. J Reprod Fertil. 1965;10:227–240. doi: 10.1530/jrf.0.0100227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton LA, Westhoff CL, Scoccia B, et al. Causes of infertility as predictors of subsequent cancer risk. Epidemiology. 2005;16:500–507. doi: 10.1097/01.ede.0000164812.02181.d5. [DOI] [PubMed] [Google Scholar]
- Buck GM, Msall ME, Schisterman EF, et al. Extreme prematurity and school outcomes. Paediatr Perinat Epidemiol. 2000;14:324–331. doi: 10.1046/j.1365-3016.2000.00276.x. [DOI] [PubMed] [Google Scholar]
- Burdge GC, Lillycrop KA. Nutrition, epigenetics, and developmental plasticity: implications for understanding human disease. Annu Rev Nutr. 2010;30:315–339. doi: 10.1146/annurev.nutr.012809.104751. [DOI] [PubMed] [Google Scholar]
- Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9:108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrell DT, Peterson CM, editors. Reproductive endocrinology and infertility: integrating modern clinical and laboratory practice. New York: Springer; 2010. [Google Scholar]
- Ceelen M, van Weissenbruch MM, Prein J, et al. Growth during infancy and early childhood in relation to blood pressure and body fat measures at age 8–18 years of IVF children and spontaneously conceived controls born to subfertile parents. Hum Reprod. 2009;24:2788–2795. doi: 10.1093/humrep/dep273. [DOI] [PubMed] [Google Scholar]
- Ceelen M, van Weissenbruch MM, Roos JC, et al. Body composition in children and adolescents born after in vitro fertilization or spontaneous conception. J Clin Endocrinol Metab. 2007;92:3417–3423. doi: 10.1210/jc.2006-2896. [DOI] [PubMed] [Google Scholar]
- Ceelen M, van Weissenbruch MM, Vermeiden JP, et al. Pubertal development in children and adolescents born after IVF and spontaneous conception. Hum Reprod. 2008;23:2791–2798. doi: 10.1093/humrep/den309. [DOI] [PubMed] [Google Scholar]
- Ceelen M, van Weissenbruch MM, Vermeiden JP, et al. Cardiometabolic differences in children born after in vitro fertilization: follow-up study. J Clin Endocrinol Metab. 2008a;93:1682–1688. doi: 10.1210/jc.2007-2432. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention ASfRM; Society for Assisted Reproductive Technology. 2010 National ART Summary Report. 2012. [Google Scholar]
- Chen JL, Lucas JE, Schroeder T, et al. The genomic analysis of lactic acidosis and acidosis response in human cancers. PLoS Genet. 2008;4:e1000293. doi: 10.1371/journal.pgen.1000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn K, Rossant J. Making the blastocyst: lessons from the mouse. J Clin Invest. 2010;120:995–1003. doi: 10.1172/JCI41229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David A, Vilensky A, Nathan H. Temperature changes in different parts of the rabbit oviduct. Preliminary report. Harefuah. 1971;80:180–182. [PubMed] [Google Scholar]
- Davies MJ, Moore VM, Willson KJ, et al. Reproductive technologies and the risk of birth defects. N Engl J Med. 2012;366:1803–1813. doi: 10.1056/NEJMoa1008095. [DOI] [PubMed] [Google Scholar]
- de Rooij SR, Painter RC, Phillips DI, et al. Hypothalamic-pituitary-adrenal axis activity in adults who were prenatally exposed to the Dutch famine. Eur J Endocrinol. 2006a;155:153–160. doi: 10.1530/eje.1.02193. [DOI] [PubMed] [Google Scholar]
- de Rooij SR, Painter RC, Phillips DI, et al. Impaired insulin secretion after prenatal exposure to the Dutch famine. Diabetes Care. 2006b;29:1897–1901. doi: 10.2337/dc06-0460. [DOI] [PubMed] [Google Scholar]
- de Rooij SR, Painter RC, Phillips DI, et al. Cortisol responses to psychological stress in adults after prenatal exposure to the Dutch famine. Psychoneuroendocrinology. 2006c;31:1257–1265. doi: 10.1016/j.psyneuen.2006.09.007. [DOI] [PubMed] [Google Scholar]
- de Rooij SR, Painter RC, Roseboom TJ, et al. Glucose tolerance at age 58 and the decline of glucose tolerance in comparison with age 50 in people prenatally exposed to the Dutch famine. Diabetologia. 2006d;49:637–643. doi: 10.1007/s00125-005-0136-9. [DOI] [PubMed] [Google Scholar]
- de Rooij SR, Wouters H, Yonker JE, et al. Prenatal undernutrition and cognitive function in late adulthood. Proc Natl Acad Sci USA. 2010;107:16881–16886. doi: 10.1073/pnas.1009459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delle Piane L, Lin W, et al. Effect of the method of conception and embryo transfer procedure on midgestation placenta and fetal development in an IVF mouse model. Hum Reprod. 2010;25:2039–2046. doi: 10.1093/humrep/deq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh RS, Ostrup O, Ostrup E, et al. DNA methylation in porcine preimplantation embryos developed in vivo and produced by in vitro fertilization, parthenogenetic activation and somatic cell nuclear transfer. Epigenetics. 2011;6:177–187. doi: 10.4161/epi.6.2.13519. [DOI] [PubMed] [Google Scholar]
- Dey SK, Lim H, Das SK, et al. Molecular cues to implantation. Endocr Rev. 2004;25:341–373. doi: 10.1210/er.2003-0020. [DOI] [PubMed] [Google Scholar]
- DiCarlantonio G, Talbot P. Inhalation of mainstream and sidestream cigarette smoke retards embryo transport and slows muscle contraction in oviducts of hamsters (Mesocricetus auratus) Biol Reprod. 1999;61:651–656. doi: 10.1095/biolreprod61.3.651. [DOI] [PubMed] [Google Scholar]
- Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- Doherty AS, Mann MR, Tremblay KD, et al. Differential effects of culture on imprinted H19 expression in the preimplantation mouse embryo. Biol Reprod. 2000;62:1526–1535. doi: 10.1095/biolreprod62.6.1526. [DOI] [PubMed] [Google Scholar]
- Ecker DJ, Stein P, Xu Z, et al. Long-term effects of culture of preimplantation mouse embryos on behavior. Proc Natl Acad Sci USA. 2004;101:1595–1600. doi: 10.1073/pnas.0306846101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards LJ, Williams DA, Gardner DK. Intracellular pH of the mouse preimplantation embryo: amino acids act as buffers of intracellular pH. Hum Reprod. 1998a;13:3441–3448. doi: 10.1093/humrep/13.12.3441. [DOI] [PubMed] [Google Scholar]
- Edwards LJ, Williams DA, Gardner DK. Intracellular pH of the preimplantation mouse embryo: effects of extracellular pH and weak acids. Mol Reprod Dev. 1998b;50:434–442. doi: 10.1002/(SICI)1098-2795(199808)50:4<434::AID-MRD7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Eriksson J, Forsen T, Tuomilehto J, et al. Fetal and childhood growth and hypertension in adult life. Hypertension. 2000;36:790–794. doi: 10.1161/01.hyp.36.5.790. [DOI] [PubMed] [Google Scholar]
- Eriksson JG, Forsen T, Tuomilehto J, et al. Catch-up growth in childhood and death from coronary heart disease: longitudinal study. BMJ. 1999;318:427–431. doi: 10.1136/bmj.318.7181.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson JG, Forsen TJ, Kajantie E, et al. Childhood growth and hypertension in later life. Hypertension. 2007;49:1415–1421. doi: 10.1161/HYPERTENSIONAHA.106.085597. [DOI] [PubMed] [Google Scholar]
- Fabbri R, Porcu E, Marsella T, et al. Human oocyte cryopreservation: new perspectives regarding oocyte survival. Hum Reprod. 2001;16:411–416. doi: 10.1093/humrep/16.3.411. [DOI] [PubMed] [Google Scholar]
- Favetta LA, StJohn EJ, King WA, et al. High levels of p66shc and intracellular ROS in permanently arrested early embryos. Free Radic Biol Med. 2007;42:1201–1210. doi: 10.1016/j.freeradbiomed.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Fernandez-Gonzalez R, Moreira P, et al. Long-term effect of in vitro culture of mouse embryos with serum on mRNA expression of imprinting genes, development, and behavior. Proc Natl Acad Sci USA. 2004;101:5880–5885. doi: 10.1073/pnas.0308560101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Gonzalez R, Moreira PN, Perez-Crespo M, et al. Long-term effects of mouse intracytoplasmic sperm injection with DNA-fragmented sperm on health and behavior of adult offspring. Biol Reprod. 2008;78:761–772. doi: 10.1095/biolreprod.107.065623. [DOI] [PubMed] [Google Scholar]
- Ferraretti AP, Goossens V, deMouzon J, et al. Assisted reproductive technology in Europe, 2008: results generated from European registers by ESHRE. Hum Reprod. 2012;27:2571–2584. doi: 10.1093/humrep/des255. [DOI] [PubMed] [Google Scholar]
- Feuer S, Camarano L, Rinaudo P. Impact of ART on adult health: insights on molecular mechanisms from animal models. Mol Hum Reprod. doi: 10.1093/molehr/gas066. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnstrom O, Kallen B, Lindam A, et al. Maternal and child outcome after in vitro fertilization–a review of 25 years of population-based data from Sweden. Acta Obstet Gynecol Scand. 2011;90:494–500. doi: 10.1111/j.1600-0412.2011.01088.x. [DOI] [PubMed] [Google Scholar]
- Fischer B, Bavister BD. Oxygen tension in the oviduct and uterus of rhesus monkeys, hamsters and rabbits. J Reprod Fertil. 1993;99:673–679. doi: 10.1530/jrf.0.0990673. [DOI] [PubMed] [Google Scholar]
- Fischer JJ, Toedling J, Krueger T, et al. Combinatorial effects of four histone modifications in transcription and differentiation. Genomics. 2008;91:41–51. doi: 10.1016/j.ygeno.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Forsen T, Eriksson JG, Tuomilehto J, et al. Growth in utero and during childhood among women who develop coronary heart disease: longitudinal study. BMJ. 1999;319:1403–1407. doi: 10.1136/bmj.319.7222.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortunato A, Tosti E. The impact of in vitro fertilization on health of the children: an update. Eur J Obstet Gynecol Reprod Biol. 2011;154:125–129. doi: 10.1016/j.ejogrb.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Sibley C, Reik W, et al. Imprinted genes, placental development and fetal growth. Horm Res. 2006;65(Suppl 3):50–58. doi: 10.1159/000091506. [DOI] [PubMed] [Google Scholar]
- Fox CA, Meldrum SJ, Watson BW. Continuous measurement by radio-telemetry of vaginal pH during human coitus. J Reprod Fertil. 1973;33:69–75. doi: 10.1530/jrf.0.0330069. [DOI] [PubMed] [Google Scholar]
- Frieden C, Gilbert HR, Bock PE. Phosphofructokinase. III. Correlation of the regulatory kinetic and molecular properties of the rabbit muscle enzyme. J Biol Chem. 1976;251:5644–5647. [PubMed] [Google Scholar]
- Gardner DK, Lane M. Culture of viable human blastocysts in defined sequential serum-free media. Hum Reprod. 1998;13(Suppl 3):148–159. doi: 10.1093/humrep/13.suppl_3.148. discussion 160. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Lane M. Towards a single embryo transfer. Reprod Biomed Online. 2003;6:470–481. doi: 10.1016/s1472-6483(10)62170-0. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Leese HJ. The role of glucose and pyruvate transport in regulating nutrient utilization by pre-implantation mouse embryos. Development. 1988;104:423–429. doi: 10.1242/dev.104.3.423. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Leese HJ. Concentrations of nutrients in mouse oviduct fluid and their effects on embryo development and metabolism in vitro. J Reprod Fertil. 1990;88:361–368. doi: 10.1530/jrf.0.0880361. [DOI] [PubMed] [Google Scholar]
- Gieseke C, Talbot P. Cigarette smoke inhibits hamster oocyte pickup by increasing adhesion between the oocyte cumulus complex and oviductal cilia. Biol Reprod. 2005;73:443–451. doi: 10.1095/biolreprod.105.041152. [DOI] [PubMed] [Google Scholar]
- Gillet E, Martens E, Martens G, et al. Prelabour caesarean section following ivf/icsi in older-term nulliparous women: too precious to push? J Pregnancy. 2011:362518. doi: 10.1155/2011/362518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giritharan G, Delle Piane L, Donjacour A, et al. In vitro culture of mouse embryos reduces differential gene expression between inner cell mass and trophectoderm. Reprod Sci. 2012;19(3):243–252. doi: 10.1177/1933719111428522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giritharan G, Li MW, De Sebastiano F, et al. Effect of ICSI on gene expression and development of mouse preimplantation embryos. Hum Reprod. 2010;25:3012–3024. doi: 10.1093/humrep/deq266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giritharan G, Talbi S, Donjacour A, et al. Effect of in vitro fertilization on gene expression and development of mouse preimplantation embryos. Reproduction. 2007;134:63–72. doi: 10.1530/REP-06-0247. [DOI] [PubMed] [Google Scholar]
- Gomes Sobrinho DB, Oliveira JB, et al. IVF/ICSI outcomes after culture of human embryos at low oxygen tension: a meta-analysis. Reprod Biol Endocrinol. 2011;9:143. doi: 10.1186/1477-7827-9-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Noda Y, Mori T, Nakano M. Increased generation of reactive oxygen species in embryos cultured in vitro. Free Radic Biol Med. 1993;15:69–75. doi: 10.1016/0891-5849(93)90126-f. [DOI] [PubMed] [Google Scholar]
- Guerin P, El Mouatassim S, Menezo Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum Reprod Update. 2001;7:175–189. doi: 10.1093/humupd/7.2.175. [DOI] [PubMed] [Google Scholar]
- Hancock JT, Desikan R, Neill SJ. Role of reactive oxygen species in cell signalling pathways. Biochem Soc Trans. 2001;29:345–350. doi: 10.1042/0300-5127:0290345. [DOI] [PubMed] [Google Scholar]
- Hansen M, Kurinczuk JJ, Bower C, et al. The risk of major birth defects after intracytoplasmic sperm injection and in vitro fertilization. N Engl J Med. 2002;346:725–730. doi: 10.1056/NEJMoa010035. [DOI] [PubMed] [Google Scholar]
- Harlow GM, Quinn P. Development of preimplantation mouse embryos in vivo and in vitro. Aust J Biol Sci. 1982;35:187–193. doi: 10.1071/bi9820187. [DOI] [PubMed] [Google Scholar]
- Harrison M, Langley-Evans SC. Intergenerational programming of impaired nephrogenesis and hypertension in rats following maternal protein restriction during pregnancy. Br J Nutr. 2009;101:1020–1030. doi: 10.1017/S0007114508057607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorne GM, Wick K, Elder K, et al. Effect of cell number at freezing upon survival and viability of cleaving embryos generated from stimulated IVF cycles. Hum Reprod. 1990;5:857–861. doi: 10.1093/oxfordjournals.humrep.a137198. [DOI] [PubMed] [Google Scholar]
- Harvey MB, Arcellana-Panlilio MY, Zhang X, et al. Expression of genes encoding antioxidant enzymes in preimplantation mouse and cow embryos and primary bovine oviduct cultures employed for embryo coculture. Biol Reprod. 1995;53:532–540. doi: 10.1095/biolreprod53.3.532. [DOI] [PubMed] [Google Scholar]
- Hassa H, Gurer F, Tanir HM, et al. Effect of cigarette smoke and alpha-tocopherol (vitamin E) on fertilization, cleavage, and embryo development rates in mice: an experimental in vitro fertilization mice model study. Eur J Obstet Gynecol Reprod Biol. 2007;135:177–182. doi: 10.1016/j.ejogrb.2007.05.020. [DOI] [PubMed] [Google Scholar]
- He K, Zhao H, Wang Q, et al. A comparative genome analysis of gene expression reveals different regulatory mechanisms between mouse and human embryo pre-implantation development. Reprod Biol Endocrinol. 2010;8:41. doi: 10.1186/1477-7827-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmerhorst FM, Perquin DA, Donker D, et al. Perinatal outcome of singletons and twins after assisted conception: a systematic review of controlled studies. BMJ. 2004;328:261. doi: 10.1136/bmj.37957.560278.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentemann M, Mousavi K, Bertheussen K. Differential pH in embryo culture. Fertil Steril. 2011;95:1291–1294. doi: 10.1016/j.fertnstert.2010.10.018. [DOI] [PubMed] [Google Scholar]
- Hille ET, den Ouden AL, Saigal S, et al. Behavioural problems in children who weigh 1000 g or less at birth in four countries. Lancet. 2001;357:1641–1643. doi: 10.1016/S0140-6736(00)04818-2. [DOI] [PubMed] [Google Scholar]
- Holemans K, Aerts L, Van Assche FA. Evidence for an insulin resistance in the adult offspring of pregnant streptozotocin-diabetic rats. Diabetologia. 1991;34:81–85. doi: 10.1007/BF00500377. [DOI] [PubMed] [Google Scholar]
- Holm P, Walker SK, Seamark RF. Embryo viability, duration of gestation and birth weight in sheep after transfer of in vitro matured and in vitro fertilized zygotes cultured in vitro or in vivo. J Reprod Fertil. 1996;107:175–181. doi: 10.1530/jrf.0.1070175. [DOI] [PubMed] [Google Scholar]
- Holman RC, Stoll BJ, Clarke MJ, et al. The epidemiology of necrotizing enterocolitis infant mortality in the United States. Am J Public Health. 1997;87:2026–2031. doi: 10.2105/ajph.87.12.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsthemke B, Ludwig M. Assisted reproduction: the epigenetic perspective. Hum Reprod Update. 2005;11:473–482. doi: 10.1093/humupd/dmi022. [DOI] [PubMed] [Google Scholar]
- Hunter RH, Nichol R. A preovulatory temperature gradient between the isthmus and ampulla of pig oviducts during the phase of sperm storage. J Reprod Fertil. 1986;77:599–606. doi: 10.1530/jrf.0.0770599. [DOI] [PubMed] [Google Scholar]
- Hvidtjorn D, Schieve L, Schendel D, et al. Cerebral palsy, autism spectrum disorders, and developmental delay in children born after assisted conception: a systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2009;163:72–83. doi: 10.1001/archpediatrics.2008.507. [DOI] [PubMed] [Google Scholar]
- ICMART. International Committee Monitoring Assisted Reproductive Technology (ICMART) World Report: preliminary 2008 data; ESHRE Annual Meeting; Istanbul, Turkey. 2012. [Google Scholar]
- Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006;20:811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- Jackson RA, Gibson KA, Wu YW, et al. Perinatal outcomes in singletons following in vitro fertilization: a meta-analysis. Obstet Gynecol. 2004;103:551–563. doi: 10.1097/01.AOG.0000114989.84822.51. [DOI] [PubMed] [Google Scholar]
- Jaddoe VW, Witteman JC. Hypotheses on the fetal origins of adult diseases: contributions of epidemiological studies. Eur J Epidemiol. 2006;21:91–102. doi: 10.1007/s10654-005-5924-5. [DOI] [PubMed] [Google Scholar]
- Jansson T, Lambert GW. Effect of intrauterine growth restriction on blood pressure, glucose tolerance and sympathetic nervous system activity in the rat at 3–4 months of age. J Hypertens. 1999;17:1239–1248. doi: 10.1097/00004872-199917090-00002. [DOI] [PubMed] [Google Scholar]
- Johnson MH, Nasr-Esfahani MH. Radical solutions and cultural problems: could free oxygen radicals be responsible for the impaired development of preimplantation mammalian embryos in vitro? Bioessays. 1994;16:31–38. doi: 10.1002/bies.950160105. [DOI] [PubMed] [Google Scholar]
- Kallen B, Finnstrom O, Lindam A, et al. Cancer risk in children and young adults conceived by in vitro fertilization. Pediatrics. 2010;126:270–276. doi: 10.1542/peds.2009-3225. [DOI] [PubMed] [Google Scholar]
- Kallen B, Finnstrom O, Nygren KG, et al. In vitro fertilization in Sweden: child morbidity including cancer risk. Fertil Steril. 2005;84:605–610. doi: 10.1016/j.fertnstert.2005.03.035. [DOI] [PubMed] [Google Scholar]
- Katari S, Turan N, Bibikova M, et al. DNA methylation and gene expression differences in children conceived in vitro or in vivo. Hum Mol Genet. 2009;18:3769–3778. doi: 10.1093/hmg/ddp319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman DL, Mitchell JA. Intrauterine oxygen tension during the oestrous cycle in the hamster: patterns of change. Comp Biochem Physiol Comp Physiol. 1994;107:673–678. doi: 10.1016/0300-9629(94)90368-9. [DOI] [PubMed] [Google Scholar]
- Kermack WO, McKendrick AG, McKinlay PL. Death-rates in Great Britain and Sweden: expression of specific mortality rates as products of two factors, and some consequences thereof. J Hyg (Lond) 1934;34:433–457. doi: 10.1017/s0022172400043230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorasanizadeh S. Recognition of methylated histones: new twists and variations. Curr Opin Struct Biol. 2011;21:744–749. doi: 10.1016/j.sbi.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Khosla S, Dean W, Brown D, et al. Culture of preimplantation mouse embryos affects fetal development and the expression of imprinted genes. Biol Reprod. 2001;64:918–926. doi: 10.1095/biolreprod64.3.918. [DOI] [PubMed] [Google Scholar]
- Khuc K, Blanco E, Burrows R, et al. Adolescent metabolic syndrome risk is increased with higher infancy weight gain and decreased with longer breast feeding. Int J Pediatr. 2012;2012:478610. doi: 10.1155/2012/478610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y, Yanagimachi R. Intracytoplasmic sperm injection in the mouse. Biol Reprod. 1995;52:709–720. doi: 10.1095/biolreprod52.4.709. [DOI] [PubMed] [Google Scholar]
- Knoll M, Shaoulian R, Magers T, et al. Ciliary beat frequency of hamster oviducts is decreased in vitro by exposure to solutions of mainstream and sidestream cigarette smoke. Biol Reprod. 1995;53:29–37. doi: 10.1095/biolreprod53.1.29. [DOI] [PubMed] [Google Scholar]
- Kolahi KS, Donjacour A, Liu X, et al. Effect of substrate stiffness on early mouse embryo development. PLoS One. 2012;7:e41717. doi: 10.1371/journal.pone.0041717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuivasaari-Pirinen P, Raatikainen K, Hippelainen M, et al. Adverse outcomes of IVF/ICSI pregnancies vary depending on aetiology of infertility. ISRN Obstet Gynecol. 2012;2012:451915. doi: 10.5402/2012/451915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa M, Fissore RA. ICSI-generated mouse zygotes exhibit altered calcium oscillations, inositol 1,4,5-trisphosphate receptor-1 down-regulation, and embryo development. Mol Hum Reprod. 2003;9:523–533. doi: 10.1093/molehr/gag072. [DOI] [PubMed] [Google Scholar]
- Kwong WY, Wild AE, Roberts P, et al. Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development. 2000;127:4195–4202. doi: 10.1242/dev.127.19.4195. [DOI] [PubMed] [Google Scholar]
- Labouesse M. Forces and tension in development. 2011/04/20. San Diego: Elsevier; 2011. [DOI] [PubMed] [Google Scholar]
- Lane M, Gardner DK. Lactate regulates pyruvate uptake and metabolism in the preimplantation mouse embryo. Biol Reprod. 2000;62:16–22. doi: 10.1095/biolreprod62.1.16. [DOI] [PubMed] [Google Scholar]
- Lane M, Gardner DK. Ammonium induces aberrant blastocyst differentiation, metabolism, pH regulation, gene expression and subsequently alters fetal development in the mouse. Biol Reprod. 2003;69:1109–1117. doi: 10.1095/biolreprod.103.018093. [DOI] [PubMed] [Google Scholar]
- Leese HJ, Baumann CG, Brison DR, et al. Metabolism of the viable mammalian embryo: quietness revisited. Mol Hum Reprod. 2008;14:667–672. doi: 10.1093/molehr/gan065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leese HJ, Hugentobler SA, Gray SM, et al. Female reproductive tract fluids: composition, mechanism of formation and potential role in the developmental origins of health and disease. Reprod Fertil Dev. 2008;20:1–8. doi: 10.1071/rd07153. [DOI] [PubMed] [Google Scholar]
- Leese HJ. The formation and function of oviduct fluid. J Reprod Fertil. 1988;82:843–856. doi: 10.1530/jrf.0.0820843. [DOI] [PubMed] [Google Scholar]
- Leese HJ. Metabolism of the pre-implantation embryo: 40 years on. Reproduction. 2012;143:417–427. doi: 10.1530/REP-11-0484. [DOI] [PubMed] [Google Scholar]
- Lems W, Hopkins B, Samson JF. Mental and motor development in preterm infants: the issue of corrected age. Early Hum Dev. 1993;34:113–123. doi: 10.1016/0378-3782(93)90046-w. [DOI] [PubMed] [Google Scholar]
- Levental KR, Yu H, Kass L, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Vu TH, Ulaner GA, et al. IVF results in de novo DNA methylation and histone methylation at an Igf2-H19 imprinting epigenetic switch. Mol Hum Reprod. 2005;11:631–640. doi: 10.1093/molehr/gah230. [DOI] [PubMed] [Google Scholar]
- Maas DH, Storey BT, Mastroianni L., Jr Oxygen tension in the oviduct of the rhesus monkey (Macaca mulatta) Fertil Steril. 1976;27:1312–1317. doi: 10.1016/s0015-0282(16)42201-6. [DOI] [PubMed] [Google Scholar]
- Macdonald RR, Lumley IB. Endocervical pH measured in vivo through the normal menstrual cycle. Obstet Gynecol. 1970;35:202–206. [PubMed] [Google Scholar]
- Maffini MV, Rubin BS, Sonnenschein C, et al. Endocrine disruptors and reproductive health: the case of bisphenol-A. Mol Cell Endocrinol. 2006:254–255. 179–186. doi: 10.1016/j.mce.2006.04.033. [DOI] [PubMed] [Google Scholar]
- Magers T, Talbot P, DiCarlantonio G, et al. Cigarette smoke inhalation affects the reproductive system of female hamsters. Reprod Toxicol. 1995;9:513–525. doi: 10.1016/0890-6238(95)02002-0. [DOI] [PubMed] [Google Scholar]
- Maher ER, Afnan M, Barratt CL. Epigenetic risks related to assisted reproductive technologies: epigenetics, imprinting, ART and icebergs? Hum Reprod. 2003;18:2508–2511. doi: 10.1093/humrep/deg486. [DOI] [PubMed] [Google Scholar]
- Mahsoudi B, Li A, O’Neill C. Assessment of the long-term and transgenerational consequences of perturbing preimplantation embryo development in mice. Biol Reprod. 2007;77:889–896. doi: 10.1095/biolreprod.106.057885. [DOI] [PubMed] [Google Scholar]
- Mandelbaum J, Anastasiou O, Levy R, et al. Effects of cryopreservation on the meiotic spindle of human oocytes. Eur J Obstet Gynecol Reprod Biol. 2004;113(Suppl 1):S17–S23. doi: 10.1016/j.ejogrb.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Manipalviratn S, DeCherney A, Segars J. Imprinting disorders and assisted reproductive technology. Fertil Steril. 2009;91:305–315. doi: 10.1016/j.fertnstert.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann MR, Lee SS, Doherty AS, et al. Selective loss of imprinting in the placenta following preimplantation development in culture. Development. 2004;131(15):3727–3735. doi: 10.1242/dev.01241. [DOI] [PubMed] [Google Scholar]
- Markey CM, Luque EH, Munoz De Toro M, et al. In utero exposure to bisphenol A alters the development and tissue organization of the mouse mammary gland. Biol Reprod. 2001;65:1215–1223. doi: 10.1093/biolreprod/65.4.1215. [DOI] [PubMed] [Google Scholar]
- Mastroianni L, Jr, Jones R. Oxygen tension within the rabbit fallopian tube. J Reprod Fertil. 1965;9:99–102. doi: 10.1530/jrf.0.0090099. [DOI] [PubMed] [Google Scholar]
- Matson PL, Graefling J, Junk SM, et al. Cryopreservation of oocytes and embryos: use of a mouse model to investigate effects upon zona hardness and formulate treatment strategies in an in-vitro fertilization programme. Hum Reprod. 1997;12:1550–1553. doi: 10.1093/humrep/12.7.1550. [DOI] [PubMed] [Google Scholar]
- McBride W. Thalidomide and congenital abnormalities. Lancet. 1961;278:1358. [Google Scholar]
- McCance RA, Widdowson EM. The determinants of growth and form. Proc R Soc Lond B Biol Sci. 1974;185:1–17. doi: 10.1098/rspb.1974.0001. [DOI] [PubMed] [Google Scholar]
- McDonald SD, Han Z, Mulla S, et al. Preterm birth and low birth weight among in vitro fertilization twins: a systematic review and meta-analyses. Eur J Obstet Gynecol Reprod Biol. 2010;148:105–113. doi: 10.1016/j.ejogrb.2009.09.019. [DOI] [PubMed] [Google Scholar]
- McLaughlin CC, Baptiste MS, Schymura MJ, et al. Maternal and infant birth characteristics and hepatoblastoma. Am J Epidemiol. 2006;163:818–828. doi: 10.1093/aje/kwj104. [DOI] [PubMed] [Google Scholar]
- Miozzo M, Simoni G. The role of imprinted genes in fetal growth. Biol Neonate. 2002;81:217–228. doi: 10.1159/000056752. [DOI] [PubMed] [Google Scholar]
- Mitchell JA, Yochim JM. Measurement of intrauterine oxygen tension in the rat and its regulation by ovarian steroid hormones. Endocrinology. 1968;83:691–700. doi: 10.1210/endo-83-4-691. [DOI] [PubMed] [Google Scholar]
- Moll AC, Imhof SM, Cruysberg JR, et al. Incidence of retinoblastoma in children born after in-vitro fertilisation. Lancet. 2003;361:309–310. doi: 10.1016/S0140-6736(03)12332-X. [DOI] [PubMed] [Google Scholar]
- Nadal A, Ropero AB, Laribi O, et al. Nongenomic actions of estrogens and xenoestrogens by binding at a plasma membrane receptor unrelated to estrogen receptor alpha and estrogen receptor beta. Proc Natl Acad Sci USA. 2000;97:11603–11608. doi: 10.1073/pnas.97.21.11603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neitzke U, Harder T, Schellong K, et al. Intrauterine growth restriction in a rodent model and developmental programming of the metabolic syndrome: a critical appraisal of the experimental evidence. Placenta. 2008;29:246–254. doi: 10.1016/j.placenta.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Ombelet W, Peeraer K, De Sutter P, et al. Perinatal outcome of ICSI pregnancies compared with a matched group of natural conception pregnancies in Flanders (Belgium): a cohort study. Reprod Biomed Online. 2005;11:244–253. doi: 10.1016/s1472-6483(10)60965-0. [DOI] [PubMed] [Google Scholar]
- Ooi SK, O’Donnell AH, Bestor TH. Mammalian cytosine methylation at a glance. J Cell Sci. 2009;122:2787–2791. doi: 10.1242/jcs.015123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea TM, Klinepeter KL, Dillard RG. Prenatal events and the risk of cerebral palsy in very low birth weight infants. Am J Epidemiol. 1998;147:362–369. doi: 10.1093/oxfordjournals.aje.a009458. [DOI] [PubMed] [Google Scholar]
- Palermo G, Joris H, Derde MP, et al. Sperm characteristics and outcome of human assisted fertilization by subzonal insemination and intracytoplasmic sperm injection. Fertil Steril. 1993;59:826–835. doi: 10.1016/s0015-0282(16)55867-1. [DOI] [PubMed] [Google Scholar]
- Parikh NI, Cnattingius S, Mittleman MA, et al. Subfertility and risk of later life maternal cardiovascular disease. Hum Reprod. 2012;27:568–575. doi: 10.1093/humrep/der400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petridou ET, Sergentanis TN, Panagopoulou P, et al. In vitro fertilization and risk of childhood leukemia in Greece and Sweden. Pediatr Blood Cancer. 2012;58:930–936. doi: 10.1002/pbc.23194. [DOI] [PubMed] [Google Scholar]
- Phillips KP, Leveille MC, Claman P, et al. Intracellular pH regulation in human preimplantation embryos. Hum Reprod. 2000;15:896–904. doi: 10.1093/humrep/15.4.896. [DOI] [PubMed] [Google Scholar]
- Polyzos A, Schmid TE, Pina-Guzman B, et al. Differential sensitivity of male germ cells to mainstream and sidestream tobacco smoke in the mouse. Toxicol Appl Pharmacol. 2009;237:298–305. doi: 10.1016/j.taap.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Pommerenke WT, Breckenridge MA. Biochemical studies of the female genital tract. Ann NY Acad Sci. 1952;54:786–795. doi: 10.1111/j.1749-6632.1952.tb39953.x. [DOI] [PubMed] [Google Scholar]
- Prevention CfDCa. Services USDoHaH. Atlanta: 2011. 2009 Assisted reproductive technology success rates: National summary and fertility clinic reports. [DOI] [PubMed] [Google Scholar]
- Putney LK, Barber DL. Expression profile of genes regulated by activity of the Na-H exchanger NHE1. BMC Genomics. 2004;5:46. doi: 10.1186/1471-2164-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puumala SE, Nelson HH, Ross JA, et al. Similar DNA methylation levels in specific imprinting control regions in children conceived with and without assisted reproductive technology: a cross-sectional study. BMC Pediatr. 2012;12:33. doi: 10.1186/1471-2431-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rancourt RC, Harris HR, Michels KB. Methylation levels at imprinting control regions are not altered with ovulation induction or in vitro fertilization in a birth cohort. Hum Reprod. 2012;27:2208–2216. doi: 10.1093/humrep/des151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reefhuis J, Honein MA, Schieve LA, et al. Assisted reproductive technology and major structural birth defects in the United States. Hum Reprod. 2009;24:360–366. doi: 10.1093/humrep/den387. [DOI] [PubMed] [Google Scholar]
- Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature. 2007;447:425–432. doi: 10.1038/nature05918. [DOI] [PubMed] [Google Scholar]
- Rinaudo P, Schultz RM. Effects of embryo culture on global pattern of gene expression in preimplantation mouse embryos. Reproduction. 2004;128:301–311. doi: 10.1530/rep.1.00297. [DOI] [PubMed] [Google Scholar]
- Rinaudo PF, Giritharan G, Delle Piane LAD . Mice conceived by in vitro fertilization (IVF) dysplay reduced growth curve and glucose intolerance. Glasgow, Scotland: Society of Gynecologic Investigation; 2009. p. 107A. [Google Scholar]
- Rinaudo PF, Giritharan G, Talbi S, et al. Effects of oxygen tension on gene expression in preimplantation mouse embryos. Fertil Steril. 2006;86(4 Suppl):1252–1265. e1251–e1236. doi: 10.1016/j.fertnstert.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Rivera RM, Stein P, Weaver JR, et al. Manipulations of mouse embryos prior to implantation result in aberrant expression of imprinted genes on day 9.5 of development. Hum Mol Genet. 2008;17:1–14. doi: 10.1093/hmg/ddm280. [DOI] [PubMed] [Google Scholar]
- Romundstad LB, Romundstad PR, Sunde A, et al. Increased risk of placenta previa in pregnancies following IVF/ICSI; a comparison of ART and non-ART pregnancies in the same mother. Hum Reprod. 2006;21:2353–2358. doi: 10.1093/humrep/del153. [DOI] [PubMed] [Google Scholar]
- Saigal S, Rosenbaum P, Szatmari P, et al. Learning disabilities and school problems in a regional cohort of extremely low birth weight (less than 1000 G) children: a comparison with term controls. J Dev Behav Pediatr. 1991;12:294–300. [PubMed] [Google Scholar]
- Saigal S. Follow-up of very low birthweight babies to adolescence. Semin Neonatol. 2000;5:107–118. doi: 10.1053/siny.1999.0003. [DOI] [PubMed] [Google Scholar]
- Saunders KM, Parks JE. Effects of cryopreservation procedures on the cytology and fertilization rate of in vitro-matured bovine oocytes. Biol Reprod. 1999;61:178–187. doi: 10.1095/biolreprod61.1.178. [DOI] [PubMed] [Google Scholar]
- Schulz LC. The Dutch Hunger Winter and the developmental origins of health and disease. Proc Natl Acad Sci USA. 2010;107:16757–16758. doi: 10.1073/pnas.1012911107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer C, Esteves TC, Arauzo-Bravo MJ, et al. ART culture conditions change the probability of mouse embryo gestation through defined cellular and molecular responses. Hum Reprod. 2012;27:2627–2640. doi: 10.1093/humrep/des223. [DOI] [PubMed] [Google Scholar]
- Scott KA, Yamazaki Y, Yamamoto M, et al. Glucose parameters are altered in mouse offspring produced by assisted reproductive technologies and somatic cell nuclear transfer. Biol Reprod. 2010;83:220–227. doi: 10.1095/biolreprod.109.082826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seller MJ, Bnait KS. Effects of tobacco smoke inhalation on the developing mouse embryo and fetus. Reprod Toxicol. 1995;9:449–459. doi: 10.1016/0890-6238(95)00037-b. [DOI] [PubMed] [Google Scholar]
- Shevell T, Malone FD, Vidaver J, et al. Assisted reproductive technology and pregnancy outcome. Obstet Gynecol. 2005;106:1039–1045. doi: 10.1097/01.AOG.0000183593.24583.7c. [DOI] [PubMed] [Google Scholar]
- Shi W, Haaf T. Aberrant methylation patterns at the two-cell stage as an indicator of early developmental failure. Mol Reprod Dev. 2002;63:329–334. doi: 10.1002/mrd.90016. [DOI] [PubMed] [Google Scholar]
- Shrode LD, Tapper H, Grinstein S. Role of intracellular pH in proliferation, transformation, and apoptosis. J Bioenerg Biomembr. 1997;29:393–399. doi: 10.1023/a:1022407116339. [DOI] [PubMed] [Google Scholar]
- Steel AJ, Sutcliffe A. Long-term health implications for children conceived by IVF/ICSI. Hum Fertil (Camb) 2009;12:21–27. doi: 10.1080/14647270802499201. [DOI] [PubMed] [Google Scholar]
- Stromberg B, Dahlquist G, Ericson A, et al. Neurological sequelae in children born after in-vitro fertilisation: a population-based study. Lancet. 2002;359:461–465. doi: 10.1016/S0140-6736(02)07674-2. [DOI] [PubMed] [Google Scholar]
- Summers MC, Biggers JD. Chemically defined media and the culture of mammalian preimplantation embryos: historical perspective and current issues. Hum Reprod Update. 2003;9:557–582. doi: 10.1093/humupd/dmg039. [DOI] [PubMed] [Google Scholar]
- Tachi N, Aoyama M. Effects of cigarette smoke exposure on early stage embryos in the rat. Bull Environ Contam Toxicol. 1989;43:467–472. doi: 10.1007/BF01701884. [DOI] [PubMed] [Google Scholar]
- Tae JC, Kim EY, Lee WD, et al. Sterile filtered paraffin oil supports in vitro developmental competence in bovine embryos comparable to co-culture. J Assist Reprod Genet. 2006;23:121–127. doi: 10.1007/s10815-006-9024-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y, Tsutsumi O, Ikezuki Y, et al. Estrogen receptor-mediated effects of a xenoestrogen, bisphenol A, on preimplantation mouse embryos. Biochem Biophys Res Commun. 2000;270:918–921. doi: 10.1006/bbrc.2000.2548. [DOI] [PubMed] [Google Scholar]
- Takai Y, Tsutsumi O, Ikezuki Y, et al. Preimplantation exposure to bisphenol A advances postnatal development. Reprod Toxicol. 2001;15:71–74. doi: 10.1016/s0890-6238(00)00119-2. [DOI] [PubMed] [Google Scholar]
- Tosti E, Fortunato A, Settimi A. The impact of in vitro fertilization on the health of hte mother and the offspring. Curr Women Health Rev. 2006;2:239–247. [Google Scholar]
- Tremblay KD, Schultz RM, Doherty AS, et al. Effect of embryo culture on imprinted gene expression in the preimplantation mouse embryo. In: Goldberg E, editor. The testis: from stem cell to sperm function. New York: Springer-Verlag; 2000. [Google Scholar]
- Tucker MJ, Cohen J, Massey JB, et al. Partial dissection of the zona pellucida of frozen-thawed human embryos may enhance blastocyst hatching, implantation, and pregnancy rates. Am J Obstet Gynecol. 1991;165:341–344. doi: 10.1016/0002-9378(91)90088-9. discussion 344–345. [DOI] [PubMed] [Google Scholar]
- Van Langendonckt A, Vansteenbrugge A, Donnay I, et al. Three year results of in vitro production of bovine embryos in serum-poor bovine oviduct conditioned medium. An overview. Reprod Nutr Dev. 1996;36:493–502. doi: 10.1051/rnd:19960505. [DOI] [PubMed] [Google Scholar]
- Vitthala S, Gelbaya TA, Brison DR, et al. The risk of monozygotic twins after assisted reproductive technology: a systematic review and meta-analysis. Hum Reprod Update. 2009;15:45–55. doi: 10.1093/humupd/dmn045. [DOI] [PubMed] [Google Scholar]
- Wadsworth ME, Cripps HA, Midwinter RE, et al. Blood pressure in a national birth cohort at the age of 36 related to social and familial factors, smoking, and body mass. Br Med J (Clin Res Ed) 1985;291:1534–1538. doi: 10.1136/bmj.291.6508.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenaar K, Ceelen M, van Weissenbruch MM, et al. School functioning in 8- to 18-year-old children born after in vitro fertilization. Eur J Pediatr. 2008;167:1289–1295. doi: 10.1007/s00431-008-0677-2. [DOI] [PubMed] [Google Scholar]
- Walters E, Edwards RG. On a fallacious invocation of the Barker hypothesis of anomalies in newborn rats due to mothers’ food restriction in preimplantation phases. Reprod Biomed Online. 2003;7:580–582. doi: 10.1016/s1472-6483(10)62075-5. [DOI] [PubMed] [Google Scholar]
- Wang WH, Meng L, Hackett RJ, et al. Limited recovery of meiotic spindles in living human oocytes after cooling-rewarming observed using polarized light microscopy. Hum Reprod. 2001;16:2374–2378. doi: 10.1093/humrep/16.11.2374. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zang C, Rosenfeld JA, et al. Combinatorial patterns of histone acetylations and methylations in the human genome. Nat Genet. 2008;40:897–903. doi: 10.1038/ng.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins AJ, Lucas ES, Torrens C, et al. Maternal low-protein diet during mouse pre-implantation development induces vascular dysfunction and altered renin-angiotensin-system homeostasis in the offspring. Br J Nutr. 2010;103:1762–1770. doi: 10.1017/S0007114509993783. [DOI] [PubMed] [Google Scholar]
- Watkins AJ, Platt D, Papenbrock T, et al. Mouse embryo culture induces changes in postnatal phenotype including raised systolic blood pressure. Proc Natl Acad Sci USA. 2007;104:5449–5454. doi: 10.1073/pnas.0610317104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins AJ, Ursell E, Panton R, et al. Adaptive responses by mouse early embryos to maternal diet protect fetal growth but predispose to adult onset disease. Biol Reprod. 2008;78:299–306. doi: 10.1095/biolreprod.107.064220. [DOI] [PubMed] [Google Scholar]
- Webb BA, Chimenti M, Jacobson MP, et al. Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer. 2011;11:671–677. doi: 10.1038/nrc3110. [DOI] [PubMed] [Google Scholar]
- Wennerholm UB, Soderstrom-Anttila V, Bergh C, et al. Children born after cryopreservation of embryos or oocytes: a systematic review of outcome data. Hum Reprod. 2009;24:2158–2172. doi: 10.1093/humrep/dep125. [DOI] [PubMed] [Google Scholar]
- Wilding M, Coppola G, Dale B, et al. Mitochondria and human pre-implantation embryo development. Reproduction. 2009;137:619–624. doi: 10.1530/REP-08-0444. [DOI] [PubMed] [Google Scholar]
- Wilkins KM, Warnock JK, Serrano E. Depressive symptoms related to infertility and infertility treatments. Psychiatr Clin North Am. 2010;33:309–321. doi: 10.1016/j.psc.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Williams CA, Angelman H, Clayton-Smith J, et al. Angelman syndrome: consensus for diagnostic criteria. Angelman Syndrome Foundation. Am J Med Genet. 1995;56:237–238. doi: 10.1002/ajmg.1320560224. [DOI] [PubMed] [Google Scholar]
- Wright K, Brown L, Brown G, et al. Microarray assessment of methylation in individual mouse blastocyst stage embryos shows that in vitro culture may have widespread genomic effects. Hum Reprod. 2011;26:2576–2585. doi: 10.1093/humrep/der201. [DOI] [PubMed] [Google Scholar]
- Wyman A, Pinto AB, Sheridan R, et al. One-cell zygote transfer from diabetic to nondiabetic mouse results in congenital malformations and growth retardation in offspring. Endocrinology. 2008;149:466–469. doi: 10.1210/en.2007-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga K, Rice C, Krenn J, et al. Effects of nicotine on early pregnancy in the rat. Biol Reprod. 1979;20:294–303. doi: 10.1095/biolreprod20.2.294. [DOI] [PubMed] [Google Scholar]
- Zaitseva I, Zaitsev S, Alenina N, et al. Dynamics of DNA-demethylation in early mouse and rat embryos developed in vivo and in vitro. Mol Reprod Dev. 2007;74:1255–1261. doi: 10.1002/mrd.20704. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Reinberg D. Transcription regulation by histone methylation: interplay between different covalent modifications of the core histone tails. Genes Dev. 2001;15:2343–2360. doi: 10.1101/gad.927301. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Baltz JM. Bicarbonate/chloride exchange and intracellular pH throughout preimplantation mouse embryo development. Am J Physiol. 1996;271:C1512–C1520. doi: 10.1152/ajpcell.1996.271.5.C1512. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Chauvet PJ, Alper SL, et al. Expression and function of bicarbonate/chloride exchangers in the preimplantation mouse embryo. J Biol Chem. 1995;270:24428–24434. doi: 10.1074/jbc.270.41.24428. [DOI] [PubMed] [Google Scholar]


