Abstract
The numerous pores in the basement membrane (BM) of the intestinal villi are essential for the communication of enterocytes with cells in the lamina propria, an important mechanism for the induction of intestinal immune responses. The intestinal epithelial barrier is affected by the mycotoxin deoxynivalenol (DON) from both the apical (luminal) and basolateral (serosal) side. The pig is the most susceptible species to the anorectic and immune-modulating effects of DON, which is most prevalent in crops. We analysed in pigs the effect of DON-contaminated feed on the composition and perforation of the BM and the presence of CD16+ cells or their dendrites in the epithelium. In addition to in vivo experiments, in vitro studies were carried out. Using microarray analyses, the effects of DON on IPEC-J2 cells were studied with the focus on the BM. Our in vivo results showed in the control pigs: (1) a significant increased pore number (p ≤ 0.001) in the jejunum in comparison to ileum, (2) no difference in the pore size, and (3) comparable frequency of intraepithelial CD16+ cells/dendrites in the jejunum and ileum. There was a marked trend that DON feeding increases: (1) the pore number in jejunum, and (2) the number of CD16+ cells/dendrites in the epithelium (Tukey–Kramer; p = 0.055 and p = 0.067, respectively). The in vivo results were extended with microarray analyses of epithelial cell (IPEC-J2 cells). The down-regulation of genes like syndecan, fibulin 6 and BM-40 was observed. These proteins are important factors in the BM composition and in formation of pores. Our results provide evidence that already low basolateral concentrations of DON (50 ng/mL) influence the production of the BM protein laminin by epithelial cells. Thus, DON affects the composition of the BM.
Keywords: DON, Basement membrane, Pores, Immune cells in the lamina epithelialis mucosae, laminin, Microarray
Introduction
The intestinal mucosa represents a pivotal border between the organism and its environment. The large contact surface of the gut allows efficient nutrient absorption, acts as an important barrier for pathogens and toxins, and participates in the innate immune response (Mariani et al. 2009; Pitman and Blumberg 2000). The intestine is lined by a confluent polarised monolayer of enterocytes (=lamina epithelialis mucosae). Enterocytes and sub-epithelial area (Lamina propria) are separated by the basement membrane (BM). Different studies using transmission electron microscopy have shown that the epithelial BM has many small pores. Through these pores, cells can migrate from the lamina propria into the epithelium and can directly contact the enterocytes or sample antigens (Palay and Karlin 1959; Toner and Ferguson 1971; Rescigno et al. 2001). In the lamina propria, different cell types are present such as T cells, B- cells or CD16+ monocytes. During inflammation, lymphocytes and monocytes are recruited into the lamina propria from the systemic circulation.
The mycotoxin deoxynivalenol (DON) is a trichothecene mycotoxin and is primarily produced by the plant pathogens Fusarium graminearum and F. culmorum, DON is an important food contaminant. Pigs are regarded as especially sensitive to the adverse effects of DON which mainly include a reduction in feed intake and immune-modulating properties. In the gastrointestinal tract, DON comes into contact with the epithelial surface, is rapidly absorbed (Prelusky et al. 1988) and might reach the basolateral region of the intestinal mucosa and the BM via the systemic (serosal) route. It has been shown that DON interferes with the intestinal epithelium and affects tight junction proteins in porcine intestinal cell lines (Diesing et al. 2011).
The effects of high DON concentrations on the enterocyte layer result in cell death and loss of the integrity of the epithelial barrier (Diesing et al. 2011). Low concentrations of DON (50 ng/mL) were observed in the peripheral blood of pigs after DON-contaminated feed intake (Dänicke et al. 2004). In the present study, we aimed to analyse possible effects of low DON-concentrations on the basolateral region of the intestinal epithelium in vivo. The basement membrane was analysed with the focus on pore size and pore numbers. Furthermore, the effect of low DON concentrations on the immune system was studied in terms of the migration of CD16+ cells from the lamina propria into the epithelium. In addition, in vitro experiments using the DON-exposed porcine intestinal epithelial cell line J2 (IPEC-J2) and microarray analyses were performed with the focus on influence of DON on the BM proteins.
Materials and methods
In vivo experiment in pigs
The animal experiment was conducted according to the European Community regulations concerning the protection of experimental animals and the guidelines of the Regional Council of Braunschweig, Lower Saxony, Germany (File number 509.42502/09-01.03) and is described in detail in Dänicke et al. (2012).
Experimental design
Fattening pigs (Deutsches Bundeshybridzuchtprogramm) were either fed a control diet (CON) or a deoxynivalenol-contaminated diet (DON). Diets were based on wheat, barley and triticale, whereby the triticale batch of the control diet was exchanged for a batch naturally contaminated with deoxynivalenol. The starter and finishing diet thus contained 2.2 and 2.9 mg DON/kg feed in the compound feed, respectively (Dänicke et al. 2012). Barrows were housed individually in slatted floor pens during the entire trial (11 weeks) and 5 animals of each group were slaughtered after the finishing period at a final body weight of 111 kg. Tissue samples were taken from the mid-jejunum, 3 cm distal from the pylorus, and the terminal ileum, approximately 5 cm proximal to the ileo-caecal junction. Samples were snap-frozen in liquid nitrogen and stored at −80 °C until further analysis.
Immunofluorescence of intestinal samples
In each group, 10 cryo-sections were cut from every gut segment (Leica CM3050S cryotom), with sections 1, 5 and 10 being used for the analysis (3 sections/gut segment/animal). This resulted in 15 sections per group (thickness: 5 μm; 3 sections/slide). For confocal microscopy, tissue sections were cut at 16 μm thickness. Tissue sections were fixed with methanol:acetone (mixture 1:1, 90 s), permeated with 0.3 % triton (10 min; Triton X100; Sigma, Germany) and blocked with 10 % normal goat serum (10 min; Axxora, USA). Sections were incubated (1 h, humid chamber) with primary antibodies mouse anti-CD16 (2 h, RT, 1:100, mouse IgG1, monoclonal; AbD Serotec, Germany) and rat anti-laminin beta 1 (2 h, RT, 1:800, rat IgG1, monoclonal, clone LT3; Abcam, Germany). After washing (3 × 5 min with 0.1 M phosphate-buffered saline, PBS) tissue sections were incubated with the corresponding secondary antibodies goat anti-mouse IgG1 TexasRed and goat anti-rat IgG Alexa488 (both: 1 h, RT, 1:200; Invitrogen, Germany). A secondary antibody control was performed in the same procedure without the primary antibodies by using 0.1 M PBS instead of them. Nuclei were stained with 4´,6-diamidino-2-phenylindole (DAPI, 5 min, 1:10 in 0.1 M PBS; Partec, Germany) and sections embedded in Vectashield/glycerol mounting medium (1:1; Vector Laboratories, USA) containing 2 % 1,4-Diazabicyclo[2.2.2]octan (DABCO).
Epifluorescence microscopy was performed on a Zeiss Axioplan2, photographs were taken with an AxioCam HRc camera (Zeiss, Germany) and analysed with Axiovision software 4.8 (Zeiss). Six pictures were taken of each section. The pore size and the number of per 1,000 μm BM were determined based on the laminin staining (Fig. 1). At the same time intraepithelial CD16+ cells or dendrites/1,000 μm (CD16+ cells/dendrites in the lamina epithelialis mucosae) were counted in every section of the epithelium. Confocal microscopy was performed on a Leica SP2 confocal microscope (Leica, Heidelberg, Germany). Visualisation of three-dimensional structures was performed by rayetrace rendering of optical sections and rotation (VolumeJ plugin Fiji, NIH).
Fig. 1.
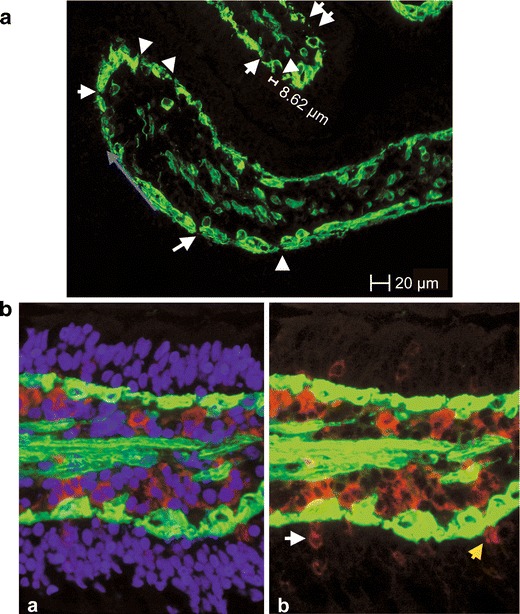
Evaluation of the in vivo experiment. a Laminin staining of the BM (green). Continuous staining was not detected, and the unstained areas of the basement membrane represent the pores of the BM. The length of the unlabelled area was taken as the diameter of the pores, given that the pores are circular. The blue arrow shows how the length of the BM was measured. b CD16+ cells (red) in close contact to the BM (laminin-stained, green); a triple-stained picture, nuclei (DAPI, blue), b CD16+ cells (white arrow) and a CD16+ dendrite (yellow arrow) in the epithelium
In vitro experiment with a porcine intestinal cell line (IPEC-J2)
Cell culture conditions
IPEC-J2 cells were used in this study (passages 78–98, ACC 705; Steube et al. 2011). Cells were cultured in Dulbecco’s modified eagle medium [DMEM/Ham’s F-12 (1:1)] supple-mented with 5 % fetal calf serum (FCS), 1 % insulin-transferrin-selenium (ITS), 16 mmol/L HEPES (all PAN-Biotech, Germany) and 5 ng/mL epidermal growth factor (EGF; BD Biosciences, Germany) and incubated at 39 °C and 5 % CO2. The cells were seeded at a density of 2.0 x 105/well (6-wells, 1 μm pore size; ThinCertTM membrane inserts; Greiner Bio-one, Germany) and cultured for 7 days until trans-epitelial electrical resistance (TEER) measurements (Millicell Electrical resistance system; Millipore, France) proved the confluence of differentiated cells (TEER value of 1 kOhm/well).
Incubation with DON
DON (D0156; Sigma-Aldrich, Germany) was diluted in absolute ethanol (99.6 %; Roth, Germany) to a 0.2 mg/mL stock solution and working dilutions were prepared in cell culture medium. Application of low (50 ng/mL) and intermediate (200 ng/mL) DON-concentration were chosen to simulate physiological DON exposition and high (2,000 ng/mL) DON concentration represented an acute intoxication. DON was applied either in the basolateral (bl) or apical (ap) compartment of the culture.
Sample collection
After 72 h of incubation with low and high DON concentrations from either apical or basolateral sites, cells were collected from membranes with a cell scraper. Total RNA was isolated using TRIzol Reagent (Invitrogen) according to the manufacturer’s protocol. The cells were lysed, chloroform was added, and the RNA was recovered from the aqueous phase by precipitation with isopropyl alcohol, dried, and dissolved in DEPC water (Roche, Germany). After DNaseI treatment the RNA was cleaned up with the RNeasy Kit (Qiagen, Germany). The quantity of RNA was measured using the NanoDrop ND-1000 spectrophotometer (Peqlab, Germany). The RNA samples were stored at −80 °C until processing. Samples for western blot analysis were washed with PBS and collected in 500 μL pre-warmed SDS-gel loading buffer (80 °C; 1 M Tris base pH 6.8; 1 % Glycerol, 10 % SDS, 0.1 % Bromophenol blue; 0.05 % ß-mercaptoethanol). Cells were harvested, denatured at 95 °C for 5 min, and stored at −80 °C for further analysis.
Microarray analysis
The microarray (n = 3) and data analysie were performed as described by Diesing et al. (2012). In brief, cells were seeded on uncoated 6-well inserts (1 μm pore-sized ThinCert™ inserts; Greiner Bio-one) and cultured for 7 days. IPEC-J2 cells were treated with 200 ng/mL and 2,000 ng/mL DON applied in the apical compartment of the inserts. To analyse the possible effect of DON on the basolateral region of the cells, the same concentrations were also applied to the basolateral compartment of the inserts. Total RNA was isolated using TRIzol Reagent (Invitrogen) and 500 ng of purified RNA was analysed by Gene Chip® Porcine Genome Array (Affymetrix, UK). Analysis of the read-out was performed by Affymetrix GCOS 1.3 software using global scaling to a target signal of 500.
qPCR
The qPCR was performed as described in Diesing et al. 2011. Real-time PCR amplification was performed for LAMA3 and LAMC1 (LAMA3 left: 5’-gaatggctgtcctgaccact-3’, LAMA3 right: 5’- cggatggtgtctgaaatcaa-3’; LAMC1 left: 5’-acagacaaggtggctgctg, LAMC1 right: 5’-gctaaagtgaaggcacatgga-3’) under the following conditions on an icycler (BioRad, Germany): 1.5 min at 95 °C, 5 min at 95 °C followed by 40 cycles of 30 s at 95 °C and 60 s at optimal primer annealing temperature. Melting curve analysis (50–95 °C) was used for assessing amplification specificity. The reaction volume of 25 μL contained 12.5 μL Maxima Mastermix (Fermentas, Germany) with SYBR® Green and Fluorescein as internal standard, 300 nM of the respective primers (2.5 μL each), 0.5 μL UNG (Uracil-DNA-glycosilase), 6.5 μL nuclease free water and 1 μL cDNA (60 ng/mL). The analyses consisted of at least 5 independent experiments. Each experimental sample was assayed using triplicates for each primer pair. The ΔΔCT-method was used for the calculation of the differences in the expression profiles. The relative difference of the expression between control and treated cells, which was normalised to the reference gene β-actin, resulted in the set phrase: ratio = 2-ΔΔCT.
Western blot analysis
Western blot analyses were performed as described in Nossol et al. 2011. The Qubit® Protein Assay Kit was used to measure the protein concentration (Invitrogen). Samples (40 μg) were loaded on 8 % SDS polyacrylamide gel in parallel with the pre-stained protein ladder (SM1811, 10–250 kD; Fermentas) and subsequently transferred to PVDF membrane by semidry electroblotting. A rat-anti-laminin beta 1 antibody (1:100; clone LT3; Abcam,) was used to analyse the laminin content after DON incubation. The secondary antibody goat anti-rat IgG HRP (1:2,000) was purchased from Cell Signalling (Germany). Actin served as loading control (mouse-anti β-actin antibody, 1:30,000; Cell signalling). The secondary antibody for β-actin was purchased with the BM Chemiluminescence Western Blotting Kit mouse/rabbit (Roche, Germany). Blots were analysed on an Alpha-Ease® FC Imaging System (Alpha Innotech, Canada).
Statistical analysis
In vivo experiment in pigs
The data were analysed according to a complete two-way design by using the procedure “MIXED” of the SAS software package (v.9.1; SAS Institute, Cary, NC, USA) with “treatment”, “intestinal segment” and “treatment × intestinal segment” as fixed factors. Possible correlations within individual pigs were considered by the “REPEATED” statement. Least square mean (LSMEAN) differences were evaluated by an adjusted Tukey–Kramer post hoc test (T = p ≤ 0.1(trend or tendency), *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.01).
In vitro experiment with a porcine intestinal cell line (IPEC-J2)
Statistical analyses of the microarray are described in Diesing et al. (2012). Differences between control and DON-treatment group with the focus on mRNA level in qPCR were analysed by SPSS (one-way ANOVA; Tukey; SPSS 17) (T = p ≤ 0.1(trend or tendency), *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.01).
Results
In vivo experiment in pigs
Basement membrane (BM) pore size in the jejunum and ileum
The presence of laminin was used as a marker to localise the BM in 5-μm cryo-sections in the jejunum and ileum. Analysis of BM pores in jejunum and ileum of non-treated control animals resulted in pore sizes ranging from 0.51 to 27.95 μm in the jejunum and from 0.25 to 34.26 μm in the ileum. Pores were clustered in size ranges spanning 1 μm and numbers of pores within each cluster were plotted (Fig. 2). In both segments of the intestine, large pores greater than 10 μm are rare. About 80 % of the pores were smaller than 5 μm. An average pore size of 3.6 μm was measured in the jejunum and of 3.4 μm in the ileum (Fig. 3). Similar results were found for the DON-diet group (jejunum: 3.8 μm; ileum: 4 μm). The average pore size was independent of the intestinal segment and independent of DON feeding.
Fig. 2.
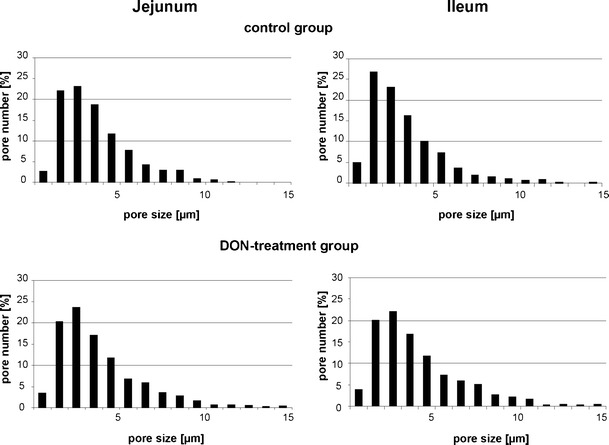
Distribution of the pore size. In the control groups of the jejunum and the ileum, a total amount of 498 (jejunum) and 931 pores (ileum) were analysed. The percentage of the different pore sizes is illustrated. In the jejunum as well as in the ileum, about 80 % of the pores had a size between 0.25 and 5 μm. In the DON-diet groups, a total amount of 788 (jejunum) and 1,035 (ileum) pores were found; 74–76 % of the pores had a size between 0.11 and 0.5 μm
Fig. 3.
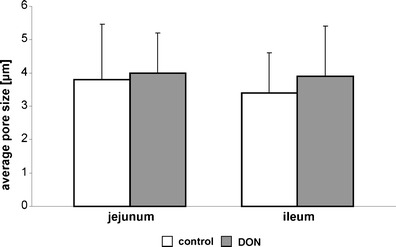
Average pore size. No differences with the focus on the pore size were found between the control and DON-diet group of the jejunum as well as of the ileum and no significant differences were found in the comparison of the intestinal segments (values represent means ± SD)
Effect of DON on the pore number/1,000 μm BM
The absolute number of pores was significantly increased in the ileum (11.3 pores/1,000 μm BM length) in comparison to the jejunum (6.4 pores/1,000 μm BM) in the control groups (Tukey–Kramer; p < 0.001; Fig. 4). The DON-diet triggered in the jejunum a trend (Tukey–Kramer; p = 0.055) to a higher number of pores (9.7 pores/1,000 μm BM) in comparison to the control group (6.4 pores/1,000 μm BM). In DON-exposed pigs, the pore numbers in the jejunum were comparable in the jejunum and ileum.
Fig. 4.
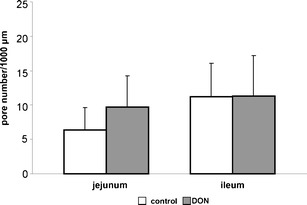
Average pore number. The dataset was analysed with an Ftest (for details see “Materials and methods”). Significant differences were found between jejunum and ileum. This was also confirmed with a t test and an increased number of pores were found in the control group of the ileum in comparison to the control group of the jejunum (p < 0.001). A marked effect was found with the F test between the groups (p = 0.097). This result was approved with a t test and a tendency of a higher pore number in the DON-diet group in the jejunum was observed in comparison to the control (control: 6.4; DON-diet group: 9.7; p = 0.055). No differences were found between control and DON-diet group in the ileum (control: 11.26; DON-diet group: 11.36)
Three-dimensional reconstruction of pores
Cryo-sections (16 μm) of the ileum were labelled using immunofluorescence for laminin (green, basement membrane), CD 16 (red, general monocyte/macrophage/natural killer cell marker) and DAPI (blue, nuclei), microscopical sections were reconstructed and rendered to obtain the three-dimensional structure of the villus tip. Two views of a villus tip are shown (Fig. 5a, b); the picture in Fig. 5b is rotated by 180° along the longitudinal axis through the villus. Symbols (square, star and circle) mark the position of three individual cells in both views. Red arrows mark characteristic pores. Two nuclei (star and circle) were found within a pore structure indicating a kind of passage through the basement membrane. CD16+ cells were found in close contact to the basement membrane (e. g. square marked cell).
Fig. 5.
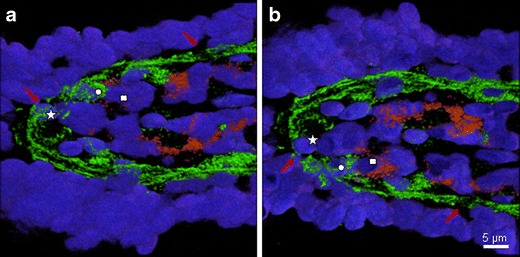
Three-dimensional reconstruction of the pores. Confocal microscopical pictures of a cryosection (16 μm) showing the laminin-labelled BM (green), CD16+ cells (red), and cell nuclei (DAPI, blue) studied. The optical sections were reconstructed and rendered to obtain the three-dimensional structure of the villus tip. Two views of the villus are given (a, b), the picture in (b) is rotated by 180° along the longitudinal axis. Symbols (square, star and circle) mark the position of the same three individual cells in both views. Red arrows mark characteristic pores. Two nuclei (star and circle) were found within a pore structure showing cells on the passage through the basal membrane. CD16+ cells were found in close contact to the basement membrane (see square for example)
Effect of DON on the number of CD16+ cells or dendrites per 1,000 μm BM
The frequency of CD16+ cells or dendrites of CD16+ cells/1,000 μm BM was determined. In the first statistical analysis, the dataset was characterised using a two-way design to detect any effect of the intestinal segment and treatment. No differences were found between the treatments (F test, p = 0.509). A marked effect was observed between the intestinal segments (trend; p = 0.087) and the interaction of ‘treatment × intestinal segment’ (trend; p = 0.091). In the next step, the least square mean differences were analysed with a Tukey–Kramer post hoc test. A tendency of an up-regulation of CD16+ cells/dendrites was found in the DON-treatment group of the jejunum in comparison to the DON-group of the ileum (jejunum: 4.9; ileum: 2.8; p = 0.067; Fig. 6). No differences between control and DON-diet groups were observed in the ileum. The comparison of both control groups showed no significant differences.
Fig. 6.
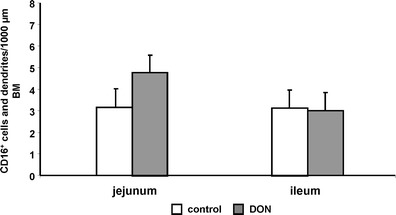
Average number of CD16+ cells or dendrites. No differences were found between the groups (F test; p = 0.509). A marked effect was observed with focus on gut segment (T; p = 0.087) and the interaction of group × gut segment (T; p = 0.091). A higher number of CD16+ cells/dendrites were detected in the DON-diet group of the jejunum in comparison to control (control: 3.4; DON group: 4.9). The comparison of the control and DON-diet group of the ileum resulted in no significant differences. A tendency of a higher number of CD16+ cell/dendrites were found in the comparison of the two DON-diet groups (t test; T, p = 0.067)
In vitro experiment in a porcine intestinal cell line
Effect of DON on genes of the BM in IPEC-J2: analysis via microarray
Microarray analyses were performed to examine the effect of DON on genes of the BM proteins. Based on the observed differences in vivo, microarray analyses in the jejunum-derived, porcine IPEC-J2 cells were designed. Laminin and collagen are important proteins of the basement membrane. Three of 11 genes which encode for laminin were differently regulated by DON: (1) LAMA3 showed a significant up-regulation in the DON-treatment group 200 ng/mL bl; (2) asignificant down-regulated was observed in LAMB3 in 200 ng/mL bl; and (3) LAMC1 was significantly up-regulated in 2,000 ng/mL ap. An up-regulation of COL4A1 was found in the DON-treatment groups: 2,000 ng/mL ap, 200 ng/mL bl and 2,000 ng/mL bl. Syndecan was down-regulated in the 2,000 ng/mL ap DON-group. In contrast, syntenin (a syndecan binding protein) showed an up-regulation in 2,000 ng/mL ap and 200 ng/mL bl. A significant down-regulation of the basement membrane protein BM-40 was detected in the DON-treatment group with low concentrations independent of the application route. Fibulin 6 was down-regulated in 200 ng/mL bl DON-treated cells (0.35; Table 1). No significant changes were found in fibulin 1, 2 and 5. Nidogen also showed no changes. All integrins were up-regulated with the exception of the integrin α-V precursor in the DON-treatment 200 ng/mL bl group. Integrin α-8 precursor showed the highest fold change (6.03, 200 ng/mL bl; Table 1) and was also up-regulated in 200 ng/mL ap. Metalloproteinase (MMP) 16 is up-regulated in the 200 ng/mL bl group. MMP-11 and MMP24 were down-regulated in the same group. TIMP-3 was significant up-regulated in the 2000 ng/mL bl group.
Table 1.
Microarray analysis showing the significant up- or down-regulation of genes in comparison to the control; the n-fold changes are indicated
| product | Fold change | ||||
|---|---|---|---|---|---|
| Gene name | 200 | 2000 | 200 | 2000 | |
| ap | ap | bl | bl | ||
| Laminin α-3 chain precursor | LAMA3 | 1.44* | |||
| Laminin β-3 chain precursor | LAMB3 | 0.79* | |||
| Laminin γ-1 chain precursor | LAMC1 | 1.44* | |||
| Collagen α 1(XI) chain precursor | COL11A1 | 1.89* | 0.61* | ||
| Collagen α 1(III) chain precursor | COL3A1 | 1.91** | |||
| Collagen α 1(IV) chain precursor | COL4A1 | 1.7* | 1.77* | 1.65* | |
| Collagen alpha 1(XVI) chain precursor | COL16A1 | 1.26* | 1.28** | ||
| collagen, type XXIV, alpha 1 | COL24A1 | 0.61* | |||
| Goodpasture antigen-binding protein (GPBP) (Collagen type IV α 3 binding protein) | COL4A3BP | 0.55** | |||
| Syndecan-4 precursor (SYND4) | SDC4 | 0.83* | |||
| Syntenin 2 (Syndecan binding protein 2) | SDCBP2 | 2.1*** | 2.1** | ||
| SPARC precursor (Secreted protein acidic and rich in cysteine) (Osteonectin) (Basement membrane protein BM-40). | SPARC | 0.89** | 0.47* | ||
| fibulin 6; hemicentin | NP_114141 | 0.35* | |||
| Fibrillin 1 precursor | FBN1 | 1.9** | 1.78* | ||
| Integrin α-3 precursor | ITGA3 | 1.37** | 1.3* | ||
| Integrin α-8 precursor | ITGA8 | 4.83* | 6.03* | ||
| Integrin α-9 precursor | ITGA9 | 5.28* | 2.68* | ||
| Integrin α-V precursor | ITGAV | 0.43* | 0.66* | ||
| Integrin β-4 precursor | ITGB4 | 1.34* | |||
| Integrin β-6 precursor | ITGB6 | 1.97* | 1.76* | ||
| Protocadherin 9 precursor | PCDH9 | 0.59* | |||
| Metalloproteinase inhibitor 3 precursor (TIMP-3) | TIMP3 | 1.33* | |||
| Matrix metalloproteinase-16 precursor (MMP-16) | MMP16 | 1.41* | |||
| Matrix metalloproteinase-24 precursor (MMP-24) | MMP24 | 0.64* | |||
| Stromelysin-3 precursor (MMP-11) | MMP11 | 0.5* | |||
*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.01
Analysis of Laminin expression after DON-treatment in IPEC-J2 via qPCR
As the effects of DON 200 ng/mL induced diverse effects in the laminin precursors expression, additional qPCR analyses were carried out in IPEC-J2 and the laminin α-3 chain precursor (LAMA3) and laminin γ-1 chain precursor (LAMC1) were studied. To check the effect of an even lower DON concentration (comparable to the level that has been observed in the peripheral blood), DON 50 ng/mL bl was used instead of DON 200 ng/mL bl. No significant differences in the LAMA3 and the LAMC1 mRNA-level were found in the DON-treatment groups in comparison to control (Fig. 7).
Fig. 7.
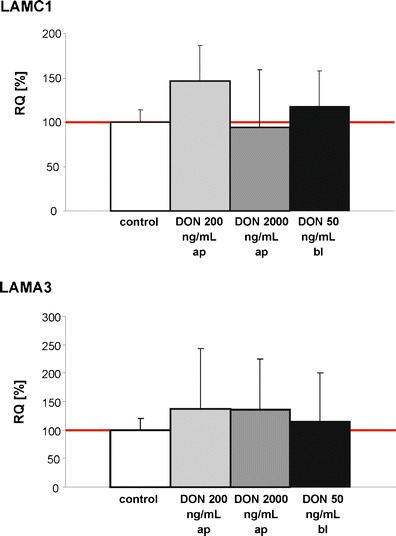
Analysis of the laminin expression on mRNA-level using to qPCR. IPEC-J2 cells were treated with DON 200 ng/mL ap and 2,000 ng/mL ap which resemble the chronic and the acute DON-exposure. The low concentration of 50 ng/mL was used to reflect the DON-concentration found in the peripheral blood after feeding DON-diet in pigs (Dänicke et al. 2004). No differences were found in the comparison of control and DON-treatment groups (RQ relative quantification)
Laminin content after DON-treatment in IPEC-J2
IPEC-J2 cells were treated with different DON-concentration: 200 and 2,000 ng/mL ap and 50 ng/mL bl. In contrast to the qPCR results, western blot analysis showed a marked decrease of the laminin production in the following order: IPEC-J2 CON > DON 200 ng/mL ap > DON 2000 ng/mL ap > DON 50 ng/mL bl (Fig. 8).
Fig. 8.
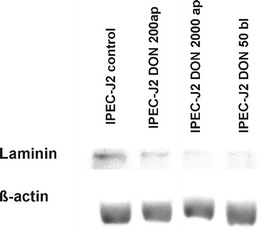
Analysis of the laminin content. IPEC-J2 cells were analysed by western blot after DON-treatment (DON 0 ng/mL; DON 200 ng/mL ap; DON 2,000 ng/mL ap and DON 50 ng/mL bl). A marked reduction of the laminin content was found in the DON-treatment groups in comparison to the control
Discussion
Effect of DON on the pore distribution in the BM
In the present study, a laminin antibody was used to label the BM. On the basis of this protein location, the number of pores in the membrane was determined and their size was measured morphologically. In the jejunum, the pore number was lower then in the ileum. Takeuchi and Gonda (2004) examined Wistar rats and divided the villus in 4 sections (position 1: top of the villus; position 4: basal position of the villus); they detected larger pores in the upper three areas of the villi, being about 1 μm in diameter. They found a higher density of pores in the ileal Peyer’s patches and they suggested that the frequency of pores in the BM corresponds to the passage of immuno-competent cells (Takeuchi and Gonda 2004). There is evidence in the present experiments of differences in the numbers of pores between the control and DON-treatment group in the jejunum but not in the ileum (based on the trend analysis; Tukey–Kramer-test, p = 0.055). The jejunum seems to be more sensitive to DON than the ileum. This has also been observed by Klunker et al. (2013). In their study, they showed that the apical ZO-1 expression was severely damaged in DON treated animals in the mid jejunum, but not in the ileum. So far, there is no explanation for this interesting observation.
Effect of DON on the composition of the BM
The basement membrane is a 50- to 100-nm-thick layer of specialised extracellular matrix found as the basal part of the intestinal epithelium. This BM provides the border between the epithelial monolayer and the connective tissue of the underlying lamina propria. The BM provides the structural support of the epithelial cells and enables the contact of immune cells with the epithelium. The four major components are collagen type IV, laminin, enactin and perlecan; collagen type IV and laminin self-assemble into supra-structures (Martinez-Hernandez and Amenta 1983).
The mRNA expression of integrins, collagen and matrix-metalloproteinase precursors is influenced by DON. TIMP-3, an important gene which is responsible for the degradation of proteins of the basement membrane, is significantly up-regulated in 200 ng/mL bl. All these genes have an effect on the adhesion of the epithelial cells to the BM, as well as laminin and collagen IV (Martinez-Hernandez and Amenta 1983).
Laminin is the most abundant non-collagenous protein in the BM and, therefore, we analysed the effect of DON on the laminin expression in vivo and in vitro. In vivo, we found a trend of a higher pore number in the DON-diet group of the jejunum in comparison to control. We suggest that this is result of a change in the distribution of laminin and/or a change in the protein production of basement membrane proteins. To examine this hypothesis, we treated IPEC-J2 (epithelial cells) in vitro, and a marked reduction of the production of laminin was observed in the following sequence: Control (DON 0 ng/mL) > DON 200 ng/mL ap > DON 2,000 ng/mL ap > DON 50 ng/mL bl, indicating that DON influences the laminin-beta-1-production in vivo and in vitro. DON is able to induce the reduction of the laminin production after both apical and basolateral exposition. Low doses occurring in vivo (50 ng/mL) from basolateral have the same or even higher effect in comparison to acute DON exposition (2,000 ng/mL) from the intestinal lumen (Diesing et al. 2012). An amount of 50 ng/mL DON was detected in the peripheral blood in DON-fed pigs, reflecting our basolateral concentration inducing the marked reduction of laminin (Dänicke et al. 2004). Therefore, the present in vitro results are comparable with the effects in vivo, where DON is reaching the intestinal epithelial cells via the blood stream in low concentration from the basolateral side.
Effect of DON on the migration of immune cells into the lamina epithlialis mucosae
Different studies showed that lymphocytes are frequently present in the pores of the BM of intestinal villi, suggesting the busy traffic across the BM (Meader and Landers 1967; Komuro 1985). Takahashi-Iwanaga et al. (1999) found no large pores allowing such a cell passage. In contrast, Saito et al. 1998 showed large pores containing lymphocytes in the basement membrane covering cryptopatches in the mouse. In our experiments, more than 80 % of the pores have a size smaller than 5 μm and thus enable at least a partial protrusion of immune cells, as shown in mice (Rescigno et al. 2001).
The changes in the laminin production and distribution in the DON-diet group in vivo causes an increase of the pore number in the jejunum, which is the basis for an increased migration of CD16+ cells into the epithelium. We suggest that DON exposition may trigger the immune system by increasing the contact frequency of epithelial cells and immune cells based on the changed BM composition and the resulting higher number of pores. Finally, these putative DON effects may improve the antigen sampling in the intestinal epithelium.
Another explanation for an increased number of CD16+ cells in the lamina epithelialis mucosae after DON-diet is the different composition of the BM that leads to a change in the adhesion of the cells, possibly resulting in apoptosis of epithelial cells. Diesing et al. (2011) showed that high DON concentrations on the enterocyte border comprise cell death and loss of the epithelial barrier integrity. The phagocytic macrophages enter the epithelium and incorporate apoptotic enterocytes (Takahashi-Iwanaga et al. 1999). DON acting from basolateral also affects immune and other cells within the connective tissue of the lamina propria. In comparison to epithelial cells, cellular components of the immune system seem to be more sensitive to DON-mediated effects. In the murine macrophage cell line RAW264.7, low concentrations of DON (250 ng/mL) triggered phosphorylation of AKT and ERK, finally leading to activation of caspase 3 and internucleosomal DNA fragmentation (Zhou et al. 2005), but lower concentrations than 250 ng/mL have not yet been studied.
In conclusion, our study showed for the first time a higher pore number of the BM in the jejunum in comparison to the ileum. Furthermore, DON affects the frequency of pores in the BM of the jejunum but not in the ileum in pigs. We suggest that DON changes the composition of the basement membrane by influencing the regulation of important genes of the BM in intestinal epithelial cells and reduces the production of laminin. Here, low and physiological concentrations of DON (comparable to blood level in DON-fed pigs) show identical results compared with high concentrations. Finally, DON increases the number of CD16+ cells migrating from the lamina propria into the epithelium of the jejunum. This may lead to a change in the antigen sampling in the gut. In addition, epithelial cells can lose contact to the BM because of changes in the epithelial cell–BM adherence, inducing a higher number of CD16+ cells in the epithelium. Further studies are essential to examine these processes in detail.
Acknowledgement
The authors are indebted to Anke Schmidt, Andrea Kröber, Romina Wolter, Sandra Vorwerk, Sybille Röhl and Brigitte Ketzler for their technical assistance. This research was financially supported by the Deutsche Forschungsgemeinschaft (DFG RO 743/3-2).
Conflict of interest
None
References
- Dänicke S, Valenta H, Spilke J. Effects of long-term storage on Fusarium toxin concentrations in wheat–sources of error of the analytical results. Arch Anim Nutr. 2004;58:507–515. doi: 10.1080/00039420400019936. [DOI] [PubMed] [Google Scholar]
- Dänicke S, Brosig B, Klunker LR, Kahlert S, Kluess J, Doll S, Valenta H, Rothkötter HJ. Systemic and local effects of the Fusarium toxin deoxynivalenol (DON) are not alleviated by dietary supplementation of humic substances (HS) Food Chem Toxicol. 2012;50:979–988. doi: 10.1016/j.fct.2011.12.024. [DOI] [PubMed] [Google Scholar]
- Diesing AK, Nossol C, Danicke S, Walk N, Post A, Kahlert S, Rothkötter HJ, Kluess J. Vulnerability of polarised intestinal porcine epithelial cells to mycotoxin deoxynivalenol depends on the route of application. PLoS ONE. 2011;6:e17472. doi: 10.1371/journal.pone.0017472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diesing AK, Nossol C, Ponsuksili S, Wimmers K, Kluess J, Walk N, Post A, Rothkötter HJ, Kahlert S. Gene regulation of intestinal porcine epithelial cells IPEC-J2 is dependent on the site of deoxynivalenol toxicological action. PLoS ONE. 2012;7:e34136. doi: 10.1371/journal.pone.0034136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klunker LR, Kahlert S, Panther P, Diesing AK, Reinhardt N, Brosig B, Kersten S, Danicke S, Rothkotter HJ, Kluess JW. Deoxynivalenol and E.coli lipopolysaccharide alter epithelial proliferation and spatial distribution of apical junction proteins along the small intestinal axis. J Anim Sci. 2013;91:276–285. doi: 10.2527/jas.2012-5453. [DOI] [PubMed] [Google Scholar]
- Komuro T. Fenestrations of the basal lamina of intestinal villi of the rat. Scanning and transmission electron microscopy. Cell Tissue Res. 1985;239:183–188. doi: 10.1007/BF00214918. [DOI] [PubMed] [Google Scholar]
- Mariani V, Palermo S, Fiorentini S, Lanubile A, Giuffra E. Gene expression study of two widely used pig intestinal epithelial cell lines: IPEC-J2 and IPI-2I. Vet Immunol Immunopathol. 2009;131:278–284. doi: 10.1016/j.vetimm.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A, Amenta PS. The basement membrane in pathology. Lab Invest. 1983;48:656–677. [PubMed] [Google Scholar]
- Meader RD, Landers DF. Electron and light microscopic observations on relationships between lymphocytes and intestinal epithelium. Am J Anat. 1967;121:763–773. doi: 10.1002/aja.1001210318. [DOI] [PubMed] [Google Scholar]
- Nossol C, Diesing AK, Walk N, Faber-Zuschratter H, Hartig R, Post A, Kluess J, Rothkötter HJ, Kahlert S. Air-liquid interface cultures enhance the oxygen supply and trigger the structural and functional differentiation of intestinal porcine epithelial cells (IPEC) Histochem Cell Biol. 2011;136:103–115. doi: 10.1007/s00418-011-0826-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palay SL, Karlin LJ. An electron microscopic study of the intestinal villus. II. The pathway of fat absorption. J Biophys Biochem Cytol. 1959;5:373–384. doi: 10.1083/jcb.5.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman RS, Blumberg RS. First line of defense: the role of the intestinal epithelium as an active component of the mucosal immune system. J Gastroenterol. 2000;35:805–814. doi: 10.1007/s005350070017. [DOI] [PubMed] [Google Scholar]
- Prelusky DB, Hartin KE, Trenholm HL, Miller JD. Pharmacokinetic fate of 14C-labeled deoxynivalenol in swine. Fundam Appl Toxicol. 1988;10:276–286. doi: 10.1016/0272-0590(88)90312-0. [DOI] [PubMed] [Google Scholar]
- Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- Saito H, Kanamori Y, Takemori T, Nariuchi H, Kubota E, Takahashi-Iwanaga H, Iwanaga T, Ishikawa H. Generation of intestinal T cells from progenitors residing in gut cryptopatches. Science. 1998;280:275–278. doi: 10.1126/science.280.5361.275. [DOI] [PubMed] [Google Scholar]
- Steube KG, Koelz AL, Uphoff CC, Drexler HG, Kluess J, Steinberg P. The necessity of identity assessment of animal intestinal cell lines: A case report. Cytotechnology. 2011 doi: 10.1007/s10616-011-9420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi-Iwanaga H, Iwanaga T, Isayama H. Porosity of the epithelial basement membrane as an indicator of macrophage-enterocyte interaction in the intestinal mucosa. Arch Histol Cytol. 1999;62:471–481. doi: 10.1679/aohc.62.471. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Gonda T. Distribution of the pores of epithelial basement membrane in the rat small intestine. J Vet Med Sci. 2004;66:695–700. doi: 10.1292/jvms.66.695. [DOI] [PubMed] [Google Scholar]
- Toner PG, Ferguson A. Intraepithelial cells in the human intestinal mucosa. J Ultrastruct Res. 1971;34:329–344. doi: 10.1016/S0022-5320(71)80076-X. [DOI] [PubMed] [Google Scholar]
- Zhou HR, Islam Z, Pestka JJ. Induction of competing apoptotic and survival signaling pathways in the macrophage by the ribotoxic trichothecene deoxynivalenol. Toxicol Sci. 2005;87:113–122. doi: 10.1093/toxsci/kfi234. [DOI] [PubMed] [Google Scholar]


