Abstract
Hypertension and stroke are highly prevalent risk factors for cognitive impairment and dementia. Alzheimer's disease (AD) and vascular dementia (VaD) are the most common forms of dementia, and both conditions are preceded by a stage of cognitive impairment. Stroke is a major risk factor for the development of vascular cognitive impairment (VCI) and VaD; however, stroke may also predispose to AD. Hypertension is a major risk factor for stroke, thus linking hypertension to VCI and VaD, but hypertension is also an important risk factor for AD. Reducing these two major, but modifiable, risk factors—hypertension and stroke—could be a successful strategy for reducing the public health burden of cognitive impairment and dementia. Intake of long-chain omega-3 polyunsaturated fatty acids (LC-n3-FA) and the manipulation of factors involved in the renin–angiotensin system (e.g. angiotensin II or angiotensin-converting enzyme) have been shown to reduce the risk of developing hypertension and stroke, thereby reducing dementia risk. This paper will review the research conducted on the relationship between hypertension, stroke, and dementia and also on the impact of LC-n3-FA or antihypertensive treatments on risk factors for VCI, VaD, and AD.
Keywords: animal models, cerebral hemodynamics, cognitive impairment, dementia, diet, hypertension
Introduction
The term dementia comprises several symptoms, for example, a progressive loss of memory and behavioral changes, which together interfere with independent performance of tasks of daily life.1 As a result of increasing life expectancy, dementia is developing into one of the major public health problems in our aging society. This is driven mostly by the increasing prevalence of Alzheimer's disease (AD) with increasing age. Alzheimer's disease and vascular dementia (VaD) are the number one and number two disorders in terms of prevalence, and together they are responsible for most cases of dementia.2, 3 These disorders are preceded by a stage in which the individual shows cognitive decline but is still able to maintain independent functioning. In AD, this stage is referred to as mild cognitive impairment (MCI) due to AD, whereas in VaD this prodromal stage is termed vascular cognitive impairment (VCI). In this review, the term AD will mostly reflect the continuum of mild cognitive impairment due to AD and dementia due to AD, and the term VaD will reflect the continuum of VCI and VaD.
Historically, AD and VaD have been considered as separate entities, and this separation remains driven by clinical classification criteria. Therefore, the inclusion of AD in a review discussing vascular aspects of dementia may seem confusing. However, there is considerable overlap between these disorders, and the underlying interactions between VaD and AD will be explained and summarized. Furthermore, two major risk factors for VaD and AD, hypertension and stroke, will be further discussed to demonstrate their impact on both types of dementia. Studies involving long-chain omega-3 polyunsaturated fatty acids (LC-n3-FA) supplementation and manipulation of the renin–angiotensin system (RAS) have shown that these novel therapeutic approaches have the potential to lower the effect of hypertension and stroke. Therefore, these new preventive strategies against two major risk factors for cognitive impairment and dementia will be discussed.
Materials and Methods
Search Strategy and Selection of the Papers
We searched both the PubMed and Web of Science databases for original and review articles published in English from 1987 until 22 August 2013. The main search topics concerned the classification of AD and VaD, risk factors for both types of dementia, impact of hypertension and stroke on both AD and VaD, and also preventive strategies against dementia, such as long-chain omega-3 polyunsaturated fatty acids and the RAS. The search strategy was based on these search terms: dementia, AD, VaD, VCI, murine and human studies, hypertension, stroke, preventive strategies against dementia (long-chain omega-3 polyunsaturated fatty acids and the RAS). Moreover, to identify potentially relevant new papers, we filtered our total list of relevant papers by hand. Based on the title and abstract, we selected the studies. If these two components were not sufficient for selection, we purchased and evaluated the total publication.
Results
Vascular Dementia, Classification, and Etiology
About 5% to 20% of dementia cases in the population are based on VaD,4 which is a common disorder in the elderly but also prevalent among younger adults.5 The concept of VaD consists of two main elements: the presence of a dementia syndrome and an underlying vascular cause.6 To characterize VaD, criteria of State of California Alzheimer's Disease Diagnostic and Treatment Centers and the National Institute for Neurological Disorders and Stroke-Association Internationale pour la Recherche et I'Enseignement en Neurosciences are commonly used.4, 6, 7 Dementia caused by ischemic or hemorrhagic cerebrovascular disease (CVD) or by ischemic–hypoxic brain lesions of cardiovascular origin is also included in VaD.5, 8 Among recurrent or first-ever stroke patients, poststroke dementia is a frequent sequel, ranging from 6% to 31.8%.9, 10, 11
Definitions of vascular dementia and vascular cognitive impairment
The dementia stage of VaD can also be understood as the most severe form of VCI.4, 12 Vascular cognitive impairment is a syndrome characterized by the presence of clinical stroke or vascular brain injury and cognitive impairment affecting more than one cognitive domain.13 The VCI–VaD continuum can be divided into familial and sporadic forms.13 The most frequent subtype of familial VaD, caused by genetic mutations, is ‘cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy' (CADASIL)13 caused by mutations in the Notch3 gene.14 Sporadic VaD has three major subtypes: multi-infarct dementia, strategic infarct dementia, and subcortical vascular encephalopathy (synonymous with Binswanger's disease).13 O'Brien15 has published an alternative classification of the VaD subtypes:5, 15 multi-infarct dementia (cortical VaD); small vessel dementia (subcortical VaD); strategic infarct dementia; hypoperfusion dementia; hemorrhagic dementia; AD with CVD; and the familial variant of VaD, CADASIL.
Stroke and Vascular dementia
Many stroke patients show a gradual but continuous deterioration after a single-stroke lesion.16 This deterioration is characterized clinically by cognitive and behavioral dysfunction. Stroke research has traditionally focused on motor impairment (e.g. limb paresis), where a number of patients show partial recovery indicating the brain's capacity for repair or compensation after injury.17 However, this research has paid little attention to cognitive and behavioral deficits induced by stroke. After stroke, recovery from these deficits is often absent, and, as indicated, in many patients stroke leads to progressive deterioration even in the absence of new stroke lesions. Novel research indicates that stroke-induced lesions in brain networks are responsible for this absence of recovery or even for progressive disease, leading to an increased mortality rate.18 However, it is still not fully understood how stroke, cognitive decline, and dementia are interconnected. Stroke may predispose older adults to developing VaD.
Alzheimer's Disease, Definition and Etiology
In 1906, Alois Alzheimer mentioned arteriosclerotic changes in cerebral blood vessels of the postmortem brain of his 55-year old patient Auguste D(eter) besides the neuropathologic hallmarks, amyloid plaques and neurofibrillary tangles.19, 20 The production of Aβ peptides is increased in familial forms of AD and is thought to be the primary driving force in non-familial (sporadic) AD pathogenesis.21 This amyloid cascade hypothesis is still the dominant theory for the pathogenesis of AD, but remains under debate, as other researchers casted doubt that the Aβ plaques and the NFTs are really the main cause of the neurodegeneration in AD.22 Experimental results showed that the density of senile Aβ plaques can be the same in patients affected by AD and in non-affected patients.23, 24 Recently, the focus of the research on amyloid beta has shifted towards the oligomerization of Aβ, as several studies showed that these oligomers and fibrils are in fact the toxic forms of Aβ-peptides.25
Cerebral amyloid angiopathy and Alzheimer's disease
The accumulation of Aβ in the walls of arteries and arterioles in the leptomeninges and cerebral cortex is called cerebral amyloid angiopathy (CAA).26 Cerebral amyloid angiopathy has been linked to hemorrhages (microbleeds), most clearly shown in a mouse model for CAA.27 Because CAA is found both in sporadic AD patients and in cognitively normal individuals without prodromal AD,28, 29 the exact relationship between AD and CAA remains uncertain.
Risk Factors for Vascular Dementia
The assumption has been made that risk factors for VCI and VaD would be the same as those for stroke.30 The risk factors for stroke can be divided into three major classes: non-modifiable (e.g. age, sex, genetic factors, etc.); modifiable (e.g. hypertension, diabetes, hyperlipidemia, atrial fibrillation, smoking, obesity, etc.); and potentially modifiable (e.g. alcohol abuse, infection).31
Hypertension has been shown to be the most common modifiable risk factor for stroke worldwide.32, 33 Large-scale, placebo-controlled clinical trials have shown an association between hypertension and stroke,34, 35 and a linear relationship between blood pressure and stroke mortality has been revealed.36 More specifically, a rise of only 1 mm Hg in systolic blood pressure in treated hypertensive patients increased stroke-related death by 2%.36 A community-based prospective cohort study revealed that incremental increases in blood pressure were linked to an increase in microinfarcts in initially non-demented persons (65 to 80 years of age), but not in the older age group.37
A history of stroke leads to a twofold increase in the risk of dementia in the population older than 65 years,38, 39 and this effect was also confirmed in animal studies.40 Combining confirmed AD pathology and cerebral infarcts after autopsy with the test results of cognitive function revealed that AD patients with cerebral infarcts showed more cognitive impairment than patients without cerebral lesions.41, 42 In the population-based Rotterdam Scan Study, 1,015 participants underwent neuropsychological testing and cerebral magnetic resonance imaging and were monitored for dementia during the study period.43 In this study, silent brain infarcts doubled the risk for dementia.43 Furthermore, in subjects without dementia, presence of these infarcts increased the chances of a decline in global cognitive function.43 Thus, an increased risk for incident stroke is associated with cognitive decline and dementia.44
Even individuals who are stroke and dementia free, but have a higher risk of developing stroke, have more cognitive deficits compared with individuals with lower stroke risk.45 As already mentioned for VaD, stroke may also predispose older adults to developing AD. The mechanisms behind cognitive decline after the occurrence of a stroke could help us develop new treatments to prevent the onset of dementia. Furthermore, new preventative treatments for stroke are needed to counteract both stroke and dementia. A possible way to reduce stroke would be to reduce modifiable risk factors such as lifestyle, hypertension, diabetes, hyperlipidemia, atrial fibrillation, smoking, obesity, etc.
Risk Factors for Alzheimer's Disease
Several vascular risk factors for the development of AD have been demonstrated—for example, hypertension, diabetes mellitus, atherosclerosis, atrial fibrillation, coronary artery disease, smoking, obesity, and metabolic syndrome.46, 47 Many studies have shown the association between increased blood pressure in mid-life and cognitive decline or AD in late life,48, 49 although conflicting studies have been reported as well. Associated with increased levels of cardiovascular risk factors,50 the apolipoprotein E ɛ4 allele represents a strong genetic risk for AD.12 Notably, among all vascular risk factors, hypertension seems to be the most powerful risk factor for AD.51 Furthermore, recent studies demonstrated that in the elderly a history of stroke can double the prevalence of AD.52 The combination of the latter results demonstrates the impact of these two risk factors for AD.
The risk factors for AD are almost the same as those for VaD. Therefore, in the next paragraph the overlap and interactions of VaD and AD, as well as between their risk factors, are discussed.
Overlap and Interactions of VaD and AD, and their Risk Factors
In the first instance, it may seem difficult to see the overlap between VaD and AD, as these entities are strictly separated in terms of their clinical criteria. As a first illustration of why VaD and AD can no longer be strictly separated in this way, Biessels et al53 have shown in their systematic review that diabetes as a risk factor may directly influence both vascular and neurodegenerative pathology. Biessels et al53 suggested from mechanistic studies that vascular disease and alterations in glucose, insulin, and amyloid metabolism were connected and may underlie the pathophysiology of both AD and VaD.
There is now more and more awareness that vascular risk factors have a key role in the pathogenesis of AD.54 In aged subjects, a relation between vascular risk factors and AD has been found.55, 56 In addition, other epidemiologic and clinical studies identified that AD and VaD share common risk factors such as hypertension, diabetes mellitus, hyperlipidemia, and arrhythmia.57, 58, 59, 60, 61, 62 This overlap in major risk factors for these clinically and pathologically different conditions (VaD and AD) may seem confusing when it comes to understanding pathogenesis. However, a simple practical consequence is, for example, that hypertension is a major risk factor for cognitive decline and dementia in the elderly, regardless of whether this is due to stroke, VaD, or AD, or combinations of these disorders. Another overlap between VaD and AD is found in the neurovascular unit. The neurovascular unit is the collective term for neurons, glia, and perivascular and vascular cells.12 This unit is responsible for the strong increase in cerebral blood flow after cognitive activation. In AD and VCI–VaD patients, the neurovascular unit is disrupted, and this could lead to insufficient perfusion during cognitive activation, contributing to further neuronal dysfunction.63, 64, 65, 66, 67
In addition, alterations in the cerebral microvascular structure are related to AD and VCI.68, 69 In animal models, hypertension, aging, and diabetes, the major risk factors for AD and VCI, interfere with endothelium-dependent responses in the microcirculation and in the functional hemodynamic response of the brain.12, 64, 70, 71 Furthermore, hypertension promotes atherosclerosis in cerebral arteries65 and induces lipohyalinosis, affecting the blood supply to the white matter.65 These changes can result in lacunar infarction or brain hemorrhage.65 Notably, studies have shown that vascular lesions lead to a decrease in the threshold for the clinical manifestation of AD.12 In demented and non-demented Japanese–American men, Petrovitch et al72 showed that cerebrovascular lesions increase dementia frequency in patients with low neuritic plaque frequency. From this finding, they concluded that the preservation of late-life cognitive function is dependent on the prevention of cerebrovascular lesions.72 To support the idea that vascular lesions or CVD lower the threshold for dementia due to AD and α-synucleinopathies, Toledo et al73 demonstrated that CVD is commonly found in aged subjects with dementia, whereas it is even more common in AD patients, especially in younger patients. Furthermore, Toledo et al73 found that the presence of CVD increases the risk for dementia in patients with α-synucleinopathies and also in those being affected by AD.73 These latter studies clearly show the relation between cerebrovascular impairment and dementia, especially AD and VaD.
Hypertension—an overlapping risk factor for vascular dementia and Alzheimer's Disease
Hypertension and Alzheimer's Disease: One of the most common cardiovascular risk factors, arterial hypertension, has been shown to increase the risk for both AD and VaD.49, 74, 75, 76, 77, 78, 79, 80, 81 Skoog and Gustafson80 demonstrated a relationship between hypertension and amyloid plaques, neurofibrillary tangles, and brain atrophy. Furthermore, Skoog and Gustafson80 showed that increased blood pressure appeared decades before the onset of AD, followed by a decrease in blood pressure years before the start of AD. This phenomenon of hypertension followed by a gradual reduction in blood pressure may be caused by difficulties in maintaining blood pressure homeostasis due to a damaged central nervous system.80, 82 Studies that link blood pressure with dementia may be complicated by this non-stationary course of blood pressure in the trajectory of dementia.
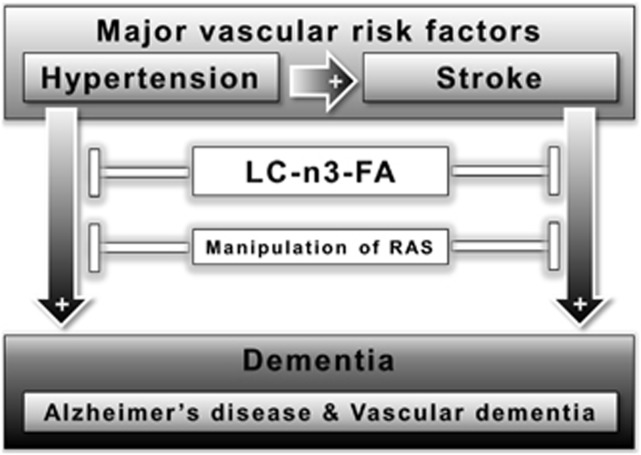
Long-standing hypertension promotes atherosclerosis and vascular remodeling, including increases in wall thickness. Arterial stiffness and severe atherosclerosis can lead to an increase in pulse pressure (the difference between systolic and diastolic blood pressure).83 In a community-based study, increased pulse pressure correlated with a higher risk for AD in older adults.83 In the Rotterdam study, the presence of atherosclerotic plaques or wall thickening has been associated with dementia and its two major subtypes AD and VaD.75 Based on that study, arterial stiffening has been suggested to be a key player in the pathogenesis of dementia.84 One hypothesis to explain how hypertension is a risk factor for dementia, which follows from these studies, is that hypertension could lead to atherosclerosis and arterial stiffness, which in turn promotes the development of dementia (Figure 1).
Figure 1.
Proposed connections between risk factors, dementia, and possible therapies affecting the major vascular risk factors for Alzheimer's disease (AD) and vascular dementia (VaD), hypertension, and stroke. Hypertension and stroke are major risk factors for dementia, in particular AD and VaD, whereas hypertension itself is also a risk factor for stroke. Despite all research and drug development efforts, no curative pharmacological therapies are available for dementia and also no definitive treatments are attainable for VaD. In human and animal studies, long-chain omega-3 polyunsaturated fatty acids (LC-n3-FA) and the manipulation of factors involved in the renin–angiotensin system (RAS) have shown to be beneficial for lowering the risk of developing dementia like AD and VaD.
Disentangling the possible causal relationships between hypertension, CVD, and Alzheimer's disease in human studies is complex, if not impossible, because vascular risk factors like hypertension may take years or decades to lead to marked cerebrovascular and cognitive symptoms, and because Alzheimer pathology is thought to be present years to decades before clinical symptoms appear. Therefore, animal models are needed to elucidate these mechanisms and translate them to preventive or therapeutic interventions. Poulet et al85, 86 developed an animal model to resemble hypertension-related ‘Alzheimer-like pathology'.,86 These mice were subjected to high blood pressure, and this resulted in accumulation of amyloid aggregates.85, 86 Hypertension was induced via a coarctation of the aortic arch between the two carotid arteries, causing changes in the CBF, leakage of the blood–brain barrier and neurodegenerative changes.85, 86
However, there may also be a reverse association between AD and hypertension. To investigate the impact of Aβ on blood pressure, in spontaneously hypotensive Sprague–Dawley rats (mean arterial blood pressure⩽100 mm Hg) the intra-arterial infusion of Aβ increased the mean arterial blood pressure compared with vehicle distilled water infusion.87 This finding suggests that Aβ may be able to induce hypertension, possibly through direct systemic vascular effects, without parenchymal Aβ deposition and before dementia onset.87 Taken together, animal studies suggest that Aβ may be responsible for high blood pressure as well as for cerebrovascular impairment. Furthermore, neurodegeneration is due to energetic deficiency and can be explained by Aβ-induced cerebrovascular impairment by, e.g., impairing glucose transport in the hippocampal and cortical neurons.88 Hypothetically, these blood pressure and vascular effects of Aβ, which predate cognitive effects, may interact with common vascular disease (e.g., essential hypertension), potentially further elevating blood pressure levels and synergistically inducing cerebrovascular lesions.
Hypertension and Vascular Dementia: Whereas the link between hypertension and VaD, through the well-established relationship between hypertension and stroke, may seem self-evident, it is often overlooked that in many cases of VaD there is no clear history of stroke. To be more precise, cortical stroke, which is often symptomatic and therefore more easily recognized, is not the most prevalent cause of VaD. VaD is most often caused by lacunar infarcts (e.g., multi-infarct dementia) or severe white matter disease, both related to small vessel disease, and these are frequently clinically unrecognized as acute stroke. As an illustration of this often clinically silent course, lacunar infarcts and white matter disease are frequently found in studies in elderly subjects without known cognitive disorders.42, 89 Depending on the location of these vascular lesions, their extent, and the patient's cognitive reserve, these silent lesions can, however, have sufficient impact to cause VCI or VaD (Figure 1).
Can stroke cause vascular dementia and Alzheimer's disease?
To strengthen the overlap and the connections between AD and VaD, accumulation of amyloid precursor protein and Aβ 1 to 42, hallmarks of AD, has been demonstrated in patients with multi-infarct dementia, which is the most prevalent form of VaD.90, 91, 92 Also, animal studies using models of cerebral ischemia indicated a relationship between the amyloid precursor protein and cerebral ischemia.90, 91, 93 A highly sensitive fluorescent RT-PCR assay revealed a significant increase in the peripheral blood expression of amyloid precursor protein mRNA levels among patients who suffered from stroke recently.94 A correlation between the density of cortical microinfarcts and the degree of CAA was found in a postmortem analysis of human brains.95 Although CAA may occur unrelated to AD, this example serves to explain that AD patients with CAA may present with stroke and cerebrovascular comorbidity.
Endothelin-induced ischemia mimicking small lacunar infarcts in the APP23 AD mouse model increased AD-like pathology and inflammatory markers of AD in the cortex and hippocampus of these transgenic mice.40 In another murine study Garcia-Alloza et al96 demonstrated that stroke accelerates amyloid deposition via interference with amyloid clearance pathways. Garcia-Alloza et al96 examined this association by using a transgenic AD mouse model (APP/PS1) subjected to microstrokes in the middle cerebral artery territory, an experimental stroke model using Rose Bengal dye.96 A fast increase in amyloid plaque burden and CAA was measured in the region surrounding the infarction.96 As discussed in that paper, these changes were transient –and this may explain why these authors did not find amyloid plaques in postinfarct brain tissue in humans in earlier work.
An association between cerebral hypoperfusion, caused by CAA,97, 98 and cortical microinfarct was demonstrated by Okamoto et al.95 Chronic cerebral hypoperfusion due to bilateral common carotid artery stenosis in a CAA mouse model showed that the deposition of Aβ in leptomeningeal vessels was accelerated in combination with the development of microinfarcts.95 Notably, in a rat model of AD and cerebral ischemia, the accumulation of amyloid increased the infarct size, neuroinflammation, and also cognitive deficits in these rats.99
Hemodynamic changes in vascular dementia and Alzheimer's disease: A meta-analysis of transcranial Doppler studies has shown that both AD and VaD patients have evident changes in cerebrovascular hemodynamics (mostly reduced CBF and increased cerebrovascular resistance), albeit much more pronounced in VaD patients.100 A study in transgenic mice overexpressing APP has demonstrated the impact of Aβ on cerebrovascular regulation.101 Using quantitative autoradiography, resting CBF was shown to be reduced in the cerebral cortex and in the hippocampus.101 These APP-overexpressing mice also showed a disturbance in cerebrovascular autoregulation, as they were incapable of maintaining a stable CBF during moderate hypotension or hypertension.102 Whether this observation can be translated to human AD remains uncertain.103
Preventive Strategies—Long-Chain Omega-3 Polyunsaturated Fatty Acids and the Renin–Angiotensin System
Despite all research and drug development efforts, no curative pharmacological therapy is available for dementia and also no definitive treatments are attainable for VaD.5 Therefore, to reduce the huge burden of disease, development of preventive strategies is urgent. Considering that vascular disorders contribute importantly to dementia as described above, such preventive strategies could consist of pharmaceutical interventions aimed at vascular dysfunctions (for example, treatment of hypertension and hyperlipidemia). In addition, current studies focus more and more on lifestyle. As already mentioned above, there is growing awareness that lifestyle components, e.g. diet and exercise, can influence modifiable risk factors such as hypertension, type 2 diabetes, and obesity. These modifiable risk factors affect the vascular system, thereby influencing the risk for dementia, including AD.49, 104, 105, 106, 107 Modifying these risk factors via a change in lifestyle may potentially delay the onset of dementia and lead to a decrease in the prevalence and public health burden of dementia.44, 107 Aarsland et al108 demonstrated in a systematic review that physical exercise may prevent the development of VaD. Ravaglia et al109 found in a population-based cohort study that physical activity is associated with a lower risk for VaD but not for AD. In the review by Dichgans et al,110 the lifestyle risk factors for VaD, AD, dementia (unspecified), and cognitive impairment are summarized well. Based on epidemiologic studies, they show that VaD and AD share common lifestyle risk factors such as smoking, decreased physical activity, and obesity.110 Furthermore, they also state from the existing epidemiologic studies that insufficient evidence for diet as a potential risk factor for VaD exists, whereas concerning AD an improved dietary behavior was associated with a lower risk for cognitive decline.110
Of the many important lifestyle factors, in this review we focus on (the supplementation of) LC-n3-FAs and its impact on cognition and the development of dementia. In addition, we also concentrate on the impact of these LC-n3-FAs on risk factors for VaD and AD, such as hypertension and stroke, to show that diet could have an impact on the development of both VaD and AD.
In a randomized, double-blind, placebo-controlled trial (van de Rest et al111) no effect of supplementation with eicosapentaenoic acid combined with DHA for 26 weeks on mental well-being in independently living older individuals could be detected. In another randomized, double-blind, placebo-controlled clinical trial (Freund-Levi et al112) the administration of LC-n3-FA in patients with mild-to-moderate AD did not slow down the cognitive decline. However, in a small subgroup of patients with very mild AD, this administration led to beneficial effects.112 A 24-week supplementation with 900 mg/day DHA led to improved learning and memory function in age-related cognitive decline.113
Many of these clinical trials had a relatively short duration of supplementation of LC-n3-FA or focused only on moderate or advanced AD patients. A systematic review with a meta-analysis in animal models of AD focusing on the effects of long-term LC-n3-FA supplementation on cognitive impairment, amyloid-β pathology, and neuronal loss revealed reduced Aβ burden, improved cognitive function, and decreased neuronal loss.114 A very recent, double-blind, randomized interventional study has shown that the intake of LC-n3-FA in healthy older adults significantly increased executive functions.115 Furthermore, Witte et al115 demonstrated that this intake had also beneficial effects on microstructural integrity and gray matter volume in the frontal, temporal, parietal, and limbic areas, and on carotid intima media thickness and diastolic blood pressure.
Long-chain omega-3 polyunsaturated fatty acids and blood pressure
As already mentioned in the previous paragraph, Witte et al115 were able to show that in healthy older adults the LC-n3-FA supplementation led to a significant decrease in diastolic blood pressure. In addition, a meta-analysis of 31 placebo-controlled trials in 1,356 subjects revealed a dose-response effect of LC-n3-FA leading to a decrease in blood pressure.116 This beneficial blood pressure-lowering effect was even most prominent in hypertensive subjects and in patients with atherosclerosis or hypercholesterolemia.116 This is in accordance with another study showing a reduction in blood pressure in untreated hypertensive patients with daily administration of LC-n3-FA.117 However, the use of LC-n3-FA as antihypertensive treatment in humans needs to be analyzed in long-term studies. Long-term administration of DHA inhibits the development of hypertension in stroke-prone spontaneously hypertensive rats, a model for hypertension and stroke118 Notably, DHA also prolonged the life span of spontaneously hypertensive rats.118 However, not only DHA but other factors also may lower blood pressure. A meta-analysis of 25 randomized controlled trials revealed that an increased intake of dietary fibers also reduces blood pressure in hypertensive patients.119 Reducing dietary salt intake may also be beneficial, as high salt intake increased the mortality rate, raised blood pressure, and increased the number of cerebral aneurysms in spontaneously hypertensive rats.120 Also in humans, evidence supports the idea that reducing dietary salt intake can reduce hypertension-related disease.121
Long-chain omega-3 polyunsaturated fatty acids and stroke
As mentioned above, fish consumption is recommended to reduce the risk for cardiovascular diseases.122 Therefore, it is likely to reduce the risk of developing stroke as well. A meta-analysis performed by He et al123 revealed that very low fish consumption protects against the incidence of ischemic stroke. De Goede et al124 demonstrated a relationship between higher eicosapentaenoic acid–DHA and lower stroke risk for women, whereas for men these associations were not statistically significant. Intraperitoneal pretreatment with DHA helped to reduce brain infarctions in Sprague–Dawley rats.125 Moreover, Ozen et al126 demonstrated a protective effect against cerebral ischemia in rats fed with a standard diet plus LC-n3-FA, including eicosapentaenoic acid and DHA. These rats had a reduced number of apoptotic neurons in the prefrontal cortex. This effect of DHA may be mediated by neuroprotectin 1. Docosahexaenoic acid is the precursor of neuroprotectin 1, and aspirin activates the synthesis of aspirin-triggered neuroprotectin 1.127 After the occlusion of the middle cerebral artery in Sprague–Dawley rats, inducing an experimental stroke, the administration of synthetic neuroprotectin 1 attenuated cerebral ischemic injury.127 In women, intake of LC-n3-FA was associated with a lowered risk for total stroke, whereas dietary cholesterol was positively associated with risk for total stroke and cerebral infarction.128 Increased intake of a Mediterranean-style diet was associated with a lowered risk for ischemic stroke, myocardial infarction, and vascular death.129 The reduction in cardiovascular morbidity was most prominent for stroke, and was attributed to high intake of olive oil or nuts. However, not only this diet originating around the Mediterranean Sea but other factors also seem to have an influence on stroke incidence. A population-based case–control study performed in southern Sweden demonstrated that stroke risk decreased with fat-fish intake, and especially in women the consumption of lean fish increased the stroke risk.130 Additionally, a deficient intake of alpha-linolenic acid, the plant-derived LC-n3-FA, may also be a risk factor for the development of stroke.131 This is in line with a murine study on rapeseed oil-enriched diets (rapeseed oil is a rich source of alpha-linolenic acid). After middle cerebral artery occlusion, the rapeseed oil-fed groups demonstrated a decreased mortality rate, lowered levels of lipid peroxidation, and a reduced infarct size.132 A meta-analysis by Arab et al133 showed that individuals consuming three cups of either green or black tea daily had a 21% lower risk for stroke than those with a daily consumption of less than one cup of tea. In summary, in human and animal studies, LC-n3-FA have shown beneficial effects on stroke and also on hypertension, lowering the risk for dementia, see Tables 1 and 2 and also Figure 1.
Table 1. Animal studies: summary of intervention trials (LC-n3-FA and the manipulation of factors involved in the RAS).
| Study | Species | Model | Sex | Supplement+Dose | Route of administration | Start supplementation | Duration of supplementation | Results |
|---|---|---|---|---|---|---|---|---|
| LC-n3-FA | ||||||||
| 127 | Rat | Focal cerebral I/R | M | 333 μg/kg of AT-NPD1 in SS 333 μg/kg of AT-NPD1 in Me Vehicle | Intravenously | — | 3 hours before ischemia | • AT-NPD1 reduces brain infarction size+brain edema• Improved tissue matrix• White matter protection |
| 118 | Rat | SHRSP | ? | 1% DHA 5% DHA Control diet | Diet | 6 weeks of age | 14 weeks | • DHA has antihypertensive action• DHA↑acetylcholine levels• DHA↑life span |
| 132 | Mouse | Focal cerebral I/R | M | 5% RSO 10% RSO 20% RSO 5% palm oil | Diet | — | for 4 or 6 weeks before ischemia | • Groups 2+3: decreased mortality rate, lowered levels of lipid peroxidation, and a reduced infarct size |
| 126 | Rat | Focal cerebral I/R | M | Control Marincap capsule (EPA 18%, DHA 12%) | Gavage | 8–12 weeks of age | 2 weeks before ischemia | • ↓In apoptotic neurons in the prefrontal cortex in rat group given marincap capsules |
| 125 | Rat | Focal cerebral I/R | M | 100 nmol/kg 500 nmol/kg Vehicle | Intraperitoneally | — | Once 1 hour before ischemia Once 3 days before ischemia Daily for 6 weeks before ischemia | • In all DHA-injected animal groups: reduced infarct volume |
| Manipulation of the RAS | ||||||||
| 141 | Mouse | ApoE KO+HCD+focal cerebral I/R | M | Telmisartan (0.3 mg/kg per day) | Osmotic mini pump | 14 weeks of age | For 2 weeks before ischemia | • Telmisartan↓ischemic brain area, neurologic deficit, reduction of cerebral blood flow in the penumbra |
| 139 | Mouse | Aβ 1–40 injection | M | C21 (1.0 μg/kg per day) C21 (10.0 μg/kg per day) Vehicle | Intraperitoneally | 10–12 weeks of age | for 2 weeks before MWM | • C21 prevented cognitive decline in this AD mouse model |
| 137 | Mouse | APP23 mice | M | Olmesartan (1.0 mg/kg per day) Vehicle | Orally | 8 weeks of age | Daily for 4–5 weeks | • ARB olmesartan decreased oxidative stress in cerebral microvessels |
| 137 | Mouse | Aβ 1–40 injection | M | Olmesartan (0.5 mg/kg per day) Olmesartan (1.0 mg/kg per day) Vehicle | Orally | 7-8 weeks of age | for 4 weeks before Aβ injection | • Olmesartan completely prevented Aβ-induced vascular dysregulation and ↓ impairment of the hippocampal synaptic plasticity |
| 138 | Mouse | Aβ 1–40 injection | M | Telmisartan (0.35 mg/kg per day) Telmisartan (0.35 mg/kg per day)+GW9662 Vehicle | Orally | 8 weeks of age | for 4 weeks before Aβ injection | • ↓of Aβ 1–40 concentration by telmisartan• Telmisartan ↑ CBF• Telmisartan improved Aβ-induced cognitive decline |
| 144 | Mouse | Tg2576 AD mouse model | M | Valsartan (10.0 mg/kg per day) Valsartan (40.0 mg/kg per day) Water | Orally | 6 months of age | for 5 months | • Valsartan ↓ AD-type neuropathology• Valsartan ↓ development of Aβ-mediated cognitive deterioration |
| 135 | Mouse | Aβ 25–35 injection | M | Perindopril (0.1, 0.3 or 1 mg/kg per day) Imidapril (0.3, 1 or 3 mg/kg per day) Enalapril (1, 3 or 10 mg/kg per day) Vehicle | Orally | 5-6 weeks of age | After Aβ 25–35 injection daily for 5 consecutive days | • Perindopril ameliorated the cognitive impairment in the AD model mice through inhibition of brain ACE activity |
ACE, angiotensin-converting enzyme; AD, Alzheimer's disease; APP, amyloid precursor protein; ARB, angiotensin receptor blocker; AT-NPD1, aspirin-triggered NPD1; C21, compound 21; CBF, cerebral blood flow; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; HCD, high-cholesterol diet; I/R, ischemia reperfusion; KO, knockout; LC-n3-FA, long-chain omega-3 polyunsaturated fatty acids; ME, methyl ester; MWM, Morris water maze; NPD1, neuroprotectin 1; RAS, renin–angiotensin system; RSO, rapeseed oil; SS, sodium salt.
Table 2. Human studies: summary of intervention trials (LC-n3-FA, and the manipulation of factors involved in the RAS).
| Study | Age | Subjects | Sex | N | Supplement+dose/intake | Route of administration | Duration of supplementation | Results |
|---|---|---|---|---|---|---|---|---|
| LC-n3-FA | ||||||||
| 131 | 20–65 years | Healthy subjects | M+F | 20,069 | • Habitual diet was assessed with a validated 178-item food frequency questionnaire | Orally | — | • Higher ALA intake ↓ risk of stroke |
| 124 | 20–65 years | Healthy subjects | M+F | 20,069 | • Intake of EPA–DHA was registered | Orally | — | • Women: higher EPA–DHA and fish intake ↓ stroke risk• Men: no association |
| 112 | 74±9 years | Patients with mild-to-moderate AD | M+F | 204 | 1.7 g/day DHA 0.6 g/day EPA Placebo | Orally | 6 months | • No delay in the rate of cognitive decline (MMSE/ cognitive portion of ADAS)• In very mild AD patients ↓ in cognitive decline (MMSE) |
| 129 | 69±10 years | Healthy subjects | M+F | 2,568 | • Mediterranean-style diet registered by questionnaire | Orally | — | • Mediterranean-style diet ↓ ischemic stroke, myocardial infarction, and vascular death |
| 128 | 49–83 years | Healthy subjects | F | 34,670 | • Intake of LC-n3-FA was registered• Intake of cholesterol was registered | Orally | — | • LC-n3-FA↓risk of stroke• Cholesterol↑ risk of stroke |
| 130 | 43–85 years | Healthy +Stroke patients | M+F | 5,191 | • Fish intake was reported retrospectively | Orally | — | • Fat fish ↓ risk of ischemic stroke• Women: lean fish ↑ risk of stroke |
| 111 | ≥65 y | Nondepressed, older individuals | M+F | 302 | 1.8 g/day EPA+DHA 0.4 g/d EPA+DHA Placebo | Orally | 26 weeks | • Plasma concentrations of EPA+DHA ↑ in both test groups• No effect on mental well-being |
| 115 | 50–75 years | Healthy subjects | F | 30 | (1.32 g EPA+ 0.88 g DHA+15 mg vitamin E)/day Placebo | Orally | 26 weeks | • ↑Executive functions• positive effects on white matter microstructure, gray matter volume, and vascular markers• ↓in diastolic blood pressure |
| 113 | ⩾55 years | Healthy subjects | M+F | 485 | 0.9 g/day DHA Placebo | Orally | 24 weeks | • ↑learning and memory function in ARCD |
| Manipulation of the RAS | ||||||||
| 136 | 57±10 years | Patients with manifested atherosclerotic disease | M+F | 575 | • Blood pressure+use of antihypertensive drugs was registered | — | — | • Untreated hypertension, poorly controlled hypertension, and high BP levels associated with ↓ pCBF• treatment with ARBs leads to less decline in pCBF than other antihypertensives |
| 143 | 70–89 y | Hypertensive patients | M+F | 1,518 | Candesartan (8 mg/day) Placebo | Orally | 3–5 y | • ARB candesartan↓relative risk of stroke |
| 142 | 1. 70±9 y 2. 65±11 y | 1. Patients with CAD2. Patients without CAD | M+F | 1. 707 2. 2,324 | • Blood pressure+use of antihypertensive drugs+occurrence of cardiovascular and cerebrovascular events was registered | — | — | • Valsartan prevented more cardio-cerebrovascular events than conventional non-ARB treatment in high-risk hypertensive patients• Valsartan ↓ prevalence of stroke |
AD, Alzheimer's disease; ADAS, Alzheimer's disease assessment scale; ALA, alpha-linolenic acid; ARB, angiotensin receptor blocker; ARCD, age-related cognitive decline; BP, blood pressure; CAD, coronary artery disease; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; LC-n3-FA, long-chain omega-3 polyunsaturated fatty acids; MMSE, mini–mental state examination; pCBF, parenchymal cerebral blood flow; RAS, renin–angiotensin system.
Manipulation of the renin–angiotensin system—another possible therapy
The renin–angiotensin system is important not only in the cardiovascular system but also in the central nervous system. Angiotensin II binds to two main receptors, type 1 (AT1) and type 2 (AT2). Angiotensin type 2 receptors are found in the cerebral regions involved in the control and learning of motor activity.134
In Aβ-injected mice, the cognitive impairment was ameliorated by perindopril, a centrally active angiotensin-converting enzyme inhibitor.135 In these mice, perindopril inhibited the cerebral, but not the peripheral, ACE activity, demonstrating a beneficial effect on AD as well as on hypertension.135 In this animal study, neither imidapril nor enalapril were able to reverse the cognitive impairment in spontaneous alteration and object recognition tests of the AD model mice.135 In the SMART-MR study, patients treated with ARBs had less decline in CBF compared with patients treated with other hypertensive drugs (e.g. β-blockers, diuretics, calcium channel blockers, or ACE inhibitors).136 In line with this human study, in young AD transgenic mouse models (APP23 mouse), treatment with the ARB olmesartan decreased oxidative stress in cerebral microvessels.137 In the same study, Takeda et al137 used an acute mouse model induced by intracerebroventricular administration of Aβ 1 to 40, where pretreatment with a low dose of olmesartan completely prevented Aβ-induced vascular dysregulation and also partially reduced the impairment of the hippocampal synaptic plasticity. This preventive effect on cognitive decline by treatment with ARBs in AD was also demonstrated by another animal study performed by Tsukuda et al,138 using intracerebroventricular injection of Aβ 1 to 40 in male ddY mice. In these mice, the ARB telmisartan decreased the cerebral Aβ 1 to 40 concentration and enhanced cerebral blood flow.138 Furthermore, pretreatment with this ARB reduced the cognitive effects of Aβ 1 to 40 to control level.138 Using a similar model, Jing et al139 demonstrated that the direct stimulation of the AT2 receptor by a newly generated AT2 receptor agonist, Compound 21 (C21), prevented cognitive decline. In an observational study on cognitive function and systolic blood pressure reduction (OSCAR), in more than 60,000 hypertensive patients, the specific use of the ARB eprosartan led to a reduction in blood pressure and also improved the cognitive function, revealing a positive correlation between cognitive decline or dementia and blood pressure levels.140
Angiotensin receptor blockers do not only have the potential to reduce cognitive decline in animal and human studies (Tables 1 and 2). In line with the described beneficial effect of ARBs on dementia, the Systolic Hypertension in the Elderly Program and the Systolic Hypertension in Europe study demonstrated that antihypertensive treatment lowered the risk of developing stroke.34, 35 In addition to the effect of an ARB in hypertension, an animal study showed a beneficial effect of ARBs on stroke.141 After an middle cerebral artery occlusion in apolipoprotein E-deficient mice—an atherosclerosis mouse model—treated with a cholesterol-high diet, the administration of the ARB telmisartan did not significantly decrease blood pressure but decreased the ischemic area and also the atherosclerotic formation in the proximal aorta and led to improved cerebral blood flow in the penumbra of these treated mice.141 In patients from the Kyoto heart study, who had coronary artery disease, treatment with the ARB, valsartan, lowered the prevalence of stroke compared with non-treated subjects.142 Antihypertensive treatment with the ARB candesartan in elderly patients with isolated systolic hypertension significantly reduced the relative risk of developing stroke in comparison with other types of antihypertensive treatment, despite little difference in blood pressure reduction.143
The potential mechanisms that could explain the link between hypertension and the development of AD need further investigation. Most human studies are epidemiologic studies that show cross-sectional or longitudinal associations between hypertension and AD, but mechanistic studies or interventional studies are sparse (Table 2). In animal studies, the focus of research has been mainly on the effect of low-dose blood pressure-lowering medication on Aβ accumulation and cognition. These preclinical studies indicate the possible potential for antihypertensive drugs against AD pathology and cognitive decline in AD patients.144 Indeed, in a systematic review Shah et al145 mentioned the need for large randomized clinical trials to explore the connection between blood pressure-lowering medications and dementia in humans.
Conclusions
Many studies have shown that AD, VaD, and stroke share hypertension as a common risk factor, whereas stroke in itself is also a risk factor for the development of AD or VaD (Figure 1). Hypertension combined with aging decreases CBF and results in changes in the cerebrovascular structure and function.43, 44 Together, this leads to cognitive decline, white matter changes, increase in Aβ pathology, and dysfunction of the blood–brain barrier, a combination of factors that may well reflect the multifactorial causes of AD in the elderly population.
Notably, an increase in Aβ (regardless of the underlying cause) could lead to changes in cerebrovascular structure and function, and, if combined with treatment for vascular disease, could represent a vicious cycle of aggravated CVD, increasing Aβ pathology, which in turn enhances vascular disease. As detailed, Aβ may increase blood pressure, decrease the amount of vascular endothelial cells, impair vascular function, and decrease CBF, resulting in further neurodegeneration. Thus, elevated Aβ levels due to any cause could explain the association between hypertension, CVD, and AD. Studying the causal relationships between hypertension, CVD, and AD in humans is complex because of the long latency between pathologic changes and clinical symptoms, which may span decades both in vascular disease and AD. Thus, properly designed animal studies that can be validly translated are needed to enlighten the underlying mechanisms and translate them to preventive or therapeutic interventions. In murine studies, induced hypertension led to an increased accumulation of Aβ, neuroinflammation, changed CBF, and disturbed blood–brain barrier. Moreover, animal models for stroke also showed an increased AD-like pathology, such as enhanced amyloid deposition, neuroinflammation, and cognitive deficits. If we consider hypertension and stroke as major risk factors for dementia, both for VaD and AD, there are two possible therapies for dementia. On the one hand, research has revealed that the supplementation with LC-n3-FA reduces the risk of developing hypertension and stroke, thereby also lowering the risk of developing dementia (Figure 1, Tables 1 and 2). On the other hand, the manipulation of factors involved in the RAS-like angiotensin II receptor blockers or ACE inhibitors showed beneficial effects in animal and human studies (Figure 1, Tables 1 and 2).
As a sequel, future research needs to focus more on the role of other (both medical and lifestyle) treatment strategies to lower the risk factors for dementia. Such research could elucidate the importance of reducing the rate of hypertension and stroke. Until now, studies on stroke have concentrated on motor impairment. Therefore, more research is needed that aim at the cognitive and behavioral deficits induced by stroke. This would also help to understand the connection between stroke, cognitive decline, and dementia and to elucidate the differences and overlap between VaD and AD.
The authors declare no conflict of interest.
Footnotes
This study was supported by a grant (no11528) from The Internationale Stichting Alzheimer Onderzoek (ISAO) and the EU 7th framework LipiDiDiet project (FP7/2007-2013) under grant agreement no211696.
References
- Ritchie K, Lovestone S. The dementias. Lancet. 2002;360:1759–1766. doi: 10.1016/S0140-6736(02)11667-9. [DOI] [PubMed] [Google Scholar]
- Morris JC. The nosology of dementia. Neurol Clin. 2000;18:773–788. doi: 10.1016/s0733-8619(05)70225-5. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Gorelick PB. Converging pathogenic mechanisms in vascular and neurodegenerative dementia. Stroke. 2003;34:335–337. doi: 10.1161/01.str.0000054050.51530.76. [DOI] [PubMed] [Google Scholar]
- Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- Chen N, Yang M, Guo J, Zhou M, Zhu C, He L. Cerebrolysin for vascular dementia. Cochrane Database Syst Rev. 2013;1:CD008900. doi: 10.1002/14651858.CD008900.pub2. [DOI] [PubMed] [Google Scholar]
- Tang WK, Chan SSM, Chiu HFK, Ungvari GS, Wong KS, Kwok TCY, et al. Impact of applying NINDS-AIREN criteria of probable vascular dementia to clinical and radiological characteristics of a stroke cohort with dementia. Cerebrovasc Dis. 2004;18:98–103. doi: 10.1159/000079256. [DOI] [PubMed] [Google Scholar]
- Chui HC, Victoroff JI, Margolin D, Jagust W, Shankle R, Katzman R. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer's Disease Diagnostic and Treatment Centers. Neurology. 1992;42:473. doi: 10.1212/wnl.42.3.473. [DOI] [PubMed] [Google Scholar]
- Román GC. Vascular dementia revisited: diagnosis, pathogenesis, treatment, and prevention. Med Clin North Am. 2002;86:477–499. doi: 10.1016/s0025-7125(02)00008-1. [DOI] [PubMed] [Google Scholar]
- Madureira S, Guerreiro M, Ferro JM. Dementia and cognitive impairment three months after stroke. Eur J Neurol. 2001;8:621–627. doi: 10.1046/j.1468-1331.2001.00332.x. [DOI] [PubMed] [Google Scholar]
- Pohjasvaara T, Erkinjuntti T, Ylikoski R, Hietanen M, Vataja R, Kaste M. Clinical determinants of poststroke dementia. Stroke. 1998;29:75–81. doi: 10.1161/01.str.29.1.75. [DOI] [PubMed] [Google Scholar]
- Inzitari D, Di Carlo A, Pracucci G, Lamassa M, Vanni P, Romanelli M, et al. Incidence and determinants of poststroke dementia as defined by an informant interview method in a hospital-based stroke registry. Stroke. 1998;29:2087–2093. doi: 10.1161/01.str.29.10.2087. [DOI] [PubMed] [Google Scholar]
- Gorelick PB, Scuteri A, Black SE, DeCarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal DR, Grinberg LT, Attems J. Vascular dementia: different forms of vessel disorders contribute to the development of dementia in the elderly brain. Exp Gerontol. 2012;47:816–824. doi: 10.1016/j.exger.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, et al. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383:707–710. doi: 10.1038/383707a0. [DOI] [PubMed] [Google Scholar]
- O'Brien JT. Vascular cognitive impairment. Am J Geriatr Psychiatry. 2006;14:724–733. doi: 10.1097/01.JGP.0000231780.44684.7e. [DOI] [PubMed] [Google Scholar]
- Grant I, Adams K. Neuropsychological Assessment of Neuropsychiatric and Neuromedical Disorders. Oxford University Press: USA; 2009. [Google Scholar]
- Thieme H, Bayn M, Wurg M, Zange C, Pohl M, Behrens J. Mirror therapy for patients with severe arm paresis after stroke—a randomized controlled trial. Clin Rehabil. 2012;27:314–324. doi: 10.1177/0269215512455651. [DOI] [PubMed] [Google Scholar]
- Rutten-Jacobs LC, Arntz RM, Maaijwee NA, Schoonderwaldt HC, Dorresteijn LD, van Dijk EJ, et al. Long-term mortality after stroke among adults aged 18 to 50 years. Cochrane Database Syst Rev. 2013;309:1136–1144. doi: 10.1001/jama.2013.842. [DOI] [PubMed] [Google Scholar]
- Alzheimer A. Über einen eigenartigen schweren Erkrankungsprozeß der Hirnrinde. Neurologisches Centralblatt. 1906;23:1129–1136. [Google Scholar]
- Maurer K, Volk S, Gerbaldo H. Auguste D and Alzheimer's disease. Lancet. 1997;349:1546–1549. doi: 10.1016/S0140-6736(96)10203-8. [DOI] [PubMed] [Google Scholar]
- Gouras GK, Tsai J, Naslund J, Vincent B, Edgar M, Checler F, et al. Intraneuronal A[beta]42 accumulation in human brain. Am J Pathol. 2000;156:15–20. doi: 10.1016/s0002-9440(10)64700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre JC. Is Alzheimer's disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol. 2004;3:184–190. doi: 10.1016/S1474-4422(04)00683-0. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18:351–357. doi: 10.1016/s0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E, Bohl J, Reintjes R. Age, neurofibrillary changes, A[beta]-amyloid and the onset of Alzheimer's disease. Neurosci Lett. 1996;210:87–90. doi: 10.1016/0304-3940(96)12668-9. [DOI] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid [beta]-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- Vinters HV. Cerebral amyloid angiopathy. A critical review. Stroke. 1987;18:311–324. doi: 10.1161/01.str.18.2.311. [DOI] [PubMed] [Google Scholar]
- Winkler DT, Bondolfi L, Herzig MC, Jann L, Calhoun ME, Wiederhold K-H, et al. Spontaneous hemorrhagic stroke in a mouse model of cerebral amyloid angiopathy. J Neurosci. 2001;21:1619–1627. doi: 10.1523/JNEUROSCI.21-05-01619.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriagada PV, Marzloff K, Hyman BT. Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer's disease. Neurology. 1992;42:1681. doi: 10.1212/wnl.42.9.1681. [DOI] [PubMed] [Google Scholar]
- Price JL, Davis PB, Morris JC, White DL. The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer's disease. Neurobiol Aging. 1991;12:295–312. doi: 10.1016/0197-4580(91)90006-6. [DOI] [PubMed] [Google Scholar]
- Gorelick PB. Risk factors for vascular dementia and alzheimer disease. Stroke. 2004;35:2620–2622. doi: 10.1161/01.STR.0000143318.70292.47. [DOI] [PubMed] [Google Scholar]
- Yu J-G, Zhou R-R, Cai G-J. From hypertension to stroke: mechanisms and potential prevention strategies. CNS Neurosci Ther. 2011;17:577–584. doi: 10.1111/j.1755-5949.2011.00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahathevan R, Brodtmann A, Donnan GA. Dementia, stroke, and vascular risk factors; a review. Int J Stroke. 2012;7:61–73. doi: 10.1111/j.1747-4949.2011.00731.x. [DOI] [PubMed] [Google Scholar]
- Tu JV. Reducing the global burden of stroke: INTERSTROKE. Lancet. 2010;376:74–75. doi: 10.1016/S0140-6736(10)60975-0. [DOI] [PubMed] [Google Scholar]
- SHEP Cooperative Research Group Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP) JAMA. 1991;265:3255–3264. [PubMed] [Google Scholar]
- Staessen JA, Fagard R, Thijs L, Celis H, Arabidze GG, Birkenhäger WH, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. Lancet. 1997;350:757–764. doi: 10.1016/s0140-6736(97)05381-6. [DOI] [PubMed] [Google Scholar]
- Palmer AJ, Bulpitt CJ, Fletcher AE, Beevers DG, Coles EC, Ledingham JG, et al. Relation between blood pressure and stroke mortality. Hypertension. 1992;20:601–605. doi: 10.1161/01.hyp.20.5.601. [DOI] [PubMed] [Google Scholar]
- Schneider JA. High blood pressure and microinfarcts: a link between vascular risk factors, dementia, and clinical Alzheimer's disease. J Am Geriatr Soc. 2009;57:2146–2147. doi: 10.1111/j.1532-5415.2009.02521.x. [DOI] [PubMed] [Google Scholar]
- Savva GM, Stephan BCM. Group tAsSVDSR. Epidemiological studies of the effect of stroke on incident dementia. Stroke. 2010;41:e41–e46. doi: 10.1161/STROKEAHA.109.559880. [DOI] [PubMed] [Google Scholar]
- Béjot Y, Aboa-Eboulé C, Durier J, Rouaud O, Jacquin A, Ponavoy E, et al. Prevalence of early dementia after first-ever stroke. Stroke. 2011;42:607–612. doi: 10.1161/STROKEAHA.110.595553. [DOI] [PubMed] [Google Scholar]
- Whitehead SN, Massoni E, Cheng G, Hachinski VC, Cimino M, Balduini W, et al. Triflusal reduces cerebral ischemia induced inflammation in a combined mouse model of Alzheimer's disease and stroke. Brain Res. 2010;1366:246–256. doi: 10.1016/j.brainres.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Heyman A, Fillenbaum GG, Welsh-Bohmer KA, Gearing M, Mirra SS, Mohs RC, et al. Cerebral infarcts in patients with autopsy-proven Alzheimer's disease: CERAD, part XVIII. Consortium to establish a registry for Alzheimer's disease. Neurology. 1998;51:159–162. doi: 10.1212/wnl.51.1.159. [DOI] [PubMed] [Google Scholar]
- Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA. 1997;277:813–817. [PubMed] [Google Scholar]
- Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MMB. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88:1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias MF, Sullivan LM, D'Agostino RB, Elias PK, Beiser A, Au R, et al. Framingham stroke risk profile and lowered cognitive performance. Stroke. 2004;35:404–409. doi: 10.1161/01.STR.0000103141.82869.77. [DOI] [PubMed] [Google Scholar]
- Kalaria R. Similarities between Alzheimer's disease and vascular dementia. J Neurol Sci. 2002;203–204:29–34. doi: 10.1016/s0022-510x(02)00256-3. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Akinyemi R, Ihara M. Does vascular pathology contribute to Alzheimer changes. J Neurol Sci. 2012;322:141–147. doi: 10.1016/j.jns.2012.07.032. [DOI] [PubMed] [Google Scholar]
- Kivipelto M, Helkala EL, Laakso MP, Hänninen T, Hallikainen M, Alhainen K, et al. Midlife vascular risk factors and Alzheimer's disease in later life: longitudinal, population based study. BMJ. 2001;322:1447–1451. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoog I, Nilsson L, Persson G, Lernfelt B, Landahl S, Palmertz B, et al. 15-year longitudinal study of blood pressure and dementia. Lancet. 1996;347:1141–1145. doi: 10.1016/s0140-6736(96)90608-x. [DOI] [PubMed] [Google Scholar]
- Eichner JE, Dunn ST, Perveen G, Thompson DM, Stewart KE, Stroehla BC, et al. Polymorphism and cardiovascular disease: a HuGE review. Am J Epidemiol. 2002;155:487–495. doi: 10.1093/aje/155.6.487. [DOI] [PubMed] [Google Scholar]
- Kennelly SP, Lawlor BA, Kenny RA. Blood pressure and the risk for dementia—A double edged sword. Ageing Res Rev. 2009;8:61–70. doi: 10.1016/j.arr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Sun X, He G, Qing H, Zhou W, Dobie F, Cai F, et al. Hypoxia facilitates Alzheimer's disease pathogenesis by up-regulating BACE1 gene expression. Proc Natl Acad Sci USA. 2006;103:18727–18732. doi: 10.1073/pnas.0606298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5:64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- Panza F, D'Introno A, Colacicco AM, Basile AM, Capurso C, Kehoe PG, et al. Vascular risk and genetics of sporadic late-onset Alzheimer's disease. J Neural Transm. 2004;111:69–89. doi: 10.1007/s00702-003-0071-1. [DOI] [PubMed] [Google Scholar]
- Latchman DS. Herpes simplex virus and risk of Alzheimer's disease. Lancet. 1997;349:1101. doi: 10.1016/S0140-6736(05)62323-9. [DOI] [PubMed] [Google Scholar]
- Breteler MMB, Claus JJ, Grobbee DE, Hofman A. Cardiovascular disease and distribution of cognitive function in elderly people: the Rotterdam Study. BMJ. 1994;308:1604–1608. doi: 10.1136/bmj.308.6944.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner HB, Tang M-X, Luchsinger J, Lantigua R, Stern Y, Mayeux R. The relationship of hypertension in the elderly to AD, vascular dementia, and cognitive function. Neurology. 2000;58:1175–1181. doi: 10.1212/wnl.58.8.1175. [DOI] [PubMed] [Google Scholar]
- Kilander L, Andrén B, Nyman H, Lind L, Boberg M, Lithell H. Atrial fibrillation is an independent determinant of low cognitive function. Stroke. 1998;29:1816–1820. doi: 10.1161/01.str.29.9.1816. [DOI] [PubMed] [Google Scholar]
- Ott A, Stolk RP, Hofman A, van Harskamp F, Grobbee DE, Breteler MMB. Association of diabetes mellitus and dementia: the Rotterdam study. Diabetologia. 1996;39:1392–1397. doi: 10.1007/s001250050588. [DOI] [PubMed] [Google Scholar]
- Kivipelto M, Helkala E-L, Laakso MP, Hänninen T, Hallikainen M, Alhainen K, et al. Midlife vascular risk factors and Alzheimer's disease in later life: longitudinal, population based study. BMJ. 2001;322:1447–1451. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launer LJ, Ross GW, Petrovitch H, Masaki K, Foley D, White LR, et al. Midlife blood pressure and dementia: the Honolulu–Asia aging study. Neurobiol Aging. 2000;21:49–55. doi: 10.1016/s0197-4580(00)00096-8. [DOI] [PubMed] [Google Scholar]
- Nagata KEN, Sato M, Satoh Y, Watahiki Y, Kondoh Y, Sugawara M, et al. Hemodynamic aspects of Alzheimer's Disease. Ann N Y Acad Sci. 2002;977:391–402. doi: 10.1111/j.1749-6632.2002.tb04843.x. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Davisson RL. Hypertension and cerebrovascular dysfunction. Cell Metab. 2008;7:476–484. doi: 10.1016/j.cmet.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Park L, Capone C. Threats to the mind: aging, amyloid, and hypertension. Stroke. 2009;40:S40–S44. doi: 10.1161/STROKEAHA.108.533638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacchigna S, Lambrechts D, Carmeliet P. Neurovascular signalling defects in neurodegeneration. Nat Rev Neurosci. 2008;9:169–181. doi: 10.1038/nrn2336. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. The Blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Kalaria RN. Linking cerebrovascular defense mechanisms in brain ageing and Alzheimer's disease. Neurobiol Aging. 2009;30:1512–1514. doi: 10.1016/j.neurobiolaging.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Selnes OA, Vinters HV. Vascular cognitive impairment. Nat Clin Pract Neurol. 2006;2:538–547. doi: 10.1038/ncpneuro0294. [DOI] [PubMed] [Google Scholar]
- Modrick ML, Didion SP, Sigmund CD, Faraci FM. Role of oxidative stress and AT1 receptors in cerebral vascular dysfunction with aging. Am J Physiol Heart Circ Physiol. 2009;296:H1914–H1919. doi: 10.1152/ajpheart.00300.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L, Anrather J, Girouard H, Zhou P, Iadecola C. Nox2-derived reactive oxygen species mediate neurovascular dysregulation in the aging mouse brain. J Cereb Blood Flow Metab. 2007;27:1908–1918. doi: 10.1038/sj.jcbfm.9600491. [DOI] [PubMed] [Google Scholar]
- Petrovitch H, Ross GW, Steinhorn SC, Abbott RD, Markesbery W, Davis D, et al. AD lesions and infarcts in demented and non-demented Japanese-American men. Ann Neurol. 2005;57:98–103. doi: 10.1002/ana.20318. [DOI] [PubMed] [Google Scholar]
- Toledo JB, Arnold SE, Raible K, Brettschneider J, Xie SX, Grossman M, et al. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer's Coordinating Centre. Brain. 2013;136:2697–2706. doi: 10.1093/brain/awt188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias MF, Wolf PA, D'Agostino RB, Cobb J, White LR. Untreated blood pressure level is inversely related to cognitive functioning: the Framingham Study. Am J Epidemiol. 1993;138:353–364. doi: 10.1093/oxfordjournals.aje.a116868. [DOI] [PubMed] [Google Scholar]
- Hofman A, Ott A, Breteler MMB, Bots ML, Slooter AJC, van Harskamp F, et al. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer's disease in the Rotterdam Study. Lancet. 1997;349:151–154. doi: 10.1016/S0140-6736(96)09328-2. [DOI] [PubMed] [Google Scholar]
- Kilander L, Nyman H, Boberg M, Hansson L, Lithell H. Hypertension is related to cognitive impairment. Hypertension. 1998;31:780–786. doi: 10.1161/01.hyp.31.3.780. [DOI] [PubMed] [Google Scholar]
- Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ. The association between midlife blood pressure levels and late-life cognitive function. The Honolulu-Asia Aging Study. JAMA. 1995;274:1846–1851. [PubMed] [Google Scholar]
- Ruitenberg A, Skoog I, Ott A, Aevarsson O, Witteman JCM, Lernfelt B, et al. Blood pressure and risk of dementia: results from the rotterdam study and the Gothenburg H-70 Study. Dement Geriatr Cogn Disord. 2001;12:33–39. doi: 10.1159/000051233. [DOI] [PubMed] [Google Scholar]
- Sharp SI, Aarsland D, Day S, Sønnesyn H. Alzheimer's society vascular dementia systematic review G. Ballard C . Hypertension is a potential risk factor for vascular dementia: systematic review. Int J Geriatr Psychiatry. 2011;26:661–669. doi: 10.1002/gps.2572. [DOI] [PubMed] [Google Scholar]
- Skoog I, Gustafson D. Update on hypertension and Alzheimer's disease. Neurol Res. 2006;28:605–611. doi: 10.1179/016164106X130506. [DOI] [PubMed] [Google Scholar]
- Yoshitake T, Kiyohara Y, Kato I, Ohmura T, Iwamoto H, Nakayama K, et al. Incidence and risk factors of vascular dementia and Alzheimer's disease in a defined elderly Japanese population. Neurology. 1995;45:1161–1168. doi: 10.1212/wnl.45.6.1161. [DOI] [PubMed] [Google Scholar]
- Gentile MT, Poulet R, Pardo AD, Cifelli G, Maffei A, Vecchione C, et al. β-Amyloid deposition in brain is enhanced in mouse models of arterial hypertension. Neurobiol Aging. 2009;30:222–228. doi: 10.1016/j.neurobiolaging.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Qiu C, Winblad B, Viitanen M, Fratiglioni L. Pulse pressure and risk of Alzheimer disease in persons aged 75 years and older: A community-based, longitudinal study. Stroke. 2003;34:594–599. doi: 10.1161/01.STR.0000060127.96986.F4. [DOI] [PubMed] [Google Scholar]
- Hanon O, Haulon S, Lenoir H, Seux M-L, Rigaud A-S, Safar M, et al. Relationship between arterial stiffness and cognitive function in elderly subjects with complaints of memory loss. Stroke. 2005;36:2193–2197. doi: 10.1161/01.STR.0000181771.82518.1c. [DOI] [PubMed] [Google Scholar]
- Carnevale D, Lembo G. 'Alzheimer-like' pathology in a murine model of arterial hypertension. Biochem Soc Trans. 2011;39:939–944. doi: 10.1042/BST0390939. [DOI] [PubMed] [Google Scholar]
- Poulet R, Gentile MT, Vecchione C, Distaso M, Aretini A, Fratta L, et al. Acute hypertension induces oxidative stress in brain tissues. J Cereb Blood Flow Metab. 2005;26:253–262. doi: 10.1038/sj.jcbfm.9600188. [DOI] [PubMed] [Google Scholar]
- Arendash GW, Su GC, Crawford FC, Bjugstad KB, Mullan M. Intravascular β-amyloid infusion increases blood pressure: implications for a vasoactive role of β-amyloid in the pathogenesis of Alzheimer's disease. Neurosci Lett. 1999;268:17–20. doi: 10.1016/s0304-3940(99)00239-6. [DOI] [PubMed] [Google Scholar]
- Matsubayashi K, Shimada K, Kawamoto A, Ozawa T. Incidental brain lesions on magnetic resonance imaging and neurobehavioral functions in the apparently healthy elderly. Stroke. 1992;23:175–180. doi: 10.1161/01.str.23.2.175. [DOI] [PubMed] [Google Scholar]
- Chodosh EH, Foulkes MA, Kase CS, Wolf PA, Mohr JP, Hier DB, et al. Silent stroke in the NINCDS Stroke Data Bank. Neurology. 1988;38:1674. doi: 10.1212/wnl.38.11.1674. [DOI] [PubMed] [Google Scholar]
- Jendroska K, Poewe W, Daniel SE, Pluess J, Iwerssen-Schmidt H, Paulsen J, et al. Ischemic stress induces deposition of amyloid beta immunoreactivity in human brain. Acta Neuropathol. 1995;90:461–466. doi: 10.1007/BF00294806. [DOI] [PubMed] [Google Scholar]
- Jendroska K, Hoffmann OM, Patt S. Amyloid β Peptide and Precursor Protein (APP) in Mild and Severe Brain Ischemia. Ann N Y Acad Sci. 1997;826:401–405. doi: 10.1111/j.1749-6632.1997.tb48492.x. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Bhatti SU, Palatinsky EA, Pennington DH, Shelton ER, Chan HW, et al. Accumulation of the beta amyloid precursor protein at sites of ischemic injury in rat brain. Neuroreport. 1993;4:211–214. doi: 10.1097/00001756-199302000-00025. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Takeda M, Niigawa H, Hariguchi S, Nishimura T. Amyloid β-protein precursor deposition in rat hippocampus lesioned by ibotenic acid injection. Neurosci Lett. 1992;136:95–98. doi: 10.1016/0304-3940(92)90656-r. [DOI] [PubMed] [Google Scholar]
- Pottier C, Wallon D, Lecrux AR, Maltete D, Bombois S, Jurici S, et al. Amyloid-β Protein Precursor Gene Expression in Alzheimer's Disease and Other Conditions. J Alzheimers Dis. 2012;28:561–566. doi: 10.3233/JAD-2011-111148. [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Yamamoto T, Kalaria R, Senzaki H, Maki T, Hase Y, et al. Cerebral hypoperfusion accelerates cerebral amyloid angiopathy and promotes cortical microinfarcts. Acta Neuropathol. 2012;123:381–394. doi: 10.1007/s00401-011-0925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Alloza M, Gregory J, Kuchibhotla KV, Fine S, Wei Y, Ayata C, et al. Cerebrovascular lesions induce transient β-amyloid deposition. Brain. 2011;134:3697–3707. doi: 10.1093/brain/awr300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto Y, Ihara M, Fujita Y, Ito H, Takahashi R, Tomimoto H. Cortical microinfarcts in Alzheimer's disease and subcortical vascular dementia. Neuroreport. 2009;20:990–996. doi: 10.1097/WNR.0b013e32832d2e6a. [DOI] [PubMed] [Google Scholar]
- Suter O-C, Sunthorn T, Kraftsik R, Straubel J, Darekar P, Khalili K, et al. Cerebral hypoperfusion generates cortical watershed microinfarcts in Alzheimer disease. Stroke. 2002;33:1986–1992. doi: 10.1161/01.str.0000024523.82311.77. [DOI] [PubMed] [Google Scholar]
- Whitehead SN, Cheng G, Hachinski VC, Cechetto DF. Progressive increase in infarct size, neuroinflammation, and cognitive deficits in the presence of high levels of amyloid. Stroke. 2007;38:3245–3250. doi: 10.1161/STROKEAHA.107.492660. [DOI] [PubMed] [Google Scholar]
- Sabayan B, Jansen S, Oleksik AM, van Osch MJP, van Buchem MA, van Vliet P, et al. Cerebrovascular hemodynamics in Alzheimer's disease and vascular dementia: a meta-analysis of transcranial Doppler studies. Ageing Res Rev. 2012;11:271–277. doi: 10.1016/j.arr.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Niwa K, Kazama K, Younkin SG, Carlson GA, Iadecola C. Alterations in cerebral blood flow and glucose utilization in mice overexpressing the amyloid precursor protein. Neurobiol Dis. 2002;9:61–68. doi: 10.1006/nbdi.2001.0460. [DOI] [PubMed] [Google Scholar]
- Niwa K, Kazama K, Younkin L, Younkin SG, Carlson GA, Iadecola C. Cerebrovascular autoregulation is profoundly impaired in mice overexpressing amyloid precursor protein. Am J Physiol Heart Circ Physiol. 2002;283:H315–H323. doi: 10.1152/ajpheart.00022.2002. [DOI] [PubMed] [Google Scholar]
- Claassen JAHR, Zhang R. Cerebral autoregulation in Alzheimer's disease. J Cereb Blood Flow Metab. 2011;31:1572–1577. doi: 10.1038/jcbfm.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmer RA, Gunderson EP, Barrett-Connor E, Charles P, Quesenberry J, Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ. 2005;330:1360. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchsinger J, Mayeux R. Cardiovascular risk factors and Alzheimer's disease. Curr Atheroscler Rep. 2004;6:261–266. doi: 10.1007/s11883-004-0056-z. [DOI] [PubMed] [Google Scholar]
- Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurol. 2006;5:735–741. doi: 10.1016/S1474-4422(06)70537-3. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Maestre GE, Arizaga R, Friedland RP, Galasko D, Hall K, et al. Alzheimer's disease and vascular dementia in developing countries: prevalence, management, and risk factors. Lancet Neurol. 2008;7:812–826. doi: 10.1016/S1474-4422(08)70169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarsland D, Sardahaee FS, Anderssen S, Ballard C, The Alzheimer's Society Systematic Review. Is physical activity a potential preventive factor for vascular dementia? A systematic review. Aging Ment Health. 2010;14:386–395. doi: 10.1080/13607860903586136. [DOI] [PubMed] [Google Scholar]
- Ravaglia G, Forti P, Lucicesare A, Pisacane N, Rietti E, Bianchin M, et al. Physical activity and dementia risk in the elderly: findings from a prospective Italian study. Neurology. 2008;70:1786–1794. doi: 10.1212/01.wnl.0000296276.50595.86. [DOI] [PubMed] [Google Scholar]
- Dichgans M, Zietemann V. Prevention of vascular cognitive impairment. Stroke. 2012;43:3137–3146. doi: 10.1161/STROKEAHA.112.651778. [DOI] [PubMed] [Google Scholar]
- van de Rest O, Geleijnse JM, Kok FJ, van Staveren WA, Hoefnagels WH, Beekman AT, et al. Effect of fish-oil supplementation on mental well-being in older subjects: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2008;88:706–713. doi: 10.1093/ajcn/88.3.706. [DOI] [PubMed] [Google Scholar]
- Freund-Levi Y, Eriksdotter-Jönhagen M, Cederholm T, Basun H, Faxén-Irving G, Garlind A, et al. Ω-3 fatty acid treatment in 174 patients with mild to moderate alzheimer disease: omegad study: a randomized double-blind trial. Arch Neurol. 2006;63:1402–1408. doi: 10.1001/archneur.63.10.1402. [DOI] [PubMed] [Google Scholar]
- Yurko-Mauro K, McCarthy D, Rom D, Nelson EB, Ryan AS, Blackwell A, et al. Beneficial effects of docosahexaenoic acid on cognition in age-related cognitive decline. Alzheimers Dement. 2010;6:456–464. doi: 10.1016/j.jalz.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Hooijmans CR, Pasker-de Jong PCM, de Vries RBM, Ritskes-Hoitinga M. The effects of long-term omega-3 fatty acid supplementation on cognition and Alzheimer's pathology in animal models of Alzheimer's Disease: A Systematic Review And Meta-Analysis. J Alzheimers Dis. 2012;28:191–209. doi: 10.3233/JAD-2011-111217. [DOI] [PubMed] [Google Scholar]
- Witte AV, Kerti L, Hermannstädter HM, Fiebach JB, Schreiber SJ, Schuchardt JP, et al. Long-chain omega-3 fatty acids improve brain function and structure in older adults. Cereb Cortex. 2013. [DOI] [PubMed]
- Morris MC, Sacks F, Rosner B. Does fish oil lower blood pressure? A meta-analysis of controlled trials. Circulation. 1993;88:523–533. doi: 10.1161/01.cir.88.2.523. [DOI] [PubMed] [Google Scholar]
- Appel Lj, Miller ER, 3rd, Seidler AJ, Whelton PK. Does supplementation of diet with 'fish oil' reduce blood pressure? A meta-analysis of controlled clinical trials. Arch Intern Med. 1993;153:1429–1438. [PubMed] [Google Scholar]
- Kimura S, Saito H, Minami M, Togashi H, Nakamura N, Ueno K, et al. Docosahexaenoic acid attenuated hypertension and vascular dementia in stroke-prone spontaneously hypertensive rats. Neurotoxicol Teratol. 2002;24:683–693. doi: 10.1016/s0892-0362(02)00219-2. [DOI] [PubMed] [Google Scholar]
- Whelton SP, Hyre AD, Pedersen B, Yi Y, Whelton PK, He J. Effect of dietary fiber intake on blood pressure: a meta-analysis of randomized, controlled clinical trials. J Hypertens. 2005;23:475–481. doi: 10.1097/01.hjh.0000160199.51158.cf. [DOI] [PubMed] [Google Scholar]
- Zhang W, Liu A-J, Yi-Ming W, Liu J-G, Shen F-M, Su D-F. Pressor and non-pressor effects of sodium loading on stroke in stroke-prone spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 2008;35:83–88. doi: 10.1111/j.1440-1681.2007.04746.x. [DOI] [PubMed] [Google Scholar]
- Kuller LH, Shemanski L, Manolio T, Haan M, Fried L, Bryan N, et al. Relationship between ApoE, MRI findings, and cognitive function in the cardiovascular health study. Stroke. 1998;29:388–398. doi: 10.1161/01.str.29.2.388. [DOI] [PubMed] [Google Scholar]
- Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, et al. Guidelines for the primary prevention of stroke. Stroke. 2011;42:517–584. doi: 10.1161/STR.0b013e3181fcb238. [DOI] [PubMed] [Google Scholar]
- He K, Song Y, Daviglus ML, Liu K, Van Horn L, Dyer AR, et al. Fish consumption and incidence of stroke. Stroke. 2004;35:1538–1542. doi: 10.1161/01.STR.0000130856.31468.47. [DOI] [PubMed] [Google Scholar]
- de Goede J, Verschuren WMM, Boer JMA, Kromhout D, Geleijnse JM. Gender-specific associations of Marine n-3 fatty acids and fish consumption with 10-year incidence of stroke. PLoS ONE. 2012;7:e33866. doi: 10.1371/journal.pone.0033866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H-C, Kao T-K, Ou Y-C, Yang D-Y, Yen Y-J, Wang C-C, et al. Protective effect of docosahexaenoic acid against brain injury in ischemic rats. J Nutr Biochem. 2009;20:715–725. doi: 10.1016/j.jnutbio.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Ozen O, Cosar M, Sahin O, Fidan H, Eser O, Mollaoglu H, et al. The protective effect of fish n-3 fatty acids on cerebral ischemia in rat prefrontal cortex. Neurol Sci. 2008;29:147–152. doi: 10.1007/s10072-008-0926-1. [DOI] [PubMed] [Google Scholar]
- Bazan NG, Eady TN, Khoutorova L, Atkins KD, Hong S, Lu Y, et al. Novel aspirin-triggered neuroprotectin D1 attenuates cerebral ischemic injury after experimental stroke. Exp Neurol. 2012;236:122–130. doi: 10.1016/j.expneurol.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson SC, Virtamo J, Wolk A. Dietary fats and dietary cholesterol and risk of stroke in women. Atherosclerosis. 2012;221:282–286. doi: 10.1016/j.atherosclerosis.2011.12.043. [DOI] [PubMed] [Google Scholar]
- Gardener H, Wright CB, Gu Y, Demmer RT, Boden-Albala B, Elkind MS, et al. Mediterranean-style diet and risk of ischemic stroke, myocardial infarction, and vascular death: the Northern Manhattan Study. Am J Clin Nutr. 2011;94:1458–1464. doi: 10.3945/ajcn.111.012799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudin A, Wennberg M. Fish Consumption and ischemic stroke in Southern Sweden. Nutr J. 2011;10:109. doi: 10.1186/1475-2891-10-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Goede J, Verschuren WMM, Boer JMA, Kromhout D, Geleijnse JM. Alpha-linolenic acid intake and 10-Year incidence of coronary heart disease and stroke in 20,000 middle-aged men and women in The Netherlands. PLoS ONE. 2011;6:e17967. doi: 10.1371/journal.pone.0017967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguemeni C, Delplanque B, Rovère C, Simon-Rousseau N, Gandin C, Agnani G, et al. Dietary supplementation of alpha-linolenic acid in an enriched rapeseed oil diet protects from stroke. Pharmacol Res. 2010;61:226–233. doi: 10.1016/j.phrs.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Arab L, Liu W, Elashoff D. Green and black tea consumption and risk of stroke. Stroke. 2009;40:1786–1792. doi: 10.1161/STROKEAHA.108.538470. [DOI] [PubMed] [Google Scholar]
- Mogi M, Li J-M, Iwanami J, Min L-J, Tsukuda K, Iwai M, et al. Angiotensin II type-2 receptor stimulation prevents neural damage by transcriptional activation of methyl methanesulfonate sensitive 2. Hypertension. 2006;48:141–148. doi: 10.1161/01.HYP.0000229648.67883.f9. [DOI] [PubMed] [Google Scholar]
- Yamada K, Uchida S, Takahashi S, Takayama M, Nagata Y, Suzuki N, et al. Effect of a centrally active angiotensin-converting enzyme inhibitor, perindopril, on cognitive performance in a mouse model of Alzheimer's disease. Brain Res. 2010;1352:176–186. doi: 10.1016/j.brainres.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Muller M, van der Graaf Y, Visseren FL, Mali WPTM, Geerlings MI. for the SSG. Hypertension and longitudinal changes in cerebral blood flow: the SMART-MR study. Ann Neurol. 2012;71:825–833. doi: 10.1002/ana.23554. [DOI] [PubMed] [Google Scholar]
- Takeda S, Sato N, Takeuchi D, Kurinami H, Shinohara M, Niisato K, et al. Angiotensin receptor blocker prevented β-amyloid-induced cognitive impairment associated with recovery of neurovascular coupling. Hypertension. 2009;54:1345–1352. doi: 10.1161/HYPERTENSIONAHA.109.138586. [DOI] [PubMed] [Google Scholar]
- Tsukuda K, Mogi M, Iwanami J, Min L-J, Sakata A, Jing F, et al. Cognitive deficit in Amyloid-β–injected mice was improved by pretreatment with a low dose of telmisartan partly because of peroxisome proliferator-activated receptor-γ activation. Hypertension. 2009;54:782–787. doi: 10.1161/HYPERTENSIONAHA.109.136879. [DOI] [PubMed] [Google Scholar]
- Jing F, Mogi M, Sakata A, Iwanami J, Tsukuda K, Ohshima K, et al. Direct stimulation of angiotensin II type 2 receptor enhances spatial memory. J Cereb Blood Flow Metab. 2012;32:248–255. doi: 10.1038/jcbfm.2011.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlyakhto E. Observational study on cognitive function and systolic blood pressure reduction (OSCAR): preliminary analysis of 6-month data from>10 000 patients and review of the literature. Curr Med Res Opin. 2007;23:S13–S18. doi: 10.1185/030079907X260719. [DOI] [PubMed] [Google Scholar]
- Iwai M, Inaba S, Tomono Y, Kanno H, Iwanami J, Mogi M, et al. Attenuation of focal brain ischemia by telmisartan, an angiotensin II Type 1 receptor blocker, in atherosclerotic apolipoprotein E-deficient mice. Hypertens Res. 2008;31:161–168. doi: 10.1291/hypres.31.161. [DOI] [PubMed] [Google Scholar]
- Shiraishi J, Sawada T, Koide M, Yamada H, Matsubara H. Cardio-Cerebrovascular protective effects of valsartan in high-risk hypertensive patients with coronary artery disease (from the Kyoto Heart Study) Am J Cardiol. 2012;109:1308–1314. doi: 10.1016/j.amjcard.2011.12.025. [DOI] [PubMed] [Google Scholar]
- Papademetriou V, Farsang C, Elmfeldt D, Hofman A, Lithell H, Olofsson B, et al. Stroke prevention with the angiotensin II type 1-receptor blocker candesartan in elderly patients with isolated systolic hypertension. The study on cognition and prognosis in the elderly (SCOPE) J Am Coll Cardiol. 2004;44:1175–1180. doi: 10.1016/j.jacc.2004.06.034. [DOI] [PubMed] [Google Scholar]
- Wang J, Ho L, Chen L, Zhao Z, Zhao W, Qian X, et al. Valsartan lowers brain β-amyloid protein levels and improves spatial learning in a mouse model of Alzheimer disease. J Clin Invest. 2007;117:3393–3402. doi: 10.1172/JCI31547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K, Qureshi SU, Johnson M, Parikh N, Schulz PE, Kunik ME. Does use of antihypertensive drugs affect the incidence or progression of dementia? A systematic review. Am J Geriatr Pharmacother. 2009;7:250–261. doi: 10.1016/j.amjopharm.2009.11.001. [DOI] [PubMed] [Google Scholar]