Abstract
Background
The high rates of HIV and Hepatitis C (HCV) infection among opioid abusers is a serious public health problem, and efforts to enhance knowledge regarding risks for HIV/hepatitis infection in this population are important. Abuse of prescription opioids (PO), in particular, has increased substantially in the past decade and is associated with increasing rates of injection drug use and HCV infection.
Methods
This study describes the effects of a brief HIV/HCV educational intervention delivered in the context of a larger randomized, double-blind clinical trial evaluating the relative efficacy of 1-, 2-, and 4-week outpatient buprenorphine tapers and subsequent oral naltrexone maintenance for treating PO dependence. HIV- and HCV-related knowledge and risk behaviors were characterized pre- and post-intervention in 54 primary PO abusers.
Results
The educational intervention was associated with significant improvements in HIV (p<.001) and HCV (p<.001) knowledge. Significant improvements (p<.001) were observed on all three domains of the HIV questionnaire (i.e., general knowledge, sexual risk behaviors, drug risk behaviors) and on 21 and 11 individual items on the HIV and HCV questionnaires, respectively. Self-reported likelihood of using a condom also increased significantly (p<.05) from pre- to post-intervention. No additional changes in self-reported risk behaviors were observed.
Conclusion
These results suggest that a brief, easy-to-administer intervention is associated with substantial gains in HIV and HCV knowledge among PO abusers and represents the necessary first step towards the dissemination of a structured prevention HIV and HCV intervention for PO abusers.
Keywords: HIV, hepatitis C, noninjection, injection, education, intervention, opioids, prescription opioids, treatment, buprenorphine
1. INTRODUCTION
Elevated rates of HIV and Hepatitis C (HCV) infection among substance abusers present a serious public health problem (Neaigus et al., 2007; Scheinmann et al., 2007; Des Jarlais and Semaan, 2008). The Centers for Disease Control and Prevention (CDC) estimates that approximately 50,000 and 21,000 Americans are newly infected with HIV and HCV respectively each year (CDC, 2010, 2011). HIV and HCV are each responsible for approximately 15,000 annual deaths (CDC, 2008, 2011), and deaths from HCV-related liver complications are markedly increasing among opioid-dependent individuals (Larney et al., 2012). HIV and HCV acquisition among substance users have historically been associated with injection drug use and risky sexual behaviors. More recent research, however, has also found a correlation between noninjection drug use and increased risk for HIV and HCV (Strathdee and Sherman, 2003; Neaigus et al., 2007; Scheinmann et al., 2007), making risk-behavior prevention critical for all substance abusers.
This increased risk for HIV and HCV is particularly serious among prescription opioid (PO) abusers. POs are now the second most commonly abused drugs in U.S. (CDC, 2012; SAMHSA, 2013). The annual cost of PO abuse, which includes lost workplace productivity, health care, treatment, and criminal justice costs, is estimated at $55 billion (Birnbaum et al., 2011; Hansen et al., 2011). Annual costs related to HIV- and HCV-related medical complications in PO abusers are estimated to be $750 million (Hansen et al., 2011), and evidence suggests that HIV and HCV risk behaviors may be increasing in this population. For example, injection rates among PO abusers tripled over a 5-year period (Bruneau et al., 2012), and PO abuse has been independently related to risky injection behaviors, such as sharing syringes (Havens et al., 2011). Increased injection use among PO abusers has also been independently associated with HCV infection (Bruneau et al., 2012; Havens et al., 2013), and a recent large-scale longitudinal study reported the proportion of HCV-positive PO abusers increased from 21% to 75% over 5 years (Bruneau et al., 2012). Despite this, PO abusers generally perceive themselves to be at lower risk for acquiring HIV and also have lower likelihood of ever being tested (Pollini et al., 2011).
Given the rise in PO abusers and their risk for HIV and HCV acquisition, efforts to identify intervention strategies for reducing disease transmission in this population are crucial. Brief psychosocial interventions focused on increasing knowledge about disease contraction and prevention are an appealing method for delivering HIV and HCV risk information because they can be done within a single session and do not require specialized counselor training. Risk reduction interventions have been associated with a decrease in self-reported HIV risk behaviors (Copenhaver et al., 2006; Meader et al., 2010) and HCV injection risk behaviors in both substance abusers (Garfein et al., 2007; Tucker et al., 2004) and the general population (Shah et al., 2013). To our knowledge, no interventions have simultaneously targeted HIV and HCV knowledge and risk behavior among PO abusers.
We have previously reported the results of two randomized, cross-over educational interventions aimed at increasing HIV knowledge among cocaine-dependent outpatients (Heil et al., 2005; Herrmann et al., 2013). In these studies, participants in the experimental group completed a 1-hour counselor-delivered intervention on HIV risk behavior while the control group completed a session on the psychopharmacology of cocaine. Following an assessment of HIV knowledge, control participants were then crossed over to receive the educational condition. Results in both studies showed significant knowledge increases in both groups following receipt of the active versus control intervention (Heil et al., 2005; Herrmann et al., 2013).
For the present study, we expanded this intervention to include information on HCV and to emphasize noninjection drug use risk behaviors among PO abusers undergoing detoxification from opioids. The goal of the present study was to characterize baseline knowledge of HIV and HCV transmission risks in a PO-dependent sample and to evaluate within-subject changes in self-reported knowledge and risk behaviors as a function of a single session, 1-hour intervention. A full description of that randomized controlled trial comparing three durations of buprenorphine detoxification for primary PO dependent adults is available elsewhere (Sigmon et al., in press); thus, only methods directly relevant to the present report will be presented here.
2. METHODS
2.1 Participants
To be eligible for the parent trial, participants (n=70) had to be ≥18 years old, meet DSM-IV-TR criteria for opioid dependence, provide an opioid-positive urine sample, be interested in detoxification, report a PO as their primary drug of abuse, and report using it illicitly (e.g., without a valid prescription). Participants who were pregnant and/or nursing, who had a significant and uncontrolled psychiatric and/or medical illness, or who required ongoing narcotic treatment for a chronic pain condition were excluded.
As described below, HIV and HCV knowledge pre-tests were administered at the intake screening for the parent study, and post-tests were administered following completion of the HIV/HCV intervention. Analyses include only the participants who completed both the pre- and post-intervention knowledge assessments (n=54), which represents 77% of participants who completed the screening visit for the parent study. However, the HIV/HCV intervention was completed by 100% of participants who began the intervention. There were no significant differences in demographic or drug use characteristics between participants who did and did not begin the HIV/HCV intervention. The institutional review board approved the study and voluntary informed consent was collected as part of their primary study participation. Participants’ baseline characteristics are presented in Table 1.
Table 1.
Demographic and Drug Use Characteristics (N=54)
| Demographics | |
| Male (%) | 70 |
| Caucasian (%) | 98 |
| Age (years) | 27 ± 6.86 |
| Fagerström Test for Nicotine Dependence (0-10) | 4.9 ± 2.25 |
| Michigan Alcoholism Screening Test (0-53) | 8.8 ± 8.70 |
| Opioid Use Characteristics | |
| Primary oxycodone user (%) | 70 |
| Mgs used per day | 107 ± 80.50 |
| Primary route of administration | |
| Oral | 13 |
| Intranasal | 70 |
| Injection | 17 |
| Ever used heroin (%) | 57 |
| Ever received opioid treatment (%) | 39 |
| Percent Abusing Other Drugs | |
| Marijuana | 98 |
| Alcohol | 91 |
| Cocaine | 81 |
| Benzodiazepines | 56 |
| Amphetamines | 37 |
| Psychosocial Functioning | |
| Beck Depression Inventory (0-63) | 20.2 ± 11.10 |
| Addiction Severity Index Scores (0-1) | |
| Medical | 0.21 ± 0.29 |
| Employment | 0.57 ± 0.28 |
| Alcohol | 0.07 ± 0.08 |
| Drug | 0.36 ± 0.07 |
| Legal | 0.16 ± 0.22 |
| Family/Social | 0.17 ± 0.22 |
| Psychiatric | 0.25 ± 0.21 |
Values presented as Mean ± Standard Deviation unless otherwise indicated.
2.2 Primary Treatment Trial
All participants were enrolled in a double-blind, 12-week, randomized controlled trial evaluating the relative efficacy of buprenorphine taper durations and subsequent naltrexone therapy for treating PO dependence. Participants were stabilized briefly onto buprenorphine and then randomized to receive a 1, 2, or 4-week double-blind buprenorphine taper. Those who successfully tapered without resumption of illicit opioid use were transitioned to naltrexone for the remainder of the study. All participants received individualized behavioral therapy based on the Community Reinforcement Approach and provided urine specimens for toxicology testing throughout the trial.
2.3 HIV and HCV Educational Intervention
A master’s level therapist delivered the HIV/HCV intervention during a single, 1-hour session in the first or second week of the study. Participants completed questionnaires assessing baseline levels of HIV and HCV infection and risk behavior knowledge during the screening visit for the primary study. The intervention began by providing corrective feedback on their responses. Next, participants watched a 23-minute video (“HIV/AIDS: The Untold Truth & Myths,” Nimco, Inc.) that provided detailed information on the HIV virus and disease transmission; participants then discussed video content and questions with the therapist. An HIV pamphlet (“HIV and AIDS: What you need to know,” Channing Bete Company, Inc.) and three pamphlets discussing Hepatitis A, B, and C (“About Viral Hepatitis”, Channing Bete Company, Inc.; “The Hepatitis Information You Need to Know: Intravenous Drug Users”, American Liver Foundation; “The ABCs of Hepatitis”, Hepatitis Foundation International) were reviewed with participants, and the ways in which HIV and HCV differ were emphasized. The therapist then reviewed materials discussing correct injection techniques and sexual transmission risks for infection, which included depictions of proper male and female condom application. Finally, participants were provided with condoms and information on resources for free testing for HIV and Hepatitis B and C antibodies, as well as assistance in contacting testing facilities if interested.
2.4 Measures
2.4.1 Study assessments
The intake included a clinic-developed drug history questionnaire, the Addiction Severity Index (McLellan et al., 1985), the Beck Depression Inventory (Beck et al., 1961), the Michigan Alcoholism Screening Test (Selzer, 1971), the Fagerström Test for Nicotine Dependence (Heatherton et al., 1991), and a Timeline Follow-Back (TLFB) assessment of past-month opioid and other drug use (Sobell and Sobell, 1992).
2.4.2 HIV and HCV knowledge assessments
Assessments were administered at study intake (pre-intervention) and immediately after the intervention (post-intervention). Participants were not permitted to use the pamphlets to answer the questions, and corrective feedback was only provided as part of the intervention. HIV knowledge was assessed using the Marsch HIV/AIDS knowledge test, a 50-item assessment of HIV/AIDS knowledge in three areas: general knowledge, sexual risk, and drug risk behaviors (Marsch et al., 2005). HCV knowledge was assessed using a 15-item knowledge questionnaire developed by our group for this intervention. Available responses on both questionnaires were “True,” “False,” or “Don’t know” (Herrmann et al., 2013) and correct answers were summed to provide a total overall score. Finally, participants also completed a visual analog scale (VAS) item, “How much do you know about how the AIDS virus is transmitted?”, that was rated on a scale of 0 (nothing) to 100 (a lot), at pre- and post-intervention.
2.4.3 HIV and HCV risk behavior assessments
Risk behavior assessments included the HIV Risk-Taking Behaviour Scale (HRBS; Darke et al., 1991), an 11-item survey that asked about self-reported risk behaviors in the past month and week. Items were rated on Likert scales (0-6) that were summed to yield an injection and sexual risk behavior subscale for each time point. Participants also completed the Risk Assessment Battery (RAB; Navaline et al., 1994), a 22-item survey that asked about self-reported risk behaviors in the past 6 months (intake) and the past month (all other assessments). Items were rated on Likert scales (0-3) and were summed into injection and sexual risk behavior subscales, a total score, and a total scaled score. Participants also completed a series of VAS risk items that assessed risk on a scale of 0 (no chance) to 100 (very high chance) including “Chance of contracting HIV/AIDS,” “Refrain from using a dirty needle,” “Refrain from unprotected sex,” and “Use a condom the next time you have sex.” “Chance of contracting Hepatitis C” was added to the VAS midway through data collection and was only completed by a subset of participants (n=22). Finally, participants completed four questions developed by us to target additional noninjection risk behaviors (i.e., “How many times did you have sex under the influence of any drug?”, “Did you have sex with a person who abuses alcohol on a regular basis?”, “Did you have sex with a person who abuses drugs on a regular basis?”, “Have you ever tested positive for the following sexually transmitted diseases (chlamydia, genital herpes, genital HPV, gonorrhea, syphilis, and trichomoniasis)?”). These assessments were completed at study intake and weeks 4, 6, 8, 12, and 24. Due to attrition from the primary trial, only results from study intake and the assessment timepoint closest to the educational intervention (post-intervention for VAS, and Week 4 for HRBS and RAB) were analyzed.
2.5 Data Analyses
Means (SD) and percentages were used to descriptively characterize baseline demographic and drug use. Total percent correct on the Marsch and HCV questionnaires was computed for each participant at the pre- and post-intervention assessments to create a total percent accuracy score; scores were then compared using paired t-tests. Marsch questionnaire items were further categorized based on general, sexual, or drug use knowledge and summed percent accuracy scores for each subscale were compared between pre and post intervention using paired t-tests. For both the Marsch and HCV scales, higher values indicate greater accuracy. McNemar’s test was used to determine whether accuracy changed significantly on individual items from pre- to post-intervention on the Marsch and HCV knowledge questionnaires, with P≤.01 considered significant to protect against multiple comparisons.
Given that opioid treatment has been associated with reductions in HIV risk behaviors (Marsch, 1998; Sullivan et al., 2008), participants were dichotomized on self-reported history of treatment for opioid dependence and t-tests were used to compare groups in pre-test performance on knowledge questionnaires and risk assessments. Significant associations were examined further using linear regressions to determine whether pre-test performance and history of opioid treatment were independently related with post-test performance.
Risk behaviors, as measured by VAS ratings, HRBS, and RAB subscale scores, were compared using paired t-tests. HRBS subscale scores for individual drug and sex risk behaviors were computed by summing answers within each category and the HRBS total score was computed by summing the total drug and sex risk scores (Ward et al., 1990). RAB subscale scoring instructions were obtained from the scale developer. Six and 9 items were summed for the individual drug and sex risk scores, respectively, and total scaled scores were computed by dividing the total score by 40 (the highest risk potential). For the HRBS, and RAB scales, higher values indicated a higher frequency of risky behaviors. McNemar’s tests were conducted to identify any significant changes in individual risk behaviors, including having sex with a regular alcohol user, having sex with a regular drug user, and condom use, from pre to post intervention. Statistical analyses were conducted using SPSS (version 19.0) and P<.05 was considered statistically significant unless otherwise specified.
3. RESULTS
3.1 Participant demographics
Participants were predominately male, Caucasian, and 27(6.86) years old (Table 1). All participants self-identified as heterosexual. The majority of participants reported oxycodone as their primary drug of abuse and used an average of 107 (80.5) milligrams per day. The second most commonly abused drug was illicit buprenorphine (24%), though participants generally reported abusing an average of 3(1.34) opioids in addition to their primary PO. The primary route of opioid administration at study intake was intranasal (70%), though injection (17%) and oral (13%) routes were also reported. Fifty-seven percent of participants reported having used heroin in the past, and only 39% had previously received treatment for opioids. A summary of other baseline risk behaviors is presented in Table 2.
Table 2.
HIV Risk Behaviors at Treatment Intake (N=54)
| Ever injected (%) | 43 |
| Age first IV use (Mean ± Standard Deviation) | 21 ± 6.26 |
| Partner abuses opioids (%) | 26 |
| Partner abuses other drugs (%) | 80 |
| Partner primary route of administration (%) | |
| Oral | 18 |
| Intranasal | 54 |
| Injection | 18 |
| Worried about catching HIV (%) | 35 |
| Worried has been exposed to HIV (%) | 15 |
| Majority of sex relations under the influence of drugs (%) | 37 |
| Sex with regular alcohol abuser (%) | 13 |
| Sex with regular drug abuser (%) | 25 |
| Previous sexually transmitted disease diagnosis (%) | |
| Chlamydia | 13 |
| Genital HPV | 6 |
| Genital Herpes | 4 |
| Gonorrhea | 2 |
| Trichomoniasis | 2 |
| Unspecified sexually transmitted disease | 2 |
| Syphilis | 0 |
Values presented as Mean ± Standard Deviation unless otherwise indicated.
3.2 HIV and HCV knowledge
The educational intervention was associated with significant improvements in HIV and HCV knowledge. Mean total scores on the Marsch HIV knowledge questionnaire increased from 72% to 94% pre- to post-intervention, representing a 31% increase in accuracy (t(53)= -9.2, p<.001; Figure 1, top panel). When subscale scores were examined to evaluate specific knowledge domains, there were significant increases in the general knowledge (78% to 95%; t (53)=-6.5, p<.001), sexual risk (69% to 95%; t (53)=-10.2, p<.001), and drug risk (67% to 91%; t(53)=-7.8, p<.001) scales from pre- to post-intervention, respectively (Figure 1, bottom panel; Table 3).
FIGURE 1.
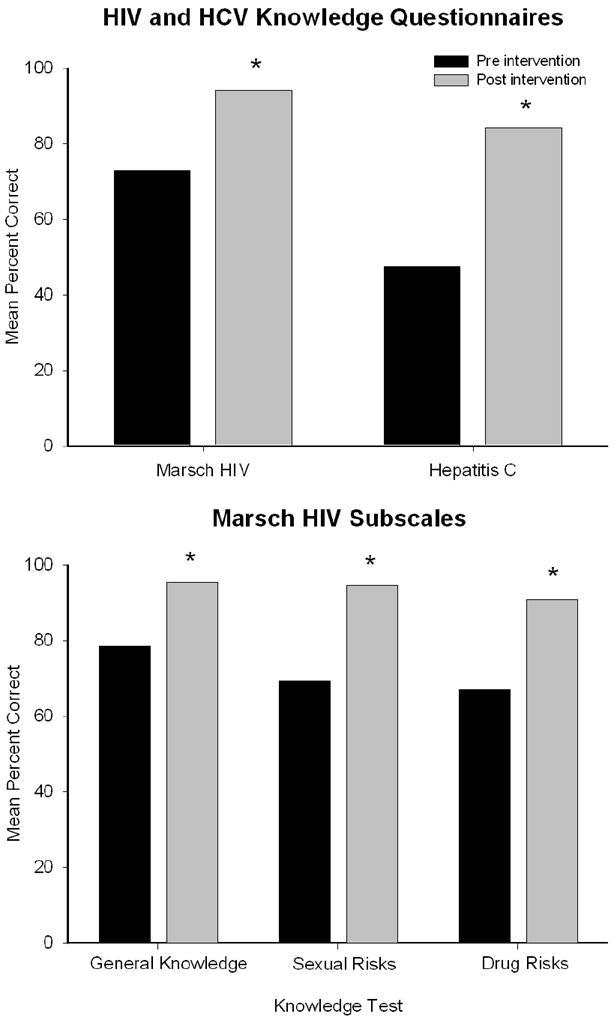
Mean percent correct on the total scores of the Marsch and HCV knowledge questionnaires (top panel) and for the individual Marsch knowledge domains (bottom panel). Asterisks indicate significant differences (p<.01) between the pre- (black) and post- (gray) intervention time points.
Table 3.
Significant Pre-Post Test Improvements on Knowledge Tests
| Item | Pretest % | Posttest % | % Change | P-value |
|---|---|---|---|---|
| Marsch HIV/AIDS Knowledge Test | ||||
| Total Score | 73% | 94% | 21% | <.001 |
| General Knowledge (Total Score) | 79% | 96% | 17% | <.001 |
| 1 The HIV virus can be transmitted by mosquitoes or bugs (F) | 39% | 93% | 54% | <.001 |
| 2 AIDS is not always fatal (F) | 48% | 94% | 46% | <.001 |
| 3 An infected mother can transmit HIV to an infant via breastfeeding (milk) (T) | 52% | 96% | 44% | <.001 |
| 4 People with AIDS can get severe illnesses which are not ususally a threat to people without AIDS (T) | 67% | 93% | 26% | 0.001 |
| 5 If a person is infected with the HIV virus, he/she will feel sick within a few days to a week after infection (F) | 76% | 98% | 22% | 0.002 |
| 6 AIDS can be transmitted when an infected person coughs or sneezes on another person (F) | 80% | 98% | 19% | 0.006 |
| 7 A person who becomes infected with the HIV virus may not test positive for the virus for up to 4 weeks to 6 months after infection (T) | 78% | 96% | 19% | 0.006 |
| Sexual Risk Knowledge (Total Score) | 69% | 95% | 25% | <.001 |
| 8 Vaseline can be safely used to lubricate a condom (F) | 35% | 93% | 57% | <.001 |
| 9 A person who has had a sexually transmitted disease is at an increased risk for HIV (T) | 37% | 93% | 56% | <.001 |
| 10 Condoms that are not long enough to cover the whole penis may not be able to prevent the transmission of the HIV virus (T) | 50% | 94% | 44% | <.001 |
| 11 Using oil-based lubricants, such as hand lotion, cold cream, food products, or baby oil, with a condom will weaken the condom and increase the likelihood that it may break during sex (T) | 50% | 94% | 44% | <.001 |
| 12 Latex condoms are better than natural skin or lambskin condoms in preventing the spread of HIV (T) | 59% | 94% | 35% | <.001 |
| 13 A person can not get AIDS from pre-ejaculatory fluids (F) | 54% | 87% | 33% | <.001 |
| 14 The HIV virus is present in vaginal secretions (T) | 61% | 93% | 31% | <.001 |
| Drug Risk Knowledge (Total Score) | 67% | 91% | 24% | <.001 |
| 15 Boiling drug works for 15 minutes before each use will reduce the likelihood of becoming infected with the AIDS virus (T) | 26% | 87% | 61% | <.001 |
| 16 Using bleach to clean drug works after each use greatly reduces the risk of getting AIDS (T) | 43% | 87% | 44% | <.001 |
| 17 Transferring a drug from one syringe to another can transmit the HIV virus (T) | 67% | 98% | 31% | <.001 |
| 18 Drug users can increase their chances of getting AIDS by sharing water, in which needles or syringes were dipped, with another user (T) | 63% | 94% | 31% | <.001 |
| 19 It is safe to re-use bleach after someone else has used it to clean their drug works (F) | 67% | 94% | 28% | 0.001 |
| 20 The HIV virus can be transmitted from one person to another by sharing drug works, such a cookers or cottons (T) | 69% | 94% | 26% | 0.003 |
| 21 Needles bought on the street in a sterile wrapper cannot transmit the HIV virus (F) | 50% | 78% | 28% | 0.004 |
| Hepatitis C Knowledge Test | ||||
| Total Score | 48% | 84% | 37% | <.001 |
| 1 The hepatitis virus can live for 7 days in dried blood (F) | 9% | 63% | 54% | <.001 |
| 2 People who contract hepatitis C usually feel sick within 1-2 days of contracting the virus (F) | 43% | 96% | 54% | <.001 |
| 3 There is a vaccine available for hepatitis C (F) | 37% | 87% | 50% | <.001 |
| 4 Hepatitis C is spread by breast feeding, sneezing, hugging, coughing, or casual contact (F) | 9% | 59% | 50% | <.001 |
| 5 Symptoms of hepatitis may include flu-like symptoms (loss of appetite, nausea, vomiting, fever, weakness, and mild abdominal pain) (T) | 48% | 98% | 50% | <.001 |
| 6 It is harder to kill hepatitis C by cleaning a syringe with bleach than HIV (T) | 37% | 76% | 39% | <.001 |
| 7 You can die from hepatitis C (T) | 56% | 94% | 39% | <.001 |
| 8 It is dangerous to continue drinking alcohol after learning you have contracted hepatitis C (T) | 59% | 94% | 35% | <.001 |
| 9 Most individuals with hepatitis C exhibit no recognizable signs or symptoms (T) | 43% | 78% | 35% | <.001 |
| 10 Hepatitis can be contracted through the sharing of intranasal equipment (i.e., straws) (T) | 63% | 94% | 31% | <.001 |
| 11 You can be treated for hepatitis C, but it is very hard to permanently cure (T) | 67% | 93% | 26% | 0.001 |
Only items that showed significant (P<.01) pre-post changes are presented; answers provided in parentheses (T=True, F=False)
Mean total scores on the HCV knowledge test also showed a similar pattern, with scores increasing from 47% to 84%, representing a 78% increase in accuracy (t (53)=-11.1, p<.001; Figure 1, top panel). The VAS item “How much do you know about how the AIDS virus is transmitted?” also showed significant differences from pre- to post-intervention (67% vs. 80%, respectively; t(53)=-2.88, p<.01; Table 4).
Table 4.
Self-Reported Risk Behaviors
| Item | Intake | Follow-up | P-value | ||
|---|---|---|---|---|---|
|
| |||||
| Score | N | Score | Na | ||
|
|
|||||
| HIV Risk-Taking Behaviour Scaleb | |||||
| Sex Behavior Subscale Total Last Month (0-25) | 5.04 | 54 | 3.80 | 36 | 0.23 |
| Sex Behavior Subscale Total Last Week (0-25) | 4.16 | 54 | 3.20 | 36 | 0.32 |
| Drug-Use Subscale Last Month (0-30) | 4.67 | 54 | 6.33 | 36 | 0.42 |
| Drug-Use Subscale Last Week (0-30) | 2.00 | 54 | 6.33 | 36 | 0.13 |
| Total Score Last Month (0-55) | 5.27 | 54 | 5.65 | 36 | 0.76 |
| Total Score Last Week (0-55) | 4.30 | 54 | 4.84 | 36 | 0.66 |
| Risk Assessment Batteryb | |||||
| Sex Risk Past Month (0-18) | 4.00 | 54 | 3.74 | 36 | 0.47 |
| Drug Risk Past Month (0-22) | 0.32 | 54 | 0.19 | 36 | 0.23 |
| Total Risk Past Month (0-40) | 4.32 | 54 | 3.92 | 36 | 0.32 |
| Total Scaled Score Past Month (0-1) | 0.11 | 54 | 0.10 | 36 | 0.35 |
| Visual Analog Scale (No chance (0)- Very High Chance (100)) c | |||||
| Chance of Contracting HIV | 15.08 | 53 | 21.34 | 53 | 0.03 |
| Restrain from using dirty needle | 64.72 | 54 | 73.93 | 54 | 0.17 |
| Restrain from unprotected sex | 59.67 | 54 | 62.56 | 54 | 0.53 |
| Knowledge about AIDS transmission | 67.02 | 54 | 80.00 | 54 | 0.01 |
| Likelihood of using condom next time | 54.27 | 52 | 62.42 | 52 | 0.05 |
| Chance of contracting Hepatitis Cd | 26.91 | 22 | 34.64 | 22 | 0.32 |
| Intoxicated during sex (% endorse)b | 77% | 54 | 96% | 36 | 0.09 |
| Sex with person who abuses alcohol (% endorse)b | 13% | 54 | 24% | 36 | 0.99 |
| Sex with person who abuses drugs (% endorse)b | 25% | 54 | 29% | 36 | 0.07 |
Sample sizes are decreased due to study attrition at the 4-week assessment, which could occur 2-4 weeks following the knowledge post-test.
Assessed at 4-week intervention
Assessed at post-intervention
Introduced mid-way through data collection resulting in smaller overall sample size
Examination of individual items using the McNemar’s test identified significant pre- to post-intervention increases on 32 individual items, representing 49% of all questions (Table 3). This translates to 21 (42% of possible questions) and 11 (73% of possible questions) items from the Marsch and HCV questionnaires, respectively. As shown in Table 3, the percent of participants improving on these items ranged from 20-63%. Further analysis of all 65 potential questions identified only 3 items on which more than 10% of participants did more poorly at the post- vs. pre-intervention timepoint.
History of being previously treated for opioid dependence was independently associated with higher pre-test Marsch Total Score (78% vs. 68% of total score; t (52)= -2.51, p=.02) and higher scores on the general knowledge (82% vs. 72%; t(52)=-2.21, p=.02) and drug (72% vs. 63%; t(52)=-2.10, p=.02) subscales, but not the sexual risk subscale (73% vs. 66%; t(52)=-1.50, p=.14), compared to participants with no previous treatment, respectively. Prior treatment was also associated with a higher pre-test HCV total score (58% vs. 41%; t(52)=-2.70, p=.01), and a higher VAS rating on the item assessing perceived chance of contracting AIDS (24% vs. 11%; t(52)=-3.07, p<.01). However, only pre-test HCV score was significantly associated with post-test performance (b=.31, t=3.78, p<.01) and prior treatment was not significantly associated with any baseline risk behaviors.
3.3 Self-reported risk behaviors
There was a significant change between pre- and post-intervention assessments on the VAS item “Likelihood of using condom before having sex” (54% to 62%; t(53)=-2.01, p =.05). No changes were observed on remaining VAS scores or risk behavior scales (Table 4). McNemar’s test did not reveal any significant changes from pre- to post-intervention in the percent of people having sex with regular drug and alcohol users or reported condom utilization.
4. DISCUSSION
A brief educational intervention was associated with significant increases in HIV and HCV knowledge in primary PO abusers. These results are consistent with prior studies conducted with cocaine-dependent outpatients (Heil et al., 2005; Herrmann et al., 2013) and extend the utility of this intervention to PO abusers. These data are also consistent with recent meta-analyses that indicate educational interventions can increase HIV and HCV knowledge (Meader et al., 2010; Shah et al., 2013). Inspection of individual items revealed significant pre-to post-intervention gains on 49% of questions, with percent increases in accuracy ranging from 20-63% per item. Only 3 items showed significant losses from pre- to post-intervention, suggesting that gains were not likely a function of guessing. Our knowledge questionnaires were also modified from the original forced choice (True-False) answers to include the response “I don’t know,” which has been shown to significantly decrease guessing and provide more reliable estimates of knowledge (Harris and Changas, 1994; Pennington et al., 2001; Herrmann et al., 2013).
The largest gains observed in this study were for HCV knowledge, which may be because participants presented with relatively high levels of HIV knowledge (72% pre-test accuracy). In contrast, they had 47% accuracy on the pre-test HCV assessment, which is consistent with HCV knowledge among methadone-maintained patients (Du et al., 2012). The lower level of knowledge regarding basic HCV risk behaviors in this population is alarming and may partially explain why PO abuse is highly associated with HCV acquisition (Bruneau et al., 2012; Havens et al., 2013). This is the first intervention, to our knowledge, to directly target HCV knowledge and related behaviors in a PO-dependent population. The large gains in HCV knowledge associated with the intervention are encouraging and suggest that even a brief intervention may substantially impact knowledge about HCV risks in these patients. Interestingly, despite the robust increases in HIV and HCV knowledge, participants still perceived themselves to be at low risk for HIV infection, consistent with prior research on the perception of HIV risk among PO abusers (Pollini et al., 2011). No significant changes were observed regarding perceived chance of contracting HCV, though data suggest this item might have been underpowered to detect an effect. Drug users do engage in less risky behavior if they perceive themselves to be at high risk for HIV infection (Tsui et al., 2012); therefore, this finding is a potentially important clinical target for future interventions.
Having been previously treated for opioid dependence was significantly associated with providing higher pre-test scores on the Marsch total, general knowledge, and drug risk subscales, the HCV total score, and the VAS rating of risk of contracting AIDS, though linear regressions did not reveal any unique contribution of previous treatment to any post-test score values. Nevertheless, these data are encouraging because they suggest participants may be receiving some level of education regarding HIV/HCV risks in other treatment areas, though more research is needed to permit causal associations between prior treatment and baseline knowledge.
Participants also endorsed a significantly greater likelihood of using a condom after the intervention, which suggests the intervention produced greater recognition of the risks associated with unprotected sex. This result should be considered preliminary, however, because the lack of control group precludes any causal attributions. No additional changes in rates of risk behaviors were detected. There are several possible reasons for this. First, as shown in Table 4, participant attrition from the primary study resulted in sample size discrepancies between the pre- and post-intervention for these questions and may have left them underpowered to detect an effect. Second, participants endorsed a low baseline incidence of risk behaviors on the HRBS and RAB in general, which may have produced a floor effect that did not leave room for subsequent reductions. Third, as the HIV/HCV intervention was evaluated in the context of an opioid detoxification trial, changes in risk behaviors could have been confounded with aspects of participants’ treatment response. It is also not clear whether changes in HIV risk behaviors that occur as a function of methadone and buprenorphine maintenance would extend to opioid detoxification settings (Marsch, 1998; Sullivan et al., 2008). Finally, the 4-week assessment time period may have been too limited to fully characterize changes in risk behaviors that may occur over an extended duration.
Approximately half of the PO abusers in this study reported having injected drugs, which is consistent with another large-scale report suggesting a meaningful incidence of injection use among PO abusers (Bruneau et al., 2012). However, a large percentage of participants in this study also engaged in noninjection risk behaviors. Noninjection behaviors associated with increased risk of infection include potential sharing of snorting equipment (Koblin et al., 2003), transitioning between noninjecting and injecting routes of administration (Abelson et al., 2006; Crofts et al., 1996; Darke et al., 1994a, 1994b; Des Jarlais et al., 1992; Friedman et al., 1995; Fuller et al., 2002; Griffiths et al., 1992, 1994; Irwin et al., 1996; Neaigus et al., 2001b; Strang et al., 1992), having sex with other regular drug users (Bravo et al., 2003; Neaigus et al., 2001a; Purcell et al., 2006; Roy et al., 2004), having sex while under the influence of a drug (Celentano et al., 2006), and having been previously diagnosed with a sexually transmitted disease (Hwang et al., 2000; Kalichman et al., 2005). The prevalence of some of these risk behaviors was identified in our sample by adding questions to the risk assessment batteries. To our knowledge, this is the only study to examine these risks in PO abusers. These data underscore the need to develop measures that target noninjection risk behaviors, which will enable development of improved clinical interventions, a more comprehensive understanding of risk profile, and better outcome measures for detecting clinical effects of risk interventions.
The present study has several limitations. First, it was a pre- to post-intervention, within-subject evaluation conducted within the context of a larger treatment trial. The lack of an HIV/HCV intervention control condition limits interpretation of the data and precludes casual inferences regarding the contribution of the intervention to changes in HIV and HCV knowledge and reductions in risk behaviors. However, results from our prior controlled studies evaluating a similar intervention in cocaine-dependent patients (Heil et al., 2005; Herrmann et al., 2013) strongly suggest that improvements in knowledge were attributable to the intervention. Second, attrition from the primary study resulted in smaller sample sizes at follow-up and prevented an evaluation of whether knowledge increases were sustained over time or whether risk behaviors changed after the intervention. Third, we did not conduct HIV and/or HCV testing and only collected self-reported HIV status; thus, we cannot determine whether knowledge and/or risk behaviors varied as a function of disease status or whether disease status changed as a function of the intervention. Fourth, the knowledge questionnaires have not been statistically validated. Finally, the study sample was relatively homogenous (e.g., predominately male, Caucasian, rural geographic region), and identified exclusively as heterosexual, which may limit the generalization of these results into more diverse samples.
Despite these limitations, these data are encouraging and suggest that a brief intervention delivered within the context of an ongoing treatment may significantly improve knowledge of HIV/HCV risk behaviors and reduce the likelihood of having unprotected sex among PO abusers. These data extend prior studies to the growing population of PO abusers and expand the focus to include HCV and noninjection risk behaviors. Overall, these results support the use of brief educational interventions with PO abusers and suggest this can be accomplished using relatively low-cost methods. For instance, this intervention was developed using low cost, commercially available materials, and was administered by a counselor who had no specific expertise in HIV/HCV risks and did not complete any specialized training. Taken together, the prevalence of PO abuse, combined with the increasing incidence of injection drug use, makes the prevention of HIV and HCV in this population an important public health target. This study represents a strong first step towards the development of a brief but effective HIV/HCV educational intervention for PO abusers.
Acknowledgments
This study was supported by research grant R01 DA019989 (Sigmon) and training grant T32 DA007242 (Higgins) from the National Institute on Drug Abuse. We thank Bruce Brown, Betsy Bahrenberg, John Brooklyn, Abby Cooper-Marshall, Allison Newth, Allison Necheles, and Matt Scanlin for their assistance conducting this study.
Role of Funding Source: Funding for this study was provided by NIDA Grants R01 DA019989 (Sigmon) and NIDA T32 DA007242 (Higgins). NIDA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Contributors: Authors Dunn and Sigmon developed the HIV/HCV intervention. Authors Dunn, Saulsgiver, and Patrick delivered the intervention. Authors Heil, Higgins, and Sigmon developed the larger treatment intervention in which the HIV/HCV intervention was delivered. Author Dunn was the primary author of the manuscript, and conducted the literature searches and statistical analyses, and developed the tables and Figure 1. Authors Saulsgiver, Patrick, Heil, Higgins, and Sigmon made important intellectual contributions to the content and organization of the manuscript and provided editing advice and suggestions. All authors contributed to and have approved the final manuscript.
Conflict of Interest: None of the authors have any conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abelson J, Treloar C, Crawford J, Kippax S, van Beek I, Howard J. Some characteristics of early-onset injection drug users prior to and at the time of their first injection. Addiction. 2006;101:548–555. doi: 10.1111/j.1360-0443.2006.01379.x. [DOI] [PubMed] [Google Scholar]
- American Liver Foundation. The Hepatitis Information You Need to Know: Intravenous Drug Users. American Liver Foundation; New York, NY: 2003. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Birnbaum HG, White AG, Schiller M, Waldman T, Cleveland JM, Roland CL. Societal costs of prescription opioid abuse, dependence, and misuse in the United States. Pain Med. 2011;12:657–667. doi: 10.1111/j.1526-4637.2011.01075.x. [DOI] [PubMed] [Google Scholar]
- Bravo MJ, Barrio G, de la Fuente L, Royuela L, Domingo L, Silva T. Reasons for selecting an initial route of heroin administration and for subsequent transitions during a severe HIV epidemic. Addiction. 2003;98:749–760. doi: 10.1046/j.1360-0443.2003.00393.x. [DOI] [PubMed] [Google Scholar]
- Bruneau J, Roy E, Arruda N, Zang G, Jutras-Aswad D. The rising prevalence of prescription opioid injection and its association with hepatitis C incidence among street-drug users. Addiction. 2012;107:1318–1327. doi: 10.1111/j.1360-0443.2012.03803.x. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) CDC grand rounds: prescription drug overdoses - a U.S. epidemic. MMWR. 2012;61:10–13. [PubMed] [Google Scholar]
- CDC. [04/23/2013];HIV Surveillance Report: Diagnoses of HIV infection and AIDS in the United States and Dependent Areas. 2011 23 at: http://www.cdc.gov/hiv/topics/surveillance/basic.htm. [Google Scholar]
- CDC. [04/23/2013];Viral Hepatitis Surveillance- United States 2010. 2010 at: http://www.cdc.gov/hepatitis/statistics/
- CDC. Disease burden from Viral hepatitis A, B, and C in the United States. Division of Viral Hepatitis, National Center for HIV/AIDS, Viral Hepatitis, STD and TB Prevention; 2008. [04/23/2013]. at: http://www.cdc.gov/hepatitis/Statistics/index.htm. [Google Scholar]
- CDC. Cases of HIV infections and AIDS in the United Stated and Dependant Areas. [04/23/2013];HIV/AIDS Surveillance Report. 2011 19 http://www.cdc.gov/hiv/topics/surveillance/resources/reports/2007report/default.htm. [Google Scholar]
- Celentano DD, Valleroy LA, Sifakis F, MacKellar DA, Hylton J, Thiede H, McFarland W, Shehan DA, Stoyanoff SR, LaLota M, Koblin BA, Katz MH, Torian LV Young Men’s Survey Study Group. Associations between substance use and sexual risk among very young men who have sex with men. Sex Transm Dis. 2006;33:265–271. doi: 10.1097/01.olq.0000187207.10992.4e. [DOI] [PubMed] [Google Scholar]
- Channing Bete Company Inc. HIV and AIDS – What you need to know. South Deerfield, MA: 2008. [Google Scholar]
- Channing Bete Company Inc. About Viral Hepatitis. South Deerfield, MA: 1986. [Google Scholar]
- Copenhaver MM, Johnson BT, Lee IC, Harman JJ, Carey MP SHARP Research Team. Behavioral HIV risk reduction among people who inject drugs: meta-analytic evidence of efficacy. J Subst Abuse Treat. 2006;31:163–171. doi: 10.1016/j.jsat.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofts N, Louie R, Rosenthal D, Jolley D. The first hit: circumstances surrounding initiation into injecting. Addiction. 1996;91:1187–1196. doi: 10.1046/j.1360-0443.1996.918118710.x. [DOI] [PubMed] [Google Scholar]
- Darke S, Cohen J, Ross J, Hando J, Hall W. Transitions between routes of administration of regular amphetamine users. Addiction. 1994a;89:1077–1083. doi: 10.1111/j.1360-0443.1994.tb02784.x. [DOI] [PubMed] [Google Scholar]
- Darke S, Hall W, Heather N, Ward J, Wodak A. The reliability and validity of a scale to measure HIV risk-taking behaviour among intravenous drug users. AIDS. 1991;5:181–185. doi: 10.1097/00002030-199102000-00008. [DOI] [PubMed] [Google Scholar]
- Darke S, Swift W, Hall W, Ross M. Predictors of injecting and injecting risk-taking behaviour among methadone-maintenance clients. Addiction. 1994b;89:311–316. doi: 10.1111/j.1360-0443.1994.tb00897.x. [DOI] [PubMed] [Google Scholar]
- Des Jarlais DC, Casriel C, Friedman SR, Rosenblum A. AIDS and the transition to illicit drug injection--results of a randomized trial prevention program. Br J Addict. 1992;87:493–498. doi: 10.1111/j.1360-0443.1992.tb01950.x. [DOI] [PubMed] [Google Scholar]
- Des Jarlais DC, Semaan S. HIV prevention for injecting drug users: the first 25 years and counting. Psychosom Med. 2008;70:606–611. doi: 10.1097/PSY.0b013e3181772157. [DOI] [PubMed] [Google Scholar]
- Du J, Wang Z, Xie B, Zhao M. Hepatitis C knowledge and alcohol consumption among patients receiving methadone maintenance treatment in Shanghai, China. Am J Drug Alcohol Abuse. 2012;38:228–232. doi: 10.3109/00952990.2011.643974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SR, Jose B, Deren S, Des Jarlais DC, Neaigus A. Risk factors for human immunodeficiency virus seroconversion among out-of-treatment drug injectors in high and low seroprevalence cities. The National AIDS Research Consortium. Am J Epidemiol. 1995;142:864–874. doi: 10.1093/oxfordjournals.aje.a117726. [DOI] [PubMed] [Google Scholar]
- Fuller CM, Vlahov D, Ompad DC, Shah N, Arria A, Strathdee SA. High-risk behaviors associated with transition from illicit non-injection to injection drug use among adolescent and young adult drug users: a case-control study. Drug Alcohol Depend. 2002;66:189–198. doi: 10.1016/s0376-8716(01)00200-9. [DOI] [PubMed] [Google Scholar]
- Garfein RS, Golub ET, Greenberg AE, Hagan H, Hanson DL, Hudson SM, Kapadia F, Latka MH, Ouellet LJ, Purcell DW, Strathdee SA, Thiede H. A peer-education intervention to reduce injection risk behaviors for HIV and hepatitis C infection among young injection drug users. AIDS. 2007;21:1923–1932. doi: 10.1097/QAD.0b013e32823f9066. [DOI] [PubMed] [Google Scholar]
- Griffiths P, Gossop M, Powis B, Strang J. Extent and nature of transitions of route among heroin addicts in treatment--preliminary data from the Drug Transitions Study. Br J Addict. 1992;87:485–491. doi: 10.1111/j.1360-0443.1992.tb01949.x. [DOI] [PubMed] [Google Scholar]
- Griffiths P, Gossop M, Powis B, Strang J. Transitions in patterns of heroin administration: a study of heroin chasers and heroin injectors. Addiction. 1994;89:301–309. doi: 10.1111/j.1360-0443.1994.tb00896.x. [DOI] [PubMed] [Google Scholar]
- Hansen RN, Oster G, Edelsberg J, Woody GE, Sullivan SD. Economic costs of nonmedical use of prescription opioids. Clin J Pain. 2011;27:194–202. doi: 10.1097/AJP.0b013e3181ff04ca. [DOI] [PubMed] [Google Scholar]
- Harris DK, Changas PS. Revision of Palmore’s second facts on aging quiz from a true–false to a multiple-choice format. Educ Gerontol. 1994;20:741–754. [Google Scholar]
- Havens JR, Lofwall MR, Frost SD, Oser CB, Leukefeld CG, Crosby RA. Individual and network factors associated with prevalent hepatitis C infection among rural Appalachian injection drug users. Am J Public Health. 2013;103:e44–52. doi: 10.2105/AJPH.2012.300874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havens JR, Oser CB, Leukefeld CG. Injection risk behaviors among rural drug users: implications for HIV prevention. AIDS Care. 2011;23:638–645. doi: 10.1080/09540121.2010.516346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heil SH, Sigmon SC, Mongeon JA, Higgins ST. Characterizing and improving HIV/AIDS knowledge among cocaine-dependent outpatients. Exp Clin Psychopharmacol. 2005;13:238–243. doi: 10.1037/1064-1297.13.3.238. [DOI] [PubMed] [Google Scholar]
- Hepatitis Foundation International. The ABC’s of Hepatitis. Hepatitis Foundation, International; Cedar Grove, NJ: 2001. [Google Scholar]
- Herrmann ES, Heil SH, Sigmon SC, Dunn KE, Washio Y, Higgins ST. Characterizing and improving HIV/AIDS knowledge among cocaine-dependent outpatients using modified materials. Drug Alcohol Depend. 2013;127:220–225. doi: 10.1016/j.drugalcdep.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang LY, Ross MW, Zack C, Bull L, Rickman K, Holleman M. Prevalence of sexually transmitted infections and associated risk factors among populations of drug abusers. Clin Infect Dis. 2000;31:920–926. doi: 10.1086/318131. [DOI] [PubMed] [Google Scholar]
- Irwin KL, Edlin BR, Faruque S, McCoy HV, Word C, Serrano Y, Inciardi J, Bowser B, Holmberg SD. Crack cocaine smokers who turn to drug injection: characteristics, factors associated with injection, and implications for HIV transmission. The Multicenter Crack Cocaine and HIV Infection Study Team. Drug Alcohol Depend. 1996;42:85–92. doi: 10.1016/0376-8716(96)01262-8. [DOI] [PubMed] [Google Scholar]
- Kalichman SC, Cain D, Knetch J, Hill J. Patterns of sexual risk behavior change among sexually transmitted infection clinic patients. Arch Sex Behav. 2005;34:307–319. doi: 10.1007/s10508-005-3119-5. [DOI] [PubMed] [Google Scholar]
- Koblin BA, Factor SH, Wu Y, Vlahov D. Hepatitis C virus infection among noninjecting drug users in New York City. J Med Virol. 2003;70:387–390. doi: 10.1002/jmv.10407. [DOI] [PubMed] [Google Scholar]
- Larney S, Randall D, Gibson A, Degenhardt L. The contributions of viral hepatitis and alcohol to liver-related deaths in opioid-dependent people. Drug Alcohol Depend. 2012;131:252–257. doi: 10.1016/j.drugalcdep.2012.11.012. [DOI] [PubMed] [Google Scholar]
- Marsch LA. The efficacy of methadone maintenance interventions in reducing illicit opiate use, HIV risk behavior and criminality: a meta-analysis. Addiction. 1998;93:515–532. doi: 10.1046/j.1360-0443.1998.9345157.x. [DOI] [PubMed] [Google Scholar]
- Marsch LA, Bickel WK, Badger GJ, Stothart ME, Quesnel KJ, Stanger C, Brooklyn J. Comparison of pharmacological treatments for opioid-dependent adolescents: a randomized controlled trial. Arch Gen Psychiatry. 2005;62:1157–1164. doi: 10.1001/archpsyc.62.10.1157. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr HL, O’Brien CP. New data from the Addiction Severity Index. Reliability and validity in three centers. J Nerv Ment Dis. 1985;173:412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- Meader N, Li R, Des Jarlais DC, Pilling S. Psychosocial interventions for reducing injection and sexual risk behaviour for preventing HIV in drug users. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD007192.pub2. CD007192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navaline HA, Snider EC, Petro CJ, Tobin D, Metzger D, Alterman AI, Woody GE. Preparations for AIDS vaccine trials. An automated version of the Risk Assessment Battery (RAB): enhancing the assessment of risk behaviors. AIDS Res Hum Retroviruses. 1994;10(Suppl. 2):S281–S283. [PubMed] [Google Scholar]
- Neaigus A, Gyarmathy VA, Zhao M, Miller M, Friedman SR, Des Jarlais DC. Sexual and other noninjection risks for HBV and HCV seroconversions among noninjecting heroin users. J Infect Dis. 2007;195:1052–1061. doi: 10.1086/512081. [DOI] [PubMed] [Google Scholar]
- Neaigus A, Miller M, Friedman SR, Des Jarlais DC. Sexual transmission risk among noninjecting heroin users infected with human immunodeficiency virus or hepatitis C virus. J Infect Dis. 2001a;184:359–363. doi: 10.1086/322020. [DOI] [PubMed] [Google Scholar]
- Neaigus A, Miller M, Friedman SR, Hagen DL, Sifaneck SJ, Ildefonso G, des Jarlais DC. Potential risk factors for the transition to injecting among non-injecting heroin users: a comparison of former injectors and never injectors. Addiction. 2001b;96:847–860. doi: 10.1046/j.1360-0443.2001.9668476.x. [DOI] [PubMed] [Google Scholar]
- Pennington HR, Pachana NA, Coyle SL. Use of the facts on aging quiz in New Zealand: validation of questions, performance of a student sample, and effects of a don’t know option. Educ Gerontol. 2001;27:409–416. [Google Scholar]
- Pollini RA, Banta-Green CJ, Cuevas-Mota J, Metzner M, Teshale E, Garfein RS. Problematic use of prescription-type opioids prior to heroin use among young heroin injectors. Subst Abuse Rehabil. 2011;2:173–180. doi: 10.2147/SAR.S24800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell DW, Mizuno Y, Metsch LR, Garfein R, Tobin K, Knight K, Latka MH. Unprotected sexual behavior among heterosexual HIV-positive injection drug using men: associations by partner type and partner serostatus. J Urban Health. 2006;83:656–668. doi: 10.1007/s11524-006-9066-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy KM, Goldberg DJ, Hutchinson S, Cameron SO, Wilson K, MacDonald L. Hepatitis C virus among self declared non-injecting sexual partners of injecting drug users. J Med Virol. 2004;74:62–66. doi: 10.1002/jmv.20146. [DOI] [PubMed] [Google Scholar]
- Scheinmann R, Hagan H, Lelutiu-Weinberger C, Stern R, Des Jarlais DC, Flom PL, Strauss S. Non-injection drug use and Hepatitis C Virus: a systematic review. Drug Alcohol Depend. 2007;89:1–12. doi: 10.1016/j.drugalcdep.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selzer ML. The Michigan alcoholism screening test: the quest for a new diagnostic instrument. Am J Psychiatry. 1971;127:1653–1658. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- Sigmon SC, Dunn KE, Saulsgiver K, Patrick M, Badger GJ, Heil SH, Brooklyn J, Higgins ST. A randomized, double-blind evaluation of buprenorphine taper duration in primary prescription opioid abusers. JAMA Psychiatry. doi: 10.1001/jamapsychiatry.2013.2216. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah HA, Abu-Amara M. Education provides significant benefits to patients with Hepatitis B and Hepatitis C infection: a systematic review. Clin Gastroenterol Hepatol. 2013 doi: 10.1016/j.cgh.2013.04.024. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Humana Press; Totowa, NJ: 1992. p. 41. [Google Scholar]
- Strang J, Des Jarlais DC, Griffiths P, Gossop M. The study of transitions in the route of drug use: the route from one route to another. Br J Addict. 1992;87:473–483. doi: 10.1111/j.1360-0443.1992.tb01948.x. [DOI] [PubMed] [Google Scholar]
- Strathdee SA, Sherman SG. The role of sexual transmission of HIV infection among injection and non-injection drug users. J Urban Health. 2003;80:iii7–14. doi: 10.1093/jurban/jtg078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA), Center for Behavioral Health Statistics and Quality. The NSDUH Report: Nonmedical use of Prescription-Type Drugs by County Type. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2013. [Google Scholar]
- Sullivan LE, Moore BA, Chawarski MC, Pantalon MV, Barry D, O’Connor PG, Schottenfeld RS, Fiellin DA. Buprenorphine/naloxone treatment in primary care is associated with decreased human immunodeficiency virus risk behaviors. J Subst Abuse Treat. 2008;35:87–92. doi: 10.1016/j.jsat.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui H, Lau JT, Xiang W, Gu J, Wang Z. Should associations between HIV-related risk perceptions and behaviors or intentions be positive or negative? PLoS One. 2012;7:e52124. doi: 10.1371/journal.pone.0052124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker T, Fry CL, Lintzeris N, Baldwin S, Ritter A, Donath S, Whelan G. Randomized controlled trial of a brief behavioral intervention for reducing hepatitis C virus risk practices among injecting drug users. Addiction. 2004;99:1157–1166. doi: 10.1111/j.1360-0443.2004.00809.x. [DOI] [PubMed] [Google Scholar]
- Ward J, Darke S, Hall W. The HIV Risk-Taking Behaviour Scale (HRBS) Manual. National Drug and Alcohol Research Centre; Sydney: 1990. [Google Scholar]


