Abstract
Hypertension in the elderly substantially contributes to cerebromicrovascular damage and promotes the development of vascular cognitive impairment. Despite the importance of the myogenic mechanism in cerebromicrovascular protection, it is not well understood how aging affects the functional adaptation of cerebral arteries to high blood pressure. Hypertension was induced in young (3 months) and aged (24 months) C57/BL6 mice by chronic infusion of angiotensin II (AngII). In young hypertensive mice, the range of cerebral blood flow autoregulation was extended to higher pressure values, and the pressure-induced tone of middle cerebral artery (MCA) was increased. In aged hypertensive mice, autoregulation was markedly disrupted, and MCAs did not show adaptive increases in myogenic tone. In young mice, the mechanism of adaptation to hypertension involved upregulation of the 20-HETE (20-hydroxy-5,8,11,14-eicosatetraenoic acid)/transient receptor potential cation channel, subfamily C (TRPC6) pathway and this mechanism was impaired in aged hypertensive mice. Downstream consequences of cerebrovascular autoregulatory dysfunction in aged AngII-induced hypertensive mice included exacerbated disruption of the blood–brain barrier and neuroinflammation (microglia activation and upregulation of proinflammatory cytokines and chemokines), which were associated with impaired hippocampal dependent cognitive function. Collectively, aging impairs autoregulatory protection in the brain of mice with AngII-induced hypertension, potentially exacerbating cerebromicrovascular injury and neuroinflammation.
Keywords: blood–brain barrier, CBF, dementia, inflammation, myogenic, TRPC
Introduction
Hypertension has deleterious effects on the brain and the cerebral circulation. The population in the western world is aging and as hypertension affects most elderly people (⩾ 65 years of age), these individuals are more likely to develop cerebrovascular pathologies.1 There is increasing evidence that in the elderly hypertension-induced microvascular injury promotes the development of vascular cognitive impairment,2, 3 which is the second most common cause of cognitive impairment after Alzheimer's disease.
Epidemiologic studies provide evidence that in addition to the increased prevalence of hypertension in aging, the deleterious cerebrovascular effects of hypertension are also exacerbated in elderly patients whereas young individuals appear to be more protected from cerebromicrovascular damage induced by hypertension.4, 5, 6 Although the available human data suggest that advanced age and hypertension have synergistic effects, there are no studies addressing the specific age-related mechanisms through which aging increases the vulnerability of the cerebromicrovasculature to hypertension leading to vascular cognitive impairment.7
Studies on young animals show that cerebral arteries exhibit functional and structural adaptation to hypertension, which protects the distal portion of the cerebral microcirculation from pressure overload.8, 9 Among these adaptive responses the increased pressure-induced myogenic constriction of cerebral arteries is of great significance.10, 11, 12 Previous studies showed that in young hypertensive animals increased pressure sensitivity of the myogenic machinery leads to an increased resistance at the level of the cerebral arteries, keeping pressure in the thin-walled, injury-prone arterioles and capillaries in the normal range with little change in tissue blood supply and oxygenation. As a result of this adaptive response, the range of cerebral blood flow (CBF) autoregulation is extended to higher pressure values both in hypertensive experimental animals and in hypertensive patients.8, 9, 13 Studies in animal models of hypertension and stroke14 suggest that pathologic loss of autoregulatory protection contributes to cerebromicrovascular injury. Despite the paramount importance of the myogenic mechanism in cerebromicrovascular protection, it is not well understood how aging affects the functional adaptation of the cerebral arteries in hypertension.
The present study was designed to test the hypothesis that aging impairs functional adaptation of cerebral arteries to high blood pressure. A prediction based on this hypothesis is that age-related loss of autoregulatory protection exacerbates hypertension-induced microvascular damage and neuroinflammation leading to cognitive decline. To test our hypotheses, we induced hypertension in young and aged C57BL/6 mice by chronic infusion of angiotensin II (AngII) and assessed changes in arterial myogenic constriction and autoregulation of CBF, blood–brain barrier (BBB) function, microvascular density, markers of neuroinflammation, and cognitive function.
Materials and methods
For detailed description of methods, please refer to Supplementary Experimental Procedures.
Mice
Young (3 months, n=80) and aged (24 months, n=80) male C57/BL6 mice were purchased from the National Institute on Aging. A cohort of young (3 months old) and aged (24 months old) α-smooth muscle actin (αSMA)-GFP transgenic mice (to visualize pericytes) was also used. All procedures were approved by the Institutional Animal Use and Care Committees of the participating institutions and in accordance with the ARRIVE guidelines.
Angiotensin II-Induced Hypertension
To induce hypertension, cohorts of young and aged mice received AngII (1,000 ng/min per kg) infusions via a subcutaneously implanted osmotic minipump for 4 weeks. During the 4-week experimental period, systolic blood pressure of mice in each experimental group was measured by the tail cuff method.
Behavioral Studies
On week 4 after minipump implantation, mice were assessed for learning capacity using an elevated plus maze-based learning protocol.
Cerebrovascular Autoregulation
On day 28 after implantation in anesthetized, ventilated mice, cortical blood flow was measured by laser speckle flowmetry. Blood pressure was elevated or decreased in 20 mm Hg steps by intravenous infusion of phenylephrine (1 to 2 μg/kg per minute) or via controlled exsanguination (100 to 400 μL of arterial blood), respectively. The range of blood pressure studied was 40 to 160 mm Hg. Changes in CBF were expressed relative to CBF corresponding to a systolic pressure of 80 mm Hg. Then, animals were killed and tissues were isolated for subsequent in vitro functional studies and biochemical assays.
Assessment of Pressure- and Flow-Induced Responses in Isolated Middle Cerebral Arteries
To assess cerebrovascular autoregulatory function in isolated middle cerebral arteries (MCAs), both the static and dynamic components in the vascular myogenic response were assessed.
20-hydroxy-5,8,11,14-eicosatetraenoic acid (20-HETE) is a potent vasoconstrictor metabolite of arachidonic acid produced by cytochrome P450 Ω-hydroxylases, and has a central role in regulation of CBF by contributing to pressure- and flow-dependent responses of cerebral arteries.15, 16, 17 To assess the role of 20-HETE in age- and hypertension-induced changes in the myogenic response, MCAs were incubated with the cytochrome P450 ω-hydroxylase inhibitor HET0016 (10−6 mol/L) and the myogenic response was reassessed.
Previous studies showed that activation of transient receptor potential cation channel, subfamily C (TRPC6) channels mediated, at least in part, 20-HETE-induced vasomotor responses. To assess the role of TRPC channels in age- and hypertension-induced changes in the myogenic response, MCAs were incubated with SKF96365 (5 × 10−6 mol/L, for 15 minutes), a potent blocker of TRPC channels and the myogenic response was reassessed.
In isolated MCAs, we also assessed flow-induced constriction, which is an important local vasoregulatory mechanism involved in protection of the cerebral microcirculation and regulation of blood flow. Vascular responses to ATP and adenosine (10−8 and 10−7 mol/L) were also obtained.
At the end of each experiment, the pressure-passive diameter curves were obtained in maximally dilated MCAs.
Quantitative Real-Time RT-PCR
A quantitative real-time RT-PCR technique was used to analyze mRNA expression of Cyp4a12, Cyp4a10, Cyp4a14, Trpc6, Trpc3, Trpc7, Trpm4, and Cacna1 in MCAs of mice from each experimental group.
Assessment of the Integrity of the Blood–Brain Barrier
Sodium fluorescein tracer assay
To quantify BBB permeability, we used the sodium fluorescein tracer assay. In brief, after the 4-week experimental period in a separate cohort of anesthetized mice, the small water-soluble tracer sodium fluorescein (5 mL/kg, 2% in physiologic saline) was administered intravenously by retroorbital injection. After 30 minutes of circulation of the tracer, animals were transcardially perfused with 1 × heparin containing phosphate-buffered saline. Then, the mice were decapitated and the brains were removed. From each brain, the hippocampus, the white matter, and the frontal cortex were isolated and homogenized. The extravasated sodium fluorescein was quantified spectrophotofluorometrically using a microplate reader and normalized to tissue weight.
Demonstration of IgG extravasation by immunofluorescence
As an additional marker for increased hippocampal BBB permeability, IgG extravasation was also shown by immunofluorescent labeling and confocal microscopy.
Western Blotting
Immunoblotting studies for TRPC6 in MCA homogenates and for the tight junction proteins (ZO-1, occludin, and claudin-5) in hippocampal homogenates were performed. The relative abundance of studied proteins was determined with densitometry using β-actin as a loading control.
Pericyte Coverage
Pericytes have key roles in maintenance of the BBB and in preservation of the structural integrity of the cerebral microcirculation.18 To assess age- and hypertension-induced changes in pericyte coverage of microvessels, in brain sections from young and aged αSMA-GFP transgenic mice with or without AngII-induced hypertension, immunolabeling for the endothelial marker CD31 was performed. Using stereological methods, the number of pericytes (identified as GFP-positive cells with a typical pericyte morphology, including long processes that extend over capillaries, and perivascular localization) per CD 31-positive capillary surface area was calculated.
Capillary Density Analysis
Immunofluorescent labeling for CD31 was used to identify microvessels in the brain. Using stereological methods, capillary density in CA1, CA3, and dentate gyrus of the hippocampus, retrosplenial cortex, primary somatosensory cortex (S1), and corpus callosum was quantified as the length of blood vessels <10 μm in diameter per volume of tissue using the Neurolucida software (MBF Bioscience, Williston, VT, USA).
Neuroinflammation
Microglia activation was quantified in hippocampal sections by immunofluorescent labeling for CD68 (a marker of activated microglia/macrophage) and Iba-1 (a microglia marker).
In addition to quantifying microglia activation, we analyzed relative abundance of several neuroinflammatory cytokines/chemokines through quantitative real-time PCR with RNA isolated from snap-frozen brain samples.
To further characterize neuroinflammation in our cohorts, protein levels of selected micoglia-derived proinflammatory factors (MCP-1, TNFα, and IP-10) were analyzed in homogenates of the hippocampi using a fluorescent bead assay. For normalization purposes, the sample protein content was used.
Determination of Hippocampal Protein 3-Nitrotyrosine Content
As a marker of hippocampal oxidative/nitrosative stress, 3-nitrotyrosine (3-NT; a marker for peroxynitrite action) was assessed in homogenates of hippocampi.
Statistical Analysis
Data were analyzed by two-way analysis of variance followed by Tukey post hoc tests and Pearson's correlation analysis, as appropriate. A P value of <0.05 was considered as statistically significant. Data are expressed as mean±s.e.m.
Results
Impaired Cerebrovascular Autoregulation in Aged Hypertensive Mice
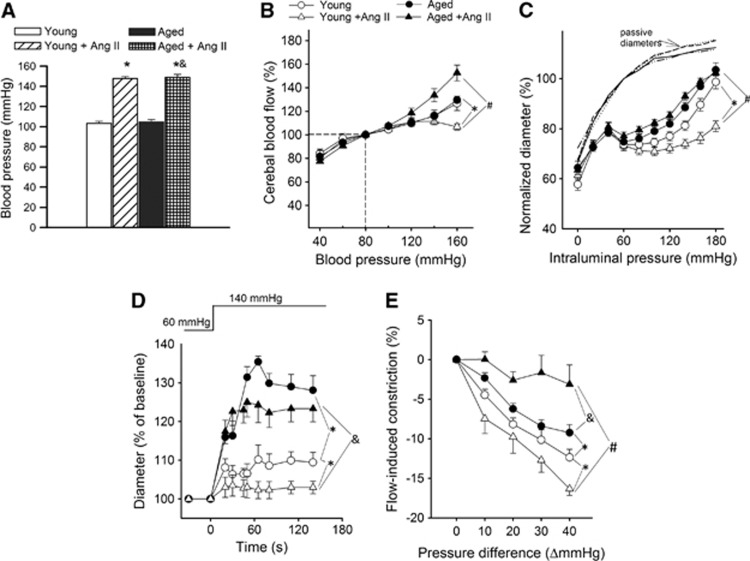
Blood pressure was significantly increased in both young and aged mice receiving AngII infusion, as shown in Figure 1A. In young control mice, CBF was independent of blood pressure in the range of 60 to 120 mm Hg, which indicates that autoregulation was present (Figure 1B). No differences in autoregulation were observed among young and aged normotensive mice (Figure 1B). In young hypertensive mice, however, the relationship between CBF and blood pressure was substantially altered. There was a progressive expansion of the autoregulated range, indicating an adaptive response (Figure 1B), which was completely absent in aged hypertensive mice. In aged hypertensive mice, the relationship between blood pressure and CBF was essentially linear, which indicates total dependence of CBF on blood pressure and loss of autoregulation (Figure 1B).
Figure 1.
Aging impairs adaptation of cerebrovascular autoregulation to hypertension. (A) Effect of chronic infusion of angiotensin II (AngII) on systolic blood pressure in young and aged mice. Data are mean±s.e.m. (n=20 to 25 for each group) *P<0.05 vs. Young; &P<0.05 vs. Aged. (B) Relationship between cerebral blood flow (CBF) and systolic blood pressure in young control, young hypertensive (Young+AngII), aged control, and aged hypertensive (Aged+AngII) mice. Data are mean±s.e.m. (n=8 in each group). In young control mice, CBF is statistically different from the value of 100 mm Hg at pressure values of <60 and >140 mm Hg, indicating the autoregulatory range. In young hypertensive mice, there was a progressive expansion of the autoregulated range, indicating an adaptive response (*P<0.05 vs. Young), which was completely absent in aged hypertensive mice (#P<0.05 vs. Young+AngII). (C) Steady-state changes in diameter of middle cerebral arteries (MCAs) isolated from each experimental group of mice in response to increases in intraluminal pressure, representing the static component of the myogenic response. Vascular diameters are expressed as a percentage of the maximally dilated passive diameter of each vessel at 80 mm Hg. (D) Changes in the diameter of MCAs to a sudden increase in intraluminal pressure (from 60 to 140 mm Hg), representing the dynamic component of the myogenic response. (E) Flow-induced constriction of MCAs (induced by increasing intraluminal flow by creating a pressure gradient through the vessels). Data are mean±s.e.m. (n=8 to 16). *P<0.05 vs. Young; #P<0.05 vs. Young+AngII; &P<0.05 vs. Aged.
Aging Impairs Autoregulatory Function of Cerebral Arteries: Role of Myogenic and Flow-Induced Constriction
Figure 1C shows myogenic constriction developed in isolated MCAs at intraluminal pressures of 20 to 180 mm Hg. In MCAs of young control mice, increases in intravascular pressure increased myogenic constriction up to 60 mm Hg, and myogenic tone was maintained at almost the same level up to ∼120 mm Hg, which overlaps the autoregulatory range of CBF. At higher pressures, myogenic tone then tended to decrease and arteries dilated gradually (Figure 1C). Myogenic constriction in MCAs from young hypertensive mice was significantly enhanced and the myogenic tone was maintained at almost the same level up to ∼160 mm Hg, which corresponds to the increased autoregulatory range of CBF in these animals. Middle cerebral arteries of aged mice developed a slightly decreased myogenic constriction and did not exhibit a similar hypertension-induced adaptive increase in myogenic constriction, which was observed in young mice (Figure 1C). The pressure-passive diameter curves were similar in MCAs from each group (Supplementary Figure S1). We also investigated the time-dependent behavior of the myogenic response. In MCAs of young control mice, a step increase in intraluminal pressure from 60 to 140 mm Hg resulted only in slight increase in diameter (Figure 1D). In MCAs of young hypertensive mice, the myogenic constriction was enhanced, and no increase in diameter was observed. In contrast, MCAs of aged mice could not maintain resting tone and did not exhibit a hypertension-induced adaptive increase in myogenic constriction (Figure 1D).
Increases in intraluminal flow elicited vasoconstriction in MCAs of young mice and this response was enhanced by hypertension (Figure 1E). In contrast, there was no adaptive increase in flow-induced constriction in MCAs of aged hypertensive mice (Figure 1E).
Role of 20-HETE and TRPC6 in Functional Maladaptation of Aged Cerebral Arteries to Hypertension
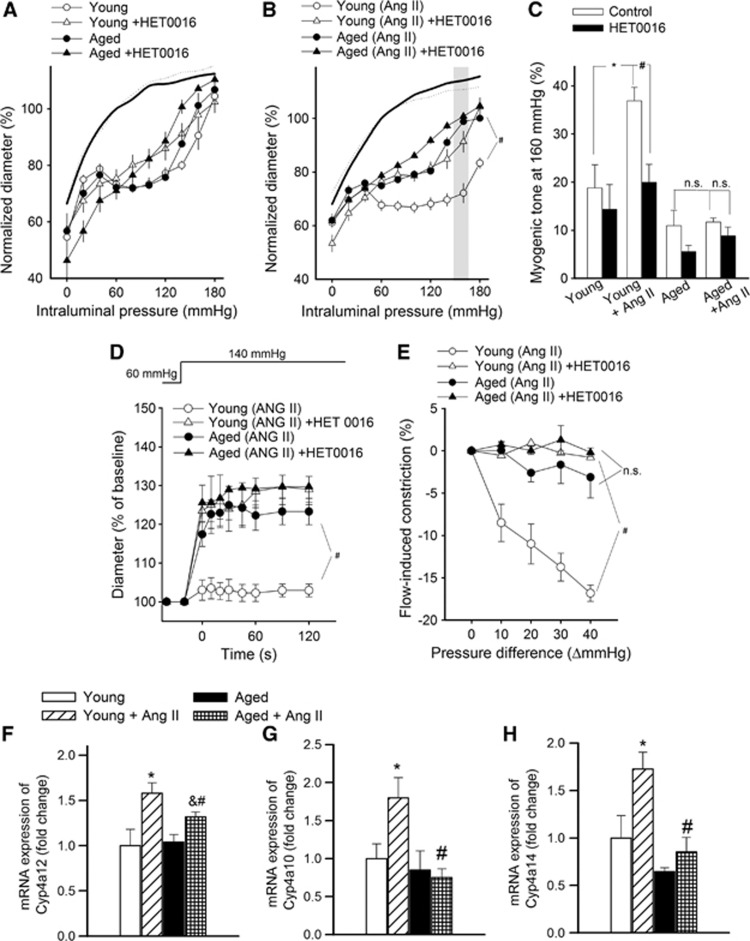
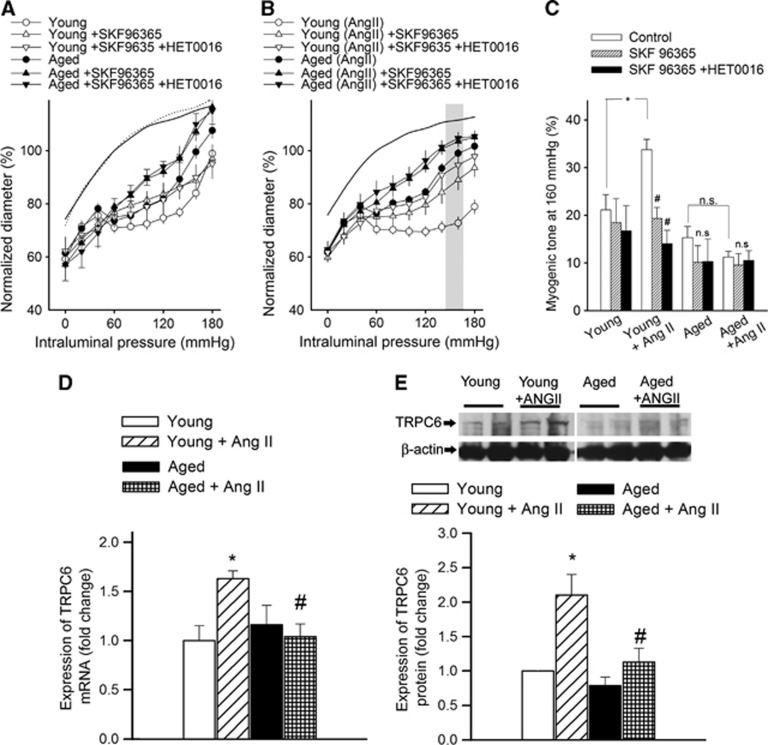
To assess the role of 20-HETE in functional adaptation of cerebral arteries to hypertension, we tested the effect of HET0016 on myogenic constriction at a pathophysiologically relevant pressure range (⩾160 mm Hg; Figures 2A to 2C). Because we found that in mouse arteries the vasoconstrictor effect of 20-HETE is significantly inhibited by the TRPC6 inhibitor SKF96365 (Supplementary Figure S2) we also assessed the role of TRPC6 channels in 20-HETE-dependent functional adaptation of cerebral arteries to hypertension using this inhibitor. We found that in MCAs of young hypertensive mice increased myogenic tone was significantly inhibited by both HET0016 (Figure 2C) and SKF96365 (Figure 3C) eliminating the difference between the four groups, whereas neither HET0016 nor SKF96365 significantly affects myogenic tone in MCAs of aged hypertensive mice. HET0016 (Figures 2D and 2E) and SKF96365 (not shown) also inhibited the enhanced dynamic component of myogenic constriction and flow-induced constriction in MCAs of young hypertensive mice, whereas it had no significant effects in MCAs of aged hypertensive mice. Hypertension was associated with upregulated expression of the CYP4A arachidonic acid ω-hydroxylases Cyp4a12, Cyp4a10, and Cyp4a14 (Figures 2F to 2H) and TRPC6 channels (Figures 3D and 3E) in MCAs of young mice, whereas these adaptive responses were significantly impaired or absent in MCAs of aged hypertensive mice. Hypertension did not increase significantly the mRNA expression of Trpc3, Trpc7, Trpm4 (transient receptor potential cation channel, subfamily M, member 4) and Cacna1 (L-type calcium channel) in MCAs of young and aged mice (data not shown).
Figure 2.
Role of 20-hydroxy-5,8,11,14-eicosatetraenoic acid (20-HETE) mediation in cerebrovascular autoregulatory dysfunction in aged hypertensive mice. (A, B) The effect of HET0016 (10−6 mol/L), an inhibitor of 20-HETE synthesis, on myogenic constriction of middle cerebral arteries (MCAs) isolated from young control, young hypertensive (Young+AngII), aged control, and aged hypertensive (Aged+AngII) mice in response to increases in intraluminal pressure. Vascular diameters are expressed as a percentage of the passive diameter of the vessels at 80 mm Hg. (C) Myogenic tone of MCAs in the absence and presence of HET0016 at an intraluminal pressure of 160 mm Hg. Data are mean±s.e.m. (n=8 in each group). *P<0.05 vs. young and #P<0.05 vs. Young+AngII. (D, E) Effect of HET0016 on the early rapid phase of the myogenic response (D; induced by a sudden increase in intraluminal pressure from 60 to 140 mm Hg) and flow-induced constriction (E; induced by increasing intraluminal flow by creating a pressure gradient through the vessels; see Materials and methods) of MCAs. Data are mean±s.e.m. (n=8 to 16). #P<0.05 vs. Young+AngII. (F to H) QRT-PCR data showing mRNA expression of cytochrome P450 4A enzymes in MCAs. Data are mean±s.e.m. (n=6 in each group). *P<0.05 vs. Young; #P<0.05 vs. Young+AngII and &P<0.05 vs. Aged. AngII, angiotensin II.
Figure 3.
Role of transient receptor potential cation channel, subfamily C (TRPC6) in cerebrovascular autoregulatory dysfunction in aged hypertensive mice. (A, B) The effect of SKF96365 (5 μmol/L), a TRPC channel blocker, and HET0016 (10−6 mol/L), an inhibitor of 20-hydroxy-5,8,11,14-eicosatetraenoic acid (20-HETE) synthesis, on myogenic constriction of middle cerebral arteries (MCAs) isolated from young control, young hypertensive (Young+AngII), aged control, and aged hypertensive (Aged+AngII) mice. Vascular diameters are expressed as a percentage of the passive diameter of the vessels at 80 mm Hg. (C) Myogenic tone of MCAs in the absence and presence of SKF96365 and HET0016 at an intraluminal pressure of 160 mm Hg. Data are mean±s.e.m. (n=8 in each group). *P<0.05 vs. Young and #P<0.05 vs. Young+AngII. (D, E) mRNA (D; QRT-PCR data) and protein expression (E; western blotting, one representative western blot (of three) is presented showing two samples from each group (for each group, n=6 vessels from 6 animals) of TRPC6 in MCAs. Data are mean±s.e.m. (n=6 in each group). *P<0.05 vs. Young and #P<0.05 vs. Young+AngII.
Aging Exacerbates Hypertension-Induced Blood–Brain Barrier Disruption
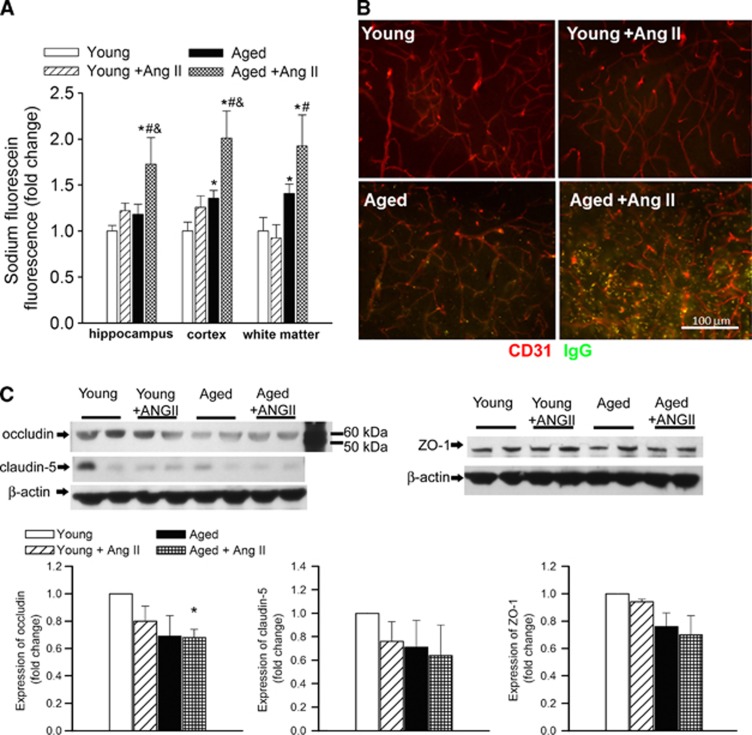
Using a sodium fluorescein tracer assay, we tested the hypothesis that cerebrovascular autoregulatory dysfunction in aged hypertensive mice leads to BBB disruption. As predicted by our model, we found that aging exacerbates hypertension-induced fluorescein leakage in the hippocampi, cortex, and white matter (Figure 4A). Aging per se also tended to increase the fluorescein leakage, but these changes reached statistical significance only in the cortex and white matter. We hypothesized that the aging hypertensive brain also cannot maintain the BBB against endogenous circulating macromolecules, some of which exert significant proinflammatory and, potentially, neurotoxic effects. Immunostaining for plasma-derived IgG revealed significant perivascular IgG deposits in the hippocampus of aged hypertensive mice (Figure 4B). Leakage of IgG in the hippocampus of young hypertensive mice was significantly reduced and there was no detectable IgG leakage in young control mice. Blood–brain barrier breakdown in neurodegenerative disorders often indicates disruption of the tight junctions due to reduced expression of tight junction proteins.19 In that regard, it is significant that in the hippocampi of aged hypertensive mice their expression also tended to be downregulated (Figure 4C), although these alterations per se are unlikely to explain exacerbation of hypertension-induced BBB disruption in aging.
Figure 4.
Aging exacerbates hypertension-induced disruption of the blood–brain barrier. (A) Hypertension- and aging-induced changes in sodium fluorescein content in the hippocampus, cortex, and white matter of young control, young hypertensive (Young+AngII), aged control, and aged hypertensive (Aged+AngII) mice. Data are mean±s.e.m. *P<0.05 vs. Young, #P<0.05 vs. Young+AngII; and &P<0.05 vs. Aged (n=6 to 10). (B) Confocal microscopy analysis of plasma-derived IgG (green) and CD31-positive microvessels (red) in the hippocampus of young and aged mice with or without AngII-induced hypertension. Note the increased presence of extravascular IgG deposits in the hippocampus of aged hypertensive mice. (C) Expression of occludin, claudin-5, and ZO-1 in the hippocampi of young and aged normotensive and hypertensive mice. Upper panels: original western blots. β-Actin was used as a loading control. One representative western blot (of three) is presented showing two samples from each group (for each group, n=6 vessels from 6 animals). Bar graphs are summary densitometric values. Data are mean±s.e.m. *P<0.05 vs. Young. n=6 animals per group. AngII, angiotensin II.
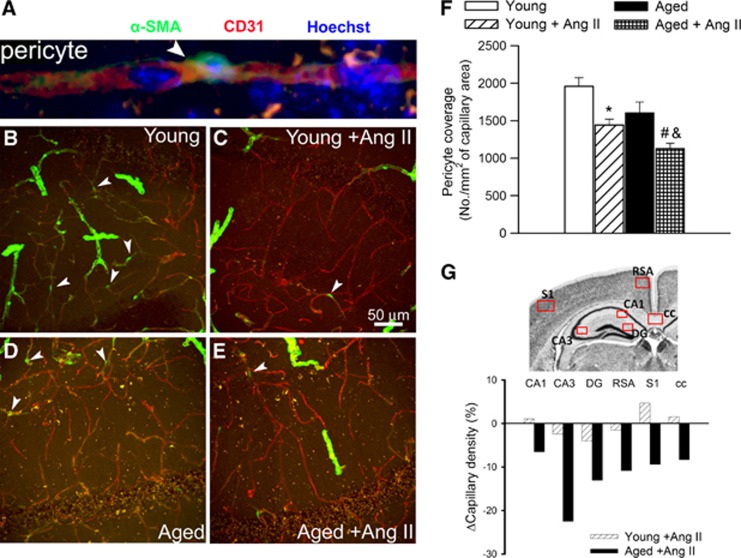
Aging Exacerbates Hypertension-Induced Pericyte Loss and Microvascular Rarefaction
Because recent studies show that pericyte loss can compromise BBB integrity,20 we assessed age- and hypertension-induced changes in pericyte coverage of hippocampal microvessels (Figures 5A to 5E). In young mice, hypertension resulted in a significant decline in the relative number of pericytes (Figure 5F) and capillary pericyte coverage (by ∼29%). In aged mice, hypertension-induced decreases in pericyte number (Figure 5F) and pericyte coverage (by ∼41%) were exacerbated. We also determined whether pericyte loss influences brain capillary density in the aged hypertensive mice. Capillaries were identified by their expression of CD31, using a lumen diameter of 10 μm or less as a standard identifier. As shown in Figure 5G, relative hypertension-induced decreases in capillary length density in CA1, CA3, and dentate gyrus of the mouse hippocampus, retrosplenial cortex, primary somatosensory cortex, and corpus callosum of aged mice were significantly greater than in young mice.
Figure 5.
Hypertension-induced changes in pericyte coverage of hippocampal capillaries and hippocampal capillary density. (A) Representative confocal image showing perivascular localization of an α-smooth muscle actin (αSMA) expressing pericyte (green, arrowhead) surrounding CD31-positive capillary endothelial cells (red) in the CA1 region of the mouse hippocampus. Hoechst 33342 was used for nuclear counterstaining. (B to E) Representative confocal microscopy analysis of αSMA expressing pericyte coverage (green, arrowheads) of CD31-positive capillaries (red) in the CA1 region of the hippocampi of young control (B), young hypertensive (Young+AngII) (C), aged control (D), and aged hypertensive (Aged+AngII) (E) animals. Note that αSMA expressing pericytes (arrowheads) and vascular smooth muscle cells surrounding the terminal arterioles exhibit different morphologies. (F) Summary data showing hypertension-dependent loss of pericyte coverage in the hippocampus (see Supplementary Experimental Procedures for details). *P<0.05 vs. Young; #P<0.05 vs. Young+AngII; and &P<0.05 vs. Aged. (G) Hypertension-induced relative changes of capillary density in the CA1 and CA3 regions of the hippocampus, dentate gyrus (DG), retrosplenial cortex (RSA), primary somatosensory cortex (S1), and corpus callosum (cc) of young and aged mice. Boxes indicate brain regions that were included in the evaluation of capillary density (see Supplementary Experimental Procedures for details). AngII, angiotensin II.
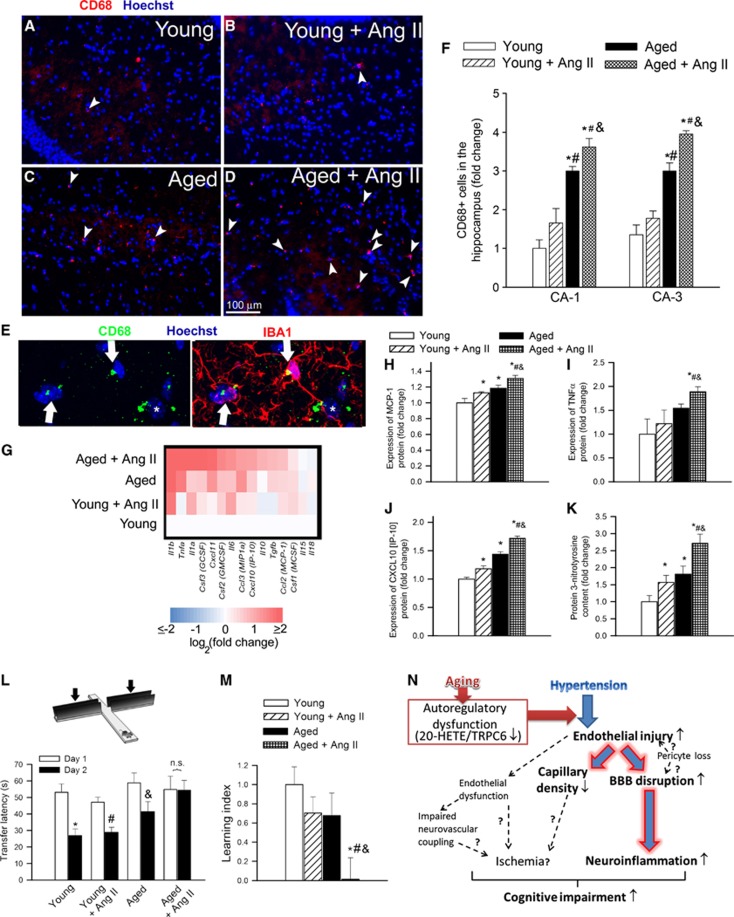
Aging Exacerbates Hypertension-Induced Inflammation and Oxidative Stress in the Hippocampus
Previous studies suggest that leakage of plasma-derived factors through the damaged BBB has the potential to induce neuroinflammation by activating microglia.19 In the hippocampi of young mice, the number of activated microglia was low and hypertension-induced changes in microglia activation did not reach statistical significance. We found that aging is associated with a relative increase in the number of activated microglia in the hippocampi. Importantly, hypertension-induced microglia activation was exacerbated in the hippocampi of aged mice (Figures 6A to 6F). Sustained activation of microglia was associated with an increased expression of several proinflammatory cytokines and chemokines (Figure 6G) and other inflammatory mediators (data not shown) in the hippocampi of aged hypertensive mice. These findings were corroborated by demonstration of increased protein expression of MCP-1, TNFα, and IP-10 (Figures 6I and 6J), which are known to be secreted by activated microglia, in the hippocampi of aged hypertensive mice.
Figure 6.
Aging exacerbates hypertension-induced neuroinflammation and cognitive decline. (A to D) CD68-positive (red fluorescence, arrowheads) activated microglia in the hippocampus CA1 region from young control (A), young hypertensive (Young+AngII) (B), aged control (C1 and aged hypertensive (Aged+AngII) (D) animals (blue fluorescence: nuclei). Panel (E) shows that in the hippocampus of aged hypertensive mice most CD68-positive cells are positive for IBA1 (arrows), yet there are CD68+/IBA1− cells present as well (star). Panel (F) depicts summary data of relative changes in the number of CD68-positive activated microglia in the CA1 and CA3 regions of hippocampus (fold change). Data are mean±s.e.m. *P<0.05 vs. Young; #P<0.05 vs. Young+AngII; and &P<0.05 vs. Aged. (G) Hypertension in aging is associated with a proinflammatory shift in cytokine expression profiles in the mouse hippocampus. The heat map is a graphic representation of normalized mRNA expression of cytokines and chemokines depicted by color intensity, from highest (bright red) to lowest (bright blue) expression (n=6 in each group). Aged hypertensive mice have the highest expression of inflammatory markers. (H to K) Relative hippocampal levels of microglia-derived proinflammatory cytokines MCP-1 (H), TNFα (I) and IP-10 (J) and 3-nitrotyrosine (3-NT) (a marker for peroxynitrite action; K). Data are mean±s.e.m. *P<0.05 vs. Young; #P<0.05 vs. Young+AngII; and &P<0.05 vs. Aged. (L, M) Aging exacerbates hypertension-induced cognitive impairment (elevated plus maze-based learning protocol; see Supplementary Experimental Procedures for details). For old hypertensive mice, transfer latency was similar on days 1 and 2 (corresponding to a learning index: ∼0), indicating that these mice had significantly impaired hippocampal cognitive function. Data are mean±s.e.m. *P<0.05 vs. Young; #P<0.05 vs. Young+AngII; and &P<0.05 vs. Aged. (N) Proposed scheme depicting the mechanisms by which age-related cerebrovascular autoregulatory dysfunction exacerbates hypertension-induced microvascular damage and blood–brain barrier (BBB) disruption. Future studies should elucidate the links (dashed lines; question marks) among endothelial dysfunction, microvascular injury, regional ischemia, neuroinflammation, and cognitive impairment. AngII, angiotensin II; 20-HETE, 20-hydroxy-5,8,11,14-eicosatetraenoic acid.
Neuroinflammation is frequently associated with increased oxidative stress. Consistent with the presence of hypertension-related oxidative/nitrosative stress in the brain,21 hippocampal 3-NT content was increased in young hypertensive mice (Figure 6K). Aging exacerbated hypertension-induced increases in hippocampal 3-NT content (Figure 6K), confirming that the effects of age and hypertension are synergistic.
Aging Exacerbates Hypertension-Induced Decline in Hippocampal Cognitive Function
To determine whether compromised BBB integrity, microvascular rarefaction, oxidative/nitrosative stress, and chronic low-grade neuroinflammation are sufficient to induce neuronal dysfunction, we studied hippocampally dependent learning and memory. For young control mice, transfer latency on day 2 was significantly decreased compared with day 1 (Figure 6L), indicating an intact learning effect (learning index: 1). The learning indexes for young hypertensive mice (∼0.7) and aged mice (∼0.67) tended to decrease, compared with young control mice, although the differences did not reach statistical significance. For old hypertensive mice, transfer latency was similar on days 1 and 2 (corresponding to a learning index of ∼0), indicating that these mice had a significantly impaired hippocampal cognitive function.
Discussion
The results of this study suggest that functional maladaptation of aged cerebral arteries to high blood pressure is due to the dysregulation of 20-HETE/TRPC pathway. We also show that autoregulatory dysfunction in aged mice with AngII-induced hypertension associates with exacerbation of BBB disruption, neuroinflammation, and cognitive decline.
In the cerebral circulation, larger pial arteries have a significant role in cerebrovascular resistance, thus myogenic constriction of proximal branches of the cerebrovascular tree (i.e., MCA) are uniquely important for protection of the cerebral microcirculation.22 In healthy young animals, pressure-induced myogenic constriction of the cerebral arteries (Figure 1C) acts as a critical homeostatic mechanism, which assures that increased arterial pressure does not penetrate the distal portion of the microcirculation and causes damage to the thin-walled arteriolar and capillary microvessels in the brain.10, 11 In hypertensive young mice (Figures 1B and 1C) and rats,12, 23 the myogenic constriction of cerebral arteries is enhanced and the range of cerebrovascular autoregulation is extended, which represent functional adaptation of these vessels to higher systemic blood pressure, protecting the cerebral microcirculation.8, 9, 10, 11, 12 Here, we report for the first time that cerebral arteries of aged mice do not exhibit a hypertension-induced adaptive increase in myogenic constriction observed in young mice (Figure 1C). Recent studies suggest that in addition to the myogenic response flow-induced constriction of cerebral arteries may also contribute to cerebrovascular autoregulatory function.15, 16 This is the first study to show that in young AngII-treated hypertensive mice flow-induced arterial constriction is also enhanced (Figure 1E), representing another component of functional arterial adaptation to high blood pressure. This adaptive response is impaired in aged AngII-treated hypertensive mice (Figure 1E). Taken together, AngII-induced hypertension in aging is associated with dysfunction of cerebrovascular autoregulatory mechanisms that protect the brain. Interestingly, aging per se is associated with a mild impairment of both flow-induced constriction and the dynamic component of the myogenic response (Figure 1D), suggesting that sudden increases in blood pressure (e.g., during Valsalva maneuver) may temporarily penetrate the distal portion of the cerebral microcirculation even in normal aging.
Several lines of evidence support the concept that in young animals activation of a 20-HETE/TRPC-dependent pathway underlies functional adaptation of cerebral arteries to hypertension, and that this adaptive response is dysfunctional in aging. In young hypertensive mice, 20-HETE mediates increased myogenic constriction in the higher pressure range (which overlaps the blood pressure of these animals). It is likely that this is due to an adaptive upregulation of cytochrome P450 4A ω-hydroxylases (Figure 2). Similarly, in young hypertensive rats pressure-induced 20-HETE production in cerebral arteries is also significantly increased.24 Here, we report for the first time that this 20-HETE-dependent adaptive response is impaired in aged AngII-infused hypertensive mice (Figure 2). Consistent with these findings, expression of Cyp4A12 (which is thought to be the major source of 20-HETE in cerebral arteries25) and the expression of CypA10 and CypA14 (which are also able to produce 20-HETE and other vasoconstrictor arachidonic acid metabolites in mouse tissues26) are significantly decreased in aged hypertensive MCAs compared with young hypertensive animals (Figure 2). The aforementioned results warrant further studies to characterize age-related differences in hypertension-induced changes in arachidonic acid metabolism in the cerebral vasculature (including assessment of protein expression and activity of 20-HETE producing enzymes). Previous studies show that activation of TRPC6 channels mediates 20-HETE-induced increases in intracellular Ca2+ levels in vascular smooth muscle cells27 and contributes to autoregulation and myogenic constriction of cerebral arteries.28 While in cerebral arteries of young mice AngII-induced hypertension upregulates TRPC6 expression and TRPC mediation of the myogenic constriction (Figure 3), this adaptive response is impaired in aged AngII-infused hypertensive mice. Importantly, seminal studies by Golovina and coworkers and by other laboratories show that TRPC6 channels are also upregulated in resistance arteries of Milan hypertensive rats, rodents with deoxycorticosterone acetate salt-induced hypertension and Dahl salt-sensitive rats, contributing to increased constrictor responses to agonists and pressure.29, 30, 31 Studies in the aforementioned models of hypertension suggest that a functional crosstalk exists between plasma membrane Na+/Ca2+ exchanger-1 and vascular TRPC6 channel activity.29, 32, 33, 34 Given the proposed role of TRPC6 channels in the pathogenesis of hypertension, future studies should determine whether aging affects TRPC6 channel expression/activity in systemic resistance arteries and the kidney, as well.
Recently, we proposed that flow-induced constriction of cerebral arteries may participate in the autoregulatory mechanisms in response to changes in systemic blood pressure.15, 16 Although the exact mechanism of flow-induced constriction remains elusive, there is evidence that 20-HETE-dependent pathways have an important role in flow-induced constriction both in cerebral arteries of experimental animals (Figure 2) and in humans.15, 16 Thus, dysregulation of 20-HETE dependent pathways is likely to simultaneously impair both the myogenic and the flow-induced components of cerebrovascular autoregulation in aged hypertensive mice. Because inhibition of the 20-HETE/TRPC6 pathway does not completely abolish myogenic constriction in cerebral arteries of young hypertensive mice, we cannot exclude a potential role for other mechanisms in functional maladaptation to hypertension in aging as well, including pathways involved in cellular calcium homeostasis in the vascular smooth muscle cells. The age-related mechanism(s) that are responsible for dysregulation of cytochrome P450 4A ω-hydroxylases and TRPC channels are presently unknown and may include an age-related IGF-1 deficiency.35 In support of this concept, there are data extant showing that IGF-1 deficiency in mice is associated with impaired hypertension-induced adaptive changes in cerebral arterial myogenic tone (Toth and Ungvari, unpublished observation 2012), downregulation of Cyp4a12b expression (GEO data sets GDS2019 and GDS1053), and cognitive decline, mimicking the aging phenotype.
Here, we provide evidence that autoregulatory dysfunction in aged AngII-infused hypertensive mice is associated with significant cerebromicrovascular damage. A lack of proper autoregulatory protection likely allows high blood pressure to penetrate the distal portion of the cerebral microcirculation, which leads to a significant BBB disruption in the hippocampus and other brain regions in aged AngII-infused hypertensive mice (Figure 4). The mechanisms of hypertension-induced BBB disruption are likely multifaceted. Pericytes are important cellular constituents of the BBB19 and recent studies show that pericyte deficiency in Pdgfrβ+/− mice leads to a significant impairment of BBB function20 and development of a vascular cognitive impairment-like syndrome. Thus, it is possible that the increased hypertension-induced loss of pericyte coverage reported in our studies (Figure 5) contributes to BBB disruption in AngII-infused aged mice. The mechanisms of increased pericyte loss in aged AngII-infused hypertensive mice are presently unknown. Pericytes are sensitive to oxidative damage, thus it is possible that exacerbated hypertension-induced oxidative stress (Figure 6) contributes to increased pericyte loss in aged AngII-infused mice. Pericytes have also key roles in preservation of the structural integrity of the cerebral microcirculation;18 thus, loss of pericytes is also likely to contribute to microvascular rarefaction in brain of aged AngII-infused hypertensive mice. Future studies should elucidate whether decreased capillary density in aged AngII-infused hypertensive mice is associated with reduced hippocampal blood flow leading to ischemic foci, which would have a direct deleterious effect on cognitive function. In that regard, it is significant that AngII-induced hypertension also impairs endothelial regulation of the cerebral microcirculation by increasing oxidative/nitrosative stress in endothelial cells.36, 37
Through the damaged BBB in aged hypertensive mice plasma constituents, including IgG (Figure 4B), can enter the brain. There is growing evidence that plasma-derived factors can affect neuronal function by multiple mechanisms,19, 38 including the induction of neuroinflammation (e.g., activation of microglia by IgG via the IgG Fc receptors). Here, we provide evidence that in aged AngII-infused hypertensive mice BBB disruption and increased extravasation of IgG are associated with an exacerbated neuroinflammatory response as shown by the increased number of activated microglia and macrophages in the hippocampi (Figures 6A to 6F). We also found that in the hippocampi of aged hypertensive mice there is an increased presence and expression of inflammatory mediators, which are known to be secreted by activated microglia. Microglia-derived proinflammatory cytokines, chemokines, proteases (i.e., matrix metalloproteinases), and reactive oxygen species were shown to promote neuronal dysfunction.39, 40 On the basis of the aforementioned studies, one can hypothesize that exacerbation of neuroinflammation is causally linked to hypertension-induced impairment of hippocampal function in aged mice (Figure 6). This concept is supported by clinical studies showing that treatment of hypertension resulted in a 19% to 50% reduction in dementia incidence (PROGRESS41 and Syst-Eur42). The SCOPE study also found evidence that treatment with candesartan (an AT1 receptor antagonist) may prevent cognitive decline in elderly patients with hypertension.43
Limitations of the Study
In theory, knockout models would be useful to characterize the role of TRPC6 channels in functional adaptation of MCAs to hypertension. However, the existing TRPC6−/− mouse models exhibit significant compensatory changes in cellular expression of plasma membrane ion channels. For example, in TRPC6−/− mice vascular TRPC3 and TRPC7 expression is significantly upregulated.44 Due to the potential confounding effects of lifelong compensatory upregulation of nonTRPC6-dependent pathways, we chose to use a pharmacological approach to acutely inhibit TRPC channels to study the interaction of hypertension and aging in regulation of MCA myogenic tone. Boulay et al45 observed a complete block of heterologously expressed TRPC6 after administration of SKF96365 in vitro, thus we chose this pharmacological agent to inhibit TRPC6 activity in the present study. Yet, we are aware of the fact that SKF96365 can also inhibit TRPC3/7 channels. Although hypertension does not appear to upregulate cerebrovascular expression of TRPC3 and TRPC7 channels, we cannot exclude the possibility that activation of these channel(s) contributes to functional adaptation of MCAs to hypertension. The definitive approach to determine channel activity would be using the patch clamp technique. The lack of such measurements is a limitation of our study.
Perspectives
In the present study, we show that functional adaptation of cerebral arteries to high pressure in AngII-infused aged mice is impaired and that age-related autoregulatory dysfunction in these animals is associated with BBB disruption and neuroinflammation. Future studies should elucidate the specific age-related mechanism that underlies impaired adaptation of aged cerebral vessels to hypertension, including the role of age-related changes in endocrine factors.35 Aging may increase the sensitivity of the cerebral microcirculation to the proinflammatory effects of high pressure itself and these effects may be potentiated by AngII. Future studies can test this hypothesis. Both hypertension and aging lead to endothelial dysfunction (Supplementary Figure S3) and impaired neurovascular coupling.37 The dynamic interaction between these mechanisms and myogenic autoregulation warrants further investigation. Our findings suggest that in the elderly sudden, acute increases in blood pressure (e.g., during Valsalva maneuver) are more likely transmitted to the thin-walled cerebral microvessels, which likely contribute to the increased prevalence of cerebral microbleeds in elderly hypertensive patients.46 Because accumulating evidence supports a causal role of cerebral microbleeds in cognitive decline, future studies should elucidate the link between age-related changes in 20-HETE/TRPC pathway-dependent autoregulatory protection and incidence of cerebral microbleeds in hypertensive patients.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This work was supported by grants from the American Heart Association (to PT, AC, and ZU), the American Federation for Aging Research (to AC), the Oklahoma Center for the Advancement of Science and Technology (to AC, ZU, and WES), OTKA KI08444 and the Nemzeti Fejlesztési Ügynökség (SPOR-4.2.1/b-10/2/KONV-2010-0012, and SROP-4.2.2.a-11/1/KONV-2012-0024 and -0017 to AK and ZU), the NIH (AG031085 to AC; NCCAM-R01-AT006526 to ZU; AG038747, NS056218, and P01 AG11370 to WES) and the Ellison Medical Foundation (to WES).
Supplementary Material
References
- Aronow WS, Fleg JL, Pepine CJ, Artinian NT, Bakris G, Brown AS, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation. 2011;123:2434–2506. doi: 10.1161/CIR.0b013e31821daaf6. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Park L, Capone C. Threats to the mind: aging, amyloid, and hypertension. Stroke. 2009;40:S40–S44. doi: 10.1161/STROKEAHA.108.533638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Reitz C, Luchsinger JA, Manly JJ, Schupf N, Muraskin J, et al. Long-term blood pressure fluctuation and cerebrovascular disease in an elderly cohort. Arch Neurol. 2010;67:564–569. doi: 10.1001/archneurol.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz C, Tang MX, Manly J, Mayeux R, Luchsinger JA. Hypertension and the risk of mild cognitive impairment. Arch Neurol. 2007;64:1734–1740. doi: 10.1001/archneur.64.12.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuller LH, Lopez OL, Jagust WJ, Becker JT, DeKosky ST, Lyketsos C, et al. Determinants of vascular dementia in the cardiovascular health cognition study. Neurology. 2005;64:1548–1552. doi: 10.1212/01.WNL.0000160115.55756.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, et al. National institute of neurological disorders and stroke-canadian stroke network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–2241. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev. 1990;2:161–192. [PubMed] [Google Scholar]
- Strandgaard S, Jones JV, MacKenzie ET, Harper AM. Upper limit of cerebral blood flow autoregulation in experimental renovascular hypertension in the baboon. Circ Res. 1975;37:164–167. doi: 10.1161/01.res.37.2.164. [DOI] [PubMed] [Google Scholar]
- Harper SL, Bohlen HG. Microvascular adaptation in the cerebral cortex of adult spontaneously hypertensive rats. Hypertension. 1984;6:408–419. doi: 10.1161/01.hyp.6.3.408. [DOI] [PubMed] [Google Scholar]
- Kontos HA, Wei EP, Navari RM, Levasseur JE, Rosenblum WI, Patterson JL., Jr. Responses of cerebral arteries and arterioles to acute hypotension and hypertension. Am J Physiol. 1978;234:H371–H383. doi: 10.1152/ajpheart.1978.234.4.H371. [DOI] [PubMed] [Google Scholar]
- Osol G, Halpern W. Myogenic properties of cerebral blood vessels from normotensive and hypertensive rats. Am J Physiol. 1985;249:H914–H921. doi: 10.1152/ajpheart.1985.249.5.H914. [DOI] [PubMed] [Google Scholar]
- Kontos HA, Wei EP, Raper AJ, Rosenblum WI, Navari RM, Patterson JL., Jr. Role of tissue hypoxia in local regulation of cerebral microcirculation. Am J Physiol. 1978;234:H582–H591. doi: 10.1152/ajpheart.1978.234.5.H582. [DOI] [PubMed] [Google Scholar]
- Smeda JS. Cerebral vascular changes associated with hemorrhagic stroke in hypertension. Can J Physiol Pharmacol. 1992;70:552–564. doi: 10.1139/y92-070. [DOI] [PubMed] [Google Scholar]
- Koller A, Toth P. Contribution of flow-dependent vasomotor mechanisms to the autoregulation of cerebral blood flow. J Vasc Res. 2012;49:375–389. doi: 10.1159/000338747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Rozsa B, Springo Z, Doczi T, Koller A. Isolated human and rat cerebral arteries constrict to increases in flow: Role of 20-HETE and TP receptors. J Cereb Blood Flow Metab. 2011;31:2096–2105. doi: 10.1038/jcbfm.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebremedhin D, Lange AR, Lowry TF, Taheri MR, Birks EK, Hudetz AG, et al. Production of 20-HETE and its role in autoregulation of cerebral blood flow. Circ Res. 2000;87:60–65. doi: 10.1161/01.res.87.1.60. [DOI] [PubMed] [Google Scholar]
- Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci. 2011;14:1398–1405. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capone C, Faraco G, Park L, Cao X, Davisson RL, Iadecola C. The cerebrovascular dysfunction induced by slow pressor doses of Angiotensin II precedes the development of hypertension. Am J Physiol Heart Circ Physiol. 2011;300:H397–H407. doi: 10.1152/ajpheart.00679.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraci FM, Heistad DD. Regulation of large cerebral arteries and cerebral microvascular pressure. Circ Res. 1990;66:8–17. doi: 10.1161/01.res.66.1.8. [DOI] [PubMed] [Google Scholar]
- New DI, Chesser AM, Thuraisingham RC, Yaqoob MM. Cerebral artery responses to pressure and flow in uremic hypertensive and spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2003;284:H1212–H1216. doi: 10.1152/ajpheart.00644.2002. [DOI] [PubMed] [Google Scholar]
- Dunn KM, Renic M, Flasch AK, Harder DR, Falck J, Roman RJ. Elevated production of 20-HETE in the cerebral vasculature contributes to severity of ischemic stroke and oxidative stress in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2008;295:H2455–H2465. doi: 10.1152/ajpheart.00512.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- Muller DN, Schmidt C, Barbosa-Sicard E, Wellner M, Gross V, Hercule H, et al. Mouse cyp4a isoforms: enzymatic properties, gender- and strain-specific expression, and role in renal 20-hydroxyeicosatetraenoic acid formation. Biochem J. 2007;403:109–118. doi: 10.1042/BJ20061328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R, Jensen LJ, Jian Z, Shi J, Hai L, Lurie AI, et al. Synergistic activation of vascular trpc6 channel by receptor and mechanical stimulation via phospholipase c/diacylglycerol and phospholipase a2/omega-hydroxylase/20-hete pathways. Circ Res. 2009;104:1399–1409. doi: 10.1161/CIRCRESAHA.108.193227. [DOI] [PubMed] [Google Scholar]
- Welsh DG, Morielli AD, Nelson MT, Brayden JE. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ Res. 2002;90:248–250. doi: 10.1161/hh0302.105662. [DOI] [PubMed] [Google Scholar]
- Linde CI, Karashima E, Raina H, Zulian A, Wier WG, Hamlyn JM, et al. Increased arterial smooth muscle ca2+ signaling, vasoconstriction, and myogenic reactivity in milan hypertensive rats. Am J Physiol Heart Circ Physiol. 2012;302:H611–H620. doi: 10.1152/ajpheart.00950.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae YM, Kim A, Lee YJ, Lim W, Noh YH, Kim EJ, et al. Enhancement of receptor-operated cation current and trpc6 expression in arterial smooth muscle cells of deoxycorticosterone acetate-salt hypertensive rats. J Hypertens. 2007;25:809–817. doi: 10.1097/HJH.0b013e3280148312. [DOI] [PubMed] [Google Scholar]
- Pulina MV, Zulian A, Baryshnikov SG, Linde CI, Karashima E, Hamlyn JM, et al. Cross talk between plasma membrane Na(+)/Ca (2+) exchanger-1 and TRPC/Orai-containing channels: key players in arterial hypertension. Adv Exp Med Biol. 2013;961:365–374. doi: 10.1007/978-1-4614-4756-6_31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulian A, Baryshnikov SG, Linde CI, Hamlyn JM, Ferrari P, Golovina VA. Upregulation of Na+/Ca2+ exchanger and TRPC6 contributes to abnormal Ca2+ homeostasis in arterial smooth muscle cells from milan hypertensive rats. Am J Physiol Heart Circ Physiol. 2010;299:H624–H633. doi: 10.1152/ajpheart.00356.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H. Upregulation of the renin-angiotensin-aldosterone-ouabain system in the brain is the core mechanism in the genesis of all types of hypertension. Int J Hypertens. 2012;2012:242786. doi: 10.1155/2012/242786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang BS, Ahmadi S, Ahmad M, White RA, Leenen FH. Central neuronal activation and pressor responses induced by circulating ANG II: role of the brain aldosterone-"ouabain" pathway. Am J Physiol Heart Circ Physiol. 2010;299:H422–H430. doi: 10.1152/ajpheart.00256.2010. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Csiszar A. The emerging role of IGF-1 deficiency in cardiovascular aging: Recent advances. J Gerontol A Biol Sci Med Sci. 2012;67:599–610. doi: 10.1093/gerona/gls072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girouard H, Park L, Anrather J, Zhou P, Iadecola C. Angiotensin II attenuates endothelium-dependent responses in the cerebral microcirculation through Nox-2-derived radicals. Arterioscler Thromb Vasc Biol. 2006;26:826–832. doi: 10.1161/01.ATV.0000205849.22807.6e. [DOI] [PubMed] [Google Scholar]
- Girouard H, Park L, Anrather J, Zhou P, Iadecola C. Cerebrovascular nitrosative stress mediates neurovascular and endothelial dysfunction induced by angiotensin II. Arterioscler Thromb Vasc Biol. 2007;27:303–309. doi: 10.1161/01.ATV.0000253885.41509.25. [DOI] [PubMed] [Google Scholar]
- Fernandez-Vizarra P, Lopez-Franco O, Mallavia B, Higuera-Matas A, Lopez-Parra V, Ortiz-Munoz G, et al. Immunoglobulin g fc receptor deficiency prevents Alzheimer-like pathology and cognitive impairment in mice. Brain. 2012;135:2826–2837. doi: 10.1093/brain/aws195. [DOI] [PubMed] [Google Scholar]
- Gao HM, Liu B, Zhang W, Hong JS. Critical role of microglial NADPH oxidase-derived free radicals in the in vitro MPTP model of Parkinson's disease. FASEB J. 2003;17:1954–1956. doi: 10.1096/fj.03-0109fje. [DOI] [PubMed] [Google Scholar]
- Kaneko YS, Nakashima A, Mori K, Nagatsu T, Nagatsu I, Ota A. Microglial activation in neuroinflammation: implications for the etiology of neurodegeneration. Neurodegener Dis. 2012;10:100–103. doi: 10.1159/000332936. [DOI] [PubMed] [Google Scholar]
- PROGRESS Collaborative Group Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358:1033–1041. doi: 10.1016/S0140-6736(01)06178-5. [DOI] [PubMed] [Google Scholar]
- Forette F, Seux ML, Staessen JA, Thijs L, Birkenhager WH, Babarskiene MR, et al. Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet. 1998;352:1347–1351. doi: 10.1016/s0140-6736(98)03086-4. [DOI] [PubMed] [Google Scholar]
- Trenkwalder P. The study on cognition and prognosis in the elderly (scope)—recent analyses. J Hypertens Suppl. 2006;24:S107–S114. doi: 10.1097/01.hjh.0000220415.99610.22. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Mederos YSM, Gollasch M, Gross V, Storch U, Dubrovska G, et al. Increased vascular smooth muscle contractility in TRPC6−/− mice. Mol Cell Biol. 2005;25:6980–6989. doi: 10.1128/MCB.25.16.6980-6989.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulay G, Zhu X, Peyton M, Jiang M, Hurst R, Stefani E, et al. Cloning and expression of a novel mammalian homolog of drosophila transient receptor potential (Trp) involved in calcium entry secondary to activation of receptors coupled by the Gq class of G protein. J Biol Chem. 1997;272:29672–29680. doi: 10.1074/jbc.272.47.29672. [DOI] [PubMed] [Google Scholar]
- Poels MM, Ikram MA, van der Lugt A, Hofman A, Krestin GP, Breteler MM, et al. Incidence of cerebral microbleeds in the general population: The Rotterdam Scan Study. Stroke. 2011;42:656–661. doi: 10.1161/STROKEAHA.110.607184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.