Abstract
Growing evidence suggests that endogenous lactate is an important substrate for neurons. This study aimed to examine cerebral lactate metabolism and its relationship with brain perfusion in patients with severe traumatic brain injury (TBI). A prospective cohort of 24 patients with severe TBI monitored with cerebral microdialysis (CMD) and brain tissue oxygen tension (PbtO2) was studied. Brain lactate metabolism was assessed by quantification of elevated CMD lactate samples (>4 mmol/L); these were matched to CMD pyruvate and PbtO2 values and dichotomized as glycolytic (CMD pyruvate >119 μmol/L vs. low pyruvate) and hypoxic (PbtO2 <20 mm Hg vs. nonhypoxic). Using perfusion computed tomography (CT), brain perfusion was categorized as oligemic, normal, or hyperemic, and was compared with CMD and PbtO2 data. Samples with elevated CMD lactate were frequently observed (41±8%), and we found that brain lactate elevations were predominantly associated with glycolysis and normal PbtO2 (73±8%) rather than brain hypoxia (14±6%). Furthermore, glycolytic lactate was always associated with normal or hyperemic brain perfusion, whereas all episodes with hypoxic lactate were associated with diffuse oligemia. Our findings suggest predominant nonischemic cerebral extracellular lactate release after TBI and support the concept that lactate may be used as an energy substrate by the injured human brain.
Keywords: cerebral blood flow, cerebral metabolism, cerebral microdialysis, lactate, traumatic brain injury
Introduction
Glucose is the obligate energy substrate for the brain. Abundant experimental evidence however suggests that lactate is also an important energetic substrate for the brain. More than a decade ago, Pellerin and Magistretti1 showed that astrocytes, in response to glutamate-dependent activity, can uptake glucose and convert it into lactate, which in turn may efficiently be oxidized by neurons via the tricarboxylic acid cycle. This process, named astrocyte–neuron lactate shuttle, challenged the dogma stating that lactate was just a waste by-product issued from anaerobic metabolism. Lactate production by astrocytes to supply neuronal demand was later confirmed in vitro.2, 3 Moreover, endogenous lactate was shown to act as preferential substrate,4 particularly in conditions of hypoxia.5 Experimental evidence further suggests that exogenously administered lactate exerts significant neuroprotection after acute injury6 and improves cognitive recovery in animal models of traumatic brain injury (TBI)7 and cerebral ischemia.8, 9 Recent evidence suggests that lactate may also have an important role in the regulation of memory processing10 and axonal regeneration.11
Traumatic brain injury is a major cause of physical and cognitive disability. The principal aim of TBI management is to prevent and treat secondary cerebral damage, which may add further burden to patient outcome. Although cerebral ischemia may be observed after TBI and cause an increase in extracellular cerebral lactate, recent data found aerobic utilization12, 13 and uptake14 of endogenous lactate by the injured human brain, pointing towards alternative mechanisms of lactate release other than hypoxia/ischemia. These findings suggest a potential role of endogenous lactate in the regulation and early adaptation of brain metabolism after TBI and indicate that lactate metabolism might have a crucial role in the pathophysiology of acute brain injury.
The objective of this study was to examine cerebral lactate metabolism in the early phase of severe TBI in humans, using an approach that combined cerebral microdialysis (CMD) and brain tissue oxygen tension (PbtO2) monitoring to measure regional brain metabolism with perfusion computed tomography (PCT) to assess global brain perfusion. Our study hypothesis was that elevation of lactate in brain interstitial tissue of patients with TBI is frequent and is predominantly nonhypoxic/ischemic, i.e., it is associated with aerobic glycolysis and normal brain perfusion.
Material and Methods
Patients
This was an observational study that included a cohort of 24 patients with severe TBI admitted to the Department of Intensive Care Medicine, Lausanne University Hospital (CHUV), Lausanne, Switzerland, between October 2009 and December 2012. Inclusion criteria were patients with severe TBI (defined by a Glasgow Coma Scale ≤8) and abnormal CT scan (Marshall score of 2 or more) at admission, having an indication for advanced intracranial monitoring consisting of CMD, PbtO2, and intracranial pressure (ICP) probes as a part of standard patient care. Exclusion criteria for advanced intracranial monitoring were expected brain death within 48 hours and an age <18 or >60 years. The study complies with the ARRIVE (Animal Research: Reporting In Vivo Experiments) guidelines. Approval for the study was obtained by the Ethical Committee, University of Lausanne, Lausanne, Switzerland. Waiver of consent was obtained as monitoring was part of standard patient care. For the purpose of this study, only patients who had at least 24 hours of valid intracranial monitoring data were considered. Eight patients were excluded because of monitoring malfunctioning (mainly, failure in collecting patient CMD fluid) or <24 hours monitoring duration.
General Management
Severity of the lesions was assessed by an admission noncontrast CT and classified using the Marshall score.15 All patients were treated according to a standard protocol for the management of severe TBI following an institutional algorithm and according to international guidelines.16, 17, 18 Sedation/analgesia consisted of propofol and sufentanyl. All patients were mechanically ventilated to maintain partial pressure of oxygen in arterial blood at 90 to 100 mm Hg and PaCO2 (partial pressure of carbon dioxide in arterial blood) at 35 to 40 mm Hg. Brain physiologic targets were set to maintain ICP <20 mm Hg, cerebral perfusion pressure (CPP=mean arterial pressure (MAP, measured via an intra-arterial catheter)−ICP) >60 mm Hg, and to avoid PbtO2 <20 mm Hg. Blood glucose was targeted at 6 to 8 mmol/L. All patients received antiepileptic prophylaxis with phenytoin or levetiracetam for 7 days. First-step measures to treat intracranial hypertension (ICP >20 mm Hg) included deep sedation/analgesia, moderate hyperventilation (PaCO2, 30 to 35 mm Hg), osmotherapy (with hypertonic saline or mannitol), and controlled normothermia (35°C to 37°C).
Measure of Cerebral Metabolism and Oxygenation
Regional cerebral metabolism was measured using a CMA 70 microdialysis catheter with a 20 kDa cutoff (M Dialysis AB®, Stockholm, Sweden). The microdialysis probe was perfused with artificial cerebrospinal fluid via a CMA 106 pump (M Dialysis AB®) at a rate of 0.3 μL/min. Microdialysis samples were collected every 60 minutes and analyzed at the bedside; brain extracellular concentrations of glucose, lactate, pyruvate, and glutamate were measured using a kinetic enzymatic analyzer (ISCUS flex; M Dialysis AB®). Brain tissue oxygen tension was measured using a Licox® catheter (Integra Neurosciences, Plainsboro, NJ, USA). The PbtO2 probe was calibrated to patient's temperature and its correct functioning was confirmed after an oxygen challenge (fraction of inspired oxygen 100% for 5 minutes, accompanied by a PbtO2 increase of at least 50%). Parenchymal ICP was measured using a Codman® probe (Codman ICP Monitoring System, Raynham, MA, USA).
All the three probes were inserted through a triple-lumen bolt (Integra Neurosciences, Plainsboro, NJ, USA) by the neurosurgeon in the operating room, and placed into subcortical white matter, usually into the right frontal lobe. In all patients, a follow-up noncontrast head CT was performed at ≈24 hours to confirm the correct placement of the intracranial monitoring.
Measurement of Brain Perfusion
Brain PCT was performed during the first control CT (at 24 to 48 hours after TBI), immediately following the unenhanced series, using a multidetector row CT Lightspeed (GE Medical Systems, Milwaukee, WI, USA). Scanning was initiated 5 seconds after injection of 50 mL of iohexol (300 mg/mL of iodine; GE Healthcare Europe, Glattbrugg, Switzerland), and perfused at a rate of 5 mL/s with the following parameters: 80 kV, 240 mA, 0.4 rotations/s, and total duration of 50 seconds. The series evaluated two adjacent 5-mm-thick sections of brain parenchyma.
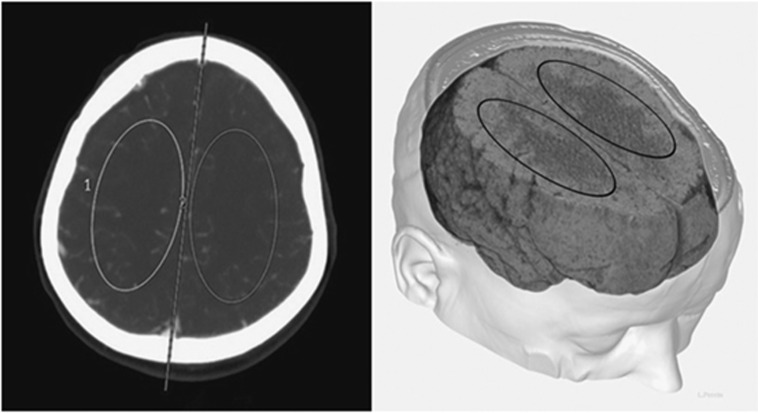
Postprocessing of PCT data was performed by an experienced neuroradiologist (JBZ), masked to CMD and PbtO2 variables, using a dedicated software (Brilliance Workspace Portal® Philips Medical Systems, Cleveland, OH, USA), which uses the central volume principle using deconvolution to measure the mean transit time; cerebral blood volume is calculated from the time–enhancement curves, and cerebral blood flow (CBF) is derived from the equation: CBF=cerebral blood volume/mean transit time. Two main regions of interest (ROIs, one for each hemisphere) of approximately 250 mm2 were selected above the ventricular system and included anterior and middle cerebral artery territories (Figure 1). Three-dimensional reconstruction was processed with Carestream Vue PACS (Carestream Health, Rochester, NY, USA) using a series of the thin-slice enhanced brain CT. Because intracranial probes were located in the white matter, ROIs were drawn in areas of predominant white matter to allow concordant measurement of global supratentorial perfusion in the same type of tissue. ROI's characteristics were selected in line with our previous studies,19 providing an accurate quantitative assessment of CBF.
Figure 1.
Schematic illustration to show the position and shape of the region of interests (ROIs) on perfusion computed tomography (CT), in relation to the brain anatomy. The left side of the panel shows how ROIs were selected for the measurement of cerebral blood flow in predominant white matter tissue on a contrast-enhanced CT. The right side of the panel is a three-dimensional representation of ROIs in relation to the brain anatomy.
Once the postprocessing completed, each PCT was categorized as oligemic, normal, or hyperemic according to CBF values, as follows: oligemia was defined by a CBF <32.5 mL/100 g/min, hyperemia by a CBF >69 mL/100 g/min, and normal perfusion by CBF values between 32.5 and 69 mL/100 g/min. This definition corresponded to values previously reported by our group.20
Data Collection and Processing
Data were collected for a maximum of 7 days from intracranial monitoring start. All brain physiologic variables (PbtO2, ICP, and CPP) were recorded every 60 seconds by a computerized medical chart system (Metavision® iMDsoft, Tel-Aviv, Israel). After data extraction, artifacts (e.g., periods of disconnection from monitoring devices or flushing of arterial line) and values outside obvious range were manually eliminated. Data from the first hour after intracranial monitoring insertion were discarded, as they correspond to probes' equilibration time. Hourly CMD samples were matched to physiologic variables using STATA software (STATA® Corporation, College Station, TX, USA) as follows: as CMD samples represent metabolites accumulated during the previous hour, lactate samples were matched to hypoxic episodes, defined by a PbtO2 <20 mm Hg for at least 5 minutes at any time during the hour previous to CMD sampling. The same process was used to determine and match episodes of ICP (>20 mm Hg) and low CPP (<60 mm Hg). Lactate elevations were defined by a CMD lactate >4 mmol/L: this threshold was used in accordance with our previous study13 and in line with a recent large prospective study in TBI patients.21 Lactate elevations were then categorized—according to the levels of PbtO2 and CMD pyruvate—as hypoxic (PbtO2 <20 mm Hg) vs. nonhypoxic and as glycolytic (CMD pyruvate >119 μmol/L) vs. low pyruvate. Although variable thresholds of PbtO2 (<10, <15, and <20 mm Hg) have been described,16 the threshold for brain hypoxia selected in this study was <20 mm Hg (i.e., moderate brain hypoxia22), consistent with our previous study.13 Hypoxia/ischemia causes a failure to regenerate the tricarboxylic acid cycle and nicotinamide adenine dinucleotide, with subsequent reduction of pyruvate concentrations as a consequence of anaerobic metabolism. Conversely, the normal level of brain extracellular pyruvate is a marker of aerobic glycolysis. Based on previous reports in subjects undergoing brain surgery23 and studies performed in poor-grade subarachnoid hemorrhage patients,24 we defined the threshold of normal pyruvate as >119 μmol/L, and categorized elevated CMD lactate as glycolytic when CMD pyruvate was >119 μmol/L, which was also consistent with our previous study.13 Conservative margins were applied to the thresholds such that samples were only counted as having elevated lactate if the concentration was ≥4.1 mmol/L, and that glycolytic pyruvate samples were only counted if the concentration was ≥119.5 μmol/L.
To examine the relationship of cerebral lactate metabolism with brain perfusion, CMD and PbtO2 variables were matched to PCT results as follows: we matched CMD/PbtO2 data at the time of PCT, plus data from the 3 hours previous and after PCT time (total of seven epochs). We considered this time window around the PCT to be representative of patient brain state.
Statistical Analysis
Data analysis was performed using JMP-10® (SAS Institute). Demographic data were presented in means and standard deviation (s.d.), except when stated otherwise. Patient percentages of both cerebral metabolic lactate patterns (hypoxic and glycolytic), along with episodes of hypoxia, ICP, and low CPP, were calculated both for (a) the total duration of monitoring and (b) daily, over the first 5 days after TBI. Individual brain physiologic data were presented as means per patient percentages with s.d. or standard error of the mean (s.e.m.). Comparison between the values of CBF for each PCT was assessed by a one-way analysis of variance. Associations between brain/systemic physiologic variables and cerebral perfusion were performed using Wilcoxon's test with Bonferroni corrections for continuous variables and Fisher's exact test for categorical variables. Statistical significance was set at P<0.05.
Results
Patient Characteristics
A total of 24 patients were studied. Mean age was 36±15 years, the median admission Glasgow Coma Scale was 5 (range 3 to 8), and the median Marshall score was 3 (range 2 to 5). Patient characteristics are summarized in Table 1. Means of cerebral and systemic physiologic variables around PCT time were within normal ranges in all patients. Furthermore, we observed no significant differences in those variables between the three different PCT conditions (oligemia, normal, and hyperemia), except for PaCO2, which was significantly higher in the hyperemic perfusion group vs. normal perfusion group. However, values of PaCO2 in the hyperemic group remained within normal ranges (35 to 40 mm Hg) (Table 2).
Table 1. Patient characteristics.
| Variable | Value |
|---|---|
| Patient number | 24 |
| Age (years) | 36±15 |
| Gender, female/male | 6/18 |
| Median admission Glasgow Coma Scale | 5 (3–8) |
| Time from TBI to monitoring (h) | 15±12 |
| Marshall CT classification | |
| 2 | 12 |
| 3 | 7 |
| 4 | 2 |
| 5 | 3 |
| Glasgow outcome score at 6–12 monthsa | |
| 1 (death) | 3 |
| 2 (vegetative) | 1 |
| 3 (severe disability) | 6 |
| 4 (moderate disability) | 7 |
| 5 (complete recovery) | 6 |
CT, computed tomography; TBI, traumatic brain injury.
Data are shown as mean±s.d., except when stated otherwise.
One outcome missing.
Table 2. Main cerebral and systemic physiologic variables around perfusion CT time, according to brain perfusion pattern.
|
Oligemia |
Normal |
Hyperemia |
||
|---|---|---|---|---|
| Variable | n=6 | n=5 | n=5 | P value |
| Intracranial pressure (mm Hg) | 10±7 | 11±4 | 10±9 | NS |
| Cerebral perfusion pressure (mm Hg) | 70±6 | 72±9 | 70±9 | NS |
| PaO2 (mm Hg) | 158±73 | 117±24 | 111±20 | NS |
| PaO2/FiO2 | 298±124 | 338±61 | 316±140 | NS |
| PaCO2 (mm Hg) | 36±3 | 36±3 | 39±3a | 0.03 |
| SaO2 (%) | 99±1 | 99±1 | 98±1 | NS |
| Hemoglobin (g/L) | 106±18 | 117±19 | 110±19 | NS |
CT, computed tomography; FiO2, fraction of inspired oxygen; NS, not significant; PaCO2, partial pressure of carbon dioxide in arterial blood; PaO2, partial pressure of oxygen in arterial blood; SaO2, saturation level of oxygen in hemoglobin; TBI, traumatic brain injury.
Results are expressed as mean±s.d. Time from TBI to perfusion CT was 53±30 hours. Associations between brain/systemic physiologic variables and cerebral perfusion were performed using Wilcoxon's test with Bonferroni corrections for continuous variables.
P<0.05 for comparison between hyperemia and normal brain perfusion.
In all patients, intracranial monitoring was started within 6 hours from hospital admission and on average 15±12 hours from brain trauma. Duration of intracranial monitoring lasted 5±3 days. Cerebral microdialysis and PbtO2 probes were placed in the right frontal lobe in the majority (19/24) of patients, and in the left frontal lobe in 5 patients. After review of the CT scans, intracranial probes were predominantly placed in areas of normal brain parenchyma (21 patients), whereas in three patients they were located in brain areas around a contusion.
Cerebral Lactate Metabolism
A total of 1,447 CMD/PbtO2 matched samples were analyzed. Patterns of elevated brain extracellular lactate were examined individually. Brain lactate was frequently elevated (>4 mmol/L). Over the entire duration of monitoring, 41±8% of total individual CMD lactate samples were >4 mmol/L and the large majority of patients (21/24; 88%) had episodes with elevated CMD lactate. In contrast, episodes with brain hypoxia (defined by at least 5 minutes of PbtO2 <20 mm Hg) were less frequently observed and only accounted for 19±7% of total individual samples.
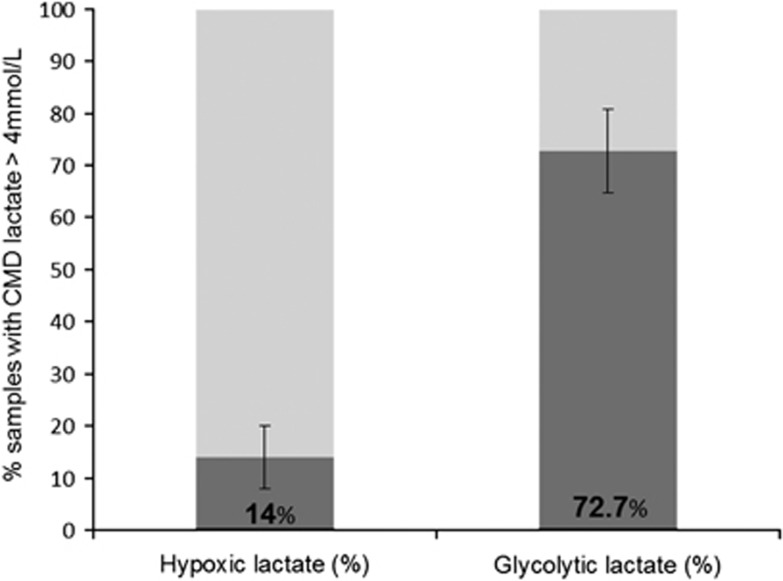
When further examining cerebral lactate metabolism, we found that the majority (73±8%) of individual lactate elevations were attributable to activated cerebral glycolysis, whereas only a minority of them (14±6%) were associated with brain hypoxia (Figure 2, total duration of monitoring). Compared with hypoxic lactate, episodes with glycolytic lactate had higher levels of lactate, pyruvate, and PbtO2 (Table 3). Episodes with hypoxic lactate pattern had higher lactate-to-pyruvate ratio (LPR) and glutamate, but this difference was not statistically significant. Of note, mean values of lactate, pyruvate, and LPR were comparable between normal (n=21) vs. pericontusional (n=3) brain tissue (CMD lactate 3.4±1.5 vs. 3.2±0.8 mmol/L, CMD pyruvate 124±48 vs. 128.3±46.6 μmol/L, LPR 28±8 vs. 28±10; all P>0.2). Brain tissue oxygen tension was higher in pericontusional vs. normal tissue (32.4±6.3 vs. 30±7.7 mm Hg, P<0.01).
Figure 2.
Cerebral lactate metabolic patterns in patients with traumatic brain injury (TBI) during the total duration of monitoring. Histograms represent individual cerebral metabolic lactate patterns (hypoxic vs. glycolytic) during the entire monitoring duration (average 5 days; n=24 patients). Hypoxic lactate=cerebral microdialysis (CMD) lactate >4 mmol/L with PbtO2 <20 mm Hg; glycolytic lactate=CMD lactate >4 mmol/L with pyruvate >119 μmol/L. Data are mean±s.e.m.
Table 3. Differences in cerebral microdialysis markers and PbtO2 between hypoxic vs. glycolytic lactate pattern (total duration of intracranial monitoring).
| Variable | Hypoxic lactate | Glycolytic lactate | P value |
|---|---|---|---|
| Lactate (mmol/L) | 4.7±0.7 | 6.1±2.2 | 0.05 |
| Pyruvate (μmol/L) | 106.8±13.2 | 186.4±46.3 | <0.0001 |
| LPR | 40±11 | 33±11 | 0.15 |
| Glutamate (μmol/L) | 20.3±33.4 | 16.2±24.3 | 0.68 |
| PbtO2 (mm Hg) | 16.7±3.4 | 27.2±5.3 | <0.0001 |
LPR, lactate-to-pyruvate ratio; PbtO2, brain tissue oxygen tension.
Data are expressed as mean±s.d.
Among episodes with elevated CMD lactate and pyruvate >119 μmol/L, 75% of them had normal pyruvate (120 to 213 μmol/L) and 25% of them had high pyruvate (>213 μmol/L):23, 24 compared with episodes with elevated lactate and normal pyruvate, those with elevated lactate and high pyruvate displayed higher values of CMD lactate (5.0±0.9 vs. 7.7±2.4 mmol/L, P<0.001), pyruvate (162.6±23.2 vs. 256.2±29.2 μmol/L, P<0.001), and glutamate (9.6±17.4 vs. 48.6±35.5 mmol/L, P<0.001), together with slightly lower PbtO2 (27.9±5.3 vs. 26.4±5.1 mm Hg, P=0.02), whereas LPR (31±6 vs. 30±8) was comparable.
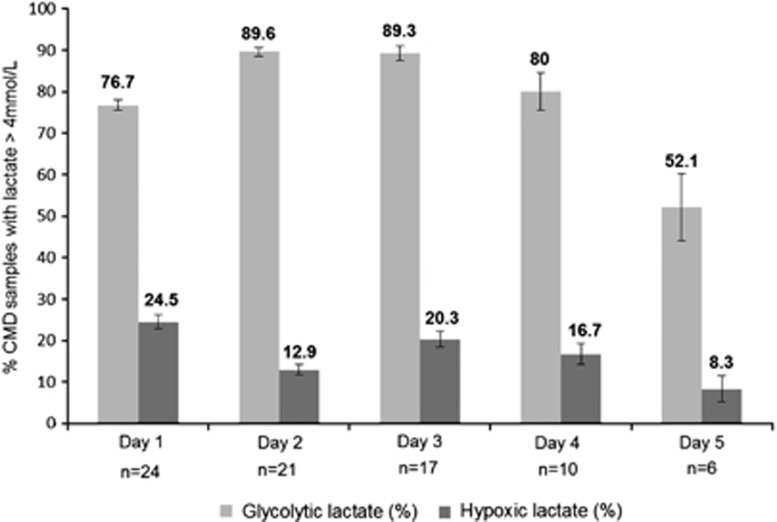
Trends over the first 5 days of both brain lactate patterns (glycolytic vs. hypoxic) are illustrated in Figure 3: a pattern of increased cerebral glycolytic lactate was consistently found from day 1 up to day 5, whereas the hypoxic lactate pattern was less frequent and predominantly observed on day 1. Absolute concentrations of each intracerebral variable for glycolytic vs. hypoxic lactate episodes across patients over the first 5 days are also shown in Table 4.
Figure 3.
Cerebral lactate metabolic patterns in patients with traumatic brain injury (TBI) over the first 5 days of monitoring. Histograms represent individual cerebral metabolic lactate patterns (hypoxic vs. glycolytic) during the first 5 days after TBI. Hypoxic lactate=cerebral microdialysis (CMD) lactate >4 mmol/L with PbtO2 <20 mm Hg; glycolytic lactate=CMD lactate >4 mmol/L with pyruvate >119 μmol/L. Data are mean±s.e.m.
Table 4. Mean values of cerebral microdialysis markers and PbtO2 for hypoxic vs. glycolytic lactate episodes over the first 5 days of intracranial monitoring.
|
Day 1 (n=24 pts) |
Day 2 (n=21 pts) |
Day 3 (n=17 pts) |
Day 4 (n=10 pts) |
Day 5 (n=6 pts) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Hypoxic | Glycolytic | Hypoxic | Glycolytic | Hypoxic | Glycolytic | Hypoxic | Glycolytic | Hypoxic | Glycolytic |
| Lactate (mmol/L) | 5.0±0.9 | 5.7±2.0 | 4.5±0.6 | 5.6±1.8 | 4.7±0.9 | 6.1±2.8 | 4.5±0.9 | 6.0±2.1 | 5.0±0.9 | 4.5±0.5 |
| Pyruvate (μmol/L) | 113.4±7.4 | 191.2±44.3 | 116.9±4.6 | 178.1±34.8 | 109.8±5.9 | 193.6±55.4 | 117.3±4.1 | 185.4±54.5 | 84.8±15.3 | 177.2±65.2 |
| LPR | 36±5 | 30±6 | 34±3 | 31±7 | 34±7 | 31±5 | 34±2 | 32±4 | 51±16 | 25±5 |
| Glutamate (mmol/L) | 21.2±16.8 | 11.2±13.9 | 19.1±11.3 | 9.9±15.9 | 13.9±15.0 | 2.6±4.9 | 75.1±7.2 | 17.7±37.8 | 79.2±11.7 | 2.5±2.9 |
| PbtO2 (mm Hg) | 14.9±3.7 | 29.0±6.2 | 17.0±2.3 | 27.9±5.8 | 17.0±3.1 | 25.4±2.9 | 18.3±1.7 | 27.0±6.0 | 16.1±1.4 | 25.3±4.8 |
LPR, lactate-to-pyruvate ratio; PbtO2, brain tissue oxygen tension; pts, patients.
Data are expressed as mean±s.d.
Glycolytic Lactate was Associated with Normal to Supranormal Brain Perfusion
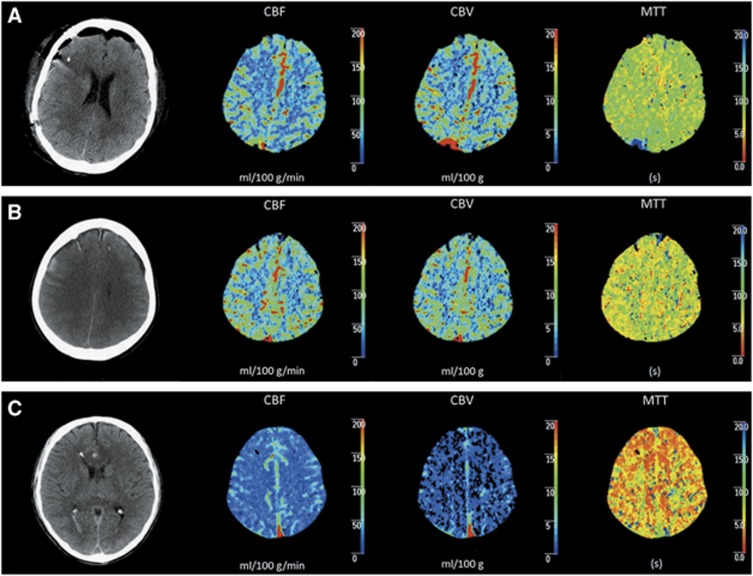
Perfusion CT was available for 16 patients and was performed on average 53±30 hours after TBI. A total of 83 MD samples and PbtO2 episodes were matched to PCT data. Samples with glycolytic lactate elevations (n=13) were always associated with normal (n=8, 62%) to supranormal (n=5, 38%) brain perfusion (Table 5). Figure 4 shows illustrative examples of increased cerebral glycolytic lactate with normal (A) and with supranormal (B) brain perfusion.
Table 5. Relationship between regional cerebral metabolic patterns and global brain perfusion.
|
Perfusion CT data |
|||
|---|---|---|---|
| Oligemia | Normal | Hyperemia | |
| CBF valuesa | |||
| CBF, ipsilateral | 28.3 (9–32.4) | 45.3 (37.1–55.7) | 76.9 (69.5–84.3) |
| CBF, contralateral | 29.7 (8.3–31.6) | 46.8 (42.7–51.2) | 76.3 (73.7–78.8) |
| Cerebral lactateb | |||
| Glycolytic lactate (n=13) | 0 | 8 (62%) | 5 (38%) |
| Hypoxic lactate(n=11) | 11 (100%) | 0 | 0 |
CBF, cerebral blood flow; CT, computed tomography.
CBF is expressed in mL/100 g/min. Ipsilateral CBF=CBF measured on the same side as intracranial probes; contralateral CBF=CBF measured on the opposite side of intracranial probes. Perfusion CT values are presented as medians (minimum–maximum).
Cerebral lactate data are presented as the number of samples (percentages).
Figure 4.
The relationship between regional cerebral lactate metabolic patterns and global brain perfusion. Illustrative examples of the relationship between brain perfusion (measured by perfusion computed tomography (CT)) and cerebral lactate metabolism, showing cerebral glycolytic lactate with normal (A) or supranormal (B) brain perfusion vs. cerebral hypoxic lactate and oligemic brain perfusion (C). Frame 1, unenhanced brain CT showing placement of intracranial monitoring; Frames 2, 3, and 4: perfusion CT maps depicting cerebral blood flow (CBF) (mL/100 g/min), cerebral blood volume (CBV) (mL/100 g), and mean transit time (MTT) (s), respectively.
Glycolytic Lactate was Associated with Normal Intracranial Pressure and Cerebral Perfusion Pressure
Over the entire duration of monitoring, episodes with elevated ICP (>20 mm Hg) and with low CPP (<60 mm Hg) represented, respectively, only 10±4% and 9±2% of all examined episodes. Importantly, episodes of cerebral glycolytic lactate increase were mainly associated with normal levels of ICP and CPP (93±4% and 88±6% of individual samples, respectively).
Hypoxic Lactate was Associated with Global Reduction of Brain Perfusion
We also found that hypoxic lactate elevations (n=11) were always associated with oligemic brain perfusion (Table 5). One illustrative example of a pattern with brain hypoxia and oligemic PCT is shown in Figure 4C. Oligemic brain perfusion was also associated with reduced cerebral extracellular pyruvate (90% of samples had CMD pyruvate <119 μmol/L). This indicates that hypoxic lactate production was associated with concomitant cerebral energy depletion, thereby suggesting a pattern of anaerobic lactate production.
Discussion
In this study, we used combined monitoring of regional cerebral metabolism (using CMD) and oxygenation (using PbtO2) with brain PCT to examine brain lactate metabolism and the patterns of increased cerebral lactate in subjects with severe TBI. Our findings can be summarized as follows: (1) cerebral extracellular lactate was frequently increased in the acute phase after TBI; (2) increased cerebral lactate was predominantly associated with activated glycolysis rather than hypoxia; and (3) cerebral glycolytic lactate was associated with normal or supranormal brain perfusion. These data collected from humans with acute brain injuries support experimental evidence that lactate may be released aerobically and could be used as an energy substrate by the injured human brain.
Endogenous Aerobic Lactate in Humans with Severe Traumatic Brain Injury
More than 20 years ago, Schurr and co-workers25 were the first to show that lactate might support synaptic function and was not solely the end-product of anaerobic metabolism. Growing evidence has since then accumulated to confirm that lactate has an important role in neuronal function and may act as an important energy substrate, both in normal conditions and in situations of increased neuronal activation/distress. More recently, several in vitro and in vivo experimental studies showed a neuroprotective effect of lactate against neuronal injury.26
In this study, we found that cerebral extracellular lactate was frequently above normal ranges (CMD >4 mmol/L), whereas brain hypoxia (PbtO2) was not a common finding. When further examining the pattern of brain lactate elevation, we found that increased cerebral lactate was mainly associated with normal pyruvate and normal brain oxygen levels, thereby suggesting that the predominant mechanism underlying lactate increase was activated glycolysis rather than ischemia/hypoxia. This was observed throughout the acute phase of TBI. First, these data from humans with acute brain injury appear to confirm experimental evidence, suggesting aerobic lactate utilization by the brain in response to a sustained increase of energy demand. Second, they are in line with recent data collected from patients with TBI12 and subarachnoid hemorrhage,13 suggesting lactate utilization by the injured brain.
Lactate increase in the presence of normal to supranormal levels of extracellular pyruvate and normal brain tissue oxygenation suggests that elevation of lactate in brain interstitial tissue does not principally take place in the setting of an anaerobic metabolism, where pyruvate and oxygen levels are decreased. Moreover, nonischemic lactate elevation occurred almost exclusively in the absence of low CPP and elevated ICP. Our findings support the concept that endogenously released lactate may potentially be used as energy substrate by brain cells in conditions of increased energy demand, such as those encountered in the early phase after TBI.
Episodes of Cerebral Glycolytic Lactate Correlate with Normal to Supranormal Cerebral Blood Flow
To further corroborate our findings, we used PCT to examine the relationship between the patterns of increased cerebral lactate and global brain perfusion. Perfusion CT has been previously validated by our group in the setting of severe TBI in humans,20, 27 and has been shown to equal xenon CT for the assessment of cerebral perfusion.28 The great advantage of PCT, compared with xenon CT or positron emission tomography scan, is that it can easily be performed during the early phase of acute brain injury and without increasing intensive care unit resources. To the best of our knowledge, this is the first clinical study in humans with acute brain injury to use combined CMD/PbtO2 monitoring and PCT to examine cerebral energy metabolism, and which paid specific attention to brain lactate metabolism.
Using this approach, we found that a pattern of increased cerebral glycolytic lactate was always associated with normal to supranormal brain perfusion. This is an important observation that supports the findings described above, and that indeed seems to confirm that increased lactate can be observed in the brain in the absence of ischemia/hypoxia. Our findings are in line with previous observations29 and altogether suggest that increased lactate in the brain of humans with severe TBI seems predominantly nonischemic. Emerging evidence suggests that nonischemic mechanisms are involved in the pathophysiology of secondary cerebral damage after TBI.14, 30, 31 The findings of nonischemic glycolytic cerebral lactate increase appear to support this notion. Indeed, when looking more in detail into glycolytic lactate pattern as a function of pyruvate levels (see p 11), we found that 25% of them had CMD pyruvate >213 μmol/L (‘hyperglycolytic'): compared with samples with elevated lactate and normal pyruvate (CMD pyruvate 120 to 213 μmol/L), the hyperglycolytic samples had significantly higher levels of lactate, pyruvate, and glutamate. This suggests that glutamate-induced activated glycolysis might take a part in these processes. However, more exact mechanisms of how this occurs still remain to be determined: among these, mitochondrial dysfunction could potentially be involved in glycolytic lactate increase.32
Episodes of Cerebral Hypoxic Lactate Correlate with Reduced Cerebral Blood Flow
We also found that a cerebral hypoxic pattern was always associated with an oligemic perfusion on PCT. We believe this is an important finding both from a methodological and a clinical standpoint, as it confirms the validity of PCT and PbtO2 as markers of inadequate brain perfusion in humans with TBI.33 Moreover, as regional microdialysis and PbtO2 reflected overall brain perfusion, neuromonitoring may be considered not only as a regional but also as a global surrogate of brain metabolism and oxygenation. Additional studies are underway at our institution to further explore this issue.
Implications for Traumatic Brain Injury Pathophysiology and Management
A better understanding of brain lactate metabolism after severe TBI could influence patient management in the future. As lactate production with concomitant glycolysis suggests the potential use of lactate as a fuel, it is reasonable to consider targeting neuroenergetics as a novel therapeutic approach to support the injured human brain, thereby preventing energy dysfunction or failure. Studies have shown the potential benefit of modulating cerebral lactate metabolism in humans. Smith et al34 first showed that exogenous lactate, administered intravenously as a sodium lactate infusion, can be metabolized by the normal brain with a concomitant decrease in glucose consumption rate, thereby suggesting the use of lactate with sparing of glucose. Additional investigation showed lactate use by the brain during intense exercise35 and found that in such conditions the contribution of plasma lactate to cerebral energy metabolism may be up to 60%.36
In TBI patients, the administration of sodium lactate was shown to be more effective than mannitol to control ICP.37 Our observations, along with these studies, encourage future investigation to examine the potential neuroprotective effect of exogenous lactate administration in patients with TBI and in other forms of acute brain injury.
Methodological Limitations
Our study has several limitations. First, it was single-centered and included a relatively small cohort. Only a subset of patients had available data on PCT with concomitant CMD and PbtO2 measurement. However, we used a standardized protocol for TBI management and analyzed a homogenous population with predominantly diffuse injury. Furthermore, the neuroradiologist who analyzed the PCT was masked to neuromonitoring data, adding validity to our results. Second, although most catheters were placed in visually normal brain areas, some were in pericontusional tissue, which may affect data, as observed in studies comparing the two types of placement.22 Nevertheless, we did not find a substantial difference in cerebral lactate metabolism between these two groups. Third, CMD only allows the assessment of regional brain lactate metabolism, unlike PET or magnetic resonance spectroscopy. However, the advantage of CMD and PbtO2 is that they allow continuous monitoring of the dynamic changes in brain neurochemistry and cerebral tissue oxygenation at the bedside. Measurement of PbtO2 is also local and does not provide information about overall oxygen consumption. However, PbtO2 measurement is considered as a good indicator of overall cerebral tissue oxygenation and our data support this notion, as low PbtO2 was associated with low global cerebral perfusion. Fourth, CMD and PbtO2 data might be altered by sedation/analgesia,23 which was not accounted for in the analysis. However, all the thresholds were derived from studies conducted on sedated brain-injured patients and appear consistent with these reports.13, 21, 24 Finally, the thresholds of brain physiologic abnormalities were somehow arbitrary and certainly deserve further discussion. In a large cohort of patients with severe TBI, Timofeev et al21 recently set a threshold of CMD lactate >4 mmol/L to define elevated lactate, which we also used here and in our previous study on brain lactate metabolism to categorize normal vs. abnormal cerebral lactate. Regarding brain hypoxia, as described in Materials and methods, several thresholds have been described (ranging from 10 to 20 mm Hg16): the threshold of 20 mm Hg (moderate brain hypoxia) was selected here mainly to be consistent with our previous study.13 The threshold of normal pyruvate (CMD >119 μmol/L) was defined based on our previous study and in line with Samuelsson et al:24 apart from the methodological limitation of selecting such a threshold, it also must be stressed that pyruvate is at the crossroads of multiple biochemical pathways, being both synthesized and consumed, and therefore interpretation of its concentration may be complex, particularly as microdialysis only measures the extracellular pool.30 For all these reasons, we wish to underline that the combination of CMD lactate >4 mmol/L with CMD pyruvate >119 μmol/L should be regarded as indicating glycolysis in this study.
Conclusions
In patients with severe TBI, our study indicates that increased cerebral lactate appears to be predominantly associated with activated glycolysis rather than hypoxia. We further showed that glycolytic lactate increase was always associated with normal to supranormal cerebral perfusion. These data provide new insights into the role and regulation of cerebral lactate metabolism in humans with TBI and support experimental evidence that lactate is produced aerobically and may be used as an energy substrate by the injured human brain. Our findings support the notion that in certain circumstances, increased lactate may be viewed as an adaptive response to increased energy needs rather than as a marker of tissue hypoxia/ischemia.
Acknowledgments
We thank Dr Ludovic Perrin for his help with PCT data analysis.
The authors declare no conflict of interest.
Footnotes
This work was supported by grants from the Swiss National Science Foundation (Grant No. 320030_138191) and the European Critical Care Research Network, European Society of Intensive Care Medicine.
References
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis—a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Pellegri G, Bittar PG, Charnay Y, Bouras C, Martin JL, et al. Evidence supporting the existence of an activity-dependent astrocyte–neuron lactate shuttle. Dev Neurosci. 1998;20:291–299. doi: 10.1159/000017324. [DOI] [PubMed] [Google Scholar]
- Schurr A, Miller JJ, Payne RS, Rigor BM. An increase in lactate output by brain tissue serves to meet the energy needs of glutamate-activated neurons. J Neurosci. 1999;19:34–39. doi: 10.1523/JNEUROSCI.19-01-00034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzier-Sore AK, Voisin P, Canioni P, Magistretti PJ, Pellerin L. Lactate is a preferential oxidative energy substrate over glucose for neurons in culture. J Cereb Blood Flow Metab. 2003;23:1298–1306. doi: 10.1097/01.WCB.0000091761.61714.25. [DOI] [PubMed] [Google Scholar]
- Schurr A, Payne RS, Miller JJ, Rigor BM. Brain lactate, not glucose, fuels the recovery of synaptic function from hypoxia upon reoxygenation—an in vitro study. Brain Res. 1997;744:105–111. doi: 10.1016/s0006-8993(96)01106-7. [DOI] [PubMed] [Google Scholar]
- Ros J, Pecinska N, Alessandri B, Landolt H, Fillenz M. Lactate reduces glutamate-induced neurotoxicity in rat cortex. J Neurosci Res. 2001;66:790–794. doi: 10.1002/jnr.10043. [DOI] [PubMed] [Google Scholar]
- Holloway R, Zhou Z, Harvey HB, Levasseur JE, Rice AC, Sun D, et al. Effect of lactate therapy upon cognitive deficits after traumatic brain injury in the rat Acta Neurochir (Wien) 2007149919–927.discussion 927. [DOI] [PubMed] [Google Scholar]
- Berthet C, Castillo X, Magistretti PJ, Hirt L. New evidence of neuroprotection by lactate after transient focal cerebral ischaemia—extended benefit after intracerebroventricular injection and efficacy of intravenous administration. Cerebrovasc Dis. 2012;34:329–335. doi: 10.1159/000343657. [DOI] [PubMed] [Google Scholar]
- Berthet C, Lei H, Thevenet J, Gruetter R, Magistretti PJ, Hirt L. Neuroprotective role of lactate after cerebral ischemia. J Cereb Blood Flow Metab. 2009;29:1780–1789. doi: 10.1038/jcbfm.2009.97. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, et al. Astrocyte–neuron lactate transport is required for long-term memory formation. Cell. 2011;144:810–823. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487:443–448. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher CN, Carpenter KL, Grice P, Howe DJ, Mason A, Timofeev I, et al. The human brain utilizes lactate via the tricarboxylic acid cycle: a 13C-labelled microdialysis and high-resolution nuclear magnetic resonance study. Brain. 2009;132:2839–2849. doi: 10.1093/brain/awp202. [DOI] [PubMed] [Google Scholar]
- Oddo M, Levine JM, Frangos S, Maloney-Wilensky E, Carrera E, Daniel RT, et al. Brain lactate metabolism in humans with subarachnoid hemorrhage. Stroke. 2012;43:1418–1421. doi: 10.1161/STROKEAHA.111.648568. [DOI] [PubMed] [Google Scholar]
- Glenn TC, Kelly DF, Boscardin WJ, McArthur DL, Vespa P, Oertel M, et al. Energy dysfunction as a predictor of outcome after moderate or severe head injury—indices of oxygen, glucose, and lactate metabolism. J Cereb Blood Flow Metab. 2003;23:1239–1250. doi: 10.1097/01.WCB.0000089833.23606.7F. [DOI] [PubMed] [Google Scholar]
- Marshall LF, Marshall SB, Klauber MR, Van Berkum Clark M, Eisenberg H, Jane JA, et al. The diagnosis of head injury requires a classification based on computed axial tomography. J Neurotrauma. 1992;9 (Suppl 1:S287–S292. [PubMed] [Google Scholar]
- Bratton SL, Chestnut RM, Ghajar J, McConnell Hammond FF, Harris OA, Hartl R, et al. Guidelines for the management of severe traumatic brain injury. X. Brain oxygen monitoring and thresholds. J Neurotrauma. 2007;24 (Suppl 1:S65–S70. doi: 10.1089/neu.2007.9986. [DOI] [PubMed] [Google Scholar]
- Bratton SL, Chestnut RM, Ghajar J, McConnell Hammond FF, Harris OA, Hartl R, et al. Guidelines for the management of severe traumatic brain injury. IX. Cerebral perfusion thresholds. J Neurotrauma. 2007;24 (Suppl 1:S59–S64. doi: 10.1089/neu.2007.9987. [DOI] [PubMed] [Google Scholar]
- Bratton SL, Chestnut RM, Ghajar J, McConnell Hammond FF, Harris OA, Hartl R, et al. Guidelines for the management of severe traumatic brain injury. VIII. Intracranial pressure thresholds. J Neurotrauma. 2007;24 (Suppl 1:S55–S58. doi: 10.1089/neu.2007.9988. [DOI] [PubMed] [Google Scholar]
- Wintermark M, Chiolero R, van Melle G, Revelly JP, Porchet F, Regli L, et al. Relationship between brain perfusion computed tomography variables and cerebral perfusion pressure in severe head trauma patients. Crit Care Med. 2004;32:1579–1587. doi: 10.1097/01.ccm.0000130171.08842.72. [DOI] [PubMed] [Google Scholar]
- Wintermark M, van Melle G, Schnyder P, Revelly JP, Porchet F, Regli L, et al. Admission perfusion CT—prognostic value in patients with severe head trauma. Radiology. 2004;232:211–220. doi: 10.1148/radiol.2321030824. [DOI] [PubMed] [Google Scholar]
- Timofeev I, Carpenter KL, Nortje J, Al-Rawi PG, O'Connell MT, Czosnyka M, et al. Cerebral extracellular chemistry and outcome following traumatic brain injury—a microdialysis study of 223 patients. Brain. 2011;134:484–494. doi: 10.1093/brain/awq353. [DOI] [PubMed] [Google Scholar]
- Longhi L, Pagan F, Valeriani V, Magnoni S, Zanier ER, Conte V, et al. Monitoring brain tissue oxygen tension in brain-injured patients reveals hypoxic episodes in normal-appearing and in peri-focal tissue. Intens Care Med. 2007;33:2136–2142. doi: 10.1007/s00134-007-0845-2. [DOI] [PubMed] [Google Scholar]
- Reinstrup P, Stahl N, Mellergard P, Uski T, Ungerstedt U, Nordstrom CH.Intracerebral microdialysis in clinical practice—baseline values for chemical markers during wakefulness, anesthesia, and neurosurgery Neurosurgery 200047701–709.discussion 709–710. [DOI] [PubMed] [Google Scholar]
- Samuelsson C, Hillered L, Zetterling M, Enblad P, Hesselager G, Ryttlefors M, et al. Cerebral glutamine and glutamate levels in relation to compromised energy metabolism—a microdialysis study in subarachnoid hemorrhage patients. J Cereb Blood Flow Metab. 2007;27:1309–1317. doi: 10.1038/sj.jcbfm.9600433. [DOI] [PubMed] [Google Scholar]
- Schurr A, West CA, Rigor BM. Lactate-supported synaptic function in the rat hippocampal slice preparation. Science. 1988;240:1326–1328. doi: 10.1126/science.3375817. [DOI] [PubMed] [Google Scholar]
- Belanger M, Allaman I, Magistretti PJ. Brain energy metabolism—focus on astrocyte–neuron metabolic cooperation. Cell Metab. 2011;14:724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Wintermark M, Chiolero R, Van Melle G, Revelly JP, Porchet F, Regli L, et al. Cerebral vascular autoregulation assessed by perfusion-CT in severe head trauma patients. J Neuroradiol. 2006;33:27–37. doi: 10.1016/s0150-9861(06)77225-x. [DOI] [PubMed] [Google Scholar]
- Wintermark M, Thiran JP, Maeder P, Schnyder P, Meuli R. Simultaneous measurement of regional cerebral blood flow by perfusion CT and stable xenon CT—a validation study. Am J Neuroradiol. 2001;22:905–914. [PMC free article] [PubMed] [Google Scholar]
- Bergsneider M, Hovda DA, Shalmon E, Kelly DF, Vespa PM, Martin NA, et al. Cerebral hyperglycolysis following severe traumatic brain injury in humans—a positron emission tomography study. J Neurosurg. 1997;86:241–251. doi: 10.3171/jns.1997.86.2.0241. [DOI] [PubMed] [Google Scholar]
- Hutchinson PJ, O'Connell MT, Seal A, Nortje J, Timofeev I, Al-Rawi PG, et al. A combined microdialysis and FDG-PET study of glucose metabolism in head injury Acta Neurochir (Wien) 200915151–61.discussion 61. [DOI] [PubMed] [Google Scholar]
- Vespa P, Bergsneider M, Hattori N, Wu HM, Huang SC, Martin NA, et al. Metabolic crisis without brain ischemia is common after traumatic brain injury—a combined microdialysis and positron emission tomography study. J Cereb Blood Flow Metab. 2005;25:763–774. doi: 10.1038/sj.jcbfm.9600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzeo AT, Beat A, Singh A, Bullock MR. The role of mitochondrial transition pore, and its modulation, in traumatic brain injury and delayed neurodegeneration after TBI. Exp Neurol. 2009;218:363–370. doi: 10.1016/j.expneurol.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Bhatia A, Gupta AK. Neuromonitoring in the intensive care unit. II. Cerebral oxygenation monitoring and microdialysis. Intens Care Med. 2007;33:1322–1328. doi: 10.1007/s00134-007-0660-9. [DOI] [PubMed] [Google Scholar]
- Smith D, Pernet A, Hallett WA, Bingham E, Marsden PK, Amiel SA. Lactate—a preferred fuel for human brain metabolism in vivo. J Cereb Blood Flow Metab. 2003;23:658–664. doi: 10.1097/01.WCB.0000063991.19746.11. [DOI] [PubMed] [Google Scholar]
- van Hall G, Stromstad M, Rasmussen P, Jans O, Zaar M, Gam C, et al. Blood lactate is an important energy source for the human brain. J Cereb Blood Flow Metab. 2009;29:1121–1129. doi: 10.1038/jcbfm.2009.35. [DOI] [PubMed] [Google Scholar]
- Boumezbeur F, Petersen KF, Cline GW, Mason GF, Behar KL, Shulman GI, et al. The contribution of blood lactate to brain energy metabolism in humans measured by dynamic 13C nuclear magnetic resonance spectroscopy. J Neurosci. 2010;30:13983–13991. doi: 10.1523/JNEUROSCI.2040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichai C, Armando G, Orban JC, Berthier F, Rami L, Samat-Long C, et al. Sodium lactate versus mannitol in the treatment of intracranial hypertensive episodes in severe traumatic brain-injured patients. Intens Care Med. 2009;35:471–479. doi: 10.1007/s00134-008-1283-5. [DOI] [PubMed] [Google Scholar]