Abstract
Recanalization of an occluded vessel with recombinant tissue plasminogen activator is an effective strategy for treating acute ischemic stroke. Recombinant tissue plasminogen activator is administered as alteplase, a formulation containing many excipients including L-arginine, the substrate for nitric oxide production. Most studies fail to compare the effects of alteplase on brain injury to its L-arginine carrier solution. This study aimed to verify the previously reported detrimental effects of alteplase after cerebral ischemia and delineate the contribution of L-arginine. Male Wistar rats, subjected to 90 minutes of intraluminal middle cerebral artery occlusion (MCAO), were administered alteplase, the carrier solution or saline upon reperfusion. Neither alteplase nor the carrier affected cerebral blood flow (CBF) restoration throughout the first 60 minutes of reperfusion. Alteplase treatment was associated with increased mortality after MCAO. Twenty-four hours after MCAO, neurologic function and infarct volume did not differ between rats treated with alteplase, the carrier solution, or saline. Irrespective of treatment group, infarct volume was correlated with CBF during reperfusion, neuroscore, and peri-infarct depolarizations. These results suggest that alteplase treatment, independent of thrombolysis, does not cause increased ischemic injury compared with its appropriate carrier solution, supporting the continued use of alteplase in eligible ischemic stroke patients.
Keywords: alteplase, cerebral ischemia, infarct, L-arginine, middle cerebral artery occlusion, recombinant tissue plasminogen activator
Introduction
Recanalization of an occluded vessel is the single most effective strategy to improve patient outcome after acute ischemic stroke. Currently, there is only one approved thrombolytic treatment for ischemic stroke, recombinant tissue plasminogen activator (rtPA). Recombinant tissue plasminogen activator converts plasminogen to plasmin to break down the fibrin clot, leading to the restoration of blood flow.1 Alteplase, a formulation of rtPA, has been shown to improve functional recovery in ischemic stroke patients2 and provide benefit when administered within 4.5 hours of symptom onset.3 After this time period, however, administration of alteplase can enhance the risk of hemorrhaging within the brain,4 which limits its clinical utility to 4.5 hours post stroke.
Alteplase is now widely used as a first-line therapy for eligible ischemic stroke patients, but it may also have neurotoxic effects.5, 6 Wang et al.7 first showed that alteplase administration increased infarct volume in wild-type mice 24 hours after mechanical middle cerebral artery occlusion (MCAO), suggesting these neurotoxic effects were independent of its thrombolytic activity. Some subsequent studies failed to replicate this finding,8, 9 but a meta-analysis of animal studies investigating the effect of rtPA in mechanical models of ischemic injury revealed that rtPA increases infarct volume after ischemia, particularly in mice.5 Potential neurotoxic mechanisms proposed include the serine protease activity of rtPA, the activation of excitotoxic N-methyl-D-aspartate receptors, and the production of nitric oxide (NO).5, 6
Alteplase is the licensed formulation of rtPA, but it also contains other excipients including L-arginine, phosphoric acid, and polysorbate 80 (Genentech, South San Francisco, CA, USA; Table 1). The L-arginine component of alteplase is used to provide stability to the rtPA protein,10 but L-arginine is also the precursor to NO formation,11 and NO can have both neurotoxic and neuroprotective effects after cerebral ischemia.12 The L-arginine present in alteplase may contribute to any rtPA effects, including augmentation of ischemic injury.5 However, very few of the studies assessing alteplase neurotoxicity used appropriate control solutions containing L-arginine.5 Therefore, we wished to verify the neurotoxic effect of alteplase after cerebral ischemia, and identify any effect of alteplase on other physiologic parameters, while delineating the contribution that L-arginine makes to these effects.
Table 1. The components of the five treatment groups.
| Saline | Alteplase 1 | Alteplase 10 | Carrier 1 | Carrier 10 |
|---|---|---|---|---|
| 0.9% saline | 1 mg/mL rtPA | 10 mg/mL rtPA | — | — |
| — | 35 mg/mL L-arginine | 350 mg/mL L-arginine | 35 mg/mL L-arginine | 350 mg/mL L-arginine |
| — | 10 mg/mL phosphoric acid | 100 mg/mL phosphoric acid | 10 mg/mL phosphoric acid | 100 mg/mL phosphoric acid |
| — | ⩽0.11 mg/mL polysorbate 80 | ⩽1.1 mg/mL polysorbate 80 | ⩽0.11 mg/mL polysorbate 80 | ⩽1.1 mg/mL polysorbate 80 |
rtPA, recombinant tissue plasminogen activator.
Materials and Methods
Animals
All procedures conformed to the Animal (Scientific Procedures) Act 1986 (UK), and were approved by the University of Oxford Animal Ethics Committee and the Home Office (UK). Male Wistar rats (243 to 338 g) (Harlan, Bicester, UK) were used. All animals were housed in a 12-hour light–dark cycle and had ad libitum access to food and water.
Middle Cerebral Artery Occlusion
The intraluminal filament MCAO model was performed as previously described.13 Anesthesia was induced with 4% isoflurane and maintained with 1.5% to 2% isoflurane carried in 30% oxygen and 70% nitrous oxide throughout all surgical procedures. A 4−0 filament with a silicone tip (Doccol Corporation, Sharon, MA, USA) was advanced into the right external carotid artery stump and up the internal carotid artery to occlude the origin of the right MCA. The filament was retracted after 90 minutes to produce reperfusion. Further details of the MCAO procedure are in the Supplementary methods. Before MCAO, the tail artery was cannulated with polyethylene tubing (OD 0.8 mm), and blood gases (pH, pO2, pCO2, and % hematocrit) and mean arterial blood pressure were assessed. There were no significant differences in blood gases or mean arterial blood pressure between any of the treatment groups (Supplementary Table S1).
Drug Administration
During the MCAO period, the left external jugular vein was cannulated with polyethylene tubing (OD 0.8 mm). At the onset of reperfusion (upon filament retraction), one of the five treatments was administered at a volume of 1 mL/kg with 10% volume administered as intravenous bolus whereas the remaining 90% volume was infused intravenously over 60 minutes. The five treatment groups and their components are outlined in Table 1. All treatment solutions were generously supplied by Genentech (South San Francisco, CA, USA), randomized, and masked to the investigator.
Cerebral Blood Flow
Before MCAO, a laser Doppler probe (Oxford Optronix, Oxford, UK) to measure cerebral blood flow (CBF) was positioned over the thinned skull approximately 4 mm lateral and 1.5 mm caudal to bregma overlying the MCA territory. Occlusion was confirmed if a drop in CBF to <30% of baseline was observed. CBF was continuously monitored throughout the ischemic and reperfusion/drug administration periods.
Neuroscore
Twenty-four hours after the onset of MCAO, animals were blindly assessed for neurologic deficit using six sub-tests investigating activity, limb symmetry, motor function, and sensory stimulation, modified from Garcia et al.14 Each test was scored out of 2 or 3, with a maximum overall score of 15 (severe neurologic deficit) and a minimum score of 0 (no neurologic deficit). Further details regarding the neuroscore assessment are in the Supplementary methods.
Infarct Analysis
Following neurologic assessment, all animals were decapitated and the brain was sliced into 2-mm thick sections. Each brain slice was stained with 2% 2,3,5-triphenyl-tetrazolium chloride (TTC) for 30 minutes at 37°C in the dark. 2,3,5-Triphenyl-tetrazolium chloride stains metabolic tissue deep red/pink whereas infarcted tissue remains white. Infarct area (mm2) and infarct volume (mm3) were calculated from the photographed sections (ImageJ, NIH, Bethesda, MD, USA). Further details regarding infarct analysis are in the Supplementary methods.
Data Analysis
All data are presented as mean±standard error of the mean except for non-parametric data, which are presented as a median. For comparisons between groups and within groups, two-way analysis of variance (ANOVA) with repeated measures was used. For comparisons between multiple groups, one-way ANOVA with Tukey's post hoc test for pairwise comparisons was carried out. For non-parametric data, comparisons between multiple groups were carried out using a Kruskal–Wallis test followed by Dunn's test for pairwise comparisons. For correlations between two variables, Pearson's (parametric) or Spearman's (non-parametric) correlation coefficient (R) was used. A P-value ⩽0.05 was considered statistically significant. A power calculation was used to determine sample size for each group. We estimated a 40% increase in infarct volume (alteplase versus saline),5 a 20% decrease in infarct volume (carrier versus saline),5 and a standard deviation of 40% of the mean within each group. With α=0.05, the sample size to achieve power (1-β)=0.8 was n=9 per group.
Results
Mortality
Sixty animals were initially included in this study. There were six deaths, three of which were in the alteplase 1 group (21% mortality), two were in the alteplase 10 group (15% mortality), and one in the carrier 1 group (8% mortality) (Table 2). The other two groups had no fatalities. There were ten animals excluded for other reasons (see Table 2), leaving 44 animals included in the final analysis (all groups n=9, except the carrier 10 group, which had n=8).
Table 2. The effect of alteplase on mortality and intracerebral hemorrhage after 90 minutes of MCAO.
| Saline | Alteplase 1 | Alteplase 10 | Carrier 1 | Carrier 10 | |
|---|---|---|---|---|---|
| Animals | 10 | 14 | 13 | 12 | 11 |
| Weight (s.e.m.) | 284 (8) | 280 (5) | 270 (7) | 282 (6) | 281 (6) |
| Deaths | 0 | 3 | 2 | 1 | 0 |
| % mortality | 0 | 21.4 | 15.4 | 8.3 | 0 |
| ICHa | 0 | 2 | 1 | 1 | 1 |
| % ICH | 0 | 14.3 | 7.7 | 8.3 | 9.1 |
| Excluded for other reasonsb | 1 | 2 | 2 | 1 | 2 |
| Animals included in final analysis | 9 | 9 | 9 | 9 | 8 |
CBF, cerebral blood flow; ICH, intracerebral hemorrhage; MCAO, middle cerebral artery occlusion; s.e.m., standard error of the mean.
Note that some animals with ICH also died before 24 hours (n=3) and so those animals will be in both the ‘death' and ‘ICH' rows. The two animals that survived 24 hours with ICH were excluded from the analysis.
Other reasons for exclusion other than death and ICH include: occlusion was not confirmed by a 70% drop in CBF (n=3), severe blood loss from surgery (n=1), unsuccessful venous cannulation (n=1), and filament insertion through the wrong artery (n=3).
Intracerebral Hemorrhage
Of the 27 animals treated with either concentration of alteplase, 3 (11%) had evidence of an intracerebral hemorrhage (ICH) as defined by visual observation during dissection, whereas 2 out of 23 animals (9%) had signs of an ICH with carrier treatment (Table 2). No animals had an ICH in the saline-treatment group. Therefore, there appears to be a tendency towards a higher risk of ICH with alteplase or carrier treatment compared with saline.
Cerebral Blood Flow
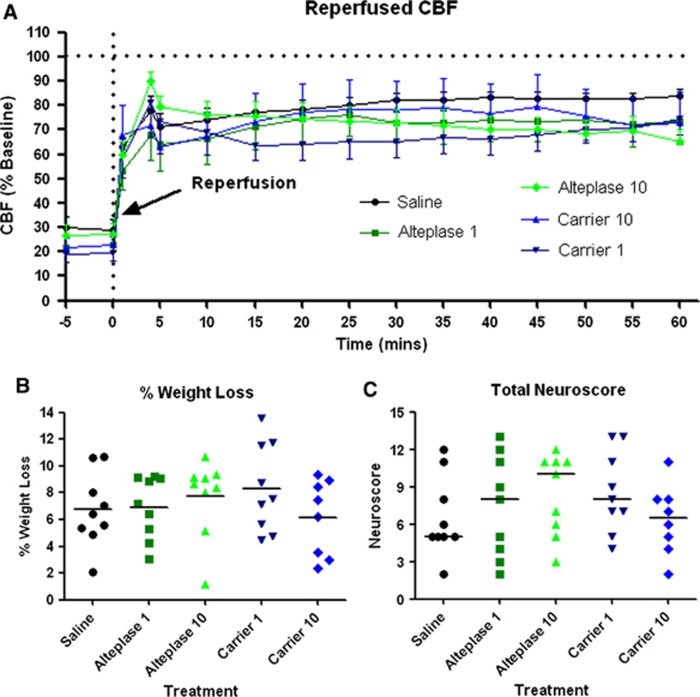
For all included animals, upon MCAO, CBF dropped below 30% of baseline (Supplementary Figure S1A). During the first 60 minutes of reperfusion, no differences in CBF were observed between any of the treatment groups (Figure 1A; ANOVA: P=0.825). Even though MCA recanalization had occurred because of filament retraction, the level of the CBF never recovered to pre-MCAO levels suggesting that the cortical MCA territory had significant hypoperfusion (65% to 80% of baseline) in all treatment groups (Figure 1A). We also assessed whether alteplase or its carrier could alter CBF in naïve animals under physiologic conditions (see Supplementary Methods and Supplementary Table S2). During administration of alteplase 10, carrier 10 or saline, there was no significant difference in CBF between any treatment groups (Supplementary Figure S1B; ANOVA: P=0.356).
Figure 1.
The effect of alteplase on cerebral blood flow (CBF), percentage weight loss, and neuroscore after cerebral ischemia. (A) Cerebral blood flow was continuously monitored during the first 60 minutes of reperfusion while treatments were administered. Reperfused CBF never recovered to pre-middle cerebral artery occlusion (MCAO) CBF levels. There was no significant effect of treatment on reperfused CBF. (B) The percentage weight loss for each animal was assessed 24 hours after MCAO onset. There was no significant effect of treatment (horizontal bar indicates the mean of each group). (C) Total neuroscore was calculated through a battery of six sub-tests 24 hours after MCAO onset. There were no significant effects of treatment group on total neuroscore (horizontal bar indicates the median of each group).
Weight Loss
Percentage weight loss over the 24 hours since MCAO can be used as an indicator of health of the animal. Figure 1B shows that mean weight loss ranged from 6% to 8%, but was not significantly different between groups (ANOVA: P=0.544).
Temperature
Rectal temperature was also measured 24 hours after the onset of MCAO to determine if the treatments had any effect on core temperature. At this timepoint, there were no significant differences between any groups (Supplementary Figure S1C; ANOVA: P=0.187).
Neuroscore
Neurologic deficit is a strong indicator of injury sustained due to MCAO. Functional neuroscore assessment, as measured by six sub-tests, revealed there were no significant differences between groups (Figure 1C; Kruskal–Wallis: P=0.559). In addition, there were no significant differences in any one sub-test between any groups (Supplementary Figure S2).
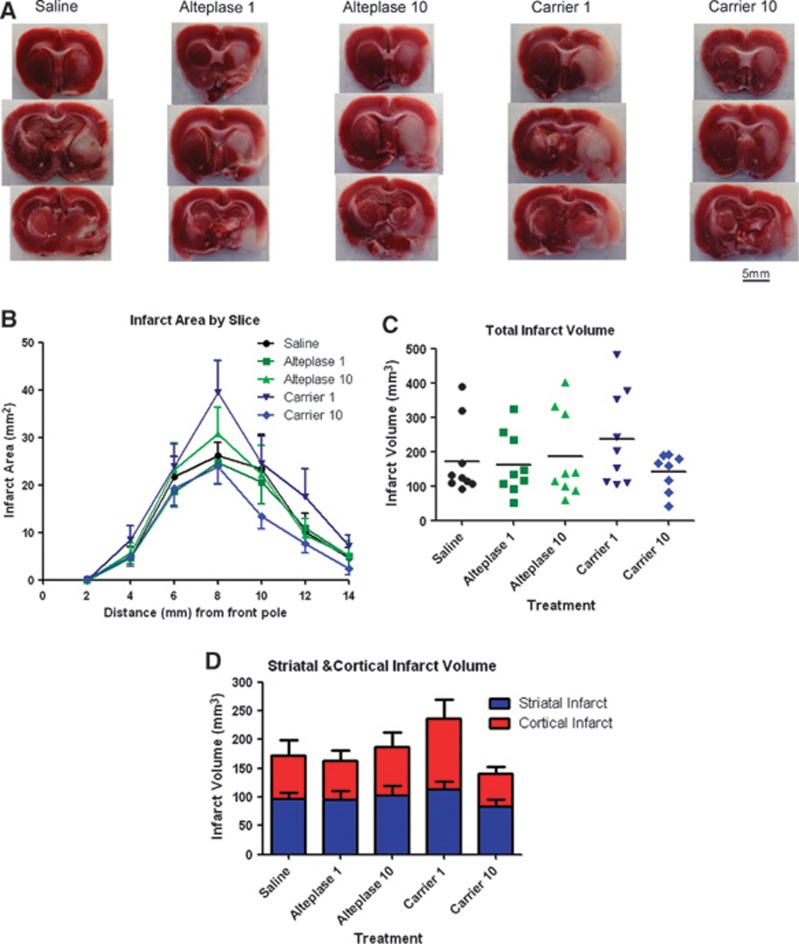
Infarct Volume
Histologic assessment of infarct volume can directly determine the extent of ischemic injury. 2,3,5-Triphenyl-tetrazolium chloride-stained brains from each treatment group revealed an infarct in both the striatal and cortical regions 24 hours after MCAO (Figure 2A). Examining the infarct area by slice showed no significant differences between any treatment groups at any slice level throughout the brain (Figure 2B). As a result, infarct volume revealed no significant differences between any treatment groups (Figure 2C, ANOVA: P=0.435). Subdividing the infarct into striatal and cortical regions showed that there were no significant effects of treatment on infarct volume in either region (Figure 2D; cortex ANOVA: P=0.389; striatum ANOVA: P=0.703).
Figure 2.
The effect of alteplase on infarct area and infarct volume 24 hours after cerebral ischemia. (A) Infarct area was assessed 24 hours after middle cerebral artery occlusion (MCAO) onset in 2 mm brain slices. 2,3,5-Triphenyl-tetrazolium chloride was used to stain viable tissue pink leaving infarcted tissue white. Pictured are representative slices for each treatment group 6 mm (top), 8 mm (middle), and 10 mm (bottom) from the frontal pole of the brain. (B) Infarct area was calculated for each slice. There was no significant difference in the infarct area between treatment groups in any slice. (C) Infarct volume was calculated as the area under the curve for infarct area versus slice thickness. There was no significant difference between treatment groups for infarct volume. (D) Infarct volume can be divided up between striatal and cortical infarct volumes. For each brain region, there was no significant difference between treatment groups.
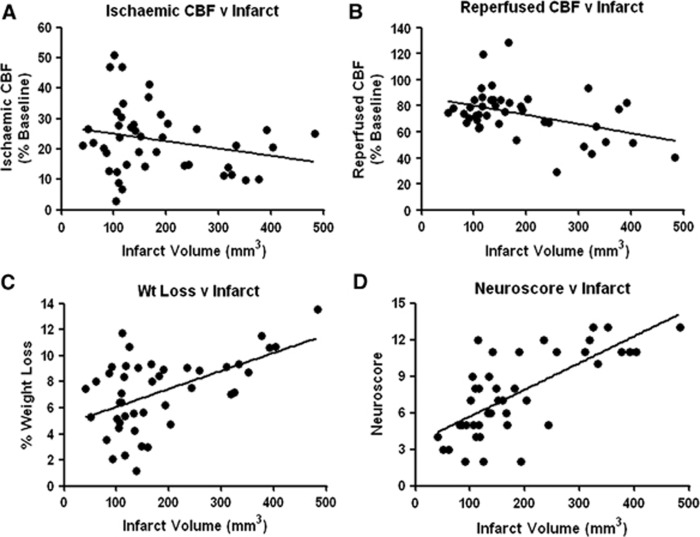
Correlations
If specific physiologic parameters correlated with infarct volume, these correlates could potentially be used as an indicator of injury. For transient MCAO irrespective of treatment group, average CBF during 90 minutes of ischemia did not correlate with infarct volume (R=−0.239, P=0.118; Figure 3A). However, average CBF during the first 60 minutes of reperfusion was inversely correlated with infarct volume (R=−0.414, P=0.006; Figure 3B). Percentage weight loss 24 hours post MCAO was a strong predictor of infarct volume (R=0.531, P⩽0.001; Figure 3C). Likewise, neuroscore at 24 hours was significantly correlated with infarct volume (R=0.665, P⩽0.001; Figure 3D). The cortical infarct volume was significantly correlated with CBF during reperfusion (R=−0.457, P=0.002), weight loss at 24 hours (R=0.559, P⩽0.001) and neuroscore at 24 hours (R=0.576, P⩽0.001). The striatal infarct volume was significantly correlated only with weight loss (R=0.38, P=0.011) and neuroscore at 24 hours (R=0.673, P⩽0.001).
Figure 3.
Correlations between infarct volume and other parameters. (A) There was no significant correlation between average cerebral blood flow (CBF) during 90 minutes middle cerebral artery occlusion (MCAO) and infarct volume. (B) Average CBF during the first 60 minutes of reperfusion was inversely correlated with infarct volume. (C) The percentage weight loss at 24 hours after MCAO was significantly correlated with infarct volume. (D) Total neuroscore was significantly correlated with infarct volume.
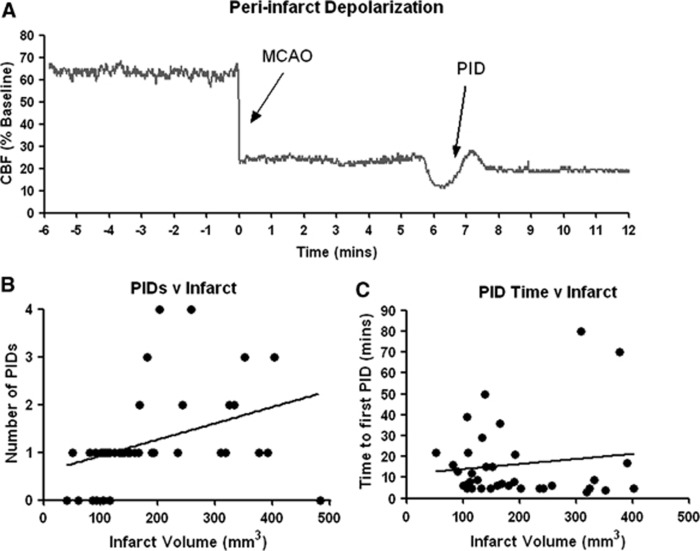
Peri-infarct depolarization (PID) is a depolarizing wave that silences synaptic activity of neurons as it propagates across the gray matter after insults such as cerebral ischemia.15 Peri-infarct depolarizations also trigger changes in CBF, and can be detected during ischemia by observing a gradual hypoperfusion followed by a hyperemia,16 after which CBF can return to its baseline (Figure 4A). In the present study, on average 1.2 PIDs occurred during 90 minutes of MCAO, typically lasting 2 to 3 minutes each; the largest number of PIDs observed in a single animal was four. Peri-infarct depolarizations were never observed during the first 60 minutes of reperfusion. Peri-infarct depolarizations may contribute to ischemic injury17 and so, we assessed whether the number of PIDs during ischemia, or the time until the first PID occurred, correlated with infarct volume. The number of PIDs during the ischemic period was significantly correlated with infarct volume (R=0.557, P⩽0.001; Figure 4B) but the time to the first PID was not correlated with infarct volume (R=0.131, P=0.447; Figure 4C).
Figure 4.
Correlations between infarct volume and peri-infarct depolarizations (PIDs). (A) Peri-infarct depolarizations were observed in the majority of animals during the ischemic period only. Peri-infarct depolarizations were characterized by a gradual hypoperfusion followed by a hyperemia lasting 2 to 3 minutes, of which an example is shown. (B) The number of PIDs during the ischemic period was counted and was significantly correlated with infarct volume. (C) The time taken from MCAO onset (minutes) before the first PID was observed had no significant correlation with infarct volume.
Discussion
A large number of ischemic stroke patients benefit from rtPA through improvement of neurologic deficit and disability,18 but rtPA also produces a number of complications including ICH, secondary embolization, and angioedema.19 Another complication of rtPA is neurotoxicity, which has been observed in animal models of cerebral ischemia, particularly those that use mechanical occlusion of a cerebral artery, showing that these neurotoxic effects are independent of the thrombolytic action of rtPA.5, 7 Concordantly, a retrospective clinical study revealed that alteplase treatment could lead to a worse clinical outcome through increasing blood–brain barrier disruption,20 and it is rtPA's toxic action on the neurovascular unit21, 22 that may produce both neurotoxic and hemorrhagic complications. The present study revealed no significant effect of alteplase on infarct volume after transient rat MCAO (Figure 2). However, 18.5% of all alteplase-treated animals died (with 60% of these deaths having ICH), compared with 4% of carrier-treated animals and 0% of saline-treated animals. This suggests that while infarct volume was not affected in rats that survived alteplase treatment, there was a higher risk of acute mortality associated with alteplase treatment, in line with what is observed clinically.18
When administering alteplase, it is impossible to differentiate the actions of rtPA from the effects of the other excipients used in the carrier solution including L-arginine. Therefore, in any rtPA study, an appropriate control would be to administer the excipient solution containing L-arginine, which itself may have significant biologic effects. A meta-analysis investigating the effect of rtPA on infarct volume after cerebral ischemia identified only 2 out of 29 contrasts between rtPA and control using an L-arginine control solution, and these contrasts showed no significant effect.5 Here we showed that the carrier solution containing L-arginine did not affect infarct volume, CBF, or behavioral outcome after MCAO compared with alteplase or saline. However, other studies have shown that early administration (less than 3 hours) of L-arginine alone reduced cerebral injury, whereas delayed administration (greater than 11 hours) exacerbated ischemic injury.5 From these results it is clear that L-arginine does have biologic activity and certainly needs to be controlled for in studies investigating rtPA.
Even though rtPA possesses potent thrombolytic activity to reperfuse the ischemic brain, there is evidence that rtPA may exert effects on CBF independent of its thrombolytic action. After mechanical rat MCAO, alteplase increased infarct volume while producing an initial hyperperfusion followed by a hypoperfusion in the penumbra compared with saline.23 These flow changes may be due to alteplase downregulating endothelial NO synthase protein expression after cerebral ischemia.24 However, our study demonstrated no difference in CBF after reperfusion between alteplase, carrier, or saline treatments within the MCA territory (Figure 1A). Notably, the animals in this study had raised pCO2, which could alter CBF independently of the treatments. However, experiments in normocapnic naïve animals showed that neither alteplase nor its carrier solution altered CBF throughout the infusion period (Supplementary Figure S1B), suggesting that CBF changes from these compounds are likely not responsible for any changes in infarct volume. In contrast to this, rtPA has previously been shown to cause dysfunction of both vascular tone and vasoreactivity,25, 26 which may lead to hemorrhagic transformation and edema after cerebral ischemia. Endogenous tissue plasminogen activator appears to have a role in controlling CBF through N-methyl-D-aspartate receptor-mediated NO production.27 However, it is unknown whether alteplase, as administered clinically, would affect neurovascular coupling, and the contribution of L-arginine to these CBF responses is equally unclear.
The present study only evaluated infarct volume at 24 hours after ischemic onset, meaning we cannot exclude the possibility that alteplase or its carrier could have affected cerebral injury beyond this time. Most previous studies that investigated alteplase after MCAO examined infarct size at 24 hours,5 and so this timepoint was chosen to make comparisons with these studies. Whether maximal infarct volume occurs at 24 hours is controversial, with some studies showing increased infarct volume beyond 24 hours,28 whereas others showed maximal infarct volume at 24 hours.29 However, the duration of ischemia may determine the pathophysiological response, and thus the temporal dynamics of a focal infarct. One study revealed that severe MCAO (90 minutes duration) produced a maximal infarct at 24 hours, whereas brief ischemia (30 minutes duration) delayed infarct expansion beyond 24 hours.30
Physiologic parameters can indicate an animal's health status, but can also predict the extent of injury resulting from cerebral ischemia. In the present study, irrespective of treatment group, infarct volume was inversely correlated with CBF during initial reperfusion but not with CBF during ischemia (Figure 3). This suggests that, for transient cerebral ischemia, blood flow throughout the initial reperfusion period determines injury, whereas it is blood flow during ischemia that is inversely correlated with injury after permanent cerebral ischemia.13 This demonstrates the importance of CBF to the outcome after cerebral ischemia, and that neuroprotective agents may actually modulate CBF to produce their effect.31 Our data revealed that other parameters such as percentage body weight loss, neuroscore, and the number of PIDs were also correlated with infarction (Figures 3 and 4). However, it may be the total duration of tissue depolarization and not its frequency that correlates with ischemic injury.32
In conclusion, alteplase had no effect on CBF, neurologic function, or infarct volume after mechanical transient MCAO in the rat. These results add to the heterogeneous data previously collected suggesting that the effect of rtPA on infarct volume after mechanical cerebral ischemia is variable and appears to depend on the species and model used.5 The scarcity of studies comparing rtPA to its L-arginine carrier solution prompted us to carry out this study, and we found no effect of the L-arginine carrier solution compared with alteplase or saline. These data support the continued use of alteplase treatment for ischemic stroke, but ascertaining the contribution of L-arginine to the effects of alteplase would be insightful given its widespread use. Therefore, any formulation of rtPA that does not contain L-arginine would be worth investigating for its thrombolytic ability as well as other effects.
Acknowledgments
The authors thank Prof James Peeling and Mr Ain Neuhaus for comments on this manuscript, Drs Nicholas Van Bruggen and Joan Greve for assistance with the study design, and Dr Keith Brooks for his technical assistance with the experiments.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
The work presented in this manuscript was funded by Fondation Leducq. The treatment compounds used in this study were generously supplied by Genentech.
Supplementary Material
References
- Korninger C, Collen D. Studies on the specific fibrinolytic effect of human extrinsic (tissue-type) plasminogen activator in human blood and in various animal species in vitro. Thromb Haemost. 1981;46:561–565. [PubMed] [Google Scholar]
- NINDS Tissue plasminogen activator for acute ischemic stroke. The national institute of neurological disorders and stroke rt-pa stroke study group. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- Bluhmki E, Chamorro A, Davalos A, Machnig T, Sauce C, Wahlgren N, et al. Stroke treatment with alteplase given 3.0–4.5 hours after onset of acute ischaemic stroke (ecass iii): Additional outcomes and subgroup analysis of a randomised controlled trial. Lancet Neurol. 2009;8:1095–1102. doi: 10.1016/S1474-4422(09)70264-9. [DOI] [PubMed] [Google Scholar]
- Hacke W, Donnan G, Fieschi C, Kaste M, von Kummer R, Broderick JP, et al. Association of outcome with early stroke treatment: pooled analysis of atlantis, ecass, and ninds rt-pa stroke trials. Lancet. 2004;363:768–774. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- Harston GW, Sutherland BA, Kennedy J, Buchan AM. The contribution of L-arginine to the neurotoxicity of recombinant tissue plasminogen activator following cerebral ischemia: A review of rtpa neurotoxicity. J Cereb Blood Flow Metab. 2010;30:1804–1816. doi: 10.1038/jcbfm.2010.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur J, Zhao Z, Klein GM, Lo EH, Buchan AM. The neurotoxicity of tissue plasminogen activator. J Cereb Blood Flow Metab. 2004;24:945–963. doi: 10.1097/01.WCB.0000137868.50767.E8. [DOI] [PubMed] [Google Scholar]
- Wang YF, Tsirka SE, Strickland S, Stieg PE, Soriano SG, Lipton SA. Tissue plasminogen activator (tpa) increases neuronal damage after focal cerebral ischemia in wild-type and tpa-deficient mice. Nat Med. 1998;4:228–231. doi: 10.1038/nm0298-228. [DOI] [PubMed] [Google Scholar]
- Klein GM, Li H, Sun P, Buchan AM. Tissue plasminogen activator does not increase neuronal damage in rat models of global and focal ischemia. Neurology. 1999;52:1381–1384. doi: 10.1212/wnl.52.7.1381. [DOI] [PubMed] [Google Scholar]
- Meng W, Wang X, Asahi M, Kano T, Asahi K, Ackerman RH, et al. Effects of tissue type plasminogen activator in embolic versus mechanical models of focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 1999;19:1316–1321. doi: 10.1097/00004647-199912000-00004. [DOI] [PubMed] [Google Scholar]
- Nguyen TH, Ward C.Stability characterization and formulation development of alteplase, a recombinant tissue plasminogen activatorIn: Wang YJ, Pearlman R (eds).. Stability and CharActerization of Protein and Peptide Drugs: Case Histories Plenum Press: New York; 199391–134. [DOI] [PubMed] [Google Scholar]
- Palmer RM, Rees DD, Ashton DS, Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988;153:1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Bright and dark sides of nitric oxide in ischemic brain injury. Trends Neurosci. 1997;20:132–139. doi: 10.1016/s0166-2236(96)10074-6. [DOI] [PubMed] [Google Scholar]
- Nagel S, Papadakis M, Chen R, Hoyte LC, Brooks KJ, Gallichan D, et al. Neuroprotection by dimethyloxalylglycine following permanent and transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2011;31:132–143. doi: 10.1038/jcbfm.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JH, Wagner S, Liu KF, Hu XJ.Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation Stroke 199526627–634.discussion 635. [DOI] [PubMed] [Google Scholar]
- Hossmann KA. Periinfarct depolarizations. Cerebrovasc Brain Metab Rev. 1996;8:195–208. [PubMed] [Google Scholar]
- Strong AJ, Anderson PJ, Watts HR, Virley DJ, Lloyd A, Irving EA, et al. Peri-infarct depolarizations lead to loss of perfusion in ischaemic gyrencephalic cerebral cortex. Brain. 2007;130:995–1008. doi: 10.1093/brain/awl392. [DOI] [PubMed] [Google Scholar]
- Hartings JA, Rolli ML, Lu XC, Tortella FC. Delayed secondary phase of peri-infarct depolarizations after focal cerebral ischemia: Relation to infarct growth and neuroprotection. J Neurosci. 2003;23:11602–11610. doi: 10.1523/JNEUROSCI.23-37-11602.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw JM, Murray V, Berge E, del Zoppo G, Sandercock P, Lindley RL, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet. 2012;379:2364–2372. doi: 10.1016/S0140-6736(12)60738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balami JS, Sutherland BA, Buchan AM. Complications associated with recombinant tissue plasminogen activator therapy for acute ischaemic stroke. CNS Neurol Disord Drug Targets. 2013;12:155–169. doi: 10.2174/18715273112119990050. [DOI] [PubMed] [Google Scholar]
- Kidwell CS, Latour L, Saver JL, Alger JR, Starkman S, Duckwiler G, et al. Thrombolytic toxicity: blood brain barrier disruption in human ischemic stroke. Cerebrovasc Dis. 2008;25:338–343. doi: 10.1159/000118379. [DOI] [PubMed] [Google Scholar]
- Su EJ, Fredriksson L, Geyer M, Folestad E, Cale J, Andrae J, et al. Activation of pdgf-cc by tissue plasminogen activator impairs blood-brain barrier integrity during ischemic stroke. Nat Med. 2008;14:731–737. doi: 10.1038/nm1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Kamiya T, Deguchi K, Inaba T, Zhang H, Shang J, et al. Dissociation and protection of the neurovascular unit after thrombolysis and reperfusion in ischemic rat brain. J Cereb Blood Flow Metab. 2009;29:715–725. doi: 10.1038/jcbfm.2008.164. [DOI] [PubMed] [Google Scholar]
- Kilic E, Bahr M, Hermann DM. Effects of recombinant tissue plasminogen activator after intraluminal thread occlusion in mice: role of hemodynamic alterations. Stroke. 2001;32:2641–2647. doi: 10.1161/hs1101.097381. [DOI] [PubMed] [Google Scholar]
- Kilic E, Kilic U, Matter CM, Luscher TF, Bassetti CL, Hermann DM. Aggravation of focal cerebral ischemia by tissue plasminogen activator is reversed by 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitor but does not depend on endothelial no synthase. Stroke. 2005;36:332–336. doi: 10.1161/01.STR.0000152273.24063.f7. [DOI] [PubMed] [Google Scholar]
- Cipolla MJ, Lessov N, Clark WM, Haley EC., Jr. Postischemic attenuation of cerebral artery reactivity is increased in the presence of tissue plasminogen activator. Stroke. 2000;31:940–945. doi: 10.1161/01.str.31.4.940. [DOI] [PubMed] [Google Scholar]
- Nassar T, Akkawi S, Shina A, Haj-Yehia A, Bdeir K, Tarshis M, et al. In vitro and in vivo effects of tpa and pai-1 on blood vessel tone. Blood. 2004;103:897–902. doi: 10.1182/blood-2003-05-1685. [DOI] [PubMed] [Google Scholar]
- Park L, Gallo EF, Anrather J, Wang G, Norris EH, Paul J, et al. Key role of tissue plasminogen activator in neurovascular coupling. Proc Natl Acad Sci USA. 2008;105:1073–1078. doi: 10.1073/pnas.0708823105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Mori T, Tateishi N, Kagamiishi Y, Satoh S, Katsube N, et al. Astrocytic activation and delayed infarct expansion after permanent focal ischemia in rats. Part I: enhanced astrocytic synthesis of s-100beta in the periinfarct area precedes delayed infarct expansion. J Cereb Blood Flow Metab. 2002;22:711–722. doi: 10.1097/00004647-200206000-00010. [DOI] [PubMed] [Google Scholar]
- Liu F, Schafer DP, McCullough LD. Ttc, fluoro-jade b and neun staining confirm evolving phases of infarction induced by middle cerebral artery occlusion. J Neurosci Methods. 2009;179:1–8. doi: 10.1016/j.jneumeth.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du C, Hu R, Csernansky CA, Hsu CY, Choi DW. Very delayed infarction after mild focal cerebral ischemia: a role for apoptosis. J Cereb Blood Flow Metab. 1996;16:195–201. doi: 10.1097/00004647-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Sutherland BA, Papadakis M, Chen RL, Buchan AM. Cerebral blood flow alteration in neuroprotection following cerebral ischaemia. J Physiol. 2011;589:4105–4114. doi: 10.1113/jphysiol.2011.209601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkhuizen RM, Beekwilder JP, van der Worp HB, Berkelbach van der Sprenkel JW, Tulleken KA, Nicolay K. Correlation between tissue depolarizations and damage in focal ischemic rat brain. Brain Res. 1999;840:194–205. doi: 10.1016/s0006-8993(99)01769-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.