Abstract
There are substantial racial/ethnic disparities in cardiovascular disease in the U.S., but few mechanisms have emerged as feasible intervention targets. A growing body of research suggests that racial/ethnic differences in sleep deficiency, including extreme sleep duration, sleep-disordered breathing, and insomnia, may help explain disparities in cardiovascular disease. However, little is known about the mechanisms underlying racial/ethnic disparities in sleep. In this article, we review the extant literature on sleep and cardiovascular outcomes (e.g., hypertension, stroke, cardiovascular disease) and racial/ethnic differences in these relations. We also discuss possible mechanisms that might help explain racial/ethnic sleep disparities, including neighborhood disadvantage, psychosocial and occupational stressors, acculturation, and treatment access and adherence. More research is needed to establish causal linkages among race/ethnicity, sleep, and these mechanisms, but existing evidence suggests that targeting these factors in interventions may reduce racial/ethnic sleep disparities and improve primary prevention of cardiovascular disease among all racial/ethnic groups.
Keywords: sleep, sleep duration, sleep-disordered breathing, insomnia, race, ethnicity, disparities, cardiovascular disease
Racial/ethnic disparities in cardiovascular disease (CVD) pose a serious public health problem in the U.S. Relative to non-Hispanic Whites, African Americans/Blacks are 43% more likely to develop hypertension, 95% more likely to have a stroke, and 30% more likely to die from CVD.(1) There are many potential mechanisms that likely contribute to CVD disparities (e.g., social and physical environment), but few have emerged as feasible intervention targets. Sleep disturbances and sleep disorders are disproportionately prevalent in minority groups. Therefore, sleep may help explain CVD disparities and provide novel targets to improve CV health.(2) We review the literature on the interplay among race/ethnicity, sleep duration, two common sleep disorders (sleep-disordered breathing [SDB] and insomnia), and CVD, highlighting mechanisms that may explain disparities.
What is Sleep's Purpose?
Sleep is a distinct neurophysiological state during which characteristic physiological changes occur. Brain electrical and metabolic activities and autonomic nervous system activity vary with sleep versus wake states and across sleep stages. Variations in autonomic nervous system activity, hormone levels, and metabolism influence intermediate pathways to CVD, including diurnal patterns of blood pressure and heart rate, insulin sensitivity, and fluid and salt homeostasis. Sleep serves a restorative function for the brain and body, and is an integral part of overall health and a source of physiological and psychological resilience.
Humans have evolved to require 7-9 hours per night during adulthood, with absolute variation in sleep need likely influenced by genetic and other individual factors. There is evidence that sleep disorders are associated with increased risk of accidents, injuries,(3,4) and reduced health-related quality of life and cognitive functioning (5). Sleep deficiency, “a deficit in the quantity or quality of sleep obtained versus the amount needed for optimal health and well-being,” (6) has deleterious effects on the autonomic nervous system, and thus, is particularly stressful on the CV system (see Figure 1).(7,8)
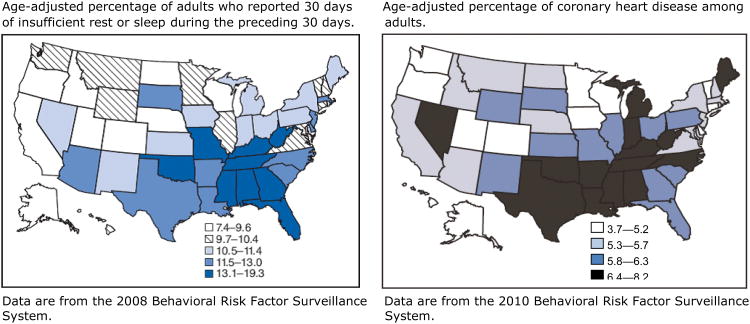
Figure 1.
Distribution of insufficient sleep and coronary heart disease across the United States.
Sleep Duration and CVD
Extreme sleep duration
A review of 15 studies on sleep duration and CV outcomes found strong evidence that short sleep duration is associated with a greater risk of stroke and developing or dying from CVD.(9) Similarly, a systematic review of 30 studies found that self-reported short sleepers are 55% more likely to be obese than those reporting ≥7 hours of sleep per night.(10) National Health Interview Survey (NHIS) data indicate that, relative to sleeping 7-8 hours/night, self-reported short (≤ 6 hours) and long sleep (>9 hours) are independently associated with increased obesity, and diagnosis of diabetes, hypertension, and CVD.(11)
Mechanisms linking short sleep and CVD likely include perturbations of the hypothalamic-pituitary-adrenal axis as well as disturbances in autonomic activity. Experimental studies testing physiological effects of acute sleep deprivation observed increased diastolic blood pressure, abnormalities in muscle sympathetic nerve activity,(12) reduced leptin, and increased ghrelin levels,(13) elevated afternoon and evening cortisol, and reduced insulin sensitivity.(14) Prospective studies have linked short sleep duration with incident hypertension (15) and coronary artery calcification. (16)
Although long sleep (> 9 hours per night) has been linked with adverse CVD outcomes, the basis for this association is unclear. Long sleep may occur in various chronic psychiatric and medical conditions, which may be unmeasured confounders. Long sleep may also be associated with excessive sedentary behavior, which may independently influence CVD. Furthermore, long sleep may be due to over-compensation for poor sleep quality stemming from a sleep disorder (e.g., SDB).(17)
Racial/ethnic differences in extreme sleep duration
Extremes of sleep duration vary across racial/ethnic groups. Compared to Whites, Blacks are nearly twice as likely to report short sleep,(18,19), and over 60% more likely to report long sleep.(20) One study estimated that Blacks sleep roughly 35-60 minutes less per night than do Whites.(21-23) A recent meta-analysis of 14 studies found that Blacks were more likely to be short sleepers than were Whites, with larger effect sizes in studies using objective as compared to. self-reported measures of sleep duration.(24) Self-report measures may underestimate the magnitude of racial differences in sleep duration.
Blacks may also be at risk for more severe consequences of extreme sleep than Whites. Analysis of NHIS data revealed that, among individuals reporting short or long sleep duration, Blacks were at greater risk of diabetes than were Whites.(25) Moreover, these effects remained significant when controlling for age, sex, and income.
Less research has examined sleep health in Hispanics, but existing evidence suggests that they, too, may be at increased risk for extreme sleep duration.(26) Relative to non-Hispanic whites, Hispanics are more likely to report short (20) and long sleep duration,(19) controlling for confounders. More work is needed to investigate whether similar effects are present among other minority groups, and for other CV outcomes.
In sum, extreme sleep durations are disproportionately common in low-income and minority groups, and are associated with intermediate CVD mechanisms, and subclinical and clinical CVD. Although there are limited longitudinal data available, it appears likely that extreme sleep duration may mediate a portion of the increased CVD burden observed in African Americans and Hispanic groups.
Sleep Disorders and CVD
Sleep disorders, such as SDB and insomnia, also influence the pathogenesis of CVD.
Sleep-disordered breathing (SDB)
SDB is the occurrence of repetitive episodes of pharyngeal obstruction during sleep manifest as recurrent hypopneas or apneas. SDB is associated with symptoms of sleep disruption, snoring, and daytime sleepiness. The physiological effects of SDB-associated recurrent intermittent hypoxemia, intrathoracic pressure swings, brain arousals, and sympathetic nervous system activation adversely impact blood pressure, endothelial function, insulin sensitivity, atherogenesis, and cardiac function.(27-29) Epidemiological studies have established SDB as a risk factor for incident hypertension,(30) heart failure, coronary artery disease, and stroke.(31,32) SDB treatment with nasal continuous positive airway pressure (CPAP) improves insulin sensitivity and metabolic indices, decreases blood pressure (33,34) and reduces rates of fatal and non-fatal CVD (35) and incident hypertension.(30)
Racial/ethnic differences in SDB
Minority group members may be at greater risk for more frequent and severe presentations of SDB than Whites. Racial differences in SDB are most evident among children and young adults, as Black children and young adults are 4-6 times more likely to have SDB than their White counterparts, (37,38) with differences emerging very early.
A cross-sectional study of 346 children age 2-6 found that the odds of snoring were three times greater among Blacks than Whites.(36) Self-reported “frequent” snoring is also more common among Hispanic and Black adults than among Whites.(37) One study showed that Hispanic and Black adults were nearly three times as likely to exhibit higher frequency of oxygen desaturation events during sleep as were Whites.(38) A cross-sectional study of 280 patients with obstructive sleep apnea found that: (a) relative to Whites, Blacks were significantly more obese and had higher rates of hypertension at the time of sleep apnea diagnosis, (b) Black females were diagnosed at a significantly younger age than were White females, and (c) Black males had significantly lower oxygen saturation level than did White males, controlling for BMI and age.(39) Thus, Blacks may experience earlier and more severe presentations of SDB than Whites, and SDB may contribute to a lifelong increased risk of CVD among minority groups.
Insomnia
Insomnia is defined as difficulty falling or remaining asleep, despite adequate opportunities to sleep, with associated daytime impairment. It reflects a condition of cognitive and/or physiological “hyperarousal,” as evidenced by its associations with worry and psychological stress, and alterations in metabolic rate, heart rate variability, and cortisol secretion.(40-43) For example, in a study of over 4300 Danish civil servants, insomnia symptoms were associated with reduced cortisol awakening response and a flattened diurnal cortisol profile.(44) Given these pathophysiological correlates, it is not surprising that there is a growing literature linking insomnia to CV morbidity. Laugsand and colleagues found that insomnia symptoms were associated with greater risk of acute myocardial infarction in a study of >50,000 adults.(45) A recent review estimated that the presence of insomnia symptoms is associated with a relative risk of CVD ranging from 1.5-3.9.(46) Recent evidence suggests that the link between insomnia and CVD may be especially strong among short sleepers. A prospective study of nearly 1400 adults found that the risk of incident hypertension was highest among those with self-reported chronic insomnia and objective short sleep duration, controlling for SDB.(47) Together, these studies indicate a robust link between insomnia and CV morbidity. Further research is needed to dissect the associations among sleep duration, insomnia, and CVD.
Racial/ethnic differences in insomnia
Racial differences in insomnia are not well-understood.(20) One cross-sectional study of 1741 adults found that self-reported chronic insomnia (i.e., symptoms >1 year) was more common among minority group members than among Whites.(48) Similarly, a survey of 92 primary care patients found that Blacks were more likely than Whites to report sleep disturbance.(49) This relationship was partially attenuated when controlling for SES, depression, and medical illness. Similar results were found in a prospective study: non-Whites suffered from more chronic insomnia, controlling for potential confounders and baseline insomnia symptoms.(50)
In contrast, other studies have found that minorities are at lower risk of insomnia. A survey of over 17,000 adults revealed that Whites reported more trouble falling asleep and staying asleep than did Blacks and Hispanics.(51) Similar findings were reported in a diary study examining self-reported chronic insomnia in 769 adults.(52)
These mixed findings may be due, in part, to methodological issues, such as differing definitions of insomnia or reliance on self-reports. Of the studies reviewed here, those with the strongest methods (e.g., use of validated self-report measures, prospective designs) found evidence of a racial difference in insomnia;(49) still, more methodologically-sound studies are needed to establish the true nature of the link between race/ethnicity and insomnia, and better understand how perceived symptoms and physiological differences in sleep vary by ethnicity/race.
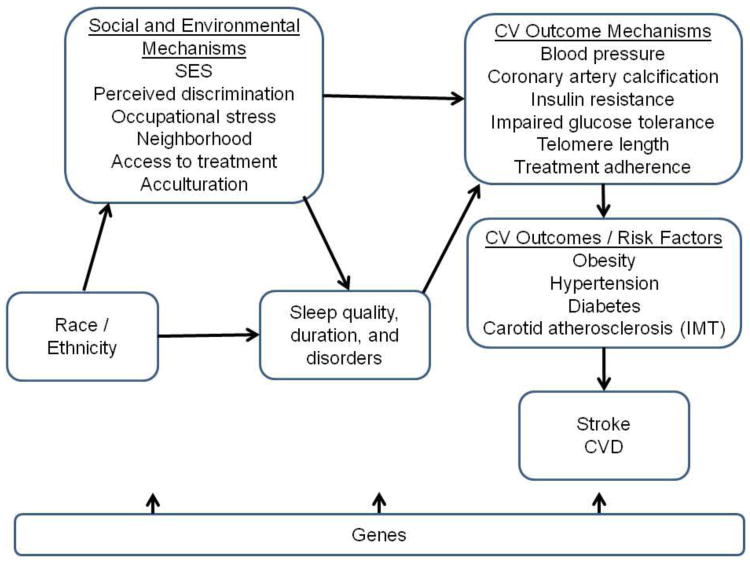
Possible Mechanisms (see Figure 2 for conceptual model)
Figure 2.
Conceptual model of relationship among social and environmental factors, sleep, and cardiovascular disease outcomes.
There is considerable evidence linking extreme sleep duration, SDB, and insomnia with CVD markers and CV outcomes. Poor sleep health is associated with physiological responses (e.g., hormonal changes, increased blood pressure, reduced insulin sensitivity), which may have harmful downstream consequences for CV health. Because there is evidence of racial differences in both extreme sleep duration and SDB, and to a slightly lesser extent, insomnia, these factors have received consideration as contributors to observed ethno-racial disparities in CV outcomes. Less is known about the mediating mechanisms that explain why racial and ethnic minorities are at greater risk for sleep deficiency than are Whites.
Neighborhood disadvantage
One possible way in which race/ethnicity influences sleep is through increased exposure to various environmental physical and emotional stressors. For example, poor urban environments have higher levels of air pollution,(53,54) which may be lead to an increased prevalence of SDB.(55) Other studies have found an association between neighborhood disorder and self-reported sleep quality.(56,57) Those living in poorer neighborhoods are more likely to be exposed to factors that may contribute to sleep deficiency, such as inopportune light exposure, noise, allergens, and irritants (e.g., environmental tobacco).(58,59) Some of these factors (such as particulate air pollution) may also contribute to abnormalities in the autonomic nervous system and increase CV morbidity. Because minority group members are more likely to live in disadvantaged neighborhoods than are Whites,(60) the adverse effects of stressors and exposures in disadvantaged neighborhoods may contribute to CVD disparities via an influence on sleep.
Occupational stressors
Minorities are also at greater risk of suffering from occupational stressors, which have been linked with sleep deficiency,(61) self-reported sleep quality, continuity,(62) and duration.(63) In the U.S., Blacks and Hispanics are more likely to work the night shift, less likely to have flexible work schedules, and more likely to work greater than 40 hours per week than are Whites.(23,64) Work-environment characteristics, such as harassment on the job and low supervisor support, are associated with increased sleep deficiency.(65) Having an inflexible manager with respect to work and family needs is associated with both shorter sleep duration on the order of one-half hour per day, and a doubling of CVD risk.(66) Shift work is associated with abnormalities in intermediate markers of CVD such as blood pressure and glucose levels, as well as with myocardial infarction, and diabetes.(67,68) Taken together, these studies suggest that minorities may suffer from greater sleep deficiency and CVD risk factors in part from increased exposure to occupational stressors that include shift work, long work hours,(23) inflexible work schedules, and possibly workplace discrimination. It also likely that workplace exposures to airborne irritants may contribute to SDB.
Psychosocial stressors
Researchers have suggested that psychosocial factors should receive greater attention as contributors to health disparities. One of the primary psychosocial stressors that is disproportionately experienced by minorities, and may be implicated in sleep disparities, is perceived racial discrimination.(69,70) Reports of lifetime discrimination were positively associated with self-reported insufficient sleep in a survey of over 7000 adults.(71) Similarly, a study of 168 Hispanics found that perceived racism was predictive of self-reported sleep disturbance.(72) Perceived discrimination has also been shown to influence sleep architecture. As mentioned previously, Blacks have less slow wave sleep than do Whites,(73) and this effect appears to be partially mediated by perceived racial discrimination.(74) More work is needed to unpack the effects of discrimination on sleep, but existing evidence suggests that the stress of discrimination influences minority group members' sleep quality and duration, and thus, may have downstream effects on CV outcomes.
Acculturation
Recent evidence suggests that the harmful effects of acculturation (75,76),(77)(78,79) among Hispanics extend to sleep duration. A nationally-representative study found that Mexican-Americans were 44% more likely to report short sleep duration than were Mexican immigrants, when controlling for confounders.(80) This effect was modestly attenuated with the addition of smoking and self-reported stress to the model, suggesting that these variables may help explain the link between acculturation and sleep duration. Acculturation has also been linked to sleep disturbance. A study of over 300 women of Mexican descent found that two measures of acculturation, language preference (Spanish or English) and socialization in the U.S. before age 18, were significant predictors of self-reported sleep disturbance.(81) These studies provide initial evidence highlighting acculturation as a mechanism by which Mexican-Americans experience greater sleep deficiency than Whites. Future studies should investigate this relation in other racial/ethnic groups and dissect the extent to which acculturation reflects the influences of a “Westernized” lifestyle or reflects stress associated with acculturation.
Treatment access and adherence
Limited access to treatment for sleep disorders has been linked with race/ethnicity, and may play a role in sleep and CVD disparities. Minority group members suffering from sleep disorders may receive later diagnoses, and have less access to treatment than Whites.(82) Moreover, there is evidence suggesting that, independent of access to treatment, adherence to sleep disorder treatments is lower among Blacks than Whites,(83,84) which may unnecessarily increase incidence and severity of CV outcomes. The HomePAP study, which provided standardized care to all participants, found that Blacks were less likely to adhere to CPAP treatment than were Whites, and this effect was partially mediated by self-reported short sleep duration.(85) Differences in socioeconomic resources, social support, and perceived benefits/risks of treatment may underlie racial differences in treatment adherence.(86) Therefore, increasing sleep duration among individuals suffering from SDB may help improve treatment adherence, and reduce disparities in CV outcomes.
Future Directions and Needed Research
Much research has investigated sleep and its relationship to racial and ethnic disparities in CVD, but many questions remain unanswered. Research is needed to investigate possible mechanisms by which race/ethnicity is related to sleep, and protective factors that could be targeted by interventions aimed at promoting healthy sleep among all groups.
Clearer definitions of “short” and “long” sleep are needed in studies investigating extreme sleep duration and CV outcomes. The lack of universal definitions for these terms poses problems for meta-analyses and reviews.(17) Moreover, more work is needed to assess individual differences in sleep need, which will help researchers better identify the presence of sleep deficiency due to insufficient sleep duration. Future studies should employ more objective measures of sleep, such as actigraphy and polysomnography, or survey measures that have been validated against objective assessments in each group of interest. These methodological improvements are critical for determining a more accurate estimate of the effects of sleep on CV outcomes across different racial/ethnic groups.
More research is also needed to examine the possible bidirectional relationships between stress and sleep, and their associations with CV outcomes. Prospective studies will be critical in establishing temporal ordering of sleep duration and sleep problems, stress, and CV outcomes for different racial/ethnic groups. In addition, more work is needed to investigate how the nature of stressors influences sleep. Stressors can take many different forms, including those that are sudden, daily, and chronic.(87) Examining how the nature of stressors differs by race/ethnicity to predict sleep health could provide insight into sleep disparities. Studies are also needed to identify key demographic, personality, environmental, and genetic moderators of the effects of race on sleep, and sleep on CVD, and to better understand whether sleep disturbances differentially contribute to CVD risk in individuals of different ethnic/racial backgrounds. For example, shift workers may be at especially high risk for CVD if they also live in a poor, urban neighborhood; or, the effects of perceived discrimination on sleep may be mitigated for individuals who possess a strong sense of racial/ethnic identity. Research examining potential genetic and environmental moderators would provide a more critical understanding of the mechanistic and possible epigenetic links among race, sleep, and CV outcomes.
Finally, improvements to housing policy and culturally-relevant intervention efforts are needed to improve the sleep habits and environment of all racial/ethnic groups. For example, policies to promote safe neighborhoods, such as increased nocturnal street light, may inadvertently create barriers to healthy sleep. Moreover, subsidized housing complexes may not provide residents with the necessary resources to establish a healthy sleep environment (e.g., temperature controls, window shading). Housing policy should include measures that require new complexes to: (a) be built away from noisy and polluted roadways; (b) be constructed so as to minimize internal noise; (c) provide heating and air conditioning control; and (d) come equipped with blinds that sufficiently block outside light.
At an individual level, intervention programs should provide education about ways to improve the home sleep environment, such as the importance of a quiet, comfortable bedroom and shutting off the television and other devices when trying to fall asleep, and of the importance of sleep to overall health. Previous education-based interventions have proven effective at improving sleep hygiene and quality in university populations.(88) Similar interventions should be tailored to minority populations and integrated within programs for primary or secondary CVD risk prevention.
Conclusion: Implications for CVD Prevention and Management
Sleep is an integral part of overall health, particularly cardiovascular health. Sleep deficiencies are linked with numerous negative health outcomes including obesity, hypertension, and diabetes. Racial and ethnic minorities tend to experience more extreme sleep duration and sleep disorders than do Whites. These disparities in sleep health may contribute to the disproportionately high rates of CVD among minorities. Research investigating the mechanisms underlying these effects is in its early stages, but there is evidence suggesting that greater exposure to stressors (e.g., neighborhood, occupational, psychosocial), acculturation, and relatively low treatment access and adherence may help explain racial differences in sleep deficiency. More work is needed to identify protective factors that could be targeted by culturally-relevant interventions. Such interventions would help promote more positive sleep health among minorities, and thus, help reduce racial/ethnic disparities in CVD. However, the existing data support the need to address sleep disorders as a means for primary and secondary cardiovascular disease reduction.
Footnotes
Conflict of Interest: Karen Emmons has a pending R01 grant for social and environmental effects on sleep and hypertension in low income families.
Orfeu Buxton received a grant from NIOSH, Sepracor Inc, NIA and NHLBI; served as a consultant for Dinsmore LLC and Matsutani America; gave expert testimony for Dinsmore LLC; and received honoraria from Tufts University School of Dental Medicine.
John H. Kingsbury and Susan Redline declare no conflicts of interest.
Human and Animal Rights and Informed Consent: This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
John H. Kingsbury, Email: John_kingsbury@dfci.harvard.edu, Center for Community-Based Research, Harvard School of Public Health and Dana-Farber, Cancer Institute, 375 Longwood Ave, 6th floor, Boston, MA 02215, 617-582-7174 (phone), 617-632-1999 (fax).
Orfeu M. Buxton, Email: Orfeu_buxton@hms.harvard.edu, Division of Sleep Medicine, Harvard Medical School and Brigham and Women's Hospital, 221 Longwood Ave, Suite BLI-438K, Boston, MA 02115, 617-525-7118 (phone), 617-507-9177 (fax).
Karen M. Emmons, Email: Karen_m_emmons@dfci.harvard.edu, Center for Community-Based Research, Harvard School of Public Health and Dana-Farber, Cancer Institute, 375 Longwood Ave, 6th floor, Boston, MA 02215, 617-632-2188 (phone), 617-632-1999 (fax).
References
Recent papers of particular interest have been highlighted as:
•Of importance
••Of major importance
- 1.Schiller JS, Lucas JW, Peregoy JA. Summary health statistics for U.S. adults: National Health Interview Survey, 2011. National Center for Health Statistics. 2012;10(256) [PubMed] [Google Scholar]
- 2.Jean-Louis G, Brown CD, Zizi F, et al. Cardiovascular disease risk reduction with sleep apnea treatment. Expert Review of Cardiovascular Therapy. 2010;8(7):995–1005. doi: 10.1586/erc.10.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanna A. Obstructive sleep apnoea, motor vehicle accidents, and work performance. Chronic Respiratory Disorders. 2013;10(1):29–33. doi: 10.1177/1479972312473134. [DOI] [PubMed] [Google Scholar]
- 4.Strohl K. Assessments of driving risk in sleep apnea. In: Safwan Badr M, editor. Essentials of Sleep Medicine. Humana Press; 2012. pp. 129–142. [Google Scholar]
- 5.Cohen-zion M, Stepnowsky C, Marler T, et al. Changes in cognitive function associated with sleep disordered breathing in older people. J Am Geriatr Soc. 2001;49(12):1622–7. doi: 10.1046/j.1532-5415.2001.t01-1-49270.x. [DOI] [PubMed] [Google Scholar]
- 6.National Institutes of Health. [Accessed March 10, 2013];Sleep Disorders Research Plan. 2011 http://go.usa.gov/14t.
- 7.Takase B, Akima T, Satomura K, et al. Effects of chronic sleep deprivation on autonomic activity by examining heart rate variability, plasma catecholamine, and intracellular magnesium levels. Biomedicine & Pharmacotherapy. 2004;58:S35–S39. doi: 10.1016/s0753-3322(04)80007-6. [DOI] [PubMed] [Google Scholar]
- 8.Meerlo P, Sgoifo A, Suchecki D. Restricted and disrupted sleep: Effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med Rev. 2008;12(3):197–210. doi: 10.1016/j.smrv.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 9••.Cappuccio FP, Cooper D, D'Elia L, et al. Sleep duration predicts cardiovascular outcomes: A systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32(12):1484–92. doi: 10.1093/eurheartj/ehr007. This systematic review of 15 prospective studies, including 474,684 participants, provides valuable findings on the relation between extreme sleep duration and cardiovascular outcomes over time. [DOI] [PubMed] [Google Scholar]
- 10.Cappuccio FP, Taggart FM, Kandala N, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31(5):619–26. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11•.Buxton OM, Marcelli E. Short and long sleep are positively associated with obesity, diabetes, hypertension, and cardiovascular disease among adults in the United States. Soc Sci Med. 2010;71(5):1027–1036. doi: 10.1016/j.socscimed.2010.05.041. This population-based study of 56,507 participants found that sleep duration was frequently more strongly associated with a variety of cardiovascular outcomes than with relevant covariates (e.g., substance use, education, geographical context). Findings provide strong evidence that a sleep duration of 7-8 hours is protective against chronic disease. [DOI] [PubMed] [Google Scholar]
- 12.Ogawa Y, Kanbayashi T, Saito Y, et al. Total sleep deprivation elevates blood pressure through arterial baroreflex resetting: a study with microneurographic technique. Sleep. 2003;26(8):986–9. doi: 10.1093/sleep/26.8.986. [DOI] [PubMed] [Google Scholar]
- 13.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141(11):846–50. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 14•.Buxton OM, Pavlova M, Reid EW, et al. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes. 2010;59(9):2126–2133. doi: 10.2337/db09-0699. This randomized, controlled study of 20 healthy participants produced valuable insight on the effects of short sleep and diabetes. Results showed that sleep restriction for one week (i.e., 5 hours/night) leads to (1) elevated and sustained self-reported sleepiness, (2) elevations of afternoon and evening cortisol, and (3) a specific pattern of changes to glucose metabolism: reduced insulin sensitivity without a compensatory change in insulin response, and overall, a significant elevation of diabetes risk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Short sleep duration as a risk factor for hypertension: Analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47(5):833–9. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 16.King CR, Knutson KL, Rathouz PJ, et al. Short sleep duration and incident coronary artery calcification. JAMA. 2008;300(24):2859–65. doi: 10.1001/jama.2008.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grandner MA, Hale L, Moore M, Patel NP. Mortality associated with short sleep duration: The evidence, the possible mechanisms, and the future. Sleep Med Rev. 2010;14(3):191–203. doi: 10.1016/j.smrv.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stamatakis KA, Kaplan GA, Roberts RE. Short sleep duration across income, education, and race/ethnic groups: Population prevalence and growing disparities during 34 years of follow-up. Ann Epidemiol. 2007;17(12):948–955. doi: 10.1016/j.annepidem.2007.07.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krueger PM, Friedman EM. Sleep duration in the United States: A cross-sectional population-based study. Am J Epidemiol. 2009;169(9):1052–63. doi: 10.1093/aje/kwp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hale L, Do DP. Racial differences in self-reports of sleep duration in a population-based study. Sleep. 2007;30(9):1096–1103. doi: 10.1093/sleep/30.9.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lauderdale DS, Knutson KL, Yan LL, et al. Objectively measured sleep characteristics among early-middle-aged adults. Am J Epidemiol. 2006;164(1):5–16. doi: 10.1093/aje/kwj199. [DOI] [PubMed] [Google Scholar]
- 22.Mezick EJ, Matthews KA, Hall M, et al. Influence of race and socioeconomic status on sleep: Pittsburgh SleepSCORE project. Psychosom Med. 2008;70(4):410–6. doi: 10.1097/PSY.0b013e31816fdf21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ertel KA, Berkman LF, Buxton OM. Socioeconomic status, occupational characteristics, and sleep duration in African/Caribbean immigrants and US White health care workers. Sleep. 2011;34(4):509–18. doi: 10.1093/sleep/34.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24•.Ruiter ME, DeCoster J, Jacobs L, Lichstein KL. Normal sleep in African-Americans and Caucasian-Americans: A meta-analysis. Sleep Med. 2011;12(3):209–214. doi: 10.1016/j.sleep.2010.12.010. This meta-analysis of 14 studies, and 4,166 adults, is one of few systematic reviews of racial differences in sleep duration. This review found that the effect of race on sleep duration was stronger in studies using objective measures of sleep duration, a finding that has implications for the design of future studies in this area. [DOI] [PubMed] [Google Scholar]
- 25.Zizi F, Pandey A, Murray-Bachmann R, et al. Race/ethnicity, sleep duration, and diabetes mellitus: Analysis of the National Health Interview Survey. Am J Med. 2012;125(2):162–7. doi: 10.1016/j.amjmed.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loredo JS, Soler X, Bardwell W, et al. Sleep health in U.S. Hispanic population. Sleep. 2010;33(7):962–7. doi: 10.1093/sleep/33.7.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haas DC, Foster GL, Nieto FJ, et al. Age-dependent associations between sleep-disordered breathing and hypertension: Importance of discriminating between systolic/diastolic hypertension and isolated systolic hypertension in the Sleep Heart Health Study. Circulation. 2005;111(5):614–21. doi: 10.1161/01.CIR.0000154540.62381.CF. [DOI] [PubMed] [Google Scholar]
- 28.Benjamin JA, Lewis KE. Sleep-disordered breathing and cardiovascular disease. Postgrad Medical Journal. 2008;84(987):15–22. doi: 10.1136/pgmj.2007.062836. [DOI] [PubMed] [Google Scholar]
- 29.Paola L, Virend S. Obstructive sleep apnea and vascular disease. Respiratory Research. 2001;2(6):315–9. doi: 10.1186/rr79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marin JM, Agusti A, Villar I, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA. 2012;307(20):2169–76. doi: 10.1001/jama.2012.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31••.Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: The sleep heart health study. American Journal of Respiratory and Critical Care Medicine. 2010;182(2):269–77. doi: 10.1164/rccm.200911-1746OC. This prospective study is the first to quantify the risk of incident stroke among individuals suffering from sleep apnea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sulit L, Storfer-Isser A, Kirchner HL, Redline S. Differences in polysomnography predictors for hypertension and impaired glucose tolerance. Sleep. 2006;29(6):777–83. doi: 10.1093/sleep/29.6.777. [DOI] [PubMed] [Google Scholar]
- 33.Sharma SK, Agrawal S, Damodaran D, et al. CPAP for the metabolic syndrome in patients with obstructive sleep apnea. N Engl J Med. 2011;365(24):2277–86. doi: 10.1056/NEJMoa1103944. [DOI] [PubMed] [Google Scholar]
- 34.Weinstock TG, Wang X, Rueschman M, et al. A controlled trial of CPAP therapy on metabolic control in individuals with impaired glucose tolerance and sleep apnea. Sleep. 2012;35(5):617–25. doi: 10.5665/sleep.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marin JM, Carrizo SJ, Vicente E, Agusti AGN. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: An observational study. Lancet. 2005;365(9464):1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 36•.Goldstein NA, Abramowitz T, Weedon J, et al. Racial/ethnic differences in the prevalence of snoring and sleep disordered breathing in young children. Journal of Clinical Sleep Medicine. 2011;7(2):163–71. This study demonstrates that racial differences in sleep-disordered breathing appear as early as ages 2-6. Interventions involving parents, pediatricians, and schools may be useful in promoting positive sleep health and reducing racial/ethnic differences in sleep-disordered breathing among children. [PMC free article] [PubMed] [Google Scholar]
- 37.O'Connor GT, Lind BK, Lee ET, et al. Variation in symptoms of sleep-disordered breathing with race and ethnicity: The Sleep Heart Health Stud. Sleep. 2003;26(1):74–9. [PubMed] [Google Scholar]
- 38.Kripke DF, Ancoli-Israel S, Klauber MR, et al. Prevalence of sleep-disordered breathing in ages 40-64 years: a population-based survey. Sleep. 1997;20(1):65–76. doi: 10.1093/sleep/20.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meetze K, Gillespie MB, Lee F. Obstructive sleep apnea: A comparison of Black and White subjects. Laryngoscope. 2002;112:1271–4. doi: 10.1097/00005537-200207000-00024. [DOI] [PubMed] [Google Scholar]
- 40.Bonnet MH, Arand DL. 24-Hour metabolic rate in insomniacs and matched normal sleepers. Sleep. 1995;18(7):581–8. doi: 10.1093/sleep/18.7.581. [DOI] [PubMed] [Google Scholar]
- 41.Spiegelhalder K, Fuchs L, Ladwig J, et al. Heart rate and heart rate variability in subjectively reported insomnia. J Sleep Res. 2011;20(1):137–45. doi: 10.1111/j.1365-2869.2010.00863.x. [DOI] [PubMed] [Google Scholar]
- 42.Bonnet MH, Arand DL. Heart rate variability in insomniacs and matched normal sleepers. Psychosom Med. 1998;60(5):610–5. doi: 10.1097/00006842-199809000-00017. [DOI] [PubMed] [Google Scholar]
- 43.Hori H, Teraishi T, Sasayama D, et al. Poor sleep is associated with exaggerated cortisol response to the combined dexamethasone/CRH test in a non-clinical population. J Psychiatr Res. 2011;45(9):1257–1263. doi: 10.1016/j.jpsychires.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 44.Hansen ÅM, Thomsen JF, Kaergaard A, et al. Salivary cortisol and sleep problems among civil servants. Psychoneuroendocrinology. 2012;37(7):1086–1095. doi: 10.1016/j.psyneuen.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 45.Laugsand LE, Vatten LJ, Platou C, Janszky I. Insomnia and the risk of acute myocardial infarction: A population study. Circulation. 2011;124(19):2073–81. doi: 10.1161/CIRCULATIONAHA.111.025858. [DOI] [PubMed] [Google Scholar]
- 46•.Spiegelhalder K, Scholtes C, Riemann D. The association between insomnia and cardiovascular diseases. Nature Sci Sleep. 2010;2:71–78. doi: 10.2147/nss.s7471. This paper critically reviews the literature on the link between insomnia and cardiovascular disease, highlights shortcomings of existing studies, and discusses areas of needed research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47•.Fernandez-Mendoza J, Vgontzas AN, Liao D, et al. Insomnia with objective short sleep duration and incident hypertension: The Penn State Cohort. Hypertension. 2012;60(4):929–35. doi: 10.1161/HYPERTENSIONAHA.112.193268. This study is the first longitudinal examination of the effects of insomnia with objective short sleep duration on incident hypertension. These findings highlight the importance of considering potentially multiplicative effects of sleep problems on cardiovascular outcomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bixler EO, Vgontzas AN, Lin H, Vela-Bueno A, Kales A. Insomnia in Central Pennsylvania. J Psychosom Res. 2002;53(1):589–592. doi: 10.1016/s0022-3999(02)00450-6. [DOI] [PubMed] [Google Scholar]
- 49.Pigeon WR, Heffner K, Duberstein P, et al. Elevated sleep disturbance among Blacks in an urban family medicine practice. Journal of the American Board of Family Medicine. 2011;24(2):161–8. doi: 10.3122/jabfm.2011.02.100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singareddy R, Vgontzas AN, Fernandez-Mendoza J, et al. Risk factors for incident chronic insomnia: A general population prospective study. Sleep Med. 2012;13(4):346–53. doi: 10.1016/j.sleep.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chapman DP, Wheaton AG, Anda RF, et al. Adverse childhood experiences and sleep disturbances in adults. Sleep Med. 2011;12(8):773–779. doi: 10.1016/j.sleep.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 52.Riedel BW, Durrence HH, Lichstein KL, et al. The relation between smoking and sleep: The influence of smoking level, health, and psychological variables. Behavioral Sleep Medicine. 2004;2(1):63–78. doi: 10.1207/s15402010bsm0201_6. [DOI] [PubMed] [Google Scholar]
- 53.Evans GW. The environment of childhood poverty. Am Psychol. 2004;59(2):77–92. doi: 10.1037/0003-066X.59.2.77. [DOI] [PubMed] [Google Scholar]
- 54.Houston D, Wu J, Ong P, Winer A. Structural disparities of urban traffic in Southern California: Implications for vehicle-related air pollution exposure in minority and high-poverty neighborhoods. Journal of Urban Affairs. 2004;26(5):565–92. [Google Scholar]
- 55.Zanobetti A, Redline S, Schwartz J, et al. Associations of PM10 with sleep and sleep-disordered breathing in adults from seven U.S. urban areas. American Journal of Respiratory and Critical Care Medicine. 2010;182(6):819–25. doi: 10.1164/rccm.200912-1797OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56•.Hale L, Hill TD, Friedman E, et al. Perceived neighborhood quality, sleep quality, and health status: Evidence from the Survey of the Health of Wisconsin. Soc Sci Med. 2013;79:16–22. doi: 10.1016/j.socscimed.2012.07.021. This cross-sectional study of 1,298 adults found that neighborhood quality was predictive of health status, and that this effect was mediated by self-reported sleep quality. These findings suggest that neighborhood quality may be one factor that is implicated in racial/ethnic sleep and CVD disparities. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hill TD, Burdette AM, Hale L. Neighborhood disorder, sleep quality, and psychological distress: Testing a model of structural amplification. Health and Place. 2009;15(4):1006–13. doi: 10.1016/j.healthplace.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 58.Marshall JD, Brauer M, Frank LD. Healthy neighborhoods: Walkability and air pollution. Environ Health Perspect. 2009;117(11):1752–1759. doi: 10.1289/ehp.0900595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Landrigan PJ, Rauh VA, Galvez MP. Environmental justice and the health of children. Mount Sinai Journal of Medicine. 2010;77:178–187. doi: 10.1002/msj.20173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Logan JR. Separate and unequal: The neighborhood gap for Blacks, Hispanics, and Asians in metropolitan. America. 2011:1–22. [Google Scholar]
- 61.Krystal AD. How the circadian rhythm affects sleep, wakefulness, and overall health: Background for understanding shift work disorder. J Clin Psychiatry. 2012;73(2):e05. doi: 10.4088/JCP.11073br1. [DOI] [PubMed] [Google Scholar]
- 62.Machi MS, Staum M, Callaway CW, et al. The relationship between shift work, sleep, and cognition in career emergency physicians. Acad Emerg Med. 2012;19(1):85–91. doi: 10.1111/j.1553-2712.2011.01254.x. [DOI] [PubMed] [Google Scholar]
- 63.Virtanen M, Ferrie JE, Gimeno D, et al. Long working hours and sleep disturbances: The Whitehall II prospective cohort study. Sleep. 2009;32(6):737–45. doi: 10.1093/sleep/32.6.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Golden L. Flexible work schedules: Which workers get them? Am Behav Sci. 2001;44(7):1157–78. [Google Scholar]
- 65.Sorensen G, Stoddard AM, Stoffel S, Buxton O, Sembajwe G, Hashimoto D, et al. The role of the work context in multiple wellness outcomes for hospital patient care workers. Journal of Occupational and Environmental Medicine. 2011;53(8):899–910. doi: 10.1097/JOM.0b013e318226a74a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Berkman LF, Buxton O, Ertel K, Okechukwu C. Managers' practices related to work–family balance predict employee cardiovascular risk and sleep duration in extended care settings. J Occup Health Psychol. 2010;15(3):316–329. doi: 10.1037/a0019721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ellingsen T, Bener A, Gehani AA. Study of shift work and risk of coronary events. Journal of the Royal Society for the Promotion of Health. 2007;127(6):265–7. doi: 10.1177/1466424007083702. [DOI] [PubMed] [Google Scholar]
- 68.Haupt CM, Alte D, Dörr M, et al. The relation of exposure to shift work with atherosclerosis and myocardial infarction in a general population. Atherosclerosis. 2008;201(1):205–211. doi: 10.1016/j.atherosclerosis.2007.12.059. [DOI] [PubMed] [Google Scholar]
- 69.Mays VM, Cochran SD, Barnes NW. Race, race-based discrimination, and health outcomes among African Americans. Annu Rev Psychol. 2007;58:201–25. doi: 10.1146/annurev.psych.57.102904.190212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Williams DR, Mohammed SA. Discrimination and racial disparities in health: evidence and needed research. J Behav Med. 2009;32(1):20–47. doi: 10.1007/s10865-008-9185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grandner M, Hale L, Jackson N, et al. Perceived racial discrimination as an independent predictor of sleep disturbance and daytime fatigue. Beh Sleep Med. 2012;10(4):235–249. doi: 10.1080/15402002.2012.654548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Steffen PR, Bowden M. Sleep disturbance mediates the relationship between perceived racism and depressive symptoms. Ethn Dis. 2006;16(1):16–21. [PubMed] [Google Scholar]
- 73.Van Dongen HPA, Vitellaro KM, Dinges DF. Individual differences in adult human sleep and wakefulness: Leitmotif for a research agenda. Sleep. 2005;28(4):479–96. doi: 10.1093/sleep/28.4.479. [DOI] [PubMed] [Google Scholar]
- 74.Thomas KS, Bardwell WA, Ancoli-Israel S, Dimsdale JE. The toll of ethnic discrimination on sleep architecture and fatigue. Health Psychology. 2006;25(5):635–42. doi: 10.1037/0278-6133.25.5.635. [DOI] [PubMed] [Google Scholar]
- 75.Cherpitel CJ, Borges G. Substance use among emergency room patients: An exploratory analysis by ethnicity and acculturation. Am J Drug Alcohol Abuse. 2002;28(2):287–95. doi: 10.1081/ada-120002975. [DOI] [PubMed] [Google Scholar]
- 76.Vega WA, Alderete E, Kolody B, Aguilar-Gaxiola S. Illicit drug use among Mexicans and Mexican Americans in California: The effects of gender and acculturation. Addiction. 1998;93(12):1839–50. doi: 10.1046/j.1360-0443.1998.931218399.x. [DOI] [PubMed] [Google Scholar]
- 77.Coonrod DV, Bay RC, Balcazar H. Ethnicity, acculturation and obstetric outcomes Different risk factor profiles in low- and high-acculturation Hispanics and in white non-Hispanics. J Reprod Med. 2004;49(1):17–22. [PubMed] [Google Scholar]
- 78.Lara M, Gamboa C, Kahramanian MI, et al. Acculturation and Latino health in the United States: A review of the literature and its sociopolitical context. Annu Rev Public Health. 2012;26(1):367–97. doi: 10.1146/annurev.publhealth.26.021304.144615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ortega AN, Rosenheck R, Alegría M, Desai RA. Acculturation and the lifetime risk of psychiatric and substance use disorders among Hispanics. The Journal of Nervous and Mental Disease. 2000;188(11):728–35. doi: 10.1097/00005053-200011000-00002. [DOI] [PubMed] [Google Scholar]
- 80.Hale L, Rivero-Fuentes E. Negative acculturation in sleep duration among Mexican Immigrants and Mexican Americans. Journal of Immigrant and Minority Health. 2011;13(2):402–07. doi: 10.1007/s10903-009-9284-1. [DOI] [PubMed] [Google Scholar]
- 81.Heilemann MV, Choudhury SM, Felix SK, Lee KA. Factors associated with sleep disturbance in women of Mexican descent. Journal of Advanced Nursing. 2012;68:2256–66. doi: 10.1111/j.1365-2648.2011.05918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boss EF, Smith DF, Ishman SL. Racial/ethnic and socioeconomic disparities in the diagnosis and treatment of sleep-disordered breathing in children. Int J Pediatr Otorhinolaryngol. 2011;75(3):299–307. doi: 10.1016/j.ijporl.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 83.Budhiraja R, Parthasarathy S, Drake CL, et al. Early CPAP use identifies subsequent adherence to CPAP therapy. Sleep. 2007;30(3):320–4. [PubMed] [Google Scholar]
- 84.Sawyer AM, Canamucio A, Moriarty H, et al. Do cognitive perceptions influence CPAP use? Patient Educ Couns. 2011;85(1):85–91. doi: 10.1016/j.pec.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Billings ME, Rosen CL, Wang R, et al. Is the relationship between race and continuous positive airway pressure adherence mediated by sleep duration? Sleep. 2013;36(2):221–7. doi: 10.5665/sleep.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fleck DE, Keck PE, Corey KB, Strakowski SM. Factors associated with medication adherence in African American and White patients with bipolar disorder. J Clin Psychiatry. 2005;66(5):646–52. doi: 10.4088/jcp.v66n0517. [DOI] [PubMed] [Google Scholar]
- 87.Wheaton B. The nature of stressors. In: Horwitz A, Scheid T, editors. A handbook for the study of mental health: Social contexts, theories, and systems. New York, NY: Cambridge University Press; pp. 1999pp. 176–197. [Google Scholar]
- 88.Brown FC, Buboltz WC, Jr, Soper B. Development and evaluation of the Sleep Treatment and Education Program for Students (STEPS) J Am College Health. 2006;54(4):231–237. doi: 10.3200/JACH.54.4.231-237. [DOI] [PubMed] [Google Scholar]