Abstract
Background and Objectives
Dyslipidaemia is a major cardiovascular risk factor associated with type 2 diabetes mellitus. Saroglitazar (ZYH1) is a novel peroxisome proliferator-activated receptor (PPAR) agonist with predominant PPARα and moderate PPARγ activity. It has been developed for the treatment of dyslipidaemia and has favourable effects on glycaemic parameters in type 2 diabetes mellitus. The objective of this phase 1 study was to evaluate the pharmacokinetics, safety and tolerability of saroglitazar in healthy human subjects.
Methods
This was a randomized, double-blind, placebo-controlled, single-centre, phase I study in healthy human volunteers, and was performed in two parts; part I evaluated single ascending oral doses of saroglitazar (0.125, 0.25, 0.5, 1, 2, 4, 8, 16, 32, 64 and 128 mg) in healthy subjects, and part II measured the effects of food and sex on the pharmacokinetics of 1 mg saroglitazar, the human equivalent efficacy dose derived from pre-clinical studies. A total of 96 subjects were enrolled in the study, which included 88 healthy male subjects in part I and 16 healthy subjects (8 males from part I of the study and 8 females) in part II.
Results
Saroglitazar was rapidly and well absorbed across all doses in the single-dose pharmacokinetic study, with a median time to the peak plasma concentration (t max) of less than 1 h (range 0.63–1 h) under fasting conditions across the doses studied. The maximum plasma concentration ranged from 3.98 to 7,461 ng/mL across the dose range. The area under the plasma concentration–time curve increased in a dose-related manner. The average terminal half-life of saroglitazar was 5.6 h. Saroglitazar was not eliminated via the renal route. There was no effect of sex on the pharmacokinetics of saroglitazar, except for the terminal half-life, which was significantly shorter in females than in males. Food had a small effect on the pharmacokinetics; however, it was not consistent in males and females. Single oral doses of saroglitazar up to 128 mg were well tolerated. No serious adverse events were reported. Adverse events were generally mild and moderate in nature. Saroglitazar did not show any clinically relevant findings in clinical laboratory investigations, physical examinations, vital signs and electrocardiograms.
Conclusion
The highest dose of saroglitazar evaluated in this study was 128 mg, several times the estimated therapeutic doses (1–4 mg). The pharmacokinetics of saroglitazar support a once daily dosage schedule. Saroglitazar was found to be safe and well tolerated in this study.
Introduction
Cardiovascular disease is the most common underlying cause of death, accounting for about 50 % of mortality in type 2 diabetes mellitus (T2DM) [1, 2]. The results of the ACCORD (Action to Control Cardiovascular risk in Diabetes) and ADVANCE (Action in Diabetes and Vascular Disease; Preterax and Diamicron Modified Release Controlled Evaluation) studies showed that intensive glycaemic control reduced microvascular complications (new or worsening nephropathy or retinopathy) but not macrovascular complications (cardiovascular death, nonfatal myocardial infarction or nonfatal stroke) [3, 4]. Diabetic dyslipidaemia is characterized by elevation of serum triglyceride (TG) levels (>150 mg/dL), reduced high-density lipoprotein (HDL) cholesterol levels (<40 mg/dL in males and <50 mg/dL in females) and normal or elevated levels of low-density lipoprotein (LDL) cholesterol (>100 mg/dL) [5, 6]. Low levels of serum HDL cholesterol have been correlated with cardiovascular disease [7–9], and identification of agents that elevate HDL cholesterol in diabetic patients is an area of active interest. The American Diabetes Association (ADA) recommends control of diabetic dyslipidaemia. It has been well established that dyslipidaemia is a major cardiovascular risk factor associated with T2DM [7].
The potential of peroxisome proliferator-activated receptor (PPAR) agonists to reduce the risk of cardiovascular disease in T2DM patients has remained an area of continuous medical interest. PPARα and PPARγ agonists are approved for lipid and glycaemic control, respectively [10, 11]. Numerous dual PPAR agonists have been developed for management of both glycaemic and lipid abnormalities in T2DM. However, none of these agents has so far been successful [11, 12]. Saroglitazar, [(S)-α-ethoxy-4-{2-[2-methyl-5-(4-methylthio) phenyl)]-1H-pyrrol-1-yl]-ethoxy})-benzenepropanoic acid magnesium salt], is the first glitazar that has been granted marketing authorization in India and is indicated for treatment of diabetic dyslipidaemia. Saroglitazar is a dual PPAR agonist with predominant PPARα and moderate PPARγ activity [13]. It was developed with an expectation to achieve optimum anti-dyslipidaemic and anti-hyperglycaemic effects, while avoiding peripheral oedema and weight gain. The structural formula of saroglitazar is given in Fig. 1.
Fig. 1.
Structural formula of saroglitazar
The purpose of this first-in-humans phase 1 study was to assess the pharmacokinetics, safety and tolerability of saroglitazar in healthy volunteers.
Methods
The study was conducted (from 16 June 2005 to 29 November 2005) in accordance with accepted standards for the protection of subject safety and welfare, and the principles of the Declaration of Helsinki and its amendments, and was in compliance with Good Clinical Practice. The study was initiated after obtaining approval from the Drug Controller General of India (DCGI; no. 12-05/05-DC, dated 27 May 2005) and an independent ethics committee (IEC), Aditya, Ahmedabad, Gujarat, India.
Study Design
This was a randomized, double-blind, placebo-controlled, single-centre study to evaluate the pharmacokinetics, safety and tolerability of single ascending oral doses of saroglitazar under fasting conditions in healthy subjects. In addition, the effects of food and sex on the pharmacokinetics of saroglitazar were also studied.
Eligible healthy subjects (aged 18–45 years, weighing 50–70 kg) with normal medical history, physical examination, electrocardiogram (ECG) and clinical laboratory findings were enrolled. Eligible healthy female subjects were enrolled if they had undergone surgical sterilization (tubectomy, hysterectomy or tubal ligation). The subjects had not received any medications within 14 days prior to the current study and had not participated in any study within 3 months prior to the current study.
In each cohort, eight subjects were randomized to receive either saroglitazar or a matching placebo (3:1) by a computer-generated block randomization (SAS Version 9.1, SAS Institute Inc., Cary, NC, USA). Subjects were admitted to the clinical pharmacology unit on the evening prior to dosing and were confined until 72 h after the last dose. The study drugs, saroglitazar (0.125–0.5 mg oral suspension or 1–128 mg tablets) or the matching placebo, were administered orally.
The study was divided into two parts: (i) a single-ascending-dose study; and (ii) a study of the effects of food and sex.
Part I: Single-Ascending-Dose Study
Saroglitazar [versus the matching placebo (3:1)] was studied in 11 cohorts; each cohort comprised eight healthy male subjects. In the first three cohorts, saroglitazar (0.125, 0.25 and 0.5 mg) or the placebo was administered as an oral suspension (0.5 mg/mL). In subsequent cohorts, saroglitazar (1, 2, 4, 8, 16, 32, 64 and 128 mg) or a matching placebo tablet was orally administered. Study drugs were administered with 240 mL of water after overnight fasting for at least 10 h (Fig. 2).
Fig. 2.
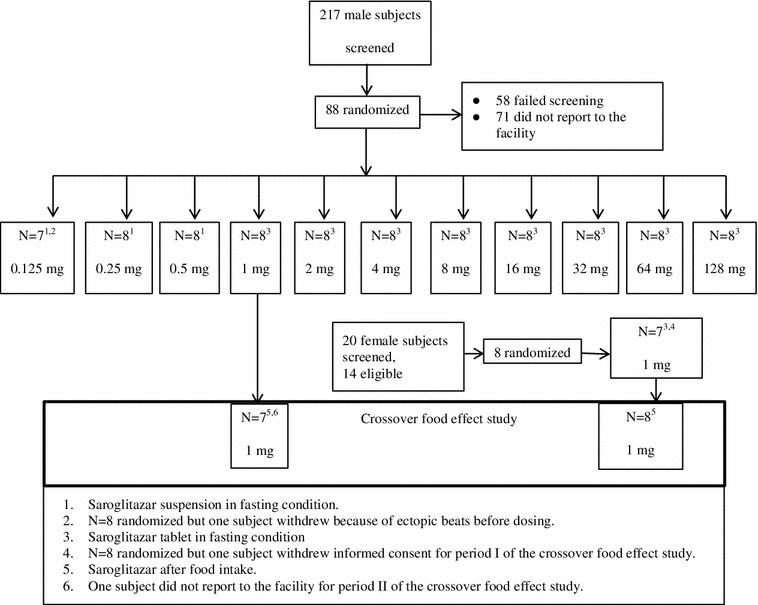
Study design and subject disposition. The pharmacokinetics of saroglitazar in male and female subjects were compared at 1 mg doses. In each group, 6 subjects received saroglitazar and 2 received placebo
Part II: Study of the Effects of Food and Sex
The effects of food and sex were studied with the saroglitazar 1 mg tablet. The food effect was studied in a crossover design. A saroglitazar tablet or a matching placebo (3:1) was administered orally with 240 mL of water after overnight fasting for at least 10 h. To assess the food effect, subjects were given a standard high-fat, high-calorie meal (approximately 800–1,000 calories, of which 150, 250 and 500–600 calories were from protein, carbohydrate and fat, respectively) 30 min prior to administration of the study drug.
Healthy female volunteers (n = 8) were enrolled to assess the effect of sex on saroglitazar pharmacokinetics. Of these, two received placebo and six received saroglitazar 1 mg. Male and female subjects were analyzed to assess the effect of sex on saroglitazar pharmacokinetics under fasting and fed conditions.
Blood Sampling for Pharmacokinetics
In part I and part II, venous blood samples (7 mL) were withdrawn in Vacutainers (ethylenediaminetetraacetic acid [EDTA]) at pre-dose, at 5, 10, 20, 30 and 45 min, and at 1, 2, 4, 6, 8, 12, 24, 48 and 72 h following study drug administration, and were placed on ice. Plasma was separated within 30 min of collection and stored frozen at −70 ± 5 °C until analysis.
Urine Sampling for Pharmacokinetics
Urine samples were collected pre-dose and at intervals of 0–1, 1–3, 3–6, 6–12, 12–24, 24–36, 36–48, 48–60 and 60–72 h following study drug administration. At the end of the interval, the urine bottles were shaken and weighed, and aliquots of the samples were stored frozen at −70 ± 5 °C until analysis.
Safety Assessments
In both parts of the above study, general safety was evaluated by the incidence of adverse events (AEs) through non-leading questions, clinical laboratory investigations (haematology, biochemistry and urinalysis), vital signs, physical examinations and 12-lead ECGs. Identification of the intensity of each AE was performed on the basis of the Common Terminology Criteria for AEs (v3.0).
Bioanalytical Methods
Saroglitazar was assayed in plasma and urine, using a sensitive and specific liquid chromatography–tandem mass spectrometry method. The assays were validated in accordance with the US Food and Drug Administration (FDA) Guidance for Bioanalytical Method Validation [14]. Plasma samples (1 mL) were processed by liquid–liquid extraction, using a mixture of diethyl ether:dichloro methane (80:20). Urine samples were processed using solid-phase extraction (Waters Oasis HLB cartridge, 30 mg, 1 mL). Glimepiride was used as the internal standard for both plasma and urine assays. Overlapping calibration curves were prepared in plasma, with ranges of 0.02–10.0, 0.1–30, 1–1,000 and 5–20,000 ng/mL. The calibration curve range for urine samples was 0.02–10.0 ng/mL. Chromatographic separation of both plasma and urine samples was achieved with an ACE C18 analytical column, using a mobile phase comprising a 70:30 mixture of ammonium acetate buffer and acetonitrile. Quantitative measurement of saroglitazar and the internal standard, glimepiride, were carried out using mass transition (m/z) values of 440 and 491, respectively. Quality-control (QC) samples were analyzed with each batch of urine and plasma samples at low levels (three times the lower limit of quantification), medium levels (the middle of the concentration range) and high levels (75–90 % of the upper limit of quantification). Inter-batch and intra-batch QC samples were within the acceptance limits.
Pharmacokinetic Data Analysis
The concentration–time data were subjected to non-compartmental pharmacokinetic analysis, using WinNonlin Professional Software, Versions 4.0.1 and 5.0.1 (Pharsight Corporation, St Louis, MO, USA). The parameters that were estimated were the maximum plasma concentration (C max), time to reach C max (t max), area under the plasma concentration–time curve (AUC), AUC from time zero to the time of the last measurable concentration (AUClast), AUC from time zero to infinity (AUC∞), terminal elimination half-life (t ½β) and percentage of the drug excreted in urine.
Statistical Analysis
Descriptive statistics (means and standard deviations) were used for pharmacokinetic and safety analysis. Dose relationships with C max and AUClast were calculated using correlation and regression analysis.
Results
A total of 96 subjects were enrolled in the study.
In the single-ascending-dose study (part I), 88 healthy male subjects were recruited and 87 completed the studies. One subject was withdrawn because of an ectopic beat before the dosing of saroglitazar 0.125 mg suspension. In the crossover food-effect study (part II), eight males (who were part of the single-ascending-dose study) and eight females participated. One of the eight female subjects withdrew her consent before dosing in the fasting study arm (period I); however, she participated in the fed study arm (period II). One of the eight male subjects did not report for the fed study arm (period II) [Fig. 2]. For the food-effect assessment, statistical analysis was performed using the five male and five female subjects who completed both study periods.
Safety and Tolerability
Saroglitazar was safe and well tolerated up to a 128 mg oral dose during the single-ascending-dose study and also during the study of the effects of food and sex. There was no serious AE observed during the study in any treatment arm. A total of 22 AEs in 11 subjects were reported during the study, included rash/itching, abdominal pain, nausea, cough, cold, headache, backache, body pain, calf pain, fever, malaise, giddiness, dyspepsia and diarrhoea; however, they were mild to moderate in intensity. None of the AEs was treatment emergent, and none required any treatment for resolution. There was no clinically relevant trend or change observed in clinical laboratory, urinalysis or ECG findings in the placebo or treatment arms during the study. There was no consistent pattern or dose dependency observed in the AEs.
Pharmacokinetics in the Single-Ascending-Dose Study (Part I)
Saroglitazar was well absorbed after oral administration under fasting conditions. The absorption was rapid, with a median t max of less than 1 h. The C max values were from 3.98 to 7,461 ng/mL across the dose range. The C max and AUClast values increased in a dose-related manner. The pharmacokinetic parameters determined in the single-ascending-dose study are presented in Table 1. The results indicated that saroglitazar is well absorbed in humans. The exposure increased in a predictable manner with increasing doses (Fig. 3). Saroglitazar concentration–time curves (mean ± standard error of the mean) after administration of 0.125, 0.25, 0.50, 1, 2, 4, 8, 16, 32, 64 and 128 mg are shown in Figs. 4 and 5. These show that the highest dose had an extended length of drug absorption.
Table 1.
Pharmacokinetic parameters (means ± standard deviations) of saroglitazar following a single oral dose of saroglitazar in healthy subjects
| Doses (mg) | C max (ng/mL) | t max (h) | AUClast (ng·h/mL) | AUC∞ (ng·h/mL) | λz (L/h) | t ½β (h) |
|---|---|---|---|---|---|---|
| 0.125a | 3.98 ± 1.14 | 0.75 ± 0.25 | 12.62 ± 3.67 | 13.22 ± 3.54 | 0.13 ± 0.07 | 6.87 ± 3.63 |
| 0.25a | 19.56 ± 3.36 | 1.17 ± 1.39 | 46.57 ± 14.68 | 49.27 ± 16.17 | 0.31 ± 0.14 | 3.08 ± 2.52 |
| 0.5a | 23.42 ± 6.32 | 0.88 ± 0.56 | 55.98 ± 15.33 | 58.97 ± 17.14 | 0.39 ± 0.13 | 2.00 ± 0.78 |
| 1 | 93.16 ± 42.12 | 1.00 ± 0.52 | 249.20 ± 104.58 | 253.82 ± 103.97 | 0.13 ± 0.03 | 5.39 ± 1.33 |
| 2 | 223.18 ± 27.42 | 1.21 ± 0.64 | 726.46 ± 237.14 | 750.57 ± 255.68 | 0.06 ± 0.03 | 14.34 ± 5.68 |
| 4 | 337.07 ± 90.99 | 0.71 ± 0.25 | 806.40 ± 160.4 | 855.96 ± 172.5 | 0.26 ± 0.10 | 2.93 ± 0.87 |
| 8 | 727.27 ± 408.63 | 0.85 ± 0.61 | 1,802.02 ± 1,009.2 | 1,937.66 ± 992.96 | 0.21 ± 0.14 | 4.47 ± 2.07 |
| 16 | 1,548.47 ± 427.29 | 0.64 ± 0.24 | 3,563.31 ± 1,094.13 | 3,689.26 ± 1,132.24 | 0.25 ± 0.09 | 3.31 ± 1.95 |
| 32 | 3,740.17 ± 1,180.28 | 1.21 ± 0.64 | 10,352.04 ± 2,217.12 | 10,479.65 ± 2,206.85 | 0.20 ± 0.07 | 3.84 ± 1.09 |
| 64 | 5,091.33 ± 1,257.22 | 1.17 ± 0.66 | 16,958.86 ± 3,648.04 | 17,419.22 ± 3,736.56 | 0.14 ± 0.08 | 7.23 ± 6.58 |
| 128 | 7,461.17 ± 3,035.09 | 1.88 ± 1.66 | 32,622.68 ± 8,282.79 | 33,385.65 ± 8,137.62 | 0.12 ± 0.07 | 7.68 ± 4.14 |
λ z first-order elimination rate constant associated with the terminal (log-linear) portion of the curve, AUC area under the plasma concentration–time curve, AUC last AUC from time zero to the time of the last measurable concentration, AUC ∞ AUC from time zero to infinity, C max maximum plasma concentration, t ½β elimination or terminal half-life, t max time to reach C max
aThe suspension was used for dosing, N = 6 (N = 5 for the 0.125 mg dose)
Fig. 3.
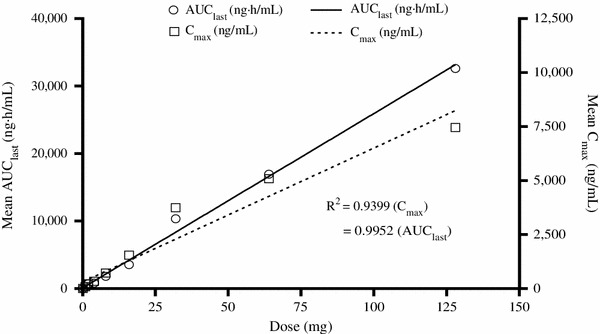
Dose linearity of the area under the plasma concentration–time curve from time zero to the time of the last measurable concentration (AUClast) and maximum plasma concentration ( C max) as a function of a single oral dose of saroglitazar administered to healthy subjects
Fig. 4.
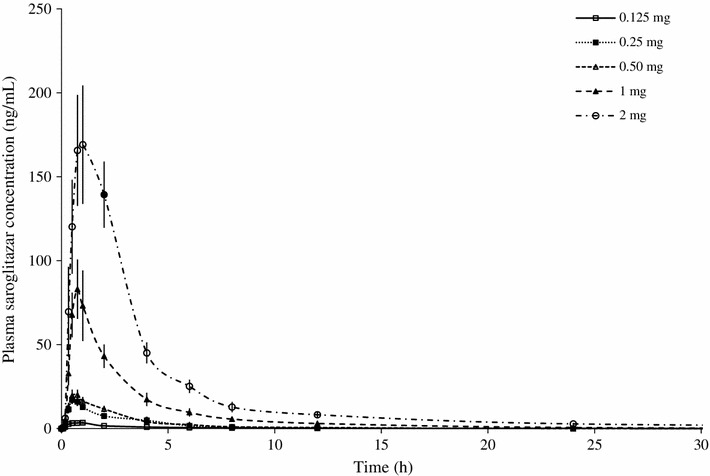
Line plot for mean (±standard error of the mean) concentration–time curves for saroglitazar after administration of 0.125, 0.25, 0.50, 1 and 2 mg doses to healthy, adult, male, human subjects under fasting conditions
Fig. 5.
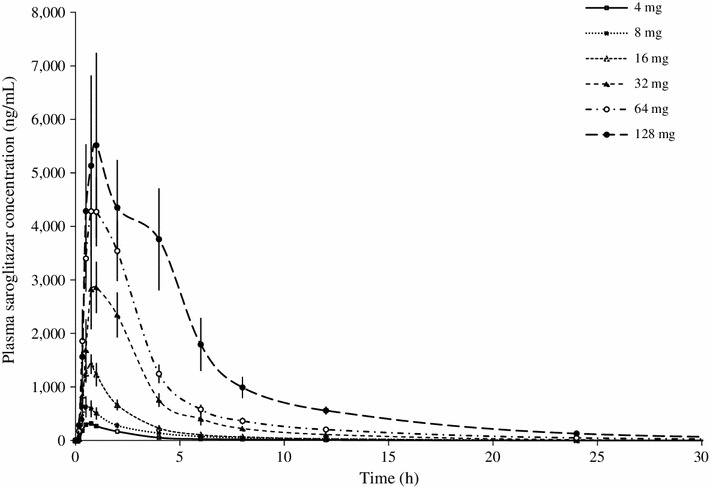
Line plot for mean (±standard error of the mean) concentration–time curves for saroglitazar after administration of 4, 8, 16, 32, 64 and 128 mg doses to healthy, adult, male, human subjects under fasting conditions
The mean elimination half-life of saroglitazar across the doses was 5.6 h. Saroglitazar was not excreted in urine, indicating that it has a non-renal route of elimination. No concentration of saroglitazar was quantifiable in urine samples in any of the subjects exposed to saroglitazar. Pre-clinical studies have shown that saroglitazar is mainly eliminated by the hepatobiliary route [15]. Taken together, these data indicate that saroglitazar might have a non-renal route of elimination in humans. On the basis of the half-life, the drug does not show a potential for accumulation following once-daily repeat dosing in normal healthy volunteers.
Effects of Food and Sex on Saroglitazar Pharmacokinetics (Part II)
The effects of food and sex on saroglitazar pharmacokinetics are presented in Table 2. The effect of sex on saroglitazar pharmacokinetics was evaluated at the 1 mg dose level under fasting conditions. The median t max was approximately 1 h post-dosing for both males and females. The mean C max and AUC values were similar in both sexes. No statistically significant differences in t max, AUC and C max values were found. The t ½β was shorter in females, and this finding was statistically significant as compared with male subjects under fasting and fed conditions.
Table 2.
Pharmacokinetic parameters (means ± standard deviations [SDs]) following a single oral dose of saroglitazar 1 mg in healthy subjects under fasting and fed conditions
| Sex | N | t max (h) | C max (ng/mL) | AUClast (ng·h/mL) | AUC∞ (ng·h/mL) | t ½β (h) |
|---|---|---|---|---|---|---|
| Male | ||||||
| Fasting | 6 | 1.00 ± 0.52 | 93.16 ± 42.12 | 249.19 ± 104.58 | 253.82 ± 103.97 | 5.39 ± 1.33 |
| Feda | 5 | 2.00b ± 0.00 | 49.42b ± 15.95 | 198.05 ± 36.86 | 202.44 ± 34.99 | 11.78b ± 4.77 |
| Female | ||||||
| Fastinga | 5 | 0.90 ± 0.14 | 96.95 ± 23.56 | 287.97 ± 101.10 | 297.36 ± 100.12 | 2.86c ± 1.08 |
| Fed | 6 | 2.38 ± 1.79 | 84.06 ± 40.74 | 275.75b ± 123.13 | 288.09b ± 128.72 | 3.77c ± 1.79 |
AUC area under the plasma concentration–time curve, AUC last AUC from time zero to the time of the last measurable concentration, AUC ∞ AUC from time zero to infinity, C max maximum plasma concentration, t ½β elimination or terminal half-life, t max time to reach C max
aOne male subject did not report during the fed period of the study, and one female subject withdrew her consent during the fasting period of the study. The means of all available data (n = 5 or 6, as available) are shown in the table
bSignificant (p < 0.05) using a paired t test for fed versus fasting for n = 5 subjects: for 5 fasting male subjects, the t max, Cmax and t ½β (mean ± SD) values were 1.00 ± 0.59 h, 99.77 ± 43.47 ng/mL and 5.00 ± 1.03 h, respectively. For 5 fed female subjects, the AUClast and AUC∞ (mean ± SD) values were 242.31 ± 102.79 ng·h/mL and 252.08 ± 104.82 ng·h/mL
cSignificant (p < 0.05) using unpaired t test for males as compared with females
The effect of food on saroglitazar pharmacokinetics was evaluated at the 1 mg dose level in male and female subjects. Administration of food had a small effect on the pharmacokinetics of the drug. In male subjects, there were no significant changes in overall exposure (AUC); however, there were significant increases in t max and t ½β, and a decrease in C max. In females, the only change that was observed was a significant reduction in AUC under fed conditions. No other pharmacokinetics parameters were affected by food.
Discussion
Saroglitazar is a novel PPAR agonist with predominant PPARα and moderate PPARγ agonist activity. It has been developed for the treatment of dyslipidaemia and glycaemic management in T2DM. PPARs are nuclear receptors, and hence the effects of saroglitazar are likely to be mediated by changes in expression of various genes involved in metabolism.
In this study, the pharmacokinetics, safety and tolerability of saroglitazar were evaluated in healthy human subjects. Saroglitazar was found to be well absorbed and showed predictable pharmacokinetic parameters when evaluated in the single-ascending-dose study. These data are consistent with high transepithelial permeability of saroglitazar (162 nm/s) as seen in the well-established human Caco-2 cell model for intestinal absorption [16]. Two different types of formulations were used in this study. Lower doses were formulated in a suspension form, which provided the required pharmaceutical properties, including content uniformity. Higher-dose strengths were prepared in tablet dosage form. Despite a switch in formulation from the suspension (0.125–0.5 mg) to tablets (1–128 mg), exposure increased in a dose-related and linear manner. There was no indication of saturation of exposure, even at the high dose. The elimination half-life in subjects treated with 2 mg appeared to be prolonged, perhaps because of slower clearance in these subjects. This finding could be due to the small sample size and inter-individual variability.
After oral administration, the saroglitazar parent drug was not detected (<20 pg/mL, the lower limit of quantification) in urine samples, indicating that saroglitazar is not eliminated via the renal route. Preclinical studies [15] have shown that saroglitazar is mainly eliminated by the enterohepatic route. Since kidney function is compromised in the advanced stage of T2DM, a non-renal route of elimination may be beneficial. Saroglitazar dose adjustment may not be required in such patients.
It should be noted that the single-ascending-dose study results were limited to healthy volunteers who were under fasting conditions and were not taking other medications. Also, there were a limited number of patients in the study of the effects of food and sex, and no correction was made for body weight differences.
This first-in-humans study with a small number of healthy volunteers demonstrated that saroglitazar was well tolerated up to a dose of 128 mg in the single-ascending-dose study. Because the estimated therapeutic dose, based on pre-clinical studies, was in the range of 1–4 mg, the highest tested dose of 128 mg provided a safety margin of over 32-fold. Therefore, further dose escalation to reach the maximum tolerated dose (MTD) was not considered. No serious AEs were observed in this study. All AEs that were observed were mild to moderate in nature. No consistent pattern or dose dependency was observed in the AEs. No clinically relevant trends or changes were observed in medical laboratory, urinalysis or ECG values over time.
Conclusion
Saroglitazar was found to be safe and well tolerated in both males and females in the SAD study. The highest dose tested in the study (128 mg) was over 32 times higher than the proposed efficacious dose. Saroglitazar was very well absorbed orally and has a non-renal route of elimination. The pharmacokinetics of saroglitazar are supportive of once daily dosing for this class.
Acknowledgments
This study was sponsored and funded by Cadila Healthcare Limited, a Zydus Group Company (Ahmedabad, Gujarat, India). The authors thank Dr Vikram Ramanathan for his contribution to the discussion and review of the manuscript, and Mr Rahul Gupta for providing statistical assistance; both are employees of Cadila Healthcare Limited. All authors are employees of Cadila Healthcare Limited. This report was prepared by the authors, who had full access to all data in the study and had final responsibility for the decision to submit the manuscript for publication.
Part of this work was presented at the 69th Scientific Sessions of the American Diabetes Association; New Orleans, LA, USA; 5–9 June 2009.
References
- 1.World Health Organization. Diabetes [factsheet no. 312] (2013) http://www.who.int/mediacentre/factsheets/fs312/en/index.html. Accessed 23 May 2013.
- 2.Morrish NJ, Wang SL, Stevens LK, et al. Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia. 2001;44(Suppl 2):S14–S21. doi: 10.1007/PL00002934. [DOI] [PubMed] [Google Scholar]
- 3.Robert GD, Graham TM. Intensive glycemic control in the ACCORD and ADVANCE trials. N Engl J Med. 2008;358:2630–2633. doi: 10.1056/NEJMe0804182. [DOI] [PubMed] [Google Scholar]
- 4.Sophia Z, Bastiaan EG, Toshiharu N, et al. Combined effects of routine blood pressure lowering and intensive glucose control on macrovascular and microvascular outcomes in patients with type 2 diabetes. Diabetes Care. 2009;32:2068–2074. doi: 10.2337/dc09-0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solano MDP, Goldberg RB. Management of diabetic dyslipidemia. Endocrinol Metab Clin N Am. 2005;34:1–25. doi: 10.1016/j.ecl.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association. Dyslipidemia management in adults with diabetes. Clinical Practice Recommendation 2004. Diabetes Care. 2004;27 (Suppl. 1):S68–71. [DOI] [PubMed]
- 7.American Diabetes Association Standards of medical care in diabetes—2013. Diabetes Care. 2013;36:S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol; Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med. 1999;341:410–418. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- 9.Steiner G. Dyslipoproteinaemias in diabetes. Clin Invest Med. 1995;18:282–287. [PubMed] [Google Scholar]
- 10.Robert RH, Michael AL, Sunder M, et al. Effects of the dual peroxisome proliferator-activated receptor-α/γ agonist aleglitazar on risk of cardiovascular disease in patients with type 2 diabetes (SYNCHRONY) Lancet. 2009;374:126–135. doi: 10.1016/S0140-6736(09)60870-9. [DOI] [PubMed] [Google Scholar]
- 11.Vamecq J, Latruffe N. Medical significance of peroxisome proliferator-activated receptors. Lancet. 1999;354:141–148. doi: 10.1016/S0140-6736(98)10364-1. [DOI] [PubMed] [Google Scholar]
- 12.Alexander T, Michael M, Enrique ZF. Dual and pan-peroxisome proliferator-activated receptors (PPAR) co-agonism. Cardiovasc Diabetol. 2005;4:14. doi: 10.1186/1475-2840-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agrawal R. The first approved agent in the glitazar class: saroglitazar. Curr Drug Targets. 2013 August 1. [Epub ahead of printing]. [DOI] [PubMed]
- 14.US Food and Drug Administration. Guidance for bioanalytical method validation, US Department of Health and Human Services. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070107.pdf. Accessed 10 Sep 2013.
- 15.Sonu S. Biliary excretion of ZYH1 in Wistar rats. Ahmedabad: Cadila Healthcare Ltd.; 2004. [Google Scholar]
- 16.Poonam G. Determination of monodirectional permeability of ZYH1 across Caco2 cell monolayer using LC-MS/MS. Ahmedabad: Cadila Healthcare Ltd.; 2011. [Google Scholar]


