Abstract
Obesity is one of the most important preventable causes of cancer and the most significant risk factor for breast cancer in postmenopausal women. Compared with lean women, obese women are more likely to be diagnosed with a larger, higher grade tumor, an increased incidence of lymph node metastases, and elevated risk of distant recurrence. However, the mechanisms connecting obesity to the pathogenesis of breast cancer are poorly defined. Here we show that during obesity, adipocytes within human and mouse breast tissues recruit and activate macrophages through a previously uncharacterized CCL2/IL-1β/CXCL12 signaling pathway. Activated macrophages in turn promote stromal vascularization and angiogenesis even prior to the formation of cancer. Recapitulating these changes using a novel humanized breast cancer model was sufficient to promote angiogenesis and prime the microenvironment prior to neoplastic transformation for accelerated breast oncogenesis. These findings provide a mechanistic role for adipocytes and macrophages prior to carcinogenesis that may be critical for prevention and treatment of obesity-related cancer.
Keywords: Breast, mammary, macrophages, stroma, obesity
Introduction
Obesity is one of the leading health concerns in the United States, responsible for increased rates of diabetes, cardiovascular disease, and cancer. It is also an important preventable cause of cancer, accounting for up to 20% of cancer deaths in women (1). Obesity is the most significant risk factor in postmenopausal women for breast cancer (2). Compared to lean women, obese women, as defined by a body mass index (BMI) >30, are more likely to be diagnosed with larger, higher grade tumors, have an increased incidence of lymph node metastases, and an elevated risk of distant recurrence, which all contribute to an increased risk of death of breast cancer (1, 3).
The mechanisms by which obesity increases breast cancer incidence and worsens prognosis remain poorly defined. One hypothesis suggests that circulating androgens produced by the adrenal glands and climactic ovaries are converted to estrogen through aromatase activity following menopause (4-6). This higher localized estrogen in obese women could promote the progression of estrogen receptor (ER) positive premalignant lesions, resulting in increased breast cancer incidence. However, obese pre- and postmenopausal women also have an increased likelihood of being diagnosed with ER-negative tumors compared to lean women (7, 8), suggesting that obesity may promote tumorigenesis through non-estrogenic mechanisms.
Obesity is associated with chronic adipose tissue inflammation, which has been implicated as an underlying cause of local and systemic insulin resistance, as well as increased aromatase activity (9, 10). Infiltration of the visceral adipose depots by immune cells appears to play a critical role in the development of inflammation, and a hallmark feature is the recruitment and accumulation of proinflammatory macrophages (11). In response to elevated circulating levels of insulin in obesity, adipocytes increase expression of CCL2 (also known as MCP-1), leading to an influx of macrophages into adipose tissue (12, 13). Consistent with this notion, CCL2 knockout mice, or its receptor, CCR2, demonstrate reduced macrophage infiltration and protection against obesity-induced inflammation in visceral adipose tissue (14, 15). Macrophages recruited to obese visceral adipose tissue increase CCL2 levels, as well as secrete cytokines and factors including TNFα, nitric oxide synthase (iNOS), IL-1, and IL-6 (16). In ovariectomized mice, subcutanteous fat depots demonstrated elevated macrophage recruitment as well as increased expression of CCL2 compared to control mice, suggesting that CCL2 may enhance inflammation in postmenopausal breast tissue (16). Chronic inflammation is a potent tumor promoter of many cancers, including breast, and expression of cytokines within tumors have been increasingly correlated with poor prognosis (17). CCL2 expression in tumors is correlated with higher histological grade and is a significant indicator of early relapse (18), as well as infiltration of tumor-associated macrophages (19). Elevated CCL2 levels from adipocytes may contribute to early events in tumorigenesis by enhancing localized inflammation through adipose macrophages. Because significant differences exist between obese subcutaneous and visceral fat depots, elucidation of the inflammatory changes within the breast stroma may have important ramifications in defining the specific risks for obesity-associated breast cancer. In addition, there is still relatively little known about how signals from the stromal microenvironment in the obese state contribute to the early events in the progression to malignancy.
The understanding of obesity on human breast cancer development has been hindered due, in part, to the limited model systems to assess complex stromal events during the premalignant stages of breast cancer formation. In recent years, we developed an innovative human-in-mouse (HIM) model to study breast cancer development and progression in vivo. This model exploits the humanized mammary fat pad of immunocompromised mice as a source of important endocrine signaling events and uses grafted human stroma to support the growth and progression of premalignant lesions (20, 21). This method results in the stepwise progression of normal human breast epithelium through distinct stages of premalignancy including hyperplasia and ductal carcinoma in situ (DCIS); these lesions as well as resulting invasive carcinomas are histologically and molecular similar to those found in humans (20, 21).
We have modified this in vivo model to study the role of obesity on breast cancer progression by engrafting human breast adipose stromal cells that model the inflammatory environment of the obese breast. Using this model, we provide new insights into the role of macrophage recruitment within subcutaneous fat depots prior to the genesis of cancer and how these changes are directly responsible for breast oncogenesis.
Materials and Methods
Cell lines and tissue culture
HL-60 and bovine retinal endothelial cells (BREC) were obtained from Drs. Richard Van Etten and Ira Herman, respectively (Tufts University). Human microvascular endothelial cells (HMVEC) were obtained from Lonza; RAW 264.7 and 293T cells were obtained from ATCC. Primary mouse heart endothelial cells were obtained from the Center for Vascular Biology Research at Beth Israel Deaconess Medical Center. HL-60 cells were grown in RPMI 1640 (Invitrogen) supplemented with 10% fetal bovine serum (FBS) and 50mM HEPES. HMVEC were grown in Endothelial Complete Media (Lonza); BREC were grown in low glucose DMEM (Invitrogen) supplemented with 5% calf serum (CS), 1% L-glutamine, and 25mM HEPES. RAW 264.7 and 293T cells were grown in high glucose DMEM supplemented with 10% FBS. All cells were grown at 37 °C and 5% CO2, and media was supplemented with 1% penicillin/streptomycin (Invitrogen). All cell lines tested negative for mycoplasma (MilliPROBE; Millipore); the identity of each cell line was not authenticated in our laboratory.
Animals and Surgery
All animal procedures were conducted in accordance with a protocol approved by the Tufts University IACUC committee. Colonies of NOD/SCID and C57Bl/6 mice were maintained in house. SCID and DTGR EGFP-Mac mice were obtained from Jax Laboratories (strain numbers: 001913 and 006000, respectively) and mated. Genotyping was perfomed by Transnetyx (Cordova, TN). Mice were given food and water ad libitum. For obesity studies, 6 week old female mice were fed either low fat maintenance chow (5% kcal from fat) or a high fat diet (60% kcal from fat, Test Diet). Weights were measured weekly for 16 weeks. Tissue was frozen for molecular analyses or embedded, sectioned, and stained for H&E at Tufts Medical Center. Details for whole mount preparation and quantification of blood vessels are provided in Supplemental Materials and Methods.
Primary Tissue Isolation and Culture
All human breast tissues were obtained in compliance with the laws and institutional guidelines, as approved by institutional IRB committee from Tufts Medical Center and Duke University. Non-cancerous breast tissues were obtained from patients undergoing elective reduction mammoplasty. Breast tissues were enzymatically digested as previously described (20, 22). Cells from the lipid layer comprising the mature adipocyte fraction (MAF) and the cell pellet containing the stromal vascular fraction (SVF) were plated in DMEM supplemented with 10% calf serum. SVF cells were immortalized with the catalytic subunit of telomerase (h-TERT) and transduced with lentiviruses containing either CSCG empty vector (SVF/EV) or CSCG-CCL2 (SVF/CCL2). Infected cells were sorted for GFP expression with a Legacy MoFlo (Beckman Coulter). For generation of SVF/CCL2 lines with shIL-1β, cells were transduced with lentiviruses containing either shscrambled or shIL-1β constructs (MISSION shRNA, Sigma) and selected in 1 ug/mL of puromycin. Details for adipocyte differentiation are provided in Supplemental Materials and Methods.
For tumorigenesis studies, epithelial organoids were dissociated to single cells and infected in suspension with lentiviruses for CSCG vector, pLenti-KrasG12V and pLenti-SV40er (21). Details for lentiviral production are provided in Supplemental Materials and Methods. To humanize mice, mammary epithelium was removed from the fourth mammary glands of 3 week-old NOD/SCID females and either SVF/EV or SVF/CCL2 cells were injected into the fat pad as described (22). Two weeks post-humanization, oncogene virus-infected cells (100,000 per gland) were co-mixed with either SVF/EV or SVF/CCL2 cells (2.5×105 per gland) in a 1:1 mixture of collagen and Matrigel (BD Biosciences) and injected into humanized fat pads. Tumors were measured twice weekly, and end stage was assessed when tumors reached 1.5 cm in diameter. For inhibitor studies, mice were treated following humanization with daily subcutaneous injections of 10mg/kg Kineret (Amgen) or 2mg/kg RS504393 (Tocris), and tissues were collected 2 weeks following tumor cell transplant or when tumors reached 1 cm in diameter.
Macrophage differentiation and treatment
Bone marrow from C57Bl/6 females was isolated and plated for 3 days in DMEM:F12 (Invitrogen) supplemented with 20% FBS. Suspended cells were differentiated into macrophages as described (23). Mouse macrophages were treated with 40% conditioned media (CM) from SVF/EV or SVF/CCL2 for 24 hours and either collected for molecular analyses or grown in DMEM supplemented with 0.5% CS for 24 hours for macrophage CM experiments.
HL-60 cells were differentiated into adherent macrophages as described (24). Macrophages were treated with DMEM supplemented with 0.5% CS and vehicle, 20ng/mL recombinant human CCL2 (rhCCL2), 10ng/mL rhIL-1β, 20ng/mL rhCCL2+10ng/mL rhIL-1β (both R&D Systems), or CM from SVF/EV, SVF/CCL2, SVF/CCL2 shscrambled, or SVF/CCL2 shIL-1β cells. For inhibitor studies, HL-60 macrophages were treated with SVF/CCL2 CM and vehicle (DMSO), 330nM RS504393 (Tocris), 40ng/mL IL-1ra (R&D Systems) or 330nM RS504393+40ng/mL IL-1ra. Macrophages were treated for 24 hours and either collected for molecular analyses or grown in DMEM supplemented with 0.5% CS for 24 hours for CM experiments.
Quantitative PCR analyses
RNA was isolated, reverse transcribed, and analyzed as described in Supplemental Materials and Methods. Primer sequences used for qPCR are listed in Table S1.
Immunohistochemistry and Immunofluorescence
F4/80 and CD31 proteins were detected as described previously (25). Details for analyses are provided in Supplementary Methods and Materials.
Western blotting
Proteins were isolated and concentrations measured as described (25). Antibody concentrations and detection methods are described in Supplementary Methods and Materials.
Statistical analyses
Results were expressed as the mean±SEM. Statistical tests included unpaired two-tailed Student t test and one-way ANOVA, followed by Neuman-Keuhls post test (for more than 2 groups). Human qPCR data was analyzed using two-tailed Pearson’s correlation. Body Mass Index (BMI) data was blinded until qPCR analyses were complete. P values of 0.05 or less were considered to denote significance. Statistical analyses were performed using Graph Pad Prism (Graph Pad Software).
Results
Stromal changes within the adipose tissue of the breast during obesity
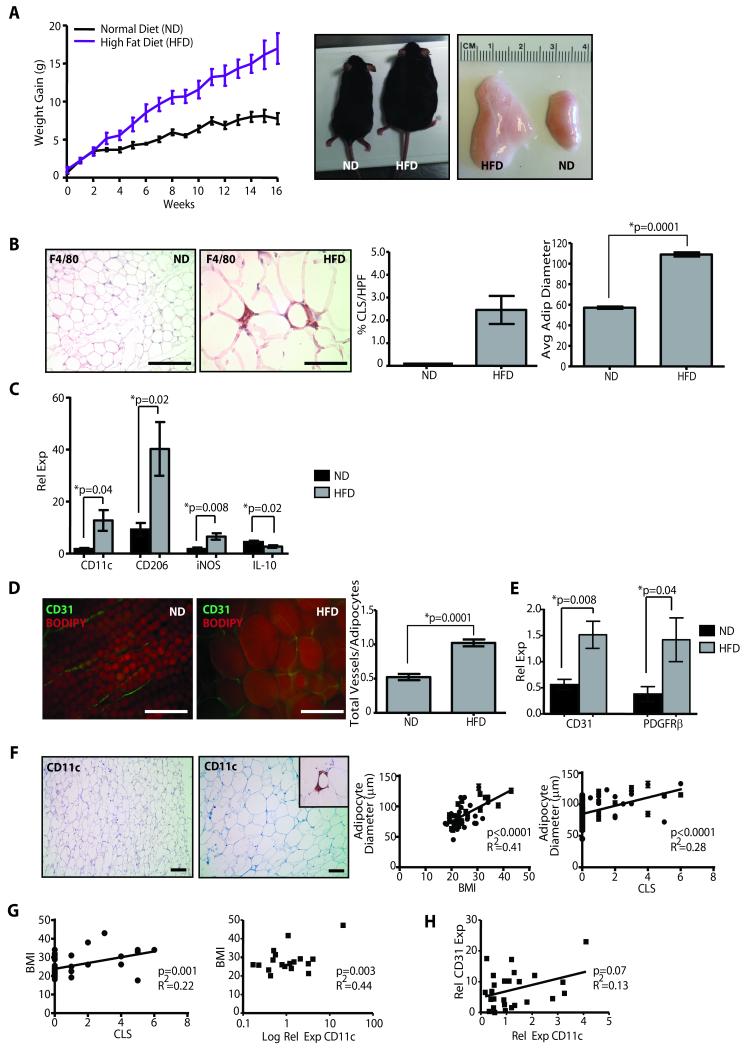
To examine the effects of obesity on mammary adipose tissue, non-ovariectomized females were fed either the normal mouse colony diet (ND) or a diet with increased calories from fat (HFD), with equivalent micronutrients and vitamins. After 16 weeks, HFD mice weighed more than twice as much as ND mice (p=0.006; Figure 1A). All fat depots expanded in obese mice compared to their lean counterparts, including the inguinal mammary glands (1.3 ± 0.4g vs 0.4 ± 0.06g, p=0.002; Figure 1A). The diameters of white adipocytes were significantly enlarged in mammary glands of obese mice (p<0.0001; Figure 1B). F4/80+ macrophages surrounded adipocytes forming crown-like structures (CLS, Figure 1B), which are typically present within visceral fat depots of genetically and diet-induced obese mice (11, 26). CLS were absent in the mammary glands of lean mice (Figure 1B). Consistent with the formation of CLS, total numbers of macrophages were significantly increased in glands of HFD mice (4.4×105 ± 0.5×105; macrophages/gland±sd) compared with ND mice (2.5×105±0.5×105; p=0.05).
Figure 1. Mammary stroma recruits inflammatory macrophages and demonstrates increased vascularity under conditions of obesity.
(A) Female C57Bl/6 mice fed a high fat diet (HFD) gained significantly more weight, resulting in increased body weight (p=0.0006, n=10 mice/group) and mammary fat pad depot size (p=0.002), compared to those fed a normal chow diet (ND). (B) In obese mice, mammary fat depots demonstrated F4/80+ crown-like structures (CLS), which were not detectable in depots of control mice, as well as significantly increased adipocyte diameters. (C) Transcripts for M1-like (CD11c, iNOS) and M2-like (CD206) macrophage markers were elevated in mammary glands from HFD mice (qPCR, n=6 mice/group). (D) Mammary glands from obese mice demonstrated a significantly increased vessel to adipocyte ratio (n=5 mice/group). (E) Transcripts for CD31 and PDGFRβ were significantly elevated in glands from HFD mice (qPCR, n=6/group). (F) In breast tissue isolated from reduction mammoplasty surgeries, increasing adipocyte diameter was correlated with increased body mass index (BMI; n=46) as well as the formation of CD11c+ CLS (n=76). (G) Increased BMI was correlated with the formation of CD11c+ CLS (n=46), as well as increased relative expression of CD11c transcripts from reduction mammoplasty tissues (n=18). (H) Elevated CD11c expression was associated with increased CD31 expression (n=28). qPCR was performed on RNA isolated from reduction mammoplasty tissue. Original magnification (B, D) 200x, (F) 100x; bar=100μm.
Macrophages have at least two polarization states: M1 polarization by LPS and IFN-γ, and M2 activation by IL-4 and IL-13 (27). Macrophages that express M1-type cytokines have been proposed to have tumoricidal functions, while those that produce M2-type cytokines may be tumor promoting (28, 29). Therefore, mammary glands were examined for expression of IL-10 and CD206 (M2) as well as CD11c and iNOS (M1) transcripts to determine the polarization state of macrophages in the obese mammary adipose tissue. Obese glands demonstrated significantly decreased IL-10 expression (p=0.02) and significantly increased iNOS and CD11c expression (p=0.008 and p=0.04; Fig. 1C), consistent with an M1 polarization phenotype of the obesity-recruited macrophages. However, CD206 was significantly elevated (p=0.02), suggesting that macrophages in obese mammary glands demonstrate a mixed polarization, consistent with reports from visceral adipose depots in mice and humans (30, 31), as well as peritumoral adipose tissue (32).
Adipose tissue is critically dependent on vascularization for growth (33). Therefore, we examined the effect of obesity on vascularity within the mammary glands. Inguinal mammary glands from HFD and ND females were stained with BODIPY to detect lipid and for the endothelial marker, CD31. CD31+ vessels surrounding adipocytes were significantly increased in glands of HFD females compared with those fed the ND (p=0.0001; Figure 1D). Transcripts from whole glands for CD31 and pericyte marker PDGFRβ were significantly increased in HFD glands compared to ND glands (Figure 1E), suggesting that both endothelial cells and pericytes were increased in the obese glands.
To determine whether obesity-induced changes in the mouse mammary gland were present in the human breast of women with increased BMI, disease-free breast tissue collected from reduction mammoplasty surgeries was examined for adipocyte size, CD11c+ macrophage recruitment, and angiogenesis. Adipocyte size strongly correlated with both increasing BMI (p<0.0001), as well as the formation of CD11c+ CLS (p<0.0001; Figure 1F). Increased BMI was strongly correlated with CD11c+ CLS formation (p=0.001) as well as increased transcripts for CD11c (p=0.003; Figure 1G). Elevated CD11c expression was also associated with elevated CD31 expression (p=0.07; Figure 1H). These results suggest that the adipose tissue of the breast during obesity exhibits increased angiogenesis and macrophage recruitment.
Generation of obesity-like humanized mammary fat pads
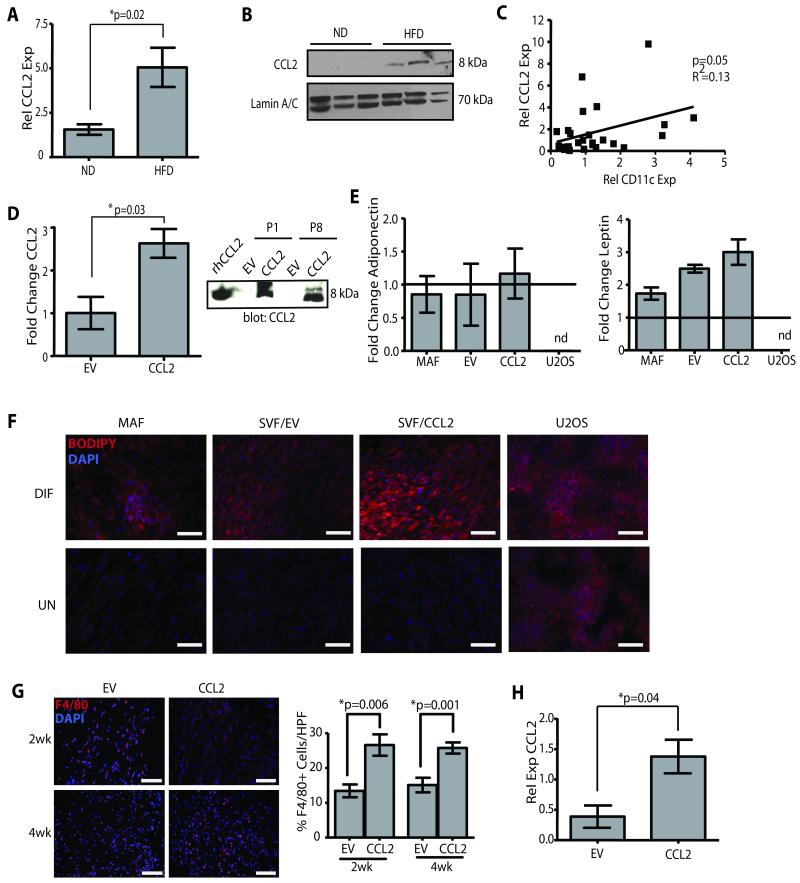
High fat diet induced obesity in the C57Bl/6 genetic background is a widely used model because of the similarities to metabolic changes in obese humans. However, C56Bl/6 mice are extremely resistant to breast oncogenesis in our hands and others (34). In order to study how the obese microenvironment of the mammary gland alters breast tumorigenesis, we utilized the human-in-mouse model to recapitulate the paracrine stromal interactions associated with obesity. First, we needed to identify factor(s) produced by the obese adipose tissue that could promote macrophage recruitment and activation. Recently, elevated CCL2 expression in subcutaneous adipose tissue was associated with the mild weight gain of ovariectomy (16), so we examined CCL2 expression in mammary glands of obese and lean mice. Obesity induced a 3-fold increase in CCL2 transcripts (p=0.02; Figure 2A), and protein (Figure 2B). This association between CCL2 and obesity was further validated in human breast tissues. CCL2 and CD11c expression were correlated (p=0.05; Figure 2C), suggesting that CD11c+ CLS, BMI, and CCL2 are associated (Figure 1G) in reduction mammoplasty tissues.
Figure 2. Overexpression of CCL2 in human adipose stroma cells results in obesity-like conditions.
(A) CCL2 transcripts were significantly increased in mammary glands of C57Bl/6 mice fed HFD diet compared to those from ND mice (qPCR, n=6 mice/group). (B) CCL2 protein was elevated in glands from obese mice. Lamin A/C was used as a loading control. (C) Increased expression of CCL2 and CD11c were significantly correlated in mammary reduction tissue (qPCR, n=28 samples). (D) SVF/CCL2 cells expressed significantly increased levels of CCL2 mRNA and protein in conditioned media compared with SVF/EV cells (n=3 experiments). Recombinant human CCL2 (rhCCL2) was used as a positive control. (E) Mature adipocyte fraction (MAF), SVF/EV, SVF/CCL2, and U2OS cells were treated with adipogenic media (DIF) or vehicle (UN). Following differentiation, changes in expression levels of adiponectin and leptin were similar to the MAF but were not detected (nd) in U2OS cells. qPCR was performed on RNA isolated from 3 experiments, and data represented as fold change following differentiation compared with vehicle-treated cells. (F) Stromal cells and U2OS cells were exposed to adipogenic media or vehicle; lipid was stained with BODIPY 558/568 and counterstained with DAPI. (G) Mammary glands from NOD/SCID mice humanized with SVF/CCL2 cells demonstrated increased F4/80+ cells compared with glands humanized with SVF/EV cells (n=3 mice/group). (H) After 4 weeks, CCL2 transcripts were significantly increased in glands from NOD/SCID mice humanized with SVF/CCL2 compared with glands humanized with SVF/EV cells. Original magnification (F, G) 200x; bar=100μm.
To create a humanized adipose stromal model that mimics the inflammatory state of obesity, we isolated the adipose stromal vascular fraction (SVF) from reduction mammoplasty breast tissues; the SVF is comprised of adipocyte stem cells, preadipocytes, mature adipocytes, and other stromal cells (35). The SVF was immortalized with human telomerase (hTERT) and transduced with either human CCL2 (SVF/CCL2) or empty vector (SVF/EV). SVF/CCL2 cells demonstrated a 3-fold increase in CCL2 transcript, as well as abundant protein secretion in conditioned media (CM) compared to SVF/EV cells (p=0.03, Figure 2D). To characterize the adipogenic differentiation potential of the SVF cells, the immortalized and non-immortalized SVF (iSVF, pSVF, respectively) lines, SVF/CCL2, and SVF/EV cells were assayed for the expression of adipocyte markers and differentiation potential and compared to the mature adipocyte fraction (MAF). Under adipogenic conditions, the MAF, SVF/CCL2, and SVF/EV cells all expressed similar levels of adiponectin (Figure 2E), PPARγ, and FABP4 (Figure S1C), induced leptin expression (p<0.05, Figure 2E), and generated large, central lipid droplets as quantified using BODIPY or Oil Red O (Figure 2F, Figure S1A, B). In contrast, confluent U2OS cells non-specifically developed diffuse lipid droplets, and expression of adiponectin and leptin was not detectable (Figure 2E). Neither SVF/CCL2 nor SVF/EV cells expressed markers of epithelial cells (cytokeratins 8 and 14), endothelial cells (CD31), hematopoietic cells (CD45), or macrophages (EMR1 and CD11b; Figure S2A), suggesting that SVF lines were enriched in adipocytes and preadipocytes.
The murine counterpart to human CCL2 protein contains an elongated C terminus (36). Since species incompatibilities between growth factor/chemokines and their receptors are well known, we evaluated whether human CCL2 (hCCL2) could enhance mouse macrophage migration in order to model the inflammatory state of obesity. RAW 264.7 cells migrated through transwell chambers in response to recombinant hCCL2 (rhCCL2, p<0.0001, Figure S2B), as well as following stimulation with SVF/CCL2 CM (Figure S2C). Migration was significantly attenuated in the presence of a CCL2 blocking antibody (p<0.0001, Figure S2C). To evaluate the ability of SVF/CCL2 cells to enhance macrophage migration in vivo, mice were humanized as previously described (20, 22) with SVF/CCL2 or SVF/EV cells. At 2 or 4 weeks following injection, SVF/CCL2 humanized fat pads demonstrated significantly increased macrophage recruitment compared to those humanized with SVF/EV cells (Figure 2G), suggesting that CCL2 secreted by SVF/CCL2 cells attracts murine macrophages. Four weeks following humanization with SVF/CCL2 cells, mammary CCL2 transcripts were elevated 3.5-fold compared to those humanized with SVF/EV cells, similar to levels in obese C57Bl/6 mammary glands (Figure 2H).
Macrophages are responsible for angiogenesis in response to the obesity-like microenvironment
Macrophages are important for angiogenesis during wound healing and tumorigenesis (37, 38), but little is known about their role during the expansion of the adipose tissue vasculature in obesity. Mammary fat pads of NOD/SCID mice were implanted with SVF/CCL2 or SVF/EV cells and examined for angiogenesis 4 weeks following humanization. SVF/CCL2 humanized fat pads demonstrated a significant 2-fold increase in CD31+ endothelial cells compared to SVF/EV fat pads (Figure 3A, B).
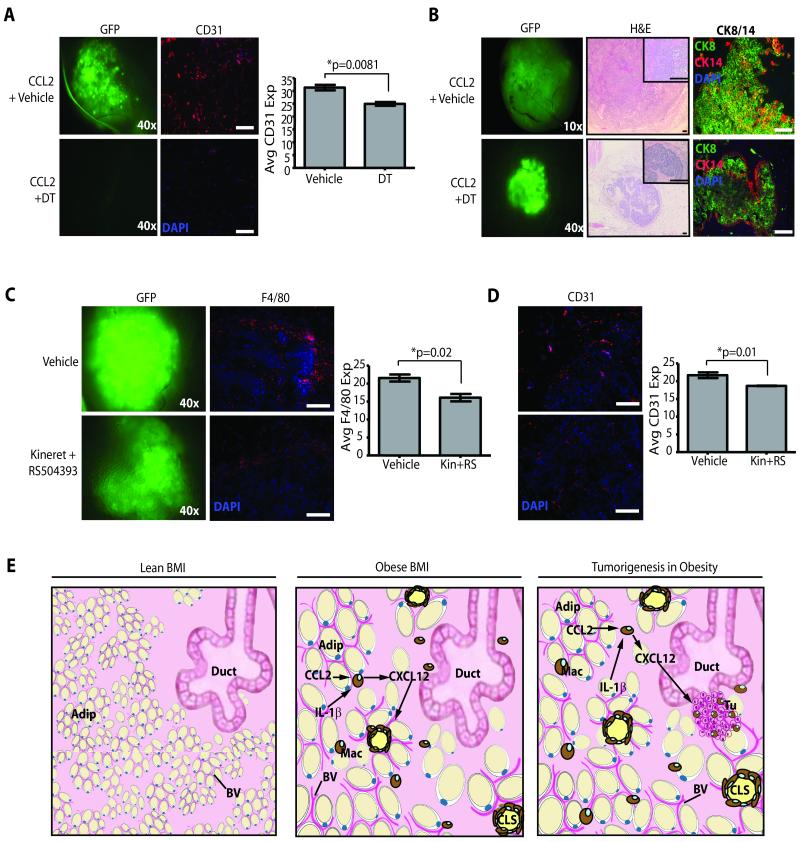
Figure 3. Macrophages promote angiogenesis in response to obesity-like stromal cells.
(A, B) Mammary glands from NOD/SCID mice humanized with SVF/CCL2 cells demonstrated significantly increased CD31+ cells after 4 weeks compared with glands humanized with SVF/EV cells as quantified by immunofluorescence (A) and flow cytometry (B). Glands from 3 mice were utilized for each time point. (C) Matrigel plugs were implanted under the flank of Mac-SCID mice. Those containing SVF/CCL2 cells recruited significantly increased numbers of F4/80+ macrophages and CD31+ endothelial cells than plugs containing SVF/EV cells. In mice treated with diphtheria toxin (DT), both F4/80+ macrophages and CD31+ endothelial cells were significantly decreased in plugs containing either SVF/CCL2 or SVF/EV cells compared with plugs from vehicle treated mice. (D) At 2 weeks following humanization, DT treatment decreased both CD11b+ and CD31+ cells compared with glands from vehicle-treated mice. Four weeks following humanization and DT administration, no significant changes in CD11b+ cells were observed, and only DT-treated SVF/CCL2 glands demonstrated significantly reduced CD31+ cells compared with those from vehicle-treated mice. Mice received DT or vehicle at 24 hours and 48 hours following humanization. Glands were collected at 2 or 4 weeks following DT administration (n=5 mice/group). Two Matrigel plugs were injected subcutaneously in each mouse. Data denoted as a fold change from vehicle treated mice in each condition. Original magnification (A) 200x; bar=100μm.
To specifically study macrophages in this process, we utilized DTGR EGFP-Mac transgenic mice (Mac) which express the simian diphtheria toxin (DT) receptor under the control of the CD11b promoter (39). DT treatment selectively ablates macrophages within 12 hours without altering other cell populations (39). For use with the humanized xenograft model, Mac mice were bred onto the SCID background (Mac-SCID) and treated with DT to assess macrophage ablation. One hour following DT treatment, the number of CD11b+ or F4/80+ macrophages was diminished within the bone marrow (Figure S2D, E). In mammary glands, CD11b+ and F4/80+ macrophages were also significantly decreased up to 48 hours following DT administration (Figure S2F, G). SCID littermates negative for the Mac transgene demonstrated a significant increase in macrophages within 24 hours of DT administration, indicating that macrophage ablation was specific to Mac transgenic mice (Figure S2F, G). Although macrophages were depleted in response to DT, transcripts for leptin and adiponectin were not significantly different following DT treatment, suggesting that the adipocytes were not altered by DT treatment (Figure S2H).
To determine whether CCL2-associated angiogenesis was mediated by macrophage recruitment, Mac-SCID mice were implanted subcutaneously with extracellular matrix (Matrigel) plugs containing SVF/EV or SVF/CCL2 cells, and DT was administered to animals 24 and 72 hours after implantation. Plugs containing SVF/CCL2 cells significantly enhanced F4/80+ (p=0.002) and CD31+ (p=0.0004) cell recruitment compared to SVF/EV cells containing plugs, indicating that CCL2 expression augments macrophage recruitment and angiogenesis (Figure 3C). DT treatment resulted in the depletion of F4/80+ and CD31+ cells recruited to Matrigel plugs (Figure 3C), implying that macrophage recruitment is necessary for angiogenesis.
Mac-SCID mice received DT at 24 and 72 hours following humanization with SVF/CCL2 or SVF/EV cells to determine the role of macrophage recruitment on angiogenesis in mammary fat pads. After 2 weeks, humanized glands from DT-treated mice demonstrated similar levels of GFP expressing SVF cells (4.9±3.4%; mean±s.d., n=10) compared with those that received vehicle (5.6±1.8%; n=10) measured by flow cytometry, suggesting that the transplanted cells were not ablated by the DT. Since SVF/CCL2 humanized gland recruit significantly more macrophages than SVF/EV humanized glands, SVF/CCL2 DT-treated mice exhibited a 2-fold reduction in the number of recruited CD11b+ cells compared to vehicle-treated cohorts, while this was not observed in glands humanized with SVF/EV cells (p=0.04: Figure 3D). DT treatment also resulted in a reduction in CD31+ cells in both SVF/EV and SVF/CCL2 humanized glands, indicating that macrophages are important for the angiogenesis induced humanization (Figure 3D). However, after 4 weeks when macrophage number returned to baseline levels in SVF/EV and SVF/CCL2 humanized glands following DT treatment, SVF/CCL2 humanized fat pads induced a greater reduction in the number of CD31+ cells (p=0.03; Figure 3D), suggesting that the magnitude and thus attenuation of the angiogenic response was greater in the presence of CCL2.
DT-treatment resulted in a long-term 2-fold reduction in CD31+ vessels in SVF/CCL2 humanized fat pads compared to vehicle-treated mice, although the percentage of CD11b+ cells was similar in all glands. This reduced angiogenesis was not observed in glands humanized with SVF/EV cells (Figure 3D). These results suggest that macrophage recruitment enhances angiogenesis, and CCL2 further augments this increase.
Macrophage/adipose CCL2/CXCL12 crosstalk promotes angiogenesis
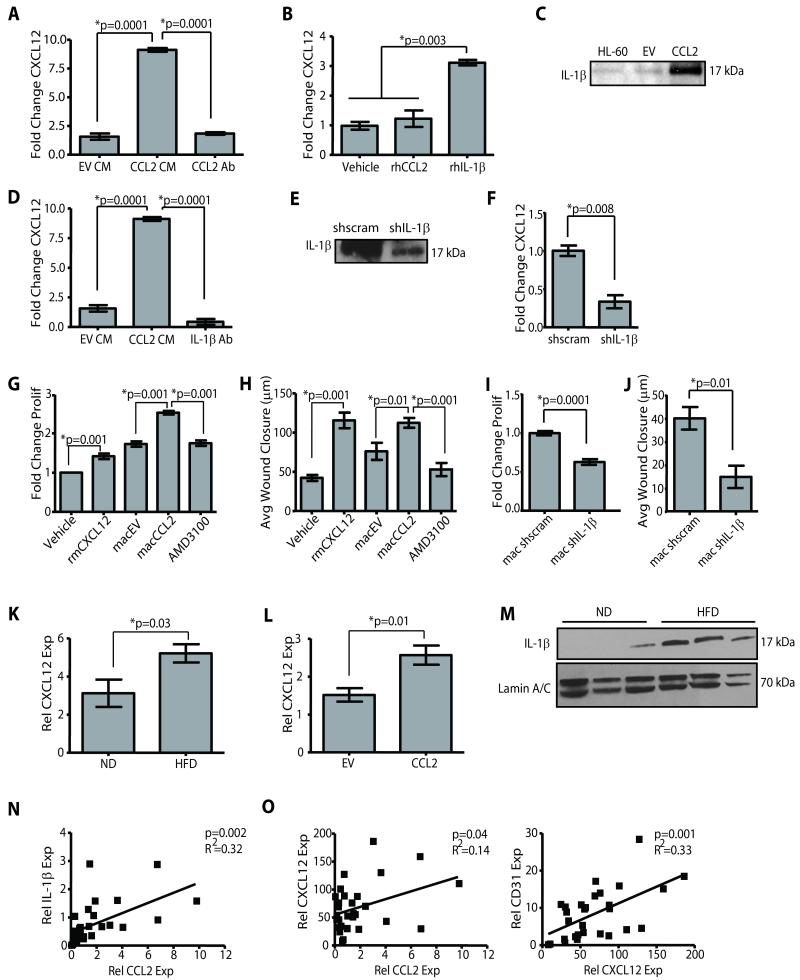
To determine the mechanism of how SVF/CCL2 mediated macrophage recruitment promotes angiogenesis, we stimulated HL-60 macrophages with SVF/EV or SVF/CCL2 CM and assessed the expression of several established mediators of angiogenesis. There were no significant changes in VEGF-A, PDGF-A, and MMP-9 expression between macrophages treated with either SVF/EV or SVF/CCL2 CM (Figure S3A, B, C). However, CXCL12 (also known as SDF-1α) expression was significantly increased in response to SVF/CCL2 CM and was attenuated in the presence of a CCL2 blocking antibody (p=0.0001; Figure 4A). Additionally, expression of CXCL12 in mouse bone marrow-derived macrophages was induced following stimulation with SVF/CCL2 CM (p<0.05; Figure S3D). However, treatment of HL-60 macrophages with rhCCL2 did not significantly upregulate CXCL12 expression (Figure 4B); this inability was not due to failure of rhCCL2 to induce downstream signaling, as ERK1/2 phosphorylation was increased (Figure S3E). These findings suggest that CCL2 is necessary but not sufficient to induce CXCL12 expression in macrophages.
Figure 4. Macrophage derived CXCL12 induces angiogenesis.
(A) SVF/CCL2 conditioned media (CM) significantly increased CXCL12 expression in HL-60 macrophages compared with SVF/EV CM or SVF/CCL2 CM + blocking antibody for CCL2 (CCL2 Ab). (B) Recombinant human IL-1β (rhIL-1β) increased CXCL12 expression in HL-60 macrophages. qPCR was performed on RNA isolated from 3 experiments. (C) SVF/CCL2 CM contained increased IL-1β protein compared to SVF/EV CM. CM from HL-60 macrophages treated with LPS was used as a positive control. (D) SVF/CCL2 CM+IL-1β blocking Ab significantly reduced CXCL12 expression in HL-60 macrophages compared to SVF/CCL2 CM. (E) CM from SVF/CCL2 shIL-1β (shIL-1β) cells demonstrated decreased IL-1β protein compared to CM from SVF/CCL2 shscrambled control cells (shscram). (F) SVF/CCL2 shIL-1β CM significantly decreased CXCL12 expression in HL-60 macrophages compared with SVF/CCL2 shscramble CM. (G, H) Human microvascular endothelial cells (HMVEC) proliferated (G) and migrated (H) in response to recombinant mouse CXCL12 (rmCXCL12), as well as in response to CM from HL-60 macrophages pre-treated with CM from SVF/CCL2 cells (macCCL2) compared to those treated with SVF/EV CM (macEV). AMD3100+macCCL2 CM significantly decreased HMVEC proliferation and migration. Proliferation data are represented as a fold change of vehicle-treated cells, and 3 experiments were performed in triplicate. (I, J) HMVEC demonstrated decreased proliferation (I) and migration (J) in response to CM from HL-60 macrophages pre-treated with CM from SVF/CCL2 shIL-1β cells (mac shIL-1β) compared to CM from SVF/CCL2 shscrambled cells (mac shscram). Proliferation data are represented as a fold change of vehicle-treated cells, and 3 experiments were performed in triplicate. (K) Glands from C57Bl/6 HFD mice demonstrated elevated CXCL12 expression compared to glands from ND mice (n=6 mice/group). (L) SVF/CCL2 humanized glands from NOD/SCID mice demonstrated significantly increased CXCL12 expression compared to SVF/EV humanized glands (n=6 mice/group). (M) IL-1β protein was increased in C57Bl/6 HFD mammary glands compared to ND glands. (N, O) Reduction mammoplasty tissue demonstrated a significant correlation between CCL2 and IL-1β expression levels (N), as well as significant correlation between expression of CXCL12 and both CCL2 and CD31 (O) qPCR was performed on RNA isolated from 28 reduction mammoplasty samples.
It has been reported that CCL2 and IL-1β can cooperate to enhance angiogenesis (40). Consistent with this notion, abundant IL-1β protein was detected in SVF/CCL2 CM (Figure 4C). Additionally, HL-60 macrophages treated with rhIL-1β exhibited a significant increase in CXCL12 expression (p=0.003; Figure 4B), and the induction of CXCL12 by SVF/CCL2 CM was abolished in the presence of an IL-1β blocking antibody (p=0.0001; Figure 4D). HL-60 macrophages treated with specific inhibitors against the CCL2 receptor, CCR2 (RS504393), or IL-1β activity (IL-1ra), significantly attenuated induction of CXCL12 following stimulation with SVF/CCL2 CM (p=0.001; Figure S3F). To further investigate the role of IL-1β in CXCL12 expression, we generated SVF/CCL2 cells with stable reduction of IL-1β protein using an shRNA construct (Figure 4E). Treatment of HL-60 macrophages with CM from SVF/CCL2 shIL-1β cells resulted in significantly lower expression of CXCL12 compared to CM from SVF/CCL2 shscrambled control cells (p=0.008; Figure 4F). These results indicate that IL-1β expressed by SVF/CCL2 cells cooperates with CCL2 to enhance the induction of CXCL12 in macrophages.
CXCL12 has been well characterized for its role in recruiting bone marrow-derived endothelial progenitor cells, although less is known about its effects on existing vasculature (41). Therefore, we examined the effects of macrophage-derived CXCL12 on endothelial cell migration and proliferation. HL-60 macrophages were stimulated with SVF/CCL2 or SVF/EV CM, and macrophage CM was collected and tested for its ability to promote angiogenesis. CM from macrophages treated with SVF/CCL2 CM (macCCL2) significantly increased migration and proliferation of human microvascular endothelial cells (HMVECs) compared with CM from macrophages treated with SVF/EV CM (macEV; Figure 4G, H, Figure S3G). The migration and proliferation of HMVECs in response to macCCL2 CM was attenuated in the presence of AMD3100, an inhibitor specific for the CXCL12 receptor (Figure 4G, H, Figure S3G). When HMVECs were treated recombinant mouse CXCL12 (rmCXCL12), migration and proliferation were significantly increased (Figure 4G, H, Figure S3G), suggesting that CXCL12 acts directly on endothelial cells. Similarly, CM from primary mouse macrophages pre-treated with SVF/CCL2 CM significantly induced migration of primary mouse vascular endothelial cells compared to CM from those pre-treated with SVF/EV CM, and migration was attenuated in the presence of AMD3100 (Figure S3I, J). Consistent with the role of CXCL12 on endothelial cells, CM from HL-60 macrophages pre-treated with CM from SVF/CCL2 shIL-1β cells (mac shIL-1β) demonstrated significantly reduced proliferation and migration compared with those treated with CM from HL-60 macrophages pre-treated with CM from SVF/CCL2 shscrambled control cells (mac shscram; Figure 4I, J, Figure S3H). The effects on migration and proliferation were specific to macrophage CM, as SVF/CCL2 CM could not significantly increase either migration or proliferation of either HMVECs or bovine retinal endothelial cells (BREC; Figure S4).
To establish whether the CCL2/IL-1β/CXCL12 crosstalk was induced during obesity, we examined mammary tissues from mice and humans. CXCL12 transcript levels were elevated in obese mammary glands compared to those from lean mice (p=0.03; Figure 4K). HFD-fed DTGr-Mac mice on the C57Bl/6 genetic background treated with DT demonstrated reduced CXCL12 expression compared to those treated with vehicle (Figure S3K). Similarly, fat pads humanized with SVF/CCL2 cells demonstrated significantly elevated CXCL12 expression compared with those humanized with SVF/EV cells (p=0.01; Figure 4L). Likewise, IL-1β protein was more abundant in mammary glands of obese mice than those in lean mice (Figure 4M). In human reduction mammoplasty tissues, CCL2 expression was strongly correlated with IL-1β expression (p=0.002; Figure 4N) and CXCL12 expression was correlated with CD31 expression (p=0.001); CXCL12 expression was also highly correlated with CCL2 expression (p=0.04; Figure 4O). Taken together, these results suggest that CCL2 and IL-1β proteins are elevated in obese adipose stroma, and cooperate to induce CXCL12 in macrophages that in turn promote angiogenesis.
Stromal changes associated with obesity accelerate tumorigenesis
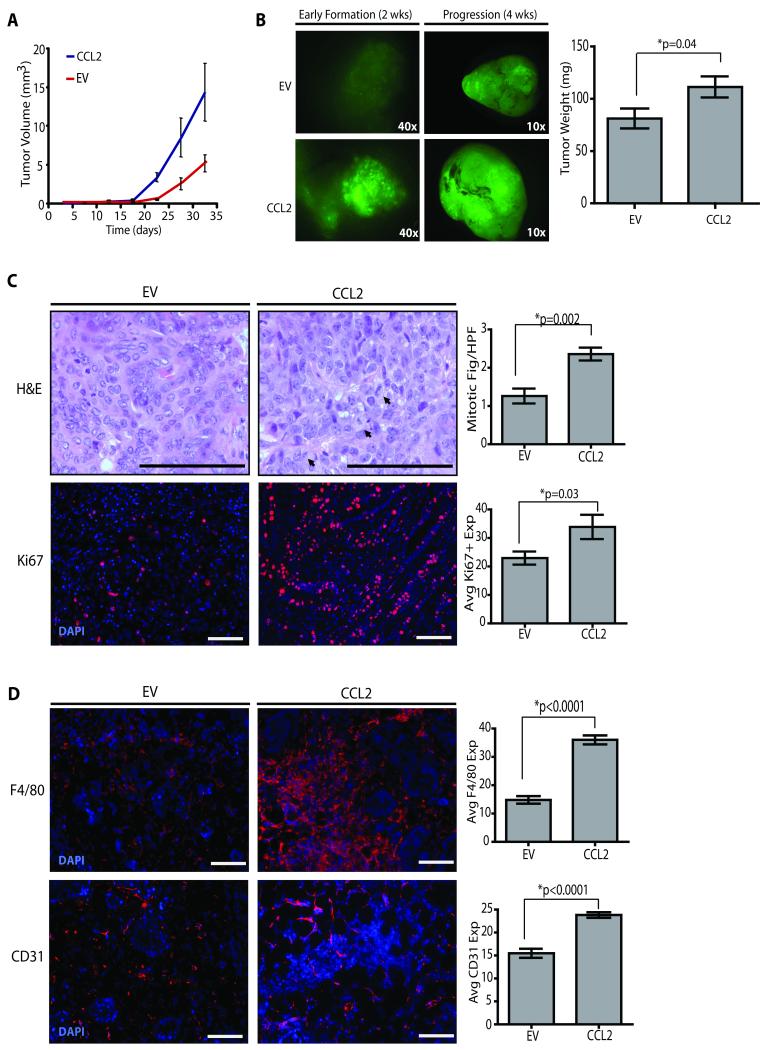
We utilized the obesity-like humanized fat pad model to determine whether changes in the adipose microenvironment associated with obesity affects breast tumor progression. Mammary fat pads were humanized with either SVF/CCL2 or SVF/EV cells and subsequently implanted with human breast epithelial cells that were transduced with lentiviruses encoding SV40/Kras oncogenes as previously described (21, 42). When infected cells were injected into glands humanized with SVF/CCL2 cells, palpable tumors formed more rapidly than in glands humanized with SVF/EV cells (Figure 5A, Table S2). Additionally, obesity-like humanized stroma led to a significant increase in tumor weight compared with those humanized with SVF/EV cells (p=0.04; Figure 5B). Although end stage tumors were histologically similar (Figure S5A), tumors that developed within SVF/CCL2 humanized fat pads exhibited significantly increased numbers of mitotic figures (p=0.002) and Ki67+ cells (p=0.03; Figure 5C), suggesting that the obese-like microenvironment generates tumors of a higher grade.
Figure 5. Obesity-like stromal cells accelerate tumorigenesis.
(A) Tumors from glands from NOD/SCID mice humanized with SVF/CCL2 cells developed earlier than those from SVF/EV humanized glands. Human mammary epithelial cells (HMEC) were isolated from 3 reduction mammoplasty tissue samples, transduced with lentiviruses encoding SV40er and KrasG12V, and transplanted into humanized glands co-mixed with either SVF/CCL2 or SVF/EV cells. (B) Tumors from SVF/CCL2 humanized glands were significantly larger in size and weight at four weeks after transplantation. N=6 tumors/group from 3 experiments with separate patient tissue samples (Table S1). (C) Tumors arising in SVF/CCL2 glands demonstrated significantly increased numbers of mitotic figures and Ki67 expression compared with those from SVF/EV glands. Mitotic figures and Ki67+ cells were quantified from 3 images from 12 tumors/group. Statistical differences were detected by t-test. (D) At 2 weeks following transplantation of transduced HMECs, glands humanized with SVF/CCL2 cells demonstrated significantly increased F4/80+ macrophage recruitment and increased CD31+ cells (n=6 tumors/group). Original magnification (C) 600x, 200x; (D) 200x; bar=100μm.
To determine whether the priming of the microenvironment by macrophages and blood vessels prior to tumor formation accelerates tumor formation, tumorigenesis was evaluated 2 weeks following implantation of human breast epithelial cells into humanized fat pads. Within the implantation site of humanized SVF/CCL2 fat pads, pre-malignant lesions exhibited significantly increased recruitment of both F4/80+ macrophages (p<0.0001) and CD31+ endothelial cells (p<0.0001) prior to the formation of carcinomas (Figure 5D). These results suggest that early macrophage recruitment and angiogenesis within the obese breast may contribute to accelerated breast cancer formation.
Macrophages are necessary for tumor progression associated with obesity
To determine whether priming of the microenvironment by macrophages is necessary for accelerated tumor growth in the obese-like stroma, glands from Mac-SCID females were humanized with SVF/CCL2 cells and transplanted with reduction mammoplasty-derived epithelial cells transduced with SV40/Kras. At 24 and 72 hours following epithelial cell implantation, mice received injections of either DT or vehicle. After 2 weeks, humanized SVF/CCL2 fat pads from vehicle-treated mice demonstrated significantly larger outgrowths compared to DT-treated mice, indicating that ablation of macrophages significantly reduced tumorigenesis (Figure 6A). Vehicle-treated mice exhibited significantly increased number of CD31+ blood vessels, suggesting that ablation of macrophages attenuates angiogenesis (p=0.0081; Figure 6A). At end stage, increased tumorigenesis was observed in vehicle-treated mice compared to the DT-treated cohort (6/8 vehicle vs 1/6 DT, p=0.03). Fat pads of the DT-treated group that did not grow expansive tumors contained small, well-circumscribed ductal carcinoma in situ (DCIS) lesions that retained expression of an outer layer of cytokeratin (CK) 14+ myoepithelial cells surrounding CK8+ luminal cells (Figure 6B). Although the pattern of CK8 and CK14 expression was different, the total staining for both markers was similar (Figure S5B). Epithelial cells in these DCIS lesions expressed both oncogenes, as well as Ki67, suggesting that DT-treatment did not ablate the transplanted cells. In contrast, expansive tumors that developed in glands of vehicle-treated females were invasive and disorganized (Figure 6B). These results suggest that in addition to increasing angiogenesis early during tumor formation, inflammatory macrophages are necessary to promote the progression of DCIS into aggressive, invasive tumors.
Figure 6. Stromal changes associated with obesity accelerate tumorigenesis.
(A) Two weeks following transplantation of virally transduced human mammary epithelial cells (HMECs), diphtheria toxin (DT)-treated mice demonstrated reduced transplant growth and significantly reduced CD31+ cells compared with vehicle-treated mice (n=6 tumors/group). (B) At end stage, glands of vehicle-treated Mac-SCID mice developed large tumors with extensive stromalization and invasive borders, with cytokeratin (CK) 8+ and CK14+ cells randomly distributed in the tumor paremchyma. Diphtheria toxin (DT)-treated mice developed small, well-circumscribed growths with CK8+ cells surrounded by a ring of CK14+ cells. (C, D) Two weeks following transplant of virally transformed HMECs, glands from mice treated with Kineret and RS504393 (n=10 transplants/group) demonstrated significantly reduced F4/80+ macrophage infiltration (C), as well as significantly reduced CD31 expression (D). (E) Obese adipose tissue (Adip) surrounding epithelial cells of the mammary gland (Duct) secretes CCL2, leading to the recruitment of inflammatory macrophages (Mac) to form crown-like structures (CLS). In response to CCL2 and IL-1β expression, macrophages secrete CXCL12, which acts on blood vessels (BV) to enhance angiogenesis. Obese adipose tissue is primed for early angiogenesis and inflammatory signaling which promotes aggressive breast malignancies (Tu). Original magnification: (A, C, D) 200x, (B) middle panel 40x, inset 200x, end panel 200x; bar=100μm.
To determine whether inhibition of CCL2 and IL-1β might be a viable prophylactic strategy in treating breast cancers associated with obesity, mice humanized with SVF/CCL2 cells and implanted with SV40/Kras transduced human breast epithelial cells were treated daily with small molecule inhibitors for CCL2 (RS504393) and of IL-1β (Kineret; IL-1ra). After 2 weeks of treatment, mice receiving inhibitors exhibited a significant reduction in recruited F4/80+ macrophages compared to fat pads of vehicle-treated mice (p=0.02; Figure 6C). Likewise, the CD31+ vasculature was significantly diminished within the premalignant lesions of inhibitor-treated mice compared to those receiving vehicle (p=0.01; Figure 6D). These results suggested that CCL2 and IL-1β inhibitors may slow the recruitment of macrophages and angiogenesis, thereby delaying tumor onset and progression. However, at end stage, there was no difference in latency among tumors in the vehicle or inhibitor treatment groups (Figure S5C). The resulting tumors in the inhibitor treatment group demonstrated significant changes in nuclear morphology including alterations in cell size, nuclear/cytoplasmic ratio, and abnormal mitoses compared with tumors from mice treated with vehicle (Figure S5D). The inhibitor-treated tumors no longer had diminished macrophage recruitment (Figure S5E) and showed a significant increase in SMA+ cancer-associated fibroblasts (Figure S5F). These results suggest that CCL2 and IL-1β inhibitors had direct effects on the tumor cells as well as the SMA+ cancer-associated fibroblasts. Although the DT experiments demonstrated that ablation of macrophages may limit tumor development, the method of drug delivery may need to target macrophages specifically to limit the response of other cell types within the tumor parenchyma.
Discussion
Obesity elevates the risk of postmenopausal breast cancer, as well as promotes the formation of aggressive tumors (3). Our findings suggest that during obesity, the enhanced production of CCL2 and IL-1β by the breast adipose tissue increases recruitment of macrophages and the formation of CLS. The recruited macrophages are in turn stimulated by CCL2 and IL-1β to secrete CXCL12 to induce angiogenesis and support the expanding tissue (Figure 6E). Under conditions of obesity, the microenvironment of the breast adipose tissue becomes a fertile soil for tumorigenesis such that a premalignant lesion can bypass the rate limiting step of inducing its own vasculature during tumor progression (43). Our studies have also demonstrated that macrophages promote progression from DCIS to aggressive tumors. Given recent work implicating CXCL12 in the expansion of cancer stem cell populations as well as metastases (44, 45), we are currently investigating whether CXCL12 administration can functionally replace macrophages in the DCIS microenvironment.
The increased levels of estrogen due to the expression of aromatase in preadipocytes (4) and in the presence of inflammation (6, 46) in obese fat tissue, likely play an important role in breast cancers associated with obesity (3). In fact, recent studies have demonstrated that increasing the levels of circulating estrogen can lead to enhanced angiogenesis and recruitment of bone marrow-derived macrophages into a growing tumor mass (25, 47). Although the tumorigenesis studies reported here were performed in ovary-intact mice, we have not excluded a role for estrogen in CCL2 mediated macrophage recruitment or angiogenesis. Nevertheless, it is likely that in obese postmenopausal breast cancer patients, limiting aromatase activity as well as adipose inflammation may be critical for effective prevention and/or treatment of breast cancer.
Angiogenesis plays a crucial role in the growth and progression of breast cancer, and anti-angiogenesis agents that target the VEGF/VEGFR pathway have become important in the treatment of cancer, in part by limiting the expansion of tumor vasculature. However, many of the anti-angiogenic therapies have had limited effects on breast cancer (48). Our studies suggest that inflammation within the obese mammary gland contributes to angiogenesis through a novel CCL2/IL-1β/CXCL12 pathway that bypasses the VEGF/VEGFR pathway. Thus, obese women may demonstrate reduced responsiveness to VEGF targeted therapies due to elevated CXCL12 expression.
Adipose tissue is also dependent on vascularization for its expansion, and recent reports confirm that disruption of neovascularization can prevent the onset of obesity in both genetic and diet-induced obesity models (49). However, the side-effects of these drugs may limit their usefulness in the treatment of obesity or in the prevention of cancer in obese at-risk women. Therefore, targeting inflammation in obese women in addition to angiogenesis might offer more promise in treating obesity or cancer. Consistent with this notion, treatment of mice with propagermanium, a CCR2 inhibitor, suppressed inflammation in the adipose tissue and increased insulin sensitivity (50). Our findings here suggest that inhibition of inflammation can limit macrophage infiltration and angiogenesis during early tumor development. Moreover, given that tumorigenesis was almost entirely prevented in mice following macrophage ablation, development of drugs that specifically target this phagocytic population may have substantial therapeutic benefits in the treatment of obesity and/or prevention of breast cancer in obese women.
Supplementary Material
Acknowledgments
We are grateful to Laura Arendt for figure design, and Dr. Stephanie Ellison-Zelski for reduction mammoplasty tissue procurement. This work was supported by grants from the Raymond and Beverly Sackler Foundation, the DOD/CDMRP BC063332, the Breast Cancer Research Foundation, NIH/NCI CA125554 and CA092644, (C.K.), NCRR K01-RR021858 (L.M.A.), and the Breast Cancer Alliance (C.K.).
Footnotes
Conflict of Interest Statement: The authors have not conflicts of interest to declare.
References
- (1).Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–91. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- (2).Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- (3).Carmichael AR. Obesity as a risk factor for development and poor prognosis of breast cancer. BJOG. 2006;113:1160–6. doi: 10.1111/j.1471-0528.2006.01021.x. [DOI] [PubMed] [Google Scholar]
- (4).Brown KA, Simpson ER. Obesity and breast cancer: mechanisms and therapeutic implications. Front Biosci (Elite Ed) 2012;4:2515–24. doi: 10.2741/e562. [DOI] [PubMed] [Google Scholar]
- (5).Morris PG, Hudis CA, Giri D, Morrow M, Falcone DJ, Zhou XK, et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res (Phila) 2011;4:1021–9. doi: 10.1158/1940-6207.CAPR-11-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Subbaramaiah K, Morris PG, Zhou XK, Morrow M, Du B, Giri D, et al. Increased levels of COX-2 and prostaglandin E2 contribute to elevated aromatase expression in inflamed breast tissue of obese women. Cancer Discov. 2012;2:356–65. doi: 10.1158/2159-8290.CD-11-0241. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- (7).Dolle JM, Daling JR, White E, Brinton LA, Doody DR, Porter PL, et al. Risk factors for triple-negative breast cancer in women under the age of 45 years. Cancer Epidemiol Biomarkers Prev. 2009;18:1157–66. doi: 10.1158/1055-9965.EPI-08-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Trivers KF, Lund MJ, Porter PL, Liff JM, Flagg EW, Coates RJ, et al. The epidemiology of triple-negative breast cancer, including race. Cancer Causes Control. 2009;20:1071–82. doi: 10.1007/s10552-009-9331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- (10).Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83:461S–5S. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- (11).Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Chen A, Mumick S, Zhang C, Lamb J, Dai H, Weingarth D, et al. Diet induction of monocyte chemoattractant protein-1 and its impact on obesity. Obes Res. 2005;13:1311–20. doi: 10.1038/oby.2005.159. [DOI] [PubMed] [Google Scholar]
- (13).Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci U S A. 2003;100:7265–70. doi: 10.1073/pnas.1133870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–24. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Rogers NH, Perfield JW, Strissel KJ, Obin MS, Greenberg AS. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology. 2009;150:2161–8. doi: 10.1210/en.2008-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Nicolini A, Carpi A, Rossi G. Cytokines in breast cancer. Cytokine Growth Factor Rev. 2006;17:325–37. doi: 10.1016/j.cytogfr.2006.07.002. [DOI] [PubMed] [Google Scholar]
- (18).Ueno T, Toi M, Saji H, Muta M, Bando H, Kuroi K, et al. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res. 2000;6:3282–9. [PubMed] [Google Scholar]
- (19).Fujimoto H, Sangai T, Ishii G, Ikehara A, Nagashima T, Miyazaki M, et al. Stromal MCP-1 in mammary tumors induces tumor-associated macrophage infiltration and contributes to tumor progression. Int J Cancer. 2009;125:1276–84. doi: 10.1002/ijc.24378. [DOI] [PubMed] [Google Scholar]
- (20).Kuperwasser C, Chavarria T, Wu M, Magrane G, Gray JW, Carey L, et al. Reconstruction of functionally normal and malignant human breast tissues in mice. Proc Natl Acad Sci U S A. 2004;101:4966–71. doi: 10.1073/pnas.0401064101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Keller PJ, Arendt LM, Skibinski A, Logvinenko T, Klebba I, Dong S, et al. Defining the cellular precursors to human breast cancer. Proc Natl Acad Sci U S A. 2012;109:2772–7. doi: 10.1073/pnas.1017626108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Proia DA, Kuperwasser C. Reconstruction of human mammary tissues in a mouse model. Nat Protoc. 2006;1:206–14. doi: 10.1038/nprot.2006.31. [DOI] [PubMed] [Google Scholar]
- (23).Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Curr Protoc Immunol. 2008:14.1.1–14.1.14. doi: 10.1002/0471142735.im1401s83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Ennaciri J, Girard D. Immune system: maturation of myeloid cells. Methods Mol Biol. 2009;550:195–203. doi: 10.1007/978-1-60327-009-0_12. [DOI] [PubMed] [Google Scholar]
- (25).Iyer V, Klebba I, McCready J, Arendt LM, Betancur-Boissel M, Wu MF, et al. Estrogen promotes ER-negative tumor growth and angiogenesis through mobilization of bone marrow-derived monocytes. Cancer Res. 2012;72:2705–13. doi: 10.1158/0008-5472.CAN-11-3287. [DOI] [PubMed] [Google Scholar]
- (26).Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–84. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Dalmas E, Clement K, Guerre-Millo M. Defining macrophage phenotype and function in adipose tissue. Trends Immunol. 2011;32:307–14. doi: 10.1016/j.it.2011.04.008. [DOI] [PubMed] [Google Scholar]
- (28).Coffelt SB, Lewis CE, Naldini L, Brown JM, Ferrara N, De PM. Elusive identities and overlapping phenotypes of proangiogenic myeloid cells in tumors. Am J Pathol. 2010;176:1564–76. doi: 10.2353/ajpath.2010.090786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006;42:717–27. doi: 10.1016/j.ejca.2006.01.003. [DOI] [PubMed] [Google Scholar]
- (30).Wentworth JM, Naselli G, Brown WA, Doyle L, Phipson B, Smyth GK, et al. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010;59:1648–56. doi: 10.2337/db09-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Shaul ME, Bennett G, Strissel KJ, Greenberg AS, Obin MS. Dynamic, M2-like remodeling phenotypes of CD11c+ adipose tissue macrophages during high-fat diet--induced obesity in mice. Diabetes. 2010;59:1171–81. doi: 10.2337/db09-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Wagner M, Bjerkvig R, Wiig H, Melero-Martin JM, Lin RZ, Klagsbrun M, et al. Inflamed tumor-associated adipose tissue is a depot for macrophages that stimulate tumor growth and angiogenesis. Angiogenesis. 2012;15:481–95. doi: 10.1007/s10456-012-9276-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Christiaens V, Lijnen HR. Angiogenesis and development of adipose tissue. Mol Cell Endocrinol. 2010;318:2–9. doi: 10.1016/j.mce.2009.08.006. [DOI] [PubMed] [Google Scholar]
- (34).Moser AR, Hegge LF, Cardiff RD. Genetic background affects susceptibility to mammary hyperplasias and carcinomas in Apc(min)/+ mice. Cancer Res. 2001;61:3480–5. [PubMed] [Google Scholar]
- (35).Leff T, Grannenman JG, editors. John Wiley and Sons; New York: 2010. Adipose Tissue in Health and Disease. [Google Scholar]
- (36).Luini W, Sozzani S, Van Damme J, Mantovani A. Species-specificity of monocyte chemotactic protein-1 and -3. Cytokine. 1994;6:28–31. doi: 10.1016/1043-4666(94)90004-3. [DOI] [PubMed] [Google Scholar]
- (37).Ding Y, Song N, Luo Y. Role of Bone Marrow-Derived Cells in Angiogenesis: Focus on Macrophages and Pericytes. Cancer Microenviron. 2012;5:225–36. doi: 10.1007/s12307-012-0106-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Rodero MP, Khosrotehrani K. Skin wound healing modulation by macrophages. Int J Clin Exp Pathol. 2010;3:643–53. [PMC free article] [PubMed] [Google Scholar]
- (39).Cailhier JF, Partolina M, Vuthoori S, Wu S, Ko K, Watson S, et al. Conditional macrophage ablation demonstrates that resident macrophages initiate acute peritoneal inflammation. J Immunol. 2005;174:2336–42. doi: 10.4049/jimmunol.174.4.2336. [DOI] [PubMed] [Google Scholar]
- (40).Nakao S, Kuwano T, Tsutsumi-Miyahara C, Ueda S, Kimura YN, Hamano S, et al. Infiltration of COX-2-expressing macrophages is a prerequisite for IL-1 beta-induced neovascularization and tumor growth. J Clin Invest. 2005;115:2979–91. doi: 10.1172/JCI23298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Petit I, Jin D, Rafii S. The SDF-1-CXCR4 signaling pathway: a molecular hub modulating neo-angiogenesis. Trends Immunol. 2007;28:299–307. doi: 10.1016/j.it.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Proia TA, Keller PJ, Gupta PB, Klebba I, Jones AD, Sedic M, et al. Genetic predisposition directs breast cancer phenotype by dictating progenitor cell fate. Cell Stem Cell. 2011;8:149–63. doi: 10.1016/j.stem.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–64. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- (44).Domanska UM, Kruizinga RC, Nagengast WB, Timmer-Bosscha H, Huls G, de Vries EG, et al. A review on CXCR4/CXCL12 axis in oncology: No place to hide. Eur J Cancer. 2012;49:219–30. doi: 10.1016/j.ejca.2012.05.005. [DOI] [PubMed] [Google Scholar]
- (45).Sun X, Cheng G, Hao M, Zheng J, Zhou X, Zhang J, et al. CXCL12/CXCR4/CXCR7 chemokine axis and cancer progression. Cancer Metastasis Rev. 2010;29:709–22. doi: 10.1007/s10555-010-9256-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Subbaramaiah K, Howe LR, Bhardwaj P, Du B, Gravaghi C, Yantiss RK, et al. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res (Phila) 2011;4:329–46. doi: 10.1158/1940-6207.CAPR-10-0381. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- (47).Gupta PB, Proia D, Cingoz O, Weremowicz J, Naber SP, Weinberg RA, et al. Systemic stromal effects of estrogen promote the growth of estrogen receptor-negative cancers. Cancer Res. 2007;67:2062–71. doi: 10.1158/0008-5472.CAN-06-3895. [DOI] [PubMed] [Google Scholar]
- (48).Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Cao Y. Adipose tissue angiogenesis as a therapeutic target for obesity and metabolic diseases. Nat Rev Drug Discov. 2010;9:107–15. doi: 10.1038/nrd3055. [DOI] [PubMed] [Google Scholar]
- (50).Tamura Y, Sugimoto M, Murayama T, Minami M, Nishikaze Y, Ariyasu H, et al. C-C chemokine receptor 2 inhibitor improves diet-induced development of insulin resistance and hepatic steatosis in mice. J Atheroscler Thromb. 2010;17:219–28. doi: 10.5551/jat.3368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.