Abstract
Nodding Syndrome is a poorly understood neurologic disorder of unknown aetiology that affects children and adolescents in Africa. Recent studies have suggested that the head nods are due to atonic seizures and Nodding Syndrome may be classified as probably symptomatic generalised epilepsy. As part of the Ugandan Ministry of Health clinical management response, a multidisciplinary team developed a manual to guide the training of health workers with knowledge and skills to manage the patients. In the absence of a known cause, it was decided to offer symptomatic care. The objective is to relieve symptoms, offer primary and secondary prevention for disability and rehabilitation to improve function. Initial management focuses on the most urgent needs of the patient and the immediate family until ‘stability’ is achieved. The most important needs were considered as seizure control, management of behavioural and psychiatric difficulties, nursing care, nutritional and subsequently, physical and cognitive rehabilitation. This paper summarises the processes by which the proposed guidelines were developed and provides an outline of the specific treatments currently being provided for the patients.
Introduction
Nodding Syndrome is a poorly understood neurologic disorder of unknown aetiology that affects children and adolescents in parts of sub-Saharan Africa. There are probably between 5000 – 10,000 affected children in East Africa1. This phenomena was first reported in Tanzania in 1960's2 and subsequent reports have come from Liberia3, South Sudan4, 5 and Northern Uganda where there are several thousand affected individuals6, 7. It however may have existed earlier in Uganda as Nakalanga syndrome8–11. The syndrome as seen in Northern Uganda and South Sudan is characterized by head nodding and complicated by a variable presence of other seizure types, cognitive and motor decline, wasting, stunting, behaviour and psychiatric difficulties. Five clinical stages with deteriorating seizures, neuro-cognitive, physical and psychiatric disability can be identified in Ugandan patients. These include a prodrome; development of head nodding and cognitive impairment; other seizure types; multiple complications and, severe disability. Recent studies have concluded that the head nods are due to atonic seizures12 and that this epidemic epilepsy syndrome may be classified as probably symptomatic generalised epilepsy (Richard Idro, unpublished). This paper is a summary of proposed guidelines for the management of Nodding Syndrome in Uganda.
Case definitions
The first International Scientific Meeting on Nodding Syndrome held in Kampala (Uganda) from 30th July to 1st August 2012 agreed on and adopted the following case definitions for Nodding Syndrome13:
A suspected case: Is reported head nodding in a previously normal person; head nodding was defined as repetitive, involuntary drops of the head to the chest on two or more occasions. This case definition is used at the community level.
- A probable case: Is a suspected case with:
- Both of the following major criteria:
- Age at onset of nodding between 3 and 18 years old
- Frequency of nodding 5 to 20 per minute
- Plus at least one of the following minor criteria:
- Other neurological abnormalities (cognitive decline, school dropout due to cognitive/behavioural problems, other seizures or neurological abnormalities)
- Clustering in space or time with similar cases
- Triggered by food and/or cold weather
- Stunting or wasting
- Delayed sexual or physical development
- Psychiatric symptoms.
- A confirmed case: Is a probable case plus a documented nodding episode that is:
- Observed by a trained healthcare worker, or
- Videotaped or,
- on EEG/EMG.
These case definitions are to be reviewed and modified as new data becomes available. The same case definitions are applied in this document.
Development of the guidelines
As part of the Ugandan Ministry of Health clinical response to the disease, in February 2012, the Director General of Health Services constituted a team of nine health workers to develop a manual on treating Nodding Syndrome14. This manual aims to guide the training of health workers and provide them with knowledge and skills to manage the patients. The multidisciplinary team comprised of a nurse, health systems and public health specialist, occupational therapist, nutritionist, paediatrician and peadiatric neurologist, physicians and psychiatrists and was later joined by a manager, an obstetrician/gynaecologist and also received input from a physiotherapist and a speech and language therapist. Initial meetings identified the most important difficulties in individuals with the syndrome and developed a problem list that guided potential interventions. Sources of data included published clinical reports from Tanzania15, 16 and South Sudan4, 17, and case investigations by the Ministries of Health, World Health Organization (WHO) and the US Centers of Disease Control (CDC) in Uganda and South Sudan5, 18, 19. Clinical evaluation reports of two sets of patients (8 patients in 2009 and 22 in 2012) brought from Kitgum district to Mulago hospital in Kampala for detailed assessments and better understanding of the syndrome provided a guiding resource for the interventions36. Lack of randomised trials for these interventions however underpins the strength of evidence. Lastly, current guidelines for specific disease conditions in the country were adapted as appropriate e.g. in the management of severe malnutrition20, 21.
Overview
In the absence of a known cause, a decision was taken to offer symptomatic care. The objective is to relieve symptoms, offer primary and secondary prevention for disability and rehabilitation to improve function. This is a long-term process to be provided by a multidisciplinary team composed of clinicians, nurses, therapists, social workers and teachers. It is suggested that initial management focuses on the most urgent needs of the patient and the immediate family until ‘stability’ is achieved. The goal is optimal functioning in adulthood. Importantly, health services should be in a stimulating, supporting and caring environment to be able to:
Identify the immediate needs of patients;
Offer or refer for inpatient care to manage complications;
Provide outpatient screening and treatment and subsequent follow up care;
Provide psychological and social support for families of children with this chronic and disabling condition; Provide rehabilitation services with occupational, physio and speech and language therapy to improve and restore some of lost functions and prevent further disability;
The most important and urgent needs were considered as seizure control, management of behavioral and psychiatric difficulties, nursing/burns/wound care, treatment for parasitic infections including microfilaria and helminthes, nutritional and subsequently, physical and cognitive rehabilitation. Other important areas included sexual and reproductive health issues as shown in table 1.
Table 1.
Main components of the management of patients with Nodding Syndrome Identification and treatment of severe complications
| Seizure control |
| Management of behavioural, social and psychiatric difficulties |
| Management of malnutrition |
| Treatment for parasitic infections including microfilaria and helminthes and vector control |
| Nursing care |
| Assessment and rehabilitation of functional difficulties and disabilities |
| Sexual and reproductive health services |
| Follow up care Surveillance, documentation, community education and engagement |
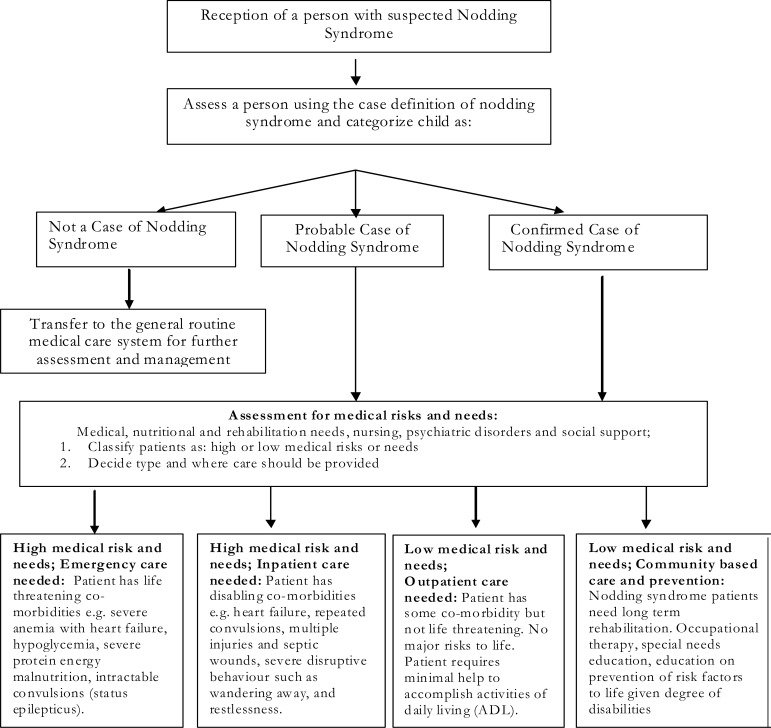
This management guideline was presented at the first International Conference on Nodding Syndrome in Kampala, Uganda, in July 2012. A hierarchical model to deliver the services was proposed to include emergency care, inpatient, outpatient and community-based care as shown in figure 1.
Figure 1.
Algorithm for the management of patients with nodding syndrome (Figure adapted from the revised training manual)
Medical management of Nodding syndrome
Emergency care
Emergency care is indicated for patients with life threatening co-morbidities including status epilepticus, severe anaemia, hypoglycaemia and hypothermia especially in severely malnourished patients, extensive burns and other severe injuries.
The initial treatment for status epilepticus should be an intravenous benzodiazepine (Diazepam 0.3mg/kg, Midazolam 0.3mg/kg or Lorazepam 0.1mg/kg). Lorazepam would be the drug of choice but there are difficulties with storage and it is not readily available in many resource limited settings. Subsequent doses of anticonvulsants should follow local protocols for status epilepticus. It should be noted that the risk of status epilepticus may be higher during febrile illnesses, pregnancy and labour.
Severely anaemic patients (haemoglobin <5g/dl) with under nutrition should be offered packed blood cell transfusion at 5 –7 mL/kg if there is concurrent congestive cardiac failure. In the absence of the latter, whole fresh blood transfusion, 10 mL/kg should be considered. In either case, transfusion should be slow for over three hours and with frusemide a diuretic.
In children with under nutrition and dehydration, give the rehydration solution for malnutrition (ReSoMal) at 5mL/kg/hr for the first 2 hours, then 5–10mL/kg ReSoMal alternating with therapeutic milk formula 75 (F75) every hour for up to 10 hours. Any impairment of consciousness should be treated as shock. Shock from dehydration and sepsis are likely to co-exist in patients with severe acute malnutrition. In this case give 10% glucose at 5 ml/kg and initiate intravenous antibiotics. Continue fluid therapy with intravenous half-strength Darrow's solution with 5% glucose, Ringer's lactate with 5% glucose or 0.45% (half-normal) saline with 5% glucose; in all cases, at 15 ml/kg. After two hours, continue rehydration with a nasogastric tube ReSoMal at 5 -10 mL/kg and alternating hourly with F75 for 10 hours20.
In the event of extensive burns or other severe injuries, the local protocol for the management of burns and trauma should be applied.
Suicidal ideation, attempted suicide and acute severe behaviour problems can occur independently or as a pre-ictal, ictal and post-ictal phases of a seizure. Emergency psychiatric management should ensure safety of both the patient and others. In a severely disturbed child, give diazepam 0.3mg/kg IV stat and repeat only once if necessary. Full psychiatric assessment is mandatory when the patient settles. In addition, assess and treat any organic and co-morbid medical condition.
Non emergency inpatient care
Patients with disabling but non emergency medical conditions and co-morbidities should be managed as inpatients. These may include:
Patients with repeated convulsions or a sudden increase in the number of tonic clonic seizures over and above the usual seizure frequency may be at risk of injury or progression to status epilepticus. In these patients, a precipitating event for the increasing seizures (e.g. acute febrile illness, poor adherence, etc.) should be sought and managed. The anticonvulsant drug dose may be titrated upwards. Caution is however advised especially with rapid increases in the dose of anticonvulsants as the pharmacokinetic properties and metabolism of these drugs in patients with Nodding Syndrome especially in the presence of malnutrition are unknown.
Patients with burns requiring admission (e.g. burns of the hands, feet or groin), those with multiple or severe injuries and septic wounds. Wound care with appropriate dressing and debridement if indicated, administration of antibiotics and a booster dose of tetanus toxoid vaccination may be provided. Consideration should also be given for the extra demand of nutrients due to open skin.
Patients with severe and disruptive behaviour such as wandering and restlessness should be managed in conjunction with psychiatry services. Psychiatric assessment should establish reasons for wandering or restlessness.
Patients with other childhood illnesses requiring inpatient care such as pneumonia, diarrhoea with severe dehydration and severe malaria. Management for these conditions should follow usual standards of care for patients without seizures disorders.
Essential investigations include thick and thin blood smears for malaria parasites, blood glucose, complete blood count, peripheral blood film and ESR, blood chemistry for liver and renal function and electrolytes and creatine kinase. In addition, a diagnostic electroencephalogram recording and a skin snip (for a diagnosis of Onchocerca volvulus and Mansonella perstans) should be obtained in all patients. Blood cultures, lumbar puncture for cerebrospinal fluid and brain imaging with brain MRI and/or CT scan should be performed as indicated. Lumber puncture is indicated in patients with features suggestive of meningitis or trypanosomiasis. In the case of repeated uncontrolled seizures and maybe new neurological signs/symptoms one should envisage serology for Taenia solium cysticercosis. Skull and limb x rays to exclude fractures from injuries, chest x rays for evidence of chest infection and x-rays of the wrist to determine bone age may also be performed.
Outpatient care
Outpatient care is offered to patients with non-life threatening co-morbidity and no major risk to life. This is the standard of care for most patients with Nodding Syndrome. These patients require only minimal help to accomplish activities of daily living. Treatment includes anti epileptic drug therapy, nutritional therapy and rehabilitation with ready to use therapeutic feeds (RUTF), nursing care, cognitive stimulation, physical, occupational and speech and language therapy, and attending to sexual and reproductive issues.
Community based care and prevention
This is care provided to cater for the long term needs and rehabilitation of patients. It includes provision of physical, occupational and speech and language therapy; adaptive services, return to school and special needs education support, prevention of risk factors for disease and injuries especially in the presence of disabilities. These interventions become even more relevant with improving seizure control and a more ambulant patient. Community education on the syndrome, health promotion services, disease surveillance and monitoring of treatment effects should form important components.
Specific therapies
Anti-epileptic drugs
In Ugandan patients, the main seizure type in Nodding Syndrome, head nodding, has been determined to be an atonic seizure12. Other seizure types including absence, tonic clonic and myoclonic seizures may develop 1–3 years after the initial signs. Some patients also develop behaviour and psychiatric difficulties, (Richard Idro, unpublished). In the choice of anti epileptic drugs, we wanted an efficacious, safe and cheap drug against epilepsies with multiple seizure types and with some activity against behavioural and psychiatric difficulties. There are no clinical trials that have examined antiepileptic medications in Nodding Syndrome. Anecdotal reports among patients with head nodding from Tanzania suggest a decrease in seizure frequency when they used phenytoin and Phenobarbitone either individually or in combination16. These two are the most common antiepileptic drugs in the affected communities. In developing the guidelines, carbamazepine was not considered because of risk of precipitating myoclonic jerks22. Instead, the types of seizures in these patients suggested sodium valproate may be a better option23, 24 Sodium valproate is also active against some behavioural difficulties like aggression and impulsive behaviour25,26. It is suggested to start with 10mg/kg/day in two divided doses and increase the dose by 5mg/kg/day increments until seizure control is achieved or the maximum dose is reached. We suggest a maximum dose of 40mg/kg/day in children. Preferably, a single brand should be used consistently as absorption characteristics may differ among brands. The pre-treatment liver function should be determined before initiation of therapy and used in monitoring treatment toxicity.
On each review visit, patients should be assessed for seizure control and for side effects of medications. These include excessive weight gain, liver toxicity (manifesting with deranged liver function, jaundice or abnormal bleeding) and skin rash. The increased risk of fetal malformations with using sodium valproate in females in the reproductive age group may limit its use27. It is important to counsel such patients to avoid pregnancy or use second line drugs. Applying these guidelines in a cohort of 22 children studied in Mulago, there was a 57% reduction in total seizures burden 2–3 weeks after initiation of sodium valproate. Reduction was observed in both clusters of head nodding and number of tonic clonic seizures. The dose range of sodium valproate was then 15–25mg/kg/day. We however do not have a recommendation on how long treatment should continue and on weaning (except advise against abrupt cessation of therapy) as it is still early days with use of this treatment regime. This information will possibly be available in future revisions of the guidelines. Additional information on the use of sodium valproate may be found at: http://www.netdoctor.co.uk/brain-and-nervous-system/medicines/epilim.html. The suggested second line anti-epileptic drug is lamotrigine. Levetiracetam is also an option28–30. To date there is no experience with the use of these two drugs in Nodding Syndrome. They may be considered in cases of severe sodium valproate toxicity31, failure of response to adequate doses of sodium valproate - 40mg/kg/day, pregnancy or girls in the reproductive age group32. Lamotrigine is a fairly safe drug but severe skin rashes or reaction may develop. For this reason, the dose should be titrated upwards very slowly. The usual maintenance dose in other childhood epilepsies is 1–10 mg/kg/day in 1–2 divided doses and the maximum daily dose is 200 mg in children under 12 years and 400mg in those 12 years or older. Levetiracetam may be associated with behaviour problems especially in children30, 33. Anecdotal reports from Tanzania indicate Phenobarbitone and probably phenytoin may be used16 (table 1). The benzodiazepines, Clonazepam and Clobazam have been suggested for short term therapy in cases of sharp increases in seizures. Clearly, clinical trials are urgently needed to determine the best treatments
Malnutrition and nutritional rehabilitation
Nodding syndrome is associated with both acute (wasting) and chronic (stunting) malnutrition. In particular, extreme short stature or stunting which is out of proportion of the degree of malnutrition is a feature in many patients. The cause of this is yet unknown. Head nodding, which may be precipitated while eating local foods, may contribute to malnutrition. Interestingly, eating non-local food has not been observed to precipitate nodding. Other factors contributing to and worsening the malnutrition include the epileptic encephalopathy with disturbed behaviours, cognitive impairment and weak jaw muscles for mastication. Anthropometric measures of the child (weight, height, head circumference and mid upper arm circumference) should be documented on initial contact and at each visit and at least every six months. Nutritional rehabilitation and supplementation with micronutrients are key components in the management.
Nodding Syndrome verification reports in Uganda have consistently shown that more than half of patients have acute malnutrition. A blanket supplementary feeding program has therefore been put in place for families of all patients. The severely malnourished child with medical complications should be treated as an inpatient and one with uncomplicated malnutrition as outpatient20, 21. Inpatient therapeutic care aims to stabilise patients with medical complications such as hypoglycaemia, hypothermia, severe dehydration, severe anaemia and life threatening infections. Administer first line antibiotics (oral amoxicillin 50–100mg/kg in three doses for seven days or Gentamicin IV/IM 3–5 mg/kg for seven days plus intravenous Ampicillin 50mg/kg six hourly for seven days. If there is no improvement within 48 hours, use Gentamicin IV or IM 7.5mg/kg daily for 7 days plus Chloramphenical 25mg/kg six hourly20.
Modest deficiencies of vitamin B6 and selenium have been described in patients with Nodding Syndrome18, 19. The combined mineral and vitamin supplements in the specialised nutritional therapeutic feeds provide most micronutrient requirements and unless the therapeutic feeds are not provided, patients may not need additional supplements. It is however suggested that treatment should initially include daily vitamin B complex and a single dose of vitamin A 200,000 IU to patients without oedematous malnutrition. Patients with oedematous malnutrition should only receive vitamin A if there are signs of severe deficiency such as corneal changes; otherwise, this is delayed until after resolution of oedema. Iron should only be administered if a patient has very severe anaemia (Hb <4 g/dl) otherwise, should be delayed until after stabilization. Following the development of the guidelines, a pilot study of seven children suggested that vitamin D deficiency may be common in patients with Nodding Syndrome; six of seven children had moderate or severe vitamin D deficiency. Anti epileptic drugs may precipitate or worsen Vitamin D deficiency. The need for supplementation with Vitamin D should be investigated urgently36.
Behaviour, Social and Psychiatric difficulties
Compared to children in the general population, children with epilepsy have almost double the prevalence of behaviour and psychiatric difficulties34, 35. Some of these behaviours can be modified positively or, rarely, negatively by sodium valproate. Patients with Nodding Syndrome and their caregivers too experience a higher burden of behaviour and psychiatric difficulties causing significant distress. These difficulties appear to be similar to those found in other epilepsies and include psychotic experiences which may manifest as wandering or aggressive behaviour, other problematic behaviours such as impulsivity, emotional and mood disorders and cognitive decline36. Depression and anxiety, including post-traumatic stress disorder, have been associated with the syndrome37. Progressive cognitive decline results in impaired intellectual development. Effects on learning may be further compounded by associated behavioural difficulties and emotional disorders34, 38. The chronicity of the syndrome makes experiences of behavior difficulties even more likely and, undoubtedly increases the burden of living with the disease. Thus, management should include an assessment for and treatment of these disorders. In other epilepsies, learning difficulties may be directly related to the epilepsy or better the ensuing epileptic encephalopathy and its management or are adverse effects of medication34, 38. The pathogenesis of cognitive decline and learning difficulties in Nodding Syndrome has not been elucidated but some of the factors in other epilepsies may be at play.
Behaviour problems in children are usually reported by the caretaker but may also be observed in the clinic. Children with such difficulties are at particular risk of getting lost, exposure to physical injury and being abused. Other issues include stigma, child abuse and neglect. There is need to document and provide a management plan for each difficulty. The psychiatry assessment should include an examination of the patient's mood, perception, cognition and thoughts. On the other hand, caretakers and families of patients may have adjustment problems, depressive and anxiety disorders. These conditions together with social stigma associated with Nodding Syndrome can affect access to and type of health care for patients.
Management should include counseling for patients and their caretakers and referral of those with severe symptoms to mental health services for drug therapy (e.g. anti depressant and anti psychotic drugs) and other interventions. Counseling of the patient and caregiver includes education about the illness, talking about and finding solutions to the emotional, social and behavioural problems, and follow up care.
Microfilaria and helminthic treatment
Nodding Syndrome has been associated with infestation with Onchocerca volvulus and Mansonella perstans. Although no casual relationship has been demonstrated, targeted responses against these filarial parasites and their vector - e.g. the black or Simulium fly - is a prudent option. Current interventions include aerial spraying against the vector and mass treatment of all people living in the affected areas with the antifilarial drug, ivermectin. Rivers in areas with prominent rapids are sprayed together with application of larvicides in areas where vegetation obstruction would limit contact of breeding sites with the insecticide. Ground application of larvicides in targeted breeding areas is also an option39, 40. The insecticide used in Uganda and elsewhere is Temephos, a non-systemic organophosphate insecticide. Ivermectin is only active against the larval forms but not the adult parasite and so, has to be administered biannually over at least 10 cycles - the lifetime of the female adult parasite. Because ivermectin is currently administered every 6 months to the whole population as part of the public health response, patients testing positive for microfilaria in health units may not require additional treatments. Secondly, due to the high prevalence of intestinal helminthes in these rural setting, a single dose of Albendazole 400mg is recommended. This may be repeated every 6 months. Caution is however advised with Albendazole if the area is endemic for neurocysticercosis as Albendazole may exacerbate latent cysts with ensuing symptomatic seizures or worsen already existing seizures41.
Nursing care
Important nursing care issues include parental education on epilepsy and seizure management, use and side effects of anti epileptic drugs and treatment adherence, hygiene, nutrition, safety, injury prevention and wound care. Children with frequent seizures require supervision to prevent injury. Parents and guardians should be educated on the importance of adherence to anti epileptic drugs, how to store them and report on adverse events of drugs. Encourage, teach and supervise self care, feeding, toileting, hygiene and monitor growth. Observe that vaccinations are up to date; promote use of insecticide treated mosquito materials for malaria prevention, and in case of any infections, treatment should be initiated at the earliest opportunity as any untreated infections increases seizures. Parents should be discouraged from restraining children with ropes. Wounds should be cleaned and dressed daily and parents taught how to keep the wounds dry and clean. Advise parents on food preparation and guide to perform daily exercises to prevent muscle atrophy, a positive attitude, storytelling and play therapy to help with concentration, memory and stimulation. Lastly, the nurse should promote education of the whole community.
Functional difficulties and disabilities Assessment
Nodding Syndrome is associated with multiple comorbidities or functional difficulties. The most common difficulties include motor, behaviour and psychiatric difficulties and declining cognitive function and difficulties with personal care. The behaviour difficulties include anxiety, fear, mood and personality change and aggression. All should be assessed for individually and the assessment repeated during follow up visits to document any progression or improvements with therapy. In addition, children with Nodding Syndrome are at an increased risk of injury, burns, drowning or getting lost. In the physical examination, the clinician should therefore conduct a survey for any injuries and evaluate for activities of daily living including feeding, self care (toileting, bathing and dressing) and mobility. Changing concerns from childhood to adult life will call for adjustments in treatment and priorities. The child's physical ability should be classified using the Gross Motor Function Classification System42, 43. This five level instrument of increasing disability is based on self-initiated movement with emphasis on sitting, transfers and mobility, and immediately gives an overview of the patients needs, helps in communication with other health workers and in monitoring improvements. The speech may be unclear and patients may be confused and disoriented especially during and up to 24 hours following a seizure. Communication difficulties may arise from impaired cognition, seizures or muscle weakness. Cranial nerve palsies are uncommon. Partly because of the decline in cognitive ability or repeated seizures, many children drop out of school. Document the presence of any of these difficulties and provide a management plan for each.
Rehabilitation
The total effect of the above difficulties is on the overall function of the child. Rehabilitation should address global development. Comprehensive rehabilitation entails assessing all aspects of function and planning for appropriate remediation. It should focus on maintaining functions where appropriate, promote compensatory adaptations for functions already lost, and prevent secondary consequences of disabilities. The aim is improving functioning of children with mild to moderate disability and achieving independence in the severely impaired. The functional goal is to achieve the most effective mode of locomotion and self-care skills, with assistive devices if necessary44.
Both fine and gross motor domains may be affected. A child may fail to walk due to poor co-ordination, weakness, lack of balance, deformity or due to contractures. Strengthening exercises, activities to improve balance and passive movement of joints can be employed. For gross motor skills, activities like jumping, playing in sand, skipping or simple games may be helpful. If balance is affected, swinging and playing on a trampoline are suggested. Mobility devices such as braces and calipers may be useful. Fine motor skills - reaching, grasping, manipulation and releasing - may be grossly affected. Activities such as stacking toys, sorting and playing with jigsaw puzzles are suggested. Aspects of cognition that can be affected include memory, concentration, judgment and orientation. Suggested activities include painting, crayoning, simple numerical skills and group activities.
In those with feeding difficulties, positioning is vital and jaw control is helpful in impaired mouth function. Sucking and blowing bubbles through a straw can help strengthen mouth control especially when a child drools. Function devices can also be employed to assist a child feed self, independently. Clothing and latrines should be adapted to suit the child's level of ability.
Interventions for speech difficulties depend on the cause. If speech difficulty is as result of muscle weakness, lip, tongue and mouth exercises can be performed. If due to cognitive dysfunction, activities that build concentration, attention, imitation and play may be helpful. If permanently impaired, augmentative and alternative communication skills such as sign language gestures and use of pictures are suggested.
Nutritional rehabilitation should continue at home beyond the duration of the Ready Therapeutic Feeds for severe malnutrition. Patients should be provided with an adequate and balanced diet. The same food which other family members eat may be sufficient but because of motor difficulties, patients tend to eat slowly and therefore supervision may be necessary. In addition, if there are problems with mouth and jaw control, the food should be smashed to ensure intake. During each clinic follow up, weights and heights should be taken to monitor progress and to identify patients who may need additional interventions.
Reproductive Health Services
Important reproductive health issues in Nodding Syndrome include prevention of sexual abuse, prevention and treatment of sexually transmitted infections (STI)/HIV/AIDS; safe motherhood and community based sexual reproductive health programmes. In addition, there is the question of maternal nutrition and antiepileptic drug use prior to and during pregnancy and breastfeeding.
a). Sexual Abuse
Because of impaired cognitive function, patients with Nodding Syndrome are prone to sexual violence. The risk is increased among those with wandering behaviour. It should be kept in mind that the perpetrator may be a family member. The healthcare provider should be able to routinely screen for sexual abuse and provide for appropriate care to victims. This should include counseling (including trauma counseling to victims and their families), psychosocial support, a HIV test and a pregnancy test if eligible and provision of post exposure HIV prophylaxis according to national guidelines. Document all cases of abuse by carefully recording the history, clinical exam and collecting forensic evidence and refer for legal services. In addition, the health worker should screen for and treat STI.
b). Safe motherhood
Safe motherhood is a concept that no woman, fetus or baby should die or be harmed by pregnancy or birth. Clinical care and services needed by the mother (antenatal, antepartum, postpartum and emergency obstetric care) and her baby should be according to routine standard of care. However, many patients with Nodding Syndrome have malnutrition and may benefit from supplemental feeding during both pregnancy and breast feeding. Labour should also be managed according to routine standards of care but the increased risk of seizures and status epilepticus in a mother in a stressful condition should be prepared for. Delivery should preferably be in a centre that can offer intravenous anticonvulsants and equipped for comprehensive emergency obstetric care (i.e. able to offer emergency caesarean section. In addition, the possibility of malformations in a baby exposed to anticonvulsants should be born in mind.
c). Antiepileptic drug use
Girls of reproductive age should be given sodium valproate with caution and were possible use drugs like lamotrigine or levetiracetam. A discussion on contraception with the family is advisable. As in all pregnancies, daily administration of folic acid is recommended during pregnancy including for those on anticonvulsant drugs. The risk of seizures may be higher during pregnancy. Partly because of physiological changes associated with pregnancy such as an increased body fluid, serum levels of anticonvulsant drugs may fall to sub-therapeutic levels. It is therefore even more important to counsel and ensure adherence. Adjust the anticonvulsant doses as required. Where possible, determining the serum levels of the anticonvulsant drugs may be useful. Because of the possible teratogenic effects of sodium valproate, patients at risk of pregnancy or intending to get pregnant should be offered second line anticonvulsants instead27. In those conceiving while on sodium valproate, check maternal serum levels of alpha fetoprotein by twelve weeks of gestation and perform ultrasonography to screen for possible congenital abnormalities and change to second line anticonvulsants.
d. Community based programmes
The health provider should be able to empower parents and the community to prevent sexual abuse and work with them to deliver reproductive health services to the patients. A programme should be put in place to prevent sexual abuse, physical violence and neglect, identify and help patient's access services. Such services may include community education by Village Health Teams or through health worker outreaches, the media, IEC materials, and by working with stake holders including the child protection services.
Follow up care
Follow up evaluation is carried out in patients who have had a full evaluation at the initial assessment and should involve the patient's family. The aim is to document any changes in the condition under treatment whether improving or worsening. At each follow up visit, patients should be assessed for seizure control, medication side-effects, nutrition, disability, psychological and social issues, and other common childhood problems such as infections and injuries. Follow up visits may initially be conducted every 2 weeks until there is seizure control and later, monthly. The initial management plan should be revised according to findings on the visit. With clinical improvement, follow up visit intervals may be increased further.
Surveillance and documentation
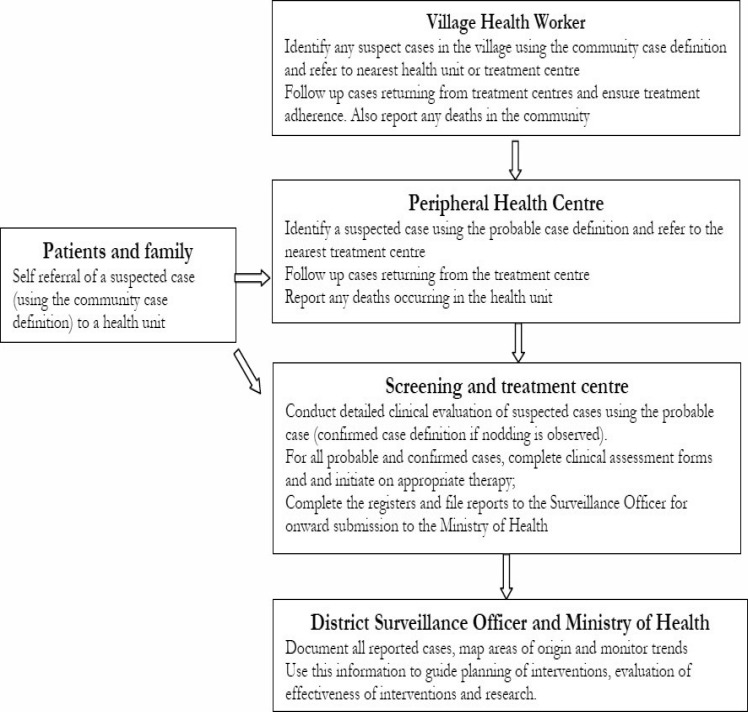
Healthcare workers should systematically document all cases of Nodding Syndrome to help characterise the epidemiology and monitor effectiveness of interventions using the Integrated Disease Surveillance and Response system45. This is a strategy of all surveillance activities and includes case detection, investigation, confirmation reporting, analysis and interpretation and Public Health Action. It spans from the community to the formal health care system. At community level, Village Health teams (VHTs) identify suspected cases using a simplified “community case definition” based on WHO August 2012 criteria for confirmed, probable and suspect cases of Nodding Syndrome and report these to the nearest health facility. The VHTs also help followup cases that have returned from the health facilities. Healthcare workers at peripheral health facilities use “suspected case definition” to detect/identify cases. The suspected cases are referred to designated screening and treatment centres where detailed clinical evaluation is carried out by trained workers using a “probable case definition” and case investigation form, figure 2. Such systematic epidemiologic and clinical data collection and analysis will facilitate better planning to improve service provision and guide development of hypothesis driven research.
Figure 2.
Surveillance for Nodding Syndrome
Conclusions
This is an evolving document and clearly revisions will in future be needed as more data become available. Already, a section on reproductive health was added during the first revision14 and more may come. To date, a total of 162 health workers have been trained on these guidelines in two sittings. These health workers are now deployed and offering care in the Nodding Syndrome screening and treatment centres in the country. Support supervision reports suggest that the medical and surveillance components of the management guidelines are being implemented confidently but there are still some difficulties with rehabilitation services partly because of limited such personnel. Because Nodding Syndrome is the subject of intensive research, cooperation and collaborations with other biomedical scientists in carrying out research is strongly encouraged.
Table 2.
Advantages, disadvantages and side effects of suggested antiepileptic drugs for nodding syndrome
| Antiepileptic | Oral Dosing | Advantage | Disadvantage | Main side effects |
| Phenobarbitone | Usual dose in children 3–5mg/kg/day Adolescents >12 yrs: 1–3 mg/kg/day in 1–2 divided doses. |
Cheap and easily available even in rural areas Anecdotal reports of some success with tonic clonic seizures. |
Central nervous system effects and behaviour difficulties in children There may be no beneficial effect on head nodding. |
Skin rash. Drowsiness, lethargy, unsteadiness, unpaired memory or cognition. Behaviour difficulties |
| Phenytoin | May require a loading dose Usual dose 4–8 mg/kg/day; max.– Daily dose: 300mg/day. |
Cheap and easily available even in rural areas |
A wide range of side effects. There may be no benefit with seizures in nodding syndrome. |
Skin rash. Nausea, vomiting, constipation, drowsiness, insomnia, nervousness, tremor, pins and needles, dizziness, headache, anorexia, gum problems, acne, excess hair growth, and coarse facial features |
| Carbamazepine | Usual dose 10–20mg/kg/day Start at 5 mg/kg/day Dinded 2 or 3 times daily Increase dose every week until optimal response is achieved; maximum recommended dose: 35 mg/kg/day. Beyond 12 years refer to adult dosing |
Available in district and regional hospitals in the affected areas. Also a mood stabiliser. Not recommended |
Risk of precipitating myoclonic seizures |
Skin rash. Dry mouth, nausea, vomiting, fluid retention, unsteadiness, dizziness, drowsiness, fatigue, and headache, low sodium in the blood blood disorders, dermatitis, and hives. |
| Sodium valpoate | Start with 10mg/kg/day in two divided doses Increase by 5–10mg/kg/day weekly doses in 2–3 doses until therapeutic levels are achieved or up to 40mg/kg/day. |
Suggested first line anti epileptic drug Broad spectrum AED Mood stabilizer |
Costly. Carries a higher risk than other AEDs of causing fetal malformations if taken during pregnancy. Pre-conceptual counselling in young girls is time consuming |
Hair loss, nausea, stomach upset, diarrhoea, and weight gain and reduced platelets in the blood. Polycystic ovaries and menstrual problems. |
| Lamotrigine |
Monotherapy in children <12 years Start with 0.3mg/kg/day and increase doses eyery 1–2 weeks. Titrate the dose up very slowly. Usual maintenance dose 4.5–7.5mg/kg/day Children> 12 years Weeks 1–2–25 mg/day Week 3–4 25mg twice daily. Increase by 50mg/day weekly doses |
Broad spectrum Alternative to sodium valpoate Mood stabilizer |
Costly. Not readily available in affected areas May cause severe skin rash and reactions Has to be introduced and titrated upwards very slowly |
Severer skin rash and hypersensitivity. Nausea, vomiting, diarrhoea, dry mouth, aggression, agitation, headache, drowsiness, dizziness, tremor, difficulty sleeping, unsteadiness, back pain, joint pain, eye movements, double vision, and blurred vision |
| Levetiracetam | Initial dose: 20 mg/kg/day in two divided doses; Increase dosage every 2 weeks by 20 mg/kg/day in two divided doses with seizure control up to 60 mg/kg/day in two divided doses. |
Alternative to sodium valpoate | Costly Not readily available in affected areas |
Anorexia, weight changes, abdominal pain, nausea, vomiting, diarrhoea, drowsiness, unsteadiness, Dizziness, headache, tremor, amnesia, aggression, agitation, depression, anxiety, and double or blurred vision. |
| Clonazepam | Initial dose: 0.01–0.03 mg/kg/day; Increase bv no more than a total 0.5 mg every third day until seizure control or adverse effects. Usual maintenance dose: 0.1–0.2 mg/kg/day. |
May be used as add on therapy with sodium valproate if poor seizure control. Short term Clobazam also suggested. |
Costly Not readily available |
Drowsiness, lethargy, unsteadiness, impaired memory or cognition. Tolerance |
Acknowledgements
The preparation of these guidelines was supported by the Ugandan Government through the Ministry of Health. Part of Dr Idro's research activities in Nodding Syndrome have been supported by the Waterloo foundation, UK. We thank Godfrey Basoita and Peace Ahabwe for their input into the components on rehabilitation.
References
- 1.Korevaar DA, Visser BJ. Reviewing the evidence on nodding syndrome, a mysterious tropical disorder. Int J Infect Dis. 2013;17(3):1149–1152. doi: 10.1016/j.ijid.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Jilek LA. [Mental Diseases and Epilepsy in Tropical Africa] Fortschr Neurol Psychiatr Grenzgeb. 1964;32:213–259. [PubMed] [Google Scholar]
- 3.Goudsmit J, van der Waals FW. Endemic epilepsy in an isolated region of Liberia. Lancet. 1983;1:528–529. doi: 10.1016/s0140-6736(83)92215-8. [DOI] [PubMed] [Google Scholar]
- 4.Lacey M. Nodding disease: mystery of southern Sudan. Lancet Neurol. 2003;2:714. doi: 10.1016/s1474-4422(03)00599-4. [DOI] [PubMed] [Google Scholar]
- 5.Blednov YA, Benavidez JM, Homanics GE, Harris RA. Behavioral characterization of knockin mice with mutations M287L and Q266I in the glycine receptor alpha1 subunit. J Pharmacol Exp Ther. 2012;340:317–329. doi: 10.1124/jpet.111.185124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaiser C, Pion S, Boussinesq M. Head nodding syndrome and river blindness: a parasitologic perspective. Epilepsia. 2009;50:2325–2326. doi: 10.1111/j.1528-1167.2009.02280.x. [DOI] [PubMed] [Google Scholar]
- 7.Wasswa H. Ugandan authorities deal with a mysterious ailment that leaves people nodding continuously. BMJ. 2012;344:e349. doi: 10.1136/bmj.e349. [DOI] [PubMed] [Google Scholar]
- 8.Jelliffe DB, Jones PR, Stroud CE. Nakalanga notes on the endemic dwarfism of Uganda. Trop Geogr Med. 1962;14:97–104. [PubMed] [Google Scholar]
- 9.Kipp W, Burnham G, Bamuhiiga J, Leichsenring M. The Nakalanga syndrome in Kabarole District, Western Uganda. Am J Trop Med Hyg. 1996;54:80–83. doi: 10.4269/ajtmh.1996.54.80. [DOI] [PubMed] [Google Scholar]
- 10.Leonard PJ, Stanfield JP. Some biochemical observations in the Nakalanga. East Afr Med J. 1965;42:692–694. [PubMed] [Google Scholar]
- 11.Marshall AJ, Cherry JK. Endocrine dysfunction in a Nakalanga dwarf. Trans R Soc Trop Med Hyg. 1961;55:188–191. doi: 10.1016/0035-9203(61)90024-4. [DOI] [PubMed] [Google Scholar]
- 12.Sejvar JJ, Kakooza AM, Foltz JL, Makumbi I, Atai-Omoruto AD, Malimbo M, Ndyomugyenyi R, Alexander LN, Abang B, Downing RG, et al. Clinical, neurological, and electrophysiological features of nodding syndrome in Kitgum, Uganda: an observational case series. Lancet Neurol. 2013;12:166–174. doi: 10.1016/S1474-4422(12)70321-6. [DOI] [PubMed] [Google Scholar]
- 13.WHO, author. International Scientific Meeting on Nodding Syndrome. 2012. pp. 1–42. 1–42. [Google Scholar]
- 14.Ministry of Health U, author. Nodding Syndrome; Health Workers Training Manual. Draft Edition; February 2012 edition. Kampala: Ministry of Health; 2012. pp. 1–107. 1–107. [Google Scholar]
- 15.Winkler AS, Friedrich K, Meindl M, Kidunda A, Nassri A, Jilek-Aall L, Matuja W, Schmutzhard E. Clinical characteristics of people with head nodding in southern Tanzania. Trop Doct. 2010;40:173–175. doi: 10.1258/td.2010.090373. [DOI] [PubMed] [Google Scholar]
- 16.Winkler AS, Friedrich K, Konig R, Meindl M, Helbok R, Unterberger I, Gotwald T, Dharsee J, Velicheti S, Kidunda A, et al. The head nodding syndrome-clinical classification and possible causes. Epilepsia. 2008;49: 2008–2015. doi: 10.1111/j.1528-1167.2008.01671.x. [DOI] [PubMed] [Google Scholar]
- 17.Nyungura JL, Akim T, Lako A, Gordon A, Lejeng L, Gibson W. Investigation into the Nodding syndrome in Witto Payam, Western Equatoria State, 2010. Southern Sudan Medical Journal. 2011;4:3–6. [Google Scholar]
- 18.Donnelly J. CDC planning trial for mysterious nodding syndrome. Lancet. 2012;379:299. doi: 10.1016/s0140-6736(12)60126-3. [DOI] [PubMed] [Google Scholar]
- 19.Uganda: Nodding disease (Situation as of 14 February, 2012) [ http://www.afro.who.int/en/clusters-a-programmes/dpc/epidemic-apandemic-alert-and-response/outbreak-news/3548-uganda-nodding-disease-situation-as-of-14-february-2012.html]
- 20.Ministry of Health U, author. Integrated Management of Acute Malnutrition Guidelines. Kampala: 2010. pp. 1–137. 1–137. [Google Scholar]
- 21.Ministry of Health U, author. Handbook for managers and Health workers Uganda. 2002. Management of the child with severe illness or severe malnutrition:Guidelines for referral facility quality of care improvement. [Google Scholar]
- 22.Guerrini R, Belmonte A, Genton P. Antiepileptic drug-induced worsening of seizures in children. Epilepsia. 1998;39(Suppl 3):S2–10. doi: 10.1111/j.1528-1157.1998.tb05118.x. [DOI] [PubMed] [Google Scholar]
- 23.Guerrini R. Valproate as a mainstay of therapy for pediatric epilepsy. Paediatr Drugs. 2006;8:113–129. doi: 10.2165/00148581-200608020-00004. [DOI] [PubMed] [Google Scholar]
- 24.Thilothammal N, Banu K, Ratnam RS. Comparison of phenobarbitone, phenytoin with sodium valproate: randomized, double-blind study. Indian Pediatr. 1996;33:549–555. [PubMed] [Google Scholar]
- 25.Saxena K, Mora L, Torres E, Hall R, Delizonna L, Torres A, Steiner H. Divalproex sodium-ER in outpatients with disruptive behavior disorders: a three month open label study. Child Psychiatry Hum Dev. 2010;41:274–284. doi: 10.1007/s10578-009-0167-4. [DOI] [PubMed] [Google Scholar]
- 26.Lindenmayer JP, Kotsaftis A. Use of sodium valproate in violent and aggressive behaviors: a critical review. J Clin Psychiatry. 2000;61:123–128. doi: 10.4088/jcp.v61n0207. [DOI] [PubMed] [Google Scholar]
- 27.Vajda FJ, O'Brien T J, Hitchcock A, Graham J, Cook M, Lander C, et al. Critical relationship between sodium valproate dose and human teratogenicity: results of the Australian register of anti-epileptic drugs in pregnancy. J Clin Neurosci. 2004;11:854–858. doi: 10.1016/j.jocn.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 28.De Los Reyes EC, Sharp GB, Williams JP, Hale SE. Levetiracetam in the treatment of Lennox- Gastaut syndrome. Pediatr Neurol. 2004;30:254–256. doi: 10.1016/j.pediatrneurol.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 29.McKee JR, Sunder TR, Vuong A, Hammer AE. Adjunctive lamotrigine for refractory epilepsy in adolescents with mental retardation. J Child Neurol. 2006;21:372–379. doi: 10.1177/08830738060210051401. [DOI] [PubMed] [Google Scholar]
- 30.Carreno M. Levetiracetam. Drugs Today (Barc) 2007;43:769–794. doi: 10.1358/dot.2007.43.11.1136902. [DOI] [PubMed] [Google Scholar]
- 31.Perucca E. Pharmacological and therapeutic properties of valproate: a summary after 35 years of clinical experience. CNS Drugs. 2002;16:695–714. doi: 10.2165/00023210-200216100-00004. [DOI] [PubMed] [Google Scholar]
- 32.Koch S, Jager-Roman E, Losche G, Nau H, Rating D, Helge H. Antiepileptic drug treatment in pregnancy: drug side effects in the neonate and neurological outcome. Acta Paediatr. 1996;85:739–746. doi: 10.1111/j.1651-2227.1996.tb14137.x. [DOI] [PubMed] [Google Scholar]
- 33.Callenbach PM, Arts WF, ten Houten R, Augustijn P, Gunning WB, Peeters EA, et al. Addon levetiracetam in children and adolescents with refractory epilepsy: results of an open-label multicentre study. Eur J Paediatr Neurol. 2008;12:321–327. doi: 10.1016/j.ejpn.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Network SIG, author. Diagnosis and management of epilepsies in children and young people. Edinburgh: Royal College of Physicians; 2005. [Google Scholar]
- 35.Agrawal N, Govender S. Epilepsy and neuropsychiatric comorbidities. Advances in Psychiatric Treatment. 2011;17:44–53. [Google Scholar]
- 36.Idro R, Opoka R O, Aanyu H T, Kakooza-Mwesige A, Piloya-Were T, Namusoke H, Tumwine JK. Nodding syndrome in Ugandan children—clinical features, brain imaging and complications: a case series. BMJ open. 2013;3(5):e002540. doi: 10.1136/bmjopen-2012-002540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Musisi S, Nakimuli-Mpungu E, Akena D, Bangirana P, Kinyanda E. Psychological Manifestations of Nodding Syndrome in Northern Uganda: A Case report. African Journal of Traumatic Stress. 2011;2(1):56–57. [Google Scholar]
- 38.Lodhi S, Agrawal N. Neurocognitive problems in epilepsy. Advances in Psychiatric Treatment. 2012;18:232–240. doi: 10.1192/aptbp110007930. [DOI] [Google Scholar]
- 39.Traore S, Wilson MD, Sima A, Barro T, Diallo A, Ake A, et al. The elimination of the onchocerciasis vector from the island of Bioko as a result of larviciding by the WHO African Programme for Onchocerciasis Control. Acta Trop. 2009;111:211–218. doi: 10.1016/j.actatropica.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 40.Garms R, Lakwo TL, Ndyomugyenyi R, Kipp W, Rubaale T, Tukesiga E, et al. The elimination of the vector Simulium neavei from the Itwara onchocerciasis focus in Uganda by ground larviciding. Acta Trop. 2009;111:203–210. doi: 10.1016/j.actatropica.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 41.Stephenson LS, Latham MC, Kinoti SN, Kurz KM, Brigham H. Improvements in physical fitness of Kenyan schoolboys infected with hookworm, Trichuris trichiura and Ascaris lumbricoides following a single dose of albendazole. Trans R Soc Trop Med Hyg. 1990;84:277–282. doi: 10.1016/0035-9203(90)90286-n. [DOI] [PubMed] [Google Scholar]
- 42.Beckung E, Hagberg G. Correlation between ICIDH handicap code and Gross Motor Function Classification System in children with cerebral palsy. Dev Med Child Neurol. 2000;42:669–673. doi: 10.1017/s0012162200001237. [DOI] [PubMed] [Google Scholar]
- 43.Wood E, Rosenbaum P. The gross motor function classification system for cerebral palsy: a study of reliability and stability over time. Dev Med Child Neurol. 2000;42:292–296. doi: 10.1017/s0012162200000529. [DOI] [PubMed] [Google Scholar]
- 44.Bakheit AM, Bower E, Cosgrove A, Fox M, Morton R, Phillips S, et al. Opinion statement on the minimal acceptable standards of healthcare in cerebral palsy. Disabil Rehabil. 2001;23:578–582. doi: 10.1080/09638280010029912. [DOI] [PubMed] [Google Scholar]
- 45.Lukwago L, Nanyunja M, Ndayimirije N, Wamala J, Malimbo M, et al. The implementation of Integrated Disease Surveillance and Response in Uganda: a review of progress and challenges between 2001 and 2007. Health Policy Plan. 2012 doi: 10.1093/heapol/czs022. [DOI] [PMC free article] [PubMed] [Google Scholar]