Abstract
Dentin matrix metalloproteinases (MMPs) are involved in collagen degradation of resin-dentin interfaces. This study evaluated if collagen degradation can be prevented by chlorhexidine after different dentin demineralization procedures. Human dentin demineralization was performed with phosphoric acid (PA), EDTA, or acidic monomers (ClearfilSEBond and XENOV). Specimens were stored (24 h, 1 wk or 3 wk) in the presence or absence of chlorhexidine. In half of the groups, active MMP-2 was incorporated into the storing solution. C-terminal telopeptide determination (ICTP) was performed in the supernatants. Collagen degradation was higher in PA and EDTA-demineralized dentin. Chlorhexidine reduced collagen degradation in these groups only for 24 h. When dentin was demineralized with SEBond or Xeno, collagen degradation was reduced up to 30%, but addition of exogenous MMP-2 significantly increased collagen degradation. In self-etchant treated dentin the inhibitory effect of chlorhexidine on MMPs lasted up to 3 wk. Treating dentin with EDTA, PA or self-etching agents produces enough demineralization to permit cleavage of the exposed collagen. Monomers infiltration may exert protection on demineralized collagen, probably through immobilization of MMPs. The partial inhibitory action of CHX on MMP activity produced by self-etching adhesives was prolonged compared to the short-acting in PA or EDTA-treated dentin.
Keywords: Matrix metalloproteinases, dentin, collagen degradation, self-etching adhesives, chlorhexidine
INTRODUCTION
Matrix metalloproteinases (MMPs) are proteinases that participate in extracellular matrix (ECM) degradation (1). They constitute a large family of zinc- and calcium-dependent endopeptidases that participate in many physiologic and pathologic processes (2). The MMPs are secreted as proenzymes (zymogens) and are activated via step-wise mechanisms. They can be activated by proteinases or in vitro by chemical agents, such as thiol-modifying agents, oxidized glutathione, chaotropic agents, reactive oxygen species and heat treatment. Low pH and heat treatment can also lead to activation. These agents and procedures most likely work through the disruption of the cysteine-zinc binding (2).
Odontoblasts synthesize MMPs that participate in tooth development (3), dentin caries progression (4,5) and hybrid layer degradation in resin-dentin bonded restorations (6,7,8). Under physiological conditions, the activity of MMPs is precisely regulated at the level of transcription of the precursor zymogens via interaction with specific ECM components and inhibition by endogenous inhibitors (TIMPS) (2).
In vitro (9) and in vivo (6,10) collagen degradation occurs within incompletely resin-infiltrated hybrid layers in bonded dentin over time, has been reported and attributed to the action of host-derived MMPs (11). Controversy exists over the interaction of phosphoric acid with MMPs. It has been reported that phosphoric acid increased dentin MMP activity (7,12) while MAZZONI et al. (8) reported that phosphoric acid (PA) decreased dentin MMP activity under different experimental conditions. Self-etching adhesives have also been shown to increase (13,14,15) or not affect (12,16) MMPs activity.
The use of chlorhexidine digluconate (CHX) as a therapeutic primer (after PA-demineralization) to stabilize the adhesive interface over time has been previously shown (6,7). Even though, the relationship between the collagenolytic activity of dentin and the role of MMPs in hybrid layer degradation has been reported, the exact mechanism of CHX inhibition of MMPs require further clarification. Aqueous 2% CHX prevented decline in bond strength and even at lower concentrations (19,12). CHX is an amphiphilic molecule that binds to several proteins by a cation-chelating mechanism. CHX may inhibit MMPs catalytic activity by Zn2+ or Ca2+ binding (12). Consensus seems to exist about the use of CHX on demineralized human dentin to preserve the collagen matrix in hybrid layers by inhibiting activated MMPs (6,7,17). However, the effect of CHX in self-etching adhesive-treated dentin has not been determined.
This study tested the null hypotheses that collagenolytic activity of MMPs in dentin is not altered by different dentin etching procedures or chlorhexidine application.
MATERIALS AND METHODS
A human dentin beam degradation assay was performed. Ten extracted non-carious human third molars were obtained with informed consent from donors younger than 25 yr of age, under a protocol approved by the Institution review board. The teeth were stored in 0.01% (w/v) thymol solution at 4°C and used within one week after extraction. A dentin disk (0.75 mm ± 0.08 mm thick) was obtained from the mid-coronal portion of each tooth using a diamond saw under water cooling. Four dentin beams (0.75 mm × 0.75 mm × 5.0 mm) were obtained from each dentin disk as described by Carvalho et al. (18). Thus, 40 beams were available.
Eight dentin beams were included in each of five groups: 1) mineralized dentin, 2) demineralized beams created by 10% PA (pH 1.0) for 12 h at 25°C (PA-demineralized) (19); 3) demineralized beams created by 0.5 M EDTA (pH 7.4) (EDTA-treated) for 6 d at 25°C (19); 4) immersion of mineralized beams in Clearfil SE Bond primer (Kuraray Medical Inc., Tokyo, Japan) for 12 h (SE-treated) (20); and 5) immersion of mineralized beams in Xeno V (Dentsply DeTrey, Konstanz, Germany) for 12 h (XE-treated) (20). The demineralization process was performed at 25°C in the dark. After removing beams from demineralizing self-etchants, polymerization was performed. The samples were irradiated in different positions for 40 s until the entire area was exposed. The light was tested for light output (600 mW/cm2) by means of a Demetron radiometer (Model 100, Demetron Research Corp., Danbury, CT).
Dentin beams were rinsed in deionized water under constant stirring at 4°C for 72 h to remove PA, EDTA and the unpolymerized self-etching primer/adhesive comonomers. They were dried over anhydrous calcium sulphate for 8 h. The dry mass of each dentin beam was measured with a digital microbalance -accuracy: 0.01 mg- (Analytical Digital Microbalance Model HM-202, A&D Engineering Inc., San Jose, CA, USA). Specimens were rehydrated in 0.9% NaCl containing 10 U/ml of penicillin G and 300 μg/ml of streptomycin (pH 7.0) for 24 h.
Mineralized dentin (10 mg) or demineralized dentin (2 mg) (two dentin beams) were placed in Eppendorf tubes. Two incubation media were utilized: 1) artificial saliva (AS) containing 50 mM HEPES, 5 mM CaCl2.2H2O, 0.001 mM ZnCl2,150 mM NaCl and 100 U/ml penicillin, 1000 μg/mL streptomycin (pH 7.2); 2) 40 mM CHX in AS (pH 7.4). Active MMP-2 was added at a dilution of 1:10 in buffer solution (50 mM borate, 5 mM CaCl2, 20% glycerol, 0.005% Brij 35) to half of the specimens in each group (pH 7.5). The final concentration of active MMP-2 was 0.01 μg/μl. The sources of the chemicals used in this study are listed in Table 1.
Table 1.
Chemicals used in the experiment.
| Chemicals (batch number) | Manufacturer |
|---|---|
| 50% Ortho-Phosphoric acid. (0000124351) | Panreac Química, Barcelona, Spain |
| Ethylenediaminetetreaacetic acid disodium salt-2-hydrate. (44006RLS) | |
| Clearfil™ SE Primer: HEMA 10-30%; 10-Methacryloyloxydecyl dihydrogen phosphate; Hydrophilic aliphatic dimethacrylate; dl-Camphorquinone; Water; Accelerators; Dyes; others. (01352A) | Kuraray Medical Inc., Tokyo, Japan |
| Xeno V: Bifunctional acrylate; acidic acrylate; functionalized phosphoric acid ester; acrylic acid; water, terciary butanol; initiator; stabilizer; Acetone<50%; UDMA<15%; Dipentaeryhritol pentaacrylate phosphate <15%; DMA <10%; TMA <10%; Other DMA <10% (0812000831) | Dentsply DeTrey, Konstanz, Germany |
| 0.9% Sodium chloride (5211B1) | Braun Medical, Barcelona, Spain |
| 20 % Chlorhedixine digluconate (9418600021) | Guinma, Valencia, Spain |
| Anhydrous calcium sulphate, Drierite, 6 mesh (14796TJ) | Sigma Aldrich, St. Louis, MO, USA |
| Borate -di-Sodium tetra-borate anhydrous- (0000173320) | Panreac Química, Barcelona, Spain |
| Brij® (35 0000173320) | |
| Calcium chloride anhydrous (0000208286) | |
| Glycerol (0000200971) | |
| HEPES buffer, Sterile, pH 8.0 −1 M- (8D007674) | Applichem, Darmstadt, Germany |
| MMP-2 active, Recombinant CHO cells −0.1 mg/mL- (D00065439) | Calbiochem, Gibbstown, NJ,USA |
| Penicillin G potassium salt (1386789) | Sigma-Aldrich, St. Louis, MO, USA |
| Sodium chloride 2-hydrate powder (0000159010) | |
| Streptomycin sulphate salt (026K8901) | |
| Zinc chloride (0000144046) |
Dentin beam specimens were incubated in 500 μl of media at 37°C for 24 h, 1 wk or 3 wk. Supernatants of the media after each storage period were withdrawn after agitation and analyzed for the release of collagen degradation product (C-terminal telopeptide of type I collagen -ICTP-) using a radioimmunoassay kit (ICTP-RIA Cat. No. 68601, Orion Diagnostica Oy, FI-02101 Espoo, Finland). A standard curve was constructed with ICTP ranging from 0.01 to 250 μg/l. Each measurement was performed in triplicate. The amount of liberated ICTP from the PA-demineralized dentin in the absence of CHX and in the presence of active MMP-2 after 3 wk was set to 100%. Collagen degradation in the other groups was calculated in each single experiment as a percent of this value (21).
The ICTP concentration values were analyzed by ANOVA and Student-Newman-Keuls multiple comparison. Differences between storage times were analyzed by Friedman's and Wilcoxon pair-wise comparisons tests. Significance was considered at P<0.05
RESULTS
Mean ICTP values were affected by the conditioning media (F = 164.70; P < 0.001), dentin demineralization treatment (F = 382.23; P < 0.001) and by storage time (F = 125.34; P< 0.001). Interactions were also significant (P < 0.001). The power of the ANOVA was 0.90.
The total amount of ICTP liberated from the dentin beams is listed in Table 2 and the percentage of collagen degradation in the different incubation media and time points in the absence or presence of active MMP-2 are presented in Figs. 1, 2 and 3.
Table 2.
Mean and standard deviation of ICTP concentration (μg/l) in conditioning media after human dentin explants storage in the different experimental groups.
| Dentin treatment + medium / storage time | 24 h | 1 wk | 3 wk |
|---|---|---|---|
| Mineralized +AS | 0.87 (0.11) 1a A | 4.08 (1.06) 2a A | 4.04 (0.97) 2a A |
| Mineralized +AS+ CHX | 0.80 (0.05) 1a A | 2.34 (0.80)1,2b A | 3.78 (1.59) 2a A |
| Mineralized +MMP2+AS | 4.46 (0.77) 1b A | 6.74 (0.56) 1c A | 9.02 (1.63) 2b A |
| Mineralized +MMP2+AS+CHX | 2.33 (0.59) 1c A | 4.55 (0.73) 2a A | 8.01 (1.49) 3b A |
| PA-demineralized +AS | 70.01 (16.67) 1a B | 160.32(20.95)2a B | 178.23(22.51)2a B |
| PA-demineralized + AS+ CHX | 30.82 (8.29) 1b B | 203.12(19.00)2ab B | 200.56(16.55)2ab B |
| PA-demineralized +MMP2+AS | 210.12 (11.40) 1c B | 220.76 (16.46)1b B | 225.00(13.52)1c B |
| PA-demineralized+ MMP2+AS+CHX | 152.32 (27.32) 1d B | 195.34(21.07)2b B | 205.56(12.60) 2b B |
| EDTA-treated +AS | 80.46 (8.70) 1a B | 155.51 (7.12) 2a B | 160.34(16.32) 2a B |
| EDTA-treated +AS+ CHX | 16.76 (1.40) 1b C | 181.01(23.60) 2a C | 179.88 (14.57) 2a C |
| EDTA-treated+MMP2+AS | 212.78 (11.14) 1c B | 215.86 (6.24) 1c B | 220.43 (12.35) 1b B |
| EDTA-treated +MMP2+AS+CHX | 60.75 (6.03) 1d C | 212.21 (6.40) 2c B | 206.76 (10.90) 2b B |
| SE-treated+AS | 12.24 (1.11) 1a C | 41.65(2.73) 2a C | 49.97 (4.16) 3a C |
| SE-treated+AS+ CHX | 6.24 (0.33) 1b D | 25.33(4.40) 2b D | 30.38 (2.55) 2b D |
| SE-treated+MMP2+AS | 150.36 (11.21) 1c C | 152.78 (7.81) 1c D | 147.84 (9.40) 1c C |
| SE-treated+MMP2+AS+CHX | 45.05 (4.20) 1d D | 140.43(13.54) 2c C | 137.96(11.33) 2c C |
| XE-treated+AS | 27.96 (0.91) 1a D | 73.96 (4.75) 2a D | 69.32(6.55) 2a D |
| XE-treated+AS+ CHX | 6.64 (0.40) 1b D | 28.84 (3.07) 2b D | 32.33(2.14) 2b D |
| XE-treated+MMP2+AS | 155.67 (4.35) 1c C | 150.34(12.65) 1c D | 140.51(5.84) 1c C |
| XE-treated+MMP2+AS+CHX | 41.79 (2.33) 1d D | 165.75(15.36) 2c D | 161.67(10.21) 2c C |
AS: Artificial saliva, CHX: Chlorhexidine, MMP2: Metalloproteinase 2, PA: Phosphoric acid, EDTA: Ethylenediaminetetreaacetic acid, SE: Clearfil SE Bond primer, XE: Xeno V.
For each horizontal row: values with identical numbers indicate no significant difference using Friedman's and Wilcoxon pair-wise comparisons tests (p>0.05).
For each vertical column: values with identical low case letters indicate no significant difference between solutions within the same dentin treatment and values with identical upper case letters indicate no significant difference between treatments within the same solution, using Student-Newman-Keuls test (p<0.05).
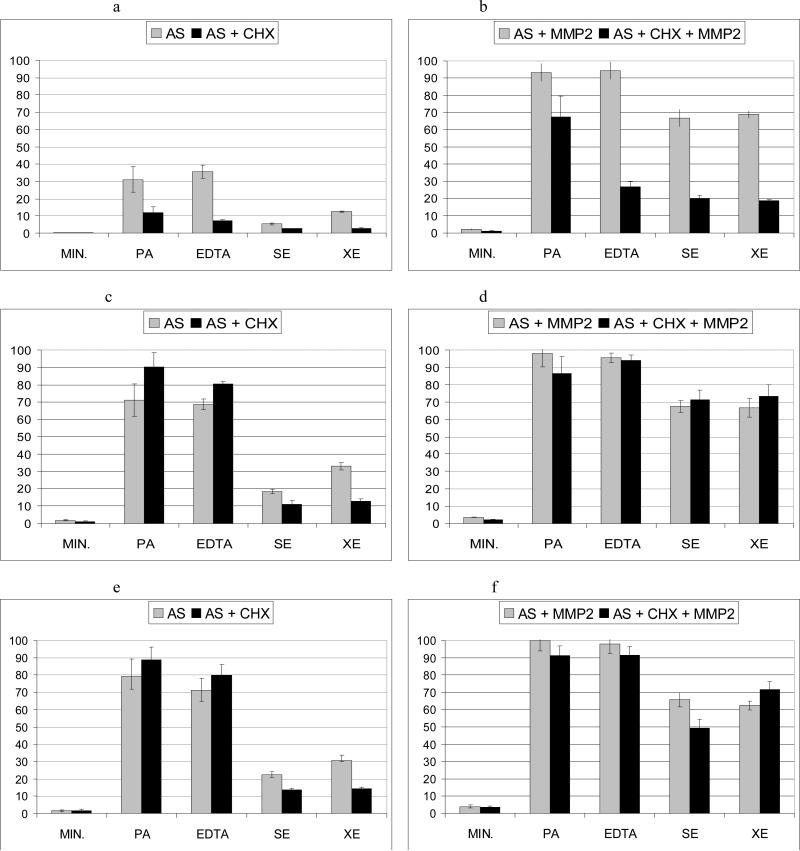
Figure 1.
Mean and SD of collagen degradation (%) of mineralized and treated human dentin explants after different storage times. Without (left), and with (right) addition of active MMP-2. 1a and 1b after 24 h; 1c and 1d after 1 wk; 1e and 1f after 3 wk.
AS: artificial saliva; MMP2: active metalloproteinase 2; CHX: digluconate chlorhexidine; MIN: mineralized dentin; PA: phosphoric acid demineralized dentin; EDTA: EDTA-demineralized dentin; SE: Clearfil SE Bond-treated dentin; XE: Xeno V-treated dentin.
The amount of ICTP liberated from the PA-demineralized dentin in the absence of chlorhexidine, and in the presence of active MMP-2 after 3 weeks was set to 100%, and the collagen degradation in the rest of the groups was calculated in each single experiment as a percent of this value.
Mineralized dentin beams released only negligible amounts of ICTP: 0.87 μg/L (24 h) to 4.04 μg/L (3 wk) that represented 0.4 to 1.8% of collagen degradation, respectively. When exogenous MMP-2 was added to the incubation media, the amount of ICTP liberated from the beams increased significantly (p<0.05) and ranged from 4.46 μg/L (24 h) to 9.02 μg/L (3 wk), which represented 1.9 to 4 % of collagen degradation.
The amount of collagen degradation was significantly higher in both PA- and EDTA-demineralized dentin (p < 0.001) compared to mineralized dentin without significant differences between them. The total amount of ICTP liberated after storage of PA-demineralized dentin in artificial saliva ranged from 70.01 μg/l (24 h) to 178.23 μg/l (3 wk), and corresponded to 31 and 79% of collagen degradation, respectively. For EDTA-demineralized dentin incubated in artificial saliva, the ICTP release values ranging from 80.46 μg/l (24 h) to 160.34 μg/l (3 wk) and correspond to 36% and 71% of collagen degradation, respectively. In both groups, the ICTP values increased between 24 h and 1 wk and then remained stable.
For PA-demineralized dentin, when exogenous active MMP-2 was added, ICTP values ranged from 210.12 μg/l - 93% collagen degradation after 24h to 225.44 μg/l - 100% collagen degradation after 3 wk. Similar values were obtained for EDTA-demineralized dentin. The addition of MMP-2 into the incubation media surrounding EDTA-demineralized dentin generated ICTP values from 212.78 μg/l (94% collagen degradation after 24 h) to 220.43 μg/l (97.7% collagen degradation after 3 wk). No significant differences were found in the amount of ICTP liberated from dentin between the three storage periods in these groups.
ICTP values for SE- and XE-treated dentin were significantly higher than those of mineralized dentin at the three study periods. The total amount of ICTP liberated after storage of SE-treated dentin in AS ranged from 12.24 μg/l (24 h) to 49.97 μg/l (3 wk), and corresponded to 5.4 and 22.2 % of collagen degradation, respectively. For XE-treated dentin 27.96 μg/l of ICTP at 24 h and 69.32 μg/l at 3 wk were obtained (12.4 and 30.8 % collagen degradation). These values did increase significantly after 1 wk of incubation, and remained stable after the 3 weeks storage period. However, SE- and XE-treated dentin beams liberated significantly lower amounts of ICTP than PA- and EDTA-demineralized dentin at 24 h of incubation in artificial saliva. When exogenous MMP-2 was added to the incubation media, ICTP values were augmented significantly (p<0.05), ranging from 150 μg/l (collagen degradation: 66.6%, 24 h) for SE-treated dentin, and 155 μg/l (collagen degradation: 68.8%, 24 h) for XE-treated dentin to 147.84 μg/l and 140.51 μg/l (3 wk), respectively; that is, 66% and 62% of collagen degradation. When exogenous MMP-2 was added, the amount of collagen degradation was similar during the 3 storage periods.
CHX digluconate significantly (p<0.05) reduced the percentage of collagen degradation in PA-demineralized dentin (up to 19% or 26% reduction in collagen degradation in the absence or presence of active MMP-2, respectively). Reduction of collagen degradation in the presence of chlorhexidine was also found in EDTA-treated dentin (28% without and 67% reduction in collagen degradation with exogenous MMP-2 addition). But, this inhibition was only observed at the 24 h evaluation period. For SE-treated and XE-treated dentin, CHX digluconate significantly reduced collagen degradation (collagen degradation was reduced in SE-treated dentin up to 2.7% or 47%; and in XE-treated dentin up to 9.5% or 50%, in the absence or presence of active MMP-2 respectively). The inhibitory effect of CHX was maintained at least up to 3 wk of storage (differences on collagen degradation between groups with or without CHX were 8.7% for SE-treated dentin and 16.4% for XE-treated dentin). However, when exogenous MMP-2 was added into the storing solution, the protective effect of CHX was only found at the 24 h evaluation period.
DISCUSSION
The ICTP is the carboxy-terminal telopeptide region of type I collagen which is liberated during degradation of mature type I collagen. The telopeptide is produced through the action of matrix metalloproteinases. Determination of ICTP is one of the most reliable techniques to quantify enzymatic activity on type I collagen (22,23). Assessment of other collagen fragments such as hydroxyproline (19) or even CTX-epitope of C-telopeptide may not be as sensitive or specific (24). It has been stated that among known collagenolytic proteinases relevant to hard tissue resorption, only MMPs can generate ICTP and, this release is inhibited by MMP-inhibitors but not by cysteine proteinase inhibitors. Thus, determination of ICTP is preferred to other less specific collagen fragments for detecting the activity of MMPs (24).
Dentin beams were employed as it is important to confirm the activity of MMP inhibitors against the native substrate and, where possible, in the presence of TIMPs (25). Active MMP-2 was added to half of the specimens in each group, because using dentin beams, only low concentrations of ICTP were liberated, reducing the sensitivity in identifying potentially less effective inhibitors. Active MMP addition may also help to overcome the problem of variations in MMP concentration in dentin beams derived from different donors.
In dentin substrates, MMP activity has been assessed by gelatin zymography (15,26), but profiling is limited. In the present study, monitoring substrate degradation was employed. The technique is only limited by the overlapping substrate specificity of metzincin subfamilies, so quantification of individual proteinases activity is not possible (22).
The results of this study do not support the null hypothesis that collagenolytic activity of MMPs in dentin is not altered by dentin etching or CHX application. The amount of collagen degradation obtained from the mineralized dentin beams (ca. 5%, even in the presence of exogenous active MMP-2) confirm that dentin collagen is not degraded if it remains in its mineralized state (22).
EDTA-demineralized dentin presented similar collagen degradation values than PA-demineralized dentin. Ultrastructural features of collagen in EDTA and PA demineralized dentin beams were found to be similar for both groups (19). As EDTA has been shown to be an effective Zn2+ and Ca2+ chelator (27), it was expected to inhibit MMP activity. However, EDTA (or Ca-EDTA) has to be present in excess, relative to Zn2+, in order to observe the chelating efficiency of EDTA (27). In our study, most traces of EDTA were removed from dentin by prolongued water rinsing, so no residual EDTA in EDTA-demineralized dentin beams was able to produce MMP inhibition. The similarity of the ICTP values in PA- and EDTA-treated dentin suggests there was no residual inhibition of EDTA. Further research has to be conducted to test MMP activity after the addition of excess EDTA to the storage media.
The proteolytic activity of MMPs in SE or XE-treated dentin was significantly lower than that of PA- and EDTA-demineralized dentin at 24 h (producing percent collagen degradation of 5.4%, 12.4%, 31% and 35.5% for SE- XE- and EDTA-treated, and PA-demineralized dentin respectively). These results are in agreement with DE MUNCK et al. (12) who found, by gelatin zymography, that no release/activation of MMP-2 and MMP-9 occurred after the application of self-etch adhesives to powdered dentin. Hydroxyethyl methacrylate (HEMA) was also shown to inhibit the activity of MMP-2 by zymography (16). The possible coordination of the hydroxyl group of HEMA with the bivalent cation (Zn2+) that is present in the catalytic domain of MMPs was suggested as main inhibitory mechanism (16,28). The results in the present study are consistent with recent findings in which adsorption of soluble MMPs to biomedical polymers (especially to resins such as polymethylmethacrylate and polyHEMA) resulted in reversible blocking of the MMPs (29,30). The durability of this adsorption/blocking mechanism remains to be determined. It is possible that the resins may molecularly immobilize the catalytic sites of MMPs in a manner that is analogous to molecular imprinting of enzyme-template complexes (31). The difference between resin immobilization of MMPs and true molecular imprinting is that the template (collagen, MMPs, etc.) is not removed. That is, the adhesive resin encapsulates the MMPs and occupies their catalytic sites.
In our study, ICTP concentration remained stable (up to 3 wk). SE and XE- treated dentin beams liberated significantly less ICTP than EDTA or PA-treated dentin groups. The same trend was observed after exogenous MMP-2 addition, even when ICTP values were higher. The results suggest the existence of a concentration-dependant blocking mechanism. DE MUNCK et al. (12) opined that the absence of MMP activity in the incubation medium in their study after mild self-etching blends application may be due to a minor decalcification effect of those adhesives on dentin. However, ICTP values significantly increased after addition of active MMPs, showing that collagen is sufficiently demineralized to be cleaved by active MMP-2.
CHX digluconate reduced collagen degradation in PA-etched dentin (up to 56% and up to 27.6% in the absence and presence of exogenous MMP-2, respectively). CHX digluconate has been identified as a zinc chelator (32,33). The inhibitory effect of CHX on dentin MMPs was previously shown (6,7,19), but our data demonstrate that this effect may be limited to a 24 h period in non-resin infiltrated PA and EDTA-treated dentin. These results are consistent with the recent findings that CHX pre-treatment of etch-&-rinse adhesive-bonded acid-etched dentin failed to prevent hybrid layer degradation after 9 months of initial inhibition of endogenous collagenolytic activities in dentin (34). CHX in solution may produce digluconate anions which may result in gradual precipitation in the presence of other mono- and divalent cations derived from body fluids (35). Moreover, as CHX only binds electrostatically to demineralized dentin collagen (36), it may slowly diffuse out of a collagen matrix via a competitive desorption mechanism in the presence of other cations.
The liberation of ICTP in XE and SE-treated dentin was inhibited by CHX digluconate at 24 h, and this inhibitory effect was maintained during the entire study period. CHX salts in solution bind to hydrophilic polymers; carboxyl groups from polyHEMA provide anionic binding sites for the cationic CHX molecules during their sorption from solution (37). These polymers may also be permeable to CHX (38,39). Polymers have been previously proposed as CHX delivery materials (40,41).
When active exogenous MMP-2 was added to the storage solution of SE- and XE-treated dentin beams in the presence of CHX, the observed inhibitory effect on collagen degradation was limited to 24 h. It has to be mentioned that active MMPs have to be diluted in Brij 35. Brij 35 is a detergent solution (nonionic polyoxyethylene surfactant) that is able to remove unreacted reagents from permeable solids, as it is usually employed to aid in reagent drainage. A clearance of chlorhexidine from monomers may have occurred. Detergents (42) and nonionic chemical cleaners are known to deactivate CHX (43).
Within the limits of this study, it may be concluded that the collagenolytic activity of dentin MMPs exist when dentin is demineralized. Collagen cleavage by MMPs is higher in EDTA or PA-treated dentin than in dentin treated by self-etching adhesives. The self-etching adhesives examined in the present study are able to produce enough decalcification in dentin to permit collagen cleavage by MMPs. The presence of hydrophilic monomers such as HEMA may have inhibited collagen degradation in the SE- and XE-treated groups, probably through adsorption of MMPs, and facilitate a prolonged inhibitory effect of CHX on MMPs within the demineralized dentin. Whether, this mechanism operates at resin-dentin bonded interfaces remains to be determined.
Acknowledgments
This work was supported by grants CICYT/FEDER MAT2008-02347, JA-P07-CTS2568 and JA-P08-CTS-3944 (PI Manuel Toledano), R21 DE019213-02 (PI. Franklin R. Tay) and R01 DE015306-06 (PI. David H. Pashley) from the National Institute of Dental and Craniofacial Research.
Footnotes
Authors have no financial affiliation or involvement with any commercial organization with direct financial interest in the materials discussed in this manuscript. Any other potential conflict of interest is disclosed (including patents, ownership, stock ownership, consultancies, speaker's fee, etc).
References
- 1.Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem. 1999;274:21491–94. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 2.Visse R, Nagasse H. Matrix metalloproteinase and tissue inhibitors of metalloproteinases: structure, function and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 3.Bourd-Boittin K, Fridman R, Fanchon S, Septier D, Goldberg M, Menashi S. Matrix metalloproteinase inhibition impairs the processing, formation and mineralization of dental tissues during mouse molar development. Exp Cell Res. 2005;304:493–505. doi: 10.1016/j.yexcr.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 4.Tjäderhane L, Larjava H, Sorsa T, Uitto VJ, Larmas M, Salo T. The activation and function of host matrix metalloproteinase in dentin matrix during breakdown in carious lesions. J Dent Res. 1998;77:1622–1629. doi: 10.1177/00220345980770081001. [DOI] [PubMed] [Google Scholar]
- 5.Chaussain-Miller C, Fioretti F, Goldberg M, Menashi S. The role of matrix metalloproteinases (MMPs) in human caries. J Dent Res. 2006;85:22–32. doi: 10.1177/154405910608500104. [DOI] [PubMed] [Google Scholar]
- 6.Hebling J, Pashley DH, TjÄderhane L, Tay FR. Chlorhexidine arrests subclinical degradation of dentin hybrid layers in vivo. J Dent Res. 2005;84:741–746. doi: 10.1177/154405910508400811. [DOI] [PubMed] [Google Scholar]
- 7.Breschi L, Mazzoni A, Nato F, Carrilho M, Visintini E, Tjäderhane L, Ruggeri A, Jr, Tay FR, Dorigo ES, Pashley DH. Chlorhexidine stabilizes the adhesive interface: a 2-year in vitro study. Dent Mater. 2010;26:320–325. doi: 10.1016/j.dental.2009.11.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazzoni A, Pashley DH, Nishitani Y, Breschi L, Manello F, Tjäderhane L, Toledano M, Pashley EL, Tay FR. Reactivation of inactivated endogenous proteolytic activities in phosphoric acid-etched dentine by etch-and-rinse adhesives. Biomater. 2006;27:4470–4476. doi: 10.1016/j.biomaterials.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto M, Ohno H, Sano H, Kaga M, Oguchi H. In vitro degradation of resin dentin bonds analyzed by microtensile bond test, scanning and transmission electron microscopy. Biomater. 2003;24:3795–3803. doi: 10.1016/s0142-9612(03)00262-x. [DOI] [PubMed] [Google Scholar]
- 10.Koshiro K, Inoue S, Sano H, De Munck J, Van Meerbeek B. In vivo degradation of resin-dentin bonds produced by a self-etch and an etch-and-rinse adhesive. Eur J Oral Sci. 2005;113:341–348. doi: 10.1111/j.1600-0722.2005.00222.x. [DOI] [PubMed] [Google Scholar]
- 11.Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, Ito S. Collagen degradation by host-derived enzymes during aging. J Dent Res. 2004;83:216–221. doi: 10.1177/154405910408300306. [DOI] [PubMed] [Google Scholar]
- 12.De Munck J, Van Den Steen PE, Mine A, Van Landuyt KL, Poitevin A, Opdenakker G, Van Meerbeek B. Inhibition of enzymatic degradation of adhesive-dentin interfaces. J Dent Res. 2009;88:1101–1106. doi: 10.1177/0022034509346952. [DOI] [PubMed] [Google Scholar]
- 13.Tay FR, Pashley DH, Loushine RJ, Weller RN, Monticelli F, Osorio R. Self-etching adhesives increase collagenolytic activity in radicular dentin. J Endod. 2006;32:862–868. doi: 10.1016/j.joen.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Nishitani Y, Yoshiyama M, Wadgaonkara B, Breschi L, Mannelo F, Mazzoni A, Carvalho RM, Tjäderhane L, Tay FR, Pashley DH. Activation of gelatinolytic/collagenolytic activity in dentin by self-etching adhesives. Eur J Oral Sci. 2006;114:160–166. doi: 10.1111/j.1600-0722.2006.00342.x. [DOI] [PubMed] [Google Scholar]
- 15.Lehmann N, Debret R, Romeas A, Magloire H, Degrange M, Bleicher F, Sommer P, Seux D. Self-etching increases matrix metalloproteinase expression in the dentin-pulp complex. J Dent Res. 2009;88:77–82. doi: 10.1177/0022034508327925. [DOI] [PubMed] [Google Scholar]
- 16.Carvalho RV, Ogliari FA, Souza AP, Silva AF, Petzhold CL, Line SR, Piva E, Etges A. 2-hydroxyethyl methacrylate as an inhibitor of matrix metalloproteinase-2. Eur J Oral Sci. 2009;117:64–67. doi: 10.1111/j.1600-0722.2008.00591.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhou J, Tan J, Chen L, Li D, Tan Y. The incorporation of chlorhexidine in a two-step self-etching adhesive preserves dentin bond in vitro. J Dent. 2009;37:807–812. doi: 10.1016/j.jdent.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 18.Carvalho RM, Yoshiyama M, Pashley EL, Pashley DH. In vitro study on the dimensional changes of human dentine after demineralization. Arch Oral Biol. 1996;41:369–377. doi: 10.1016/0003-9969(95)00115-8. [DOI] [PubMed] [Google Scholar]
- 19.Carrilho MR, Tay FR, Donnelly AM, Agee KA, Tjäderhane L, Mazzoni A, Breschi L, Foulger S, Pashley DH. Host-derived loss of dentin matrix stiffness associated with solubilization of collagen. J Biomed Mater Res B Appl Biomater. 2009;90:373–380. doi: 10.1002/jbm.b.31295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oliveira SS, Marshall SJ, Habelitz S, Gansky SA, Wilson RS, Marshall GW., Jr. The effect of a self-etching primer on the continuous demineralization of dentin. Eur J Oral Sci. 2004;112:376–383. doi: 10.1111/j.1600-0722.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 21.Piecha D, Weik J, Kheil H, Becher G, Timmermann A, Jaworski A, Burger M, Hofmann MW. Novel selective MMP-13 inhibitors reduce collagen degradation in bovine articular and human osteoarthritis cartilage explants. Inflamm Res. 2010;59:379–389. doi: 10.1007/s00011-009-0112-9. [DOI] [PubMed] [Google Scholar]
- 22.Klein T, Geurink PP, Overkleeft S, Kauffman HK, Bischoff R. Functional proteomics of zinc-dependent metalloproteinases using inhibitors probes. Chem Med Chem. 2009;4:164–170. doi: 10.1002/cmdc.200800284. [DOI] [PubMed] [Google Scholar]
- 23.Okabe R, Nakatsuka K, Inaba M, Miki M, Naka H, Masaki H, Moriguchi A, Nishizawa Y. Clinical evaluation of the Elecsys β-CrossLaps serum assay, a new assay for degradation products of type I Collagen C-telopeptides. Clin Chem. 2001;47:1410–1414. [PubMed] [Google Scholar]
- 24.Garnero P, Ferreras M, Karsdal MA, Nicamhlaoibh R, Risteli J, Borel O, Quist P, Delmas PD, Foged NT, Delaissé JM. The type I collagen fragments ICTP and CTX reveal distinct enzymatic pathways of bone collagen degradation. J Bone Miner Res. 2003;18:859–67. doi: 10.1359/jbmr.2003.18.5.859. [DOI] [PubMed] [Google Scholar]
- 25.Monovich LG, Tommasi RA, Fujimoto RA, Blancuzzi V, Clark K, Cornell WD, Doti R, Doughty J, Fang J, Farley D, Fitt J, Ganu V, Goldberg R, Goldstein R, Lavoie S, Kulathila R, Macchia W, Parker DT, Melton R, O'byme E, Pastor G, Pellas T, Quadros E, Reel N, Roland DM, Sakane Y, Singh H, Skiles J, Somers J, Toscano K, Wigg A, Zhou S, Zhu L, Shieh WC, Xue S, Mcquire LW. Discovery of potent, selective, and orally active carboxylic acid based inhibitors of matrix metalloproteinase-13. J Med Chem. 2009;52:3523–3538. doi: 10.1021/jm801394m. [DOI] [PubMed] [Google Scholar]
- 26.Mazzoni A, Mannello F, Tay FR, Tonti GA, Papa S, Mazzotti G, Di Lenarda R, Pashley DH, Breschi L. Zymographic analysis and characterization of MMP-2 and -9 forms in human sound dentin. J Dent Res. 2007;86:436–440. doi: 10.1177/154405910708600509. [DOI] [PubMed] [Google Scholar]
- 27.Azuma T, Kondo T, Ikeda S, Imai H, Yamada M. Effects of EDTA saturated with Ca2+ (Ca-EDTA) on pig, bovine and mouse oocytes at the germinal vesicle stage during maturation culture and the involvement of chelation of Zn2+ in pronuclear formation induction by Ca-EDTA. Reproduction. 2002;124:235–240. [PubMed] [Google Scholar]
- 28.Mccall KA, Huang C, Fierke CA. Function and mechanism of zinc metalloenzymes. J Nutr. 2000;130(Suppl):1437–1446. doi: 10.1093/jn/130.5.1437S. [DOI] [PubMed] [Google Scholar]
- 29.Renò F, Traina V, Cannas M. Adsorption of matrix metalloproteinases on biomedical polymers: a new aspect in biological acceptance. J Biomater Sci Polymer Edn. 2008;19:19–29. doi: 10.1163/156856208783227631. [DOI] [PubMed] [Google Scholar]
- 30.Skarja GA, Brown AL, Ho RK, May MH, Sefton MV. The effect of a hydroxamic acid-containing polymer on active matrix metalloproteinases. Biomater. 2009;30:1890–1897. doi: 10.1016/j.biomaterials.2008.12.040. [DOI] [PubMed] [Google Scholar]
- 31.Jiang X, Jiang N, Zhang H, Liu M. Small organic molecular imprinted materials: their preparation and application. Anal Bioanal Chem. 2007;389:355–368. doi: 10.1007/s00216-007-1336-6. [DOI] [PubMed] [Google Scholar]
- 32.Tallant C, Marrero A, Gomis-Rüth FX. Matrix metalloproteinases: Fold and function of their catalytic domains. Biochim Biophys Acta. 2010;1803:20–28. doi: 10.1016/j.bbamcr.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Mohammadi Z, Abbott PV. The properties and applications of chlorhexidine in endodontics. Int Endod J. 2009;42:288–302. doi: 10.1111/j.1365-2591.2008.01540.x. [DOI] [PubMed] [Google Scholar]
- 34.Sadek FT, Braga RR, Muench A, Liu Y, Pashley DH, Tay FR. Ethanol wet bonding challenges current anti-degradation strategy. J Dent Res. 2010:89. doi: 10.1177/0022034510385240. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zerella J, Fouad A, Spångberg L. Effectiveness of a calcium hydroxide and chlorhexidine digluconate mixture as disinfectant during retreatment of failed endodontic cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:756–761. doi: 10.1016/j.tripleo.2005.05.072. [DOI] [PubMed] [Google Scholar]
- 36.Kim J, Uchiyama T, Carrilho M, Agee KA, Mazzoni A, Breschi L, Carvalho RM, Tjäderhane L, Looney S, Wimmer C, Tezvergil-Mutluay A, Tay FR. Chlorhexidine binding to mineralized versus demineralized dentin powder. Dent Mater. 2010;26:771–778. doi: 10.1016/j.dental.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plaut BS, Meakin BJ, Davies DJ, Richardson NE. The mechanism of interaction between chlorhexidine digluconate and poly(2-hydroxyethylmethacrylate). J Pharm Pharmacol. 1981;33:82–88. doi: 10.1111/j.2042-7158.1981.tb13716.x. [DOI] [PubMed] [Google Scholar]
- 38.Plaut BS, Meakin BJ, Davies DJ. The influence of various counter ions on the interaction of chlorhexidine with the hydrophilic contact lens polymer poly(2-hydroxyethylmethacrylate). J Pharm Pharmacol. 1980;32:453–459. doi: 10.1111/j.2042-7158.1980.tb12968.x. [DOI] [PubMed] [Google Scholar]
- 39.Plaut BS, Meakin BJ, Davies DJ. On the anomalous sorption behaviour of chlorhexidine with poly(2-hydroxyethylmethacrylate). J Pharm Pharmacol. 1980;32:525–532. doi: 10.1111/j.2042-7158.1980.tb12989.x. [DOI] [PubMed] [Google Scholar]
- 40.Yue IC, Poff J, Cortés ME, Sinisterra RD, Faris CB, Hildgen P, Langer R, Shastri VP. A novel polymeric chlorhexidine delivery device for the treatment of periodontal disease. Biomater. 2004;25:3743–3750. doi: 10.1016/j.biomaterials.2003.09.113. [DOI] [PubMed] [Google Scholar]
- 41.Hiraishi N, Yiu CKY, King NM, Tay FR. Chlorhexidine release and antibacterial properties of chlorhexidine-incorporated polymethyl methacrylate-based resin cement. J Biomed Mater Res Part B: Appl Biomater. 2010;94B:134–140. doi: 10.1002/jbm.b.31633. [DOI] [PubMed] [Google Scholar]
- 42.Kaiser N, Klein D, Karanja P, Greten Z, Newman J. Inactivation of chlorhexidine gluconate on skin by incompatible alcohol hand sanitizing gels. Am J Infect Control. 2009;37:569–573. doi: 10.1016/j.ajic.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 43.Szögyi M, Cserhäti T, Lelkes L. Differential Scaning calorimetry to study the possible ternary complex formation between chlorhexidine, phosphatidylcholine and some nonionic tenzides. J Biochem Biophys Methods. 1991;23:31–43. doi: 10.1016/0165-022x(91)90048-2. [DOI] [PubMed] [Google Scholar]