Abstract
Heat-shock protein 70 (Hsp70) protects against cerebral ischemia, which is attributed to its chaperone activity. However, recent reports also describe pro-inflammatory actions of Hsp70 via activation of Toll-like receptors (TLR). Using membrane-permeable transactivator of transcription (TAT)-Hsp70, we analyzed TAT-Hsp70-induced neuroprotection and its underlying mechanism after cerebral ischemia in mice. Infusion of TAT-Hsp70 reduced infarct volume and enhanced blood–brain barrier integrity on day 3 poststroke, when given no later than 12 hours. The latter was associated with reduction of microglial activation, although upregulation of pro-inflammatory TLR-2/4 was observed both in verum and in control animals. Nevertheless, protein abundance and nuclear translocation of downstream nuclear factor kappa B (NF-κB) as well as proteasomal degradation of the NF-κB regulator Ikappa B alpha (IκB-α) were significantly reduced by TAT-Hsp70. TAT-Hsp70-induced neuroprotection and functional recovery were restricted to 4 weeks only. However, TAT-Hsp70 provided an appropriate extracellular milieu for delayed intravenous transplantation of adult neural precursor cells (NPCs). Thus, NPCs that were grafted 28 days poststroke induced long-term neuroprotection for at least 3 months, which was not due to integration of grafted cells but rather due to paracrine effects of transplanted NPCs. Conclusively, TAT-Hsp70 ameliorates postischemic inflammation via proteasome inhibition, thus providing an appropriate extracellular milieu for delayed NPC transplantation and culminating in long-term neuroprotection.
Keywords: cerebral ischemia, Hsp70, neural precursor cells, neuroregeneration, proteasome, stroke
Introduction
Heat-shock proteins are stress responsive proteins that are upregulated upon multiple noxious events such as heat, inflammation, and ischemia.1, 2 Among these proteins, Hsp70 has been studied in a great number of disease models.1, 2 Heat-shock protein 70 has been reported to convey neuroprotection leading to reduction of both apoptotic cell injury and inflammation after hypoxic-ischemic brain injury.1, 2, 3, 4 The majority of this work, however, refers to the intracellular form of Hsp70. Recently, there is evidence that Hsp70 is secreted into the extracellular matrix where it initiates pro-inflammatory actions, albeit these results are still controversial.1, 5 Nevertheless, these issues have to be addressed critically, if Hsp70 is supposed to be regarded as a therapeutic approach for stroke in clinical settings.
Although beneficial effects of Hsp70 in experimental stroke have been shown before, these studies were often restricted to models using transgenic Hsp70-overexpressing mice or viral vectors,6, 7, 8 making Hsp70 not attractive for clinical application under these conditions. As proteins like Hsp70 do not pass the intact cell membrane or blood–brain barrier (BBB), we have previously fused Hsp70 to the transactivator of transcription (TAT) domain of the human immunodeficiency virus.9 Upon fusion of proteins like Hsp70 to TAT, high transduction rates of both cells and tissues are ensured.10, 11 As such, intravenous application of TAT-Hsp70 during early reperfusion in mice induced neuroprotection via anti-apoptotic effects of the chaperone and via enhanced neuroregeneration.9 The latter was limited to increased numbers of endogenous neural precursor cells (NPCs) that expressed an immature neuronal phenotype, which were unlikely to significantly contribute to tissue recovery. However, the mechanisms underlying TAT-Hsp70-induced neuroprotection and whether the increased survival of NPCs may be used for therapeutic approaches remained unknown at that time.
In the present study, we show that TAT-Hsp70 reduces postischemic brain injury via anti-inflammatory actions within the rodent brain, albeit neuroprotection is limited to an observation period of 4 weeks only. Rather, the reduced initial anti-inflammatory response due to TAT-Hsp70 offers an attractive extracellular milieu, in which late intravenous transplantation of subventricular zone (SVZ)-derived NPCs on day 28 ensures long-term neuroprotection for as long as 3 months poststroke. A combinational therapeutic approach using early intravenous TAT-Hsp70 infusion to provide an appropriate cellular environment for late transplantation of NPCs might therefore be a feasible strategy in experimental stroke treatment.
Materials and Methods
Animals and Experimental Groups
Experimental procedures were in accordance with the National European Institutes of Health guidelines for the care and use of laboratory animals and approved by local authorities (Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen, Germany). For all experiments, male C57BL/6 mice (11 to 13 weeks, 25 to 27 g; Charles River, Germany) were used. Mice were randomized to different treatment groups and treatment was masked to the experimenters. Animals underwent a defined treatment paradigm (Table 1), which included systemic intravenous infusion of TAT-Hsp70 (1 nmol) or TAT-HA (1 nmol, control) during the beginning of reperfusion (0 hour) or thereafter, i.e., 6 hours, 12 hours, or 24 hours after induction of stroke. As previous work of our group observed significant modulation of postischemic neuroregeneration due to additional injections of TAT-Hsp70,9 mice surviving 28 days or 84 days received additional injections of TAT-Hsp70 or TAT-HA (1 nmol each) on day 14 poststroke. For mimicking clinically relevant situations, some animals underwent systemic thrombolysis (rt-PA; 10 mg/kg body weight) or NaCl infusion as control during reperfusion followed by TAT-Hsp70/TAT-HA infusion 12 hours poststroke. All injections have been performed via cannulation of the femoral vein. Animals surviving for 3 days after induction of stroke showed survival rates of 100% for each experimental condition. Survival rates for 28-day survivors were 83.3% (TAT-HA) and 76.9% (TAT-Hsp70). Detailed survival rates for mice surviving 84 days were as follows: 100% (TAT-HA), 84.6% (TAT-Hsp70), 91.6% (TAT-HA+NPCs), 91.6% (TAT-Hsp70+NPCs), 100% (TAT-HA+NaCl), and 100% (TAT-Hsp70+NaCl). Numbers of mice used for statistical analysis are given in the corresponding figure legends. Assessment of cell proliferation was performed on animals surviving either 28 days or 84 days via 11 consecutive intraperitoneal injections of 5-bromo-2-desoxyuridine (BrdU; 50 mg/kg body weight) on days 8 to 18.
Table 1. Experimental treatment paradigm.
| TAT-Hsp70 (reperfusion) | TAT-Hsp70 (6 hours) | TAT-Hsp70 (12 hours) | TAT-Hsp70 (24 hours) | TAT-HA (reperfusion) | TAT-HA (6 hours) | TAT-HA (12 hours) | TAT-HA (24 hours) | |
|---|---|---|---|---|---|---|---|---|
| No additional Treatment | Survival 3 days | Survival 3 days | Survival 3, 28, 84 days | Survival 3 days | Survival 3 days | Survival 3 days | Survival 3, 28, 84 days | Survival 3 days |
| + rt-PA (reperfusion) | ND | ND | Survival 3 days | ND | ND | ND | Survival 3 days | ND |
| + NaCl (reperfusion) | ND | ND | Survival 3 days | ND | ND | ND | Survival 3 days | ND |
| + NPCs (day 28) | ND | ND | Survival 84 days | ND | ND | ND | Survival 84 days | ND |
| + NaCl (day 28) | ND | ND | Survival 84 days | ND | ND | ND | Survival 84 days | ND |
Hsp70, heat-shock protein 70; ND, not determined; TAT, transactivator of transcription.
Assessment of the therapeutic time window for treatment with TAT-Hsp70 was done by injecting TAT-Hsp70 (1 nmol) or TAT-HA (1 nmol, control) intravenously into the femoral vein. TAT fusion proteins were given either during the beginning of reperfusion (0 hour) or at 6 hours, at 12 hours or at 24 hours after induction of stroke. Please note that animals surviving for 28 days or 84 days received additional injections of TAT-Hsp70/TAT-HA on day 14 poststroke. Whereas the majority of animals received no additional treatments, some animals received intravenous thrombolysis via rt-PA (10 mg per kg body weight; NaCl served as control) during the beginning of reperfusion before treatment with fusion proteins at 12 hours poststroke. Other animals first received injections with TAT-Hsp70/TAT-HA at 12 hours and on day 14 before intravenous transplantation of neural precursor cells (NPCs, 1 × 106 cells per transplantation; NaCl served as control) on day 28 poststroke.
Preparation of Recombinant Proteins
TAT-Hsp70 and TAT-HA, which are also included in the TAT-Hsp70 construct serving as control, were prepared under native conditions as described before.12 Briefly, the recombinant genes were expressed in Escherichia coli strain BL21 (DE3) pLysS (Novagen, Madison, WI, USA) and proteins were isolated in 10 mmol/L Tris, pH 10, 20% glycerol, 274 mmol/L NaCl, 0.1% Pluronic, 0.02% Tween-80. Bacterial debris was removed by centrifugation and the cell extracts were purified by affinity chromatography using Ni-tris-carboxymethyl-ethylene-diamine.13 Protein was eluted by stepwise addition of binding buffer containing increasing concentrations of imidazole. The column eluate was purified from imidazole by gel filtration (SephadexTM G-25 M, GE Healthcare Bio-Sciences AB, Freiburg, Germany). According to this procedure, TAT-Hsp70 is highly stable after cell transduction as no protein degradation is detected 24 hours after protein application, suggesting that the intracellular half-life time is at least a few days.12
Adult Subventricular Zone-Derived Neural Precursor Cells
Neural precursor cells were isolated from the SVZ of 6 to 8-week old male transgenic green fluorescence protein positive (GFP+) animals (C57BL/6-Tg ACTB-EGFP, 1Osb/J; JAX Laboratory, Bar Harbor, ME, USA) as described.14 The SVZ was microdissected under stereomicroscopic control (Zeiss, Jena, Germany) and minced into small pieces, followed by mechanical trituration and dissociation into a single-cell suspension. Thereafter, cells were cultured in serum-free basic DMEM (Dulbecco's Modified Eagle Medium)-F12 (PAA, Pasching, Austria) supplemented with epidermal growth factor (2 μg/mL), basic fibroblast growth factor (2 μg/mL), and penicillin–streptomycin (Invitrogen, Darmstadt, Germany). Cells were incubated with 5% CO2 at 37°C. The growth factors were supplemented every 2 to 3 days and cells were passaged via accutase (Invitrogen) digestion for 30 minutes at 37°C with a re-suspension every 10 minutes. Thereafter, cells were centrifuged and resuspended in conditioned medium. Neurosphere passages were done every 7 to 10 days and cells used for transplantation were derived from passage 3 to 8. These NPCs were intravenously infused (1 × 106 cells in 100 μL saline; saline served as control) via femoral vein cannulation 28 days after induction of stroke.
Induction of Transient Focal Cerebral Ischemia
Cerebral ischemia was induced using middle cerebral artery occlusion.15 Animals were anesthetized (0.8% to 1.5% isoflurane, 30% O2, remainder N2O), and rectal temperature was maintained at 36.5°C to 37.0°C employing a feedback-controlled heating system under continuous control of blood flow changes by means of a laser Doppler flow system (Perimed, Järfälla, Sweden). Occlusion of the left middle cerebral artery was achieved using a 7–0 silicon-coated nylon monofilament (180 μm tip diameter; Doccol, Sharon, MA, USA), which was withdrawn after 60 minutes to induce transient focal cerebral ischemia. Laser Doppler flow recordings continued for additional 15 minutes to monitor appropriate reperfusion.
Analysis of PostStroke Brain Injury and Immunohistochemistry
Infarct volumes were analyzed on day 3 via staining with 2,3,5-triphenyltetrazolium chloride (2%), followed by a computer-based analysis of infarct volumes using the freely available software ImageJ. Postischemic brain edema was measured as the increase of ipsilateral hemispheric volume in comparison to the contralateral hemisphere.
For immunohistochemical analysis, animals were intraperitoneally injected with chloral hydrate (420 mg/kg body weight) and transcardially perfused with 4% paraformaldehyde at the time points given. The brains were removed, shock-frozen in liquid nitrogen, and 16-μm thick coronal cryostat sections were prepared. Quantitative analyses for immunohistochemical stainings were performed defining regions of interest within the ischemic basal ganglia. Stereotactic coordinates were 0.14 mm anterior, 2.5 to 3.25 mm ventral, and 1.5 to 2.25 mm lateral from bregma. Three sections per animal and regions of interest were used.
For quantitative analysis of proliferating BrdU+ cells, sections were exposed to blocking solution and subsequently stained with a monoclonal mouse anti-BrdU antibody (1:400; Roche, Mannheim, Germany) or a monoclonal rat anti-BrdU antibody (1:400; Abcam, Cambridge, UK). As quenching of fluorescence signal of GFP+ NPCs occurs during section processing, a polyclonal rabbit anti-GFP antibody (1:2500; Abcam) was used to enhance GFP signal intensity.
Differentiation analysis of GFP+ or BrdU+ cells was done using double staining against BrdU/GFP and a polyclonal goat anti-doublecortin antibody (1:50; Santa Cruz Biotechnology, Heidelberg, Germany), a polyclonal rat anti-glial fibrillary acidic protein antibody (1:500; Zymed, Darmstadt, Germany), a monoclonal mouse anti-CNPase antibody (1:400; Millipore, Billerica, MA, USA), a monoclonal mouse anti-NeuN antibody (1:1000; Millipore, Oxfordshire, UK) or a monoclonal mouse anti-nestin antibody (1:500; Millipore). All antibodies were incubated for 18 hours at 4°C. Thereafter, the sections were incubated for 1 hour at room temperature. For double staining with BrdU, the following secondary antibodies were used: goat anti-mouse Cy-3 (1:400; Dianova, Hamburg, Germany) or goat anti-rat Alexa 594 (1:400; Dianova) for BrdU staining, goat anti-rat Alexa 488 (1:250; Molecular Probes, Darmstadt, Germany) or donkey anti-goat Alexa 488 (1:250; Molecular Probes) for glial fibrillary acidic protein or doublecortin staining, and goat anti-mouse Alexa 488 (1:100; Jackson Immunoresearch, Suffolk, UK) for CNPase staining and goat anti-mouse Alexa 488 (1:400; Molecular Probes) for NeuN staining. For double staining with GFP, the secondary antibodies were as follows: goat anti-mouse Cy-3 (1:100; Jackson ImmunoResearch) for NeuN, CNPase, and nestin staining as well as goat anti-rat Cy-3 antibody (1:200, Abcam) for glial fibrillary acidic protein staining. Double staining against doublecortin was done using a donkey anti-goat Cy-3 secondary antibody (1:500; Dianova). Photos for differentiation analysis with subsequent 3D-reconstruction were made using a Zeiss microscope (Germany) equipped with an ApoTome and the corresponding AxioVision software (Zeiss).
Further assessment of brain injury was performed on day 3 by means of terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling as described before.14 Analysis of brain injury on days 3, 28, and 84 was performed by determination of neuronal density, i.e., after quantitative analysis of NeuN+ cells within defined regions of interest as stated above. Assessment of microglial activation was performed using a rat biotin-conjugated lectin Ib4 (1:100; Vector, Peterborough, UK). Cell numbers for each specific staining were recalculated and are given as total amount of cells per square millimeter.
Evans Blue Extravasation
Mice received intravenous bolus injections of 2% Evans Blue (2 mL/kg body weight) via tail vein cannulation 22 hours poststroke to assess the integrity of the BBB.16 Animals were killed 2 hours later and transcardially perfused with phosphate-buffered saline. The left (ischemic) hemispheres were weighed, homogenized in 2 mL of 50% trichloroacetic acid, and centrifuged at 10,000 r.p.m. for 20 minutes. The extracted Evans Blue dye was further diluted with ethanol, and the fluorescence signal was measured with a luminescence spectrophotometer (exc.=620 nm, em.=680 nm). An external standard (62.5 to 500 ng/mL) was used for calculation of Evans Blue contents, which is given as (μg) Evans Blue per (g) tissue.
Assessment of PostStroke Functional Recovery
Motor coordination deficits were analyzed using the rotarod, tightrope, and the corner turn test.17 Behavioral tests were performed in a masked manner. One day before induction of stroke, animals were trained before the beginning of the actual tests on day 7, 14, 28, 56, and 84. Using the rotarod test, animals were put on an accelerating treadmill (TSE Systems, Bad Homburg, Germany; 3 cm diameter) with an accelerating speed of 4 to 40 r.p.m. The maximum speed was achieved after 260 seconds, and maximum testing time was 300 seconds. The time until animals dropped was registered and statistically analyzed. For the tightrope test, animals were placed on a 60 cm long rope grasping the string with their forepaws. Maximum test time was 60 seconds, and results were scored from 0 (minimum) to 20 (maximum) according to a validated score.17 Rotarod and tightrope tests were performed twice at each time point and means were calculated. For the corner turn test, two vertical boards were attached at one side with an angle of 30°, and each mouse was tested for the side chosen over 10 trials per test day. Whereas healthy animals leave the corner without side preference, mice suffering from stroke preferentially turn to the left, non-impaired body side.18, 19 The laterality index was calculated according to the following formula: number of right turns/10.
For assessment of cognitive deficits, a modified water maze test was performed on days 26 to 28, days 54 to 56, and on days 82 to 84 poststroke as has been described.20 A transparent plexiglass platform (11 × 11 cm) was submerged in a swimming pool with the top located 1 cm below the water surface. A full experiment consisted of 24 trials per time point; four trials in the morning and four trials in the afternoon of each test day with a maximum testing time of 90 seconds per trial. For trials 1 to 16 and 21 to 24 the platform was always located in the center of the same quadrant and animals were always put into the same of one of the remaining quadrants. For assessment of new learning strategies (task switch), the platform was set into another quadrant for the trials 17 to 20. Thereafter, the platform was relocated at its original position for the remaining four trials (21 to 24). The time needed to reach the platform was statistically analyzed using a computer-based system (TSE Systems). Data are given as means of four trials each.
Western Blotting and NF-κB p65 Activation Kit
Western blotting was performed on day 3 after induction of stroke in the left (ischemic) hemispheres of individual mice, which were complemented with lysis buffer (50 mmol/L Tris, pH 8.0, 150 mmol/L NaCl, 1% Triton X-100, and protease inhibitors), homogenized, and centrifuged. Contralateral right (non-ischemic) hemispheres served as control. Lysates were centrifuged and supernatants were used for SDS-PAGE. Equal amounts of protein (60 μg) were diluted in 6 × sample buffer, boiled, and loaded onto 12% polyacrylamide gels. Proteins were transferred onto Polyvinylidene difluoride membranes, which were immersed in blocking solution (5% milk in TBS-T (0.1% Tween 20+TBS); 1 hour at room temperature) and then incubated overnight at 4°C with a rabbit polyclonal antibody against TLR-2 (1:1000, Santa Cruz Biotechnology), a rabbit polyclonal antibody against TLR-4 (1:1000, Santa Cruz Biotechnology), a mouse monoclonal antibody against NF-κB p65 (1:200, Santa Cruz Biotechnology), or a mouse monoclonal antibody against Ikappa B alpha (IκB-α) (1:1000, Santa Cruz Biotechnology). Thereafter, membranes were incubated with a peroxidase-coupled goat anti-rabbit or goat anti-mouse secondary antibody (1:2000; Santa Cruz Biotechnology), washed several times, immersed in enhanced chemiluminescence solution and exposed to enhanced chemiluminescence-Hyperfilm (Amersham, Hamburg, Germany). Membranes were scanned and used for densitometric quantitative analysis of protein abundance referred to actin expression.
For assessment of NF-κB translocation (i.e., activation of NF-κB), an NF-κB activation kit (FIVEphoton Biochemicals, San Diego, CA, USA) was used, thus discriminating between both cytosolic and nuclear fractions of NF-κB p65. After the manufacturer's protocol, the kit was performed in the left (ischemic) brain hemispheres on day 3 poststroke within animals that had been treated with either TAT-HA or TAT-Hsp70 at 12 hours poststroke.
Enzyme Linked Immunosorbent Assay for Measurement of Growth Factors
Detection of selected growth factors was done in the ischemic hemispheres of mice on day 84 after induction of stroke. Levels of growth factors such as vascular endothelial growth factor (R&D Systems, Minneapolis, MN, USA), nerve growth factor (Promega, Mannheim, Germany), brain-derived neurotrophic factor (Promega), glial cell line-derived neurotrophic factor (Promega), basic fibroblast growth factor (R&D Systems), and epidermal growth factor (R&D Systems) were measured using commercial mouse ELISA kits according to the manufacturer's instructions.
Measurement of Proteasome Activity
Proteasome activity was determined in brain homogenates of the left ischemic hemispheres on day 3 after induction of cerebral ischemia. Hemispheres were lysed using a buffer containing 100 mmol/L Tris-HCl, 145 mmol/L NaCl, 10 mmol/L EDTA, and 0.5% Triton X-100 at pH 7.5 as has been described before.21 The substrate Suc-LLVY-AMC (50 μmol/L; Bachem, Heidelberg, Germany) was used for detection of the chymotrypsin-like activity of the proteasome. A volume of 200 μL of lysis buffer was incubated with 90 μL of a buffer containing 50 mmol/L Tris, 20 mmol/L KCl, 1 mmol/L magnesium acetate, 2 mmol/L dithiothreitol, 1 mmol/L leupeptin, 1 μg/mL aprotinin (Sigma-Aldrich, Taufkirchen, Germany) and 1 mmol/L PMSF (Merck, Darmstadt, Germany). As Suc-LLVY-AMC is not specific for determination of proteasome activity,21 some samples/lysates were given the proteasome inhibitor MG-132 (1 μmol/L; Sigma-Aldrich) immediately before the beginning of the measurement, as previously described.21 Determination of substrate cleavage was performed at 37°C in a fluorescence microtiter plate reader with λexc.=355 nm and λem.=460 nm; AMC (Sigma-Aldrich) was used for initial calibration. Proteasome activities are given as arbitrary units per minute per milligrams of protein. The Bradford assay was used for measurement of protein content.
For assessment of direct effects of TAT fusion proteins on the proteasome, the chymotrypsin-like activity of isolated 20S proteasomes was estimated by hydrolysis of Suc-LLVY-AMC as described previously.22 Briefly, proteasomes (100 ng) were pre-incubated with either TAT-HA or TAT-Hsp70 (0.1, 0.5, and 1 μmol/L each) in 20 mmol/L Tris and 1 mmol/L EDTA (pH 7.2) for 10 minutes at room temperature. Control proteasomes received phosphate-buffered saline instead of fusion proteins. The measurement was started by addition of 50 μmol/L Suc-LLVY-AMC in the same buffer and incubated for 20 minutes at 37°C.
Statistics
All data are given as mean±standard deviation (s.d.). For comparison between two groups, the student t-test was used, whereas for comparison between multiple groups a one-way analysis of variance followed by the Tukey's post hoc test was performed. A P value of <0.05 was considered to be statistically significant.
Results
Delayed Intravenous Infusion of TAT-Heat-Shock Protein 70 Protects Against Cerebral Ischemia
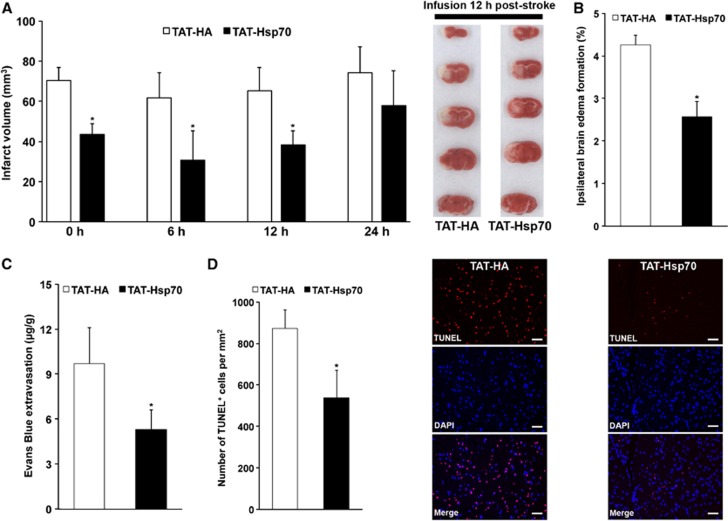
We have previously shown that infusion of TAT-Hsp70 immediately after reperfusion is neuroprotective.9 However, such early therapeutic interventions are unlikely under clinical settings. We therefore analyzed the therapeutic time window for TAT-Hsp70-induced neuroprotection after middle cerebral artery occlusion in mice. Analysis of infarct volumes on day 3 after stroke revealed an acute neuroprotection when TAT-Hsp70 was given no later than 12 hours poststroke (Figure 1A). We observed infarct volumes of 38.3±6.9 mm3 in mice that had been treated with TAT-Hsp70 12 hours poststroke as compared with infarct volumes of 65.2±11.7 mm3 in control animals that had been treated with TAT-HA.
Figure 1.
Delayed intravenous infusion of TAT-heat-shock protein 70 (Hsp70) is neuroprotective. (A) Analysis of infarct volumes on day 3 after induction of stroke using 2,3,5-triphenyltetrazolium chloride (TTC) staining (n=7 per condition). TAT-Hsp70 or TAT-HA (control) was intravenously given during the beginning of reperfusion (0 hour), 6 hours, 12 hours, or 24 hours after stroke onset. TTC (2,3,5-triphenyltetrazolium chloride) stainings depicted represent typical orientation of ischemic lesions when TAT-Hsp70 or TAT-HA were given 12 hours poststroke. (B) Measurement of brain edema from mice used for (A) by subtracting the increase of ipsilateral hemispheric volume in comparison to the contralateral hemisphere. TAT-Hsp70 or TAT-HA was given 12 hours poststroke. (C) Integrity of blood–brain barrier on day 3 by means of Evans Blue extravasation. Measurements were performed on day 3 poststroke on animals that had received TAT-Hsp70 or TAT-HA 12 hours after stroke onset (n=6 per condition). (D) Assessment of brain injury on day 3 poststroke via TUNEL staining. Mice (n=7 per condition) received intravenous infusion of TAT-Hsp70 or TAT-HA 12 hours poststroke. *Significantly different from controls, P<0.05. Scale bar, 50 μm. DAPI, 4,6-diamidino-2-phenylindole; TAT, transactivator of transcription; TUNEL, TdT-mediated dUTP nick end labeling.
In line with the reduced infarct volumes, further analysis of postischemic brain injury yielded reduced brain edema in mice treated with TAT-Hsp70 (Figure 1B). Integrity of the BBB was also enhanced in those animals that had been treated with the anti-apoptotic fusion protein when compared with control animals (Figure 1C). Analysis of TdT-mediated dUTP nick end labelling positive cells on day 3 showed reduced numbers of TdT-mediated dUTP nick end labelling positive cells in TAT-Hsp70-treated mice (Figure 1D), further suggesting that TAT-Hsp70 is neuroprotective when given no later than 12 hours poststroke. In view of the high clinical relevance of a delayed protein delivery, further experiments evaluating the therapeutic potential of TAT-Hsp70 and its underlying mechanisms were therefore performed using the 12-hour treatment paradigm.
TAT-Heat-Shock Protein 70 Reduces rt-PA-Mediated Brain Toxicity
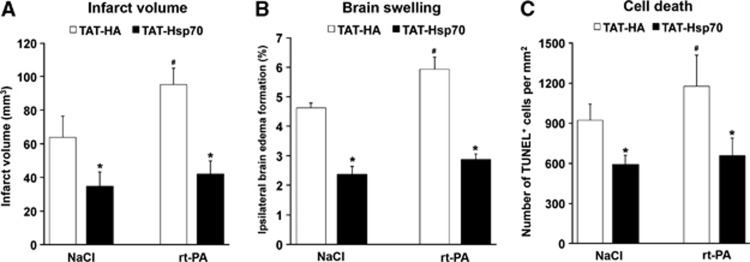
In order to assess the therapeutic potential of TAT-Hsp70 under clinically relevant situations, mice receiving thrombolytic rt-PA immediately after reperfusion were intravenously treated with TAT-Hsp70 12 hours poststroke. Using this experimental paradigm, application of TAT-Hsp70 resulted in significant reduction of infarct volume in animals that were treated with either NaCl or rt-PA (Figure 2A). Noteworthy, TAT-Hsp70 diminished rt-PA-induced brain injury on day 3 poststroke (Figure 2A). In line with the reduction of infarct volume, application of TAT-Hsp70 resulted in reduced brain swelling (Figure 2B) and reduced DNA fragmentation evaluated by TdT-mediated dUTP nick end labelling staining (Figure 2C). These data suggest that TAT-Hsp70 should even be neuroprotective when given after systemic thrombolysis.
Figure 2.
TAT-heat-shock protein 70 (Hsp70) reduces rt-PA-mediated brain toxicity. Animals were intravenously treated with either rt-PA (10 mg/kg body weight (BW)) or NaCl (control) at the beginning of reperfusion followed by intravenous infusion of TAT-Hsp70 or TAT-HA 12 hours after stroke onset. (A) Analysis of infarct volumes on day 3 after induction of stroke using 2,3,5-triphenyltetrazolium chloride (TTC) staining (n=8 per condition). (B) Measurement of brain edema from mice used for (A) by subtracting the increase of ipsilateral hemispheric volume in comparison to the contralateral hemisphere. (C) Analysis of brain injury using TUNEL staining on day 3 poststroke. Animals (n=6 per condition) were treated as stated above. *Significantly different from controls, P<0.05. #Significantly different from mice treated with both NaCl and TAT-HA. TAT, transactivator of transcription; TUNEL, TdT-mediated dUTP nick end labeling.
TAT-Heat-Shock Protein 70 Induces Longer-Lasting but not Long-Term Neuroprotection
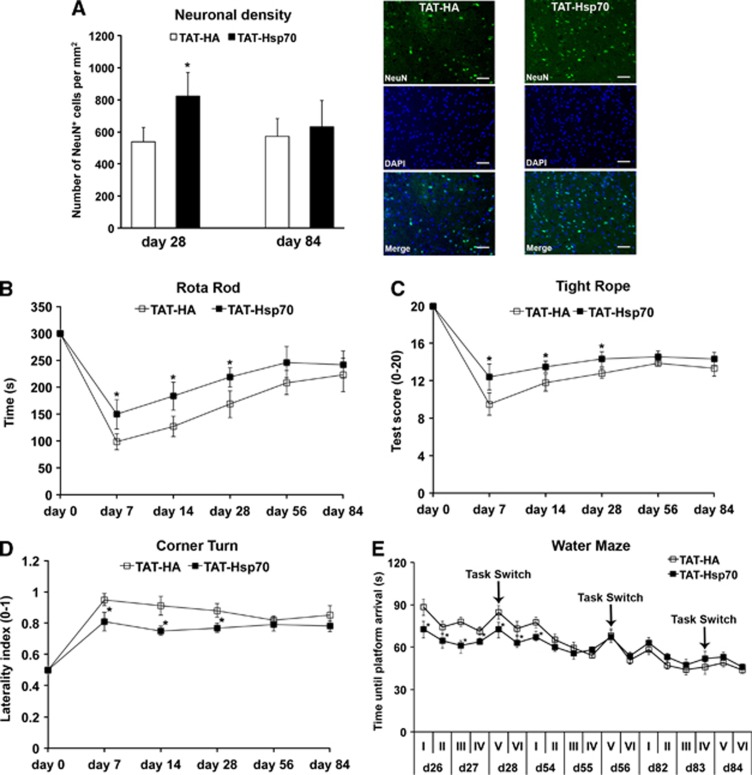
Intravenous delivery of TAT-Hsp70 induced neuroprotection that persisted until day 28 when initiated during early reperfusion (12 hours after middle cerebral artery occlusion) and repeated once on day 14 after stroke (Figure 3A). The latter was associated with reduced functional impairment (Figures 3B–E). Noteworthy, differences in water maze test performance were not attributed to impaired motor coordination as average swimming speed of all runs between TAT-HA controls and TAT-Hsp70-treated mice did not differ from each other (17.2±3.1 cm/second vs. 15.8±4.7 cm/second). However, TAT-Hsp70 treatment did not yield sustained neuroprotection with functional improvement on day 84 poststroke (Figure 3). Neuronal density on day 84 was 572±111 in TAT-HA-treated animals as compared with 632±162 per mm2 in mice treated with TAT-Hsp70. Behavioral data revealed that therapeutic effects because of TAT-Hsp70 treatment disappeared between day 56 and day 84 poststroke (Figures 3B–E).
Figure 3.
Analysis of long-term neuroprotection and functional impairment after stroke. (A) Neuronal density determined on day 28 (n=9 to 10 per condition) and on day 84 (n=11 per condition) in animals that had received intravenous injections of TAT-heat-shock protein 70 (Hsp70) or TAT-HA 12 hours poststroke plus additional injections on day 14. Representative photos taken for day 28 poststroke from animals that had received either TAT-Hsp70 or TAT-HA. (B) Motor coordination (n=11 per condition) as assessed using the rotarod test with a maximum test time of 300 seconds on the rod. (C) Further analysis of postischemic motor coordination by means of the tightrope assay (n=11 per condition), where animals were scored from 0 (minimum) to 20 (maximum). (D) Corner turn test (n=11 per condition) depicting the postischemic laterality index (0 to 1) of test mice. High values of laterality indices refer to more pronounced postischemic injury. (E) Assessment of cognitive impairment in stroke mice was performed using a modified water maze test (n=11 per condition) with a maximal testing time of 90 seconds per trial. Animals that did not reach the platform were scored 90 seconds. A total of 24 trials per time point was performed. Four trials were always grouped as one run, which is given in roman numbers I to VI per time point (i.e., days 26 to 28, days 54 to 56, and days 82 to 84). The platform was always located at the same position except for run V, where the position was changed (‘task switch'). The platform was placed back on its original position for the final run VI of each time point. *Significantly different from controls, P<0.05. Scale bar, 50 μm. DAPI, 4,6-diamidino-2-phenylindole; TAT, transactivator of transcription.
TAT-Heat-Shock Protein 70 Induces Acute Neuroprotection via Anti-Inflammatory Actions
As extracellular Hsp70 has been shown to also induce pro-inflammatory cell cascades,1, 5 we wondered how treatment with TAT-Hsp70 affects postischemic microglial activation. Analysis of Ib4+ cells on day 3 poststroke (Figure 4A) revealed significantly reduced Ib4+ cells within the ischemic striatum of mice treated with TAT-Hsp70 (204.0±62.1/ mm2) as compared with controls (338.0±37.2/ mm2). Although numbers of Ib4+ cells declined over time in both experimental groups, the amount of Ib4+ cells was still significantly reduced in TAT-Hsp70-treated mice as compared with controls (16.3±3.6/ mm2 vs. 41.2±6.8/ mm2). We observed no Ib4+ cells in any experimental group on day 84 poststroke (data not shown).
Figure 4.
TAT-heat-shock protein 70 (Hsp70) induces acute neuroprotection via anti-inflammatory actions. (A) Quantitative analysis of microglial activation within the ischemic striatum using Ib4 staining on day 3 after induction of stroke (n=7). Photos depicted show typical representation of Ib4 staining on day 3 in mice treated with either TAT-HA or TAT-Hsp70. (B) Western blot analysis (n=5) in lysates derived from both contralateral (‘No ischemia') and ipsilateral (‘Ischemia') hemispheres from mice treated with either TAT-HA or TAT-Hsp70. Western blots were performed on day 3 poststroke against Toll-like receptors 2/4 (TLR-2/TLR-4), the nuclear factor kappa B p65 and against Ikappa B alpha. (C) Densitometric analyses from western blots obtained in (B). (D) Measurement of proteasomal chymotrypsin-like activity (n=5 per condition) in left ischemic brain homogenates 3 days after stroke using Suc-LLVY-AMC as substrate. As Suc-LLVY-AMC is not a specific proteasome substrate, some samples were incubated with the proteasome inhibitor MG-132 (1 μmol/L) immediately before the recording of substrate cleavage to verify the measurement of proteasomal activity. Data are given as arbitrary fluorescence units per minute per mg of total protein. *Significantly different from controls, P<0.05. Scale bar, 40 μm. DAPI, 4,6-diamidino-2-phenylindole; TAT, transactivator of transcription.
Hsp70 is a ligand of the Toll-like receptors (TLR) 2/4, which are located on the cell membrane inducing pro-inflammatory cellular pathways via NF-κB signaling upon activation.23 Noteworthy, protein abundance of both TLR-2 and TLR-4, which were upregulated upon cerebral ischemia, did not differ between both treatment groups (Figures 4B and 4C). Analysis of expression patterns of NF-κB p65, however, revealed reduced protein abundance in mice treated with TAT-Hsp70, which was associated with increased protein abundance of the physiologic NF-κB inhibitor IκB-α in these animals (Figures 4B and 4C). As NF-κB p65 expression patterns do not necessarily reflect activation states of the transcription factor, nuclear translocation of NF-κB p65 (indicating activation) was performed on day 3 poststroke. Western blotting of cytosolic/nuclear fractions of ischemic brain homogenates revealed significantly enhanced detection levels of NF-κB p65 in nuclear fractions of TAT-HA-treated mice, whereas NF-κB p65 levels of TAT-Hsp70-treated mice were essentially restricted to cytosolic fractions (Supplementary Figure 1).
Regulation of NF-κB by means of IκB-α depends on proteasomal degradation.24 As such, we analyzed whether TAT-Hsp70 affected proteasomal activity within the ischemic brain. Activity of the chymotrypsin-like activity of the proteasome was significantly reduced on day 3 poststroke in mice that had been treated with TAT-Hsp70 (Figure 4D). However, proteasomal activity of isolated 20S proteasomes was not directly affected by TAT-Hsp70 (not shown). These data suggest that TAT-Hsp70 reduces postischemic proteasomal activity affecting the NF-κB pathway, which is associated with reduced microglial activation.
TAT-Heat-Shock Protein 70 Provides a Favorable PostIschemic Milieu for Systemic Neural Precursor Cells Transplantation
As TAT-Hsp70-induced anti-inflammatory actions (Figure 4) associated with longer-lasting neuroprotection (Figure 3), we wondered whether the therapeutic potential of TAT-Hsp70 might be increased when combined with transplantation of adult NPCs. In this sense, systemic transplantation of NPCs is well known to induce neuroprotection.14, 25, 26 However, cell-based strategies are hampered because of low survival rates of transplanted NPCs.14, 20 We therefore hypothesized that the favorable anti-inflammatory and anti-apoptotic milieu induced by TAT-Hsp70 delivery might enhance the success of NPC transplantation, even when cells are transplanted at later stages of the disease.
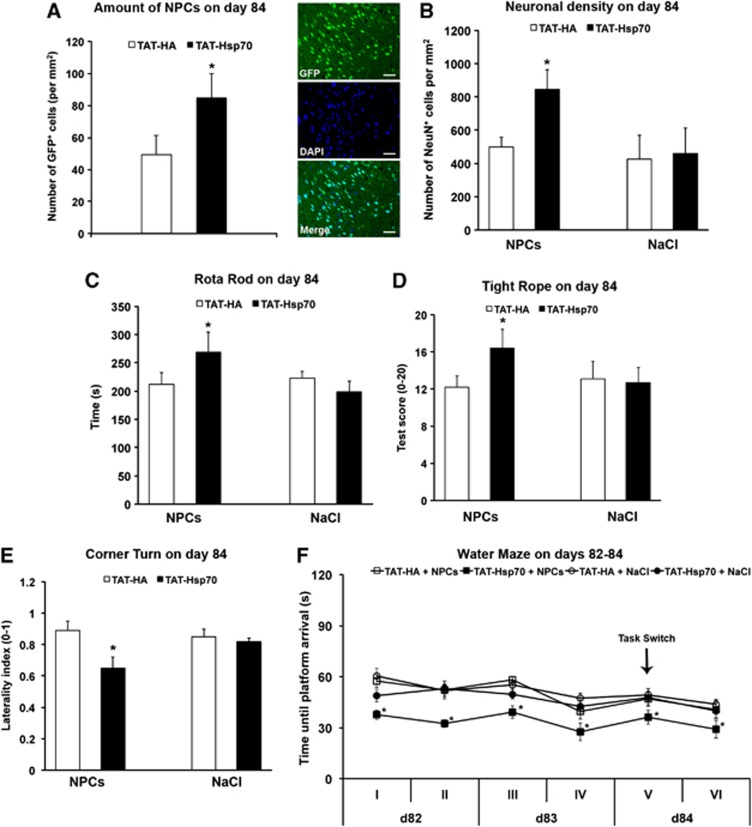
Hence, SVZ-derived GFP+ NPCs were systemically (intravenously) transplanted on day 28 in mice, which had been treated intravenously with either TAT-Hsp70 or TAT-HA at 12 hours and on day 14 poststroke as described above. Tracking of GFP+ cells on day 84 within the ischemic hemisphere revealed significantly increased numbers of NPCs in mice that had been treated with the TAT-Hsp70 fusion protein as compared with mice, which had been treated with TAT-HA (Figure 5A). We observed 49.2±12.3 GFP+ NPCs within the ischemic hemisphere of TAT-HA-treated mice as compared with 84.7±15.1 cells per mm2 in animals treated with TAT-Hsp70. In order to assess ‘maximal' intracerebral NPC numbers that can be achieved under the experimental protocol used, NPCs were also injected on day 28 in otherwise non-treated stroke mice. To exclude relevant secondary cell death of grafted cells, mice were killed 72 hours after transplantation and the amount of transplanted intracerebral NPCs was analyzed on day 31 poststroke. Thus, we observed 154.4±31.9 NPCs/ mm2, suggesting a significant decline of intracerebral NPC numbers over time until day 84, which is significantly attenuated by treatment with TAT-Hsp70 (Figure 5A).
Figure 5.
TAT-heat-shock protein 70 (Hsp70) provides favorable extracellular milieu for postischemic neural precursor cell (NPC) transplantation. Mice received TAT-Hsp70 or TAT-HA during reperfusion plus additional infusion on day 14 followed by intravenous/systemic transplantation of 1 × 106 green fluorescence protein positive (GFP+) NPCs on day 28 poststroke. (A) Assessment of numbers of GFP+ NPCs (n=12 per condition) within the ischemic hemisphere on day 84 for animals that had been treated with either TAT-Hsp70 or TAT-HA. Photo depicted shows representative orientation of NPCs within the ischemic basal ganglia on day 84 in mice that had been treated with TAT-Hsp70. (B) Analysis of postischemic brain injury (n=11 to 12) on day 84 using NeuN staining. Animals received intravenous infusion of TAT-Hsp70 or TAT-HA as stated above plus additional injection of NPCs or NaCl (control) on day 28 poststroke. (C–E) Motor coordination tests (n=11 to 12) were performed on day 84 using the rotarod, the tightrope, and the corner turn test. For the rotarod test, maximum test time was 300 seconds, whereas for the tightrope test, animals were scored from 0 (minimum) to 20 (maximum). Using the corner turn test, the postischemic laterality index (0 to 1) for the four different treatment groups was determined on day 84. High values of laterality indices refer to more pronounced postischemic injury. (F) Assessment of cognitive impairment in stroke mice (n=11 to 12) was performed using a modified water maze test on days 82 to 84 with a maximal testing time of 90 seconds per trial. Animals that did not reach the platform were scored 90 seconds. A total of 24 trials were performed, and four trials were always grouped as one run. The platform was always located at the same position except for run V, where the position was changed (‘task switch'). The platform was placed back on its original position for the final run VI. *Significantly different from controls, P<0.05. Scale bar, 40 μm. DAPI, 4,6-diamidino-2-phenylindole.
In line with the increased intracerebral numbers of grafted NPCs in mice that had been treated with TAT-Hsp70, these animals showed increased postischemic neuronal survival and improved neurologic outcome when compared with mice that had been treated with TAT-HA and NPCs (Figures 5B–E). Again, significant differences in the water maze test are unlikely to be biased because of impaired motor coordination, as average swimming speed of mice did not differ between each other (data not shown). Interestingly, transplantation of NPCs in mice that had been treated with TAT-HA did not improve neurologic recovery. These data suggest a synergistic action of TAT-Hsp70 and adult NPCs, indicating that the favorable TAT-Hsp70-induced milieu may be used for successful cell transplantation later on.
Differentiation Patterns of Endogenous and Grafted Neural Precursor Cells are not Affected by TAT-Heat-Shock Protein 70
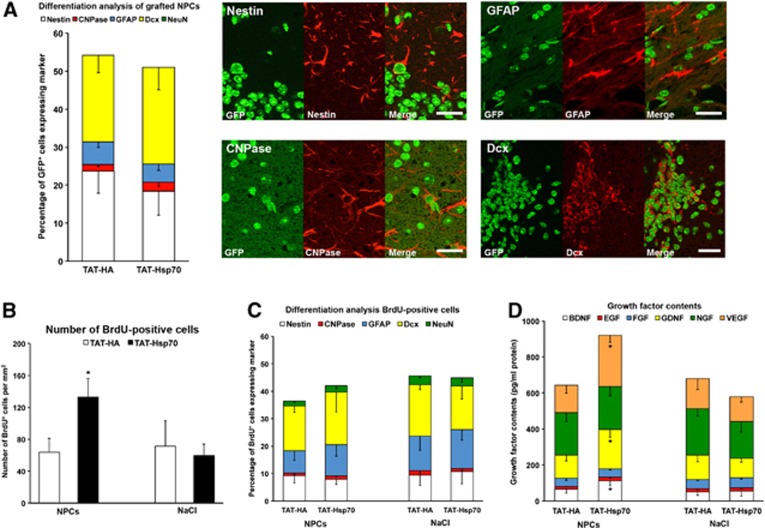
After the observation that the favorable extracellular milieu induced by TAT-Hsp70 treatment could be used for late intravenous cell transplantation enabling sustained neuroprotection in the ischemic brain, we wondered whether NPC-induced neuroprotection was a consequence of neuronal differentiation of grafted NPCs. Differentiation analysis of transplanted GFP+ NPCs on day 84 revealed no mature neuronal phenotype as evidenced by the absence of NeuN in grafted cells (Figure 6A). Rather, transplanted NPCs expressed the neural marker nestin, the immature neuronal marker doublecortin, the astroglial marker glial fibrillary acidic protein or the oligodendroglial marker CNPase (Figure 6A). However, TAT-Hsp70 treatment did not influence the differentiation pattern (Figure 6A). Assessment of postischemic proliferation of new-born endogenous cells via BrdU staining (given intraperitoneally on days 8 to 18, i.e., before transplantation of GFP+ NPCs) showed significantly increased numbers of BrdU+ cells within the ischemic striatum of animals that had been treated with both TAT-Hsp70 and subsequent NPC transplantation (Figure 6B). Again, differentiation analysis of these endogenous new-born cells yielded no significant difference between TAT-HA and TAT-Hsp70 delivery (Figure 6C). As transplantation of NPCs in mice that had been treated with TAT-Hsp70 did not result in sufficiently high mature neuronal differentiation rates of either grafted or endogenous NPCs, we hypothesized that beneficial effects of grafted cells are most likely due to indirect mechanisms. We therefore analyzed the contents of selected growth factors within the ischemic hemisphere of mice that had been treated with TAT-HA or TAT-Hsp70 followed by additional treatment with either NPCs or NaCl. Notably, the combined delivery of TAT-Hsp70 and NPCs increased concentrations of BDNF, VEGF and GDNF (Figure 6D), whereas Monotherapies failed to do so. Thus paracrine effects were involved in the effects observed by transplanted NPCs.
Figure 6.
Differentiation patterns of endogenous and grafted neural precursor cells (NPCs) are not affected by TAT-heat-shock protein 70 (Hsp70) treatment. (A) Quantitative differentiation analysis (n=12 per condition) of green fluorescence protein positive (GFP+) transplanted NPCs on day 84 within the ischemic hemisphere of mice that had been treated with either TAT-Hsp70 or TAT-HA before. Representative photos were taken on day 84 within the ischemic striatum of animals that had received a combinational therapy consisting of both TAT-Hsp70 and NPCs. (B) Quantitative analysis (n=11 to 12 per condition) of 5-bromo-2-desoxyuridine positive (BrdU+) endogenous cells on day 84 within the ischemic hemisphere. Animals had been treated with either TAT-Hsp70 or TAT-HA (during reperfusion plus day 14) followed by additional infusion (day 28) of either NPCs or NaCl as control. (C) Quantitative differentiation analysis of BrdU+ cells from (B) within the ischemic hemisphere on day 84 poststroke. (D) Analysis of growth factor contents in ischemic hemispheres (n=5 per condition) on day 84 from animals that had been treated as described in (B). *Significantly different from controls, P<0.05. Scale bars, 20 μm. GFAP, glial fibrillary acidic protein.
Discussion
Neuroprotection in various models of experimental stroke by overexpression of Hsp706, 7, 8 or by application of pharmacological Hsp70-inducers like geldanamycin2 has been shown before. As these models are not suitable for translational approaches partly because of toxicity of Hsp70 inducers, we have previously used the TAT-Hsp70 fusion protein inducing neuroprotection against stroke.9 However, observation periods in that study where TAT-Hsp70 was given during early reperfusion were limited to 4 weeks. Consequently, we now further evaluated the therapeutic potential of TAT-Hsp70 and analyzed mechanisms underlying TAT-Hsp70-induced neuroprotection.
Intravenous infusion of TAT-Hsp70 reduced postischemic brain injury, which was associated with reduced brain swelling, enhanced integrity of the BBB, and reduced DNA fragmentation at 3 days after induction of stroke. However, TAT-Hsp70 cannot be delivered beyond 12 hours poststroke to be protective, a time point when opening of the BBB is critically involved in ischemic damage.27 Although thrombolysis remains the only causal therapy for ischemic stroke, rt-PA is neurotoxic on its own.28 Interestingly, TAT-Hsp70 also yielded neuroprotection when animals where first treated with rt-PA, making Hsp70 an interesting tool for further translational investigations in the future.
Hsp70 induces neuroprotection via its chaperone activity resulting in reduction of apoptotic cell injury.1 Further studies have shown that Hsp70-induced tissue rescue is not limited to its chaperone activity but also involves reduced inflammatory tissue responses, thus reducing cell injury within the ischemic brain.1, 2 However, extracellular Hsp70 has been described to also act as a pro-inflammatory mediator, but the exact role of this effect remains elusive. Infusion of TAT-Hsp70 significantly reduced microglial activation 3 days after stroke, suggesting that modulation of postischemic inflammation is one key factor for acute neuroprotection in our stroke model next to the aforementioned stabilization of the BBB. Hence, reports on pro-inflammatory actions of Hsp70 might be partly attributed to endotoxin contaminations during preparation of recombinant Hsp70 in some studies, as has been discussed previously.2
Hsp70 is a ligand of TLR such as TLR-2 and TLR-4, which are involved in pro-inflammatory cell signaling via the NF-κB pathway23 and which are upregulated upon cerebral ischemia.29 Consequently, mice deficient of TLR have been shown to develop reduced postischemic brain injury.30, 31 In our model, cerebral ischemia induced upregulation of TLR-2 and TLR-4 in both TAT-Hsp70-treated mice and control animals at 3 days after stroke, which is in line with the previous reports.32 Although we did not find a difference in TLR protein expression between mice treated with TAT-Hsp70 or TAT-HA, NF-κB p65 protein abundance that is downstream of TLR signaling was significantly reduced in mice, which had been treated with TAT-Hsp70. Expression patterns of NF-κB p65, however, do not necessarily reflect activation states of the transcription factor. We therefore analyzed activation of NF-κB p65, demonstrating enhanced nuclear translocation within the mice treated with TAT-HA, thus supporting our hypothesis that TAT-Hsp70 prevents postischemic NF-κB p65 activation. In association with decreased NF-κB p65 expression and reduced nuclear translocation, abundance of the physiologic NF-κB regulator IκB-α was significantly enhanced in TAT-Hsp70-treated mice. IκB-α is regulated by proteasomal degradation,24 and determination of proteasomal activity in TAT-Hsp70-treated mice revealed reduced activity of the chymotrypsin-like activity of the 20S proteasome, resulting in the aforementioned high expression of IκB-α. Although there is evidence suggesting that heat-shock proteins like Hsp90 are necessary for assembly of the 26S proteasome,33 there is, to our knowledge, no report on a direct negative impact on proteasomal activity by Hsp70. Likewise, TAT-Hsp70 as used in our study did not affect in vitro 20S proteasome activity directly. The observed reduced proteasomal activity must therefore be a consequence of different hitherto unknown mediators.
TAT-Hsp70 induced neuroprotection and reduced inflammation within the ischemic brain despite high levels of TLR-2/4. In spite of numerous reports on TLRs mediating postischemic inflammation, there is also evidence demonstrating that deficiency of TLR-2 might rather aggravate brain injury than protect against stroke.34 In this context, inhibition of postischemic proteasome activation is likely to be crucial for TAT-Hsp70-induced neuroprotection, albeit a direct inhibitory effect by TAT-Hsp70 itself can be excluded as suggested above. Proteasome inhibition has been shown to be a promising tool to protect from ischemic brain injury, in part by inhibiting pro-inflammatory NF-κB signaling.35, 36, 37 Using the novel proteasome inhibitor BSc2118 that extends the therapeutic time window towards 12 hours poststroke, we have previously shown that proteasome inhibition is a valuable concept in stroke treatment.38 However, stroke treatment by proteasome inhibition remains delicate depending on both timing and dosages used, where inhibition of the 20S proteasome can also induce rather than protect from ischemic brain injury.39
Monotherapy with TAT-Hsp70 did not result in long-term neuroprotection when given 12 hours and 14 days after stroke, which might be in part attributed to the injection paradigm chosen. Nevertheless, benefit via anti-inflammatory TAT-Hsp70-induced actions due to extended injection protocols is not likely, since inflammation at later stages of the disease is of minor relevance. In line with this, we observed no Ib4+ cells on day 84 in any experimental group. Whether additional non-inflammatory TAT-Hsp70-induced actions after extended injections paradigms could contribute to long-term neuroprotection was, however, beyond the scope of the present work.
Consequently, we wondered if the therapeutic potential of TAT-Hsp70 might be enhanced when combined with a neuroregenerative approach. Hence, treatment with TAT-Hsp70 was augmented with intravenous delivery of adult NPCs. Despite numerous reports on postischemic neuroprotection due to intravenous NPC transplantation,25, 26 the therapeutic success of stem cell transplantation depends on sufficiently high survival rates of grafted cells, which are limited because of an unfavorable extracellular micromilieu.20 In this context, infusion of TAT-Hsp70 provides a favorable extracellular brain milieu due to the reduction of inflammation as well as maintenance and protection of residing neural networks where survival rates of grafted NPCs are increased, thus exerting long-term neuroprotection after stroke. Transplanted cells, however, are unlikely to be integrated into the residing neural network, as neuronal differentiation rates were low. Rather, transplanted NPCs act as ‘mini-pumps' via continuous secretion of trophic mediators thus increasing survival of endogenous SVZ-derived NPCs and reducing secondary brain injury as discussed.14, 40
In conclusion, the present study demonstrates that TAT-Hsp70 induces acute postischemic neuroprotection via anti-inflammatory mechanisms, involving proteasomal inhibition that reduces NF-κB activation via stabilization of IκB-α. The favorable extracellular postischemic milieu induced by TAT-Hsp70 in turn facilitates transplantation of NPCs, providing stable and sustained neuroprotection against cerebral ischemia. We hypothesize that combinations of anti-inflammatory and cell-based therapies may particularly be promising for stroke treatment, as the reshaped micromilieu of the tissue may allow successful transplantation that enables long-term neurologic recovery.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Supplementary Material
References
- Giffard RG, Han RQ, Emery JF, Duan M, Pittet JF. Regulation of apoptotic and inflammatory cell signaling in cerebral ischemia: the complex roles of heat shock protein 70. Anesthesiology. 2008;109:339–348. doi: 10.1097/ALN.0b013e31817f4ce0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N, Kim JY, Yenari MA. Anti-inflammatory properties and pharmacological induction of Hsp70 after brain injury. Inflammopharmacology. 2012;20:177–185. doi: 10.1007/s10787-011-0115-3. [DOI] [PubMed] [Google Scholar]
- Brown IR. Heat shock proteins and protection of the nervous system. Ann N Y Acad Sci. 2007;1113:147–158. doi: 10.1196/annals.1391.032. [DOI] [PubMed] [Google Scholar]
- Xu L, Xiong X, Ouyang Y, Barreto G, Giffard R. Heat shock protein 72 (Hsp72) improves long term recovery after focal cerebral ischemia in mice. Neurosci Lett. 2011;488:279–282. doi: 10.1016/j.neulet.2010.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava P. Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev Immunol. 2002;2:185–194. doi: 10.1038/nri749. [DOI] [PubMed] [Google Scholar]
- Badin RA, Lythgoe MF, van der Weerd L, Thomas DL, Gadian DG, Latchman DS. Neuroprotective effects of virally delivered HSPs in experimental stroke. J Cereb Blood Flow Metab. 2006;26:371–381. doi: 10.1038/sj.jcbfm.9600190. [DOI] [PubMed] [Google Scholar]
- Rajdev S, Hara K, Kokubo Y, Mestril R, Dillmann W, Weinstein PR, et al. Mice overexpressing rat heat shock protein 70 are protected against cerebral infarction. Ann Neurol. 2000;47:782–791. [PubMed] [Google Scholar]
- Zheng Z, Kim JY, Ma H, Lee JE, Yenari MA. Anti-inflammatory effects of the 70 kDa heat shock protein in experimental stroke. J Cereb Blood Flow Metab. 2008;28:53–63. doi: 10.1038/sj.jcbfm.9600502. [DOI] [PubMed] [Google Scholar]
- Doeppner TR, Nagel F, Dietz GP, Weise J, Tonges L, Schwarting S, et al. TAT-Hsp70-mediated neuroprotection and increased survival of neuronal precursor cells after focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2009;29:1187–1196. doi: 10.1038/jcbfm.2009.44. [DOI] [PubMed] [Google Scholar]
- Dietz GP, Bahr M. Delivery of bioactive molecules into the cell: the Trojan horse approach. Mol Cell Neurosci. 2004;27:85–131. doi: 10.1016/j.mcn.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Nagel F, Dohm CP, Bahr M, Wouters FS, Dietz GP. Quantitative evaluation of chaperone activity and neuroprotection by different preparations of a cell-penetrating Hsp70. J Neurosci Methods. 2008;171:226–232. doi: 10.1016/j.jneumeth.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Nagel F, Falkenburger BH, Tonges L, Kowsky S, Poppelmeyer C, Schulz JB, et al. Tat-Hsp70 protects dopaminergic neurons in midbrain cultures and in the substantia nigra in models of Parkinson's disease. J Neurochem. 2008;105:853–864. doi: 10.1111/j.1471-4159.2007.05204.x. [DOI] [PubMed] [Google Scholar]
- Dietz GP, Bahr M. Synthesis of cell-penetrating peptides and their application in neurobiology. Methods Mol Biol. 2007;399:181–198. doi: 10.1007/978-1-59745-504-6_13. [DOI] [PubMed] [Google Scholar]
- Doeppner TR, Ewert TA, Tonges L, Herz J, Zechariah A, Elali A, et al. Transduction of neural precursor cells with TAT-heat shock protein 70 chaperone: therapeutic potential against ischemic stroke after intrastriatal and systemic transplantation. Stem Cells. 2012;30:1297–1310. doi: 10.1002/stem.1098. [DOI] [PubMed] [Google Scholar]
- Doeppner TR, Kaltwasser B, ElAli A, Zechariah A, Hermann DM, Bahr M. Acute hepatocyte growth factor treatment induces long-term neuroprotection and stroke recovery via mechanisms involving neural precursor cell proliferation and differentiation. J Cereb Blood Flow Metab. 2011;31:1251–1262. doi: 10.1038/jcbfm.2010.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba Y, Sasayama T, Miyake S, Koyama J, Kondoh T, Hosoda K, et al. Anti-VEGF receptor antagonist (VGA1155) reduces infarction in rat permanent focal brain ischemia. Kobe J Med Sci. 2008;54:E136–E146. [PubMed] [Google Scholar]
- Doeppner TR, Bretschneider E, Doehring M, Segura I, Senturk A, Acker-Palmer A, et al. Enhancement of endogenous neurogenesis in ephrin-B3 deficient mice after transient focal cerebral ischemia. Acta Neuropathol. 2011;122:429–442. doi: 10.1007/s00401-011-0856-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Blizzard KK, Zeng Z, DeVries AC, Hurn PD, McCullough LD. Chronic behavioral testing after focal ischemia in the mouse: functional recovery and the effects of gender. Exp Neurol. 2004;187:94–104. doi: 10.1016/j.expneurol.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Zhang L, Schallert T, Zhang ZG, Jiang Q, Arniego P, Li Q, et al. A test for detecting long-term sensorimotor dysfunction in the mouse after focal cerebral ischemia. J Neurosci Methods. 2002;117:207–214. doi: 10.1016/s0165-0270(02)00114-0. [DOI] [PubMed] [Google Scholar]
- Doeppner TR, El Aanbouri M, Dietz GP, Weise J, Schwarting S, Bahr M. Transplantation of TAT-Bcl-xL-transduced neural precursor cells: long-term neuroprotection after stroke. Neurobiol Dis. 2010;40:265–276. doi: 10.1016/j.nbd.2010.05.033. [DOI] [PubMed] [Google Scholar]
- Doeppner TR, Grune T, de Groot H, Rauen U. Cold-induced apoptosis of rat liver endothelial cells: involvement of the proteasome. Transplantation. 2003;75:1946–1953. doi: 10.1097/01.TP.0000065291.02855.6A. [DOI] [PubMed] [Google Scholar]
- Braun HA, Umbreen S, Groll M, Kuckelkorn U, Mlynarczuk I, Wigand ME, et al. Tripeptide mimetics inhibit the 20 S proteasome by covalent bonding to the active threonines. J Biol Chem. 2005;280:28394–28401. doi: 10.1074/jbc.M502453200. [DOI] [PubMed] [Google Scholar]
- Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- Rothwarf DM, Karin M. The NF-kappa B activation pathway: a paradigm in information transfer from membrane to nucleus. Sci STKE. 1999;1999:RE1. doi: 10.1126/stke.1999.5.re1. [DOI] [PubMed] [Google Scholar]
- Bacigaluppi M, Pluchino S, Peruzzotti Jametti L, Kilic E, Kilic U, Salani G, et al. Delayed post-ischaemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain. 2009;132:2239–2251. doi: 10.1093/brain/awp174. [DOI] [PubMed] [Google Scholar]
- Chu K, Kim M, Park KI, Jeong SW, Park HK, Jung KH, et al. Human neural stem cells improve sensorimotor deficits in the adult rat brain with experimental focal ischemia. Brain Res. 2004;1016:145–153. doi: 10.1016/j.brainres.2004.04.038. [DOI] [PubMed] [Google Scholar]
- Latour LL, Kang DW, Ezzeddine MA, Chalela JA, Warach S. Early blood-brain barrier disruption in human focal brain ischemia. Ann Neurol. 2004;56:468–477. doi: 10.1002/ana.20199. [DOI] [PubMed] [Google Scholar]
- Copin JC, Bengualid DJ, Da Silva RF, Kargiotis O, Schaller K, Gasche Y. Recombinant tissue plasminogen activator induces blood-brain barrier breakdown by a matrix metalloproteinase-9-independent pathway after transient focal cerebral ischemia in mouse. Eur J Neurosci. 2011;34:1085–1092. doi: 10.1111/j.1460-9568.2011.07843.x. [DOI] [PubMed] [Google Scholar]
- Marsh BJ, Williams-Karnesky RL, Stenzel-Poore MP. Toll-like receptor signaling in endogenous neuroprotection and stroke. Neuroscience. 2009;158:1007–1020. doi: 10.1016/j.neuroscience.2008.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao CX, Yang QW, Lv FL, Cui J, Fu HB, Wang JZ. Reduced cerebral ischemia-reperfusion injury in Toll-like receptor 4 deficient mice. Biochem Biophys Res Commun. 2007;353:509–514. doi: 10.1016/j.bbrc.2006.12.057. [DOI] [PubMed] [Google Scholar]
- Lehnardt S, Lehmann S, Kaul D, Tschimmel K, Hoffmann O, Cho S, et al. Toll-like receptor 2 mediates CNS injury in focal cerebral ischemia. J Neuroimmunol. 2007;190:28–33. doi: 10.1016/j.jneuroim.2007.07.023. [DOI] [PubMed] [Google Scholar]
- Tu XK, Yang WZ, Shi SS, Wang CH, Zhang GL, Ni TR, et al. Spatio-temporal distribution of inflammatory reaction and expression of TLR2/4 signaling pathway in rat brain following permanent focal cerebral ischemia. Neurochem Res. 2010;35:1147–1155. doi: 10.1007/s11064-010-0167-6. [DOI] [PubMed] [Google Scholar]
- Imai J, Maruya M, Yashiroda H, Yahara I, Tanaka K. The molecular chaperone Hsp90 plays a role in the assembly and maintenance of the 26S proteasome. The EMBO J. 2003;22:3557–3567. doi: 10.1093/emboj/cdg349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohacek I, Cordeau P, Lalancette-Hebert M, Gorup D, Weng YC, Gajovic S, et al. Toll-like receptor 2 deficiency leads to delayed exacerbation of ischemic injury. J Neuroinflammation. 2012;9:191. doi: 10.1186/1742-2094-9-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AJ, Dave JR, Tortella FC. Neuroprotection with the proteasome inhibitor MLN519 in focal ischemic brain injury: relation to nuclear factor kappaB (NF-kappaB), inflammatory gene expression, and leukocyte infiltration. Neurochem Int. 2006;49:106–112. doi: 10.1016/j.neuint.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Buchan AM, Li H, Blackburn B. Neuroprotection achieved with a novel proteasome inhibitor which blocks NF-kappaB activation. Neuroreport. 2000;11:427–430. doi: 10.1097/00001756-200002070-00041. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang ZG, Buller B, Jiang J, Jiang Y, Zhao D, et al. Combination treatment with VELCADE and low-dose tissue plasminogen activator provides potent neuroprotection in aged rats after embolic focal ischemia. Stroke. 2010;41:1001–1007. doi: 10.1161/STROKEAHA.109.577288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doeppner TR, Mlynarczuk-Bialy I, Kuckelkorn U, Kaltwasser B, Herz J, Hasan MR, et al. The novel proteasome inhibitor BSc2118 protects against cerebral ischaemia through HIF1A accumulation and enhanced angioneurogenesis. Brain. 2012;135:3282–3297. doi: 10.1093/brain/aws269. [DOI] [PubMed] [Google Scholar]
- Ge P, Luo Y, Liu CL, Hu B. Protein aggregation and proteasome dysfunction after brain ischemia. Stroke. 2007;38:3230–3236. doi: 10.1161/STROKEAHA.107.487108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TM, Andres RH, Steinberg GK. Optimizing the success of cell transplantation therapy for stroke. Neurobiol Dis. 2010;37:275–283. doi: 10.1016/j.nbd.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.