Abstract
Toxoplasma rhoptry neck protein 4 (TgRON4) is a component of the moving junction macromolecular complex that plays a central role during invasion. TgRON4 is exposed on the cytosolic side of the host cell during invasion, but its molecular interactions remain unclear. Here, we identified host cellular β-tubulin as a binding partner of TgRON4, but not Plasmodium RON4. Coimmunoprecipitation studies in mammalian cells demonstrated that the C-terminal 15-kDa region of β-tubulin was sufficient for binding to TgRON4, and that a 17-kDa region in the proximal C-terminus of TgRON4 was required for binding to the C-terminal region of β-tubulin. Analysis of T. gondii-infected lysates from CHO cells expressing the TgRON4-binding region showed that the C-terminal region of β-tubulin interacted with TgRON4 at early invasion step. Our results provide evidence for a parasite-specific interaction between TgRON4 and the host cell cytoskeleton in parasite-infected cells.
Toxoplasma gondii is an obligate intracellular parasite of the phylum Apicomplexa, which includes the human and animal parasites Plasmodium, Eimeria, Neospora, Theileria, Babesia, and Cryptosporidium. Approximately one-third of humans worldwide are chronically infected with T. gondii1,2. While the infection is usually asymptomatic, Toxoplasma causes the opportunistic disease toxoplasmosis in immunocompromised individuals and neonates with congenital infections3. Unlike most other Apicomplexa, Toxoplasma can invade and replicate in almost all of the nucleated cells of warm-blooded animals. Apicomplexan parasites have a very diverse host range, and the cell invasion machinery among these parasites is highly conserved. Host cell invasion by T. gondii involves the sequential secretion of two distinct secretory organelles, termed micronemes and rhoptries, which are characteristic of the Apicomplexa phylum4.
A key structure for host cell invasion is a tight junction structure known as the moving junction, which is formed by intimate contact between the apical tip of the parasite and the host cell membrane5. As the invasion advances, the parasite propels itself by means of an internal actomyosin motor into the host cell, thereby leading to the formation of a parasitophorous vacuole surrounded by the parasitophorous vacuole membrane inside the host cell6. The moving junction is composed of apical membrane antigen 1 (AMA1), secreted from micronemes, and rhoptry neck (RON) proteins, secreted from rhoptries. AMA1, secreted on the parasite surface, plays a central role in invasion by Plasmodium merozoites and Toxoplasma tachyzoites7,8,9. Immunoprecipitation studies with Toxoplasma have identified TgRON2, TgRON4, TgRON5, and TgRON8 as components of the moving junction complex10,11,12,13. RON2, RON4, and RON5 have also been characterized in P. falciparum14,15,16,17, and their orthologs can be found in the genomes of Apicomplexan species18,19,20, suggesting a conserved function for these proteins; RON8, however, appears to be restricted to the coccidia12. TgRON4, TgRON5, and TgRON8 are secreted into the host cell where they are exposed to the cytosolic face of the host cell membrane, whereas TgRON2 is inserted as an integral transmembrane protein into the host plasma membrane and can interact with TgAMA1 in the absence of other RON proteins13. More detailed studies have revealed that the C-terminal region of RON2 interacts with AMA1 and that the RON2-AMA1 interaction is essential for both T. gondii and P. falciparum entry into their host cells21,22. The crystal structure of the complex formed between AMA1 and RON2 has been determined23,24. In contrast to these several lines of evidence that AMA1 directly interacts with RON2 in vitro, Giovannini et al.25 have showed that AMA1 is not detected at the moving junction of invading tachyzoites. There is an ongoing active debate on the role of AMA1 in the moving junction.
The functions of other soluble RONs (TgRON4/5/8) have been studied, particularly those of TgRON8. Exogenous expression of TgRON8 in mammalian cells indicates that this protein is transported to the periphery of the host cell, corresponding to the plasma membrane or cortical cytoskeleton, via its C-terminal domain12. Moreover, functional analyses of TgRON8 indicate that both attachment and invasion are severely impaired in RON8 knockout parasites and suggest that loss of TgRON8 leads to disorganized secretion of junction components26. A molecular model has showed that RON8 forms a firm intracellular grip that commits the parasite to invasion by anchoring it to the host cytoskeleton. The precise roles of TgRON4 and TgRON5 remain unknown. In conditional TgAMA1 null mutant tachyzoites, TgRON4 showed a typical ring-like signal, indicating that TgAMA1 is dispensable for the formation of a functional moving junction25. In P. berghei, both AMA1 and RON4 play important roles in merozoite invasion of erythrocytes, but only RON4 is likely to be essential for sporozoite invasion25. Attempts to obtain knockout parasites have been unsuccessful in T. gondii tachyzoites10 and the erythrocytic stage of P. berghei25.
In this study, we identified host cellular β-tubulin as a binding partner of TgRON4. We also showed that the C-terminal region of β-tubulin is sufficient for binding to TgRON4 and the region of TgRON4 that contains amino acids 727–860 binds to the C-terminal region of β-tubulin. The results of immunoprecipitation and immunofluorescence assays suggest that the C-terminal region of β-tubulin interacts with TgRON4 during the invasion step.
Results
Preparation of Fc-fusion RON4 recombinant proteins
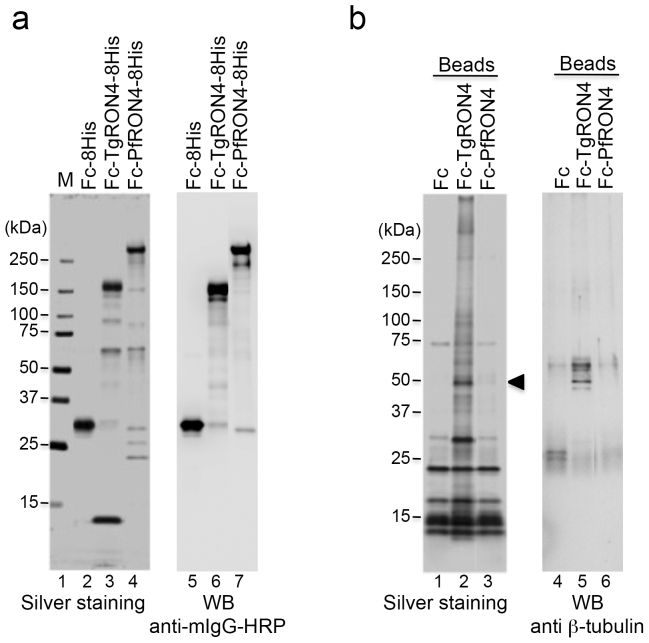
For the binding study, we prepared recombinant TgRON4 and PfRON4 proteins, which were fused with the Fc region of murine IgG2a at the N terminus and an octahistidine tag at the C terminus. Each recombinant protein was expressed using a baculovirus expression system and purified on Ni-NTA agarose from the culture supernatant of the baculovirus-infected Tn5 cells. As a negative control, Fc-8His recombinant protein was prepared. The purified Fc-TgRON4-8His recombinant protein produced a major band on SDS-PAGE with a molecular mass of approximately 164 kDa, larger than its predicted size of 133 kDa (Fig. 1a: lane 3). Expression in Drosophila Schneider 2 cells of a secreted recombinant TgRON4 (RON4S2) with a V5-his tag at the C terminus produced three bands with apparent molecular masses of approximately 110–140 kDa under reducing conditions27. Taking into account the presence of the Fc tag, the major band of Fc-TgRON4-8His probably corresponds to the larger band of RON4S2, the immature form of TgRON4. Purification of Fc-PfRON4-8His yielded a major band with a molecular mass of > 250 kDa, much greater than its predicted size of 162 kDa (Fig. 1a: lane 4). Immunoblots with anti-PfRON4 antisera in parasite extracts have previously detected a single band of ~ 200 kDa or 250 kDa16,28. The discrepancy in the predicted (134 kDa) versus these observed molecular weights is likely due to the presence of peptide repeat regions in the N terminus that are rich in charged amino acid residues. Immunoblotting with an anti-mouse Fc antibody detected each of the major bands of the recombinant TgRON4 and PfRON4 proteins (Fig. 1a).
Figure 1. Identification of TgRON4-binding host proteins.
(a) Recombinant proteins expressed using the baculovirus expression system and purified on Ni-NTA agarose. Each protein (50 ng) was separated by 5%–20% gradient SDS-PAGE and subjected to silver staining (lanes 2–4). The molecular masses (kDa) are indicated on the left. Immunoblotting with an HRP-conjugated anti-mouse Fc antibody of purified recombinant proteins (lanes 5–7). (b) Each Fc-fusion protein was crosslinked to protein G magnetic beads and incubated with membrane proteins from 293 T cells. The eluate was separated by SDS-PAGE followed by silver staining (lanes 1–3). The arrowhead indicates a 50-kDa band that is specific for incubation with TgRON4-linked beads. Immunoblotting of the eluates with an anti-β-tubulin antibody (lanes 4–6).
Identification of RON4-binding host cellular proteins
To identify host cellular proteins that interact with parasite RON4, we performed a pulldown assay using protein G magnetic beads immobilized with Fc-RON4-8His recombinant proteins. To detect TgRON4-interacting host proteins at an early invasion step, we used membrane proteins prepared from 293 T cells. Incubation of the hydrophobic proteins with TgRON4-linked beads detected a specific major band with a molecular mass of approximately 50 kDa, whereas incubation with PfRON4-linked beads detected no specific band (Fig. 1b, lanes 3, 6). Mass spectrometric analysis of the 50-kDa protein revealed tubulin beta chain (TUBB5) and tubulin beta-2C chain (TUBB2C) as shown in Table 1. A Mascot search showed same number of matched peptides and the same coverage in TUBB5 and TUBB2C, except for a slight difference in the score. The amino acid sequences of TUBB5 and TUBB2C shared 97.7% sequence identity. Immunoblotting with an anti-β-tubulin antibody detected the 50-kDa band specifically in the Fc-TgRON4-8His pulldown (Fig. 1b, lane 5).
Table 1. Identification of TgRON4-binding proteins by LC-MS/MS.
| Protein name | Molecular weight | Score | Matches | Coverage (%) |
|---|---|---|---|---|
| Tubulin beta chain (TUBB5) | 49639 | 348 | 9 | 24 |
| Tubulin beta-2C chain (TUBB2C) | 49799 | 318 | 9 | 24 |
Host cellular β-tubulin binds to the C-terminal half of TgRON4
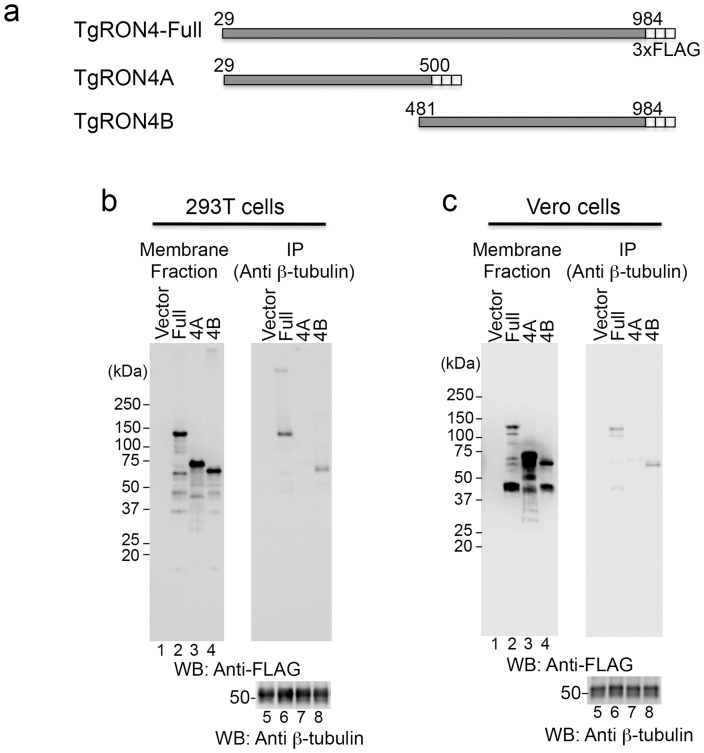
TUBB5 and TUBB2C are ubiquitously distributed and represent the major isotypes among the seven β-tubulin isotypes in all tissues except for brain29. To validate the binding specificity between TgRON4 and host cellular β-tubulin, we conducted immunoprecipitation assays using mammalian cells expressing TgRON4. We prepared deletion constructs consisting of 3xFLAG-tagged full-length TgRON4 (TgRON4-Full) lacking its putative signal sequence, the N-terminal half of TgRON4 (TgRON4A), and the C-terminal half of TgRON4 (TgRON4B) (Fig. 2a). 293T and Vero cells were transiently transfected with a control or each of these constructs, and the membrane proteins prepared from collected cells were immunoprecipitated with an anti-β-tubulin antibody. Expression of 3xFLAG-tagged TgRON4 protein was observed in the hydrophobic fraction (Fig. 2b and 2c, lanes 2–4), and some smaller bands could also be observed, especially in Vero cells. TgRON4-Full and TgRON4B coimmunoprecipitated with β-tubulin in both 293 T and Vero cells, but RON4A did not (Fig. 2b and 2c, lames 6–8). The ratios of precipitated TgRON4-Full and TgRON4B to TgRON4 in total membrane fractions were 1.7% and 0.82% in 293 T cells, 1.0% and 0.56% in Vero cells, respectively (Fig. 2b and 2c, lames 2, 4, 6, 8). The ratio of precipitated TgRON4-Full is higher than that of TgRON4B. TgRON4A may partially contributed to fully binding to β-tubulin. These results demonstrate that the C-terminus of TgRON4 mainly binds to host cellular β-tubulin.
Figure 2. The C-terminal half of TgRON4 binds to host β-tubulin.
(a) Schematic representation of TgRON4 and its deletion mutants. (b), (c) Coimmunoprecipitation of β-tubulin with deletion mutants of 3xFLAG-tagged TgRON4. 293 T (b) and Vero (c) cells were transiently transfected with the indicated TgRON4 expression plasmids. Membrane fractions were immunoprecipitated with an anti-β-tubulin antibody. The membrane fractions (3.3% input) and immunoprecipitates (IP) were analyzed by Western blotting with anti-FLAG and anti-β-tubulin antibodies.
Mapping of the TgRON4-binding region on TUBB2C
To identify the TgRON4-binding region of host cellular β-tubulin, we generated expression vectors encoding various fragments of HA-tagged TUBB2C (Fig. 3a and 3b, upper). We used the coding sequence of TUBB2C to prepare the deletion constructs, because of the slightly higher transient expression level of TUBB2C (data not shown). HA-tagged TUBB2C mutants were coexpressed with 3xFLAG-tagged TgRON4B in Vero cells and immunoprecipitated with an anti-HA antibody. All N-terminal truncated deletions of TUBB2C did not affect the binding to TgRON4B (Fig. 3a, 17–20). To determine whether the C-terminal region of TUBB2C is important for the binding to TgRON4B, we generated a C-terminal truncated mutant of HA-tagged TUBB2C. The TUBB2C mutant, consisting of amino acids 1–334, did not coprecipitate with 3xFLAG-tagged TgRON4B (Fig. 3b, lane 12), suggesting that the TgRON4-binding region is contained within amino acids 335–445.
Figure 3. Identification of the TgRON4-binding region in TUBB2C.
(a), (b) Upper: Schematic representation of TUBB2C and its N- and C-truncated mutants. Lower: The indicated HA-tagged TUBB2C proteins were coexpressed with 3xFLAG-tagged TgRON4B. The cell lysates were immunoprecipitated with an anti-HA antibody. The cell lysates and immunoprecipitates (IP) were analyzed by Western blotting with anti-HA and anti-FLAG antibodies. Owing to low expression, transient transfection of TUBB2C FR3 was scaled up (see Methods).
Mapping of the TUBB2C-binding region on TgRON4
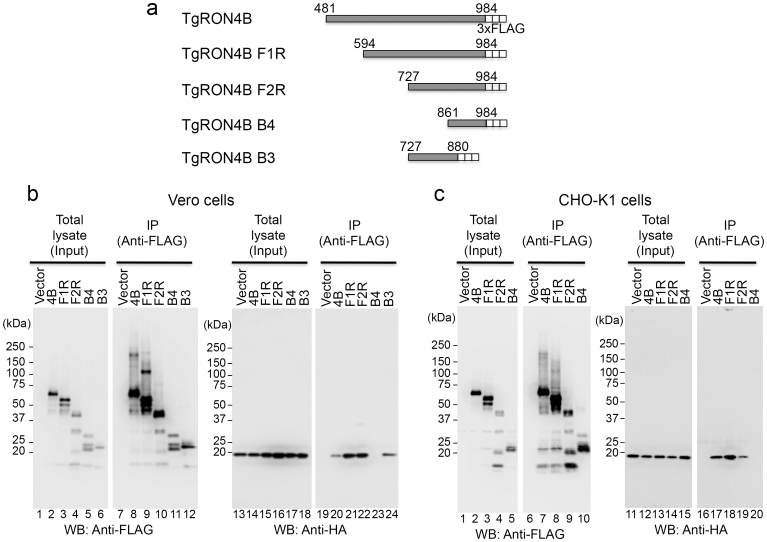
HA-tagged TUBB2C consisting of amino acids 315–445 (TUBB2C F3R) was sufficient to bind 3xFLAG-tagged TgRON4B (Fig. 3a, lane 20). To determine the TUBB2C-binding region of TgRON4, we made 3xFLAG-tagged expression constructs containing various deletions in the C-terminal half of TgRON4 (Fig. 4a). These deletion mutants were coexpressed with HA-tagged TUBB2C F3R in Vero and CHO cells and immunoprecipitated with an anti-FLAG antibody. Of the N-terminal truncated deletions, the 3xFLAG-tagged TgRON4B mutants (F1R and F2R) consisting of amino acids 594–984 and 727–984 coimmunoprecipitated with HA-tagged TUBB2C F3R in both Vero and CHO cells, indicating that they bound to the HA-tagged TUBB2C F3R (Fig. 4b lanes 21, 22 and 4c lanes 18, 19), whereas the 3xFLAG-tagged TgRON4B mutant (B4) consisting of amino acids 861–984 did not (Fig. 4b lane 23 and 4c lane 20). In Vero cells, the 3xFLAG-tagged TgRON4B mutant (B3) consisting of amino acids 727–880 bound to HA-tagged TUBB2C F3R (Fig. 4b, lane 24). Because the transient expression of TgRON4B B3 was limited in CHO cells, cotransfection was scaled up 4-fold. The 3xFLAG-tagged TgRON4B B3 mutant coimmunoprecipitated with HA-tagged TUBB2C F3R in both Vero and CHO cells (Supplemental Fig. 1 lanes 14, 16). Together, these results suggest that the TUBB2C-binding region is localized within amino acids 727–860 of TgRON4.
Figure 4. Identification of the TUBB2C-binding region in TgRON4.
(a) Schematic representation of TgRON4B and its deletion mutants. (b), (c) The indicated 3xFLAG-tagged RON4 proteins were coexpressed with HA-tagged TUBB2C F3R in Vero and CHO cells. The cell lysates were immunoprecipitated with an anti-FLAG antibody. The cell lysates and immunoprecipitates (IP) were analyzed by Western blotting with anti-HA and anti-FLAG antibodies.
Interaction between TUBB2C and TgRON4 in T. gondii-infected CHO cells
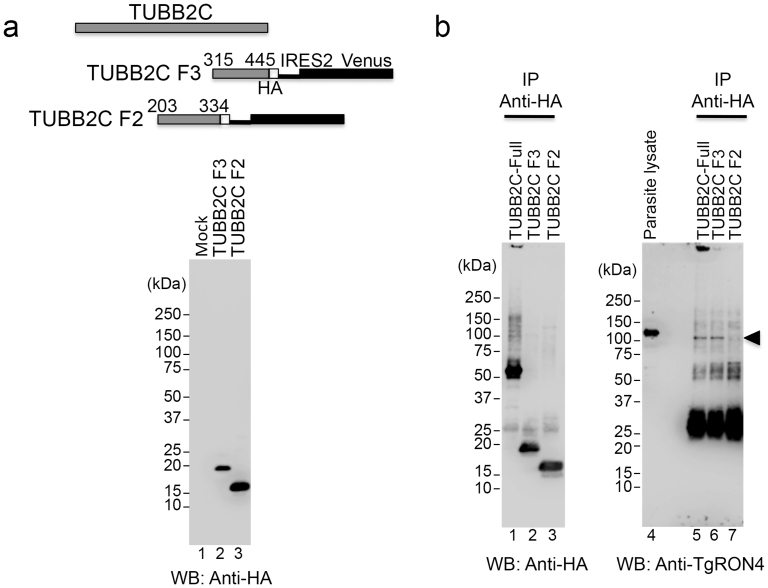
To better assess the binding of TgRON4 to TUBB2C, we generated stable CHO cell lines expressing HA-tagged TUBB2C by use of lentiviral vector-mediated gene transfer. CHO cells were infected with lentivirus containing HA-tagged TUBB2C consisting of amino acids 315–445 (F3) or 203–334 (F2) followed by the IRES2-Venus sequence. Venus-positive cells that formed colonies were picked up from the diluted cell culture and propagated. The expression of HA-tagged TUBB2C proteins was confirmed by Western blotting using an anti-HA antibody (Fig. 5a). Stable Vero cell lines expressing HA-tagged TUBB2C could not be established due to the low expression of the transgene (data not shown). As a positive control, full-length TUBB2C with an HA tag was transiently expressed in CHO cells. Monolayers of CHO cells expressing HA-tagged TUBB2C were infected with T. gondii RH parasites using a potassium buffer shift30. Parasites were allowed to invade for 2 min and then infected CHO monolayers were lysed. The total lysates were immunoprecipitated with an anti-HA antibody. An anti-TgRON4 polyclonal antibody was produced by immunization of mice with TgRON4 recombinant protein fused with the 3xFLAG tag at the N terminus and an octahistidine tag at the C terminus. Immunoblotting with the anti-TgRON4 polyclonal antibody detected a major band with a molecular mass of approximately 120 kDa in the parasite lysates (Fig. 5b, lane 4). Pulse-chase metabolic labeling and immunoprecipitation with anti-TgRON4 mAb T5 4H1 previously showed that TgRON4 is expressed as a pro-protein of ~ 120 kDa that is cleaved to yield a mature protein of ~ 110 kDa under reducing condition13. In T. gondii–infected CHO cells expressing HA-tagged TUBB2C-Full, TgRON4 coimmunoprecipitated with TUBB2C and was observed at approximately 110 kDa, corresponding to the mature form of TgRON4 (Fig. 5b, lane 5). When stable CHO cell lines expressing HA-tagged truncated TUBB2C were infected with T. gondii RH parasites by means of synchronous invasion, TUBB2C F3 coimmunoprecipitated with TgRON4, but TUBB2C F2 did not (Fig. 5b, lanes 6, 7). These results indicate that the 15-kDa region at the C-terminus of TUBB2C binds to the mature form of TgRON4.
Figure 5. Interaction between the C-terminal region of TUBB2C and TgRON4 in T. gondii-infected CHO cells.
(a) Upper: Schematic representation of HA-tagged TUBB2C F3 and F2, followed by the IRES2-Venus sequence. Immunoblotting with an anti-HA antibody of the total lysates from CHO cells expressing the lentivirus-mediated HA-tagged TUBB2C F3 (lane 2) and F2 (lane 3). (b) T. gondii RH tachyzoites were allowed to synchronously invade monolayers of CHO cells expressing the indicated HA-TUBB2C for 2 min. The total lysates of infected CHO monolayers were then immunoprecipitated with an anti-HA antibody. Immunoprecipitates (IP) were analyzed by Western blotting with anti-HA and anti-TgRON4 polyclonal antibodies. At the same time, parasite lysates were immunoblotted by using an anti-TgRON4 polyclonal antibody. The arrowhead indicates a 110-kDa band that corresponds to the mature form of TgRON4.
Localization of the TUBB2C C-terminal region and TgRON4 in the moving junction
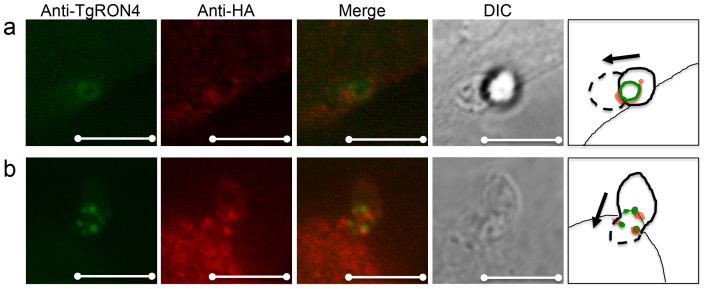
It was recently reported that host cell microtubules localize to a tight junction, called the moving junction, of early invading T. gondii parasites31. To investigate whether the 15-kDa region at the C-terminus of TUBB2C is sufficient for localization to the moving junction, we infected monolayers of CHO cells expressing HA-tagged TUBB2C F3 with T. gondii RH parasites. Parasites were allowed to synchronously invade for 2 min, after which the infected CHO monolayers were fixed with formaldehyde and permeabilized. The anti-TgRON4 polyclonal antibody recognized the moving junction on the invading parasites (Fig. 6a and 6b), and the anti-HA antibody detected TUBB2C F3 accumulation around (Fig. 6a) the moving junction, corresponding to the previous report that host microtubules are asymmetrically concentrated on one side of the moving junction31. While TUBB2C F3 did not completely overlap with TgRON4, TUBB2C F3 and TgRON4 were closely localized in invading parasites (Fig. 6b). Although early invasion of T. gondii seemed unaffected by expressing HA-tagged TUBB2C F3 (Supplemental Fig. 2), these results indicate that the 15-kDa region at the C-terminus of TUBB2C may associate with TgRON4 in invading parasites.
Figure 6. Localization of the TUBB2C C-terminal region and TgRON4 in the moving junction during Toxoplasma invasion.
CHO cells expressing HA-TUBB2C F3 were infected with T. gondii RH tachyzoites using a potassium buffer shift. At 2-min invasion, infected CHO monolayers were fixed and permeabilized. HA-TUBB2C F3 was stained with a rat anti-HA antibody and TgRON4 was stained with an anti-TgRON4 mouse polyclonal antibody. Alexa Fluor 350 goat anti-mouse IgG and Alexa Fluor 546 goat anti-rat IgG served as secondary antibodies. The HA-TUBB2C F3 accumulated around (a) or near (b) the moving junction. The cartoon illustrates the direction of the parasite invasion. Scale bar, 5 μm.
Discussion
Here, we demonstrated that TgRON4, but not Plasmodium RON4, binds to host cellular β-tubulin. Through coimmunoprecipitation studies, we identified binding regions for the interaction between TgRON4 and host cellular β-tubulin. Moreover, the TgRON4-binding region of β-tubulin coimmunoprecipitated with and localized near TgRON4 in T. gondii-infected mammalian cells.
Although host cell invasion is an active process that depends on the parasite cytoskeleton for motility32, the cortical host cytoskeleton is also reported to contribute to the anchoring of the moving junction. Actin and actin nucleators, such as Arp2/3, colocalize with the moving junction around the entering Toxoplasma tachyzoites33. Recent studies have shown that Toxoplasma toxofilin, an actin-binding protein, regulates host actin filament disassembly and turnover, facilitating parasite invasion34. TgRON8 expressed in mammalian cells traffics to the cell periphery, suggesting that it may interact with the cortical cytoskeleton12. In addition to actin filaments, the host microtubules have been implicated in interactions with invading Toxoplasma tachyzoites. Disruption of host microtubules significantly reduces early but not late invasion, indicating that host microtubules shorten the period before parasites initiate host cell invasion31. Our results show that TgRON4 is a binding partner of host β-tubulin, yet host actin was not detected in our pulldown experiment using membrane fractions from 293 T cells. Tubulin can be obtained with membrane proteins after solubilizing the membranes with Triton X-11435, similar to our preparation of membrane fractions. Our data indicate that TgRON4 specifically interacts with host β-tubulin. Toxoplasma RON proteins exported to the cytosolic side of the host cell could interact with the cortical host cytoskeleton in a molecule-specific manner.
Our results show that the C-terminal region of β-tubulin is required for binding to TgRON4 (Fig. 3 and 5). Standard X-ray crystallography studies have revealed that the C-terminal tail of the αβ-tubulin dimer cannot be accurately predicted because it is on the external surface36. Most of the known microtubule-binding proteins interact with microtubules and tubulins through acidic C-terminal tail sequences and contain more than one short basic-repeat sequence. For example, the microtubule-associated proteins of the MAP/Tau family include three to four 18 amino acid long imperfect repeats separated by 13 or 14 inter-repeat sequences37,38. Although the possibility remains that other sequence of β-tubulin in addition to the acidic C-terminal tail sequence could interact with TgRON4, the N-terminal part of the β-tubulin binding region of TgRON4 has a basic pI value.
The TgRON4 region required for binding to β-tubulin contains amino acids 727–880 (Fig. 4). The amino acid sequence of TgRON4 consists of a putative signal peptide, two perfect 44-amino acid repeats separated by 85 amino acids, and a region defined by the presence of positionally conserved cysteine residues39. The Plasmodium yoelii PyP140/RON4-specific monoclonal antibody (MAb) designated 48F8 appears to recognize free PyP140/RON4 and does not precipitate the PyAMA1/RON4 complex40. The epitope recognized by MAb 48F8 lies in residues 414 to 420 (TFKDQPE), which are located between the N-terminal repeat region and the C-terminal cysteine–rich region41. An amino acid sequence homologous to this epitope is present in amino acids 403–409 of TgRON4. Accordingly, this region could play an important role in the interaction between RON4 and AMA1. The β-tubulin-binding region of TgRON4 in this study does not overlap with the epitope. Five cysteine residues in the C-terminal region of RON4 are positionally conserved among T. gondii, P. yoelii, and P. falciparum41. The TgRON4 region that binds to β-tubulin contains two of the five cysteine residues. The significance of these cysteine residues in TgRON4 function remains unclear, but the interaction between TgRON4 and β-tubulin may contribute to the formation of disulfide bridges of TgRON4 at the invasion step.
A BLAST search with the β-tubulin-binding region of TgRON4 revealed significant homology (74.7%) with only Neospora caninum RON4 (NCLIV_030050); no homology with RON4 molecules of other apicomplexan parasites was found. This finding supports our result that Plasmodium RON4 does not bind to host β-tubulin (Fig. 1). T. gondii and N. caninum are closely related tissue-dwelling Coccidia and share many common morphological and biological features42,43. Comparative genomics reveal that these parasites have very similar genomes with largely conserved gene content and that they diverged from their common ancestor around 28 million years ago44. In contrast, the TgRON4B B4 region (Fig. 4) consisting of amino acids 861–984 that does not bind to β-tubulin shows relatively low sequence identity (35.5%) with N. caninum RON4. Conservation of the β-tubulin-binding region of RON4 suggests that this region could be physiologically important.
TgRON4 is thought to be essential for the propagation of parasite as attempts to obtain knockout parasites have been unsuccessful10. RON4 is conserved among apicomplexan parasites, but does not bear any of the known recognition motifs that have been implicated in molecular interactions. Our finding is the first to reveal a specific region of TgRON4 that binds a host cell protein in T. gondii-infected cells. Further studies of the molecular interactions of RON proteins are required to fully understand host-parasite communication at the invasion step.
Methods
The primers and antibodies used in this study are described in the Supplementary Information. Construction of β-tubulin and TgRON4 expression plasmids and invasion assay were performed as described in the Supplementary Information.
Cells and parasites
293 T cells were maintained in Dulbecco's modified Eagle's medium (DMEM; Nissui, Tokyo, Japan) supplemented with 10% FCS, glutamine, penicillin and streptomycin. Vero cells were cultured in DMEM with 5% FCS. CHO cells were maintained in Ham F-12 medium (Life Technologies, Carlsbad, CA) with 10% FCS and antibiotics. Tachyzoites of T. gondii RH strain were maintained in Vero cells as described previously45. Spodoptera frugiperda Sf9 and Trichoplusia ni Tn5 insect cells were cultured as described previously46.
Preparation of RON4 recombinant proteins
Total RNA of T. gondii RH strain was extracted using Trizol (Life Technologies) and further purified with the SV Total RNA Isolation System (Promega) according to the manufacturer's protocol. The coding sequence for TgRON4 amino acids 29–984 was amplified by RT-PCR using the sets of primers TgRON4-F/TgRON4-R. The coding sequence for Plasmodium falciparum RON4 amino acids 25–1201 was PCR amplified from P. falciparum cDNA (HB3 strain) using the sets of primers PfRON4-F/PfRON4-R. These amplified fragments were then cloned into the SpeI/SmaI sites of the baculovirus expression vector pBSV-Fc-8His46. The resultant plasmids were designated pBSV-Fc-TgRON4-8His and pBSV-Fc-PfRON4-8His, respectively. To generate pBSV-3xFLAG-8His vector, a fragment containing the 3xFLAG sequence was amplified by PCR using the p3xFLAG-CMV14 vector (Sigma) as the template with a forward primer containing a PstI site and a reverse primer containing a BamHI site. Amplified fragments were cloned into the PstI/BamHI sites of pBSV-Fc-8His, replacing the Fc tag with the 3xFLAG tag. TgRON4 cDNA was cloned into the SpeI/SmaI sites of pBSV-3xFLAG-8His. The resultant plasmid was designated pBSV-3xFLAG-TgRON4-8His. These plasmids were co-transfected with BaculoGold DNA (BD Biosciences, San Jose, CA) into Sf9 insect cells. Amplification of recombinant baculovirus was performed as described previously46. The recombinant proteins, designated Fc-8His, Fc-TgRON4-8His, Fc-PfRON4-8His, and 3xFLAG-TgRON4-8His, were purified from the culture supernatant of the infected Tn5 cells by using Ni-NTA agarose (QIAGEN) and were concentrated with a Spin-X UF 6 centrifugal filter device (Corning).
Pulldown assay
Fc-fusion proteins (2 μg each) were crosslinked to protein G magnetic beads (New England Biolabs) by using dimethyl pimelidate dihydrochloride according to the IgG crosslinking protocol of the manufacturer. Membrane proteins were prepared from 293 T cells (4.5 × 107) by using a CELlytic MEM protein extraction kit (SIGMA). Membrane proteins diluted with IP buffer (150 mM NaCl, 10 mM Tris-HCl pH 7.4, 1 mM EDTA, 1 mM EGTA, 0.2 mM sodium orthovanadate, 1% Triton X-100, 0.5% NP-40) supplemented with Complete EDTA-free protease inhibitor cocktail (Roche) were first pre-cleared by mixing them with protein G magnetic beads and then incubating them with Fc-8His-linked beads. Non-specific protein complexes were removed by magnetic separation. Supernatant was added to the Fc-TgRON4-8His or Fc-PfRON4-8His protein-linked beads and the mixture was incubated for 3 h at 4°C. The beads were then washed four times with IP buffer and eluted with SDS sample loading buffer (187.5 mM Tris-HCl pH 6.8, 6% SDS, 30% glycerol, 150 mM DTT, 0.03% BPB). The eluates were separated by 5%–20% gradient SDS-PAGE (Atto, Tokyo, Japan) for silver staining and Western blot analysis using the anti-β-tubulin antibody.
Protein identification by mass spectrometry
For protein identification, hydrophobic proteins from 1.3 × 108 293 T cells and protein G magnetic bead-immobilized Fc-TgRON4-8His recombinant protein (6 μg) were used in the pulldown assay as mentioned above. The protein bands stained with Silver Stain MS Kit (Wako, Osaka, Japan) were excised, destained, and treated with trypsin. The digested peptide mixture was then separated by using a prominence nano-liquid chromatography system (Shimadzu, Kyoto, Japan). Collected fractions were subjected to the 4700 Proteomics Analyzer MALDI-TOF/MS (Applied Biosystems, Framingham, MA). Protein identification was carried out using the MS/MS Ion Search in the Mascot Search (Matrix Science).
Transfection and immunoprecipitation
Vero and 293 T cells were transiently transfected using TransIT-LT1 Reagent (TaKaRa) as described in the manufacturer's protocol. Transient transfection into CHO cells was performed by using a TransIT-CHO Transfection kit (TaKaRa) according to the manufacturer's protocol. Cells were seeded onto 6-well tissue culture plate 24 h before transfection. Cells were then transfected with 2.5 μg of 3xFLAG-tagged TgRON4 construct per well. At 48 h post transfection, hydrophobic proteins from collected cells were prepared as described above and incubated with protein G magnetic bead-immobilized β-tubulin antibody. For co-immunoprecipitation of TgRON4 with TUBB2C, Vero and CHO cells were transfected with 1.25 μg each of HA-tagged TUBB2C and 3xFLAG-tagged TgRON4 and incubated for 48 h. After being washed, the cells were lysed in a modified RIPA buffer (50 mM Tris-HCl pH 8.0, 5 mM EDTA, 75 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.005% SDS) supplemented with Complete EDTA-free protease inhibitor cocktail. The lysates were immonoprecipitated with protein G magnetic bead-immobilized anti-HA or anti-FLAG M2 Monoclonal Antibodies (SIGMA). These immunoprecipitates were washed several times, and were then separated by 5%–20% gradient SDS-PAGE and immunoblotted to detect 3xFLAG-tagged and/or HA-tagged proteins. For the C-terminal truncated TUBB2C construct, which was expressed at low levels, co-transfection was performed with 2.25 μg of HA-tagged TUBB2C and 0.25 μg of 3xFLAG-tagged TgRON4 per well, and was scaled up 4-fold. For the 3xFLAG-tagged TgRON4B B3 construct, which was expressed at low levels in CHO cells, cotransfection was performed with 2 μg of 3xFLAG-tagged TgRON4B B3 and 0.5 μg of HA-tagged TUBB2C F3R per well, and was scaled up 4-fold.
Lentiviral transduction
The coding sequence for TUBB2C amino acids 203–334 and 315–445, which contains the HA tag sequence, was PCR amplified from HA-tagged deleted TUBB2C plasmids by using the sets of primers F2forCSII/RforCSII and F3forCSII/RforCSII, respectively (The primers are described in Supplemental Table S1). The amplified fragments were then cloned into the NotI/BamHI sites of CSII-EF-MCS-IRES2-Venus (RIKEN BioResource Center) by using the In-Fusion HD Cloning Kit. For the generation of lentiviral vectors, 293 T cells were transfected with CSII-EF-TUBB2-IRES2-Venus, pCAG-HIVgp (RIKEN), and pCMV-VSV-G-RSV-Rev (RIKEN) by using TransIT-293 Reagent (TaKaRa). After 48 h, culture supernatant was passed through a 0.45-μm filter and the lentiviral supernatant, diluted with equal volume of F-12 medium containing 10% FCS, was added to the CHO cells. At 48 h post-infection, the medium was replaced with fresh F-12 medium supplemented with 10% FCS and antibiotics. To obtain TUBB2C-expressing CHO cells, cells were plated at a low density and Venus-positive cells that formed colonies were suspended by pipetting in a penicillin cup and were transferred to a new culture plate. Expression of HA-tagged TUBB2C was confirmed by Western blotting of the total cell lysate.
Immunoprecipitation using TUBB2C-expressing CHO cells infected with T. gondii
Monolayers of T. gondii-infected Vero cells were mechanically lysed by several passages through a 25-gauge needle and tachyzoites were purified by filtration through a 5.0-μm filter. HA-tagged truncated TUBB2C-expressing CHO cells were seeded onto two PLL-coated 60-mm culture dishes 24 h before infection. As a control, transient transfection was performed with the pHA-TUBB2C full-length plasmid into CHO cells seeded onto a PLL-coated 60-mm culture dish and cells were incubated for 48 h prior to infection. A potassium buffer shift was used to synchronize invasion, as described previously30. Purified tachyzoites were suspended in Endo buffer (44.7 mM K2SO4, 10 mM MgSO4, 106 mM sucrose, 5 mM glucose, 20 mM Tris-H2SO4 pH 8.2, 3.5 mg/ml BSA). Parasites were added to cells at a multiplicity of infection (MOI) of 5 and allowed to settle at 37°C for 20 min. The Endo buffer was then exchanged for prewarmed F-12 medium with 10% FCS to initiate parasite invasion. After a 2-min invasion, the medium was removed and the infected cells were lysed in the Lysis and Separation buffer of the CELlytic MEM protein extraction kit. Total lysate was diluted with IP buffer containing protease inhibitor cocktail and incubated with anti-HA antibody-linked protein G magnetic beads. After the beads were washed, elution and SDS-PAGE were performed as described above. Anti-HA and anti-TgRON4 antibodies were used for immunoblotting, and antibody reactions were performed by using Can Get Signal immunoreaction enhancer solution (ToYoBo).
Immunofluorescence assays
CHO cells expressing TUBB2C-HA were seeded onto a 35-mm glass bottom dish (Greiner Bio-one, Frickenhausen, Germany) 24 h before infection. Parasites were added to the cells at an MOI of 10 and invading parasites were synchronized by using a potassium buffer shift. After a 2-min invasion, the infected CHO monolayers were fixed with 3.7% paraformaldehyde in PBS for 15 min and permeabilized with PBS/3% BSA/0.1% Triton-X100 for 20 min, and then blocked with PBS/3% BSA for 30 min at room temperature. The cells were then incubated in PBS/3% BSA with anti-TgRON4 antibody and anti-HA antibody for 1 h at 37°C, washed, and then incubated with the secondary antibodies Alexa Fluor 350 goat anti-mouse IgG and Alexa Fluor 546 goat anti-rat IgG (Life Technologies). Images were taken with a Leica TCS SP5 confocal microscope.
Author Contributions
H.T. and K. Kato designed the experiments. H.T. carried out the whole experiments and analyzed the data. T.S., K. Kobayashi, H.G., A. Ishiwa, F.C.R., F.M., T.I., A. Inomata, T.H. and H.A. contributed to the data analysis and discussion. H.T. and K. Kato wrote the manuscript. K. Kato supervised the study.
Supplementary Material
Supplementary information
Acknowledgments
This study was supported by Grants-in-Aid for Young Scientists, Exploratory Research, Scientific Research on Innovative Areas (3308) from the Ministry of Education, Culture, Science, Sports, and Technology (MEXT) of Japan, Bio-oriented Technology Research Advancement Institution (BRAIN), and the Program to Disseminate Tenure Tracking System from the Japan Science and Technology Agency (JST).
References
- Tenter A. M., Heckeroth A. R. & Weiss L. M. Toxoplasma gondii: from animals to humans. Int. J. Parasitol. 30, 1217–1258 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D. & Dubey J. P. Toxoplasma gondii: transmission, diagnosis and prevention. Clin. Microbiol. Infect. 8, 634–640 (2002). [DOI] [PubMed] [Google Scholar]
- Montoya J. G. & Liesenfeld O. Toxoplasmosis. Lancet 363, 1965–1976 (2004). [DOI] [PubMed] [Google Scholar]
- Carruthers V. B. & Sibley L. D. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur. J. Cell Biol. 73, 114–123 (1997). [PubMed] [Google Scholar]
- Aikawa M., Miller L. H., Johnson J. & Rabbege J. Erythrocyte entry by malarial parasites. A moving junction between erythrocyte and parasite. J. Cell Biol. 77, 72–82 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suss-Toby E., Zimmerberg J. & Ward G. E. Toxoplasma invasion: the parasitophorous vacuole is formed from host cell plasma membrane and pinches off via a fission pore. Proc. Natl. Acad. Sci. U.S.A. 93, 8413–8418 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mital J., Meissner M., Soldati D. & Ward G. E. Conditional expression of Toxoplasma gondii apical membrane antigen-1 (TgAMA1) demonstrates that TgAMA1 plays a critical role in host cell invasion. Mol. Biol. Cell 16, 4341–4349 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triglia T. et al. Apical membrane antigen 1 plays a central role in erythrocyte invasion by Plasmodium species. Mol. Microbiol. 38, 706–718 (2000). [DOI] [PubMed] [Google Scholar]
- Tyler J. S., Treeck M. & Boothroyd J. C. Focus on the ringleader: the role of AMA1 in apicomplexan invasion and replication. Trends Parasitol. 27, 410–420 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander D. L., Mital J., Ward G. E., Bradley P. & Boothroyd J. C. Identification of the moving junction complex of Toxoplasma gondii: a collaboration between distinct secretory organelles. PLoS Pathog. 1, e17 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun M. et al. The rhoptry neck protein RON4 re-localizes at the moving junction during Toxoplasma gondii invasion. Cell. Microbiol. 7, 1823–1833 (2005). [DOI] [PubMed] [Google Scholar]
- Straub K. W., Cheng S. J., Sohn C. S. & Bradley P. J. Novel components of the Apicomplexan moving junction reveal conserved and coccidia-restricted elements. Cell. Microbiol. 11, 590–603 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besteiro S., Michelin A., Poncet J., Dubremetz J. F. & Lebrun M. Export of a Toxoplasma gondii rhoptry neck protein complex at the host cell membrane to form the moving junction during invasion. PLoS pathog. 5, e1000309 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander D. L., Arastu-Kapur S., Dubremetz J. F. & Boothroyd J. C. Plasmodium falciparum AMA1 binds a rhoptry neck protein homologous to TgRON4, a component of the moving junction in Toxoplasma gondii. Eukaryot. Cell 5, 1169–1173 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J. et al. Rhoptry neck protein RON2 forms a complex with microneme protein AMA1 in Plasmodium falciparum merozoites. Parasitol. Int. 58, 29–35 (2009). [DOI] [PubMed] [Google Scholar]
- Morahan B. J., Sallmann G. B., Huestis R., Dubljevic V. & Waller K. L. Plasmodium falciparum: genetic and immunogenic characterisation of the rhoptry neck protein PfRON4. Exp. Parasitol. 122, 280–288 (2009). [DOI] [PubMed] [Google Scholar]
- Curtidor H., Patino L. C., Arevalo-Pinzon G., Patarroyo M. E. & Patarroyo M. A. Identification of the Plasmodium falciparum rhoptry neck protein 5 (PfRON5). Gene 474, 22–28 (2011). [DOI] [PubMed] [Google Scholar]
- Besteiro S., Dubremetz J. F. & Lebrun M. The moving junction of apicomplexan parasites: a key structure for invasion. Cell. Microbiol. 13, 797–805 (2011). [DOI] [PubMed] [Google Scholar]
- Kemp L. E., Yamamoto M. & Soldati-Favre D. Subversion of host cellular functions by the apicomplexan parasites. FEMS Microbiol. Rev. 37, 607–631 (2013). [DOI] [PubMed] [Google Scholar]
- Proellocks N. I., Coppel R. L. & Waller K. L. Dissecting the apicomplexan rhoptry neck proteins. Trends Parasitol. 26, 297–304 (2010). [DOI] [PubMed] [Google Scholar]
- Lamarque M. et al. The RON2-AMA1 interaction is a critical step in moving junction-dependent invasion by apicomplexan parasites. PLoS Pathog. 7, e1001276 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler J. S. & Boothroyd J. C. The C-terminus of Toxoplasma RON2 provides the crucial link between AMA1 and the host-associated invasion complex. PLoS Pathog. 7, e1001282 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonkin M. L. et al. Host cell invasion by apicomplexan parasites: insights from the co-structure of AMA1 with a RON2 peptide. Science 333, 463–467 (2011). [DOI] [PubMed] [Google Scholar]
- Vulliez-Le Normand B. et al. Structural and functional insights into the malaria parasite moving junction complex. PLoS pathog. 8, e1002755 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannini D. et al. Independent roles of apical membrane antigen 1 and rhoptry neck proteins during host cell invasion by apicomplexa. Cell Host Microbe 10, 591–602 (2011). [DOI] [PubMed] [Google Scholar]
- Straub K. W., Peng E. D., Hajagos B. E., Tyler J. S. & Bradley P. J. The moving junction protein RON8 facilitates firm attachment and host cell invasion in Toxoplasma gondii. PLoS pathog. 7, e1002007 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid I. et al. Immunological responses induced by a DNA vaccine expressing RON4 and by immunogenic recombinant protein RON4 failed to protect mice against chronic toxoplasmosis. Vaccine 29, 8838–8846 (2011). [DOI] [PubMed] [Google Scholar]
- Richard D. et al. Interaction between Plasmodium falciparum apical membrane antigen 1 and the rhoptry neck protein complex defines a key step in the erythrocyte invasion process of malaria parasites. J. Biol. Chem. 285, 14815–14822 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leandro-Garcia L. J. et al. Tumoral and tissue-specific expression of the major human β-tubulin isotypes. Cytoskeleton (Hoboken) 67, 214–223 (2010). [DOI] [PubMed] [Google Scholar]
- Kafsack B. F., Beckers C. & Carruthers V. B. Synchronous invasion of host cells by Toxoplasma gondii. Mol. Biochem. Parasitol. 136, 309–311 (2004). [DOI] [PubMed] [Google Scholar]
- Sweeney K. R., Morrissette N. S., LaChapelle S. & Blader I. J. Host cell invasion by Toxoplasma gondii is temporally regulated by the host microtubule cytoskeleton. Eukaryot. Cell 9, 1680–1689 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrowolski J. M. & Sibley L. D. Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite. Cell 84, 933–939 (1996). [DOI] [PubMed] [Google Scholar]
- Gonzalez V. et al. Host cell entry by apicomplexa parasites requires actin polymerization in the host cell. Cell Host Microbe 5, 259–272 (2009). [DOI] [PubMed] [Google Scholar]
- Delorme-Walker V. et al. Toxofilin upregulates the host cortical actin cytoskeleton dynamics, facilitating Toxoplasma invasion. J. Cell. Sci. 125, 4333–4342 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltramo D. M., Nunez M., Alonso A. D. & Barra H. S. The relationship of hydrophobic tubulin with membranes in neural tissue. Mol. Cell. Biochem. 141, 57–63 (1994). [DOI] [PubMed] [Google Scholar]
- Lowe J., Li H., Downing K. H. & Nogales E. Refined structure of αβ-tubulin at 3.5 Å resolution. J. Mol. Biol. 313, 1045–1057 (2001). [DOI] [PubMed] [Google Scholar]
- Lewis S. A., Wang D. H. & Cowan N. J. Microtubule-associated protein MAP2 shares a microtubule binding motif with tau protein. Science 242, 936–939 (1988). [DOI] [PubMed] [Google Scholar]
- Dehmelt L. & Halpain S. The MAP2/Tau family of microtubule-associated proteins. Genome Biol. 6, 204 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley P. J. et al. (2005) Proteomic analysis of rhoptry organelles reveals many novel constituents for host-parasite interactions in Toxoplasma gondii. J. Biol. Chem. 280, 34245–34258 (2005). [DOI] [PubMed] [Google Scholar]
- Narum D. L., Ogun S. A., Thomas A. W. & Holder A. A. Immunization with parasite-derived apical membrane antigen 1 or passive immunization with a specific monoclonal antibody protects BALB/c mice against lethal Plasmodium yoelii yoelii YM blood-stage infection. Infect. Immun. 68, 2899–2906 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narum D. L. et al. Identification and characterization of the Plasmodium yoelii PyP140/RON4 protein, an orthologue of Toxoplasma gondii RON4, whose cysteine-rich domain does not protect against lethal parasite challenge infection. Infect. Immun. 76, 4876–4882 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey J. P., Carpenter J. L., Speer C. A., Topper M. J. & Uggla A. Newly recognized fatal protozoan disease of dogs. J. Am. Vet. Med. Assoc. 192, 1269–1285 (1988). [PubMed] [Google Scholar]
- Dubey J. P. et al. Redescription of Neospora caninum and its differentiation from related coccidia. Int. J. Parasitol. 32, 929–946 (2002). [DOI] [PubMed] [Google Scholar]
- Reid A. J. et al. Comparative genomics of the apicomplexan parasites Toxoplasma gondii and Neospora caninum: Coccidia differing in host range and transmission strategy. PLoS Pathog. 8, e1002567 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugi T. et al. Molecular analyses of Toxoplasma gondii calmodulin-like domain protein kinase isoform 3. Parasitol. Int. 58, 416–423 (2009). [DOI] [PubMed] [Google Scholar]
- Kobayashi K. et al. Plasmodium falciparum BAEBL binds to heparan sulfate proteoglycans on the human erythrocyte surface. J. Biol. Chem. 285, 1716–1725 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information