Abstract
Objective
Little is known about prevalence of osteoporosis risk factors among American Indians and Alaska Natives (AIAN).
Methods
We included AIAN people (n = 8,039) enrolled in the Education and Research Towards Health (EARTH) Study. Prevalence ratios were used to determine cross-sectional associations of risk factors with self-reported bone fractures.
Results
There is a high prevalence of multiple risk factors for osteoporosis in AIAN, although the factors that are associated with past fracture vary by gender and geographical area. In general, women who reported a fracture reported more risk behaviors, more than two medical conditions, and low physical activity. Men with higher BMI were less likely to report a fracture. Smoking history was associated with fracture for both genders, though not significantly in all sub-groups.
Conclusion
We prevent a high prevalence of risk factors for osteoporosis for AIAN. Future research for osteoporosis risk reduction and prevention in AIAN people is indicated.
Keywords: Osteoporosis, fracture, American Indian, Alaskan Native
Osteoporosis, fracture rates, and risk factors for these conditions are understudied in American Indian (AI) and Alaska Native (AN, or AIAN) women. The prevalence of osteoporosis among AIAN people is not known. However, two studies addressed hip fracture risk or osteoporosis risk factors among AIAN women and suggest that incident fracture is similar among White and American Indian women (2.4 and 2.8%, respectively).1,2 Recent evidence suggests that the association of vitamin D levels with risk of fracture also varies by ethnicity with no association observed among AI.3 However, available data also suggest that risk factors for fracture differ by ethnicity.1 Fracture history and hormone use were most closely related to annualized fracture among American Indian women, whereas risk factors appeared to be universally associated with fracture among women regardless of ethnicity.1 To our knowledge, no one has addressed these issues among AIAN men.
Given the limited characterization of prevalence of the type and number of risk factors of osteoporosis among AIAN people, the aim of this paper was to report the prevalence of reported adult fracture and risk factors for osteoporosis in AIAN men and women. In addition we evaluate the association of self-reported fracture with risk factors for osteoporotic fracture in both AIAN women and men.
Methods
Study population
Education and Research Towards Health (EARTH) study participants were a convenience sample recruited from Southcentral, Southwestern, and Southeastern Alaska and from the Fort Defiance and Shiprock Health Service Units on the Navajo Nation between March 2004 and October 2007. Area participants eligible to participate met the following inclusion criteria: American Indian or Alaska Native eligible for Indian Health Service health care, age 18 years or older, not pregnant, not actively undergoing chemotherapy, and physically and mentally able to understand the consent form and to complete survey instruments. The 8,039 AI/AN (5,230 women and 2,809 men with complete diet and fracture data) examined in this paper were participants in the EARTH Study. The Alaska Area Institutional Review Board, the Navajo Nation Human Research Review Board, the Indian Health Service National Institutional Review Board, and the University of Utah Institutional Review Board approved this study. Regional, local, and village tribal health boards and chapters within local health boards approved and supported the study.
Data collection
The design and methods have been previously reported.4 Study visits were conducted in a variety of settings including stationary locations in areas with more dense populations, temporary study centers in remote villages, and a mobile van on the Navajo Nation. We defined urban areas using the 2000 U.S. Census definition of 50,000 people or more and rural ones as those outside of urban areas. A culturally appropriate computerized data collection and tracking system was developed using audio computer-assisted interview (ACASI) and touch screens. Participants provided information at baseline on marital status, education level, and employment status, diet, and a 12-item short form health survey (SF12).
The Health, Lifestyle, and Physical Activity questionnaire included self-report of medical conditions, including high blood pressure, heart disease, high cholesterol, stroke, gallbladder disease, kidney disease, liver disease, thyroid disease, arthritis, asthma, lung disease, diabetes, cataracts, depression, and cancer; family history of medical conditions; physical activity information; and current and past use of tobacco.5 The questionnaire assessed occurrence of fracture by asking, “Did a doctor or other health care provider ever tell you that you had a bone fracture or break as an adult, that is after age 18?” Participants who reported a fracture were asked the age at the time of fracture and the location of the fracture. Participants also were asked the health care facility where the fracture was assessed.
The diet history questionnaire was adapted from the CARDIA diet history to include foods commonly eaten by AIAN people6 and was validated for use with AIAN in this study.7 Separate versions of the diet history questionnaire were utilized using foods eaten regionally in Alaska and those eaten by Navajo. Participants were excluded in a stepwise fashion for very high (>6500 kcal/d for women, >8000 kcal/d for men) or low (<800 kcal for men, <600 kcal for women) reported non-alcohol energy intake (n=2,248). Also excluded from the present analysis were 119 for missing fracture data; 11 for missing BMI; and 604 for missing responses to having a diagnosis of arthritis.
We created a risk behavior score based on participants’ reported the use of seatbelts and float jacket use, speeding behaviors, and driving or riding in a car or boat after having more than one alcoholic beverage. We created a summary variable of osteoporosis risk factors that included smoking, more than three alcoholic drinks per day, inadequate calcium intake (less than the 1997 Dietary Reference Intake for age and gender, mg/d8), inadequate vitamin D intake (less than 1997 Dietary Reference intake for age and gender, IU/d8), two or more medical conditions and behavior risk scores of three or more. We also created a summary variable to represent the number of medical conditions listed above reported by an individual participant (none, one, or two or more) as an index of overall health.
Menopausal status was determined by women’s responses to a series of questions including whether they had experienced menopause, had their ovaries removed, or had a period in the last 12 months. If women reported that they did not know whether they had gone through menopause and were age 60 or older, then menopause was assumed.
Anthropometric measures included height and weight. Measurements were taken in duplicate with the participants wearing loose clothing and no shoes. Weight was measured using Tanita® digital scales (BWP800/BWP627A, Tanita Corporation of America Inc., Arlington Hills, IL). Two measurements were taken (a third was taken if there was greater than a two-pound difference). Standing height was measured with the Road Rod Stadiometer® (Seca, Hamburg, Germany). If the two height measurements differed by more than 1.0 inch, measurements were repeated; the average of the final two measurements was used. The final two measurements were entered and the average was used for analysis.
Statistical analysis
Data were analyzed using SAS version 9.1 (SAS Institute, Cary, NC). Descriptive statistics were used to characterize the study population. Significant differences were noted when p values was less than .05. Prevalence ratios were used to determine cross-sectional associations of risk factors with self-reported bone fractures using GENMOD; data were stratified by the self-reported age at which the fracture occurred (younger than 30, 30–39, and 40 years of age or older) relative to those of similar age without a self-reported fracture, by study center (i.e., Alaska or Navajo) and by gender. Adjustment variables included age, height, tobacco, alcohol use, calcium and vitamin D intakes (above or below recommended levels), number of medical conditions (none, one or two or more), menopausal status, BMI (<25, 25–30, 30–35, >35), geographic location of participant, and risk behavior score. Additionally, we restricted analysis to those with reported hip, wrist, or spine fractures (commonly reported osteoporotic fracture sites in other populations) to discern whether their prevalence or the osteoporotic risk factor prevalence among those with these fractures differed from all fractures.
Results
The majority of participants were women younger than 50 years of age and having at least a high school education (Table 1). A little less than half reported being married or living as married and being employed outside the home. Approximately one-third of the participants were overweight (BMI 25–29.9) and more than one-third were obese (BMI >30). A self-reported history of adult fractures was more common in Alaska than in Navajo participants. Almost half of participants reported calcium intakes greater than the Recommended Dietary Intakes,8 whereas consumption of adequate vitamin D was less common, particularly among Navajo people.
Table 1.
PARTICIPANT DEMOGRA PHIC AND LIFESTYLE CHARA CTERISTICS
| Overall
|
Alaska
|
Navajo
|
p value* | ||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Gender | |||||||
| Men | 2809 | 34.94 | 979 | 36.14 | 1830 | 34.33 | .109 |
| Women | 5230 | 65.06 | 1730 | 63.86 | 3500 | 65.67 | |
| Age (yrs) | |||||||
| <30 | 2266 | 28.19 | 762 | 28.14 | 1504 | 28.22 | .004 |
| 30–39 | 1731 | 21.54 | 593 | 21.90 | 1138 | 21.35 | |
| 40–49 | 1989 | 24.74 | 685 | 25.30 | 1304 | 24.47 | |
| 50–59 | 1275 | 15.86 | 401 | 14.81 | 874 | 16.40 | |
| 60–69 | 578 | 7.19 | 177 | 6.54 | 401 | 7.52 | |
| 70–79 | 172 | 2.14 | 75 | 2.77 | 97 | 1.82 | |
| 80+ | 27 | 0.34 | 15 | 0.55 | 12 | 0.23 | |
| Education | |||||||
| Less then H.S. | 1684 | 21.07 | 457 | 17.06 | 1227 | 23.09 | <.001 |
| H.S. or Some College | 2966 | 37.11 | 1088 | 40.61 | 1878 | 35.35 | |
| College Grad or Higher | 3342 | 41.82 | 1134 | 42.33 | 2208 | 41.56 | |
| Marital Status | |||||||
| Married/Living as married | 3565 | 44.45 | 1210 | 44.88 | 2355 | 44.23 | .010 |
| Widowed | 385 | 4.80 | 109 | 4.04 | 276 | 5.18 | |
| Divorced | 793 | 9.89 | 301 | 11.16 | 492 | 9.24 | |
| Separated | 478 | 5.96 | 149 | 5.53 | 329 | 6.18 | |
| Never married | 2799 | 34.90 | 927 | 34.38 | 1872 | 35.16 | |
| Employment Status | |||||||
| Employed | 3753 | 46.75 | 1351 | 50.04 | 2402 | 45.08 | <.001 |
| Not employed | 2087 | 26.00 | 730 | 27.04 | 1357 | 25.47 | |
| Homemaker | 758 | 9.44 | 189 | 7.00 | 569 | 10.68 | |
| Student | 610 | 7.60 | 113 | 4.19 | 497 | 9.33 | |
| Retired | 487 | 6.07 | 192 | 7.11 | 295 | 5.54 | |
| Other | 333 | 4.15 | 125 | 4.63 | 208 | 3.90 | |
| Fracture after 18 | |||||||
| % Yes | 1669 | 20.76 | 707 | 26.10 | 962 | 18.05 | <.001 |
| Smoking | |||||||
| Current | 1788 | 23.4 | 1105 | 43.4 | 683 | 23.4 | |
| Former | 493 | 6.4 | 317 | 12.4 | 176 | 3.5 | |
| Never | 5372 | 7.2 | 1126 | 44.2 | 4246 | 83.2 | <.0001 |
| BMI | |||||||
| <25 | 1534 | 19.08 | 660 | 24.36 | 874 | 16.40 | <.001 |
| 25–29.9 | 2665 | 33.15 | 878 | 32.41 | 1787 | 33.53 | |
| 30–34.9 | 2104 | 26.17 | 603 | 22.26 | 1501 | 28.16 | |
| 35–39.9 | 1097 | 13.65 | 319 | 11.78 | 778 | 14.60 | |
| 40 or more | 639 | 7.95 | 249 | 9.19 | 390 | 7.32 | |
| Moderate-to vigorous activity | |||||||
| 5 or more hr/wk | 3014 | 37.49 | 933 | 34.44 | 2081 | 39.04 | <.001 |
| <5 hr/wk | 5025 | 62.51 | 1776 | 65.56 | 3249 | 60.96 | |
| Calcium (number at or above the 1997a DRI for age/gender (mg/day) | 3930 | 48.89 | 1290 | 47.62 | 2640 | 49.53 | .105 |
| Vitamin D (Number at or above the 1997a DRI for age/gender) | 1296 | 16.13 | 922 | 34.03 | 374 | 7.02 | <.001 |
Significance of difference between Alaska Natives and Navajo.
1997 Dietary Reference Intake for Calcium and Vitamin D8
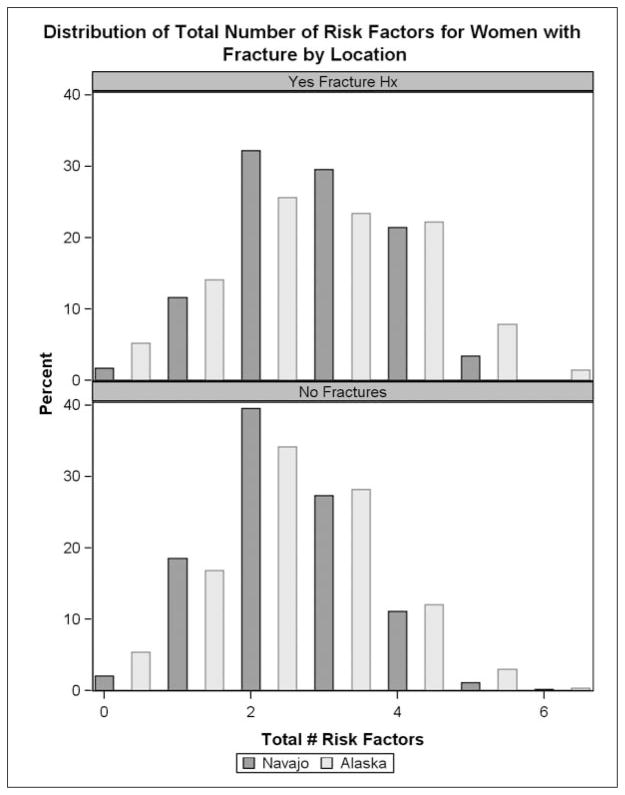
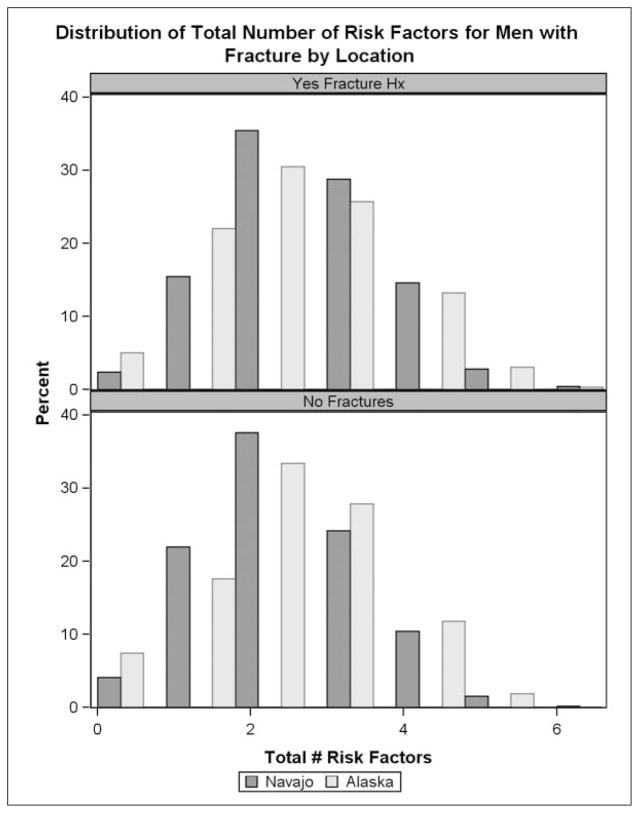
The gender specific mean (women 2.4, men 2.3) and median (2 for women and men) for reported osteoporosis risk factors were the same for Navajo and Alaska participants. The gender-specific distribution of number of reported risk factors shifted slightly upwards, with a higher number of reported risk factors among men and women with prevalent self-reported adult fractures (Figures 1 and 2).
Figure 1.
Number of osteoporosis risk factors among women.a
aOsteoporosis risk factures included: current smoking, >3 alcoholic drinks, inadequate calcium intake, inadequate vitamin D intake, menopause, 2 or more medical conditions and behavior risk scores ≥3 or more medical conditions and behavior risk scores ≥3.
Figure 2.
Number of osteoporosis risk factors among men.a
aOsteoporosis risk factures included: current smoking, >3 alcoholic drinks, inadequate calcium intake, inadequate vitamin D intake, 2 or more medical conditions and behavior risk scores ≥3.
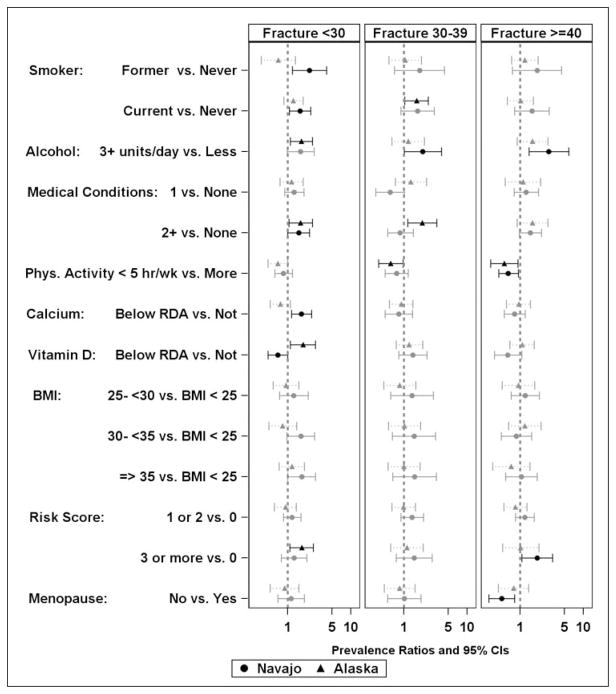
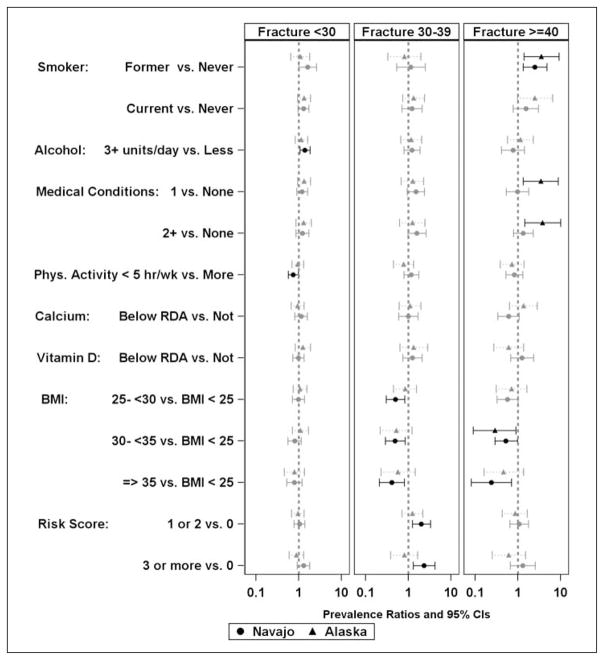
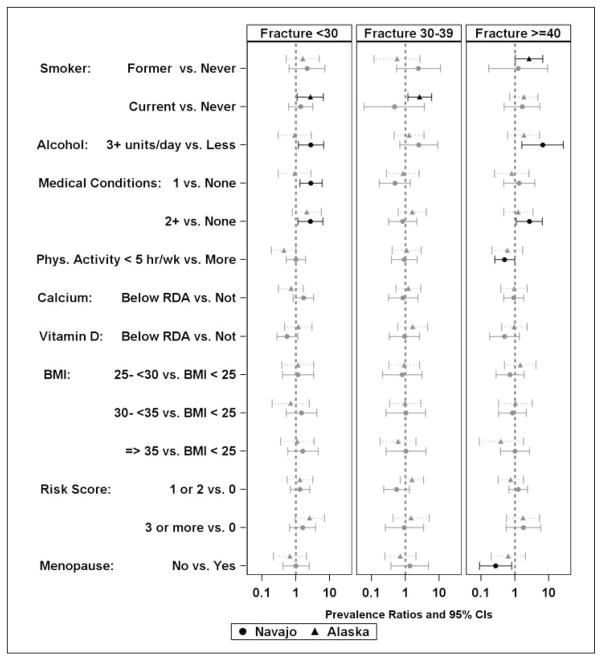
We assessed the age group stratified prevalence of fracture and documented risk factors for osteoporosis among those with reported fracture prevalence for Navajo and Alaska participants by gender and both separately and together. Although significance varied between the sites, the only risk factor with significant associations in different directions was vitamin D intake below the RDA being associated with increased risk among women from Alaska and lower risk among Navajo women less than 30 years of age (Figure 3). Both female (Figure 3) and male (Figure 4) participants reporting an adult fracture occurring before the age of 30 more commonly reported smoking (both former and current cigarette smoking in men and current cigarette smoking in women), and had higher reported consumption of alcohol (more than three alcoholic drinks per day). Additionally, in female participants younger than 30 years or 40 years old or older and men ages 30–39, two or more medical conditions and more behavior risk factors (two or more for men, three or more for women) were associated with a higher prevalent self-reported fracture. In female participants younger than 30 and 30–39 years old similar associations of risk factors were observed with the exception that consuming three or more alcoholic drinks per day was associated with greater prevalence of fracture among Navajo women, whereas two or more medical conditions (positive) and fewer than five hours of physical activity per week (negative) were significantly associated among Alaska women. In women 40 years and older, the associations were similar to those of women younger than 30 years with the exceptions that current smoking was not associated. Alcohol consumption and risky behaviors were positively associated with fracture among Navajo women. Less physical activity (less than five hours per week) and the absence of menopause were associated with less self-reported fracture for all women. In Navajo men between 30 and 39, having a BMI over 25 kg/m2 was associated with less prevalent fracture than having a BMI less than 25 kg/m2. Displaying more risky behaviors was associated with more prevalent reported fracture. In men 40 years and older, both current and cigarette smoking and a BMI between 30 and 35 kg/m2 were associated with greater prevalence of self-reported fracture for both Alaska and Navajo men whereas medical conditions were positively associated with fractures in the 40 years and older group among men in Alaska only.
Figure 3.
Probability of osteoporosis risk factors among women stratified by age at reported adult fracture.
Figure 4.
Probability of osteoporosis risk factors among men stratified by the age at reported fracture.a
aIn the group reporting fractures 40 or older, the upper confidence intervals (CIs) greater than 10 were treated as being equal to 10 to allow for equal scaling on all graphs, causing some CIs to not be symmetric around the Prevalence Ratio estimate.
CI = Confidence Intervals
BMI = Body Mass Index
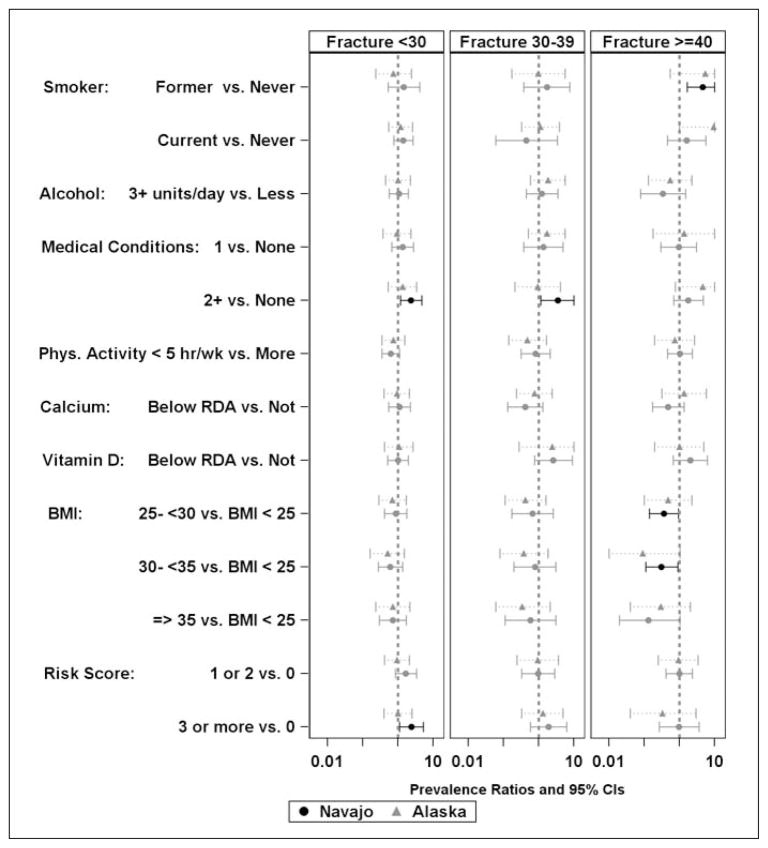
We examined the risk of prevalent hip, spine, and wrist fractures, separately from other fractures in each age strata by site and by. In women younger than 30 years old (Figure 5), current cigarette smoking, two or more medical conditions, and three or more risk behavior factors were associated with increased self-reported prevalence of fracture of the hip, spine, or wrist. In women 30–39 years old, only current cigarette smoking was associated with prevalence of self-reported fractures in women in Alaska. In women 40 years of age and older, three or more alcoholic beverages and two or more medical conditions were associated with greater risk of prevalent self-reported hip, spine, and wrist fracture in the Navajo women. In this sub-analysis, moderate-to vigorous physical activity of less than five hours per week was associated with lower prevalence of self-reported fracture in women 40 years old or older. Associations were generally similar across sites, but smoking was the only factor that reached significance among Alaska women.
Figure 5.
Probability of osteoporotic risk factor among women grouped by the age of adult fracture at the wrist, hip, or spine.a
aDark lines indicate a significant prevalence ratio.
Two or more medical conditions were associated with greater prevalence of hip, spine, and wrist fractures among Navajo men less than 30 or 30 to 39 years of age (Figure 6). Risky behaviors were associated with increased hip wrist and spine fractures among Navajo men. In Navajo men 40 years or older, former and current smoking were associated with greater prevalence of fracture, whereas having a BMI between 25 and 35 kg/m2 was associated with lower prevalence of fracture. Prevalence of self-reported hip, spine, and wrist fracture was lower among pre-menopausal women 40 years of age or older than among menopausal women of similar age.
Figure 6.
Probability of osteoporotic risk factor among men grouped according to the age of reported fracture at the wrist, hip, or spine.a
aIn the group reporting a fracture after the age of 40 the upper confidence intervals (CIs) greater than 10 were treated as being equal to 10 to allow for equal scaling on all graphs causing some CIs to not be symmetric around the prevalence ratio estimate.
CI = Confidence Intervals
BMI = Body Mass Index
Discussion
The prevalence of self-reported fracture after age 18 in EARTH participants was 20.8%; approximately 5% reported hip, spine, or wrist fracture. Prevalence rates were slightly higher among Alaska Native participants than among Navajo participants. In our study approximately half of the self-reported factures occurred before the participant was 40 years of age. Based on literature suggesting that prior fracture is a risk factor for future osteoporotic fractures,9,10 these results suggest significant risk of future osteoporotic fracture in this population.
The majority of studies on risk factors for non-traumatic fracture and osteoporosis include only White women. Prevalence data and the etiology of disparities in bone mass and bone loss rate between White and other racial/ethnic groups are not well characterized.11,12 Thus, in addition to self-report of previous fracture, we examined other traditional risk factors for low bone mineral density (BMD), including, limited activities of daily living, current tobacco use, daily alcohol use, and low calcium and vitamin D intake in the AIAN.10 Findings in the present analysis support the association of tobacco and alcohol with higher fracture risk in AIAN people. Chemicals in tobacco and more than three units of alcohol a day may directly affect bone resorption followed by bone formation, resulting in lower BMD.13 It is unclear whether alcohol consumption in modest amounts may have positive effects on bone health14 that may explain the lack of significance of this factor among some of the age groups.
In addition to modification of tobacco use and alcohol consumption, dietary consumption of calcium and vitamin D are modifiable risk factors that influence both bone accumulation and loss; however, their association with fracture risk or prevalence is less clear.15 Most dietary studies of osteoporosis have highlighted the important role of calcium and vitamin D in skeletal health.15,16 Although the estimated prevalence of low reported calcium and vitamin D intake was high in the AIAN, there was no association with prevalent adult fractures or hip, spine, and wrist fractures, perhaps relating to issues of study design and population characteristics. Among women who reported an adult fracture before the age of 30, having a low vitamin D intake was associated with increased risk of fracture among the Navajo women and lower risk of fracture in women participating in Alaska. This finding may be spurious or may be related to the lower number of women reporting adequate vitamin D intake in Alaska. Additionally, the present analyses did not include an assessment of difference in sunlight exposure as a potential source of vitamin D. Other confounders or effect modifiers (such as sodium and potassium intake) may contribute to the negative result.17,18 Alternately, or additionally, the relatively young age of the sample and the nature of fractures might explain the apparent absence of an association between dietary intake of calcium and vitamin D and prevalent fracture in this study.
Physical activity can increase BMD and decrease risk for osteoporotic fracture.19,20 Surprisingly, in our study, lower reported physical activity was associated with lower report of overall fractures, perhaps in this setting higher physical activity represented opportunity for traumatic fracture. Among younger individuals, including the majority of participants in the present study, we expect most of the fractures were traumatic rather than osteoporotic. Among women 40 years of age or older, this finding is more puzzling. Given the cross-sectional nature of the data, we are unable to discern causal path. Therefore, it is important that future studies evaluate associations with physical activity in a prospective manner to clarify the associations with bone fractures in AIAN populations.
The association of risky seatbelt, float jacket, and driving behaviors with greater prevalence of adult fracture among men and women less than 30 years of age supports the assumption that many of these fractures were traumatic in nature. However, given data to suggest that prior fracture may represent risk for future osteoporotic fracture,9,21 these data suggest that behavioral interventions may similarly be important for fracture prevention in AIAN (especially in men and women younger than 30 years of age).
Other health interventions in the AIAN may also be important for fracture prevention. The presence of two or more chronic medical conditions was associated with more self-reported fractures in at least one of the age group in both women and men. Researchers are actively studying the mechanism(s) in which chronic diseases, such as rheumatoid arthritis (RA), diabetes, peripheral vascular disease, and chronic obstructive pulmonary disease (COPD), alter inflammatory, metabolic and endocrine pathways to adversely affect bone quantity and/or quality.22–24 Of interest, adipokines are also elevated in these disease entities irrespective of BMI, and in patients receiving treatment for RA there is both significantly decreasing circulating adiponectin levels and improvement in skeletal health,25,26 providing a plausible biologic explanation for our findings of increased prevalence risk ratio for fracture with number of medical conditions simultaneously with lower relative risk with higher BMI in men. The presence of two or more medical conditions may be important to identify AIAN men and women at greatest risk for fracture and allow future directed BMD screening efforts with dual energy x-ray absorptiometry.
Our study population differs from the traditional osteoporosis screening population in that it had a relatively younger mean age and lower frequency of menopause than other studies of osteoporotic fracture risk or bone density. Nonetheless, absence of menopause in women 40 years of age or older was associated with less self-reported all fracture as well as site-specific fractures in the wrist, hip, and spine, supporting a similar age and menopause-related increase in risk among AIAN women as compared to other ethnicities. Within this age strata of women, prevalence of self-reported fracture increased with age independent of menopausal status perhaps highlighting importance of directed screening efforts in AIAN women 40 years and older.27
The World Health Organization Fracture Risk Assessment Tool (FRAX®) risk factors are traditionally used to guide osteoporosis screening.9,21 Although the femoral BMD information on this tool can be stratified by different ethnicities, the risk factors for low bone-mineral density, such as personal history of previous fracture, parental history of hip fracture, corticosteroid use, RA, current smoking, secondary osteoporosis, three or more units of alcoholic beverages is weighted equally among all people. Although it is recognized that independent of ethnicity, multiple risk factors result in more fracture, this study highlights that risk factors for fracture may vary among ethnic groups.9,21 Since the most effective approach to bone health is to prevent fractures before they occur, our study suggests that lifestyle interventions may be a cornerstone to therapy in the AIAN.27
Obesity is prevalent among AIAN people in this study. Obesity has a significant effect on the musculoskeletal system being associated with both degenerative and inflammatory conditions.28 A recent review indicates contradictory evidence, with some studies indicating that cytokines from fat mass may have beneficial effects on bone, and other studies suggesting that excessive fat mass may be inflammatory and not protect against osteoporosis or osteoporotic fracture.29 In particular, adiponectin may represent a biomarker in the relationship between visceral fat mass and bone mineral density.30 In our study, increasing BMI appeared to be associated with less self-reported fracture only in men after adjustment for number of chronic medical conditions and highlights that there may be differences among AIAN women and men in risk factors for prevalent fracture. Although the osteoporosis and risk of hip fracture is often thought of as a problem primarily for women, mortality after fracture is higher among men than among women, making secondary osteoporosis of particular concern among men.31,32
The cross-sectional study design used in the present report makes it difficult to know the etiology of the self-reported fractures and introduces potential bias. It is unknown which fractures were traumatic or osteoporotic in nature, or which fractures might be related to risky behaviors or other risk factors. Reliance on self-report of fracture, other medical conditions, physical activity patterns and dietary intake introduces potential for significant error in those measures resulting in attenuation of estimates of risk. Previous use of medications that affect bone health, such as prednisone, anti-epileptics, and hormone replacement therapies, were not considered in these analyses.
Nonetheless, our study has determined that the prevalence of multiple risk factors for osteoporosis is high in this population of AIAN people, even though the sample is relatively young on average. Thus, the traditional prescriptions of smoking cessation, responsible use of alcohol and avoiding driving or boating after its use, and appropriate of seatbelts or float jackets appear to be entirely applicable for fracture prevention in AIAN. Physical activity patterns in the AIAN should be studied for their relation to incident fracture. Directed osteoporosis screening efforts for AIAN men and women with two or more medical conditions or the presence of two or more osteoporosis risk factors may be useful in locations where dual energy x-ray absorptiometry is not possible. Further research of fracture risk in these populations should investigate the specific osteoporosis risk factors associated with fragility fracture in AIAN.
Acknowledgments
Funding: This study was funded by grants CA88958 and CA96095 and from the National Cancer Institute and AR052466 from the National Institute of Arthritis Musculoskeletal and Skin Disorders.
The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official view of the National Cancer Institute or the Indian Health Service. We would like to acknowledge the contributions and support of the Navajo Nation, the Indian Health Service, the Alaska Native Tribal Health Consortium Board of Directors, Southcentral Foundation (SCF), Southeast Alaska Regional Health Consortium (SEARHC), the Yukon-Kuskokwim Health Corporation (YKHC), Ft. Defiance and Shiprock Health Boards, Tribal Advisory Board Members including Beverley Pigman, George Ridley, Ileen Sylvester, Tim Gilbert, Fritz George, the staff on the Navajo Nation including Clarina Clark, Carmen George, the many Health Data analysts, the staff in Alaska including Jennifer Johnson, Diana Redwood, Katie Rose Hulett, Sharon Lindley, Cheri Hample, Maybelle Filler, Antoinelle Thompson, and Jayleen Wheeler.
Contributor Information
Tracy Frech, University of Utah, Division of Rheumatology.
Khe-ni Ma, University of Utah, Division of Epidemiology.
Elizabeth D. Ferrucci, The Alaska Native Tribal Health Consortium, Anchorage.
Anne P. Lanier, The Office of Alaska Native Health Research, Alaska Native Tribal Health Consortium, Anchorage, AK.
Molly McFadden, University of Utah, Division of Epidemiology.
Lillian Tom-Orme, University of Utah, Division of Epidemiology.
Martha L. Slattery, University of Utah, Division of Epidemiology.
Maureen A. Murtaugh, University of Utah, Division of Epidemiology.
Notes
- 1.Cauley JA, Wu L, Wampler NS, et al. Clinical risk factors for fractures in multi-ethnic women: the Women’s Health Initiative. J Bone Miner Res. 2007 Nov;22(11):1816–26. doi: 10.1359/jbmr.070713. [DOI] [PubMed] [Google Scholar]
- 2.Barrett-Connor E, Siris ES, Wehren LE, et al. Osteoporosis and fracture risk in women of different ethnic groups. J Bone Miner Res. 2005 Feb;20(2):185–94. doi: 10.1359/JBMR.041007. Epub 2004 Oct 18. [DOI] [PubMed] [Google Scholar]
- 3.Cauley JA, Danielson ME, Boudreau R, et al. Serum 25-hydroxyvitamin D and clinical fracture risk in a multiethnic cohort of women: the Women’s Health Initiative (WHI) J Bone Mineral Res. 2011 Oct;26(10):2378–88. doi: 10.1002/jbmr.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slattery ML, Schumacher MC, Lanier AP, et al. A prospective cohort of American Indian and Alaska Native people: study design, methods, and implementation. Am J Epidemiol. 2007 Sep 1;166(5):606–15. doi: 10.1093/aje/kwm109. Epub 2007 Jun 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical Care. 1996 Mar;34(3):220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 6.McDonald A, Van Horn L, Slattery M, et al. The CARDIA dietary history: development, implementation, and evaluation. J Am Diet Assoc. 1991 Sep;91(9):1104–12. [PubMed] [Google Scholar]
- 7.Murtaugh MA, Ma KN, Greene T, et al. Validation of a dietary history questionnaire for American Indian and Alaska Native people. Ethn Dis. 2010 Autumn;20(4):429–36. [PMC free article] [PubMed] [Google Scholar]
- 8.Institute of Medicine. Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington, DC: National Academy Press; 1997. [PubMed] [Google Scholar]
- 9.Kanis JA, Johnell O, Oden A, et al. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008 Apr;19(4):385–97. doi: 10.1007/s00198-007-0543-5. Epub 2008 Feb 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ullom-Minnich P. Prevention of osteoporosis and fractures. Am Fam Physician. 1999 Jul;60(1):194–202. [PubMed] [Google Scholar]
- 11.Pothiwala P, Evans EM, Chapman-Novakofski KM. Ethnic variation in risk for osteoporosis among women: a review of biological and behavioral factors. J Womens Health (Larchmt) 2006 Jul-Aug;15(6):709–19. doi: 10.1089/jwh.2006.15.709. [DOI] [PubMed] [Google Scholar]
- 12.Hou YL, Wu XP, Luo XH, et al. Differences in age-related bone mass of proximal femur between Chinese women and different ethnic women in the United States. J Bone Miner Metab. 2007;25(4):243–52. doi: 10.1007/s00774-007-0756-x. Epub 2007 Jun 25. [DOI] [PubMed] [Google Scholar]
- 13.Kenny AM, Prestwood KM. Osteoporosis. Pathogenesis, diagnosis, and treatment in older adults. Rheum Dis Clin North Am. 2000 Aug;26(3):569–91. doi: 10.1016/s0889-857x(05)70157-5. [DOI] [PubMed] [Google Scholar]
- 14.Tucker KL, Jugdaohsingh R, Powell JJ, et al. Effects of beer, wine, and liquor intakes on bone mineral density in older men and women. Am J Clinical Nutr. 2009 Apr;89(4):1188–96. doi: 10.3945/ajcn.2008.26765. Epub 2009 Feb 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reid IR, Mason B, Horne A, et al. Randomized controlled trial of calcium in healthy older women. Am J Med. 2006 Sep;119(9):777–85. doi: 10.1016/j.amjmed.2006.02.038. [DOI] [PubMed] [Google Scholar]
- 16.Rizzoli R. Nutrition: its role in bone health. Best Pract Res Clin Endocrinol Metab. 2008 Oct;22(5):813–29. doi: 10.1016/j.beem.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Teucher B, Dainty JR, Spinks CA, et al. Sodium and bone health: impact of moderately high and low salt intakes on calcium metabolism in postmenopausal women. J Bone Miner Res. 2008 Sep;23(9):1477–85. doi: 10.1359/jbmr.080408. [DOI] [PubMed] [Google Scholar]
- 18.Harrington M, Cashman KD. High salt intake appears to increase bone resorption in postmenopausal women but high potassium intake ameliorates this adverse effect. Nutr Rev. 2003 May;61(5 Pt 1):179–83. doi: 10.1301/nr.2003.may.179-183. [DOI] [PubMed] [Google Scholar]
- 19.Lewiecki EM. Prevention and treatment of postmenopausal osteoporosis. Obstet Gynecol Clin North Am. 2008 Jun;35(2):301–15. ix. doi: 10.1016/j.ogc.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Bailey CA, Brooke-Wavell K. Exercise for optimising peak bone mass in women. Proc Nutr Soc. 2008 Feb;67(1):9–18. doi: 10.1017/S0029665108005971. [DOI] [PubMed] [Google Scholar]
- 21.Kanis JA, McCloskey EV, Johansson H, et al. Case finding for the management of osteoporosis with FRAX—assessment and intervention thresholds for the UK. Osteoporos Int. 2008 Oct;19(10):1395–408. doi: 10.1007/s00198-008-0712-1. Epub 2008 Aug 28. [DOI] [PubMed] [Google Scholar]
- 22.Adami S. Bone health in diabetes: considerations for clinical management. Curr Med Res Opin. 2009 May;25(5):1057–72. doi: 10.1185/03007990902801147. [DOI] [PubMed] [Google Scholar]
- 23.Collins TC, Ewing SK, Diem SJ, et al. Peripheral arterial disease is associated with higher rates of hip bone loss and increased fracture risk in older men. Circulation. 2009 May 5;119(17):2305–12. doi: 10.1161/CIRCULATIONAHA.108.820993. Epub 2009 Apr 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brand C, Lowe A, Hall S. The utility of clinical decision tools for diagnosing osteoporosis in postmenopausal women with rheumatoid arthritis. BMC Musculoskelet Disord. 2008 Jan 29;9:13. doi: 10.1186/1471-2474-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomoda K, Yoshikawa M, Itoh T, et al. Elevated circulating plasma adiponectin in underweight patients with COPD. Chest. 2007 Jul;132(1):135–40. doi: 10.1378/chest.07-0227. [DOI] [PubMed] [Google Scholar]
- 26.Popa C, Netea MG, de Graaf J, et al. Circulating leptin and adiponectin concentrations during tumor necrosis factor blockade in patients with active rheumatoid arthritis. J Rheumatol. 2009 Apr;36(4):724–30. doi: 10.3899/jrheum.080626. Epub 2009 Feb 27. [DOI] [PubMed] [Google Scholar]
- 27.Lane N. Osteoporosis: is there a rational approach to fracture prevention? Bull NYU Hosp Jt Dis. 2006;64(1–2):67–71. [PubMed] [Google Scholar]
- 28.Anandacoomarasamy A, Fransen M, March L. Obesity and the musculoskeletal system. Curr Opin Rheumatol. 2009 Jan;21(1):71–7. doi: 10.1097/bor.0b013e32831bc0d7. [DOI] [PubMed] [Google Scholar]
- 29.Zhao LJ, Jiang H, Papasian CJ, et al. Correlation of obesity and osteoporosis: effect of fat mass on the determination of osteoporosis. J Bone Miner Res. 2008 Jan;23(1):17–29. doi: 10.1359/JBMR.070813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agbaht K, Gurlek A, Karakaya J, et al. Circulating adiponectin represents a biomarker of the association between adiposity and bone mineral density. Endocrine. 2009 Jun;35(3):371–9. doi: 10.1007/s12020-009-9158-2. Epub 2009 Mar 14. [DOI] [PubMed] [Google Scholar]
- 31.Kaufman JM, Goemaere S. Osteoporosis in men. Best Pract Res Clin Endocrinol Metab. 2008 Oct;22(5):787–812. doi: 10.1016/j.beem.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Bonnick SL. Osteoporosis in men and women. Clin Cornerstone. 2006;8(1):28–39. doi: 10.1016/s1098-3597(06)80063-3. [DOI] [PubMed] [Google Scholar]