Abstract
Measurement of cerebrovascular reactivity (CVR) can give valuable information about existing pathology and the risk of adverse events, such as stroke. A common method of obtaining regional CVR values is by measuring the blood flow response to carbon dioxide (CO2)-enriched air using arterial spin labeling (ASL) or blood oxygen level-dependent (BOLD) imaging. Recently, several studies have used carbogen gas (containing only CO2 and oxygen) as an alternative stimulus. A direct comparison was performed between CVR values acquired by ASL and BOLD imaging using stimuli of (1) 5% CO2 in air and (2) 5% CO2 in oxygen (carbogen-5). Although BOLD and ASL CVR values are shown to be correlated for CO2 in air (mean response 0.11±0.03% BOLD, 4.46±1.80% ASL, n=16 hemispheres), this correlation disappears during a carbogen stimulus (0.36±0.06% BOLD, 4.97±1.30% ASL). It is concluded that BOLD imaging should generally not be used in conjunction with a carbogen stimulus when measuring CVR, and that care must be taken when interpreting CVR as measured by ASL, as values obtained from different stimuli (CO2 in air versus carbogen) are not directly comparable.
Keywords: arterial spin labeling, ASL, BOLD contrast, cerebral blood flow measurement, cerebral hemodynamics, MRI
Introduction
Over the past few decades, magnetic resonance imaging (MRI) has become an indispensable tool in health care, which is used for clinical diagnosis, treatment planning, and groundbreaking research. Recently, there has been a gradual move from solely using images (susceptible to subjective interpretation) toward acquiring quantitative data that can give more specific information on a per-subject basis, and that allows for better comparison between subject populations. One quantifiable measure of localized brain physiology is cerebrovascular reactivity (CVR), a measure of the ability of vasculature in the brain to respond to a stimulus. High CVR indicates the ability of the vasculature to provide a substantial increase in cerebral blood flow (CBF) and cerebral blood volume (CBV) in response to a given stimulus, both of which increase the rate at which freshly oxygenated blood perfuses the capillary networks in the brain. Cerebrovascular reactivity varies both between subjects and across different regions of an individual's brain. Reduced CVR has long been considered as a marker for cerebrovascular pathology and as a risk factor for stroke;1, 2, 3 although CVR naturally decreases with age,4 it can be further reduced as a result of stroke,5 dementia,6, 7 diabetes,8 or severe depression.9, 10
To be clinically feasible, methods of measuring CVR must be repeatable, tolerable for patients, and relatively quick to perform. Inhalation of carbon dioxide (CO2) acts as an immediate vasodilatory stimulus with few contraindications and minimal associated cost. Mandell et al11 have shown that there is a good correlation between CVR values as measured by arterial spin labeling (ASL, a noninvasive perfusion-weighted MRI technique that is sensitive to changes in CBF) and blood oxygen level-dependent (BOLD, sensitive primarily to CBV) imaging when the fraction of inspired oxygen is kept approximately constant. Cerebrovascular reactivity can often be calculated from the MRI data taken during CO2 exposure for some other purpose, such as calibration of the BOLD response,12, 13, 14 but it is important to be aware of any potential confounding factors that might affect CVR, including variations in the concentration of oxygen inhaled throughout the course of a scan. Several authors (including Cantin et al,15 Donahue et al,16 and Hamzei et al17) have implicitly assumed that increasing the oxygen fraction of inspired air will have no effect on the measurement of CVR. The current study aimed to investigate this claim by acquiring values for CVR using ASL and BOLD during a single scan with stimuli of (1) 5% CO2 in air and (2) 5% CO2 in oxygen (carbogen-5).
Measuring Cerebrovascular Reactivity
Arterial spin labeling can be used to acquire perfusion-weighted images without the necessity of injecting a contrast agent. By magnetically tagging inflowing arterial blood in the neck it is possible to track the tagged blood's path through the cerebral vasculature. If the T1 of the labeled blood is known, then an absolute value for the CBF can be extracted. Arterial spin labeling imaging allows for fast repeat measurements and can measure speed as well as magnitude of CVR. However, it has a relatively low signal-to-noise ratio in comparison with BOLD imaging. Furthermore, ASL is still regarded as a research technique and as a result many clinical MRI scanners are not set up to perform ASL scans.
To overcome these issues, BOLD imaging has been increasingly investigated as an alternative method of measuring CVR.11 The magnitude of a BOLD signal is reduced in the presence of deoxyhemoglobin, so BOLD imaging is an indirect measure of the local rate of blood delivery to the brain. This method has been used extensively to investigate which areas of the brain are involved in particular cognitive tasks; however, it can also be used to measure the reactivity of the cerebral vasculature to an external stimulus.
In this study, subjects breathed increased concentrations of CO2, known to increase both CBF and CBV. There is some indication that a mild hypercapnic stimulus induces a small change in cerebral metabolic rate of oxygen (CMRO2) consumption in humans,18 although this effect is not yet confirmed and has been disputed by some researchers.19 Because the BOLD signal is sensitive to CBF and CBV, the BOLD response to a vasodilatory stimulus, such as CO2, can be used as a measure of CVR. However, the magnitude of the BOLD signal also depends on a number of additional parameters, including the equipment used and the chosen pulse sequence as well as resting CBF, CBV, venous oxygen saturation, and cerebral metabolic rate of oxygen. As a result, particular care must be taken when interpreting BOLD CVR data.
Some recent studies15, 16, 17 have used carbogen gas that consists of CO2 with balance oxygen for CVR quantification. However, during carbogen inhalation the arterial partial pressures of both CO2 (PaCO2) and oxygen (PaO2) are significantly increased, causing the BOLD signal to be elevated through more than one mechanism; increased blood flow due to CVR and increased oxygen saturation of venous blood. The increase in the total oxygen content of the arterial blood during carbogen breathing, in the form of an increase in the partial pressure of oxygen in the arterial plasma, induces a relative increase in the venous saturation, resulting in a greater BOLD signal difference than that which would be caused by an increase in CBF alone. Although the increase in BOLD signal in response to carbogen inhalation does contain physiologic information, and has recently been used for estimating the BOLD calibration factor and cerebral metabolic rate of oxygen,13, 20 this increase in BOLD signal does not reflect true CVR. The ASL signal however is sensitive only to CBF and is not dependent on blood oxygen saturation; thus, it may be more reliable than BOLD for measuring CVR. It is unclear whether CVR as measured by ASL will give consistent results between stimuli of CO2 in air and CO2 in oxygen.
The Bohr and Haldane effects, respectively, describe the impact of CO2 concentration on oxygen binding to hemoglobin (Hb), and the reciprocal impact of oxygen concentration on CO2 transport in the blood. In a high-oxygen/low-CO2 environment (such as the lungs), Hb has an increased affinity for oxygen and the ability to transport CO2 is reduced. These effects are reversed in low-oxygen/high-CO2 environments such as those found in the tissues. Thus, Hb's transport abilities are finely tuned to effectively transport oxygen from the lungs to the tissues, and CO2 from the tissues to the lungs. During hyperoxia, the tissues effectively become high-oxygen environments, affecting both the release of oxygen from Hb and the ability of the blood to transport CO2. During carbogen breathing, the situation is complicated even further as the lungs become a high-CO2 environment (reducing Hb's affinity for oxygen) and a high-oxygen environment (increasing Hb's affinity for oxygen), whereas the tissues are essentially in the same state as the lungs (in comparison with normal air breathing). As a result of these competing and conflicting effects, the relationship between the alveolar partial pressures and the arterial partial pressures (the Aa gradient) will change, as will the relationship between the arterial and the tissue partial pressures.
Although the Bohr and Haldane effects are individually well understood, the interaction between them when both oxygen and CO2 concentrations are simultaneously increased is exceptionally difficult to determine. Thus, the change in end-tidal measures of oxygen and CO2 between air and carbogen breathing is not necessarily indicative of the change in the arterial or tissue partial pressures, which are driving the vasoactive effects.
Materials and methods
Nine healthy, nonsmoking volunteers were recruited for this study, mean age 25±4 years, two females. Murphy et al21 showed that between 6 and 10 subjects are required for single session trials in which ASL is investigated. Research was conducted in accordance with the Good Clinical Practice guidelines specified by the University of Oxford Clinical Trials and Research Governance unit. Ethical approval for the study was obtained from the Oxfordshire Research Ethics Committee A (reference 09/H0604/95) and all subjects gave written informed consent before taking part.
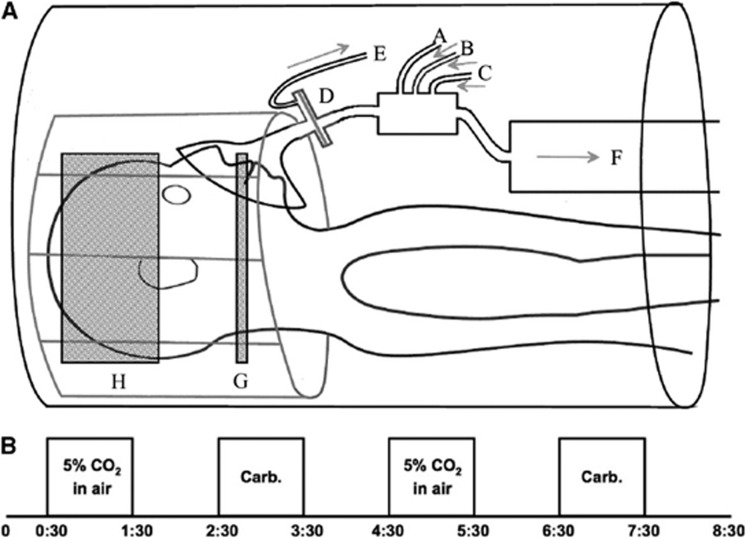
The study was performed on a 3-T Siemens Verio scanner (Siemens, Erlangen, Germany) using a 32-channel receive-only head coil. A single TI dual echo pulse sequence was used, allowing acquisition of data for both ASL and BOLD after a single radio frequency excitation (a short echo time gives an optimum signal for ASL, and a slightly longer echo time allows for BOLD imaging). A pseudo-continuous ASL scheme was used to magnetically tag spins at a labeling plane in the neck22 (tag duration (τ) of 1.4 seconds and postlabel delay time of 0.8 second). The labeling plane was placed between the upper and lower contortions of the vertebral arteries near the top of the neck, as shown in Figure 1A. An echo planar imaging readout was used for fast data acquisition, with the following parameters: repetition time=4 seconds, echo time (TE) (1)=16 ms, TE(2)=35 ms. In all, 23 slices were acquired for each subject (slice thickness 5.0 mm with an interslice gap of 0.5 mm; in plane resolution 64 × 64 voxels). A voxel size of 3.0 × 3.0 × 5.0 mm was used where this was sufficient for the field of view to cover the whole brain. In two cases, voxel dimensions were increased to 3.4 × 3.4 × 5.0 mm to ensure full brain coverage.
Figure 1.
(A) System for gas delivery. Gas mixtures flow in through tubes A, B, and C; a sampling tube E is attached to the mask side of a filter D. F is an open-ended tube acting as a reservoir of the supplied gas. G shows the approximate position of the labeling plane for the pseudo-continuous arterial spin labeling sequence, H the imaging volume. (B) Gas paradigm showing timing of blocks of 5% CO2 in air and carbogen-5 (time scale in minutes). CO2, carbon dioxide; Carb., carbogen-5.
Subjects wore a close fitting gas mask (8930 series; Hans Rudolph Inc., Kansas City, MO, USA) over the mouth and nose, which was connected to gas delivery apparatus as shown in Figure 1A. Medical air, 5% CO2 in medical air, and carbogen-5 were delivered from compressed cylinders and gas flow (through tubes labeled as A, B, and C) was managed from the MRI control room such that gas delivery was kept at 25 L/min at all times. A sampling tube (E) was attached to the mask side of the filter (D) and gas composition was monitored using oxygen and CO2 analyzers (models O2100C and CO2100C, respectively; Biopac Systems, Goleta, CA, USA). An open-ended cylinder (F) of 1.5 m length and 10 cm diameter acted as a reservoir of the supplied gas, allowing subjects to breathe normally, at their own rates. The experimental protocol is shown schematically in Figure 1B; blocks were chosen to have a 1-minute duration, as recommended by Yezhuvath et al.23
Values for end-tidal partial pressure of CO2 (PetCO2)—the concentration of CO2 measured at the very end of expiration—were extracted from respiratory data as an average of the last three full breaths taken during each stimulus block. Average baseline PetCO2 was visually determined for each subject. It has been shown that the change in PetCO2 is a good proxy for the change in PaCO2,24 so this information was used to deduce the change in PaCO2 in units of millimeters of mercury (mm Hg), the standard unit in the field of physiologic pressure measurements.
An in-house MATLAB (MathWorks, Natick, MA, USA) script was used to separate the acquired data into distinct ASL and BOLD files. Features from the FMRIB Software Library (http://www.fmrib.ox.ac.uk/fsl/)25, 26, 27 were used for brain extraction (BET, Brain Extraction Tool) and motion correction (MCFLIRT, Motion Correction using FMRIB's Linear Image Registration Tool). High-resolution (1 mm isotropic) structural scans of each subject were used to register each brain to MNI (Montreal Neurological Institute) standard space, using the MNI 152.2 mm template. This allowed each data set to be split into separate hemispheres, thus doubling the sample size to better examine any potential correlations (see for example Mandell et al11). Functional data were registered to the subjects' high-resolution structural image (7 degrees of freedom) and to MNI standard space (12 degrees of freedom) using FLIRT (FMRIB's Linear Image Registration Tool). A high-pass filter cutoff of 240 seconds and a Gaussian spatial smoothing kernel of 5 mm were applied before running a generalized linear model analysis of the functional data using FEAT, the FMRIB Expert Analysis Tool. The generalized linear model was set up with five explanatory variables representing the following: (1) tag/control signal modulation, (2) CO2 gas exposure, (3) carbogen gas stimulus, (4) interaction of CO2 exposure with tag/control signal, and (5) interaction of carbogen with tag/control signal. Gas stimuli were modeled as gamma convolutions (phase=0 second, s.d.=30 seconds, mean lag=30 seconds) with temporal derivatives added and temporal filtering applied. Values for the ASL and BOLD signal were calculated from median values for coefficient of parameter estimates within gray matter in each hemisphere.
While the percentage change in signal acquired by the ASL method may be equated to that of CBF under normoxic conditions, a correction is necessary when the fraction of inspired oxygen is increased significantly, as discussed by Bulte et al.28 This is due to the decrease in T1 of arterial blood that occurs during increased PaO2, which results in a reduction in signal difference between tag and control images with respect to baseline that has not been caused by true changes in CBF. In addition, the tagging efficiency of the ASL sequence is reduced during hypercapnia due to the increased blood flow. To correct for these effects, we solved the Buxton general kinetic model29 for continuous ASL where t>τ+Δt (all symbols have the same meaning as described in the paper by Buxton et al). An estimate for the equilibrium magnetization of blood, M0B, was made for each subject by finding M0 (of brain tissue) within a thalamus mask and applying the blood brain partition coefficient of 0.88 mL/g.30 Other parameters used for this 3-T protocol were λ=0.9;30 T1 (tissue)=1.47 seconds;28 T1b (normoxic)=1.66 seconds;31 T1b (carbogen)=1.38 seconds;28 α (air)=0.92;12 α (hypercapnia)=0.83.12 For our experimental set-up, t=2.2 seconds, τ=1.4 seconds, and Δt=0.7 second.
Results
In general, participants responded well to the protocol, with only mild discomfort reported due to the slight resistance to breathing induced by the mask and the filter. One subject (4) requested the scan to be stopped toward the end of the final carbogen block. Although sufficient data had already been taken to perform the analysis, CVR values to carbogen gas for this subject, calculated in both hemispheres, were found to be outliers (defined as being more than twice the standard deviation from the group mean). Thus, both carbogen and CO2 data from this subject were excluded from quoted group averages and further statistical tests. None of the subjects exhibited significant changes in breathing rate between the three gas mixtures.
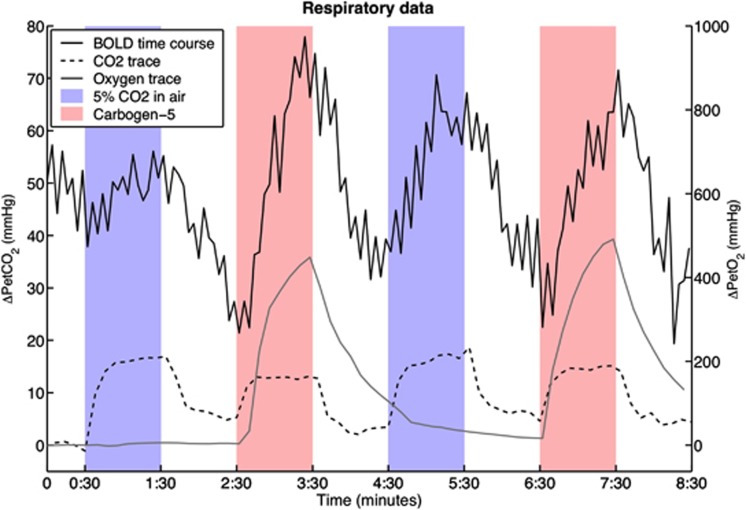
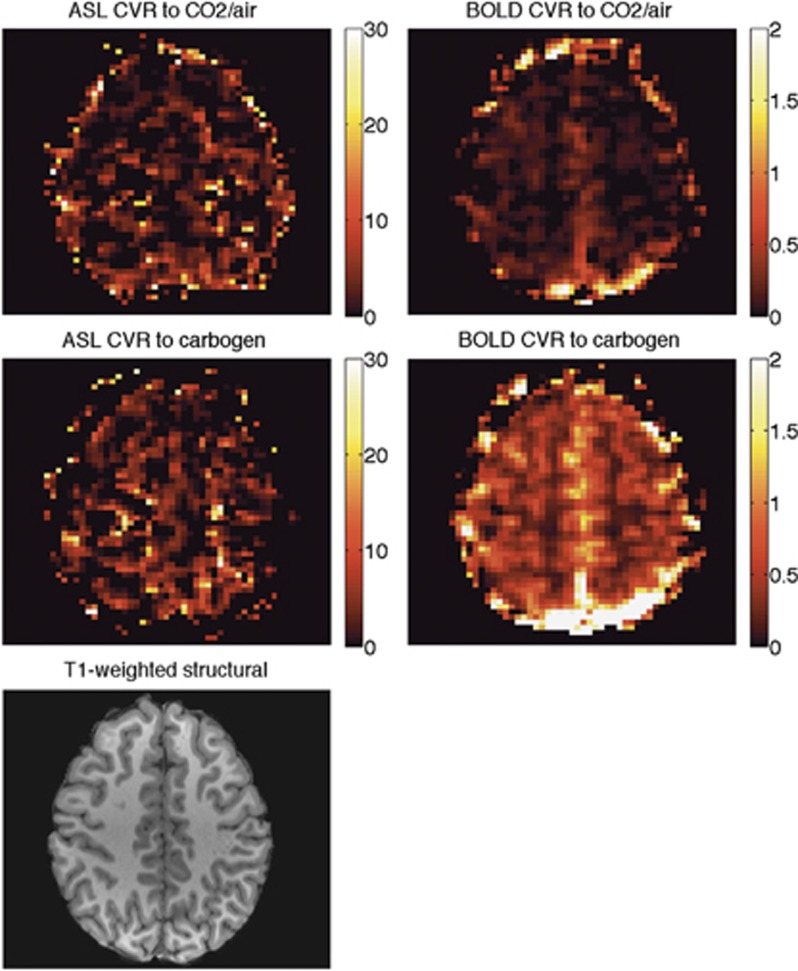
Table 1 contains respiratory data collected from the subjects during the scanning session. The PetCO2 was increased by an average of 12.1±2.2 mm Hg during breathing blocks of CO2 in air and by 10.2±2.5 mm Hg during carbogen blocks. The time courses of end-tidal pressures of CO2 and oxygen from one representative subject are shown in Figure 2, along with the BOLD signal changes. Maps of CVR for the same subject can be seen in Figure 3.
Table 1. Respiratory data.
|
CO2
in air |
Carbogen-5 |
|||
|---|---|---|---|---|
| Subject | ΔPetCO2 | ΔPetO2 | ΔPetCO2 | ΔPetO2 |
| 1 | 16.1 | 20.3 | 13.2 | 590.6 |
| 2 | 10.3 | 10.4 | 9.8 | 369.0 |
| 3 | 9.6 | 16.8 | 7.3 | 469.1 |
| 4 | 11.1 | 16.4 | 6.2 | 525.1 |
| 5 | 12.3 | 16.1 | 10.0 | 493.2 |
| 6 | 11.6 | 26.6 | 8.0 | 499.2 |
| 7 | 12.7 | 23.6 | 9.7 | 519.6 |
| 8 | 14.2 | 15.7 | 14.5 | 507.3 |
| 9 | 10.4 | 18.9 | 9.2 | 472.4 |
| Mean±s.d. | 12.1±2.2 | 18.5±5.0 | 10.2±2.5 | 490.1±61.9 |
Summary of respiratory measures (changes in end-tidal partial pressures of CO2 (ΔPetCO2) and oxygen (ΔPetO2), both given in units of mm Hg) used to calculate cerebrovascular reactivity, including mean±standard deviation (s.d.) in which the outlier (subject 4) has been excluded.
Figure 2.
Traces of end-tidal respiratory data from a representative subject (subject 3) and the corresponding BOLD signal time course of one voxel. The smooth black line is the BOLD signal change in arbitrary units, the dashed line shows the change in end-tidal partial pressure of carbon dioxide (CO2) (ΔPetCO2) with respect to baseline where each point is the maximum within an 8-second window; and the smooth gray line shows the change in end-tidal partial pressure of oxygen, defined as the minimum (also within the 8-second window). Note that the oxygen trace appears smoother because of the much smaller scale. Blue areas indicate periods of CO2 exposure, red areas indicate periods of carbogen breathing. BOLD, blood oxygen level-dependent.
Figure 3.
CVR maps of a representative subject (subject 3). The corresponding slice in the high-resolution T1-weighted structural image is also shown for comparison. Any voxels with ASL CVR >30% per mm Hg were assumed to be noise and set to 0. ASL, arterial spin labeling; BOLD, blood oxygen level-dependent; CVR, cerebrovascular reactivity.
Using a raw ASL signal, the average flow response to the CO2 in air mixture appeared to be 34.1±21.5% after performing the flow correction described above this was adjusted to 54.6±26.1%. For carbogen, the uncorrected flow increase was 21.5±18.9% and the corrected flow increase was 52.4±25.0%. Final results are summarized in Tables 1 and 2 and Figures 4 and 5. All values of CVR are reported as a percentage change in BOLD signal or in CBF during the stimulus versus baseline, per mm Hg change in PetCO2. Group mean values±standard deviations are given at the bottom of the tables. Under stimulus of 5% CO2 in air, mean values of CVR were 0.11±0.03% BOLD, 4.46±1.80% corrected ASL and under carbogen stimulus were 0.36±0.06% BOLD and 4.97±1.30% corrected ASL.
Table 2. Key results.
| CO2 in air |
Carbogen-5 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Subject | Sex | Age | Hemisphere | Uncorrected ASL CVR | Flow CVRa | BOLD CVR | Uncorrected ASL CVR | Flow CVRa | BOLD CVR |
| 1 | M | 30 | Left | 1.77 | 2.93 | 0.12 | 1.42 | 3.65 | 0.32 |
| Right | 2.54 | 3.91 | 0.12 | 2.24 | 4.80 | 0.34 | |||
| 2 | F | 21 | Left | 0.80 | 2.12 | 0.11 | 0.71 | 3.28 | 0.29 |
| Right | 0.84 | 2.18 | 0.11 | 1.93 | 4.98 | 0.32 | |||
| 3 | M | 22 | Left | 2.82 | 4.79 | 0.11 | 1.04 | 4.58 | 0.42 |
| Right | 2.13 | 3.90 | 0.12 | 0.45 | 3.76 | 0.45 | |||
| 4 | M | 32 | Left | 3.81 | 5.74 | 0.09 | 5.07 | 10.44 | 0.56 |
| Right | 3.56 | 5.45 | 0.10 | 5.62 | 11.29 | 0.59 | |||
| 5 | F | 22 | Left | 2.11 | 3.76 | 0.10 | 1.63 | 4.73 | 0.38 |
| Right | 1.52 | 2.99 | 0.10 | 1.38 | 4.38 | 0.40 | |||
| 6 | M | 27 | Left | 1.82 | 3.41 | 0.03 | 1.03 | 4.41 | 0.25 |
| Right | 1.71 | 3.31 | 0.04 | 1.13 | 4.44 | 0.27 | |||
| 7 | M | 25 | Left | 2.96 | 4.66 | 0.12 | 1.65 | 4.59 | 0.39 |
| Right | 3.53 | 5.44 | 0.10 | 2.97 | 6.51 | 0.38 | |||
| 8 | M | 21 | Left | 5.13 | 7.08 | 0.16 | 4.69 | 7.84 | 0.33 |
| Right | 6.16 | 8.43 | 0.16 | 4.38 | 7.42 | 0.34 | |||
| 9 | M | 24 | Left | 3.81 | 5.83 | 0.12 | 1.41 | 4.31 | 0.41 |
| Right | 4.41 | 6.55 | 0.12 | 2.60 | 5.90 | 0.42 | |||
| Mean±s.d. | 24±3 | 2.75±1.52 | 4.46±1.80 | 0.11±0.03 | 1.92±1.22 | 4.97±1.30 | 0.36±0.06 | ||
ASL, arterial spin labeling; BOLD, blood oxygen level-dependent; CO2, carbon dioxide.
Summary of key results, where mean±standard deviation (s.d.) was calculated excluding the outlier (subject 4). Cerebrovascular reactivity (CVR) values are given in % change per mm Hg increase in partial pressure of CO2.
These values have been corrected for the additional loss of signal due to difference in T1 of highly oxygenated versus baseline blood and change in tagging efficiency between baseline and hypercapnic states.
Figure 4.
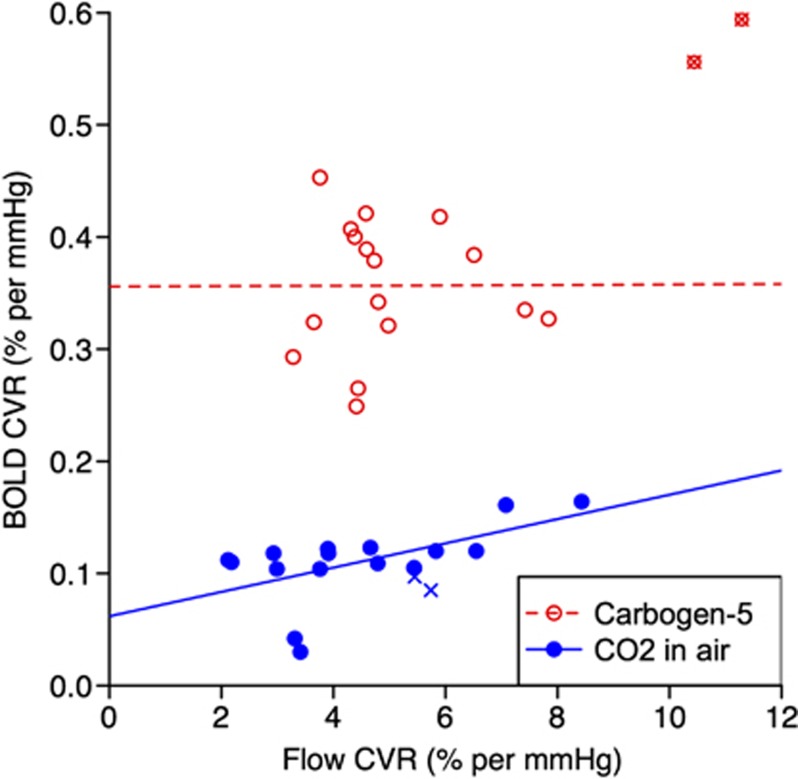
Comparison of CVR values as measured by flow and BOLD response. For carbogen-5 (red, open circles) R2<0.001, P=0.99; for 5% carbon dioxide (CO2) in air (blue, filled circles) R2=0.33, P=0.02 (excluding outlier hemispheres, marked by crosses). BOLD, blood oxygen level-dependent; CVR, cerebrovascular reactivity.
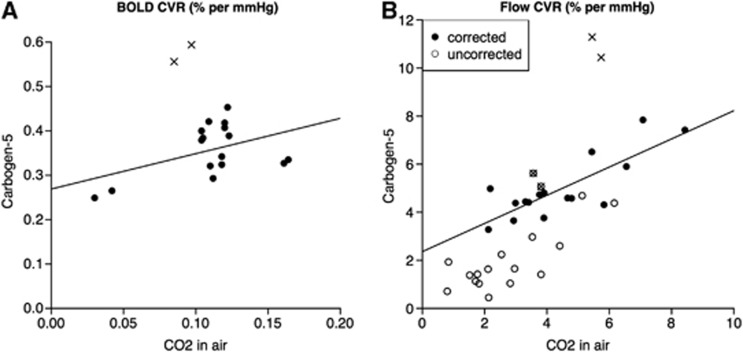
Figure 5.
Comparison of CVR values during stimuli of carbon dioxide (CO2) in air versus carbogen-5, as calculated by (A) BOLD and (B) flow response, where open circles represent raw ASL data points and filled circles have been corrected for varying T1 and tagging efficiency. The outlier hemispheres are represented by crosses, within open circles in the uncorrected case. For BOLD CVR, R2=0.21, P=0.07 and for corrected flow CVR, R2=0.66, P<0.001. ASL, arterial spin labeling; BOLD, blood oxygen level-dependent; CVR, cerebrovascular reactivity.
As shown in Figure 4, CVR values calculated from BOLD and ASL acquisitions (after correction for variable T1 and tagging efficiency) are correlated under CO2 exposure (R2=0.33, P=0.02), but not for carbogen (R2<0.001, P=0.99). Values of BOLD CVR for the two stimuli are not significantly correlated with R2=0.21, P=0.07, whereas flow CVR values are correlated with R2=0.66, P<0.001 (see Figure 5). However even though there is a correlation between the results, the actual values for CVR are not consistent between the two gas mixtures, as demonstrated by the fitted line shown in Figure 5B, the equation for which is y=0.59x+2.36. This means that flow CVR values measured in response to one gas mixture cannot be directly compared with data obtained from a different gas stimulus.
Discussion
The key findings of this experiment are that (1) the correlation observed between flow and BOLD CVR to a CO2 in air gas mixture breaks down during carbogen breathing and (2) intrasubject flow CVR as measured by ASL is affected by the partial pressure of oxygen dissolved in the blood (PO2) and is not consistent between the two gas stimuli.
Mean flow CVR to CO2 in air in our study was 4.46±1.80%. Some values obtained from gray matter in previous studies are 3.09±2.41% (25 subjects, 50 hemispheres),11 3.35±1.63% (10 subjects),32 and 4.5±1.3% (8 subjects),33 all of which were performed under comparable conditions.
Values of BOLD CVR are more difficult to compare as the absolute BOLD signal recorded is strongly dependent on several parameters, including field strength of the scanner and TE; in our study, we recorded 0.11±0.03% with TE=35 ms under similar conditions to 0.21±0.12% (25 subjects, 50 hemispheres),11 0.87±0.29% (11 subjects),32 and 0.31±0.08% (9 subjects),23 all of which used 3-T scanners and TE=30 ms.
Figure 4 shows that for the CO2 in air gas mixture, there is some correlation between CVR as measured by BOLD and ASL. This is in agreement with the findings of Mandell et al11 that the increase in BOLD signal during hypercapnia is predominantly due to changes in CBF, even in patients with steno-occlusive disease. In our study, R2=0.33 and P=0.02 which is by no means conclusive; however, the results presented by Mandell et al had values of R2=0.69 and P<0.0001 using data from 50 hemispheres in 25 people, significantly more than our 16 data points, suggesting that the trend observed in our data is genuine.
There are very good theoretical reasons why BOLD signal response to carbogen inhalation should not be used to try to measure CVR. Cerebrovascular reactivity is a physiologic response to an increase in PCO2, and when using BOLD imaging during CO2 exposure, the principal factor influencing the BOLD signal change is the concentration of deoxyhemoglobin in the blood due to an increase in CBF, giving an indirect measure of CVR. However during carbogen inhalation, PaO2 also increases significantly, causing the BOLD signal to increase from its baseline value due to (1) increased CBF due to higher PaCO2 and (2) an increase in the arterial oxygen concentration PaO2, both of which lead to higher venous oxygen saturation via independent mechanisms, as previously discussed by Prisman et al.34 A carbogen stimulus reduces the concentration of deoxyhemoglobin by more than one mechanism, and as a result it is not possible to ascertain how much of the ensuing BOLD signal increase is due to CVR alone. Consequently, increasing PO2 from baseline levels confounds the observed BOLD activation and obscures increases in signal which are due to elevated CBF alone. Indeed, after removal of the outlier the correlation between BOLD and flow CVR to carbogen gas completely disappears (see Figure 4), with R2=1.58 × 10−5 and P=0.99, showing that during a simultaneous increase in PO2 and PCO2 during a carbogen stimulus an increase in BOLD signal does not primarily reflect an increase in CBF.
Arterial Spin Labeling and Carbogen
The remaining question is whether ASL can be used to reliably measure CVR to CO2 during carbogen inhalation. From Figure 5B, there does not appear to be a simple 1:1 relationship between flow CVR to CO2 in air and flow CVR to carbogen, although with R2=0.66, P<0.001 the two methods are relatively strongly correlated. This shows that the higher oxygen content of carbogen gas has a non-negligible effect on CBF. A higher PvO2 indicates an oxygen-rich environment in which CO2 has a lower affinity to Hb (Haldane effect). In addition, the high PaCO2 causes Hb to release much of its bound oxygen (Bohr effect), further increasing PvO2. This combination of physiologic effects creates a much more complex situation than a simple increase in PaCO2 and the two stimuli should not be expected to yield comparable values, as shown by our results.
Naïvely, as the CO2 content of both gas stimuli was the same at 5%, one might assume that both gas mixtures would cause an equal increase in CBF due to CO2, and that the additional oxygen in carbogen would act as a vasoconstrictor. Thus, one might expect flow CVR to carbogen to be consistently lower than that to CO2 in air. Figure 5B shows that for our data this is not the case; in fact, average flow CVR to carbogen (4.97±1.30%) is slightly higher than that to CO2 in air (4.46±1.80%). This emphasizes the complex nature of the interaction between the Bohr and Haldane effects and the pitfalls of treating oxygen and CO2 content of gas mixtures as separate properties that can be considered in isolation.
Potential Concerns and Future Work
A potential confound of using carbogen gas in any study is that the interplay between the Bohr and Haldane effects is likely to influence the arterial–alveolar diffusion gradient in the lungs and thus affect the process of gas exchange. In this situation, recorded end-tidal partial pressure values may no longer be accurate indicators of PO2 and PCO2 of arterial blood (as has been assumed). Furthermore, arterial partial pressures will have a different relationship to partial pressures in the tissues, the latter being the actual variables of interest.
In this study, we estimated a value of the calibration constant M0B of arterial blood for each subject based on the BOLD signal within a thalamus mask. Improved estimation of this parameter may improve the T1 correction to measured blood flow and flow CVR during epochs of breathing oxygen-enriched air, such as carbogen. This could be done by an additional short calibration scan, which can acquire a value of M0 for each voxel in the brain.
We have used the Buxton model29 to correct for the changes in T1 that occur due to an increased concentration of inspired oxygen and the effect that this has on CBF quantification, also taking into account variations in tagging efficiency between normocapnic and hypercapnic states. The fitted line in Figure 5B has equation y=0.59x+2.36 (R2=0.66, P<0.001), which clearly differs from the previously assumed y=x; however, with our relatively small data set and potentially imperfect T1 correction, the possibility that a ‘true' population fit may yield a 1:1 relationship cannot be definitively excluded. This could support a recent finding by Xu et al35 that CBF appears to be unaffected by hyperoxia. The uncertainty around the flow correction and the conclusions that could be drawn from Figure 5B highlight the importance of giving proper consideration to the change in blood T1 due to varying oxygenation. However, the fact remains that neither raw ASL values nor corrected flow values (using the current method of correction) obtained from carbogen inhalation yield comparable CVR results to the traditional stimulus of CO2 exposure, a finding which is of clinical importance. An experiment investigating BOLD and ASL signal changes to a variety of inspired oxygen fractions during CO2 exposure may provide further insight into the effect of oxygen on CVR.
In conclusion, BOLD imaging should not be used in conjunction with a carbogen stimulus to measure CVR because the high oxygen content of carbogen makes it impossible to identify what proportion of the ensuing increase in BOLD signal is due to increased blood flow (as opposed to simply an increase in venous oxygen saturation). The complex and unknown interactions between the individually well-documented Bohr and Haldane effects become significant during carbogen inhalation, with the result that PetCO2 may no longer be a good proxy for tissue PCO2. In addition, hyperoxia reduces the T1 of blood so an additional correction must be applied to obtain CBF values from ASL data. We have found that intrasubject flow CVR as measured by ASL is affected by PO2 and is not consistent between CO2 in air and carbogen. Although the possibility remains that this inconsistency could be a result of imperfect estimation of tissue pressure of CO2 and CBF under a combined hyperoxia and hypercapnia regime, particular care should be taken when comparing flow CVR values obtained from stimuli containing different fractions of oxygen.
Acknowledgments
The authors are grateful to the EPSRC and the MRC for funding this research.
The authors declare no conflict of interest.
References
- Kleiser B, Widder B. Course of carotid artery occlusions with impaired cerebrovascular reactivity. Stroke. 1992;23:171–174. doi: 10.1161/01.str.23.2.171. [DOI] [PubMed] [Google Scholar]
- Yonas H, Smith HA, Durham SR, Pentheny SL, Johnson DW. Increased stroke risk predicted by compromised cerebral blood flow reactivity. J Neurosurg. 1993;79:483–489. doi: 10.3171/jns.1993.79.4.0483. [DOI] [PubMed] [Google Scholar]
- Silvestrini M, Troisi E, Matteis M, Cupini LM, Bernardi G. Effect of smoking on cerebrovascular reactivity. J Cereb Blood Flow Metab. 1996;16:746–749. doi: 10.1097/00004647-199607000-00027. [DOI] [PubMed] [Google Scholar]
- Lu H, Xu F, Rodrigue KM, Kennedy KM, Cheng Y, Flicker B, et al. Alterations in cerebral metabolic rate and blood supply across the adult lifespan. Cereb Cortex. 2011;21:1426–1434. doi: 10.1093/cercor/bhq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rother J. CT and MRI in the diagnosis of acute stroke and their role in thrombolysis. Thromb Res. 2001;103:S125–S133. doi: 10.1016/s0049-3848(01)00309-7. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Huang J, Chowdhury MH. MRI confirms mild cognitive impairments prodromal for Alzheimer's, vascular and Parkinson-Lewy body dementias. J Neurol Sci. 2007;257:97–104. doi: 10.1016/j.jns.2007.01.016. [DOI] [PubMed] [Google Scholar]
- van Straaten EC, Scheltens P, Barkhof F. MRI and CT in the diagnosis of vascular dementia. J Neurol Sci. 2004;226:9–12. doi: 10.1016/j.jns.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Fulesdi B, Limburg M, Bereczki D, Kaplar M, Molnar C, Kappelmayer J, et al. Cerebrovascular reactivity and reserve capacity in type II diabetes mellitus. J Diabetes Complications. 1999;13:191–199. doi: 10.1016/s1056-8727(99)00044-6. [DOI] [PubMed] [Google Scholar]
- Sheline YI. 3D MRI studies of neuroanatomic changes in unipolar major depression: the role of stress and medical comorbidity. Biol Psychiatry. 2000;48:791–800. doi: 10.1016/s0006-3223(00)00994-x. [DOI] [PubMed] [Google Scholar]
- Vakilian A, Iranmanesh F. Assessment of cerebrovascular reactivity during major depression and after remission of disease. Ann Indian Acad Neurol. 2010;13:52–56. doi: 10.4103/0972-2327.61278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell DM, Han JS, Poublanc J, Crawley AP, Stainsby JA, Fisher JA, et al. Mapping cerebrovascular reactivity using blood oxygen level-dependent MRI in patients with arterial steno-occlusive disease: comparison with arterial spin labeling MRI. Stroke. 2008;39:2021–2028. doi: 10.1161/STROKEAHA.107.506709. [DOI] [PubMed] [Google Scholar]
- Bulte DP, Kelly M, Germuska M, Xie J, Chappell MA, Okell TW, et al. Quantitative measurement of cerebral physiology using respiratory-calibrated MRI. Neuroimage. 2012;60:582–591. doi: 10.1016/j.neuroimage.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier CJ, Madjar C, Tancredi FB, Stefanovic B, Hoge RD. Elimination of visually evoked BOLD responses during carbogen inhalation: implications for calibrated MRI. Neuroimage. 2011;54:1001–1011. doi: 10.1016/j.neuroimage.2010.09.059. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Krainik A, David O, Salon C, Tropres I, Hoffmann D, et al. Impaired fMRI activation in patients with primary brain tumors. Neuroimage. 2010;52:538–548. doi: 10.1016/j.neuroimage.2010.04.194. [DOI] [PubMed] [Google Scholar]
- Cantin S, Villien M, Moreaud O, Tropres I, Keignart S, Chipon E, et al. Impaired cerebral vasoreactivity to CO2 in Alzheimer's disease using BOLD fMRI. Neuroimage. 2011;58:579–587. doi: 10.1016/j.neuroimage.2011.06.070. [DOI] [PubMed] [Google Scholar]
- Donahue MJ, Ayad M, Moore R, van Osch M, Singer R, Clemmons P, et al. Relationships between hypercarbic reactivity, cerebral blood flow, and arterial circulation times in patients with moyamoya disease J Magn Reson Imagingadvance online publication, 25 February 2013doi: 10.1002/jmri.24070(e-pub ahead of print). [DOI] [PMC free article] [PubMed]
- Hamzei F, Knab R, Weiller C, Rother J. The influence of extra- and intracranial artery disease on the BOLD signal in FMRI. Neuroimage. 2003;20:1393–1399. doi: 10.1016/S1053-8119(03)00384-7. [DOI] [PubMed] [Google Scholar]
- Xu F, Uh J, Brier MR, Hart J, Jr., Yezhuvath US, Gu H, et al. The influence of carbon dioxide on brain activity and metabolism in conscious humans. J Cereb Blood Flow Metab. 2011;31:58–67. doi: 10.1038/jcbfm.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Pike GB. Global cerebral oxidative metabolism during hypercapnia and hypocapnia in humans: implications for BOLD fMRI. J Cereb Blood Flow Metab. 2010;30:1094–1099. doi: 10.1038/jcbfm.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier CJ, Hoge RD. A generalized procedure for calibrated MRI incorporating hyperoxia and hypercapnia. Hum Brain Mapp. 2013;34:1053–1069. doi: 10.1002/hbm.21495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Harris AD, Diukova A, Evans CJ, Lythgoe DJ, Zelaya F, et al. Pulsed arterial spin labeling perfusion imaging at 3T: estimating the number of subjects required in common designs of clinical trials. Magn Reson Imaging. 2011;29:1382–1389. doi: 10.1016/j.mri.2011.02.030. [DOI] [PubMed] [Google Scholar]
- Dai W, Garcia D, de Bazelaire C. Alsop DC. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med. 2008;60:1488–1497. doi: 10.1002/mrm.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yezhuvath US, Lewis-Amezcua K, Varghese R, Xiao G, Lu H. On the assessment of cerebrovascular reactivity using hypercapnia BOLD MRI. NMR Biomed. 2009;22:779–786. doi: 10.1002/nbm.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young WL, Prohovnik I, Ornstein E, Ostapkovich N, Matteo RS. Cerebral blood flow reactivity to changes in carbon dioxide calculated using end-tidal versus arterial tensions. J Cereb Blood Flow Metab. 1991;11:1031–1035. doi: 10.1038/jcbfm.1991.171. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, et al. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2009;45:S173–S186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Bulte DP, Chiarelli PA, Wise RG, Jezzard P. Cerebral perfusion response to hyperoxia. J Cereb Blood Flow Metab. 2007;27:69–75. doi: 10.1038/sj.jcbfm.9600319. [DOI] [PubMed] [Google Scholar]
- Buxton RB, Frank LR, Wong EC, Siewert B, Warach S, Edelman RR. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med. 1998;40:383–396. doi: 10.1002/mrm.1910400308. [DOI] [PubMed] [Google Scholar]
- Herscovitch P, Raichle ME. What is the correct value for the brain-blood partition coefficient for water. J Cereb Blood Flow Metab. 1985;5:65–69. doi: 10.1038/jcbfm.1985.9. [DOI] [PubMed] [Google Scholar]
- Lu H, Clingman C, Golay X, van Zijl PC. Determining the longitudinal relaxation time (T1) of blood at 3.0 Tesla. Magn Reson Med. 2004;52:679–682. doi: 10.1002/mrm.20178. [DOI] [PubMed] [Google Scholar]
- Mark CI, Slessarev M, Ito S, Han J, Fisher JA, Pike GB. Precise control of end-tidal carbon dioxide and oxygen improves BOLD and ASL cerebrovascular reactivity measures. Magn Reson Med. 2010;64:749–756. doi: 10.1002/mrm.22405. [DOI] [PubMed] [Google Scholar]
- Noth U, Meadows GE, Kotajima F, Deichmann R, Corfield DR, Turner R. Cerebral vascular response to hypercapnia: determination with perfusion MRI at 1.5 and 3.0 Tesla using a pulsed arterial spin labeling technique. J Magn Reson Imaging. 2006;24:1229–1235. doi: 10.1002/jmri.20761. [DOI] [PubMed] [Google Scholar]
- Prisman E, Slessarev M, Han J, Poublanc J, Mardimae A, Crawley A, et al. Comparison of the effects of independently-controlled end-tidal PCO(2) and PO(2) on blood oxygen level-dependent (BOLD) MRI. J Magn Reson Imaging. 2008;27:185–191. doi: 10.1002/jmri.21102. [DOI] [PubMed] [Google Scholar]
- Xu F, Liu P, Pascual JM, Xiao G, Lu H. Effect of hypoxia and hyperoxia on cerebral blood flow, blood oxygenation, and oxidative metabolism. J Cereb Blood Flow Metab. 2012;32:1909–1918. doi: 10.1038/jcbfm.2012.93. [DOI] [PMC free article] [PubMed] [Google Scholar]