Abstract
Background
Chronic Obstructive Pulmonary Disease (COPD) is a multi-systemic and progressive disease. However the determinants of its impact on health related quality of life are not well-studied or understood in Nigeria.
Objectives
To assess the determinants of health related quality of life in COPD
Methods
Patients with stable COPD were recruited consecutively from the outpatient clinics of a university hospital. Health Related Quality of Life (HRQL) was assessed using the St. George's Respiratory Questionnaire (SGRQ) and the Forced Expiratory Volume in one second (FEV1), Forced Vital Capacity (FVC) were measured by a vitalograph spirometer.
Results
Fifty patients were recruited for this study (male= 60%). The mean (SD) age was 69 (9) years. The overall mean (SD) SGRQ scores was 45.9 (26.5), 50.6 (29.2), 29.7 (19.9), 38.8 (22.0) for the symptom, activity, impact and total scores respectively. After adjusting for age, sex and smoking, self-reported breathlessness independently predicted on average 25.2, 36.8, 13.65 and 22.9 points increase in SGRQ symptom, activity, impact and total scores respectively. Self-reported weight loss predicted 12.2 points increase in the impact subscale.
Conclusions
Self-reported breathlessness and weight loss are independent predictors of low HRQL score in COPD.
Keywords: Chronic obstructive pulmonary disease, Health related quality of life, St George's Respiratory Questionnaire, Nigeria
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is a growing cause of morbidity and mortality. It is projected to become the third leading cause of death worldwide by 20301, 2. Although significant progress has been made in the assessment and management of patients with COPD over the last four decades, the care of persons with this disease remains challenging because of it's progressive and multi-systemic nature.
COPD is usually assessed using outcome measures like number of hospitalization, exacerbations, mortality and lung function parameters 3, 4. However these measures do not provide a comprehensive assessment of the health status of the patients. There is growing evidence that shows that relying only on mortality and lung function measures for assessing effectiveness of treatments could be misleading in conditions like COPD that have notable non-respiratory systemic manifestations5, 6. As such, Health Related Quality of Life (HRQL) measures have achieve widespread acceptance because they provide a holistic assessment of the impact of the disease and benefits of treatments. Among these quality of life measures, the St Georges Respiratory Questionnaire (SGRQ) has been shown to be valid and specific for COPD7,8.
Quality of life (QoL) evaluation is relatively new in developing countries. It can influenced by various factors including socio-economic status and may vary between populations9, 10. We are unaware of any study in Nigeria that has assessed the quality of life of patients with COPD. The relation of QoL measures to traditional outcomes of COPD like lung function measures and the factors that predict them are not well understood.
The use of quality of life instruments holds potential use in rural and suburban health facilities in developing countries where access to spirometry and other objective measures of lung function may be lacking. The availability of adapted QoL measures may provide substantial and complementary benefit for patients with COPD and their care providers. The aim of this study was to assess the relation of SGRQ scores to lung function especially forced expiratory volume in one second (FEV1), forced vital capacity (FVC) and to determine the factors that predict its component and total scores.
Methods
Study design
It was a cross-sectional study carried out at the medical outpatient department of Obafemi Awolowo University Teaching Hospital (OAUTH), Ile-Ife. OAUTH is a tertiary hospital located in southwest Nigeria.
Study participants
Fifty (50) patients with stable COPD according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria were recruited consecutively from the outpatient clinics of Obafemi Awolowo University Teaching Hospital (OAUTH), Ile-Ife11. The inclusion criteria comprised of patients with:
chronic airflow impairment — percentage of forced expiratory volume in one second less than 80% predicted and ratio of forced expiratory volume in one second to forced vital capacity (FEV1/FVC) less than 0.7,
a bronchodilator reversibility test with change in FEV1 less than 15% and/or 200ml 20minutes after inhalation of 400micrograms of Salbutamol,
clinically stable as defined by no change in medication dosage, frequency and no exacerbation or hospital admissions in the preceding six weeks.
Those excluded were; persons with a history of asthma, atopy or nasal polyps, patients who demonstrated a remarkable response and reversibility to Salbutamol as noted by FEV1 change above 15% and/or 200ml, patients with active lung disease like Tuberculosis or Bronchiectasis and those with comorbid conditions that could contribute to dyspnoea or exercise limitation like hypertensive heart failure
Measurements
Health Status Measurement
HRQL was assessed using the SGRQ8. The SGRQ has been used extensively for assessing QoL in patients with COPD and several other chronic lung diseases12–14. It was developed by PW Jones of the St George's hospital medical school, London in 1991. It is sensitive, valid, reliable and responsive among patients with COPD. It contains 50 items with 76 weighted responses that cover three domains: symptoms - distress due to respiratory symptoms, activity - disturbances of physical activity and impact - overall impact on daily life and well-being. In addition to the domain scores, there is also a total score7. The SGRQ is scaled from zero to 100 (with zero representing the best health-related quality of life). This questionnaire which was forward and back translated in Yoruba language, was administered to each participant. However those unable to self-complete the questionnaire due to shaky hands, inability to read, or poor eye sight had the questionnaire read to them in the exact format in which they were set out without bias or undue emphasis and their responses were ticked as appropriate.
Lung function parameters
Lung function test was performed using a standardized vitalograph bellows spirometer (Vitalograph Ltd, Buckingham, England). The following parameters were measured: forced expiratory volume in one second (FEV-1) and forced vital capacity (FVC). The reference values were derived using equations from a study by Femi-Pearse et al15. The parameters were assessed before and 20 minutes after the inhalation of 400ìg of Salbutamol using a metered dose inhaler (MDI) and a spacer device. The spacer was attached to the MDI and held in the mouth. After the patient had exhaled to functional residual capacity (FRC), the canister was activated. The patient inhaled slowly to total lung capacity (TLC) and held his/her breathe for ten (10) seconds16. This was done twice for every 200µg of Salbutamol released into the spacer device.
Exercise testing and dyspnoea rating
Exercise performance was evaluated using the six minutes walk test (6-MWT) according to the American Thoracic Society guideline17. It is a simple, easy to administer and well tolerated functional walking test which is representative of the activities of daily living (ADL). This test was performed on a long, flat, straight and enclosed corridor. The walking course was 30m (100 -feet). The length of the corridor was marked every 3m and the turnaround points were also noted. A starting line, which marks the beginning and end of 60m lap, was marked on the floor using a brightly coloured marker. Patients appeared in their normal clothing on the day of the test with their shoes and walking aid, if any. The length walked was measured and the level of perceived breathlessness was measured both before and after the walk test using the visual analogue scale (VAS)
The VAS is a dynamic scale used to assess a patient's perceived breathlessness. It has been shown to be a valid measure of dyspnoea on exertion18. The visual analogue scale consisted of a 20cm horizontal line. The word ‘No Breathlessness’ was written at the left end of the scale, ‘Very Severe Breathlessness’ was at the right end of the scale. It was scored from 0 to 100. Ratings were expressed as percents of the full VAS line length. Participants were asked to grade their shortness of breath with the scale before and after the six minutes walk test.
Data collection and Analysis
A proforma was used in recording sociodemographic data from each patient. Mean values and standard deviation (SD) was computed for all continuous parameters and frequencies and proportions for categorical variables. The correlation between quality of life scores, dyspnoea rating, six minutes walking distance and physiological parameters was assessed using Pearson's linear correlation coefficient.
The St Georges respiratory questionnaire was statistically analyzed using the excel-based scoring calculator from the developer. To identify the factors that influenced HRQL in these patients, a linear regression analysis was used. The model with the best fit was assessed using the changes in the R-squared values adjusting for age, sex and smoking pack years. Ethical clearance was obtained from the hospital ethics committee and verbal informed consents were obtained from participants included in the study.
Results
Fifty patients were recruited for this study and sixty percent of the respondents were male. The mean age and standard deviation was 69 (9) years, body mass index (BMI) 22(5). The proportion of the stages of COPD were 10%, 34%, 34% and 22% for mild, moderate, severe and very severe stages of the disease respectively. The mean pre-bronchodilator FEV1 and FVC were 801ml and 1520ml while the post bronchodilator FEV1 and FVC were 817ml and 1570ml respectively. The overall mean (SD) SGRQ scores were 45.9 ± 26.5, 50.6 ± 29.2, 29.7 ± 19.9, 38.8 ± 22.0 for the symptom, activity, impact and total scales respectively (table 1).
Table 1.
General characteristics of the participants (n=50)
| Variable | Mean ± SD or n (%) |
| Age (years) | 69 ± 9 |
| Height (cm) | 162 ± 9 |
| BMI (Kg/m2) | 22 ± 5 |
| FEV1-pre (ml) | 801 ± 439 |
| FEV1-post (ml) | 818 ± 405 |
| FVC-pre (ml) | 1520 ± 679 |
| FVC-post (ml) | 1570 ± 655 |
| FEV1/FVC (%) | 51 ± 12 |
| 6 MWD (m) | 361 ± 85 |
| VAS-dyspnea before 6MWD(%) | 10 ± 15 |
| VAS-dyspnea after 6MWD (%) | 29 ± 22 |
| SGRQ score | |
| Symptom | 45.9 ± 26.5 |
| Activity | 50.6 ± 29.2 |
| Impact | 29.7 ± 19.9 |
| Total | 38.8 ± 22.0 |
| Sex (male) | 30 (60) |
| *GOLD Classification of COPD | |
| Mild | 5 (10) |
| Moderate | 17 (34) |
| Severe | 17 (34) |
| Very Severe | 11 (22) |
| Occupation | |
| Artisans | 8 (16) |
| Farmers | 17 (34) |
| Professionals | 5 (10) |
| Teachers | 5 (10) |
| Traders | 15 (30) |
FEV1 - Forced expiratory volume in one second, FVC-Forced vital capacity, pre-prebronchodilator, post-bronchodilator, VAS-visual analogue scale, SGRQ-St George's Respiratory Questionnaire, BMI-Body Mass Index; 6 MWD-6 Minutes Walking Distance. GOLD-Global initiative for Chronic Obstructive Lung Disease;
Post bronchodilator FEV1/FVC <70%. FEV1 > 80% predicted (Mild); 50% <= FEV1 < 80% predicted (Moderate); 30% <=FEV1 < 50% predicted (Severe); FEV1 < 30% predicted (Very severe).
Men had mean (SD) SGRQ scores of 42.1 (27.8), 47.5 (30.8), 27.2 (20.3), 36.0 (22.9) and for women the scores were 51.6 (23.9), 55.2 (26.7), 33.4 (19.2), 43.0 (20.4) in the symptom, activity, impact and total scales respectively. Women had worse SGRQ scores compared with men. As expected, previous heavy smokers had worse SGRQ score in the symptom subscale compared to never smokers (Data not shown). There was no current smoker in the sample.
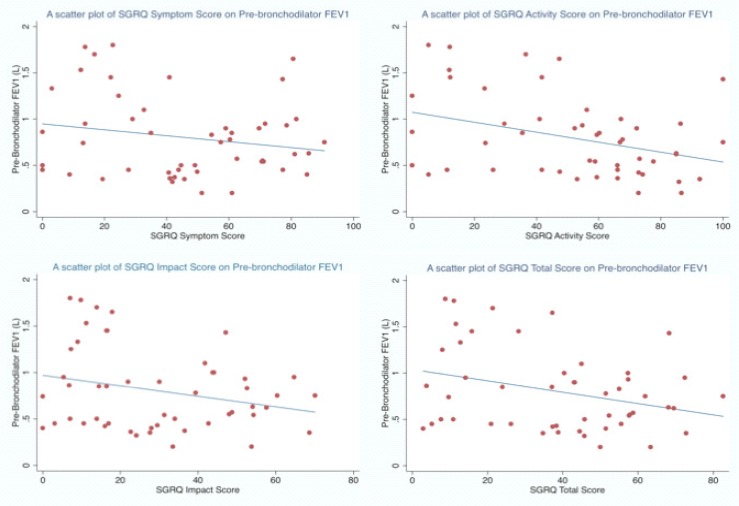
Table 2 shows the correlation coefficients between the SGRQ scores, lung function parameters, six minutes walking distance and VAS dyspnoea scores. The coefficients for FEV1 pre-bronchodilator were −0.19, −0.36, −0.26 and −0.31 for SGRQ symptoms, activity, impact and total scores respectively. The coefficients for pre-bronchodilator FVC were −0.13, −0.32, −0.18 and −0.24 respectively. The coefficients for pre-bronchodilator FEV1 and FVC were higher than the post bronchodilator values. The VAS scores post exercise showed correlation coefficients of 0.16, 0.36, 0.41 and 0.38 for SGRQ symptoms, activity, impact and total scores respectively. The activity subscale and total score demonstrated the best SGRQ correlation to FEV1, albeit weak, to lung function variables.
Table 2.
Pearson correlation coefficient of SGRQ scores
| Symptom | Activity | Impact | Total | |
| FEV1-Pre | −0.19 | −0.36* | −0.26 | −0.31* |
| FEV1-Post | −0.13 | −0.32* | −0.21 | −0.26 |
| FVC-Pre | −0.13 | −0.32* | −0.18 | −0.24 |
| FVC-Post | −0.07 | −0.24 | −0.14 | −0.18 |
| FEV1/FVC | −0.07 | −0.25 | −0.14 | −0.18 |
| 6 MWD | −0.21 | −0.24 | −0.32* | −0.30* |
| VAS -Post | 0.16 | 0.36* | 0.41** | 0.38** |
| exercise |
p <0.05
p<0.01
FEV1 - Forced expiratory volume in one second, FVC-Forced vital capacity, PRE-prebronchodilator, POST-bronchodilator, VAS-visual analogue scale, SGRQ-St George's Respiratory Questionnaire, 6 MWD - 6 Minutes Walking Distance, Significant values in bold
Table 3 shows the results of the univariate analysis of the various study parameters. Notably, the self reported dyspnoea and weight loss were important predictors for the SGRQ total and sub scores. Those reporting dyspnoea on average had 27.82, 29.46, 10.70 and 19.48 points higher scores (indicating worse quality of life) in the total, symptoms, activity and impact scales respectively.
Table 3.
Univariate regression analysis of SGRQ total and component scores on study parameters
| Total | Symptom | Activity | Impact | |
| Age (years) | 0.16 | −0.12 | 0.33 | 0.18 |
| Sex (Male) | −6.99 | −9.47 | −7.70 | −6.16 |
| Height (cm) | 0.03 | −0.24 | −0.06 | 0.15 |
| BMI (Kg/m2) | −0.14 | 0.32 | −0.06 | −0.32 |
| Smoking status | −1.32 | −1.13 | −0.52 | −1.86 |
| Smoking pack years | 0.07 | 0.44 | −0.23 | 0.08 |
| 6 MWD (m) | −0.08 | −0.07 | −0.08 | −0.08 |
| FEV1-pre | −0.02 | −0.01 | −0.02 | −0.01 |
| FEV1-post | −0.01 | −0.01 | −0.02 | −0.01 |
| FVC-pre | −0.01 | −0.01 | −0.01 | −0.01 |
| FVC-post | −0.01 | 0.00 | −0.01 | 0.00 |
| FEV1/FVC | −0.35 | −0.16 | −0.63 | −0.24 |
| VAS 1 | 0.31 | 0.38 | 0.30 | 0.28 |
| VAS 2 | 0.38 | 0.19 | 0.48 | 0.37 |
| Gold classication | 2.69 | 0.70 | 5.27 | 1.56 |
| Self-reported symptoms | ||||
| Cough | −7.16 | 4.10 | −36.03 | 5.65 |
| Sputum | 16.87 | 23.42 | 17.11 | 14.60 |
| Wheeze | 6.54 | 12.92 | 6.72 | 4.23 |
| Dyspnea | 27.82 | 29.31 | 40.93 | 19.48 |
| Weight loss | 16.38 | 15.86 | 17.26 | 15.53 |
| Chest tightness | 6.43 | 13.46 | 10.70 | 1.51 |
| Chest pain | 5.61 | 6.59 | 7.14 | 4.92 |
| Occupation | 0.34 | 0.75 | 1.01 | −0.07 |
| Cooking fuel* | −1.84 | −6.48 | −1.94 | −0.61 |
GOLD - Global initiative for chronic Obstructive Lung Disease. VAS1 - Visual Analogue Score pre-exercise, VAS 2 - Visual Analogue Score post-exercise, BMI=Body Mass Index; FEV1 - Forced Expiratory Volume in one second, FVC-Forced Vital Capacity, pre-prebronchodilator, post-postbronchodilator, SGRQ-St George's Respiratory Questionnaire, BMI-Body Mass Index, 6 MWD -6 Minutes Walking Distance.
Cooking fuel types included Firewood, Kerosene, Liquefied natural gas and Electric cooker
Significant variables at p<0.05, are in bold
Table 4 presents the result of the multivariate analysis adjusting for age, sex, smoking and FEV1. It shows that self reported breathlessness independently predicts on average 25.2, 36.8, 13.65 and 22.9 points increase in SGRQ symptom, activity, impact and total scores respectively. Adjusting for age, sex, pack years of smoking, reported symptoms like weight loss, breathlessness, VAS score post exercise and lung function, the model accounted for 24%, 42%, 27% and 35% of the variance in the SGRQ symptom, activity, impact and total score respectively.
Table 4.
Multivariate regression analyses of determinants of health related quality of life
| Coefficient | SE | 95% CI | p value | R squared | ||
| TOTAL SGRQ | ||||||
| Weight loss | 11.14 | 5.59 | −0.14 | 22.43 | 0.053 | |
| FEV1_post | 0.00 | 0.01 | −0.01 | 002 | 0.782 | Adjusted |
| Breathlessness | 22.91 | 6.20 | 10.41 | 35.41 | 0.001 | R2=0.35 |
| VAS past exercise | 0 24 | 0.12 | −0.01 | 0.49 | 0.060 | |
| SYMPTOM SGRQ | ||||||
| Weight loss | 5.17 | 7.65 | −10.26 | 20.61 | 0.502 | |
| FEV1_post | 0.00 | 0.01 | −0.02 | 0.02 | 0.845 | Adjusted |
| Breathlessness | 25.20 | 8.02 | 9.01 | 41.39 | 0.003 | R2=0.27 |
| Sputum | 18.30 | 11.94 | −5.79 | 4240 | 0.133 | |
| ACTIVITY SGRQ | ||||||
| Weight loss | 1037 | 6.98 | −3.72 | 2446 | 0.145 | |
| Breathlessness | 36.81 | 7.74 | 21.20 | 52.42 | 0.000 | Adjusted- |
| VAS past exercise | 026 | 0.15 | −0.05 | 057 | 0.099 | R2=0.42 |
| FEV1_post | 0.00 | 0.01 | −0.02 | 002 | 0.689 | |
| IMPACT SGRQ | ||||||
| Weight loss | 12.20 | 5.35 | 1.40 | 22.99 | 0.028 | |
| FEV1_post | 0.00 | 0.01 | −0.01 | 002 | 0.532 | Adjusted- |
| Breathlessness | 13.65 | 5.93 | 1.69 | 25.61 | 0.026 | R2=0.24 |
| VAS past exercise | 0.28 | 0.12 | 0.04 | 051 | 0.023 | |
CI-Confidence Interval; FEV1-Forced Expiratory Volume in one second; VAS-Visual Analogue Scale; SGRQ - St George's Respiratory Questionnaire; SE-Standard Error; Post - postbronchodilator
The model adjusted for age, sex and smoking with SGRQ ‘total’ ‘symptom’ activity and ‘Impact’ scores as dependent variables Significant variables at p<0.05, are in bold
Discussion
The SGRQ designed by Jones PW has been used widely for measuring HRQL in persons with COPD especially in drug trials and other controlled interventional studies19, 20. Recently, a shorter version derived from the SGRQ, which is described as COPD Assessment Test (CAT) was developed by the same author21. However the use and relevance of these assessment tools in patients in low-income countries where access to respiratory care, spirometry and other modalities of managing COPD are largely inaccessible, is not well studied or understood.
We found that patients with COPD had low self-perceived quality of life especially in the ‘symptom’ and ‘activity’ subscales of the SGRQ. HRQL showed a significant association with dyspnoea measures but showed weak and inverse relationship with spirometry. In addition, self reported dyspnoea and weight loss were the strongest independent predictors of HRQL.
The quality of life scores of the present study generally indicate the patients had poor health status. In healthy individuals without symptoms of respiratory diseases, SGRQ scores are usually less than 15 units 22and in clinical trials on patients with COPD, the minimum clinically important difference (MCID) for efficacy of a treatment is estimated to be 4 units23, However, in cross sectional studies of diseased individuals, a relative approach is often used.in interpretation of SGRQ scores. Scores tending towards 100 are generally indicative of worse health status while scores tending towards 0 indicate a better quality of life score7.
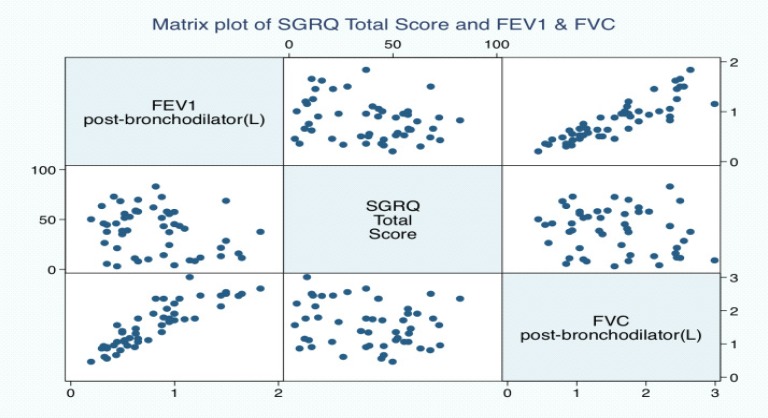
We found weak correlation between HRQL and Spirometry. The activity and total scales of the SGRQ showed the best correlation with pre-bronchodilator FEV1, but the coefficients were generally low (Figure 1a–d). A similar observation was found between SGRQ total score and post bronchodilator lung function values (Figure 2). Various correlation coefficients have been reported between HRQL and spirometry by several authors but the coefficients are usually weak 8, 25. Spirometry objectively evaluates the lung's functional and ventilatory capacity however it often fails to evaluate the non-ventilatory manifestations of COPD like weight loss, mood changes including depression. Overall, it seems that spirometry and HRQL are important tools, which evaluate different aspects of the disease especially the impact on patients daily functioning. Spirometry evaluates the functional aspects of the airway calibre and informs on the progression of the disease, a measure described as a ‘hard’ measure by PW Jones26. However HRQL provides a holistic evaluation of the total impact of the disease especially in manifestations other than airway obstruction like weight loss, mood swings which explains its description as a ‘soft’ measure26. The combination of quality of life measures to the functional measures obtained from spirometry may provide a more thorough and comprehensive evaluation of patients with COPD.
Figure 1 (a–d).
Scatter plots showing the relationship of FEV1 and SGRQ component and total scores
Figure 2.
A matrix plots of SGRQ total score and Post bronchodilator FEV1 & FVC.
The notable predictors of HRQL were self-reported breathlessness, weight loss, wheeze, sputum production and patient's subjective perception of breathlessness measured on the visual analogue scale post exercise. Age, body mass index, social class and GOLD staging of the disease did not significantly influence or predict HRQL. However, in the multivariate model, the key predictor of HRQL was self reported breathlessness. This observation shows that breathlessness during exercise or performance of daily activity is an important determinant of patient's self-perceived quality of life. Our study corroborates previous studies which showed that dyspnoea is one of the main determinants of the disease-specific HRQL and has moderate-to-strong correlation with impairment in the HRQL of patients with COPD27, 28.
In the symptom subscale, dyspnoea was the only significant explanatory variable independently accounting for 27% of the variance in the quality of life sub score. The classical symptoms of COPD like cough, sputum production and wheeze were not found to influence significantly on quality of life neither did functional variables like FEV1. This observation shows that breathlessness occupies an overriding influence on HRQL over other symptoms of the disease. In the activity subscale, breathlessness accounted for a greater proportion of the variance in the HRQL, representing 42% of the total variance in this subscale. This further shows that restriction in activity in COPD is primarily due to breathlessness. The variance in the impact subscale was also influenced independently by the perception of breathlessness, weight loss and dyspnoea score on the VAS post exercise. They accounted for 24% of the total variance in this subscale. The relatively low percentage in the accounted variance of the impact subscale model may be due to the fact that anxiety and depression were not measured in this study. Anxiety and depression have been shown to influence the impact score in COPD because they encompass the psychological and social dimension of the disease. It's been suggested that variables dependent on the psychological spheres exert a decisive influence on the way in which patients live with their disease and hence the HRQL26.
Lastly, the multiple regression analysis performed using the SGRQ total score showed that adjusting for age, sex, FEV1 and smoking, the model which includes self reported breathlessness and weight loss account for 35% of the variance in the SGRQ total score. Weight loss has been reported as an indicator of outcomes in numerous studies on COPD29, 30. In our study, it accounted in the unadjusted model for 13% of the variance in the impact subscale and 11% of the total scores (data not shown). The aetiology of weight loss in COPD is possibly multi-factorial and factors implicated include increase workload of laboured breathing, under-nutrition due to breathlessness. The resultant effect is a reduction in muscle strength, endurance and participation in social activities and under-nourishment making COPD patients prone to recurrent infection, increase hospitalization and increased mortality from the disease31.
This study has some limitations. It was a hospital-based survey and as such, the participants included in the study may be skewed towards the very sick patients. Patients often do not access health care in low resource settings until their clinical condition becomes very dire because of the cost of care. This potentially limits the ability to generalise the results of this study to all COPD patients.
Conclusion
This study showed some important implications for the care of COPD in low-income settings. Spirometry remains a core investigative modality in the management of COPD however it should be complemented with measures of quality of life. In addition the presence of reported breathlessness indicates poor health status and local clinicians may use this variable as the primary indicator of a patient's self-perceived quality of life. A simple measure of breathlessness on a linear scale post exercise may also provide significant guide into the clinical state of a patient with COPD.
This study also showed that SGRQ quality scores are weakly correlated with spirometry and it's core predictors are self-reported weight loss and breathlessness.
Acknowledgments
We wish to acknowledge the support of the Obafemi Awolowo University teaching hospital in actualizing this research. We also wish to thank the house officers and resident physicians in the respiratory unit of the hospital for their kind assistance during the course of this study.
References
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the global burden of disease study 2010. The Lancet. 2013;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopez A, Shibuya K, Rao C, Mathers C, Hansell A, Held L, Schmid V, Buist S. Chronic obstructive pulmonary disease: Current burden and future projections. European Respiratory Journal. 2006;27(2):397–412. doi: 10.1183/09031936.06.00025805. [DOI] [PubMed] [Google Scholar]
- 3.Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J. 1977;1(6077):1645. doi: 10.1136/bmj.1.6077.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peto R, Speizer F, Cochrane A, Moore F, Fletcher C, Tinker C, Higgins I, Gray R, Richards S, Gilliland J. The relevance in adults of air-flow obstruction, but not of mucus hypersecretion, to mortality from chronic lung disease. results from 20 years of prospective observation. Am Rev Respir Dis. 1983;128(3):491. doi: 10.1164/arrd.1983.128.3.491. [DOI] [PubMed] [Google Scholar]
- 5.Curtis J, Patrick D. The assessment of health status among patients with COPD. European Respiratory Journal. 2003;21(41 suppl):36s–45s. doi: 10.1183/09031936.03.00078102. [DOI] [PubMed] [Google Scholar]
- 6.Aghanwa HS, Erhabor GE. Specific psychiatric morbidity among patients with chronic obstructive pulmonary disease in a nigerian general hospital. J Psychosom Res. 2001;50(4):179–183. doi: 10.1016/s0022-3999(00)00206-3. [DOI] [PubMed] [Google Scholar]
- 7.Jones PW, Quirk F, Baveystock C. The st george's respiratory questionnaire. Respir Med. 1991;85:25–31. doi: 10.1016/s0954-6111(06)80166-6. [DOI] [PubMed] [Google Scholar]
- 8.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. the st. george's respiratory questionnaire. Am Rev Respir Dis. 1992 Jun;145(6):1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 9.Diener E, Suh E. Measuring quality of life: Economic, social, and subjective indicators. Soc Indicators Res. 1997;40(1):189–216. [Google Scholar]
- 10.Miller TR. Variations between countries in values of statistical life. Journal of Transport Economics and Policy. 2000:169–188. [Google Scholar]
- 11.Pauwels RA, Buist AS, Calverley PMA, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease NHLBI/WHO global initiative for chronic obstructive lung disease (GOLD) workshop summary. American Journal of Respiratory and Critical Care Medicine. 2001;163(5):1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 12.Beretta L, Santaniello A, Lemos A, Masciocchi M, Scorza R. Validity of the saint george's respiratory questionnaire in the evaluation of the health-related quality of life in patients with interstitial lung disease secondary to systemic sclerosis. Rheumatology. 2007;46(2):296–301. doi: 10.1093/rheumatology/kel221. [DOI] [PubMed] [Google Scholar]
- 13.Padilla A, Olveira G, Olveira C, Dorado A, Plata AJ, Gaspar I, Pérez-Frías J. Validity and reliability of the st george's respiratory questionnaire in adults with cystic fibrosis. Archivos De Bronconeumología. (English Edition) 2007;43(4):205–211. doi: 10.1016/s1579-2129(07)60052-4. [DOI] [PubMed] [Google Scholar]
- 14.Wilson CB, Jones PW, O'LEARY CJ, Cole PJ, Wilson R. Validation of the st. george's respiratory questionnaire in bronchiectasis. American Journal of Respiratory and Critical Care Medicine. 1997;156(2):536–541. doi: 10.1164/ajrccm.156.2.9607083. [DOI] [PubMed] [Google Scholar]
- 15.Femi-Pearse D, Elebute EA. Ventilatory function in healthy adult nigerians. Clin Sci. 1971 Sep;41(3):203–211. doi: 10.1042/cs0410203. [DOI] [PubMed] [Google Scholar]
- 16.Medical Section of the American Lung Association, author. Standardization of spirometry: 1994 update. Am Rev Respir Dis. 1994;152:1107–1136. [Google Scholar]
- 17.Crapo RO, Casaburi R, Coates A, Enright P, MacIntyre N, McKay R, Johnson D, Wanger J, Zeballos R, Bittner V. ATS statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 18.Grant S, Aitchison T, Henderson E, Christie J, Zare S, McMurray J, Dargie H. A comparison of the reproducibility and the sensitivity to change of visual analogue scales, borg scales, and likert scales in normal subjects during submaximal exercise. Chest Journal. 1999;116(5):1208–1217. doi: 10.1378/chest.116.5.1208. [DOI] [PubMed] [Google Scholar]
- 19.Ferrer M, Alonso J, Prieto L, Plaza V, Monso E, Marrades R, Aguar M, Khalaf A, Antó J. Validity and reliability of the st george's respiratory questionnaire after adaptation to a different language and culture: The spanish example. European Respiratory Journal. 1996;9(6):1160–1166. doi: 10.1183/09031936.96.09061160. [DOI] [PubMed] [Google Scholar]
- 20.Xu W, Collet J, Shapiro S, Lin Y, Yang T, Wang C, Bourbeau J. Validation and clinical interpretation of the st georges respiratory questionnaire among COPD patients, china. The International Journal of Tuberculosis and Lung Disease. 2009;13(2):181–189. [PubMed] [Google Scholar]
- 21.Jones P, Harding G, Berry P, Wiklund I, Chen W, Leidy NK. Development and first validation of the COPD assessment test. European Respiratory Journal. 2009;34(3):648–654. doi: 10.1183/09031936.00102509. [DOI] [PubMed] [Google Scholar]
- 22.Ferrer M, Villasante C, Alonso J, Sobradillo V, Gabriel R, Vilagut G, Masa J, Viejo J, Jimenez-Ruiz C, Miravitlles M. Interpretation of quality of life scores from the st george's respiratory questionnaire. European Respiratory Journal. 2002;19(3):405–413. doi: 10.1183/09031936.02.00213202. [DOI] [PubMed] [Google Scholar]
- 23.Jones PW. St. george's respiratory questionnaire: MCID. COPD. Journal of Chronic Obstructive Pulmonary Disease. 2005;2(1):75–79. doi: 10.1081/copd-200050513. [DOI] [PubMed] [Google Scholar]
- 24.Jones P. Interpreting thresholds for a clinically significant change in health status in asthma and COPD. European Respiratory Journal. 2002;19(3):398–404. doi: 10.1183/09031936.02.00063702. [DOI] [PubMed] [Google Scholar]
- 25.Burge PS, Calverley P, Jones PW, Spencer S, Anderson JA, Maslen T. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: The ISOLDE trial. BMJ. 2000;320(7245):1297–1303. doi: 10.1136/bmj.320.7245.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones P. Health status measurement in chronic obstructive pulmonary disease. Thorax. 2001;56(11):880–887. doi: 10.1136/thorax.56.11.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guyatt GH, Thompson PJ, Berman LB, Sullivan MJ, Townsend M, Jones NL, Pugsley SO. How should we measure function in patients with chronic heart and lung disease? J Chronic Dis. 1985;38(6):517–524. doi: 10.1016/0021-9681(85)90035-9. [DOI] [PubMed] [Google Scholar]
- 28.Okubadejo AA, Jones PW, Wedzicha JA. Quality of life in patients with chronic obstructive pulmonary disease and severe hypoxaemia. Thorax. 1996;51(1):44–47. doi: 10.1136/thx.51.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schols A, Soeters P, Mostert R, Saris W, Wouters E. Energy balance in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 1991;143(6):1248–1252. doi: 10.1164/ajrccm/143.6.1248. [DOI] [PubMed] [Google Scholar]
- 30.Landbo C, Prescott E, Lange P, Vestbo j, Almdal TP. Prognostic value of nutritional status in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 1999;160(6):1856–1861. doi: 10.1164/ajrccm.160.6.9902115. [DOI] [PubMed] [Google Scholar]
- 31.Gray-Donald K, Gibbons L, Shapiro SH, Macklem PT, Martin JG. Nutritional status and mortality in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 1996;153(3):961–966. doi: 10.1164/ajrccm.153.3.8630580. [DOI] [PubMed] [Google Scholar]