Abstract
With the advent of thrombolytic therapy and angioplasty, it has become possible to reduce myocardial infarct size through early reperfusion. Enormous effort has been expended to find therapies that can further reduce infarct size after early intervention. Animal studies have identified many cardioprotective pathways that have the potential to reduce infarct size if activated before the onset of ischemia. More recently interventions effective at the onset of reperfusion have been described. Although basic research has identified many targets, most has been conducted in rodent models which may not be directly applicable to human disease and even promising agents have been disappointing in large scale clinical trials. There are many potential explanations for this failure which is the subject of this review. Potential factors include (1) the variability inherent in the patient population whereas animal studies usually use single gender homogeneous groups maintained on standard diets in carefully controlled environments; (2) the duration of ischemia is generally shorter in animal studies, resulting in potentially more salvageable myocardium than is often the case in patients; (3) that the animals are usually young without co-morbidities whereas the patient population is generally older and has significant co-morbidities; (4) animals are not treated with medications a priori whereas the patient population is often taking medications that may affect ischemic injury; and (5) animal studies may not involve thorough assessment of effects on organs other than the heart whereas patients can experience adverse effects of treatment in other organs that can preclude clinical use.
Keywords: reperfusion injury, myocardial infarction, clinical tria
INTRODUCTION
Despite continued advances against heart disease over the past 50 years, myocardial infarction remains a leading cause of death in the United States into the 21st century. Major advances in our understanding of the process of myocardial infarction in the past 50 years include the recognition that myocyte cell death occurs in a wavefront progression, proceeding from the inner to the outer regions of the ventricle and that timely reintroduction of arterial blood flow can salvage myocardium. These observations, which were initially made in the research laboratory under carefully controlled conditions, provide the theoretical framework for reperfusion therapy. The development of reperfusion therapy and specialized coronary care units has led to a marked increase in survival and corresponding reductions in morbidity and mortality over the past 25 years. Additionally, there has been considerable research on strategies to reduce the amount of myocardium that dies during a period of ischemia, referred to as cardioprotection. Although there was initially considerable controversy concerning whether it was possible to protect the heart from ischemic injury, the description of the cardioprotective intervention called ischemic preconditioning (PC) by Murry and colleagues in 1986 1, and the subsequent confirmation of this phenomenon and the widespread applicability of the phenomenon, with different protocols and in different species, resolved the issue of whether it was possible to protect the myocardium from ischemic injury. Protection using PC was accomplished by brief intermittent episodes of ischemia and reperfusion, and this resulted in a substantial reduction in the amount of cell death resulting from a subsequent sustained period of myocardial ischemia. The discovery of this phenomenon stimulated a surge in interest in membrane receptors and/or potential subcellular signaling pathways that could potentially reveal the mechanism of IP and thereby facilitate translation of the protective mechanisms into the clinical arena. In 2003, Zhao and colleagues described another cardioprotective effect called post conditioning (PostC)2. Although both interventions include a short series of brief episodes of ischemia and reperfusion, PostC differs from IP in that it is instituted at the time of arterial reperfusion; i.e. after a sustained episode of coronary occlusion. Description of this intervention rekindled interest in finding new ways and/or new pharmacologic agents to protect myocardium from cell death much later in the course of an evolving myocardial infarct (i.e. after ischemia). The goal of this brief review is to discuss the biology of myocardial reperfusion as well the mechanisms of reperfusion injury and to discuss the obstacles and potential barriers that have limited the clinical application of reperfusion-based therapy.
Importance of the duration and the severity of ischemia in irreversible myocardial injury
Seminal studies by the Jennings group in the 1960’s highlighted the importance of the duration of the ischemic insult in determining the outcome of an episode of ischemia followed by reperfusion3. Using a canine model of regional myocardial ischemia/reperfusion (IR), they showed that left ventricular myocardium could sustain up to 15 minutes of coronary occlusion followed by reperfusion with no cell death and no permanent changes in myocyte ultrastructure. This was defined as “reversible injury”. However, when the duration of the ischemic episode was extended past 15 and up to 60 minutes, restoration of arterial blood flow was associated with a series of catastrophic changes in the ultrastructure of subendocardial myocytes that were documented by electron microscopy; marked cellular swelling, myofilament hypercontracture, large areas of membrane disruption, and calcification of the mitochondria. These changes were associated with cell death and were defined as “irreversible injury” since restoration of blood flow did not result in salvage of the tissue but rather accelerated cellular disintegration and irreversible changes in ultrastructure 4. However, this study did not assess the severity of the myocardial ischemia present in the affected area. Large animal species, including humans, have variable amounts of collateral blood flow (CBF) which can be defined as blood flow occurring through established anastomoses from vessels from adjacent vascular territories; in the case of the heart, adjacent coronary vessels. The question that needed to be answered was the quantitative relationship between the level of blood flow and the development of the irreversible ultrastructural changes and cell death. A subsequent study by the Jennings group showed that cell death was associated with severe myocardial ischemia defined as arterial collateral blood flow into the ischemic bed of less than 0.09 ml/min/gm wet weight (control tissue blood flow=1−2 ml/min/g wet wgt). If flow was greater than 0.1 ml/min/gwt, the kinetics of cell death would be slower than if flow was <0.09 ml/min/gwt. Furthermore, if flow was greater than approximately 0.25 ml/min/g wgt, there might not be any cell death in the affected region 5.
A key study in 1979 by Reimer et al showed that ischemia is present in a transmural gradient (with lowest flow in the endocardium) during total coronary artery occlusion and as a consequence, myocardial necrosis proceeds in a “wavefront” fashion starting from the subendocardium and progressing to the subepicardium as the duration of occlusion increases 6. Other factors may also slow the rate of cell death in the subepicardium, but the most important factor was the level of blood flow, and this led to the concept that infarct size needed to be correlated with the baseline predictors of infarct size, the size of the area at risk, and the level of collateral flow to determine whether or not an intervention was protective. In addition to establishing the time course of cell death and the importance of area at risk and collateral blood flow as baseline predictors of infarct size, these studies provided definitive proof that ischemic myocytes in the distribution of the affected coronary artery could be rescued by restoring arterial blood flow; this provides the experimental basis for today’s standard of care in the treatment of acute myocardial infarction.
Reperfusion injury
With the implementation of early restoration of arterial blood flow, a controversial new type of injury was described in animal studies called “reperfusion injury”. This type of injury was thought to be entirely due to the re-introduction of oxygen and blood to myocardium in the area at risk and resulted in cell death above and beyond that consequent to the preceding ischemic episode. This description stimulated a series of new investigations to determine whether agents and/or interventions administered at the time of reperfusion could increase the amount of viable myocardium above and beyond that due to restoration of arterial flow and/or interruption of the “wavefront progression” of cell death. However, for reperfusion therapy to be clinically relevant there must be a significant amount of ischemic myocardium at risk of dying but not yet dead, i.e., the heart must contain a non-completed infarct. As a result of implementation of early reperfusion to clinical practice, peak biomarker release as well as the mortality of ST segment elevation myocardial infarction (STEMI) has steadily decreased over the past 10–12 years [30 day mortality rates declined to from 10.5% to 7.8%] 7 However, the achievement of this remarkable success raises a concern; i.e. is further reduction of overall infarct size achievable and if so, will the reduction result in a measurable effect on morbidity and mortality? The answer to this important question is unclear. In addition to the amount of salvageable myocardium available, the timing of any reperfusion therapy is critical. Based upon what is known about the mechanisms of PC and PostC, to be effective, successful therapy must be instituted within 5–10 minutes of reperfusion and therefore currently only treatment during angioplasty-related opening of the infarct vessel or the perioperative period are viable options for clinically useful intervention. If the therapeutic window could be extended into the range of hours, the potential impact on morbidity and mortality could be substantially increased.
Types of reperfusion injury
Reperfusion injury is a broad term that has been applied to both reversible and lethal forms of myocyte injury. There is virtually no disagreement that reversible reperfusion injury occurs upon arterial reperfusion of ischemic tissue. The classic example of reversible reperfusion injury is myocardial stunning, which was originally described in canine hearts by Heyndrickx et al. in 1975 8. Myocardial stunning is defined as a reversible deficit in myocardial function upon reperfusion that resolves over an extended period of time (days) assuming no additional ischemic insult(s). Subsequent experimental studies by Bolli and colleagues provided evidence that stunning is due to the generation of a large burst of oxygen-derived free radicals at the onset of reperfusion 9. This was confirmed by showing that treatment with free radical scavenging agents before or at the time of reperfusion abolished the functional deficit. The mechanism of stunning is complex and thought to involve alteration(s) in the excitation-contraction efficiency and/or the myocyte contractile apparatus. It is important to note that no significant amount of cell death is associated with stunning. This form of reversible injury can be clinically relevant when a post-reperfusion functional deficit is present shortly after flow restoration in an infarct-related vessel.
The other, more potentially important, form of reperfusion injury has been called lethal reperfusion injury. This occurs when the infarct-related artery is acutely opened and the associated abrupt restoration of arterial blood flow causes additional cell death above and beyond that predicted to result from the preceding period of severe ischemia; i.e. lethal injury to otherwise viable myocytes. An alternative view would be that there are myocytes at the end of ischemia which are intact but fragile and survive or not depending on the conditions of reperfusion. Death might ensue if arterial blood is reintroduced without any protection of these fragile myocytes but they might survive if they are shielded from the effects of rapid reoxygenation and pH normalization. Traditionally, (as discussed above) infarct size is thought to be determined both by the length and severity of the preceding ischemic period. Reperfusion was thought to expose and/or accelerate irreversible metabolic and/or structural defects in ischemic myocytes but was not thought to cause additional cell death to uninjured myocytes. According to this view, lethal reperfusion injury could more accurately be called “reperfusion exposed injury”. If true lethal reperfusion injury to viable myocytes exists and is important in human infarct therapy, then modifying the conditions of reperfusion theoretically should be able to reverse or abolish the resulting cell death ultimately resulting in smaller infarcts, fewer adverse complications, and decreased morbidity and mortality. The most convincing evidence for the existence of lethal reperfusion injury comes from the experimental phenomenon PostC2. PostC involves the use of several brief episodes of ischemia/reperfusion following a long period of severe ischemia aimed at providing “controlled reperfusion” rather than the abrupt reintroduction of arterial blood typically associated with infarct vessel therapy. The mechanism of PostC is still under active investigation but current hypotheses implicate delayed pH recovery 10 and activation of reperfusion induced survival kinase (RISK) signaling pathways resulting in inhibition or reduction of opening of the mitochondrial permeability transition pore (mPTP)11. However, other experimental studies have not shown significant protection and in the process have demonstrated that PostC protection can be very model and protocol dependent12.
Potential mechanisms of lethal reperfusion injury
Over the years, a large number of experimental studies have investigated the potential mechanism(s) of lethal reperfusion injury. Knowing the mechanism(s) of lethal reperfusion injury is important to determine potential targets of effective reperfusion therapy. Although reperfusion of ischemic tissue is a complex process involving many cellular and extracellular processes, there are four major pathways that are at the hub of reperfusion-induced injury and each will be discussed in more detail in the following section.
Role of pH
As a consequence of acid generation by anaerobic glycolysis, poor washout of metabolic end products, and the loss of ionic pump activity secondary to high energy phosphate depletion (ATP), ischemic myocytes become overloaded with Na+, H+, and Ca2+ prior to reperfusion. In addition, due to degradation of energy substrates, the osmolar load of the ischemic myocytes is increased through accumulation of metabolites; primarily lactate and phosphate. The reintroduction of oxygenated, substrate containing blood results in several rapid shifts in ionic equilibrium. The intracellular acidotic pH is rapidly corrected through activity of the Na+/H+ exchanger and the Na+/HCO3− symporter resulting in a further increase in intracellular Na+ concentration. The resulting Na+ overload results in a sudden increase in intracellular Ca2+ primarily through reverse function of the Na+/Ca2+ exchanger. Large, uncontrolled increases in intracellular Ca2+ have several potentially catastrophic consequences for the cell. Interestingly, the Ca2+ shifts and resulting intracellular overload are relatively inhibited by high hydrogen ion concentrations [H+] 13 and therefore maintenance of a relatively low extracellular pH and/or prolongation of the recovery time to neutral pH (7.4) may be cytoprotective by protecting the cell from sudden large increases in calcium. Indeed, the protective effect of the “pH paradox” as well as ischemic postconditioning has been proposed to operate at least partially through damping pH recovery thereby slowing ion shifts during early reperfusion14. Therefore, interventions which delay the normally rapid recovery of extracellular pH from the acidotic conditions associated with ischemia toward neutral values may have a significant effect in limiting lethal reperfusion injury in the setting of acute myocardial infarction.
Role of calcium
A sudden increase in intracellular calcium has at least three important and potentially devastating consequences for the ischemic and post-ischemic myocyte. One important consequence is activation of calpains and/or other degradative enzymes that may weaken the cytoskeletal framework of the myocytes thereby rendering the cell membrane more susceptible to physical disruption 15–17 Several proteins have been shown to be hydrolyzed and/or damaged during ischemia or under conditions of severe anoxia including vinculin, α-actinin, α-fodrin, and ankyrin 16, 18. However, the smaller increases in calcium that occur during ischemia as well as the larger fluxes associated with reperfusion can both result in the same increase in myocyte fragility; the relative contribution of each is not clear. A second important consequence of increased calcium influx is the activation of the contractile machinery and the resulting myocyte hypercontracture. Hypercontracture occurs early after the onset of reperfusion and is the consequence of the resumption of high-energy phosphate generation (ATP) by mitochondria in the presence of normal or elevated calcium levels19. The production of hypercontracture and the associated physical stress as an important cause of cell death was first shown by Ganote et al. 20 and later confirmed by later studies that showed inhibition of hypercontracture was associated with cardioprotection 21. A third important consequence of increased intracellular calcium fluxes is the direct effects of increased calcium on mitochondrial function and membrane permeability. Increased calcium fluxes in the presence of HEP and/or mitochondrial repolarization results in increased mitochondrial calcium uptake, increased mitochondrial membrane permeability, and severe mitochondrial swelling. The abnormal membrane permeability is thought to occur primarily through a protein channel termed the mitochondrial permeability transition pore (mPTP). These channels are more likely to remain open during the conditions associated with reperfusion such as increased calcium fluxes, increased ROS, and increases in inorganic phosphate concentration10, 22. The ultimate result of increased/uncontrolled mPTP opening is a collapse of the inner mitochondrial membrane potential and uncoupling of the respiratory chain, and ultimately mitochondrial demise. In pathways proposing a critical role for the mPTP in lethal reperfusion injury, activation of protective signaling pathways leads to inhibition of mPTP opening and a resulting decrease in infarct size. Finally, a byproduct of mitochondrial swelling is the release of the enzyme cytochrome c into the cytoplasm which is a potent activator of the internal (i.e. mitochondrial) apoptotic pathway which has been described associated with reperfusion injury.
Role of cell swelling
During severe ischemia, metabolism shifts from oxygen and fatty acid-dependent Kreb’s cycle-based aerobic metabolism to glycogen-dependent anaerobic glycolysis. The capacity of anaerobic metabolism is limited and cannot maintain a stable level of ATP in the ischemic tissue. In addition, the switch also results in the generation of large amounts of lactate as the endproduct of glycolysis. The increased lactate accumulates in the intracellular and extracellular spaces and results in a relative hyper-osmotic condition in the ischemic territory. At reperfusion, there is an osmotic gradient between the blood and the hyper-osmotic ischemic bed which results in varying degrees of cell swelling. Cell swelling has been shown to induce a substantial physical stress resulting in cell death in anoxic myocardium, even in the absence of hypercontracture and/or resumption of HEP production 16. Therefore, acidotic reperfusion designed to prevent rapid restitution of normal pH and/or ischemic postconditioning may reduce or limit lethal reperfusion injury at least partially through reduction of the consequent cell swelling.
Role of oxidative stress
Reactive oxygen species (ROS) including superoxide anion (O2−), hydrogen peroxide (H2O2), and hydroxyl radical (OH·) are generated during early reperfusion, along with other radical species including nitric oxide (NO). Some level of ROS result from the normal byproduct of cellular metabolism and therefore ROS likely are involved in normal cellular signaling and housekeeping activities. Interestingly, generation of small amounts of ROS may be important in the cardioprotection associated with ischemic preconditioning, presumably through activation of protective signaling pathways. Normal cells, including myocytes, contain defense mechanisms (e.g. catalase, glutathione peroxidase, superoxide dismutase), which under homeostatic conditions are capable of defending critical organelles and proteins against the potential toxic effects of ROS. However, upon reperfusion and the associated reintroduction of oxygen there is a large burst in ROS activity that exceeds the defensive capacity of the cell and results in large concentrations of ROS which are capable of causing widespread damage. Potential targets of ROS-mediated damage include the calcium-handling machinery (sarcoplasmic reticulum (SR), ionic pumps) resulting in calcium overload, protein oxidation leading to cross-linking and eventual breakdown of critical proteins (structural and/or contractile), lipid peroxidation leading to disruption of cholesterol-containing membranes, opening of the mPTP, and activation of apoptotic pathways 23, 24. Oxidative stress may also influence potentially important, but less clearly defined mechanisms such as the recruitment of neutrophils and/or neutrophil activation in microvascular plugging and/or the no-reflow effect, the generation of peroxynitrite (ONOO−) in lipid peroxidation, and ROS-mediated disruption of cellular signaling pathways 25.
Causes of lethal reperfusion injury from experimental animal studies
Early studies focused on the role of oxidative stress and/or neutrophils in the genesis of lethal reperfusion injury. Several early studies showed positive effects (i.e. cardioprotection) of extracellular free radical scavengers, neutrophil depletion/inhibition, and other anti-oxidant-directed therapies administered either prior to or during reperfusion26–30. However, negative studies were soon published that challenged the cardioprotective efficacy by raising questions regarding the stability of extracellular free radical scavengers, the dose and timing of drug delivery, and the ability of scavengers to gain access to the intracellular space 31, 32. Later investigators used cell permeable scavengers and/or inhibitors to overcome some of the delivery and targeting issues, which generated conflicting results33, 34. As a result of these positive experimental studies, some interventions such as intravenous adenosine therapy at reperfusion reached the clinical trial stage. However, the issue is clouded by the finding that apparent reductions in infarct size with free radical scavengers and cell permeant antioxidants can be artifactual due to delayed disintegration of dead tissue33, 35.
As discussed above, one important aspect of reperfusion injury is the associated increase in intracellular Ca2+. Because early studies of myocardial infarction identified Ca2+ overload as an important component of cell death, early animal studies attempted to reduce Ca2+ overload by the use of L-type calcium channel blockers such as verapamil and nifedipine and/or more cardioselective compounds36–38. Although effective in reducing infarct size in large animal models, the hemodynamic and negative inotropic side effects of these agents precluded successful translation into the clinical arena. One of the primary causes of the increase in calcium is the consequence of reverse mode Na+/Ca2+ exchange (NCX) secondary to the rise in intracellular Na+ concentration. Because the rise in Na+ concentration is the consequence of increased proton [H+] generation during ischemia, inhibitors of Na+/H+ exchange (NHE) have been intensively investigated as potential protective agents. Several animal studies using either, specific pharmacologic inhibitors of NHE or genetically modified mice showed a reduction in the rise of Na+ and Ca2+and a reduction in ischemia/reperfusion injury39–41. Inhibition of NCX has also been shown to be protective against ischemia/reperfusion injury in experimental studies42, 43
More recently, reperfusion injury survival kinases (RISK) have been proposed as playing an important role in lethal reperfusion injury. These kinases are thought to be activated during reperfusion leading to a reduction in lethal reperfusion injury and therefore myocardial infarct size. They were originally described in the context of adenosine and insulin stimulation and were associated with cardioprotection when given at the time of reperfusion11. The signaling members associated with RISK activation were subsequently identified as phosphoinositol-3-kinase (PI3K) and extracellular related kinase (ERK) and they are thought to accomplish cardioprotection by inhibiting opening of the mPTP. Later studies suggested that these proteins (RISK) may be responsible for some of the cardioprotection associated with ischemic preconditioning. However, to date there has been no clinical trial investigating the potential role of RISK in infarct size reduction.
Ischemic postconditioning is perhaps the most intensely studied reperfusion-based intervention of the past several years. As described earlier, PostC involves the institution of brief, repetitive episodes of ischemia/reperfusion, initiated at the time of reperfusion, which has been reported to reduce infarct size2. Subsequent studies determined that the exact protocol used, i.e. the duration of the intermittent episodes of ischemia and reperfusion, as well as the species being utilized was critical to cardioprotection. In addition, later studies demonstrated that the cellular mechanisms associated with PC-induced protection overlapped with those proposed for ischemic preconditioning and that the two processes were not additive suggesting that the underlying mechanism(s) may be shared. To date, PostC has been suggested in involve reperfusion-induced activation of PI3K, protein kinase B/Akt, eNOS, PKG, PKC-ε, ERK, and mitochondrial KATP channels. However, it has been also been suggested that PostC may modify recovery of pH during reperfusion and thereby modify calcium overload and/or cell swelling or even affect the mPTP raising the possibility that it may function through a multifactorial process.
Another recently described class of pharmacologic agents shown protective in lethal ischemia/reperfusion injury is nitrite. Under the right conditions, nitrates can release nitric oxide (NO), which has been shown to be cardioprotective from ischemia/reperfusion injury in both the heart and liver44, 45
Results of clinical trials
Based on results of experimental studies, a number of clinical trials have been conducted to test whether the experimental findings can be translated into useful therapies. Several recent reviews have covered some of these trials46, 47. In general, these clinical trials have been disappointing. There may be several explanations for these failures. In the experimental studies, investigators use animal models where the animals are purchased from one supplier, maintained on the same schedule, receive the same diet, and are approximately the same age and weight. This allows for a relatively consistent degree of injury with a well defined protocol of sudden coronary occlusion followed by unimpeded reperfusion. Even with rigid adherence to these measures, infarct size in control animals demonstrates considerable biological variability, and successful investigators work diligently to limit this variability. Under these conditions, it can be possible to demonstrate a statistically significant beneficial effect of an intervention if the benefit is substantially greater than the variability. Patients, on the other hand, are highly heterogeneous, do not have a consistent diet, take medications for a wide variety of ailments, including drugs for heart disease, are variable in age and weight, do not have a defined duration of severe ischemia, do not necessarily get unimpeded restoration of blood flow with angioplasty, and therefore ischemic injury is likely to be highly variable. Furthermore, there is evidence of circadian rhythms in sensitivity to ischemia-reperfusion injury48. In animal studies this is less critical because experiments are generally performed at a consistent time, but there are some data to suggest that circadian rhythms in sensitivity to ischemia-reperfusion injury may also be true in patients49, and this will add additional variability in patient studies. With so many variables, it is much more difficult to demonstrate a statistically significant benefit of any intervention in a clinical trial than in an animal study.
Among the potential confounding variable in any clinical study is diet, medication usage, and events occurring around the time of ischemia. Diet can be important because nitrite has been shown to protect against ischemia-reperfusion injury 44, 50 and nitrite is present in many foods 51, to variable extent, and patients with high amounts of nitrite in the diet may be protected at baseline whereas those that do not have significant nitrite in the diet will not be protected. Similarly, some medications induce cardioprotection, such as nitroglycerin taken to relieve angina52–54. And angina itself may be protective if it precedes coronary thrombosis by a short time interval 55. Or if coronary thrombosis occurs gradually rather than abruptly as in the experimental studies, there could be some activation of cardioprotective signaling during the transient moderate-flow state. Insulin is cardioprotective 56 and fluctuates during the day. In animal studies, the animals may be fasted prior to the experimental procedure, making insulin levels low in the basal state, but in patients, serum insulin concentration is likely to be a confounding variable. All of these factors make it very difficult to achieve statistical significance in a clinical study. Only those clinical trials examining interventions that have a marked benefit, which exceeds the inherent variability, are likely to be successful.
Another difficulty in clinical trials is defining key baseline predictors of infarct size. Experimental studies have defined area at risk and collateral flow as prime baseline predictors of infarct size. These variables can be measured in experimental studies but not easily or reliably in patients. T2-weighted cardiac magnetic resonance imaging can be used to estimate area at risk 57, but this is an indirect measure of myocardial edema, which may approximately correlate with the ischemic zone, although this has been questioned 58. Without an accurate measure of area at risk, it is not possible to precisely determine the amount of salvage achieved with an intervention. Furthermore, in clinical studies, it is not always possible to precisely determine the onset of ischemia and the degree of stenosis in the relevant artery, or the extent of collaterals. All of these uncertainties will affect the ability to assess the effect of an intervention.
Results of clinical trials of adenosine
One of the primary mediators in many protocols of ischemic preconditioning is adenosine, and therefore, adenosine-mediated cardioprotection was one of the first preconditioning-mimetics to be tested in a clinical trial. The AMISTAD trial 59 examined whether adenosine administered during reperfusion would reduce ultimate infarct size. The first multicenter trial in 236 patients showed a 33% relative reduction in infarct size in the adenosine treated group (p = 0.03), when adenosine was administered intravenously before thrombolytic therapy. Thrombolysis was achieved by pharmacologic agents rather than angioplasty. Time from onset of symptoms to start of thrombolysis was approximately 3 hours. Infarct size was assessed by SPECT imaging. The positive outcome in this small trial provided the impetus for a larger clinical trial, the AMISTAD-II trial in 2118 patients 60. This larger trial was generally similar to the earlier trial but almost half of the patients received angioplasty rather than thrombolytic drugs. The results were disappointing. Clinical outcomes were not significantly improved, but in this trial the primary endpoints were subsequent development of congestive heart failure, re-hospitalization for heart failure, or death from any cause within six months. The secondary endpoint of infarct size reduction was assessed in a subset of patients and suggested that the highest dose of adenosine was associated with a smaller infarct size. The findings were published in 2005, and the authors suggested that a larger study was warranted. It has also been suggested that further studies should be limited to patients who can receive adenosine within 3 hours of symptom onset, because posthoc analysis of the AMISTAD-II trial suggested that only the patients receiving early treatment show evidence of benefit61. This agrees with numerous basic science studies showing that the optimal situation for cardioprotection is when there is the most salvageable myocardium. After three hours of severe ischemia, much of the ischemic tissue is already irreversibly injured, and large animal studies suggest that even ischemic preconditioning is not effective at reducing infarct size if the duration of ischemia exceeds 3 hours. This could be one explanation for the failure of these clinical trials to show a large clinical benefit.
Results of other clinical trials examining preconditioning-mimetics
There have been several clinical trials of other pharmacologic agents that have been shown to mimic the protective effect of preconditioning in animal models. These include trials of nicorandil, a K-ATP channel opener, and of atrial natriuretic peptide (ANP). This study 62 used total creatine kinase (CK) release as a measure of infarct size and found that ANP treatment reduced total CK release by ~15% when administered after reperfusion treatment. In contrast, nicorandil had no significant effect on infarct size, but the control group in this arm of the study had less total CK release than the control group in the ANP arm. Elapsed time between onset of symptoms and intervention averaged 4 hours in both arms of the study. The relatively long time interval between onset of ischemia and intervention may account for the small or absent clinical benefit, which is a limitation of most of the clinical trials to date, in that they included patients with many hours of ischemia prior to reperfusion and therefore had minimal salvageable myocardium.
Another class of potentially beneficial pharmacologic agents is nitrates, which can release nitric oxide, and could be beneficial in the acute setting and could also have a delayed preconditioning-like effect, either of which could reduce infarct size in patients undergoing myocardial ischemia. One investigation of this hypothesis 53 showed that patients who received nitroglycerin on the day before angioplasty had less ST segment elevation, less chest pain, and less contractile deficit during the first balloon inflation than the patients who did not receive nitroglycerin. Another approach to examine the possible benefit of nitrate therapy is retrospective analysis of patient records, since many patients with coronary artery disease are chronically taking nitrates for angina. Ambrosio et al 54 conducted an evaluation of 52,693 patients in the Global Registry of Acute Coronary Events, and divided the patients into those who were on chronic nitrate therapy and those who were nitrate-naïve, and they found that only 18% of nitrate users had ST elevation infarcts whereas 41% of nitrate-naïve patients presented with STEMI. Furthermore, the nitrate users had lower levels of CK-MB and troponin release than the nitrate-naïve patients. These data together suggest smaller infarcts in the nitrate users, and the authors of this study suggested that a randomized, placebo-controlled trial was warranted.
Results of other clinical trials not based on a preconditioning mechanism
In addition to approaches based on a preconditioning-type mechanism, other approaches to limit infarct size have been explored. Among these are approaches designed to limit infarct size by limitation of the ionic alterations that occur during ischemia and early reperfusion, which are thought to contribute to ischemia-reperfusion injury. As discussed earlier, during ischemia, there is an increase in cytosolic ionized sodium and calcium concentrations, in part due to sodium-proton exchange coupled with sodium-calcium exchange, and sodium-proton exchange could be accelerated during the first seconds of reperfusion due to the sudden increase in extracellular pH that occurs during reperfusion. One approach to reduce these ionic imbalances and to prevent calcium influx during late ischemia and early reperfusion is to inhibit sodium-proton exchange. There are several relatively specific NHE inhibitors that have been the focus of clinical trials including eniporide and cariporide. In the ESCAMI trial63, the effects of eniporide, an NHE-1 isoform inhibitor was studied in 959 patients. The drug was administered as a 10 minute infusion before the start of reperfusion therapy, either thrombolytic therapy or primary angioplasty. Although an initial, stage 1 dose effectiveness study suggested several potentially protective doses, in the larger stage 2 protocol, no significant effect on infarct size, assessed as the cumulative release of alpha-hydroxybutyrate dehydrogenase, the primary endpoint of the study was detected with eniporide treatment. There was also no significant effect on clinical outcomes such as death, cardiogenic shock, heart failure, or life-threatening arrhythmias. In this study, CK, CK-MB, Troponin I and troponin T were all measured, and showed good correlation with the alpha-hydroxybutyrate dehydrogenase values. They did observe better responses in patients who received primary angioplasty in the stage 1 study. No assessment of area at risk was performed in this study. Another early study of NHE inhibitors was the GUARDIAN trial, which examined the effectiveness of Cariporide, an NHE-1 selective inhibitor, in several patient populations at risk for ischemia-reperfusion injury64. These included patients presenting with unstable angina, non-Q-wave myocardial infarction, and undergoing high-risk coronary revascularization (angioplasty or bypass grafting). This study of 11,590 patients showed no significant benefit in the unstable angina or non-Q-wave MI groups after 36 days, but in the patients undergoing CABG, there was a 25% risk reduction in the Cariporide-treated group. This beneficial effect continued for 6 months after surgery64. The unstable angina and non-Q-wave MI groups may have been very heterogeneous with regard to timing of ischemia, either prior to treatment or at any point within the 36 days after treatment whereas the CABG group had a well defined ischemic episode that followed treatment by a well controlled time interval. The latter would be the optimal setting to see a beneficial effect. In view of this positive effect in this one study group, and the possibility of a chance effect with so many study arms, a second trial, the EXPEDITION study, was conducted to confirm or refute the results of the GUARDIAN study. The EXPEDITION study 65 examined 5,761 patients who were randomly assigned to cariporide or placebo 1 hour prior to high-risk coronary artery bypass surgery, and continued for 48 hours after surgery. The primary endpoints were death or myocardial infarction at 5 days, and the patients were followed for up to 6 months. The results showed a reduction in death or MI at 5 days in the cariporide group, and in particular, the incidence of MI was substantially reduced (p=0.000005). Unfortunately, there was an increase in the incidence of cerebrovascular accidents in the cariporide group, and at 6 months, there was no decrease in overall mortality in the cariporide group. The study demonstrated proof-of-principle, that the incidence of significant myocardial infarction following coronary bypass surgery could be reduced by pretreatment with a sodium-proton exchange inhibitor to minimize ionic alterations during ischemia and early reperfusion, but the adverse cerebrovascular complications makes it unlikely that this drug will be used clinically. Thus a clinically successful drug to reduce the incidence and/or severity of myocardial ischemic injury must not only protect the heart from injury but cannot have other adverse effects. It is not clear whether it will be possible to develop another sodium-proton exchange inhibitor that will not manifest this adverse effect, or whether the adverse effects could have been avoided if a shorter duration of treatment after bypass surgery had been used. In principle, the sodium-proton exchange inhibitor would only need to be present during ischemia and the first few minutes of reperfusion to be effective.
Another approach to protect against ischemia-reperfusion injury is to reduce inflammatory injury, which could contribute to ultimate infarct size, and which would be amenable to treatment since they primarily occur after the infarction process is underway. Inflammatory injury can be mediated by acute inflammatory cells entering the periphery of the evolving infarct, releasing degradative enzymes and free radicals into the local environment, where viable but injured myocytes would be present. If the adverse effects of this inflammatory process could be mitigated, ultimate infarct size might be smaller. Alternatively, inflammatory cells are necessary for the removal of dead myocytes and the initiation of the repair process. Elimination of the inflammatory cell response to an infarct might depress the repair response, and ultimately result in worse clinical outcome. Inhibition of the inflammatory response could result in a mixture of beneficial and detrimental effects, which might make it very difficult to develop an effective clinical intervention targeted to manipulate the inflammatory response. Numerous experimental studies have suggested the possibility that inhibition of inflammatory cell infiltration limits infarct size, and a number of different strategies have been employed, but the clinical trials have been negative66–68. Among the approaches are antibodies to block integrin receptors to inhibit neutrophil transmigration into the ischemic zone. This approach was effective in animal studies, but no significant reduction in infarct size was observed in patients treated with recombinant tissue plasminogen activator and an anti-CD18 antibody to block integrin receptors 69, and in another study where patients were treated with angioplasty within 6 hours of the onset of chest pain and an antibody to block the CD11/CD18 integrin receptor 70.
Another approach to attenuate inflammatory injury following myocardial infarction is to inhibit complement activation. A clinical trial of 5,745 patients randomized to receive a monoclonal antibody to sequester the C5 component of complement, or placebo, as an adjunct to angioplasty, showed no differences in primary endpoints, all-cause mortality within 30 days, or in secondary endpoints of 90 day mortality, cardiogenic shock or congestive heart failure developing through days 30 and 90. Patients were randomized within 6 hours of the onset of symptoms71.
These clinical trials of agents to attenuate inflammatory injury suggest that the effect of inflammatory injury on ultimate infarct size is too small to be detected in a heterogeneous patient population. These trials also suffer from the long ischemic time prior to reperfusion, and therefore as with other similar trials, there may have been little salvageable myocardium at the time of reperfusion. To the extent that inflammation can cause extension of the infarct into peri-infarct tissue, this should have been attenuated even with long ischemic times, but whether there is enough infarct extension to be measurable with the technical limitations involving estimation of area at risk, in a transmural infarct, is unclear. If the ischemic time was less, there could have been patchy viable myocardium in the subepicardium at the time of reperfusion, and this tissue would be most susceptible to inflammatory injury. Furthermore, inflammation has beneficial and detrimental effects, and inflammation is necessary for repair, so the effect of interventions that inhibit inflammation may impair both beneficial and detrimental effects. A more selective approach that reduces detrimental effects of inflammation without affecting the repair process might still have clinical benefit.
Results of clinical trials based on a remote preconditioning mechanism
Experimental studies have suggested that it is possible to induce a preconditioning-type effect in the myocardium by subjecting remote tissue to transient ischemia. The remote tissue can be a different vascular bed within the heart or another organ or tissue, such as a limb, which would be the easiest to translate into a clinical therapy. One approach to testing this concept was employed by Hausenloy et al. who in a randomized trial of 57 patients examined whether patients would be protected during coronary bypass surgery by several brief episodes of limb ischemia after induction of anesthesia and before the start of surgery72. They measured serum troponin T before surgery and at multiple times after surgery and observed that there was a statistically significant decrease in troponin T release in the remote ischemic preconditioning group. A similar study in a randomized trial of 45 patients 73 examined the effect of repetitive brief cycles of forearm ischemia prior to cardiac surgery using cold-blood cardioplegia and found less cumulative troponin release in the remotely preconditioning group. Another study examined the effect of remote ischemic preconditioning employed during an evolving ST-elevation myocardial infarct74. This study used arm ischemia induced by a blood pressure cuff, in 4 cycles of 5 minutes of inflation and 5 minutes of deflation, during transport to the hospital, and then angioplasty was performed in the hospital. In this study, the remote conditioning occurred after the onset of ischemia, and thus was not true preconditioning, but it was performed prior to angioplasty, and therefore whatever protective mechanisms initiated would be activated at the time of reperfusion. The results of this study of 333 patients, using myocardial perfusion imaging to estimate the proportion of the area at risk salvaged by remote preconditioning, showed that myocardial salvage was increased by remote conditioning. None of these are large trials and in most cases, the study could not be totally blinded, but the results appear promising and could be readily translated into clinical practice75. The concept of remote conditioning could potentially be employed as a prophylactic treatment, and there is evidence 76 that daily brief limb ischemia may induce protection against subsequent ischemic endothelial dysfunction, which appears to share some of the same protective pathways as cardiac myocytes, and remote conditioning could potentially be used as adjuvant therapy prior to angioplasty to activate protective pathways at the time of reperfusion.
Results of clinical trials based on a postconditioning mechanism
One of the potentially most clinically relevant interventions that has been shown to reduce in infarct size in animal studies is postconditioning, whereby a brief protocol of intermittent reperfusion prior to sustained reperfusion results in a smaller infarct than if the myocardium was reperfused abruptly and continuously. This could be employed during angioplasty, and if the correct protocol was employed, the benefit could be substantial. In an initial study by Staat et al77, 30 patients who received coronary angioplasty for ongoing myocardial infarction were randomly assigned to either a control or postconditioning protocol within 1 minute of successful reperfusion. The postconditioning group received 4 cycles of 1 minute balloon inflation and 1 minute deflation after reflow was established. Area at risk and collateral flow was estimated from left ventricular and coronary angiograms, and infarct size was assessed by measuring total CK release over 72 hours. In this small clinical study, CK release data suggested a 36% reduction in infarct size. In a subsequent study from this same group78, the chronic effects of postconditioning were examined. In this study 38 patients were enrolled. Inclusion criteria included presentation within 6 hours of onset of chest pain, no evidence of previous infarcts, suspicion for first ST-segment elevation myocardial infarct, and clinical decision to treat with angioplasty. Infarct size was assessed by CK and troponin release, and this was followed up with a 6 month assessment of infarct size using thallium computed tomography, and 1 year assessment of global and regional contractile function. The results showed an early reduction in infarct size of ~40% based on CK and TnI release in the postconditioned group, at ~6 months the imaging data suggested an ~40% reduction in perfusion defect, a surrogate marker of infarct size, and at 1 year, LV ejection fraction and wall motion was significantly better in the postconditioned group. Another long-term follow-up study of patients who had undergone postconditioning as an adjunct to angioplasty showed that postconditioning reduced infarct size as assessed by peak CK and MB-CK release, and that after a mean follow-up period of 3.4 years, LV ejection fraction remained higher in the postconditioned group79. In this study, the postconditioning protocol consisted of 4 cycles of 30 seconds of balloon occlusion and 30 seconds of deflation. These promising findings suggest that postconditioning could be a useful adjunct to angioplasty in patients undergoing acute myocardial infarction.
However, other similar studies have shown less dramatic effects. A study by Sorensson et al 80 of 76 patients who were treated with angioplasty for an acute ST-elevation myocardial infarct, randomized to standard angioplasty or postconditioning, using the same protocol as the Staat study, showed no significant overall benefit of postconditioning on infarct size, assessed by CK and TnI release, although they did observe a benefit in the patients with the largest areas at risk. In another study of postconditioning81, 79 patients undergoing angioplasty for first STEMI were randomized to postconditioning or control. Postconditioning was 4 cycles of 1 minute balloon inflation and 1 minute deflation. Inclusion criteria included presentation within 12 hours of onset. Many of the patients received intracoronary nitroglycerin or adenosine, but there was no difference between the groups. There was a trend to higher CK-MB release in the postconditioned group and TnI release was higher in the postconditioned group (p=0.05). Cardiac magnetic resonance imaging was used to assess myocardial salvage, and this showed less salvage in the postconditioned group. No significant differences in infarct size or LV ejection fraction were seen at 1 week and 6 months between the control and postconditioned group. The failure to see a positive effect of postconditioning could be due to the inclusion criteria, which allowed some patients with longer than 6 hours of ischemia to be included, and in these patients, the infarcts might have already reached nearly their maximum extent and therefore no adjunctive therapy could be beneficial. Alternatively, some of the control patients may have been protected by intracoronary injection of nitroglycerin or adenosine, and therefore, there was salvage in the control group. Regardless of the explanation, the results suggest that postconditioning may not have a substantial benefit, at least in some patients.
In addition to ischemic postconditioning, another adjunctive therapy that has shown promise in small clinical trials is intravenous infusion of cyclosporine immediately prior to angioplasty. Experimental studies suggest that a major factor in ischemia-reperfusion injury is opening of the mitochondrial permeability transition pore (mPTP) at or shortly after the onset of reperfusion. Cyclosporine has the ability to inhibit mPTP opening, and to limit infarct size in experimental animal studies. A small clinical trial of 58 patients who presented with acute STEMI was randomized to receive either saline or cyclosporine intravenously immediately prior to angioplasty82. Release of CK and TnI were measured to assess infarct size. Mean ischemic time was 5 hours in both groups. Area at risk was estimated using the circumferential extent of abnormally contracting segments. The results showed a reduction in CK release (p=0.04), a trend toward less TnI release (p=0.15), and smaller infarcts by magnetic resonance imaging. This promising result will require a larger clinical trial to validate the conclusions, but suggests a possible means to reduce infarct size at the time of angioplasty.
Potential barriers to clinical application
There are numerous barriers to clinical translation. Among the difficulties is the heterogeneous patient population, which makes it difficult to show a statistically significant benefit of a therapy, and therefore makes it unlikely that the therapy will be adopted. Some patients may be protected by pre-infarction angina, which can elicit a preconditioning-like effect, either an immediate protective effect if the interval between angina and infarction is short, or a delayed preconditioning-like effect if the episodes of angina are relatively frequent for days prior the infarct. In these patients, additional therapies designed to activate pathways involved in preconditioning are unlikely to be beneficial if the pathways are already activated.
One limitation of most clinical trials is that patients often have many hours of ischemia prior to reperfusion, and there may be relatively little salvageable myocardium left when the intervention is performed. Even a robustly protective intervention can only salvage a portion of the viable, ischemic tissue, and the protective effect might be too small to measure if there is little salvageable tissue left. An alternative approach, that would be applicable to far fewer patients, would be pretreatment prior to cardiac surgery or coronary bypass surgery. Even in this instance, the frequency of adverse effects is small with current cardioplegic solutions, and therefore a large clinical trial might be needed to show a statistically significant beneficial effect. One recently described promising approach is remote per-conditioning, where a blood pressure cuff can be used to induce limb ischemia while a patient is being taken to the hospital. This would minimize the effect of long ischemia time prior to reperfusion, and might be able to show a substantial effect in the heterogeneous patient population undergoing myocardial infarction.
Another potential difficulty with some forms of adjuvant therapy is that if it is administered at the time of angioplasty, it might not actually reach the heart at the onset of reperfusion, and the experimental studies suggest that postconditioning-type mechanisms must be initiated immediately at the time of reperfusion. Protection is often not seen if even 10 minutes elapses between reperfusion and adjuvant treatment.
Another potential conflicting variable is polymorphisms in critical effectors of protective pathways. For example, there are common polymorphisms in different ethnic groups in the enzyme mitochondrial aldehyde dehydrogenase, which is involved as a downstream effector in preconditioning. One clinical study showed that in patients with a polymorphism in this enzyme that eliminates enzymatic activity, there was no remote preconditioning effect on endothelial dysfunction, which has been used as a surrogate marker for a preconditioning effect 76. The search for polymorphisms that might affect various cardioprotective interventions is in its infancy since many critical cardioprotective pathways are redundant, and low activity in one pathway may or may not affect overall protection.
In addition to polymorphisms, aging can affect cardioprotective signaling pathways, as can comorbidities such as diabetes. Gender may also affect cardioprotective signaling. These potential confounding variables makes it more difficult to show statistically significant effects of cardioprotective interventions, even if the intervention is beneficial in some patient populations.
Emerging areas of cardioprotection
Among the newer cardioprotective agents that show promise in basic animal research studies but have had only a modest effect in clinical studies is the PKC delta peptide inhibitor developed by the Mochly-Rosen group. This inhibitor was effective when administered immediately at the start of reperfusion in transient ischemia-reperfusion protocols in animals, but the effect in a small clinical trial was small and the effect was insufficient to warrant the expense of a larger clinical trial83. Another promising area of research is adaptive autophagy. A recent small clinical trial showed that the homeostatic intracellular repair response (HIR2), shown to be cardioprotective in experimental studies, is upregulated during cardiac stress suggesting that amplification of HIR2 may be effective intervention in patients undergoing heart surgery 84.
Conclusions
Many cardioprotective interventions have been described in the basic experimental literature in animals. These animal studies are generally performed in young animals, usually male, usually without co-morbidities, in a very carefully controlled environment with genetically similar animals on the same diet. The animals are often litter-mates, making them as homogeneous as possible. The animals are not taking any medications, other than those used as part of the experimental protocol. In animal studies the experimental protocol is chosen to maximize the effect of the intervention and the procedures generally include a rigorous measurement of area at risk, which is critical for accurately measuring the effect of the intervention. Despite this rigorous approach, experimental studies are generally not blinded which may introduce unintentional bias, and there often are no explicit exclusion criteria leading to potential exclusion of unsuccessful experiments from the final analysis. On the other hand, in the clinical trials, the patients are generally older, include both males and females, often with co-morbidities, taking medications, sometimes nutritional supplements containing potentially cardioprotective agents, eating different diets, living in different environments, genetically diverse, and receiving care at different medical institutions. Significant co-morbidities include diabetes, hypertension, atherosclerosis, hyperlipidemia, and metabolic syndrome. These co-morbidities have effects on signaling pathways that are involved in cardioprotection. Furthermore the drugs used to treat these co-morbidities have effects on cardioprotective signaling pathways. Age, gender, and genetic differences in drug metabolism may also complicate response to therapy. Although there is an effort to standardize procedures between institutions involved in the clinical trial, it is often not possible to achieve complete standardization. All of these factors make the patient population in the clinical trials very heterogeneous, making the variability greater, and therefore making it more difficult to show statistical significance. Most of the experimental studies use relatively short ischemic durations to provide the most salvageable myocardium at the time of reperfusion. In the clinical trials, ischemic duration is usually long, and there may not be very much salvageable myocardium at the time of reperfusion. There is an effort to make clinical trials blinded, and the analysis is performed at a central site. There are generally predetermined exclusion criteria, but patients cannot be omitted from the analysis except through the exclusion process. These factors make it more difficult to have a successful clinical trial. Although many cardioprotective strategies that work well in experimental animals have been difficult to translate into clinical practice, there is reason for optimism that some of the current adjunctive therapies can reduce infarct size and improve cardiac function following myocardial infarction. Effective interventions are likely to be more beneficial if the time from onset of symptoms to angioplasty can be reduced, since in experimental studies, the optimal window for intervention is early during the infarction process, when the amount of potentially salvageable tissue is large. Reperfusion remains the most important intervention for reducing infarct size, but adjunctive therapies may significantly improve salvage and improve long-term outcomes in patients with coronary artery disease.
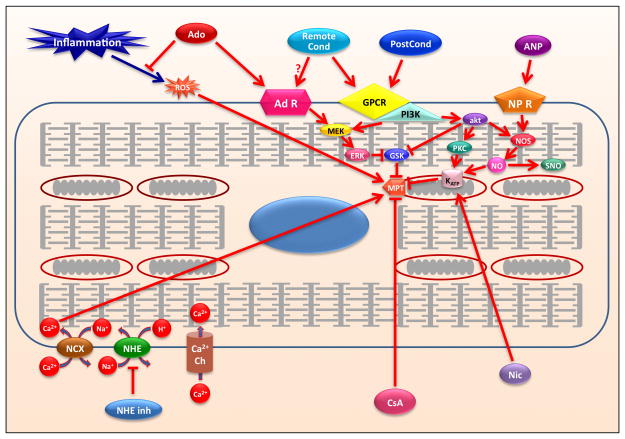
Figure 1.
Schematic representation of mechanisms involved in ischemia-reperfusion injury and cardioprotection. Abbrevations: Adenosine, Ado; Adenosine Receptors, AD R; Atrial Natriuretic Peptides, ANP; Atrial Natriuretic Peptide Receptors, NP R; Calcium channel, Ca++ Ch; Cyclosporine A, CyA; G-protein coupled receptors, GPCR; ATP-sensitive potassium channel, KATP; mitochondrial permeability transition pore, MPT; nicorandil, Nic; nitric oxide, NO; phosphatidylinositol-3-kinase, PI3K; postconditioning, PostCond; remote conditioning, Remote Cond; sodium proton exchanger, NHE; sodium calcium exchanger, NCX; S-nitrosylation, SNO.
Table 1.
Experimental Trials of Cardioprotective Interventions
| Proposed Mediator/Effector | Species | Dose or Protocol | Infarct Size reduction | Reference |
|---|---|---|---|---|
| Reduction of lactate/swelling (Ischemic preconditioning) | multiple | 4 × [5 min Ischemia + 5 min Reperfusion] | ~40% | 1 Murry et al. Circ, 1986 |
| Calcium channel blockers | dog | 0.01 mg/kg/min i.c. | 28% | 36 Lo et al. Am J Cardiol, 1985 |
| 10–20 μg/kg/min i.c. | 28–33% | 37 Higginson et al. JACC, 1991 | ||
| Free radical scavengers | rabbit | 60,000 U in perfusion buffer | n/a (dec. EPR signal) | 30 Zweier et al. JCI, 1987 |
| dog | 1 mg/kg i.v. | 46% | 29 Simpson et al. JCI, 1988 | |
| Adenosine | dog | 3.75 mg/min 60 min i.c. reperfusion | 31% | 28 Olafsson et al. Circ 1987 |
| dog | 0.15 mg/kg/ml/min i.c. 60 min + lidocaine | 27% | 27 Homeister et al. Circ 1990 | |
| dog | 0.15 mg/kg/min i.v. | 18% | 26 Pitarys et al. Circ 1991 | |
| Na+/Ca2+ exchange inhibitors | dog | 3/30 μg/kg/hr i.v. | 51/71% | 42 Kawasumi et al. J Pharm Sci, 2007 |
| rat | genetic KO of NCX | 42% | 43 Imahashi et al. Circ Res, 2005 | |
| Na+/H+ exchange inhibitors | rat | 40 μm/ml | 44% inc. in LVPD | 41 Karmazyn Am J Physiol, 1988 |
| rabbit | 5 μM | 91% dec. in CK release | 40 Liu et al. J Cardiovasc Pharm, 2010 | |
| Endothelial function (Ischemic post conditioning) | dog | 3×30 sec IR | 11% | 2 Zhao et al. Am J Physiol Heart Circ, 2003 |
| Nitrite | mice | 48 nmol LV cavity | 67% | 44 Duranski et al. JCI, 2005 |
| mice | 50 mg/liter PO suppl. | 48% | 45 Bryan et al. PNAS, 2007 |
Table 2.
Clinical Trials of Cardioprotective Interventions
| Proposed Mediator/Effector | Trials | Dose or Protocol | Endpoint | Reference |
|---|---|---|---|---|
| Adenosine | AMISTAD | 70 μg/kg/min for 3 h | 33% decrease in MI size | 59 Mahaffey et al. J Am Coll Cardiol 1999 |
| AMISTAD II | 70 μg/kg/min for 3 h | 67% decrease in MI size | 60 Ross et al. J Am Coll Cardiol 2005 | |
| Nicorandil (K-ATP opener) | J-WIND | 67 μg/kg bolus, and 1.67 μg/kg/min for 2–4 h | no effect on MI size | 62 Kitakaze et al. Lancet 2007 |
| Atrial natriuretic peptide | J-WIND | .025 μg/kg/min for 3 d | 14.7% decrease in MI size | 62 Kitakaze et al. Lancet 2007 |
| NHE inhibitors (eniprode) | ESCAMI | 50, 100, 150, 200 mg | no effect | 63 Zeymer et al. J Am Coll Cardiol 2001 |
| (cariporide) | GUARDIAN | 20, 80, 120 mg | 25% risk reduction (120 mg) | 64 Boyce et al. J Thor Cardiovasc Surg 2003 |
| (cariporide) | EXPEDITION | 180 mg (initial dose) | fewer MIs, more CVAs | 65 Mentzer et al. Annals Thor Surg 2008 |
| Inflammation (anti-CD18 Ab) | LIMIT AMI | .5 mg/kg, 2 mg/kg | no effect | 69 Baran et al. Circ 2001 |
| (anti-CD11/CD18 Ab) | HALT-MI | 3 mg/kg, 1 mg/kg | no effect | 70 Faxon et al. J Am Coll Cardiol 2002 |
| (antibody to C5) | NCT00091637 | 2 mg/kg bolus | no effect | 71 Armstrong et al. JAMA 2007 |
| Remote Preconditioning | NCT00397163 | 3 × 5 min arm ischemia | reduced troponin release | 72 Hausenloy et al. Lancet 2007 |
| NCT00397163 | 3 × 5 min arm ischemia | reduced troponin release | 73 Venugopal et al. Heart 2009 | |
| NCT00435266 | 4 × 5 min arm ischemia | salvage increased | 74 Botker et al. Lancet 2010 | |
| Postconditioning | 30 patients | 4 × 1 min ischemia | reduced CK release | 77 Staat et al. Circ 2005 |
| 38 patients | 4 × 1 min ischemia | improved EF | 78 Thibault et al. Circ 2008 | |
| 43 patients | 4 × 30 sec ischemia | smaller MI, better function | 79 Garcia et al. J Cardiovasc Trans Res 2011 | |
| CT20080014 | 4 × 1 min ischemia | no effect | 80 Sorensson et al. Heart 2010 | |
| 79 patients | 4 × 1 min ischemia | no effect | 81 Freixa et al. Eur Heart J 2012 | |
| Cyclosporine | 58 patients | 2.5 mg/kg | Decrease in MI size | 82 Piot et al. NEJM 2008 |
Acknowledgments
The authors would like to thank the National Heart Lung and Blood Institute of the NIH for their continued support.
Sources of Funding: Supported in part by NIH R21 HL098786 to RVH and NIH 5R01 HL039752 to CS.
Non-standard abbreviations and acronyms used
- PC
ischemic preconditioning
- PostC
post conditioning
- IR
ischemia/reperfusion
- CBF
coronary blood flow
- STEMI
ST segment elevation myocardial infarction
- RISK
reperfusion induced survival kinases
- ATP
adenosine triphosphate
- HEP
high energy phosphate
- ROS
reactive oxygen species
- mPTP
mitochondrial permeability transition pore
- PI3K
phosphatidylinositol-3-kinase
- eNOS
constitutive nitric oxide synthase
- PKG
protein kinase G
- PKC-E
protein kinase C epsilon
- ERK
extracellular related kinase
- ANP
atrial natriuretic peptide
- CK-(MB)
creatine kinase (MB fraction)
- NHE
sodium hydrogen exchange
- TnI
troponin I
- Ado
adenosine
- AD R
adenosine receptors
- NP R
atrial natriuretic receptor
- CyA
cyclosporine A
- GPCR
G protein coupled receptor
- KATP
ATP-sensitive potassium channel
- Nic
nicorandil
- Remote Cond
remote conditioning
- NCX
sodium calcium exchanger
- SNO
S-nitrosylation
Footnotes
Disclosures: none
References
- 1.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 2.Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: Comparison with ischemic preconditioning. American journal of physiology. Heart and circulatory physiology. 2003;285:H579–588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 3.Jennings RB, Sommers HM, Herdson PB, Kaltenbach JP. Ischemic injury of myocardium. Annals of the New York Academy of Sciences. 1969;156:61–78. doi: 10.1111/j.1749-6632.1969.tb16718.x. [DOI] [PubMed] [Google Scholar]
- 4.Kloner RA, Ganote CE, Whalen DA, Jennings RB. Effect of a transient period of ischemia on myocardial cells: Ii. Fine structure during the first few minutes of reflow. American Journal of Pathology. 1974;74:399–422. [PMC free article] [PubMed] [Google Scholar]
- 5.Kloner RA, Reimer KA, Jennings RB. Distribution of collateral flow in acute myocardial ischemic injury--effect of propranolol therapy. Cardiovascular research. 1976;10:81–90. doi: 10.1093/cvr/10.1.81. [DOI] [PubMed] [Google Scholar]
- 6.Reimer KA, Jennings RB. The “wavefront phenomenon” of myocardial ischemic cell death. Ii. Transmural progression of necrosis within the framework of ischemic bed size (myocardium at risk) and collateral flow. Laboratory Investigation. 1979;40:633–644. [PubMed] [Google Scholar]
- 7.Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. The New England journal of medicine. 2010;362:2155–2165. doi: 10.1056/NEJMoa0908610. [DOI] [PubMed] [Google Scholar]
- 8.Heyndrickx GR, Millard RW, McRitchie RJ, Maroko PR, Vatner SF. Regional myocardial functional and electrophysiological alterations after brief coronary artery occlusion in conscious dogs. Journal of Clinical Investigation. 1975;56:978–985. doi: 10.1172/JCI108178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolli R, Jeroudi MO, Patel BS, Aruoma OI, Halliwell B, Lai EK, McCay PB. Marked reduction of free radical generation and contractile dysfunction by antioxidant therapy begun at the time of reperfusion. Evidence that myocardial “stunning” is a manifestation of reperfusion injury. Circulation Research. 1989;65:607–622. doi: 10.1161/01.res.65.3.607. [DOI] [PubMed] [Google Scholar]
- 10.Cohen MV, Yang XM, Downey JM. The ph hypothesis of postconditioning: Staccato reperfusion reintroduces oxygen and perpetuates myocardial acidosis. Circulation. 2007;115:1895–1903. doi: 10.1161/CIRCULATIONAHA.106.675710. [DOI] [PubMed] [Google Scholar]
- 11.Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia-reperfusion injury: Targeting the reperfusion injury salvage kinase (risk)-pathway. Cardiovascular research. 2004;61:448–460. doi: 10.1016/j.cardiores.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz LM, Lagranha CJ. Ischemic postconditioning during reperfusion activates akt and erk without protecting against lethal myocardial ischemia-reperfusion injury in pigs. American journal of physiology. Heart and circulatory physiology. 2006;290:H1011–1018. doi: 10.1152/ajpheart.00864.2005. [DOI] [PubMed] [Google Scholar]
- 13.Panagiotopoulos S, Daly MJ, Nayler WG. Effect of acidosis and alkalosis on postischemic ca gain in isolated rat heart. The American journal of physiology. 1990;258:H821–828. doi: 10.1152/ajpheart.1990.258.3.H821. [DOI] [PubMed] [Google Scholar]
- 14.Inserte J, Ruiz-Meana M, Rodriguez-Sinovas A, Barba I, Garcia-Dorado D. Contribution of delayed intracellular ph recovery to ischemic postconditioning protection. Antioxidants & redox signaling. 2011;14:923–939. doi: 10.1089/ars.2010.3312. [DOI] [PubMed] [Google Scholar]
- 15.Ganote CE, Vander Heide RS. Cytoskeletal lesions in anoxic myocardial injury. A conventional and high-voltage electron-microscopic and immunofluorescence study. The American journal of pathology. 1987;129:327–344. [PMC free article] [PubMed] [Google Scholar]
- 16.Vander Heide RS, Ganote CE. Increased myocyte fragility following anoxic injury. Journal of molecular and cellular cardiology. 1987;19:1085–1103. doi: 10.1016/s0022-2828(87)80353-x. [DOI] [PubMed] [Google Scholar]
- 17.Steenbergen C, Hill ML, Jennings RB. Volume regulation and plasma membrane injury in aerobic, anaerobic, and ischemic myocardium in vitro. Effects of osmotic cell swelling on plasma membrane integrity. Circ Res. 1985;57:864–875. doi: 10.1161/01.res.57.6.864. [DOI] [PubMed] [Google Scholar]
- 18.Steenbergen C, Jr, Hill ML, Jennings RB. Cytoskeletal damage during myocardial ischemia: Changes in vinculin immunofluorescence staining during total in vitro ischemia in canine heart. Circulation Research. 1987;60:478–486. doi: 10.1161/01.res.60.4.478. [DOI] [PubMed] [Google Scholar]
- 19.Vander Heide RS, Angelo JP, Altschuld RA, Ganote CE. Energy dependence of contraction band formation in perfused hearts and isolated adult myocytes. The American journal of pathology. 1986;125:55–68. [PMC free article] [PubMed] [Google Scholar]
- 20.Ganote CE, Sims MA, VanderHeide RS. Mechanism of enzyme release in the calcium paradox. European heart journal. 1983;4 (Suppl H):63–71. doi: 10.1093/eurheartj/4.suppl_h.63. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Dorado D, Theroux P, Duran JM, Solares J, Alonso J, Sanz E, Munoz R, Elizaga J, Botas J, Fernandez-Aviles F, et al. Selective inhibition of the contractile apparatus. A new approach to modification of infarct size, infarct composition, and infarct geometry during coronary artery occlusion and reperfusion. Circulation. 1992;85:1160–1174. doi: 10.1161/01.cir.85.3.1160. [DOI] [PubMed] [Google Scholar]
- 22.Di Lisa F, Bernardi P. Mitochondria and ischemia-reperfusion injury of the heart: Fixing a hole. Cardiovascular research. 2006;70:191–199. doi: 10.1016/j.cardiores.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 23.Burton KP, McCord JM, Ghai G. Myocardial alterations due to free radical generation. American Journal of Physiology. 1984;246:H776–H783. doi: 10.1152/ajpheart.1984.246.6.H776. [DOI] [PubMed] [Google Scholar]
- 24.Hearse DJ, Humphrey SM, Bullock GR. The oxygen paradox and the calcium paradox: Two facets of the same problem? Journal of molecular and cellular cardiology. 1978;10:641–668. doi: 10.1016/s0022-2828(78)80004-2. [DOI] [PubMed] [Google Scholar]
- 25.Zimmet JM, Hare JM. Nitroso-redox interactions in the cardiovascular system. Circulation. 2006;114:1531–1544. doi: 10.1161/CIRCULATIONAHA.105.605519. [DOI] [PubMed] [Google Scholar]
- 26.Pitarys CJ, Virmani R, Vildibill HD, Jr, Jackson EK, Forman MB. Reduction of myocardial reperfusion injury by intravenous adenosine administered during the early reperfusion period. Circulation. 1991;83:237–247. doi: 10.1161/01.cir.83.1.237. [DOI] [PubMed] [Google Scholar]
- 27.Homeister JW, Hoff PT, Fletcher DD, Lucchesi BR. Combined adenosine and lidocaine administration limits myocardial reperfusion injury. Circulation. 1990;82:595–608. doi: 10.1161/01.cir.82.2.595. [DOI] [PubMed] [Google Scholar]
- 28.Olafsson B, Forman MB, Puett DW, Pou A, Cates CU, Friesinger GC, Virmani R. Reduction of reperfusion injury in the canine preparation by intracoronary adenosine: Importance of the endothelium and the no-reflow phenomenon. Circulation. 1987;76:1135–1145. doi: 10.1161/01.cir.76.5.1135. [DOI] [PubMed] [Google Scholar]
- 29.Simpson PJ, Todd RF, III, Fantone JC, Mickelson JK, Griffin JD, Lucchesi BR. Reduction of experimental canine myocardial reperfusion injury by a monoclonal antibody (anti mo1, anti cd11b) that inhibits leukocyte adhesion. Journal of Clinical Investigation. 1988;81:624–629. doi: 10.1172/JCI113364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zweier JL, Rayburn BK, Flaherty JT, Weisfeldt ML. Recombinant superoxide dismutase reduces oxygen free radical concentrations in reperfused myocardium. Journal of Clinical Investigation. 1987;80:1728–1734. doi: 10.1172/JCI113264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reimer KA, Jennings RB. Failure of the xanthine oxidase inhibitor allopurinol to limit infarct size after ischemia and reperfusion in dogs. Circulation. 1985;71:1069–1075. doi: 10.1161/01.cir.71.5.1069. [DOI] [PubMed] [Google Scholar]
- 32.Vander Heide RS, Reimer KA. Effect of adenosine therapy at reperfusion on myocardial infarct size in dogs. Cardiovascular research. 1996;31:711–718. doi: 10.1016/0008-6363(95)00235-9. [DOI] [PubMed] [Google Scholar]
- 33.Miki T, Cohen MV, Downey JM. Failure of n-2-mercaptopropionyl glycine to reduce myocardial infarction after 3 days of reperfusion in rabbits. Basic research in cardiology. 1999;94:180–187. doi: 10.1007/s003950050141. [DOI] [PubMed] [Google Scholar]
- 34.Horwitz LD, Fennessey PV, Shikes RH, Kong Y. Marked reduction in myocardial infarct size due to prolonged infusion of an antioxidant during reperfusion. Circulation. 1994;89:1792–1801. doi: 10.1161/01.cir.89.4.1792. [DOI] [PubMed] [Google Scholar]
- 35.Shirato C, Miura T, Ooiwa H, Toyofuku T, Wilborn WH, Downey JM. Tetrazolium artifactually indicates superoxide dismutase-induced salvage in reperfused rabbit heart. Journal of molecular and cellular cardiology. 1989;21:1187–1193. doi: 10.1016/0022-2828(89)90695-0. [DOI] [PubMed] [Google Scholar]
- 36.Lo HM, Kloner RA, Braunwald E. Effect of intracoronary verapamil on infarct size in the ischemic, reperfused canine heart: Critical importance of the timing of treatment. American Journal of Cardiology. 1985;56:672–677. doi: 10.1016/0002-9149(85)91033-1. [DOI] [PubMed] [Google Scholar]
- 37.Higginson L, Tang A, Knoll G, Calvin J. Effect of intracoronary diltiazem on infarct size and regional myocardial function in the ischemic reperfused canine heart. Journal of the American College of Cardiology. 1991;18:867–874. doi: 10.1016/0735-1097(91)90814-p. [DOI] [PubMed] [Google Scholar]
- 38.Vander Heide RS, Schwartz LM, Reimer KA. The novel calcium antagonist ro 40-5967 limits myocardial infarct size in the dog. Cardiovascular research. 1994;28:1526–1532. doi: 10.1093/cvr/28.10.1526. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Meyer JW, Ashraf M, Shull GE. Mice with a null mutation in the nhe1 na+-h+ exchanger are resistant to cardiac ischemia-reperfusion injury. Circ Res. 2003;93:776–782. doi: 10.1161/01.RES.0000094746.24774.DC. [DOI] [PubMed] [Google Scholar]
- 40.Liu H, Cala PM, Anderson SE. Na/h exchange inhibition protects newborn heart from ischemia/reperfusion injury by limiting na+-dependent ca2+ overload. Journal of cardiovascular pharmacology. 2010;55:227–233. doi: 10.1097/FJC.0b013e3181cb599f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karmazyn M. Amiloride enhances postischemic ventricular recovery: Possible role of na+-h+ exchange. American Journal of Physiology. 1988;255:H608–H615. doi: 10.1152/ajpheart.1988.255.3.H608. [DOI] [PubMed] [Google Scholar]
- 42.Kawasumi H, Satoh N, Kitada Y. Caldaret, an intracellular ca2+ handling modulator, limits infarct size of reperfused canine heart. Journal of pharmacological sciences. 2007;103:222–233. doi: 10.1254/jphs.fp0060765. [DOI] [PubMed] [Google Scholar]
- 43.Imahashi K, Pott C, Goldhaber JI, Steenbergen C, Philipson KD, Murphy E. Cardiac-specific ablation of the na+-ca2+ exchanger confers protection against ischemia/reperfusion injury. Circ Res. 2005;97:916–921. doi: 10.1161/01.RES.0000187456.06162.cb. [DOI] [PubMed] [Google Scholar]
- 44.Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, Patel RP, Yet SF, Wang X, Kevil CG, Gladwin MT, Lefer DJ. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. The Journal of clinical investigation. 2005;115:1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bryan NS, Calvert JW, Elrod JW, Gundewar S, Ji SY, Lefer DJ. Dietary nitrite supplementation protects against myocardial ischemia-reperfusion injury. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19144–19149. doi: 10.1073/pnas.0706579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gerczuk PZ, Kloner RA. An update on cardioprotection: A review of the latest adjunctive therapies to limit myocardial infarction size in clinical trials. J Am Coll Cardiol. 2012;59:969–978. doi: 10.1016/j.jacc.2011.07.054. [DOI] [PubMed] [Google Scholar]
- 47.Kloner RA, Schwartz Longacre L. State of the science of cardioprotection: Challenges and opportunities--proceedings of the 2010 nhlbi workshop on cardioprotection. Journal of cardiovascular pharmacology and therapeutics. 2011;16:223–232. doi: 10.1177/1074248411402501. [DOI] [PubMed] [Google Scholar]
- 48.Durgan DJ, Pulinilkunnil T, Villegas-Montoya C, Garvey ME, Frangogiannis NG, Michael LH, Chow CW, Dyck JR, Young ME. Short communication: Ischemia/reperfusion tolerance is time-of-day-dependent: Mediation by the cardiomyocyte circadian clock. Circ Res. 2010;106:546–550. doi: 10.1161/CIRCRESAHA.109.209346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reiter R, Swingen C, Moore L, Henry TD, Traverse JH. Circadian dependence of infarct size and left ventricular function after st elevation myocardial infarction. Circ Res. 2012;110:105–110. doi: 10.1161/CIRCRESAHA.111.254284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gonzalez FM, Shiva S, Vincent PS, Ringwood LA, Hsu LY, Hon YY, Aletras AH, Cannon RO, 3rd, Gladwin MT, Arai AE. Nitrite anion provides potent cytoprotective and antiapoptotic effects as adjunctive therapy to reperfusion for acute myocardial infarction. Circulation. 2008;117:2986–2994. doi: 10.1161/CIRCULATIONAHA.107.748814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nadtochiy SM, Redman EK. Mediterranean diet and cardioprotection: The role of nitrite, polyunsaturated fatty acids, and polyphenols. Nutrition. 2011;27:733–744. doi: 10.1016/j.nut.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bolli R. The late phase of preconditioning. Circ Res. 2000;87:972–983. doi: 10.1161/01.res.87.11.972. [DOI] [PubMed] [Google Scholar]
- 53.Leesar MA, Stoddard MF, Dawn B, Jasti VG, Masden R, Bolli R. Delayed preconditioning-mimetic action of nitroglycerin in patients undergoing coronary angioplasty. Circulation. 2001;103:2935–2941. doi: 10.1161/01.cir.103.24.2935. [DOI] [PubMed] [Google Scholar]
- 54.Ambrosio G, Del Pinto M, Tritto I, Agnelli G, Bentivoglio M, Zuchi C, Anderson FA, Gore JM, Lopez-Sendon J, Wyman A, Kennelly BM, Fox KA. Chronic nitrate therapy is associated with different presentation and evolution of acute coronary syndromes: Insights from 52,693 patients in the global registry of acute coronary events. European heart journal. 2010;31:430–438. doi: 10.1093/eurheartj/ehp457. [DOI] [PubMed] [Google Scholar]
- 55.Lorgis L, Gudjoncik A, Richard C, Mock L, Buffet P, Brunel P, Janin-Manificat L, Beer JC, Brunet D, Touzery C, Rochette L, Cottin Y, Zeller M. Pre-infarction angina and outcomes in non-st-segment elevation myocardial infarction: Data from the rico survey. PloS one. 2012;7:e48513. doi: 10.1371/journal.pone.0048513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sack MN, Yellon DM. Insulin therapy as an adjunct to reperfusion after acute coronary ischemia: A proposed direct myocardial cell survival effect independent of metabolic modulation. J Am Coll Cardiol. 2003;41:1404–1407. doi: 10.1016/s0735-1097(03)00164-5. [DOI] [PubMed] [Google Scholar]
- 57.Friedrich MG, Kim HW, Kim RJ. T2-weighted imaging to assess post-infarct myocardium at risk. JACC Cardiovascular imaging. 2011;4:1014–1021. doi: 10.1016/j.jcmg.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Croisille P, Kim HW, Kim RJ. Controversies in cardiovascular mr imaging: T2-weighted imaging should not be used to delineate the area at risk in ischemic myocardial injury. Radiology. 2012;265:12–22. doi: 10.1148/radiol.12111769. [DOI] [PubMed] [Google Scholar]
- 59.Mahaffey KW, Puma JA, Barbagelata NA, DiCarli MF, Leesar MA, Browne KF, Eisenberg PR, Bolli R, Casas AC, Molina-Viamonte V, Orlandi C, Blevins R, Gibbons RJ, Califf RM, Granger CB. Adenosine as an adjunct to thrombolytic therapy for acute myocardial infarction: Results of a multicenter, randomized, placebo-controlled trial: The acute myocardial infarction study of adenosine (amistad) trial. J Am Coll Cardiol. 1999;34:1711–1720. doi: 10.1016/s0735-1097(99)00418-0. [DOI] [PubMed] [Google Scholar]
- 60.Ross AM, Gibbons RJ, Stone GW, Kloner RA, Alexander RW. A randomized, double-blinded, placebo-controlled multicenter trial of adenosine as an adjunct to reperfusion in the treatment of acute myocardial infarction (amistad-ii) J Am Coll Cardiol. 2005;45:1775–1780. doi: 10.1016/j.jacc.2005.02.061. [DOI] [PubMed] [Google Scholar]
- 61.Kloner RA, Forman MB, Gibbons RJ, Ross AM, Alexander RW, Stone GW. Impact of time to therapy and reperfusion modality on the efficacy of adenosine in acute myocardial infarction: The amistad-2 trial. European heart journal. 2006;27:2400–2405. doi: 10.1093/eurheartj/ehl094. [DOI] [PubMed] [Google Scholar]
- 62.Kitakaze M, Asakura M, Kim J, Shintani Y, Asanuma H, Hamasaki T, Seguchi O, Myoishi M, Minamino T, Ohara T, Nagai Y, Nanto S, Watanabe K, Fukuzawa S, Hirayama A, Nakamura N, Kimura K, Fujii K, Ishihara M, Saito Y, Tomoike H, Kitamura S. Human atrial natriuretic peptide and nicorandil as adjuncts to reperfusion treatment for acute myocardial infarction (j-wind): Two randomised trials. Lancet. 2007;370:1483–1493. doi: 10.1016/S0140-6736(07)61634-1. [DOI] [PubMed] [Google Scholar]
- 63.Zeymer U, Suryapranata H, Monassier JP, Opolski G, Davies J, Rasmanis G, Linssen G, Tebbe U, Schroder R, Tiemann R, Machnig T, Neuhaus KL. The na(+)/h(+) exchange inhibitor eniporide as an adjunct to early reperfusion therapy for acute myocardial infarction. Results of the evaluation of the safety and cardioprotective effects of eniporide in acute myocardial infarction (escami) trial. J Am Coll Cardiol. 2001;38:1644–1650. doi: 10.1016/s0735-1097(01)01608-4. [DOI] [PubMed] [Google Scholar]
- 64.Boyce SW, Bartels C, Bolli R, Chaitman B, Chen JC, Chi E, Jessel A, Kereiakes D, Knight J, Thulin L, Theroux P. Impact of sodium-hydrogen exchange inhibition by cariporide on death or myocardial infarction in high-risk cabg surgery patients: Results of the cabg surgery cohort of the guardian study. The Journal of thoracic and cardiovascular surgery. 2003;126:420–427. doi: 10.1016/s0022-5223(03)00209-5. [DOI] [PubMed] [Google Scholar]
- 65.Mentzer RM, Jr, Bartels C, Bolli R, Boyce S, Buckberg GD, Chaitman B, Haverich A, Knight J, Menasche P, Myers ML, Nicolau J, Simoons M, Thulin L, Weisel RD. Sodium-hydrogen exchange inhibition by cariporide to reduce the risk of ischemic cardiac events in patients undergoing coronary artery bypass grafting: Results of the expedition study. The Annals of thoracic surgery. 2008;85:1261–1270. doi: 10.1016/j.athoracsur.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 66.Baxter GF. The neutrophil as a mediator of myocardial ischemia-reperfusion injury: Time to move on. Basic research in cardiology. 2002;97:268–275. doi: 10.1007/s00395-002-0366-7. [DOI] [PubMed] [Google Scholar]
- 67.Harlan JM, Winn RK. Leukocyte-endothelial interactions: Clinical trials of anti-adhesion therapy. Critical care medicine. 2002;30:S214–219. doi: 10.1097/00003246-200205001-00007. [DOI] [PubMed] [Google Scholar]
- 68.Vinten-Johansen J. Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovascular research. 2004;61:481–497. doi: 10.1016/j.cardiores.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 69.Baran KW, Nguyen M, McKendall GR, Lambrew CT, Dykstra G, Palmeri ST, Gibbons RJ, Borzak S, Sobel BE, Gourlay SG, Rundle AC, Gibson CM, Barron HV. Double-blind, randomized trial of an anti-cd18 antibody in conjunction with recombinant tissue plasminogen activator for acute myocardial infarction: Limitation of myocardial infarction following thrombolysis in acute myocardial infarction (limit ami) study. Circulation. 2001;104:2778–2783. doi: 10.1161/hc4801.100236. [DOI] [PubMed] [Google Scholar]
- 70.Faxon DP, Gibbons RJ, Chronos NA, Gurbel PA, Sheehan F. The effect of blockade of the cd11/cd18 integrin receptor on infarct size in patients with acute myocardial infarction treated with direct angioplasty: The results of the halt-mi study. J Am Coll Cardiol. 2002;40:1199–1204. doi: 10.1016/s0735-1097(02)02136-8. [DOI] [PubMed] [Google Scholar]
- 71.Armstrong PW, Granger CB, Adams PX, Hamm C, Holmes D, Jr, O’Neill WW, Todaro TG, Vahanian A, Van de Werf F. Pexelizumab for acute st-elevation myocardial infarction in patients undergoing primary percutaneous coronary intervention: A randomized controlled trial. JAMA : the journal of the American Medical Association. 2007;297:43–51. doi: 10.1001/jama.297.1.43. [DOI] [PubMed] [Google Scholar]
- 72.Hausenloy DJ, Mwamure PK, Venugopal V, Harris J, Barnard M, Grundy E, Ashley E, Vichare S, Di Salvo C, Kolvekar S, Hayward M, Keogh B, MacAllister RJ, Yellon DM. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: A randomised controlled trial. Lancet. 2007;370:575–579. doi: 10.1016/S0140-6736(07)61296-3. [DOI] [PubMed] [Google Scholar]
- 73.Venugopal V, Hausenloy DJ, Ludman A, Di Salvo C, Kolvekar S, Yap J, Lawrence D, Bognolo J, Yellon DM. Remote ischaemic preconditioning reduces myocardial injury in patients undergoing cardiac surgery with cold-blood cardioplegia: A randomised controlled trial. Heart. 2009;95:1567–1571. doi: 10.1136/hrt.2008.155770. [DOI] [PubMed] [Google Scholar]
- 74.Botker HE, Kharbanda R, Schmidt MR, Bottcher M, Kaltoft AK, Terkelsen CJ, Munk K, Andersen NH, Hansen TM, Trautner S, Lassen JF, Christiansen EH, Krusell LR, Kristensen SD, Thuesen L, Nielsen SS, Rehling M, Sorensen HT, Redington AN, Nielsen TT. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: A randomised trial. Lancet. 2010;375:727–734. doi: 10.1016/S0140-6736(09)62001-8. [DOI] [PubMed] [Google Scholar]
- 75.Marczak J, Nowicki R, Kulbacka J, Saczko J. Is remote ischaemic preconditioning of benefit to patients undergoing cardiac surgery? Interactive cardiovascular and thoracic surgery. 2012;14:634–639. doi: 10.1093/icvts/ivr123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Contractor H, Stottrup NB, Cunnington C, Manlhiot C, Diesch J, Ormerod JO, Jensen R, Botker HE, Redington A, Schmidt MR, Ashrafian H, Kharbanda RK. Aldehyde dehydrogenase-2 inhibition blocks remote preconditioning in experimental and human models. Basic research in cardiology. 2013;108:343. doi: 10.1007/s00395-013-0343-3. [DOI] [PubMed] [Google Scholar]
- 77.Staat P, Rioufol G, Piot C, Cottin Y, Cung TT, L’Huillier I, Aupetit JF, Bonnefoy E, Finet G, Andre-Fouet X, Ovize M. Postconditioning the human heart. Circulation. 2005;112:2143–2148. doi: 10.1161/CIRCULATIONAHA.105.558122. [DOI] [PubMed] [Google Scholar]
- 78.Thibault H, Piot C, Staat P, Bontemps L, Sportouch C, Rioufol G, Cung TT, Bonnefoy E, Angoulvant D, Aupetit JF, Finet G, Andre-Fouet X, Macia JC, Raczka F, Rossi R, Itti R, Kirkorian G, Derumeaux G, Ovize M. Long-term benefit of postconditioning. Circulation. 2008;117:1037–1044. doi: 10.1161/CIRCULATIONAHA.107.729780. [DOI] [PubMed] [Google Scholar]
- 79.Garcia S, Henry TD, Wang YL, Chavez IJ, Pedersen WR, Lesser JR, Shroff GR, Moore L, Traverse JH. Long-term follow-up of patients undergoing postconditioning during st-elevation myocardial infarction. Journal of cardiovascular translational research. 2011;4:92–98. doi: 10.1007/s12265-010-9252-0. [DOI] [PubMed] [Google Scholar]
- 80.Sorensson P, Saleh N, Bouvier F, Bohm F, Settergren M, Caidahl K, Tornvall P, Arheden H, Ryden L, Pernow J. Effect of postconditioning on infarct size in patients with st elevation myocardial infarction. Heart. 2010;96:1710–1715. doi: 10.1136/hrt.2010.199430. [DOI] [PubMed] [Google Scholar]
- 81.Freixa X, Bellera N, Ortiz-Perez JT, Jimenez M, Pare C, Bosch X, De Caralt TM, Betriu A, Masotti M. Ischaemic postconditioning revisited: Lack of effects on infarct size following primary percutaneous coronary intervention. European heart journal. 2012;33:103–112. doi: 10.1093/eurheartj/ehr297. [DOI] [PubMed] [Google Scholar]
- 82.Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, Elbelghiti R, Cung TT, Bonnefoy E, Angoulvant D, Macia C, Raczka F, Sportouch C, Gahide G, Finet G, Andre-Fouet X, Revel D, Kirkorian G, Monassier JP, Derumeaux G, Ovize M. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. The New England journal of medicine. 2008;359:473–481. doi: 10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- 83.Mochly-Rosen D, Das K, Grimes KV. Protein kinase c, an elusive therapeutic target? Nature reviews. Drug discovery. 2012;11:937–957. doi: 10.1038/nrd3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jahania SM, Sengstock D, Vaitkevicius P, Andres A, Ito BR, Gottlieb RA, Mentzer RM., Jr Activation of the homeostatic intracellular repair response during cardiac surgery. Journal of the American College of Surgeons. 2013;216:719–726. doi: 10.1016/j.jamcollsurg.2012.12.034. discussion 726–719. [DOI] [PMC free article] [PubMed] [Google Scholar]