Abstract
Autoregulation of cerebral perfusion is impaired in hypoxic–ischemic encephalopathy. We investigated whether cerebrovascular pressure reactivity (PRx), an element of cerebral autoregulation that is calculated as a moving correlation coefficient between averages of intracranial and mean arterial blood pressure (MABP) with values between −1 and +1, is impaired during and after a hypoxic–ischemic insult (HI) in newborn pigs. Associations between end-tidal CO2, seizures, neuropathology, and PRx were investigated. The effect of hypothermia (HT) and Xenon (Xe) on PRx was studied. Pigs were randomized to Sham, and after HI to normothermia (NT), HT, Xe or xenon hypothermia (XeHT). We defined PRx >0.2 as peak and negative PRx as preserved. Neuropathology scores after 72 hours of survival was grouped as ‘severe' or ‘mild.' Secondary PRx peak during recovery, predictive of severe neuropathology and associated with insult severity (P=0.05), was delayed in HT (11.5 hours) than in NT (6.5 hours) groups. Seizures were associated with impaired PRx in NT pigs (P=0.0002), but not in the HT/XeHT pigs. PRx was preserved during normocapnia and impaired during hypocapnia. Xenon abolished the secondary PRx peak, increased (mean (95% confidence interval (CI)) MABP (6.5 (3.8, 9.4) mm Hg) and cerebral perfusion pressure (5.9 (2.9, 8.9) mm Hg) and preserved the PRx (regression coefficient, −0.098 (95% CI (−0.18, −0.01)), independent of the insult severity.
Keywords: cerebrovascular pressure reactivity, hypothermia, hypoxia–ischemia, newborn, pig, xenon
Introduction
Cerebral autoregulation, implying stable cerebral blood flow (CBF) despite varying blood pressure, is impaired after perinatal asphyxia.1 Autoregulation of CBF is controlled by myogenic, metabolic, neural, and endothelial cell-related factors.2 Cerebrovascular pressure reactivity (PRx), a key element of cerebral autoregulation, reflects the ability of the smooth muscle tone of small cerebral arteries and arterioles to react to spontaneous low-frequency fluctuations in arterial blood pressure3 and quantifies the myogenic component of cerebral autoregulation. Cerebrovascular pressure reactivity is measured from the relationship between the slow waves of changing intracranial pressure (ICP) and mean arterial blood pressure (MABP).4 When PRx is preserved, minor spontaneous changes in MABP are not reflected in similar changes in the ICP waveform. There is therefore no correlation between the two, and the PRx is zero or negative. However, when cerebral autoregulation is impaired, minor changes in MABP result in similar passive changes in ICP; there is a positive correlation between MABP and ICP, and the value for PRx approaches 1.
In adults and children with traumatic brain injury, impaired PRx has been associated with a worse outcome or death.3, 5 There are no studies of PRx in perinatal asphyxia. We hypothesized that PRx, being a global estimate of pressure reactivity and a marker of cerebral autoregulation, is impaired during and after a global hypoxic–ischemic insult (HI). Hypothermia (HT) is the standard treatment after perinatal asphyxia and xenon (Xe) is currently being evaluated as a possible additional neuroprotective agent.6, 7 We investigated in our newborn pig model of global HI: (1) the course of PRx during and after the HI period; (2) whether PRx predicts neuropathology; (3) the influence of HI severity, seizures, and end-tidal CO2 (ETCO2) on PRx; and (4) the effects of HT, Xe, and the XeHT combination on PRx.
Materials and Methods
Conduct of Experiment
The protocol was conducted under UK Home Office license and approved by the University of Bristol Ethical Review Panel. This was a substudy of our original experiment, where we reported that Xe additively decreased brain injury with HT after global HI in newborn pigs.6 The present study comprised data from 51 crossbred Landrace/large white newborn pigs randomized between sham (n=3), NT (n=12), 24-hour HT (n=16), 18-hour Xe (n=7), and 18-hour Xe/24-hour HT (n=13).
Anesthesia and Ventilation
After unrestrained induction with 2% isoflurane, 65% nitrous oxide (N2O), and 33% oxygen (O2), pigs were intubated with a 3.0 mm cuffed endotracheal tube (Mallinckrodt Medical, Athlone, Ireland) and ventilated with pressure-limited time-cycled ventilation (SLE 2000; SLE, Surrey, UK) with 1 to 2% isoflurane, 70% N2O, and 28 to 29% O2 in the prone position for 60 minutes. Isoflurane and N2O was replaced by 0.7% halothane 20 minutes before HI. After HI, inhalation anesthesia was replaced by intravenous anesthesia in all animals; a bolus dose of propofol 4 mg/kg was followed by maintenance propofol (4–12 mg/kg/h) and remifentanil (20–80 μg/kg/h). Intravenous anesthesia was adjusted according to the heart rate (HR), MABP, and responsiveness of the pigs. This propofol/remifentanil combination was continued for 34 hours and then weaned until extubation.
Animal Preparation
A rectal temperature (Trec) probe (reusable YSI 400 series, Criticool, MTRE, Yavne, Israel) was inserted 6 cm into the rectum and a skin temperature probe (Criticool, MTRE) was attached to the ear lobe. Calibration was checked for each probe to within ±0.1°C over a temperature range of 20°C to 40°C against a certified mercury-in-glass thermometer (BS593; Zeal, London, UK). An ear vein was cannulated. Umbilical arterial and venous catheters were inserted allowing continuous monitoring of MABP, measurement of blood gases, and infusion of maintenance fluids and drugs. We measured MABP with Hewlett Packard invasive blood pressure unit with single-use disposable transducers (Becton & Dickinson, Oxford, UK) connected to the umbilical arterial catheter by a 0.9% saline-filled noncompliant tube. The pressure transducer was zeroed to the atmospheric pressure at the level of the heart in the mid-axillary line before commencing measurement. The maximum error with these particular transducers because of the total effects of nonlinearity, hysteresis, and sensitivity variations should have been no more than 2% of the reading or ±1 mm Hg, whichever is greater, over the operating range as it was designed to be compliant with the Association for the Advancement of Medical Instrumentation performance standard for blood pressure transducers.
An artificial fontanelle (2 × 2 cm) was made, as, unlike a human neonate, the newborn pig has no patent fontanelle. The fontanelle increases the compliance of the skull in pigs during raised intracranial pressure to simulate the human neonate. The left cerebral hemisphere was instrumented with an intraparenchymal pressure transducer (Codman, Raynham, MA, USA) ICP probe (zero drift: mean 0.1±1.6 mm Hg). The ICP probes were calibrated and inserted 1 cm into the parietal lobe via a burr hole. Animals were rested in the prone position for 60 minutes until the HI. The three sham pigs did not undergo HI and were continuously monitored.
Continuous Monitoring
Oxygen saturation (SpO2), Trec (using Criticool, Charter Kontron, Milton Keynes, UK), ETCO2 (Tidal Wave SP, Respironics Novametrix, Wallingford, CT, USA), MABP, and ICP (ICM software, Neurosurgery Unit, University of Cambridge, Cambridge, UK) were continuously monitored and recorded. A single or two-channel amplitude-integrated electroencephalogram (aEEG) (Olympic or Brainz, Natus Medical Incorporated, San Carlos, CA, USA) was continuously recorded.
Cerebrovascular Pressure Reactivity
Analog data of MABP and ICP were sampled at 50 Hz, processed through an analog to digital converter (DT9813; Data Translation, Marlboro, MA, USA), and recorded by a computer using dedicated neuroimaging software (ICM-plus).8 Cerebrovascular pressure reactivity was calculated as linear (Pearson's) moving correlation coefficients between 40 past consecutive 5-second averages of ICP and MABP. Computations were repeated with a moving window every 5 seconds.9 Complete PRx data were available from baseline to 18 hours for all groups. Because of the skewed distribution, median PRx was computed every 15 minutes from the preceding PRx data during HI and averaged over 60 minutes during the recovery period until 18 hours. We defined the positive PRx value as ‘impaired' PRx and median PRx value >0.2, which was associated with poor outcome in traumatic brain injury,9 as PRx peaks.
The ventilator settings were adjusted to maintain the SpO2 between 95% and 98% and the ETCO2 between 4.7 and 6.0 kPa. Blood gases (analyzed at 37°C) (i-STAT, Abbott,, Birmingham, UK; RapidLab 248 pH/blood gas analyzer, Siemens Healthcare Diagnostics, Surrey, UK), blood glucose (Medisense Optium, Oxon, UK), and lactate (Lactate Pro, Arkray, Kyoto, Japan) were sampled at preset time points at baseline, and during and after HI until 72 hours, as well as when clinically indicated.
Hypoxic–Ischemic Insult
The cuff of the tracheal tube was inflated and checked for leaks. The FiO2 was reduced to 5% to 7% to depress the background aEEG activity below 7 μV for 45 minutes.10, 11 The inspired oxygen fraction was regulated to the lowest value, which depressed the aEEG. When severe bradycardia or hypotension occurred, the FiO2 was transiently elevated until the HR and MABP recovered, and then reduced again depending on the tolerance of the animal. During this 45-minute HI insult, the duration in minutes and seconds of low amplitude background activity <7 μV or <3.5 μV (LAEEG), longest duration of LAEEG,10 arterial pH, and lactate were measured as markers of insult severity. The pigs were immediately randomized to NT, HT, XeNT, or XeHT after the insult.
Post Hypoxic–Ischemic Insult
After resuscitation in air, pigs were ventilated with 21% to 30% oxygen to maintain SpO2 between 95% and 98%. Inhalation anesthesia was replaced with intravenous propofol and remifentanil. Normothermia (Trec: 38.5±0.2°C, which is the normal temperature of newborn term pigs),12 was achieved using the criticool wrap with circulating water servo-controlled to Trec 38.5°C. The criticool wrap is a one-piece body-shaped garment that facilitates wrapping of the limbs and body. The wrap has water flow simultaneously through numerous channels and optimizes heat exchange through maximal surface coverage. The control unit uses the feedback from the core and skin temperature sensors, and the proprietary control algorithm responds by modifying water temperature such that the target temperature will be achieved precisely. To avoid the superficial brain being exposed to fluctuating water temperatures, the head of the piglet was not covered by the wrap.
Xenon delivery started 30 minutes after the end of the HI insult as a mixture of xenon/oxygen/nitrogen (Xe50%, O2 30%, and N2 20%) with concomitant decrease in propofol for a period of 18 hours delivered using a previously described closed-circuit rebreathing system to economize on Xe consumption.13 Hypothermia was initiated immediately after the insult by cooling the whole body to a target rectal temperature of 33.5±0.2°C (reached within 30 minutes), and maintained for 24 hours followed by rewarming at a rate of 0.5°C per hour using the servo-controlled whole-body cooling equipment (Criticool).
Intensive Care Management
Hypotension (MABP <40 mm Hg for ≥10 minutes)14 was treated in a stepwise manner after confirming adequate level of anesthesia with two separate 10 ml/kg boluses of 0.9% saline followed by dopamine (5–20 μg/kg/min), noradrenaline (20 ng to 1 μg/kg/min), and hydrocortisone (2.5 mg/kg 6 hourly or stopped early if the blood pressure recovered). Seizures were identified on continuous EEG/aEEG recordings. The protocol for treating both clinical and electrical seizures lasting >5 minutes was phenobarbital (20 mg/kg) × 2, clonazepam (100 μg/kg), and lidocaine (2 mg/kg over 10 minutes, followed by a continuous infusion of 6 mg/kg/h (6 hours), 4 mg/kg/h (12 hours), and 2 mg/kg/h (12 hours)).15 Failure to respond to treatment in 30 minutes entailed moving to the next step of this treatment protocol. At the end of 34 hours, the intracerebral probes were removed. Anesthesia was discontinued and pigs were extubated when able to breathe without support.
Neurology and Neuropathology Assessment
Clinical neurology assessment was performed using the previously validated neurology scale, during baseline and at 48 and 72 hours of the experiment.6, 10 The 11-item (scores 0, 1, or 2) clinical neurology score range from 0 to 22, with a higher score representing better neurology. The 11 items comprised assessments of respiration, consciousness, orientation, ability to walk on all four limbs, ability to control the forelimbs while raising from the lying position, tone in all limbs, activity, suck, absence of pathologic movements, vocalization, and ability to sniff (sensory stimulus provided by exposure to ‘marmite'). Dr DO, who was masked to both the clinical information and the randomization details of the pigs, independently assessed the available aEEG/EEG data for the presence of seizures. After 3 days of survival, pigs were reintubated and underwent terminal perfusion fixation of the brain under deep isoflurane anesthesia with 0.9% saline through the internal carotid arteries followed by 4% phosphate-buffered formaldehyde. A full autopsy was then performed. Hematoxylin and eosin-stained sections, serially obtained at 5 mm intervals from the right hemisphere, were assessed in six brain regions (cortex gray matter, white matter, basal ganglia, thalamus, hippocampus, and cerebellum) by a perinatal pathologist masked to the clinical details. A previously validated nine-point neuropathology score for the six brain regions, ranging from 0.0 to 4.0 with intervals of 0.5, was used.10 Average global neuropathology score, which ranged from 0.0 to 4.0, was calculated as a mean of the regional neuropathology scores.10 Score 1 involved ≤10% of the region affected with individual necrotic neurons, small patchy, complete, or incomplete infarcts. Score 2 involved 20% to 30% of the area affected with partly confluent complete or incomplete infarcts. Score 3 involved 40% to 60% of the area affected with large confluent, complete infarcts. Score 4 involved >75% of the area affected by total disintegration in cortex, large complete infarcts in thalamus, and basal ganglia with neuronal necrosis in the hippocampus.10
The ‘mild neuropathology' group was defined as pigs with average global neuropathology scores <1.5 and the ‘severe neuropathology' group as pigs with average global neuropathology scores between 1.51 and 4.0.10, 11 The group's association with clinical neurology scores supported this classification. ‘Mild' and ‘severe' neuropathology groups were associated with a median (interquartile range (IQR)) clinical neurology score of 21 (16.5, 22) and 4.5 (2.5, 16), respectively.
Statistics
Normally distributed variables were summarized as mean (s.e.m.) or mean (confidence interval (CI)) and skewed variables as median (range) and median (IQR). Difference in estimates was analyzed with Mann–Whitney U-test or Kruskal–Wallis test, depending on the number of groups. The ICP was normalized to the baseline value to better appreciate the changes in ICP during HI and recovery in the treatment group in Figures 2 and 3. Median PRx values calculated over 60 minutes were averaged over 18 hours to compute median 18-hour PRx. End-tidal CO2 was averaged over the 18-hour recovery period to assess its relation with the median 18-hour PRx. We averaged PRx during any seizures and for at least 30-minute duration before and after such seizures. Difference between the PRx during seizures and the mean pre/postseizure PRx was assessed with the Wilcoxon matched pairs test. Multiple linear regression was used to identify the significant predictors of median 18-hour PRx, MABP, ICP, cerebral perfusion pressure (CPP), and global neuropathology (Statview and SPSS version 15, SPSS, Chicago, IL, USA). A two-sided P-value of <0.05 was considered significant.
Results
Demographics and insult characteristics are shown in Table 1. There was no difference in the demographic characteristics between the groups. Arterial pH at the end of HI was significantly lower and lactate at the end of the HI was significantly higher in the NT, HT, and Xe groups compared with sham. The PO2 was significantly lower in the treatment groups compared with the sham during the insult (Table 1). The number (proportion) of animals with ‘severe neuropathology' in the different treatment groups were: NT (n=8, 66.7%), HT (n=3, 18.8%), XeNT (n=3, 42.9%), and XeHT (n=3, 23.1%), respectively. The mean (s.e.m.) average global neuropathology in the ‘severe neuropathology' group of different treatment groups was NT (3.56 (0.265)), HT (3.08 (0.083)), XeNT (2.31 (0.598)), and XeHT (2.678 (0.612)), respectively. The median (range) average global neuropathology in the ‘mild neuropathology' group of different treatment groups was NT (n=4, 33.3% 1.126 (0.583, 1.417)); HT (n=13, 81.3% 0.333 (0.083, 1.5)); XeNT (n=4, 51.7% 0.459 (0.167, 0.833)); and XeHT (n=10, 76.9% 0.291 (0.0, 0.833)), respectively. The proportions of animals requiring inotropic support in the ‘severe neuropathology' and ‘mild neuropathology' groups were NT (87.5%), HT (66.7%), XeNT (0%), and XeHT (33.3%); and NT (75%), HT (76.9%), XeNT (25%), and XeHT (70%), respectively. The need for inotropic support (P=0.35) did not affect the median PRx over 18 hours. The propofol (P=0.55) and remifentanil (P=0.21) doses used in the pigs during recovery did not have a significant effect on PRx, independent of intervention, severity of HI, and neuropathology.
Table 1. Demographic and insult characteristics of the pigs between the treatment groups.
| Variable | NT (n=12) | XeNT (n=7) | HT (n=16) | XeHT (n=13) | Sham (n=3) | Sig |
|---|---|---|---|---|---|---|
| Age, hours | 15 (11, 30) | 15.25 (2, 20) | 20 (9, 36) | 13 (4, 36) | 24 (24, 38) | NS |
| Weight, g | 1,455 (1,120, 1,710) | 1,740 (1,110, 1,965) | 1,700 (1,180, 2,310) | 1,640 (1,380, 2,435) | 1,780 (1,550, 1,950) | NS |
| Female (%) | 58% | 43% | 53% | 69% | 66% | NS |
| End insult, apH | 7.01 (6.77, 7.24) | 7.15 (7.02, 7.38) | 7.05 (6.91, 7.34) | 7.1 (6.94, 7.28) | 7.52 (7.42, 7.56) | P=0.007 |
| Insult duration, minutes | 38.82 (28.02, 46.42) | 36.37 (16, 40.05) | 33.5 (12.8, 43.5) | 32.16 (21.8, 44.6) | NS | |
| Nadir insult, pO2 kPa | 2.35 (2.67, 2.03) | 2.59 (2.06, 3.12) | 2.37 (2.13, 2.60) | 2.6 (2.13, 3.07) | 12.35 (10.39, 14.31) | P<0.0001 |
| Nadir insult, pCO2 kPa | 4.9 (4.07, 5.7) | 4.2 (3.56, 4.8) | 5.12 (4.37, 5.87) | 4.93 (4.27, 5.58) | 5.04 (4.38, 5.69) | NS |
| End insult lactate, mmol/L | 15.6 (12.4, 18.4) | 14.14 (8.62, 17.66) | 15.6 (12, 20) | 14.8 (14, 17.42) | 1.7 (1.4, 2.1) | P=0.035 |
| Pre- insult isoflurane, minutes | 5.4 (3, 9.5) | 4.75 (3.5, 6) | 5 (1.5, 8) | 5.5 (3, 8.3) | 3 (2.5, 3.5) | NS |
| PRx during HI Median (IQR) | 0.426 (0.169, 0.623) | 0.482 (0.331, 0.691) | 0.489 (0.309, 0.662) | 0.616 (0.449, 0.785) | −0.17 (−0.42, −0.02) | P=0.02 |
| Severe neuropathology | ||||||
| N | 8 | 3 | 3 | 3 | ||
| End insult, apH | 6.95 (0.14) | 6.94 (0.10) | 7.17 (0.05) | 7.05 (0.06) | NS | |
| Insult duration, minutes | 27.96 (16.9, 30.9) | 26.67 (13.2, 39.7) | 22.8 (18.1, 27.6) | 25.4 (14.6, 37.8) | NS | |
HI, hypoxia–ischemia; HT, hypothermia; IQR, interquartile range; NS, not significant; NT, normothermia; PRx, cere brovascular pressure reactivity; Xe, xenon; XeNT, combination of xenon and normothermia.
Estimates are median (range) except for end insult apH in the severe neuropathology group, which is summarized as mean (s.d.), and PRx during HI as median (IQR). End insult apH and insult duration (duration of amplitude-integrated electroencephalogram (aEEG) of ≤3.5 μV during 45 minutes of global HI insult) were markers of insult severity.
The ICP and CPP during baseline, HI, and recovery between the ‘mild' and ‘severe' neuropathology groups of different treatment groups are shown in Table 2. There was no significant difference in the ICP and CPP during recovery between the ‘mild' and ‘severe' neuropathology groups in all the treatment groups and there was no significant influence of ICP (P=0.85) or CPP (P=0.09) on PRx during recovery.
Table 2. Intracranial pressure mm Hg (median (range)) and cerebral perfusion pressure mm Hg (median (range)) during baseline, HI insult, and recovery/intervention in the different treatment groups.
|
Baseline median (range) |
HI median (range) |
Recovery and intervention median (range) |
|||||
|---|---|---|---|---|---|---|---|
| Treatment group | Neuropathology severity, N | ICP mm Hg | CPP mm Hg | ICP mm Hg | CPP mm Hg | ICP mm Hg | CPP mm Hg |
| NT | Mild (n=4) | 4.6 (1.35, 11.86) | 47.5 (38.95, 55.08) | 9.8 (5.47, 13.53) | 32.8 (27.95, 34.44) | 6.5 (2.38, 9.85) | 41.4 (37.07, 52.33) |
| NT | Severe (n=8) | 2.5 (0.97, 9.64) | 44.3 (33.52, 61.89) | 5.2 (1.56, 11.77) | 33.3 (23.18, 52.69) | 5.5 (1.51, 10.44) | 42.3 (32.29, 52) |
| HT | Mild (n=13) | 4.3 (1.0, 9.21) | 44.8 (39.82, 55.6) | 6.3 (3.44, 13.6) | 29.1 (19.74, 45.28) | 4.1 (0.97, 7.08) | 44.2 (39.88, 57.18) |
| HT | Severe (n=3) | 4.8 (0.43, 7.34) | 49.2 (44.92, 66.73) | 5.5 (5.07, 7.65) | 35.5 (25.73, 52.65) | 5.4 (2.25, 6.65) | 46.5 (43.27, 56.86) |
| XeNT | Mild (n=4) | 2.7 (1.41, 8.84) | 47.4 (40.06, 54.34) | 5.8 (2.23, 13.04) | 30.5 (16.2, 37.1) | 2.0 (1.15, 11.45) | 48.23 (38.16, 53.08) |
| XeNT | Severe (n=3) | 2.6 (2.07, 2.97) | 42.3 (39.75, 47.97) | 5.8 (5.04, 7.63) | 29.2 (29.14, 36.04) | 5.6 (3.25, 10.6) | 44.8 (38.02, 47.21) |
| XeHT | Mild (n=10) | 5.6 (1.09, 12.58) | 45.3 (30.48, 63.86) | 9.5 (1.05, 13.86) | 28.6 (18.32, 40.82) | 4.6 (1.25, 12.72) | 47.9 (30.85, 60.01) |
| XeHT | Severe (n=3) | 3.9 (3.58, 6.31) | 43.3 (40.35, 47.89) | 7.6 (6.23, 7.61) | 22.4 (22.27, 30.71) | 5.5 (4.63, 5.80) | 54.4 (44.86, 55.48) |
CPP, cerebral perfusion pressure; HI, hypoxia–ischemia; HT, hypothermia; ICP, intracranial pressure; NT, normothermia; PRx, cerebrovascular pressure reactivity; Xe, xenon; XeHT, combination of xenon and hypothermia; XeNT, combination of xenon and normothermia.
The neuropathology severity classification was based on global neuropathology score of <1.5 (mild) and from 1.51 to 4.0 (severe).
Course of Cerebrovascular Pressure Reactivity During Hypoxia–Ischemia and Recovery (Normothermia Group)
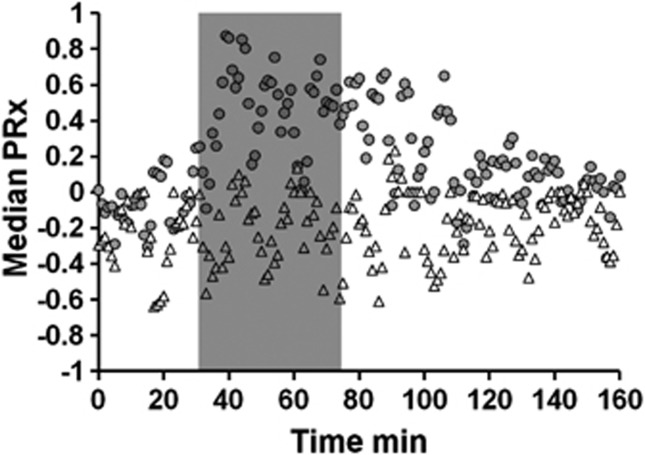
The PRx remained normal during baseline and rose to a peak of 0.87 (median) by 10 minutes after initiation of HI (Figure 1). It remained impaired throughout the HI and continued to be impaired during the post-HI recovery compared with sham. The median of PRx during HI in the sham group (−0.17) was significantly lower when compared with other treatment groups: NT (0.426), HT (0.489), XeNT (0.482), and XeHT (0.616) (Table 1). The peak occurring during HI was defined as the ‘primary peak' and the peak occurring after at least 1 hour of stable normal PRx level during recovery period was defined as the ‘secondary peak.'
Figure 1.
Cerebrovascular pressure reactivity (PRx; median) before, during, and after hypoxic–ischemic (HI) insult (gray bar). Gray circles denote the normothermic group (n=12) and the white triangles denote the sham group (n=3). Cerebrovascular pressure reactivity was calculated as linear (Pearson's) moving correlation coefficients between 40 past consecutive 5-second averages of intracranial pressure (ICP) and mean arterial blood pressure (MABP). Each data point for PRx is calculated over 1 minute. The PRx was impaired by 10 minutes of HI and continued to be impaired well into the recovery.
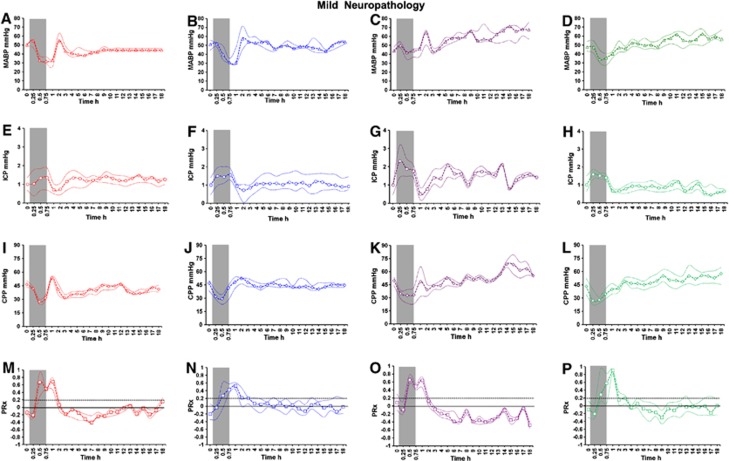
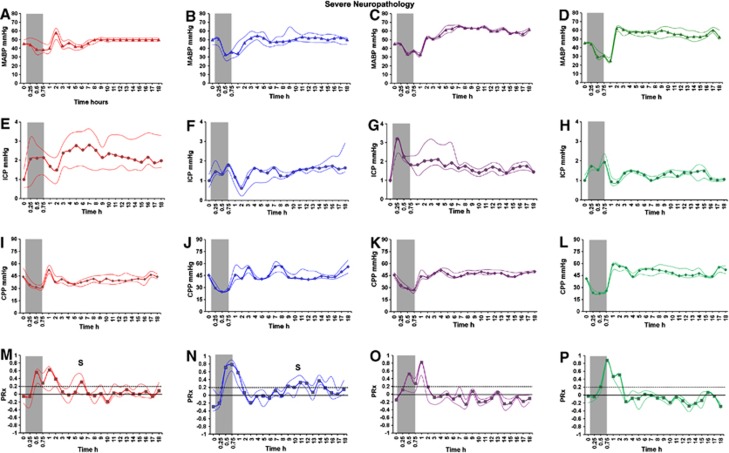
After the primary PRx peak during HI, PRx remained impaired for the first 2 to 3 hours (Figures 2M and 3M). Cerebrovascular pressure reactivity was significantly impaired during recovery and more so in the ‘severe neuropathology' group compared with the ‘mild neuropathology' group ((median (IQR): 0.013 (−0.008, 0.075) versus −0.125 (−0.209, 0.011), P=0.003; Figures 3M and 2M). In the ‘severe neuropathology' group, there was a secondary PRx peak (S) during recovery occurring at a median (IQR) of 6.5 hours (5.25, 7.75) after insult (Figure 3M).
Figure 2.
Mean arterial blood pressure (MABP; open triangle), intracranial pressure (ICP; open circle), cerebral perfusion pressure (CPP; open diamond), and cerebrovascular pressure reactivity (PRx; open square) (median (interquartile range (IQR)) during baseline, hypoxic–ischemic (HI) insult, and 18-hour recovery in the normothermic (NT, n= 4; red A, E, I, M), hypothermic (HT, n=13; blue B, F, J, N), xenon normothermia (XeNT, n=4; purple C, G, K, O), and xenon hypothermia (XeHT, n=10; green D, H, L, P) in the ‘mild neuropathology' group. The thin dotted lines denote the IQR. Gray bar indicate the HI insult. Gray interrupted line at PRx of 0.2 defines the PRx peak.
Figure 3.
Mean arterial blood pressure (MABP; filled triangle), intracranial pressure (ICP; filled circle), cerebral perfusion pressure (CPP; filled diamond), and cerebrovascular pressure reactivity (PRx; filled square) (median (interquartile range (IQR)) during baseline, hypoxic–ischemic (HI) insult, and 18-hour recovery in the normothermic (NT, n=8; red A, E, I, M), hypothermic (HT, n=3; blue B, F, J, N), xenon normothermia (XeNT, n=3; purple C, G, K, O), and xenon hypothermia (XeHT, n=3; green D, H, L, P) in the ‘severe neuropathology' group. Cerebrovascular pressure reactivity peaked during HI and developed secondary peak (S) after a latent phase in the severe neuropathology groups of NT and HT. Hypothermia significantly prolonged the latent phase. Xenon groups and the mild neuropathology groups of NT and HT did not develop secondary PRx impairment.
Mean Arterial Blood Pressure, Intracranial Pressure, and Cerebral Perfusion Pressure
The MABP decreased during HI and increased during recovery with an initial peak followed by stabilization (Figures 2 and 3). Xenon inhalation increased the MABP by (mean, 95% CI) 6.5 mm Hg (3.81, 9.35), independent of hypothermia, severity of neuropathology, and HI. The ICP increased during HI followed by stabilization during recovery (Figures 2 and 3). The ICP in the pigs with ‘severe neuropathology' displayed elevated ICP after HI in the NT group and showed a delayed increase in the HT group (Figures 3E and 3F). The CPP decreased during HI followed by an increase during recovery (Figures 3K and 3L). Both Xe (5.9 mm Hg (2.91, 8.98)) and HT (3.3 mm Hg (0.29, 6.4)) increased the CPP independent of the severity of the HI and neuropathology.
Cerebrovascular Pressure Reactivity in Relation to Intervention Hypothermia and Xenon
There was significant impairment of PRx during recovery after HI in the ‘severe neuropathology' group compared with the ‘mild neuropathology' group in the HT ((mean (s.e.m.): 0.11 (0.037) versus 0.016 (0.022) P=0.02; Figures 3N and 2N) and the XeNT groups (−0.09 (0.027) versus −0.250 (0.037), P=0.002; Figures 3O and 2O). In XeHT, there was no difference in PRx during recovery between the ‘severe' and ‘mild' neuropathology groups (Figures 3P and 2P).
The secondary PRx peak occurred significantly later in the HT compared with the NT ‘severe neuropathology' groups (median (IQR): 11.5 hours (10.25, 12.75) versus 6.5 hours (5.25, 7.75), P=0.002; Figures 3N and 3M). The secondary PRx peak was preceded by an increase in ICP in both NT and HT groups. In the ‘severe neuropathology' group, although ICP gradually increased in the HT group after 8 hours, there was persistently elevated ICP after 3 hours in the NT group (Figures 3E and 3F). Xenon under normothermia or when combined with HT attenuated the occurrence of the secondary PRx peak (Figures 3O and 3P).
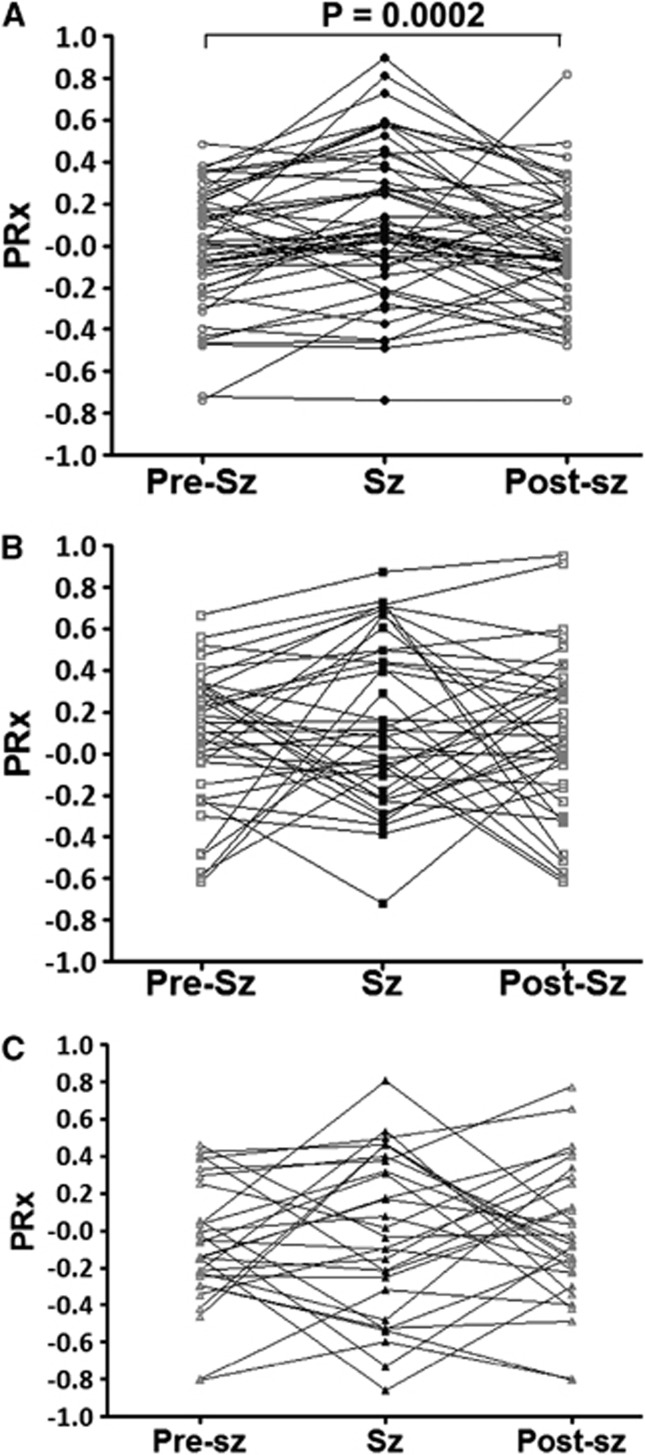
There was significant PRx impairment during seizures compared with the pre or postseizure PRx in the NT group. However, there was no significant PRx difference during or pre/post seizures in the HT and XeHT groups (Figure 4).
Figure 4.
Cerebrovascular pressure reactivity (PRx) during, before, and after seizures (Sz) in the (A) NT (normothermia; circle), (B) HT (hypothermia; square), and (C) XeHT (combination of xenon and hypothermia; triangle) groups. There was significant PRx impairment during seizures (black filled symbols) than that before and after PRx (gray open symbols) in the NT group, but there was no difference in the HT or XeHT group.
Secondary Cerebrovascular Pressure Reactivity Peak—Marker of Severe Neuropathology and Severe Hypoxia–Ischemia
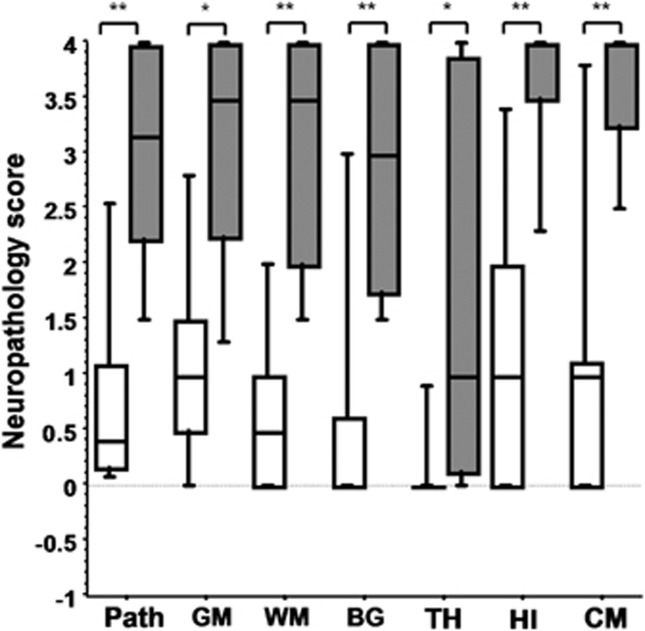
In the NT and HT groups, the presence of a secondary PRx peak predicted a severe neuropathology with a sensitivity of 91%, specificity of 100%, PPV 100%, and NPV 94%. Pigs that manifested a secondary PRx peak had significantly worse neuropathology scores in all the brain regions compared with the pigs without the secondary PRx peak in the NT and HT groups (P<0.05; Figure 5). There was no influence of gender on the neuropathology scores (P=0.64).
Figure 5.
Global neuropathology and the regional neuropathology of six brain regions (median and range) between the pigs that developed secondary cerebrovascular pressure reactivity (PRx) peak (gray box and whiskers; n=9) and the pigs without secondary PRx peak (open box and whiskers; n=19) in the normothermia (NT) and hypothermia (HT) groups. *P<0.05 and **P<0.001. BG, basal ganglia; CM, cerebellum; GM, gray matter; HI, hippocampus; Path, global neuropathology; TH, thalamus; WM, white matter.
Pigs with a secondary PRx peak had a trend toward a lower arterial pH at the end of the insult compared with the pigs without the peak (mean (s.e.m.) 6.97 (0.033) versus 7.07 (0.036), P=0.07) and the duration of EEG <3.5 μV during HI was greater in the pigs with a secondary PRx peak compared with the pigs without this peak (23.8 (2.5) versus 15.0 (2.9), P=0.05).
Factors Influencing Cerebrovacular Pressure Reactivity
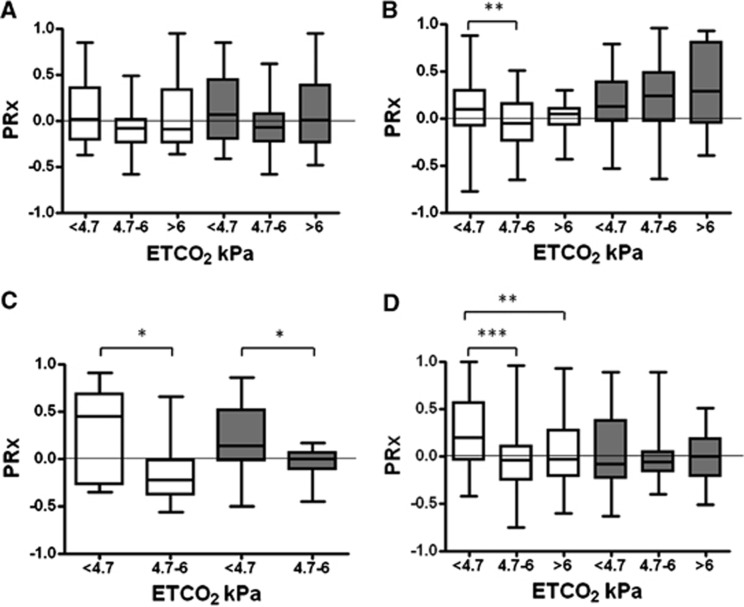
End-tidal CO2 was significantly lower at the end of HI. When the ETCO2 was in the range between 4.7 and 6 kPa, PRx was more negative, particularly in the mild neuropathology groups of all the treatment arms (Figure 6). Hypocapnia was associated with hypotension during the initial period of recovery soon after the HI; however, the later episodes of hypocapnia during recovery were not associated with hypotension. For every 1 kPa increase in ETCO2 to 8 kPa, the PRx decreased by −0.063 (95% CI (−0.082, −0.044)). In multiple linear regression, xenon (regression coefficient, −0.098 (95% CI (−0.186, −0.01)) and severity of insult (apH at the end of insult) (regression coefficient, −0.452 (95% CI (−0.77, −0.135)) predicted the median 18-hour PRx, independently of gender (P=0.85).
Figure 6.
Association between end-tidal CO2 (ETCO2) and the cerebrovascular pressure reactivity (PRx) in the four treatment groups: (A) NT (normothermia; n=12), (B) HT (hypothermia; n=16), (C) XeNT (combination of xenon and normothermia; n=7), and (D) XeHT (combination of xenon and hypothermia; n=13). End-tidal CO2 was classified as <4.7, 4.7 and 6, and >6 kPa. White and dark gray box and whiskers represent the mild and severe neuropathology groups, respectively. Cerebrovascular pressure reactivity was preserved when the ETCO2 was within the normal range of 4.7 to 6 kPa. *P<0.05, **P<0.01, and ***P<0.001.
Discussion
Cerebrovascular pressure reactivity peaked during the HI with a subsequent secondary PRx peak in the ‘severe neuropathology' groups of NT and HT. Occurrence of a secondary PRx peak was associated with severe neuropathology and greater insult severity in the NT and HT groups. The secondary PRx peak occurred significantly later in the HT compared with the NT group. Cerebrovascular pressure reactivity was preserved and there was no secondary PRx peak during Xenon inhalation, regardless of the insult severity. Cerebrovascular pressure reactivity was maintained during normocarbia and significantly impaired during hypocapnia. Seizures were associated with impaired PRx in the NT group, but not in the HT or the XeHT group.
In our newborn pig global HI encephalopathy model, PRx was significantly impaired during HI. Cerebrovascular pressure reactivity has been validated with other markers of autoregulation of cerebral blood flow such as the transcranial doppler-based mean velocity index9 and also the positron emission tomography-cerebral blood flow static rate of autoregulation.16 In our study, PRx was impaired within 10 minutes of commencing the insult and continued to be impaired during recovery particularly in the severe neuropathology group. In a newborn lamb model of asphyxia, cerebral autoregulation was impaired after 20 minutes of hypoxia.17 The difference in the timing of impairment compared with the lamb model of asphyxia is likely to be because of the difference in the species, method of insult, and the parameter measured as the marker of cerebral autoregulation. During HI, hypoxia and the ensuing accumulation of the vasodilating substances can paralyze the smooth muscle of cerebral vasculature, and the associated hypotension and hypocapnia can further impair the PRx.
The presence of a secondary PRx peak was a marker of severe neuropathology in the normothermia and hypothermia groups as shown by the significant association with worse global and regional neuropathology. A secondary PRx peak predicted pigs with ‘severe neuropathology' with a positive predictive value of 100% in the NT and HT pigs. The initial post-HI PRx impairment was followed by a latent phase of 6.5 hours with a subsequent secondary PRx peak. This timing is comparable to the secondary energy failure, delayed hyperemia, and seizures occurring after a latent phase of 6 to 15 hours, 12.8 hours, and 6 to 8 hours after primary energy failure, initial vasodilatation, and EEG suppression seen during HI in pigs and fetal sheep, respectively.18, 19, 20 The association of a secondary PRx peak with ‘severe neuropathology' is consistent with other reports such as the known association of degree of energy failure and delayed seizures with poor long-term outcome in infants21 and histologic injury in fetal sheep.20, 22
The secondary PRx peak was associated with markers of severe HI. This is consistent with the association between the degree of secondary energy failure and acute energy depletion during HI.18 The secondary PRx peak represents secondary impairment of cerebral autoregulation and is preceded by elevation of ICP. This secondary impairment of cerebral autoregulation is likely to precede the development of oedema, accumulation of cytotoxins, seizures, and hyperemia. However, the different experimental designs in different species make it difficult to identify the temporal relationship between these pathophysiological features after HI. The relationship between the secondary PRx peak and the secondary impairment of cerebral oxidative metabolism is not known. Hypothermia prolonged the latent phase by delaying the occurrence of secondary PRx peak and impairment to 11.5 hours compared with 6.5 hours in the normothermia group. This is consistent with the delay in increase in carotid blood flow at 66 hours from the end of the cerebral ischemia in the cooled fetal sheep compared with 36 hours in the sham cooled group23 and the amelioration of delayed energy failure with 12 hours of immediate HT after hypoxic ischemia.24
There were far fewer pigs with impaired PRx during seizures compared with the pre/post seizure period in the HT and XeHT pigs when compared with the NT pigs. Although seizures have been shown to decrease the cerebrovascular pressure reactivity to hypoxia in normothermic pigs,25 there has been no previous report of HT or Xe combined with hypothermia preserving the PRx during seizures. The exact mechanism behind this is unclear. Xenon50% at NT or HT preserved the cerebrovascular pressure reactivity, thus abolishing the secondary PRx peak independently of the severity of the insult. Xenon's ability to maintain the MABP and CPP has contributed to the preservation of PRx. Xenon pigs in the ‘severe neuropathology' group had received a similar insult compared with other treatment groups. Results of past studies of the effects of xenon on cerebral blood flow have been inconsistent, with some studies showing an increase,26, 27 and others showing no change28 or regional differences.29 These differences in outcome are likely to be because of differences in experimental design or early measurements taken before stabilization of xenon concentration or the conscious state of the subjects. Xenon79% for 2 hours has been reported to preserve both CO2 and pressure-regulated cerebral autoregulation in normal pigs.30 Xenon30%/50%/70% has been reported to have no effect on regional cerebral blood flow in pigs sedated with propofol.31 The coupling of cerebral glucose metabolism and cerebral blood flow was preserved during xenon60% anesthesia.32 Occurrence of severe neuropathology in xenon groups despite preservation of PRX indicates operation of other mechanisms of cell death uninfluenced by xenon or HT. As hypothermia and xenon have different effects on autoregulation, they are likely to have different methods of action, which might account for the additive neuroprotective effect.6, 33
Cerebrovascular pressure reactivity was preserved when the ETCO2 was between 4.7 and 6 kPa. For every 1 kPa increase in ETCO2, PRx decreased by −0.063. Cerebral blood flow and CO2 reactivity in preterm infants have been shown to be between 50% and 60% per kPa.34 There were two periods of hypocapnia.14(1) The first was soon after the HI during recovery. The hypocapnia resulted from the mechanical ventilation in the nonparalyzed pigs during HI, where they hyperventilated in response to metabolic acidosis and reduced CO2 production because of hypoxia. This period was associated with recovering hypotension. The basal vasodilatation secondary to steady-state hypotension could have impaired the dynamic myogenic reactivity to fluctuations in MABP and this could have increased the PRx. (2) Hypocapnia occurring during later periods of recovery were not associated with hypotension but with hyperventilation in response to severe acidosis. Impairment of PRx during this period might be because of increased vascular tone secondary to hypocapnia, which might attenuate the myogenic response to MABP fluctuations. Propofol and remifentanil dose did not affect the PRx during recovery and all pigs received these drugs. Although anesthetics can impair cerebral autoregulation, the subanesthetic concentration of xenon used in the study35 and low dose of propofol might have accounted for the lack of deteriorating effect on PRx. This is consistent with other studies reporting on cerebral vascular resistance36 or autoregulation.37
Cerebrovascular pressure reactivity, a global index and a key mechanism of cerebral autoregulation, is measured continuously as a correlation between the ICP and MABP signals, which avoids the variability associated with measuring transcranial doppler velocities because of the vessel calibre and position of the probe.38 Though the invasive nature of the measurement precludes its entry into newborn clinical practice, less invasive monitoring of autoregulation by measuring tissue oxyhemoglobin and total hemoglobin by near-infrared spectroscopy has been used in newborn babies.39, 40 We had PRx data until 18 hours; hence, we might have missed the changes with PRx during the latter part of the recovery. There were fewer pigs with severe neuropathology in the intervention (HT and Xe) arms. This is because of the availability of the data and the reduction of severe neuropathology seen in the intervention arms.
In summary, the cerebrovascular pressure reactivity peaked during the HI insult and again during recovery in the severe neuropathology groups of NT and HT. The occurrence of a secondary PRx peak was highly predictive of severe neuropathology and was directly associated with insult severity. Hypothermia on its own delayed the secondary PRx peak and adding xenon abolished this peak altogether. In the normothermic pigs, seizures and hypocapnia significantly impaired the PRx, and xenon preserved the cerebrovascular pressure reactivity, mean arterial blood pressure, and cerebral perfusion pressure independent of the insult severity and seizures. Although HT ameliorated the PRx impairment during seizures, PRx was not better preserved compared with the xenon pigs during recovery from the HI.
Acknowledgments
The authors thank Professor L Walloe for his statistical advice. They also thank Dr P Smielewski for his support with the ICM software.
The authors declare no conflict of interest.
Footnotes
This study was supported by the Sports Aiding Medical Research for Kids (SPARKS) (UK) and the Laerdal Foundation for Acute Medicine (Norway).
References
- Greisen G. Effect of cerebral blood flow and cerebrovascular autoregulation on the distribution, type and extent of cerebral injury. Brain Pathol. 1992;2:223–228. doi: 10.1111/j.1750-3639.1992.tb00695.x. [DOI] [PubMed] [Google Scholar]
- Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev. 1990;2:161–192. [PubMed] [Google Scholar]
- Zweifel C, Lavinio A, Steiner LA, Radolovich D, Smielewski P, Timofeev I, et al. Continuous monitoring of cerebrovascular pressure reactivity in patients with head injury. Neurosurg Focus. 2008;25:E2–E9. doi: 10.3171/FOC.2008.25.10.E2. [DOI] [PubMed] [Google Scholar]
- Czosnyka M, Smielewski P, Kirkpatrick P, Piechnik S, Laing R, Pickard JD. Continuous monitoring of cerebrovascular pressure-reactivity in head injury. Acta Neurochir Suppl. 1998;71:74–77. doi: 10.1007/978-3-7091-6475-4_23. [DOI] [PubMed] [Google Scholar]
- Brady KM, Shaffner DH, Lee JK, Easley RB, Smielewski P, Czosnyka M, et al. Continuous monitoring of cerebrovascular pressure reactivity after traumatic brain injury in children. Pediatrics. 2009;124:e1205–e1212. doi: 10.1542/peds.2009-0550. [DOI] [PubMed] [Google Scholar]
- Chakkarapani E, Dingley J, Liu X, Hoque N, Aquilina K, Porter H, et al. Xenon enhances hypothermic neuroprotection in asphyxiated newborn pigs. Ann Neurol. 2010;68:330–341. doi: 10.1002/ana.22016. [DOI] [PubMed] [Google Scholar]
- Edwards AD, Brocklehurst P, Gunn AJ, Halliday H, Juszczak E, Levene M, et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ. 2010;340:c363–c369. doi: 10.1136/bmj.c363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smielewski P, Czosnyka M, Steiner L, Belestri M, Piechnik S, Pickard JD. ICM+: software for on-line analysis of bedside monitoring data after severe head trauma. Acta Neurochir Suppl. 2005;95:43–49. doi: 10.1007/3-211-32318-x_10. [DOI] [PubMed] [Google Scholar]
- Czosnyka M, Smielewski P, Kirkpatrick P, Laing RJ, Menon D, Pickard JD.Continuous assessment of the cerebral vasomotor reactivity in head injury Neurosurgery 19974111–17.discussion 17-19. [DOI] [PubMed] [Google Scholar]
- Thoresen M, Haaland K, Loberg EM, Whitelaw A, Apricena F, Hanko E, et al. A piglet survival model of posthypoxic encephalopathy. Pediatr Res. 1996;40:738–748. doi: 10.1203/00006450-199611000-00014. [DOI] [PubMed] [Google Scholar]
- Tooley JR, Satas S, Porter H, Silver IA, Thoresen M. Head cooling with mild systemic hypothermia in anesthetized piglets is neuroprotective. Ann Neurol. 2003;53:65–72. doi: 10.1002/ana.10402. [DOI] [PubMed] [Google Scholar]
- Tuchsherer M, Puppe B, Tuchsherer A, Tiemann U. Early identification of neonates at risk: traits of newborn piglets with respect to survival. Thenogenology. 2000;54:371–388. doi: 10.1016/S0093-691X(00)00355-1. [DOI] [PubMed] [Google Scholar]
- Chakkarapani E, Thoresen M, Hobbs CE, Aquilina K, Liu X, Dingley J. A closed-circuit neonatal xenon delivery system: a technical and practical neuroprotection feasibility study in newborn pigs. Anesth Analg. 2009;109:451–460. doi: 10.1213/ane.0b013e3181aa9550. [DOI] [PubMed] [Google Scholar]
- Haaland K, Karlsson B, Skovlund E, Lagercrantz H, Thoresen M. Postnatal development of the cerebral blood flow velocity response to changes in CO2 and mean arterial blood pressure in the piglet. Acta Paediatr. 1995;84:1414–1420. doi: 10.1111/j.1651-2227.1995.tb13579.x. [DOI] [PubMed] [Google Scholar]
- Malingre MM, Van Rooij LG, Rademaker CM, Toet MC, Ververs TF, van Kesteren C, et al. Development of an optimal lidocaine infusion strategy for neonatal seizures. Eur J Pediatr. 2006;165:598–604. doi: 10.1007/s00431-006-0136-x. [DOI] [PubMed] [Google Scholar]
- Steiner LA, Coles JP, Johnston AJ, Chatfield DA, Smielewski P, Fryer TD, et al. Assessment of cerebrovascular autoregulation in head-injured patients: a validation study. Stroke. 2003;34:2404–2409. doi: 10.1161/01.STR.0000089014.59668.04. [DOI] [PubMed] [Google Scholar]
- Tweed A, Cote J, Lou H, Gregory G, Wade J. Impairment of cerebral blood flow autoregulation in the newborn lamb by hypoxia. Pediatr Res. 1986;20:516–519. doi: 10.1203/00006450-198606000-00007. [DOI] [PubMed] [Google Scholar]
- Lorek A, Takei Y, Cady EB, Wyatt JS, Penrice J, Edwards AD, et al. Delayed (“secondary”) cerebral energy failure after acute hypoxia-ischemia in the newborn piglet: continuous 48-hour studies by phosphorus magnetic resonance spectroscopy. Pediatr Res. 1994;36:699–706. doi: 10.1203/00006450-199412000-00003. [DOI] [PubMed] [Google Scholar]
- Marks KA, Mallard EC, Roberts I, Williams CE, Sirimanne ES, Johnston B, et al. Delayed vasodilation and altered oxygenation after cerebral ischemia in fetal sheep. Pediatr Res. 1996;39:48–54. doi: 10.1203/00006450-199601000-00007. [DOI] [PubMed] [Google Scholar]
- Williams CE, Gunn AJ, Synek B, Gluckman PD. Delayed seizures occurring with hypoxic-ischemic encephalopathy in the fetal sheep. Pediatr Res. 1990;27:561–565. doi: 10.1203/00006450-199006000-00004. [DOI] [PubMed] [Google Scholar]
- Roth SC, Baudin J, Cady E, Johal K, Townsend JP, Wyatt JS, et al. Relation of deranged neonatal cerebral oxidative metabolism with neurodevelopmental outcome and head circumference at 4 years. Dev Med Child Neurol. 1997;39:718–725. doi: 10.1111/j.1469-8749.1997.tb07372.x. [DOI] [PubMed] [Google Scholar]
- Tan WK, Williams CE, During MJ, Mallard CE, Gunning MI, Gunn AJ, et al. Accumulation of cytotoxins during the development of seizures and edema after hypoxic-ischemic injury in late gestation fetal sheep. Pediatr Res. 1996;39:791–797. doi: 10.1203/00006450-199605000-00008. [DOI] [PubMed] [Google Scholar]
- Gunn AJ, Bennet L, Gunning MI, Gluckman PD, Gunn TR. Cerebral hypothermia is not neuroprotective when started after postischemic seizures in fetal sheep. Pediatr Res. 1999;46:274–280. doi: 10.1203/00006450-199909000-00005. [DOI] [PubMed] [Google Scholar]
- Thoresen M, Penrice J, Lorek A, Cady EB, Wylezinska M, Kirkbride V, et al. Mild hypothermia after severe transient hypoxia-ischemia ameliorates delayed cerebral energy failure in the newborn piglet. Pediatr Res. 1995;37:667–670. doi: 10.1203/00006450-199505000-00019. [DOI] [PubMed] [Google Scholar]
- DiGeronimo RJ, Gegg CA, Zuckerman SL. Adenosine depletion alters postictal hypoxic cerebral vasodilation in the newborn pig. Am J Physiol. 1998;274:H1495–H1501. doi: 10.1152/ajpheart.1998.274.5.H1495. [DOI] [PubMed] [Google Scholar]
- Hartmann A, Dettmers C, Schuier FJ, Wassmann HD, Schumacher HW. Effect of stable xenon on regional cerebral blood flow and the electroencephalogram in normal volunteers. Stroke. 1991;22:182–189. doi: 10.1161/01.str.22.2.182. [DOI] [PubMed] [Google Scholar]
- Junck L, Dhawan V, Thaler HT, Rottenberg DA. Effects of xenon and krypton on regional cerebral blood flow in the rat. J Cereb Blood Flow Metab. 1985;5:126–132. doi: 10.1038/jcbfm.1985.16. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Hayman LA, Yamamoto M, Sakai F, Nakajima S. Local cerebral blood flow measured by CT after stable xenon inhalation. AJR Am J Roentgenol. 1980;135:239–251. doi: 10.2214/ajr.135.2.239. [DOI] [PubMed] [Google Scholar]
- Laitio RM, Kaisti KK, Laangsjo JW, Aalto S, Salmi E, Maksimow A, et al. Effects of xenon anesthesia on cerebral blood flow in humans: a positron emission tomography study. Anesthesiology. 2007;106:1128–1133. doi: 10.1097/01.anes.0000267596.57497.92. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Marx T, Papp-Jambor C, Schirmer U, Reinelt H. Effect of xenon on cerebral autoregulation in pigs. Anaesthesia. 2002;57:960–966. doi: 10.1046/j.1365-2044.2002.02862.x. [DOI] [PubMed] [Google Scholar]
- Fink H, Blobner M, Bogdanski R, Hanel F, Werner C, Kochs E. Effects of xenon on cerebral blood flow and autoregulation: an experimental study in pigs. Br J Anaesth. 2000;84:221–225. doi: 10.1093/oxfordjournals.bja.a013406. [DOI] [PubMed] [Google Scholar]
- Rex S, Meyer PT, Baumert JH, Rossaint R, Fries M, Bull U, et al. Positron emission tomography study of regional cerebral blood flow and flow-metabolism coupling during general anaesthesia with xenon in humans. Br J Anaesth. 2008;100:667–675. doi: 10.1093/bja/aen036. [DOI] [PubMed] [Google Scholar]
- Hobbs C, Thoresen M, Tucker A, Aquilina K, Chakkarapani E, Dingley J. Xenon and hypothermia combine additively, offering long-term functional and histopathologic neuroprotection after neonatal hypoxia/ischemia. Stroke. 2008;39:1307–1313. doi: 10.1161/STROKEAHA.107.499822. [DOI] [PubMed] [Google Scholar]
- Greisen G, Trojaborg W. Cerebral blood flow, PaCO2 changes, and visual evoked potentials in mechanically ventilated, preterm infants. Acta Paediatr Scand. 1987;76:394–400. doi: 10.1111/j.1651-2227.1987.tb10488.x. [DOI] [PubMed] [Google Scholar]
- Liu X, Dingley J, Elstad M, Scull-Brown E, Steen PA, Thoresen M. Minimum alveolar concentration (MAC) for sevoflurane and xenon at normothermia and hypothermia in newborn pigs. Acta Anaesthesiol Scand. 2013;57:646–653. doi: 10.1111/aas.12055. [DOI] [PubMed] [Google Scholar]
- Van Hemelrijck J, Fitch W, Mattheussen M, Van Aken H, Plets C, Lauwers T. Effect of propofol on cerebral circulation and autoregulation in the baboon. Anesth Analg. 1990;71:49–54. doi: 10.1213/00000539-199007000-00008. [DOI] [PubMed] [Google Scholar]
- Lagerkranser M, Stange K, Sollevi A. Effects of propofol on cerebral blood flow, metabolism, and cerebral autoregulation in the anesthetized pig. J Neurosurg Anesthesiol. 1997;9:188–1893. doi: 10.1097/00008506-199704000-00015. [DOI] [PubMed] [Google Scholar]
- Thoresen M, Haaland K, Steen PA. Cerebral Doppler and misrepresentation of flow changes. Arch Dis Child. 1994;71:F103–F106. doi: 10.1136/fn.71.2.f103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore MM, Stone BS, Shepard JA, Czosnyka M, Easley RB, Brady KM. Relationship between cerebrovascular dysautoregulation and arterial blood pressure in the premature infant. J Perinatol. 2011;31:722–729. doi: 10.1038/jp.2011.17. [DOI] [PubMed] [Google Scholar]
- Rhee CJ, Kibler KK, Brady KM, Everett AD, Graham EM, Andropoulos DB, et al. Detection of neurologic injury using vascular reactivity monitoring and glial fibrillary acidic protein. Pediatrics. 2013;131:e950–e954. doi: 10.1542/peds.2012-1702. [DOI] [PubMed] [Google Scholar]