Abstract
Adenosine monophosphate-activated protein kinase (AMPK) is an energy sensor that regulates cellular adaptation to metabolic stress. Tissue-type plasminogen activator (tPA) is a serine proteinase found in the intravascular space, where its main role is as thrombolytic enzyme, and in neurons, where its function is less well understood. Here, we report that glucose deprivation induces the mobilization and package of neuronal tPA into presynaptic vesicles. Mass spectrometry and immunohistochemical studies show that the release of this tPA in the synaptic space induces AMPK activation in the postsynaptic terminal, and an AMPK-mediated increase in neuronal uptake of glucose and neuronal adenosine 5′(tetrahydrogen triphosphate; ATP) synthesis. This effect is independent of tPA's proteolytic properties, and instead requires the presence of functional N-methyl-D-aspartate receptors (NMDARs). In agreement with these observations, positron emission tomography (PET) studies and biochemical analysis with synaptoneurosomes indicate that the intravenous administration of recombinant tPA (rtPA) after transient middle cerebral artery occlusion (tMCAO) induces AMPK activation in the synaptic space and NMDAR-mediated glucose uptake in the ischemic brain. These data indicate that the release of neuronal tPA or treatment with rtPA activate a cell signaling pathway in the synaptic space that promotes the detection and adaptation to metabolic stress.
Keywords: adenosine 5′(tetrahydrogen triphosphate) (ATP), cerebral ischemia, N-methyl-D-aspartate receptors (NMDARs), positron emission tomography (PET), tissue-type plasminogen activator (tPA)
Introduction
Adenosine 5′(tetrahydrogen triphosphate) (ATP) provides the energy required by neurons to perform basic cellular functions, such as maintenance of ionic balance and electrochemical gradients across the plasma and mitochondrial membranes. Glucose and oxygen are the two main substrates for neuronal ATP production via the oxidative phosphorylation pathway.1 Accordingly, impairment of glucose and oxygen supply to the brain during an ischemic stroke leads to rapid depletion of ATP followed by neuronal death.
Adenosine monophosphate (AMP)-activated protein kinase (AMPK) is an evolutionary conserved kinase that acts as a sensor of cellular energy status and has a pivotal function in neuronal adaptation to metabolic stress.2 It is assembled by a catalytic α-subunit and two regulatory β- and γ-subunits. Depletion of ATP in energy-compromised neurons is followed by an increase in AMP, which binds to AMPK's γ-subunit leading to AMPK activation via phosphorylation of its α-subunit at Thr172.3 Once activated, AMPK phosphorylates several downstream targets that inhibit ATP-consuming events, such as protein and glycogen synthesis, and activates ATP-generating pathways, such as glycolysis and glucose uptake.4 Additionally, AMPK activation has long-term effects via modulation of transcriptional regulators such as nuclear factor kappa-B (NF-κB) and forkhead box class O 3a (FOXO3a).
Tissue-type plasminogen activator (tPA) is a serine proteinase found in the intravascular space, where its main role is as a thrombolytic enzyme, and in the central nervous system, where it mediates several physiologic events, including axonal growth,5 long-term potentiation, motor learning,6 and regulation of the permeability of the blood–brain barrier.7 Additionally, our previous work shows that either the release of endogenous tPA or the treatment with recombinant tPA (rtPA) promotes survival in neurons exposed to an ischemic8 or excitotoxic injury.9
The in vitro and in vivo studies presented here indicate that glucose deprivation (GD) induces the rapid release of endogenous tPA from presynaptic terminals, and that the nonproteolytical interaction of this tPA with N-methyl-D-aspartate receptors (NMDARs) leads to AMPK activation in the postsynaptic dendrite, and AMPK-mediated induction of neuronal uptake of glucose and ATP synthesis. In line with these observations, our in vivo studies indicate that treatment with rtPA after the onset of cerebral ischemia induces AMPK activation in the synaptic space followed by an increase in the uptake of glucose in the ischemic brain. This effect is also observed after treatment with proteolytically inactive rtPA and requires the presence of functional NMDARs. In summary, the studies presented here show that tPA has a pivotal role in the detection and adaptation of neurons to GD and opens new venues for the use of rtPA in patients not only with ischemic stroke but also with other pathologies associated with metabolic stress.
Materials and methods
Animals and Reagents
Murine strains were male wild-type (Wt) C57BL/6J and tPA deficient (tPA−/−) mice, 8- to 12-week-old, backcrossed at least seven generations into C57BL/6J mice, and mice with a 10-fold increase in tPA expression in neurons10 (T4 mice, kindly provided by Professor JD Vassalli and Doctor R Mandani, University of Geneva, Switzerland), and their Wt littermate controls. Experiments were approved by the Institutional Animal Care and Use Committee of Emory University, Atlanta, GA, USA, and following the guidelines established by ARRIVE (Animal Research: Reporting In Vivo Experiments). Recombinant murine tPA, proteolytically inactive tPA (itPA) with an alanine for serine substitution at the active site Ser481 (S481A), human Lys plasmin, an ELISA kit that detects active tPA, and sheep anti-tPA antibodies (Cat # SASMTPA) were acquired from Molecular Innovations (Novi, MI, USA). Other reagents were human rtPA (Genentech Inc., San Francisco, CA, USA), NMDAR antagonist MK-801 (Tocris Bioscience, Minneapolis, MN, USA), 2-N (7-nitrobenz-2-oxa-1,3-diazol-4-yl-amino)-2-deoxyglucose (2-NBDG), goat Alexa-conjugated secondary antibodies and 4′-6-diamidino-2-phenylindole (DAPI; Invitrogen, Grand Island, NY, USA), 18-fluorodeoxyglucose (18FDG; PETNET Solutions), antibodies against AMPK phosphorylated at Thr172 (Cell Signaling Technology, Denvers, MA, USA), anti-microtubule associated protein (MAP-2) and NR2B subunit antibodies, advasep-7, chloromethyl ketone, and Hoechst staining (Sigma, St Louis, MO, USA), and antibodies against synaptophysin (Millipore, Temecula, CA, USA), and the NR2A subunit of the NMDAR (Abcam, Cambirdge, MA, USA), the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay (ATCC; Manassas, VA, USA), the bicinchoninic acid assay (Thermo Fisher Scientific Inc., Waltham, MA, USA), and the CellTiter-Glo Luminescent Assay (Promega, Madison, MI, USA). The AMPK-specific siRNA (E-041035-00-0005) and a non-targeting control (NTC) (D-001910-10-20) were purchased from Thermo Scientific, Waltham, MA, USA.
Neuronal Cultures and Determination of Neuronal Survival
Cerebral cortical neurons were cultured from E16 to E18 Wt and tPA−/− mice as described elsewhere.11 Briefly, the cerebral cortex was dissected, transferred into Hanks' balanced salt solution containing 100 units/mL penicillin, 100 μg/mL streptomycin, and 10 mm HEPES, and incubated in trypsin containing 0.02% DNase at 37°C for 15 minutes. Tissue was then triturated, and the supernatant was resuspended in B27-supplemented neurobasal medium containing 2 mmol/L L-glutamine and plated onto 0.1 mg/mL poly-L-lysine-coated wells. To study the effect of tPA on neuronal survival, Wt and tPA−/− cerebral cortical neurons were incubated with 5 nmol/L of tPA or vehicle control in the presence of 0 or 25 mmol/L of glucose and kept in an anaerobic chamber (Hypoxygen, Frederick, MD, USA) during 55 minutes under either normoxic or hypoxic (<0.1% oxygen) conditions. Twenty-four hours later, cell survival was quantified with the MTT assay following the manufacturer's instructions and as described elsewhere.12 Results are given as a percentage of cell survival compared with cultures maintained under normoxic conditions and 25 mmol/L of glucose. Each experiment was performed in cultures from three different animals and each observation was repeated 15 times.
Proteomics and Ingenuity Pathways Analysis
Proteomics analyses were performed as described elsewhere.8 Wild-type cerebral cortical neurons were incubated for 1 hour with 5 nmol/L of tPA or an equivalent volume of vehicle (control) followed by resolution on a 10% polyacrylamide SDS gel. Extracted peptides were loaded onto a C18 column, eluted, and detected by Orbitrap. MS/MS scans were acquired by data-dependent acquisition in an LTQ linear-ion trap mass spectrometer (Thermo Finnigan, San Jose, CA, USA) and the data were searched against a concatenated target decoy mouse reference database downloaded from the National Center for Biotechnology Information (September 2009) using the SEQUEST Sorcerer algorithm (version 3.11, SAGE-N).13 Quantitative pair-wise comparison of control and tPA-treated samples was performed using the software DQUAN. Accurate peptide mass and retention time were used to derive signal intensity for every peptide across LC-MS runs. Each peptide signal was transformed into logarithmic (log2) values and the detection of outliers determined using Dixon's Q test. The averaged log2 ratio over all peptides for a particular protein was used to determine the protein expression ratio and a standard deviation. For the Ingenuity Pathway analysis, Log2 (tPA treated/control) values of the average protein intensity ratios calculated by DQUAN were centred so that the fit gauss curve midpoint (mean) fell at zero. Log2 values 1.63 standard deviations from the mean (changed with 95% confidence, with absolute value >0.709) were considered as changing and these protein identities and quantifications were considered in the analysis.
Tissue-Type Plasminogen Activator Activity Assay
The culture media of Wt neurons was sampled after 0, 30 seconds, and 5 minutes of deprivation of either glucose (GD), or oxygen (OD), or oxygen and glucose (OGD). The concentration of tPA was quantified with an ELISA kit following the manufacturer's instructions. Results were normalized to protein concentration in each well. As a control, tPA concentration was quantified at identical time points in sister cultures maintained at normal oxygen and glucose concentrations. Each experiment was performed with cultures from three different animals and each observation was repeated 10 times.
Animal Model of Cerebral Ischemia
Transient occlusion of the middle cerebral artery (tMCAO) was induced in T4 mice and their Wt littermate controls with a 6-0 silk suture advanced from the external carotid artery into the internal carotid artery until the origin of the middle cerebral artery as described elsewhere.14 Briefly, a nylon monofilament (6-0; Ethicon, Issy Les Moulineaux, France) coated with silicone was introduced through the external carotid artery and advanced up to the origin of the middle cerebral artery. The suture was withdrawn after 1 to 60 minutes of cerebral ischemia. Cerebral perfusion in the distribution of the middle cerebral artery was monitored throughout the surgical procedure and after reperfusion with a laser Doppler (Perimed Inc., North Royalton, OH, USA), and only animals with a >70% decrease in cerebral perfusion after occlusion and complete recovery after suture withdrawn were included in this study. The rectal and masseter muscle temperatures were controlled at 37°C with a homoeothermic blanket. Heart rate, systolic, diastolic, and mean arterial blood pressures were controlled throughout the surgical procedure with an IITC 229 System (IITC-Lice Science, Woodland Hills, CA, USA). A subgroup of Wt mice was treated either immediately before or after the ischemic injury with 0.9 mg/kg/IV of either proteolytically active or chloromethyl ketone-inactivated rtPA, prepared as described elsewhere.15 Before its administration, arginin was dialyzed from rtPA using a SnakeSkin Dialysis Tubing (Thermo Scientific). A subgroup of rtPA-treated mice was injected into the lateral ventricle with 2 μL of a solution of 1 μg/μL of MK-801 at the following coordinates: bregma: −1 mm, mediolateral: 2 mm, dorsoventral: 2 mm before treatment with rtPA.16
Preparation of Cerebral Cortical Synaptoneurosomes
Synapse-enriched fractions containing the presynaptic terminal and resealed postsynaptic compartment (synaptoneurosomes) were prepared as described elsewhere.17 Briefly, the cerebral cortex was dissected either from non-ischemic Wt brains or from Wt brains 5 minutes after tMCAO and treated with 0.9 mg/kg/IV of rtPA or a comparable volume of vehicle (control), or with a combination of rtPA and MK-801 as described above (n=4). Homogenates were centrifuged at 1,000 g for 2 minutes, the supernatant S1 was transferred to a fresh tube and the pellet P1 was resuspended in 0.32 mol/L Sucrose and spun at 1,000 g for 2 minutes. The pellet was discarded and the S1 was pooled with the previous one. S1 was centrifuged at 23,000 g for 4 minutes and the S2 was discarded. The pellet P2 was resuspended in 0.32 mol/L Sucrose and the 23,000 g spin repeated. The clean P2 was resuspended in 1.5 mL (5 mL) of 0.32 mol/L Sucrose and overlayed on top of a 5, 13% ficoll (Cat# F4375; Sigma) discontinuous gradient prepared in 0.32 mol/L Sucrose. Samples were centrifuged at 50,000 g for 45 minutes. Synaptoneurosomes were collected from the 5/13 ficoll interface, diluted in 10 mL of 0.32 mol/L Sucrose and spun at 23,000 g for 15 minutes. The synaptoneurosomes pellet was washed in phosphate-buffer saline and resuspended in a small volume of DMEM for use in subsequent experiments. Protein concentration was determined using the bicinchoninic acid assay with bovine serum albumin as a standard.
Western Blot Analysis
Wild-type cerebral cortical neurons were incubated for 0 to 60 minutes with 5 nmol/L of tPA. The ischemic cerebral cortices of T4 mice and their Wt littermate controls were harvested 0 to 30 minutes after tMCAO. A subgroup of Wt mice (n=3) was treated with 0.9 mg/kg/IV of tPA followed by 5 minutes of MCAO. Synaptoneurosomes were prepared from the cerebral cortex of either nonischemic Wt brains or ischemic Wt brains 5 minutes after tMCAO and treated with 0.9 mg/kg/IV of rtPA, or a comparable volume of vehicle (control), or a combination of rtPA and MK-801. Synaptoneurosomes prepared from nonischemic brains were resuspended in DMEM buffer, equilibrated in the incubator with 5% CO2 for 10 minutes and treated during 5 minutes with 5 nmol/L of tPA or a comparable volume of vehicle (control). Brain and cell extracts were homogenized and the protein concentration was quantified using the bicinchoninic acid assay. Fifteen micrograms of protein from brains or cell extracts, or synaptoneurosomes were loaded per sample, separated by 4% to 20% precast linear gradient polyacrylamide gel (Bio-Rad, Hercules, CA, USA), transferred onto a PVDF membrane by a semidry transfer system, blocked with 5% nonfat dry milk in Tris-buffered saline pH 8.0 with 0.1% Tween-20 buffer, and immunoblotted with an antibody that recognizes AMPK phosphorylated at Thr172. Each observation was repeated four times.
Adenosine Monophosphate-Activated Protein Kinase Downregulation
Wild-type cerebral cortical neurons were treated with either AMPK-specific siRNA (E-041035-00-0005) or a nontargeting sequence (D-001910-10-20). Samples were prepared in a stock solution of 100 μmol/L in 1 × siRNA buffer and diluted in neurobasal culture medium to a final siRNA concentration of 1 μM RNA. Cells were incubated during 72 hours before being used in subsequent experiments. The downregulation efficiency was assessed by real-time PCR with TaqMan Gene Expression Assay protocol and dye-labeled primers purchased from Applied Biosystems (Gapdh: Mm99999915_g1; AMPK: Mm01296700_m1). Three replicates were run for each gene for each sample in a 96-well plate. GAPDH was used as the endogenous reference gene. The relative quantitation method (ΔΔCt) was used, with the ratio of the mRNA level for the gene of interest normalized to the level of internal control. With this protocol, we obtained an ∼60% AMPK mRNA downregulation.
Quantification of Glucose Uptake
To quantify glucose uptake in vitro, Wt neurons were treated 0 to 60 minutes with 5 nmol/L of either proteolytically active tPA or itPA, or 30 minutes with either 10 nmol/L of plasmin, or with a combination of 5 nmol/L of tPA and 10 μmol/L of MK-801. A subgroup of Wt neurons was treated with AMPK siRNA or an NTC as described above. Cells were washed twice with phosphate-buffer saline, incubated 30 minutes with 300 μmol/L of 2-NBDG in the presence of 0.5 mmol/L of glucose to minimize competition with dye uptake, and washed again with phosphate-buffer saline. Fluorescence was measured in a spectrofluorophotometer at 528/485 nm. Results were normalized to protein concentration in each well. Each experiment was repeated 10 to 16 times in cultures from four different animals. To quantify glucose uptake in vivo Wt mice (n=5) underwent tMCAO. Immediately after reperfusion, animals were treated with 0.9 mg/kg/IV of proteolytically active or inactive rtPA, or with a comparable volume of saline solution. A subgroup of mice was cotreated with rtPA and MK-801 as described above. Two hours after reperfusion, animals were injected into the tail vein with 9.25 MBq 18FDG, followed 60 minutes later by 18FDG positron emission tomography (PET) imaging in a Siemens Inveon micro PET/CT scanner (Siemens). Images with a spatial resolution of 1.8 mm full width at half maximum were acquired over 60 minutes and reconstructed with an ordered subset expectation-iterative algorithm. To quantify differences in glucose uptake, PET images were viewed in transverse sections and regions of interest were placed over the ischemic and nonischemic hemispheres. The uptake of 18FDG in each region of interest was recorded as nCi/cc and the differential uptake ratio (DUR) was calculated two separate times in seven slices per mouse using the following formula:
((nCi/cc uptake in ischemic hemisphere) – (nCi/cc uptake in nonischemic hemisphere))
(nCi/cc uptake in ischemic hemisphere)
Immunohistochemistry
Wild-type cerebral cortical neurons were maintained during 5 minutes under 0 or 25 nmol/L of glucose, fixed, permeabilized with 50 μg/mL of digitonin, blocked in 0.25% casein and 10% donkey serum in phosphate-buffer saline, and labeled with antibodies against MAP-2 (1:2,500), synaptophysin (1:2,500), and tPA (1:2,500 dilution). To study the effect of tPA on AMPK expression, neurons were incubated with 5 nmol/L of tPA or a comparable volume of vehicle (control) during 5 minutes and costained with DAPI and antibodies against pAMPK (1:1,000), MAP-2 (1:2,500 dilution), and synaptophysin (1:2,500). Conjugated secondary antibodies were goat Alexa 488 or 594. Microphotographs were obtained with a Photometric Quantix digital camera connected to a Nikon Eclipse TE300 epifluorescence microscope.
Quantification of Adenosine 5′(Tetrahydrogen Triphosphate) Synthesis
Wild-type neurons or neurons treated with AMPK siRNA or an NTC as described above were incubated with 5 nmol/L of tPA or vehicle control during 0 to 60 minutes and equilibrated 30 minutes at room temperature. Equal volumes of CellTiter-Glo reagent were added to the wells and mixed for 2 minutes in an orbital shaker. The reaction plates were then incubated at room temperature for 10 minutes to stabilize a luminescent signal and 100 μL of the lysis mixture was taken and placed into opaque-walled multiwell plates for reading with a luminometer. Plates were read with an integration time of 1 second per well. Each observation was repeated 12 times in cultures from three different animals.
Statistical Analysis
Values are expressed as percentage or mean±s.d. when appropriate. Statistical tests included the T-test followed by the Wilcoxon signed-ranked test and two-way ANOVA for comparisons between groups. P-values of <0.05 were considered as significant.
Results
Glucose Deprivation Induces the Release of Tissue-Type Plasminogen Activator from Presynaptic Terminals
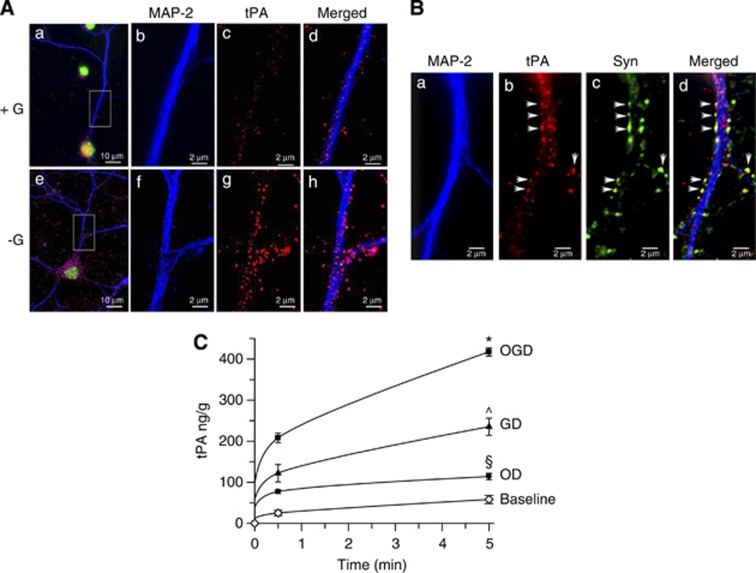
First, we studied the effect of GD on neuronal tPA. We found that GD induces a rapid increase in tPA in the neuronal soma and axons (Figure 1A), and co-staining with synaptophysin indicated that most of this tPA is localized in synaptophysin-positive presynaptic vesicles in direct contact with the postsynaptic dendrite (arrows in Figure 1B). Then, we investigated the effect of GD on the release of neuronal tPA and compared these data with the release induced by either OD alone, or combined OGD. We found that the concentration of tPA in the culture media increases from 0 ng/g, to 208±11.6 ng/g and 417.4±10.1 ng/g after 5 and 30 minutes of OGD, respectively (Figure 1C; n=10 per time point; P<0.05). Surprisingly, our data indicate that the effect of OGD on the release of neuronal tPA is primarily due to lack of glucose. Indeed, whereas 30 seconds and 5 minutes of OD increased the concentration of tPA in the culture media to 77.1±4.5 ng/g and 113.8±7.9 ng/g (∼35% of tPA released under OGD conditions), exposure to GD for similar periods of time increased the release of tPA to 122±21.2 ng/g and 235.2±20.7 ng/g, respectively (∼ 60% of tPA released under OGD conditions; P<0.05).
Figure 1.
Glucose deprivation (GD) induces the release of tissue-type plasminogen activator (tPA) from the presynaptic terminal of cerebral cortical neurons. (A) Representative micrograph of wild-type (Wt) cerebral cortical neurons maintained during 5 minutes under 25 mmol/L (+G) or 0 mmol/L (−G) of glucose and stained with antibodies against the postsynaptic dendritic marker anti-microtubule associated protein (MAP-2) (blue) and tPA (red). Green corresponds to DNA (Hoechst). Magnification × 20 in (a, e) and × 60 in (b–d) and (f–h). (B) Representative micrograph of Wt cerebral cortical neurons maintained during 5 minutes under GD and stained with antibodies against MAP-2 (blue), tPA (red), and synaptophysin (green). Arrows denote the presence of tPA-containing synaptophysin-positive presynaptic vesicles in direct contact with the postsynaptic, MAP-2-positive, dendrite. Magnification × 60. (C) Mean concentration of tPA in the culture medium of Wt cerebral cortical neurons exposed during 0 to 5 minutes to either oxygen deprivation (OD, black circles), or GD (black triangles), or oxygen and glucose deprivation (OGD; black squares), or kept under normal glucose and oxygen concentrations after medium change (baseline; white diamonds). Lines denote s.d. n=10 per condition; *P<0.05 compared with GD and OD; ^P<0.05 compared with OD and OGD; §P<0.05 compared with sister cultures maintained under physiologic conditions and with cultures exposed to either GD or OGD conditions.
Tissue-Type Plasminogen Activator Induces Adenosine Monophosphate-Activated Protein Kinase Activation in the Postsynaptic Dendrite
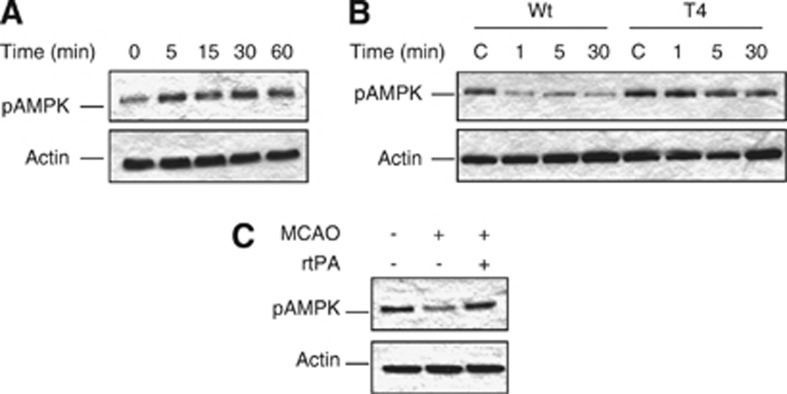
On the basis of these observations, we postulated that the release of neuronal tPA is part of an endogenous adaptive response to low glucose concentrations. To test this hypothesis, we used liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) and subsequent analysis with the Ingenuity Pathway Analysis tool to investigate whether treatment of Wt cerebral cortical neurons with tPA has an effect on the cell signaling pathways known to be activated by GD. Remarkably, we found that treatment with 5 nmol/L of tPA causes a significant increase in the abundance of neuronal AMPK (2.77 log2; P<0.05) which is a sensor of metabolic stress. Because AMPK requires phosphorylation of its α-subunit at Thr172 (pAMPK) for its activation,18 we investigated the expression of pAMPK in Wt cerebral cortical neurons after 0 to 60 minutes of incubation with 5 nmol/L of tPA. Our data indicate that tPA activates neuronal AMPK in vitro (Figure 2A). To investigate whether the release of neuronal tPA also activates AMPK in vivo, we studied the expression of pAMPK in the ischemic tissue of mice overexpressing neuronal tPA (T4) and their Wt littermate controls after 0 to 30 minutes of MCAO. We found that cerebral ischemia causes a rapid decrease in pAMPK expression in the ischemic tissue of Wt mice. In contrast, neuronal overexpression of tPA was associated with sustained AMPK activation in the ischemic brain (Figure 2B). Together, our results indicate that either incubation with tPA in vitro or release of neuronal tPA in vivo induces neuronal AMPK activation. Our data indicate that the release of neuronal tPA does not induce AMPK activation in the ischemic tissue of Wt mice in vivo. To explain this apparent discrepancy between our in vitro and in vivo data we postulated that in contrast with T4 mice, the amount of tPA released from Wt neurons in vivo is not enough to induce a sustained activation of AMPK in the ischemic tissue. To test this hypothesis, we studied the expression of pAMPK in the ischemic tissue of Wt mice treated with 0.9 mg/Kg/IV of rtPA immediately before the induction of 5 minutes of cerebral ischemia. Our results show that augmenting the concentration of tPA in the ischemic tissue increases the expression of pAMPK in Wt mice to levels comparable to those observed in T4 animals (Figure 2C).
Figure 2.
Effect of tissue-type plasminogen activator (tPA) on neuronal pAMPK expression. (A) Representative western blot analysis of adenosine monophosphate-activated protein kinase (AMPK) phosphorylated at Thr172 (pAMPK) in wild-type (Wt) cerebral cortical neurons incubated 0 to 60 minutes with 5 nmol/L of tPA. (B) Representative western blot analysis of pAMPK expression in the ischemic tissue of T4 mice and their Wt littermate controls 0 to 30 minutes after middle cerebral artery occlusion (MCAO). (C) Representative western blot analysis of pAMPK expression in the ischemic tissue of Wt mice treated with recombinant tPA (rtPA) immediately before 5 minutes of MCAO.
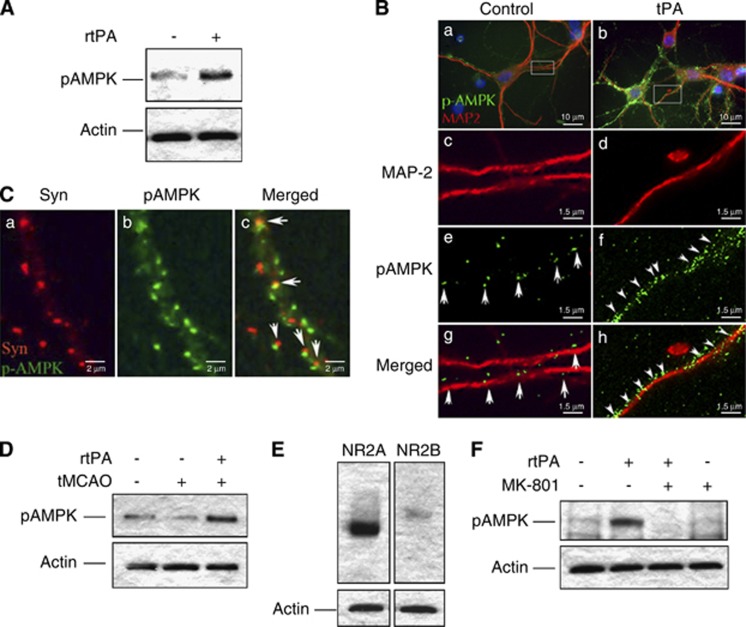
Because our data suggest that tPA has an effect on AMPK in the synaptic space, we studied the expression of pAMPK in synapse-enriched preparations containing functional presynaptic and resealed postsynaptic terminals (synaptoneurosomes), obtained from the cerebral cortex of Wt mice and incubated for 0 to5 minutes with 5 nmol/L of tPA. Our results show that tPA induces synaptic activation of AMPK not only in neuronal cultures but also in the cerebral cortex (Figure 3A). To further characterize these findings, we performed immunohistochemical staining for pAMPK in Wt cerebral cortical neurons incubated 5 minutes with 5 nmol/L of tPA. We found that treatment with tPA induces a rapid increase in the expression of pAMPK in postsynaptic dendrites (Figure 3B) and further costaining with antibodies against the presynaptic vesicular protein synaptophysin confirmed that this effect occurs in the postsynaptic terminal (note the juxtaposition of presynaptic synaptophysin-positive vesicles and postsynaptic AMPK-positive staining in Figure 3C).
Figure 3.
Tissue-type plasminogen activator (tPA) induces adenosine monophosphate-activated protein kinase (AMPK) activation in the postsynaptic terminal via N-methyl-D-aspartate receptors (NMDARs) activation. (A) Representative western blot analysis of pAMPK expression in non-ischemic wild-type (Wt) cerebral cortical synaptoneurosomes after 5 minutes of incubation with 5 nmol/L of tPA (+) or vehicle (control; −). (B) Representative microphotograph of Wt cerebral cortical neurons incubated during 5 minutes with vehicle (control; a, c, e, and g) or 5 nmol/L of tPA (b, d, f, and h), and stained with antibodies against the dendritic marker anti-microtubule associated protein (MAP-2) (red) and pAMPK (green). Magnification × 40 in (a, b) and × 60 in (c–h). (C) Representative microphotograph of Wt cerebral cortical neurons incubated 5 minutes with 5 nmol/L of tPA and stained with antibodies against synaptophysin (red) and pAMPK (green). Arrows denote examples where presynaptic synaptophysin-positive vesicles are in juxtaposition with postsynaptic pAMPK. Magnification × 100. (D) Representative western blot analysis of pAMPK expression in cerebral cortical synaptoneurosomes prepared from the cerebral cortex of Wt mice after transient middle cerebral artery occlusion (tMCAO) and treatment with either 0.9 mg/kg/IV of recombinant tPA (rtPA) or a comparable volume of saline solution. (E) Representative western blot analysis of the expression NR2A and NR2B subunits of NMDARs in synaptoneurosomes prepared from the cerebral cortex of Wt mice. (F) Representative western blot analysis of pAMPK expression in cerebral cortical synaptoneurosomes prepared from the cerebral cortex of Wt mice 5 minutes after tMCAO and treatment with 0.9 mg/kg/IV of rtPA alone or in combination with 2 μg of MK-801.
Because after its intravenous administration rtPA reaches the ischemic tissue,19 we investigated the expression of pAMPK in synaptoneurosomes prepared from the ischemic cortex of Wt mice intravenously treated with saline solution or rtPA immediately after tMCAO. We found that treatment with rtPA induces AMPK activation in the synaptic space of the ischemic tissue (Figure 3D). The postsynaptic terminal of cerebral cortical neurons is enriched in NMDARs. Because previous data indicate that several of the effects of tPA in the central nervous system are mediated by NMDARs20 we decided to study the expression of pAMPK in synaptoneurosomes isolated from the ischemic tissue of Wt mice treated immediately after tMCAO with rtPA, alone or in combination with the NMDAR antagonist MK-801. Our data indicate that these preparations express NR2A- and to a lesser extent NR2B-containing NMDARS (Figure 3E), and that these receptors are required for rtPA-induced AMPK activation (Figure 3F).
Tissue-Type Plasminogen Activator Induces Adenosine Monophosphate-Activated Protein Kinase-Mediated Neuronal Uptake of Glucose Via Non-Proteolytic Interaction with N-Methyl-D-Aspartate Receptors
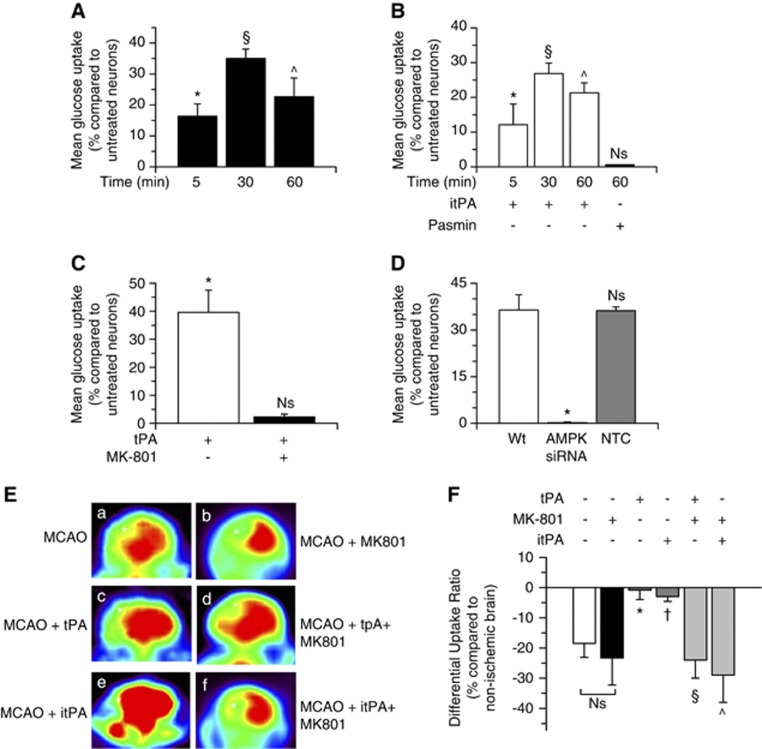
Because AMPK activation induces glucose uptake21 we decided to quantify the uptake of a fluorescent deoxyglucose analog (2-NDBG) in Wt cerebral cortical neurons after 0 to 60 minutes of incubation with 5 nmol/L of tPA. We found that compared with vehicle (control)-treated neurons, the neuronal uptake of glucose increases by 16.32±4%, 35.01±3%, and 22.66±6%, after 5, 30, or 60 minutes of incubation with tPA, respectively (Figure 4A; n=16; P<0.05). This effect was also observed after 30 or 60 minutes of treatment with 5 nmol/L of proteolytically itPA (26.85±3% and 21.29±2.9% increase in glucose uptake, respectively), but not with 10 nmol/L of plasmin (Figure 4B; n=16; P<0.05), and abrogated by either NMDARs antagonism with MK-801 (Figure 4C; n=16), or siRNA-mediated AMPK downregulation (Figure 4D; n=10; P<0.05).
Figure 4.
Tissue-type plasminogen activator (tPA) induces adenosine monophosphate-activated protein kinase (AMPK)-mediated neuronal uptake of glucose via non-proteolytic interaction with N-methyl-D-aspartate receptors (NMDARs). (A–C) Mean increase in the uptake of a fluorescent deoxyglucose analog (2-NBDG) in cerebral cortical neurons treated during the indicated periods of time with 5 nmol/L of proteolytically active (A), or inactive tPA (itPA) (B), or with plasmin (B), or with a combination of 5 nmol/L of tPA and 10 μmol/L of MK-801 (C). Lines denote s.d.. Results are given as a percent increase compared with 2-NBDG uptake (nmol/g) in neurons treated for similar length of time with vehicle (control). n=16 per experimental condition; * in (A): P<0.05 compared with untreated neurons. § in (A): P<0.05 compared with neurons treated with either vehicle (control) or tPA during 5 minutes. ^ in (A): P<0.05 compared with neurons treated with vehicle (control) or tPA during 5 or 30 minutes. *, §, and ^ in (B): P<0.05 compared with neurons incubated with vehicle (control) for similar lengths of time, and with neurons treated with plasmin. NS, nonsignificant when compared with sister cultures treated with vehicle (control). * in (C): P<0.05 compared with vehicle (control)-treated neurons and with neurons treated with a combination of tPA and MK-801. NS: non-significant compared with vehicle (control)-treated cultures. (D) Mean increase in 2-NBDG uptake in cerebral cortical neurons left untreated (wild type, Wt) or treated with AMPK siRNA or with a non-targeting control (NTC) and incubated 30 minutes with 5 nmol/L of tPA. Lines denote s.d.. Results are given as a percent increase compared with 2-N (7-nitrobenz-2-oxa-1,3-diazol-4-yl-amino)-2-deoxyglucose (2-NBDG) uptake (nmol/ng) in sister cultures treated with vehicle (control). n=10 per experimental condition; *P<0.05 compared with Wt neurons and NTC-treated neurons. NS, non-significant compared with Wt tPA-treated neurons. (E, F) Representative images of 18-fluorodeoxyglucose (18FDG)-positron emission tomography (PET) scans (E) and quantification of the differential uptake ratio (DUR) of glucose (F) in Wt mice treated immediately after transient middle cerebral artery occlusion (tMCAO) with either saline solution (a), or MK-801 (b), or proteolytically active recombinant tPA (rtPA) (c), or a combination of proteolytically active tPA and MK-801 (d), or proteolytically inactive rtPA (e), or a combination of proteolytically inactive tPA (itPA) and MK-801 (f). * and †: P<0.05 compared with DUR in mice treated with either saline solution or MK-801 alone; § and ^: P<0.05 compared with brains treated with either active or proteolytically itPA alone. NS, non-significant when animals treated with saline solution are compared with mice treated with MK-801 alone. n=7 observations per mouse. Asterisks in (E) denote the ischemic area in each animal.
To investigate whether the intravenous administration of rtPA also induces the uptake of glucose in vivo, we used 18FDG-micro PET imaging to quantify the DUR of glucose in Wt mice treated immediately after tMCAO with 0.9 mg/Kg/IV of proteolytically active or inactive rtPA, or with a combination of rtPA and MK-801. We found that cerebral ischemia induces an 18.5±4.6% decrease in the DUR of glucose, and that this effect is attenuated by treatment with either proteolytically active or inactive rtPA (0.86±3.1% and 2.9±1.6% decrease in DUR, respectively). Importantly, as observed in our in vitro studies, the beneficial effect of rtPA on the uptake of glucose was abrogated by NMDAR antagonism with MK-801 (23.3±5.7% decrease in DUR; Figure 4E and F).
The Neuroprotective Effect of Tissue-Type Plasminogen Activator Is Mediated by Its Ability to Induce Glucose Uptake
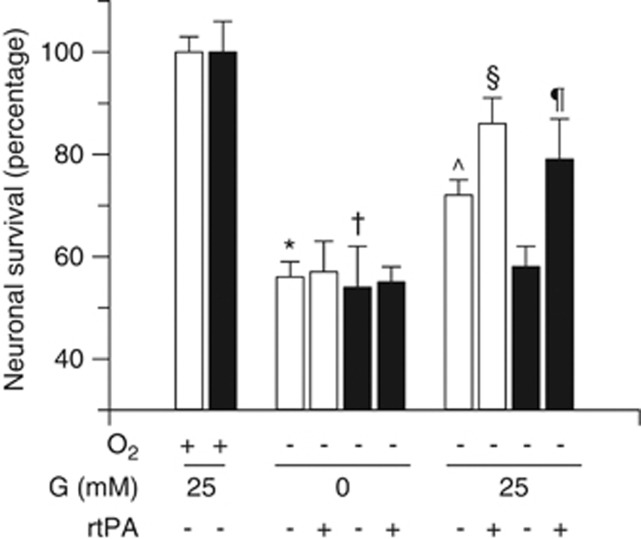
To investigate whether tPA-induced glucose uptake mediates the neuroprotective effect of tPA, we quantified cell survival in Wt and tPA−/− cerebral cortical neurons exposed to OD in the presence of 0 or 25 mmol/L of glucose. Our data indicate that compared with neurons maintained under physiologic concentrations of oxygen and glucose, exposure to combined deprivation of oxygen and glucose causes a comparable decrease in cell survival in Wt and tPA−/− neurons (56±3% and 54±8%, respectively; Figure 5; n=15). However, when 25 mmol/L of glucose was added to Wt and tPA−/− neurons exposed to OD and GD, cell survival increased in Wt (72±3%) but not in tPA−/− neurons (58±5.5%), suggesting that the release of endogenous tPA induced by aglycemia has a neuroprotective effect via its ability to promote glucose uptake. To further test this hypothesis, we quantified cell survival in Wt and tPA−/− neurons deprived from oxygen in the presence of 0 or 25 mmol/L of glucose and 0 or 5 nmol/L of tPA. We found that treatment of Wt neurons with tPA does not have an effect on cell survival when cells are kept under GD. However, the prosurvival effect of the addition of 25 mmol/L of glucose to Wt neurons (increase in cell survival from 56±3% to 72±3%) was significantly potentiated by tPA (86±5% cell survival). In contrast, cell survival remained unchanged in tPA−/− neurons after the addition of glucose alone (58±5.5%), or tPA alone (58±5.5%), but increased when cells were cotreated with 25 mmol/L of glucose and 5 nmol/L of tPA to levels comparable to those observed in Wt neurons treated with tPA and glucose (79±7.89%, Figure 5; n=15; P<0.05).
Figure 5.
The neuroprotective effect of tissue-type plasminogen activator (tPA) is mediated by its ability to induce glucose uptake. Mean cell survival in wild-type (Wt) (white bars) and tPA−/− (black bars) cerebral cortical neurons exposed to oxygen (O2) deprivation in the presence of 0 to 25 mmol/l of glucose (G), and 0 or 5 nmol/L of tPA. n=15 per experimental condition. * and †: P<0.05 compared with Wt and tPA−/− neurons maintained under normoxic and normoglycemic conditions. ^P<0.05 compared with Wt and tPA−/− neurons exposed to combined deprivation of oxygen and glucose. §P<0.05 compared with Wt neurons exposed to oxygen deprivation in the presence of 25 mmol/L of glucose but not treatment with tPA. ¶P<0.05 compared with tPA−/− neurons exposed to oxygen deprivation in the presence of 25 mmol/L of glucose but not treatment with tPA.
Tissue-Type Plasminogen Activator Induces Adenosine Monophosphate-Activated Protein Kinase-Mediated Adenosine 5′(Tetrahydrogen Triphosphate) Synthesis in Cerebral Cortical Neurons
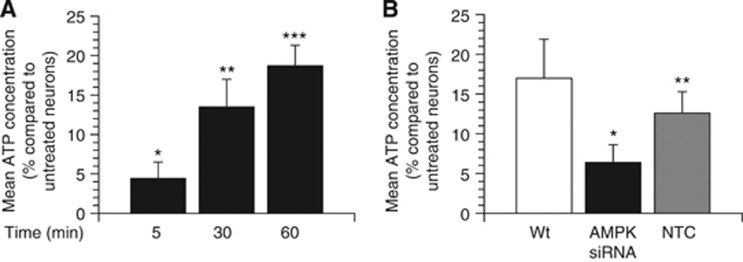
Our data indicate that tPA induces AMPK-mediated glucose uptake. Because glucose is the main substrate for neuronal ATP synthesis,1 we measured the concentration of ATP in Wt cerebral cortical neurons incubated 0 to 60 minutes with 5 nmol/L of tPA. We found that compared with vehicle (control)-treated neurons, 5, 30, or 60 minutes of incubation with tPA increases the concentration of neuronal ATP by 4.4±2.1%, 13.5±3.5%, and 18.7±2.6%, respectively (Figure 6A; n=22, P<0.05), and that this effect is attenuated by siRNA-mediated AMPK downregulation (Figure 6B; n=10; P<0.05).
Figure 6.
Tissue-type plasminogen activator (tPA) induces adenosine monophosphate-activated protein kinase (AMPK)-mediated adenosine 5′(tetrahydrogen triphosphate (ATP) synthesis in cerebral cortical neurons. Mean increase in ATP concentration in wild-type (Wt) cerebral cortical neurons incubated 5 to 60 minutes with 5 nmol/L of tPA (A), and in Wt cerebral cortical neurons treated with AMPK siRNA or a non-targeting control (NTC) and incubated 60 minutes with 5 nmol/L of tPA. Results are given as a percent increase compared with ATP concentration (nmol/g) in vehicle (control)-treated neurons. n=20 in (A) and 12 in (B) per experimental condition; * in (A): P<0.05 compared with untreated neurons. ** in (A): P<0.01 compared with control-treated neurons or with neurons treated with tPA during 5 minutes. *** in (A): P<0.001 compared with control-treated neurons or with neurons treated with tPA during 5 or 30 minutes. * in (B): P<0.05 compared with Wt or NTC-treated neurons.
Discussion
Normal brain function needs a constant supply of glucose. In fact, brain processes that consume energy use ∼25% of the total body glucose.22 Cerebral cortical neurons are highly vulnerable to hypoglycemia. Indeed, when blood glucose decreases below 2 mmol/L, the concentration of glucose in the brain decreases to zero23 leading to inhibition of synaptic activity24 and activation of a cascade of cellular events that result in neuronal death. The data presented here indicate that GD induces the release of tPA from the presynaptic terminal of cerebral cortical neurons and that this tPA activates in the postsynaptic dendrite, a cell signaling pathway that leads to ATP synthesis and neuronal adaption to metabolic stress.
Real-time imaging studies have revealed the rapid mobilization of tPA along the axon.25 Our data show that GD increases the expression of tPA in the neuronal soma and axons, and that most of the axonal tPA is packed into synaptophysin-positive presynaptic vesicles. Together, these observations indicate that lack of glucose induces the rapid mobilization of tPA from the soma to presynaptic terminals. Interestingly, we also observed an increase in tPA expression in the postsynaptic compartment (dendrites) after exposure to GD. Although we cannot rule out a direct effect of metabolic stress on tPA expression in dendrites, we consider more likely that tPA released from the presynaptic compartment is uptaken by the postsynaptic dendrite, indicating the existence of an as yet unknown recycling mechanism for tPA in the synaptic space.
We found that the release of tPA activates a cell signaling pathway in the postsynaptic terminal that promotes the adaption to conditions of metabolic stress. The AMPK is an evolutionarily preserved sensor of cellular stress and unmet metabolic demands in multiple organs, including heart, liver, adipose tissue, muscle, pancreas, and brain.21 Here, we show that either incubation of neuronal cultures with tPA in vitro or the intravenous administration of rtPA in vivo induces the rapid activation of AMPK in the postsynaptic terminal. The expression and activation of AMPK has been described in the cytoplasm and nucleus of neurons.26 However, to our knowledge this is the first report of AMPK activation in the synaptic space, more specifically in the postsynaptic dendrite. We believe that our data with preparations containing isolated functional presynaptic and postsynaptic terminals (synaptoneurosomes) is of significant clinical relevance. Indeed, these results indicate that the intravenous administration of rtPA has a direct effect on synaptic function, and that this effect is independent of tPA's ability to cleave plasminogen into plasmin, and therefore potentially devoid of harmful effects on the barrier function of the BBB.
Activation of AMPK in the heart has a protective effect.27 In contrast, its role in the ischemic brain is yet unclear. In fact, whereas some reports indicate that AMPK activation after cerebral ischemia has a deleterious effect,28 others have found that it is part of an endogenous neuroprotective response.29 Our results indicate that tPA-induced AMPK activation has an important role in the maintenance of neuronal function under conditions of metabolic stress. Importantly, our data suggest that the effect of tPA on AMPK activation in the ischemic tissue is concentration dependent. Indeed, our results indicate that the amount of tPA released from Wt neurons in vivo is not enough to induce a sustained activation of AMPK in the ischemic tissue. In contrast, an augmentation in the concentration of tPA in the ischemic brain either by treatment of Wt mice with rtPA, or by genetic overexpression of neuronal tPA in T4 animals causes a comparable increase in pAMPK expression in the ischemic tissue.
Three kinases have been reported to induce AMPK phosphorylation: liver kinase B1, transforming growth factor-β-activated kinase, and Ca+2/calmodulin-dependent protein kinase kinase β.2 Our findings indicate that the effect of tPA on AMPK activation is mediated by non-proteolytic interaction with NMDARs. These findings are in agreement with recent data indicating that at concentrations found in the ischemic brain tPA does not cleave NMDARs and that tPA's effect on NMDARs does not require the conversion of plasminogen into plasmin.9 These observations are in agreement with our in vivo data showing that treatment with rtPA, either proteolytically active or inactive, induces the uptake of glucose in the ischemic brain, and that his effect requires functional NMDARs.
Our previous work showed that the release of tPA from cerebral cortical neurons, or incubation with tPA in vitro, or the intravenous administration of rtPA in vivo has a neuroprotective effect in neurons exposed to OGD conditions and in the brain after tMCAO.8, 9, 12, 19 Surprisingly, the data presented here indicate that lack of glucose and not hypoxia is the main stimulus for the release of neuronal tPA, and that tPA needs glucose to promote survival in neurons exposed to OGD conditions. Interestingly, our data also show that glucose needs tPA to protect neurons from the deleterious effects of lack of glucose. More importantly, we found that tPA-induced AMPK-mediated glucose uptake leads to neuronal ATP synthesis, providing a mechanism whereby treatment with rtPA or release of endogenous tPA promotes neuronal adaption to situations associated with energy failure. In contrast with the necrotic core, the concentration of glucose in the area of ischemic penumbra is decreased but not absent. Therefore, based on our data, we postulate that tPA, either released from neurons in response to the ischemic insult, or given as a treatment, promotes cell survival in the area of ischemic penumbra in vivo, via its ability to induce the neuronal uptake of glucose.
In summary, based on the in vitro and in vivo data presented here, we propose a model in which endogenous tPA released from the presynaptic terminal, or rtPA intravenously administered, interacts with NMDARs in the postsynaptic compartment leading to the activation of a cell signaling pathway that induces the uptake of glucose and promotes ATP synthesis. These data indicate that tPA has a central role in the detection and adaptation to conditions of metabolic stress. This is a new role for tPA with important clinical implications for the treatment of acute ischemic stroke patients.
The authors declare no conflict of interest.
Footnotes
This work was supported in part by National Institutes of Health Grant NS-079331 (to M Yepes), VA MERIT award BX000474 (to M Yepes), Bracco Diagnostics/RSNA Research Grant RR1230 (to A Nicholson), and P30NS055077 (Proteomics Core of the Emory Neuroscience NINDS Core Facilities grant).
References
- Erecinska M, Silver IA. ATP and brain function. J Cereb Blood Flow Metab. 1989;9:2–19. doi: 10.1038/jcbfm.1989.2. [DOI] [PubMed] [Google Scholar]
- Hardie DG. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25:1895–1908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter M, Riek U, Tuerk R, Schlattner U, Wallimann T, Neumann D. Dissecting the role of 5′-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase. J Biol Chem. 2006;281:32207–32216. doi: 10.1074/jbc.M606357200. [DOI] [PubMed] [Google Scholar]
- Hardie DG. Signal transduction: How cells sense energy. Nature. 2011;472:176–177. doi: 10.1038/472176a. [DOI] [PubMed] [Google Scholar]
- Krystosek A, Seeds NW. Plasminogen activator release at the neuronal growth cone. Science. 1981;213:1532–1534. doi: 10.1126/science.7197054. [DOI] [PubMed] [Google Scholar]
- Seeds NW, Williams BL, Bickford PC. Tissue plasminogen activator induction in Purkinje neurons after cerebellar motor learning. Science. 1995;270:1992–1994. doi: 10.1126/science.270.5244.1992. [DOI] [PubMed] [Google Scholar]
- Yepes M, Sandkvist M, Moore EG, Bugge TH, Strickland DK, Lawrence DA. Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. J Clin Invest. 112:1533–1540. doi: 10.1172/JCI19212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Wu J, Nicholson AD, Echeverry R, Haile WB, Catano M, et al. Tissue-type plasminogen activator regulates the neuronal uptake of glucose in the ischemic brain. J Neurosci. 2012;32:9848–9858. doi: 10.1523/JNEUROSCI.1241-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Echeverry R, Wu J, An J, Haile WB, Cooper DS, et al. Tissue-type plasminogen activator protects neurons from excitotoxin-induced cell death via activation of the ERK1/2-CREB-ATF3 signaling pathway. Mol Cell Neurosci. 2013;52:9–19. doi: 10.1016/j.mcn.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madani R, Hulo S, Toni N, Madani H, Steimer T, Muller D, et al. Enhanced hippocampal long-term potentiation and learning by increased neuronal expression of tissue-type plasminogen activator in transgenic mice. EMBO J. 1999;18:3007–3012. doi: 10.1093/emboj/18.11.3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Polavarapu R, She H, Mao Z, Yepes M. Tissue-type plasminogen activator and the low-density lipoprotein receptor-related protein mediate cerebral ischemia-induced nuclear factor-kappaB pathway activation. Am J Pathol. 2007;171:1281–1290. doi: 10.2353/ajpath.2007.070472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverry R, Wu J, Haile WB, Guzman J, Yepes M. Tissue-type plasminogen activator is a neuroprotectant in the mouse hippocampus. J Clin Invest. 2010;120:2194–2205. doi: 10.1172/JCI41722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfried NT, Gozal YM, Donovan LE, Herskowitz JH, Dammer EB, Xia Q, et al. Quantitative analysis of the detergent-insoluble brain proteome in frontotemporal lobar degeneration using SILAC internal standards. J Proteome Res. 2012;11:2721–2738. doi: 10.1021/pr2010814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belayev L, Busto R, Zhao W, Fernandez G, Ginsberg MD. Middle cerebral artery occlusion in the mouse by intraluminal suture coated with poly-L-lysine: neurological and histological validation. Brain Res. 1999;833:181–190. doi: 10.1016/s0006-8993(99)01528-0. [DOI] [PubMed] [Google Scholar]
- Keyt BA, Berleau LT, Nguyen HV, Bennett WF. Radioiodination of the active site of tissue plasminogen activator: a method for radiolabeling serine proteases with tyrosylprolylarginyl chloromethyl ketone. Anal Biochem. 1992;206:73–83. doi: 10.1016/s0003-2697(05)80013-2. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Academic Press Inc: San Diego, CA; 2001. [Google Scholar]
- Rao A, Steward O. Evidence that protein constituents of postsynaptic membrane specializations are locally synthesized: analysis of proteins synthesized within synaptosomes. J Neurosci. 1991;11:2881–2895. doi: 10.1523/JNEUROSCI.11-09-02881.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamurthy S, Ronnett GV. Developing a head for energy sensing: AMP-activated protein kinase as a multifunctional metabolic sensor in the brain. J Physiol. 2006;574:85–93. doi: 10.1113/jphysiol.2006.110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile WB, Wu J, Echeverry R, Wu F, An J, Yepes M. Tissue-type plasminogen activator has a neuroprotective effect in the ischemic brain mediated by neuronal TNF-alpha. J Cereb Blood Flow Metab. 2012;32:57–69. doi: 10.1038/jcbfm.2011.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicole O, Docagne F, Ali C, Margaill I, Carmeliet P, MacKenzie ET, et al. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nat Med. 2001;7:59–64. doi: 10.1038/83358. [DOI] [PubMed] [Google Scholar]
- Ronnett GV, Ramamurthy S, Kleman AM, Landree LE, Aja S. AMPK in the brain: its roles in energy balance and neuroprotection. J Neurochem. 2009;1:17–23. doi: 10.1111/j.1471-4159.2009.05916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Philos Trans R Soc Lond B Biol Sci. 1999;354:1155–1163. doi: 10.1098/rstb.1999.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi IY, Lee SP, Kim SG, Gruetter R. In vivo measurements of brain glucose transport using the reversible Michaelis-Menten model and simultaneous measurements of cerebral blood flow changes during hypoglycemia. J Cereb Blood Flow Metab. 2001;21:653–663. doi: 10.1097/00004647-200106000-00003. [DOI] [PubMed] [Google Scholar]
- Fowler JC. Glucose deprivation results in a lactate preventable increase in adenosine and depression of synaptic transmission in rat hippocampal slices. J Neurochem. 1993;60:572–576. doi: 10.1111/j.1471-4159.1993.tb03187.x. [DOI] [PubMed] [Google Scholar]
- Lochner JE, Honigman LS, Grant WF, Gessford SK, Hansen AB, Silverman MA, et al. Activity-dependent release of tissue plasminogen activator from the dendritic spines of hippocampal neurons revealed by live-cell imaging. J Neurobiol. 2006;66:564–577. doi: 10.1002/neu.20250. [DOI] [PubMed] [Google Scholar]
- Turnley AM, Stapleton D, Mann RJ, Witters LA, Kemp BE, Bartlett PF. Cellular distribution and developmental expression of AMP-activated protein kinase isoforms in mouse central nervous system. J Neurochem. 1999;72:1707–1716. doi: 10.1046/j.1471-4159.1999.721707.x. [DOI] [PubMed] [Google Scholar]
- Miller EJ, Li J, Leng L, McDonald C, Atsumi T, Bucala R, et al. Macrophage migration inhibitory factor stimulates AMP-activated protein kinase in the ischaemic heart. Nature. 2008;451:578–582. doi: 10.1038/nature06504. [DOI] [PubMed] [Google Scholar]
- McCullough LD, Zeng Z, Li H, Landree LE, McFadden J, Ronnett GV. Pharmacological inhibition of AMP-activated protein kinase provides neuroprotection in stroke. J Biol Chem. 2005;280:20493–20502. doi: 10.1074/jbc.M409985200. [DOI] [PubMed] [Google Scholar]
- Kuramoto N, Wilkins ME, Fairfax BP, Revilla-Sanchez R, Terunuma M, Tamaki K, et al. Phospho-dependent functional modulation of GABA(B) receptors by the metabolic sensor AMP-dependent protein kinase. Neuron. 2007;53:233–247. doi: 10.1016/j.neuron.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]