Abstract
The mechanisms governing neuron death following NGF deprivation are incompletely understood. Here, we show that Trib3, a protein induced by NGF withdrawal, has a key role in such death via a loop involving the survival kinase Akt and FoxO transcription factors. Trib3 overexpression is sufficient to induce neuron death, and silencing of endogenous Trib3 strongly protects from death when NGF is withdrawn. Mechanism studies reveal that Trib3 interferes with phosphorylation/activity of Akt and contributes to Akt inactivation after NGF deprivation. FoxO1a, a direct Akt substrate, is dephosphorylated upon NGF withdrawal and consequently undergoes nuclear translocation and activates pro-apoptotic genes. We find that Trib3 is required for FoxO1a dephosphorylation and nuclear translocation after NGF deprivation. Conversely, Trib3 induction requires FoxO transcription factors, which show enhanced occupancy of the Trib3 promoter region following NGF withdrawal. Collectively, these findings support a mechanism in which NGF deprivation, Akt dephosphorylation/inactivation, FoxO dephosphorylation/activation and Trib3 induction are linked in a self-amplifying feed-forward loop that culminates in neuron death.
Keywords: NGF, apoptosis, Akt, Trib3, FoxO
During vertebrate development about half of neurons die due to competition for limiting supplies of target-derived trophic factors such as nerve growth factor (NGF), but the molecular mechanisms governing such death are incompletely known. Insight about these mechanisms is important in understanding how the nervous system forms and because these processes may pertain to pathological neuron degeneration. NGF-dependent sympathetic neurons are among the best characterized systems for unraveling neuron survival and death regulation by trophic factors. NGF binding to tropomyosin receptor kinase (Trk) receptors activates the phosphoinositide-3 kinase (PI3K)/Akt signaling pathway that is indispensable for maintaining the balance between neuron life and death.1, 2 When Akt is activated by NGF-stimulated phosphorylation at serine 473 (Ser473) and threonine 308 (Thr308), it phosphorylates multiple targets to promote survival.3, 4, 5 Conversely, Akt phosphorylation and activity fall without NGF, resulting in apoptotic neuron death.
Members of the forkhead box, class O (FoxO) transcription factor family are Akt targets that regulate survival and death.4, 5 Active Akt phosphorylates FoxOs, causing their cytoplasmic retention while loss of Akt activity results in dephosphorylation of FoxO proteins and their consequent nuclear translocation where they activate pro-apoptotic genes, such as Bim.6, 7 In addition to FoxOs, several other transcription factors and their associated pathways contribute to neuron death caused by NGF deprivation. These include the Jun N-terminal kinase (JNK)/c-Jun pathway, the apoptotic cell-cycle pathway, NF-Y and p53/p63/p73.8, 9, 10, 11, 12 In the case of Bim, at least four factors (c-Jun, FoxO, cell-cycle-associated B- and C-Myb and NF-Y) bind the Bim promoter after NGF deprivation and knockdown of any one suppresses Bim induction and death.6, 7, 10
Just as neuron death requires activation of multiple transcription factors, it also involves induction of multiple transcriptional targets. Besides Bim, the pro-apoptotic BH-3 domain proteins Hrk/DP5 , PUMA (p53-upregulated modulator of apoptosis) and the prolyl hydroxylase EglN3/SM20 are induced by NGF deprivation and contribute to death.13, 14, 15 Although loss or knockdown of each of these delays neuron death after NGF deprivation, none appears absolutely indispensable. This suggests that additional transcriptional targets are required for death. Trib3 (neuronal death-inducible putative kinase/Sink1/Skip3) is one such transcriptional target that is upregulated in NGF-deprived PC12 cells and sympathetic neurons.16, 17 However, it is unknown whether Trib3 has a role in death caused by NGF deprivation. Trib3 is a mammalian ortholog of the Drosophila tribbles gene.18, 19 In addition to NGF deprivation, Trib3 mRNA is upregulated by stresses, including hypoxia, ER stress and 6-hydroxydopamine treatment.20, 21, 22, 23 The range of functions attributed to Trib3 include regulating glucose homeostasis, promoting tumor cell migration, suppressing adipocyte differentiation, inhibiting ER stress and either promoting or inhibiting cell death.24, 25, 26 Trib3 is a pseudokinase with an inactive catalytic domain.17, 27 It possesses a protein binding site, the Trb domain, and has numerous binding partners depending upon cell type and conditions.24, 27, 28, 29, 30 Of particular relevance, Trib3 binds Akt and inhibits its phosphorylation at both Ser473 and Thr308.31, 32
Here, we examine the role of Trib3 in apoptosis induced by NGF deprivation. We find that Trib3 is required for neuron death in this paradigm and that it functions to inhibit Akt activity and consequently to promote FoxO dephosphorylation and nuclear translocation. We further report that Trib3 induction upon NGF deprivation requires FoxO proteins. These observaitons suggest that NGF deprivation triggers a feed-forward, self-amplifying loop involving Akt, FoxOs and Trib3 that culminates in neuron death. Trib3 thus appears to promote efficient neuron death by amplifying the loss of Akt signaling and the increase in FoxO nuclear translocation that occurs after NGF deprivation.
Results
NGF deprivation induces Trib3 mRNA and protein
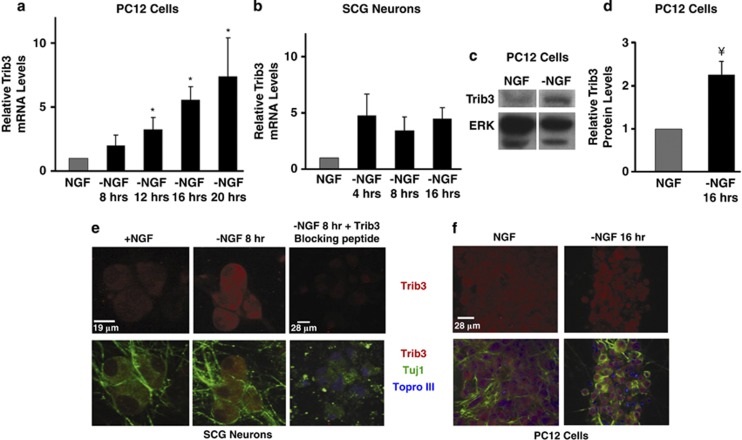
Trib3 is upregulated in NGF-deprived superior cervical ganglion (SCG) neurons and PC12 cells.16, 17 We confirmed this and found Trib3 mRNA upregulation by approximately 3–5-fold before the first evident signs of cell death caused by NGF deprivation (Figures 1a and b). In SCG neuron cultures, Trib3 mRNA was maximally induced by 4 h after NGF removal. Furthermore, 16 h of NGF deprivation yielded an approximate doubling of Trib3 protein levels in PC12 cell cultures (Figures 1c and d). Immunostaining of SCG and PC12 cell cultures also indicated increased Trib3 protein expression after NGF withdrawal. Expression was mainly cytoplasmic with some apparent nuclear staining (Figures 1e and f).
Figure 1.
Trib3 is induced in response to NGF deprivation. (a and b) Neuronal PC12 cells (a) and SCG neurons (b) were deprived of NGF for the indicated times. Total mRNA was isolated and subjected to reverse transcription followed by quantitative PCR using Trib3 primers. Rat GAPDH (glyceraldehyde 3-phosphate dehydrogenase) and α-tubulin mRNA levels were used to normalize input cDNA. The data are reported as relative increase in mRNA levels normalized to NGF control and represent mean±S.E. of three experiments for PC12 cells and±range of two experiments for SCG neurons. (c and d) Neuronal PC12 cells were deprived of NGF for 17 h. Whole-cell lysates were subjected to SDS-PAGE and western immunoblotted with anti-Trib3 and anti-ERK antisera. (c) Shows non-adjacent lanes from the same blot. (d) Shows quantification of Trib3 signal normalized to ERK signal. Values represent mean±S.E. for four independent experiments. (e and f) Immunocytochemistry of SCG neuron (e) and neuronal PC12 cell (f) cultures showing Trib3 protein upregulation after NGF deprivation. Note the absence of signal in the presence of a Trib3-blocking peptide. *P<0.05; ¥P<0.005 versus no NGF withdrawal (Student's t-test). Scale bar for SCG neurons=19 μm and for PC12 cells=28 μm
Trib3 overexpression is sufficient for neuron death in the presence of NGF
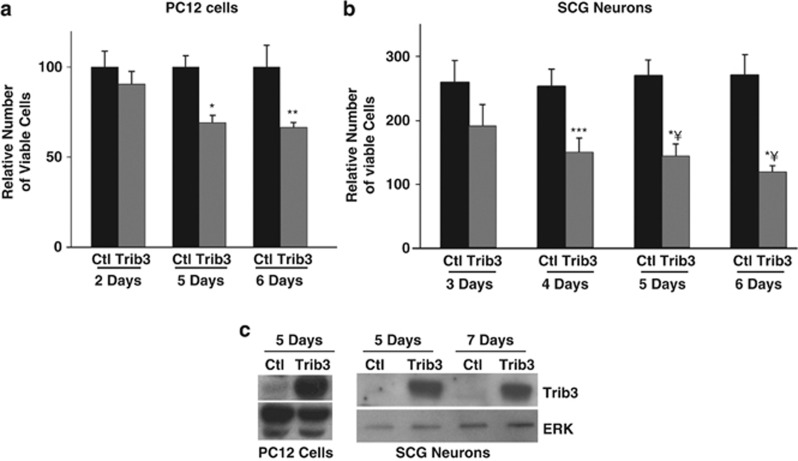
Although Trib3 is pro-apoptotic in several systems,20, 33 it was unclear whether it induces death of NGF-responsive neurons. Lentivirally induced Trib3 overexpression in PC12 cells and SCG neurons (Figure 2c) caused a steady decline in viability even in the presence of NGF (Figures 2a and b). This was not a non-specific effect, because comparable Trib3 overexpression in cultured cortical neurons did not affect viability (data not shown).
Figure 2.
Trib3 overexpression is sufficient to promote neuronal apoptosis. (a and b) Replicate cultures of neuronal PC12 cells (a) and SCG neurons (b) were infected with lentivirus expressing either Trib3+eGFP (enhanced green fluorescent protein) or eGFP alone and maintained with NGF. Cell counts were determined at various time points as described in Materials and Methods. Error bars represent mean±S.E. of three experiments for PC12 cells (a) and four experiments for SCG neurons (b) each carried out in triplicate. *P<0.005; **P<0.05; ***P<0.01; *¥P<0.0005; versus time-matched control (GFP alone) (Student's t-test). (c) Cell lysates were also prepared and subjected to immunoblotting using antibody against Trib3
Trib3 mediates neuron death caused by NGF deprivation
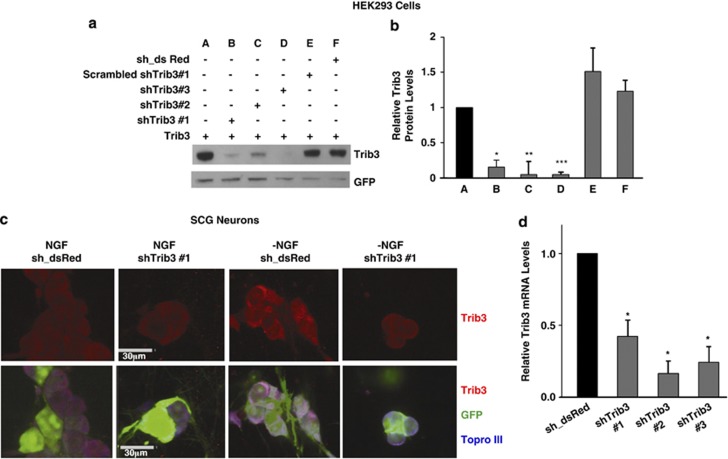
Although ours and previous work show Trib3 elevation after NGF removal, it was unknown whether Trib3 contributes to death in this situation. We therefore generated independent shRNA constructs targeting rat Trib3 and expressed them by either transfection or lentiviruses. The lentiviral constructs also expressed eGFP, and this indicated essentially complete infection of neuronal PC12 cells and SCG neurons (Figure 3c and data not shown). The shRNA constructs efficiently knocked down overexpressed rat Trib3 in HEK293 cells (Figures 3a and b) and reduced endogenous Trib3 protein expression (as judged by immunostaining) in NGF-deprived sympathetic neurons (Figure 3c) and Trib3 mRNA levels in NGF-treated SCG cultures (Figure 3d).
Figure 3.
shTrib3 constructs knock down both exogenous and endogenous Trib3. (a) HEK293 cells were co-infected with lentiviruses expressing Trib3 and shTrib3 or control shRNA constructs, and whole-cell lysates were prepared 3 days later and subjected to SDS-PAGE and immunoblotting for Trib3 or GFP (green fluorescent protein). (b) Quantification of relative Trib3 protein expression under conditions described in panel (a). Values are normalized to GFP expression to correct for infection efficiency and are expressed as means±S.E. of three experiments. Differences from Trib3 expressing samples without Trib3 shRNA: *P<0.005; **P<0.05; ***P<0.0005 (Student's t-test). (c) SCG neuronal cultures were infected with either control or shTrib3#1 expressing lentivirus for 3 days followed by 8 h of continued NGF treatment or NGF deprivation, all as indicated. Neurons were fixed and immunostained using antisera against Trib3. Bar=30 μm. (d) SCG cultures were infected with either control (sh_dsRed) or three different shTrib3 expressing lentiviruses for 3 days in the presence of NGF. Total mRNA was isolated and subjected to reverse transcription followed by quantitative PCR using Trib3 primers. Rat α-tubulin mRNA levels were used to normalize input cDNA. The data are reported as relative increase in mRNA levels normalized to NGF control and represent mean±S.E. of three experiments. *P<0.005 (Student's t-test) compared with control virus-infected neurons
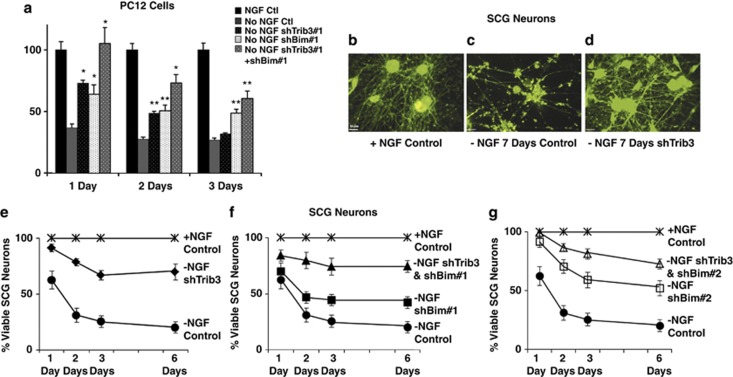
Initial neuronal PC12 cell experiments indicated that Trib3 knockdown partially protects from NGF deprivation, with 1.9-fold more cells surviving after 24 h (Figure 4a). Extension to sympathetic neurons revealed that Trib3 knockdown protects both cell bodies and neurites for at least 1 week following NGF withdrawal (Figures 4 b–d). Quantification confirmed the long-term protective effect of Trib3 knockdown (Figure 4e; similar results were achieved with shTrib3#1 and shTrib3#2, and data were therefore pooled). An average of about 65% of Trib3-depleted neurons survived 6 days after NGF deprivation versus about 25% of NGF-deprived control neurons (Figure 4e).
Figure 4.
Trib3 is required for neuronal apoptosis caused by NGF withdrawal. (a) Neuronal PC12 cells were transfected with either control shRNA, shTrib3, shBim or shTrib3+shBim constructs in the pSIREN vector and NGF was either retained or removed from the medium 48 h post transfection. Viable, transfected (GFP+) cells were counted at indicated times after NGF withdrawal. Values represent means±S.E. (four independent experiments, each in triplicate or in sets of six) and are expressed relative to cell number with NGF and control shRNA construct, *P<0.005 and **P<0.05 compared with no NGF control (Student's t-test). (b–d) Confocal images of SCG neurons infected with either control or shTrib3 lentivirus for 3 days, followed by NGF deprivation for 7 days. Note the presence of many neurites in NGF-deprived cultures infected with shTrib3. Bar=16 μm. (e–g) SCG neurons were infected with the indicated lentiviruses, including two different shRNA constructs against Trib3 and Bim, alone and in combination for 3 days before NGF deprivation. Counts of surviving neurons were performed at the indicated times, and values are given as means±S.E. (4–10 independent experiments performed in triplicate) relative to the neuron number in cultures with NGF and control shRNA on each day. Panel (e) shows pooled data for shTrib3#1 and #2 without NGF compared with control with or without NGF; (f) and (g) shows effects of shBim#1 and #2, respectively, with and without combination with shTrib3 in the absence of NGF
Bim is induced by NGF deprivation and its deletion or silencing partially protects from NGF deprivation.34, 35 We therefore compared effects of Bim and Trib3 knockdown, either separately or together. We used a previously described siRNA (expressed from a lentivirally expressed shRNA; shBim#1) that effectively silences endogenous rat Bim,12 as well as a second independent lentivirally delivered shRNA that reduces endogenous rat Bim expression by 75–80% (shBim#2; data not shown). Silencing both Bim and Trib3 led to a modest increase in protection (Figures 4a, f and g). These data indicate that Trib3 partially mediates death evoked by NGF deprivation and that Trib3 and Bim may participate along the same apoptotic pathway.
Trib3 regulates neuronal Akt phosphorylation
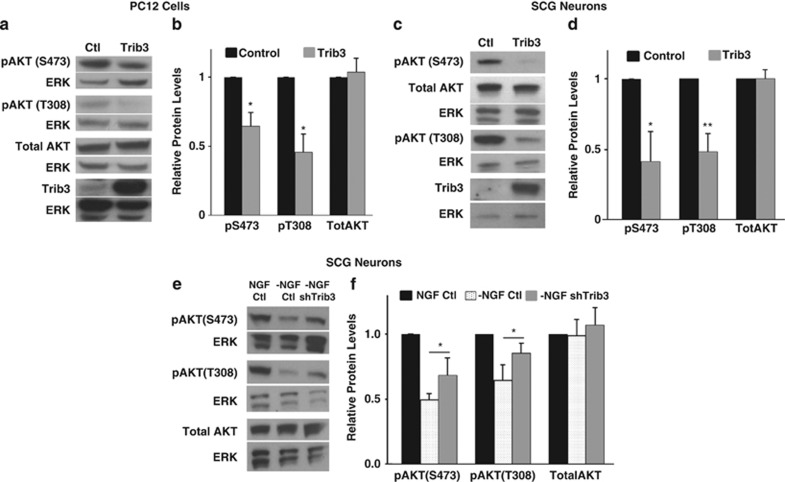
Trib3 binds Akt and blocks its phosphorylation, and therefore activity, in various non-neuronal cells.31, 32, 36 In PC12 cells, Trib3 knockdown partially reduced tunicamycin-induced dephosphorylation of Akt at Ser473.37 Active/phosphorylated Akt is an important regulator of neuron survival; NGF promotes Akt phosphorylation and NGF deprivation causes Akt dephosphorylation, which in turn contributes to death.1, 2 We therefore explored whether neuronal Akt phosphorylation is affected by Trib3. PC12 cells and sympathetic neurons were infected with Trib3-expressing lentivirus in the presence of NGF and monitored 5 days later for Akt phosphorylation at Ser473 and Thr308, sites required for full Akt activity.3 In each case, Akt phosphorylation decreased by about 40–60%, with no significant change in total Akt expression (Figures 5a–d). This loss of Akt phosphorylation/activity correlates with the observation that Trib3 overexpression causes death in both culture systems (Figure 2). We next asked whether Trib3 contributes to the loss of Akt phosphorylation occurring with NGF withdrawal. Sympathetic neurons were infected with lentivirus expressing control or shTrib3, deprived of NGF and assessed 16 h later for Akt phosphorylation at Ser473 and Thr308. The decrease in Akt phosphorylation at both sites that occurs with NGF withdrawal was significantly reversed by Trib3 knockdown (Figures 5e and f). There was no significant effect of Trib3 knockdown on total Akt (Figures 5e and f) or on phospho-Akt in the presence of NGF (data not shown). These findings support the idea that NGF deprivation elevates Trib3 expression and that Trib3 reduces Akt phosphorylation/activity and in this way contributes to neuron death.
Figure 5.
Trib3 regulates neuronal Akt phosphorylation. (a–d) Trib3 overexpression is sufficient to reduce Akt phosphorylation in (a and b) neuronal PC12 cells and (c and d) SCG neurons. Cells were infected with either control or Trib3 expressing lentivirus and maintained with NGF. Five days after infection, cell lysates were subjected to SDS-PAGE and immunoblotted using antisera against phospho-Akt (pSer473 and pThr308), Trib3, total Akt and total ERK. Representative immunoblots are shown in panels (a) and (c); graphs (b) and (d) show mean values for protein levels±S.E. for 4–6 independent experiments, in each case normalized to ERK levels. (e and f) Trib3 contributes to the decrease in Akt phosphorylation that occurs after NGF deprivation. SCG neuron cultures were infected with lentivirus expressing shTrib3 or control shRNA. Seventy-two hours post infection, NGF was removed for 16 h, and the neurons were lysed and lysates were subjected to SDS-PAGE and immunoblotted using the antisera mentioned above. Panel (e) shows representative immunoblots and (f) shows mean values for protein levels±S.E. for 5–6 independent experiments, each normalized against total ERK. *P<0.05, **P<0.005; Student's paired t-test
Trib3 regulates phosphorylation of Akt substrate FoxO1a
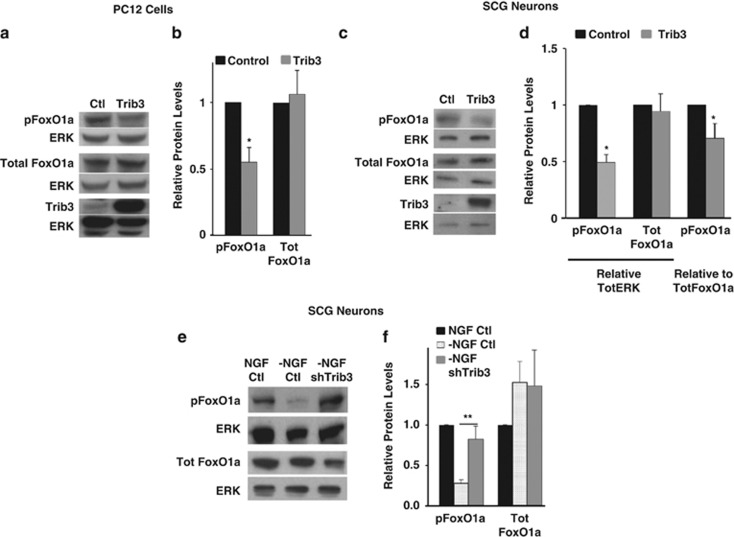
Forkhead family transcription factors are well-described Akt substrates. Akt phosphorylates FoxO1a at Ser256 and Thr24, promoting its interaction with 14-3-3 proteins and cytoplasmic sequestration.4, 5 Akt dephosphorylation/deactivation causes decreased phosphorylation of FoxO1a, its dissociation from 14-3-3 and translocation to nuclei. Nuclear FoxO1a transactivates pro-apoptotic genes such as Bim and by this means contributes to neuron death.7 Because Trib3 interferes with Akt activity, we assessed whether Trib3 also prevents FoxO1a phosphorylation at Ser256. We first examined FoxO1a phosphorylation in NGF-treated PC12 cells and sympathetic neurons infected with lentivirus expressing Trib3. This yielded an approximate 50% loss of FoxO1a phosphorylation in both systems with no significant change in total FoxO1a (Figures 6a–d).
Figure 6.
Trib3 regulates phosphorylation of the Akt substrate FoxO1a. (a–d) Trib3 overexpression is sufficient to reduce phosphorylation of FoxO1a in neuronal cells. (a and b) Neuronal PC12 cells and (c and d) SCG neurons were infected with either control or Trib3 expressing lentivirus and maintained with NGF. Five days after infection, cell lysates were subjected to SDS-PAGE and immunoblotted using antisera against phospho-FoxO1a, total FoxO1a, Trib3 and total ERK. Panels (a) and (c) show representative blots; panels (b) and (d) show mean values for protein levels±S.E. for four independent experiments, in each case normalized to total ERK levels. Panel (d) represents mean values for protein levels normalized to total FoxO1a levels, as well. (e and f) Trib3 contributes to FoxO1a dephosphorylation after NGF deprivation. SCG neurons were infected with lentivirus expressing shTrib3 or control shRNA. Seventy-two hours post infection, NGF was removed for 16 h, and the neurons were lysed and lysates were subjected to SDS-PAGE and immunoblotted using the antisera described above. Panel (e) shows representative blots; panel (f) shows mean values for protein levels±S.E. for 8 independent experiments, in each case normalized against total ERK. *P<0.05, **P<0.005; Student's paired t-test
The effects of Trib3 on Akt and FoxO1a phosphorylation suggest that Trib3 may influence phospho-FoxO1a levels during NGF deprivation. To assess this, sympathetic neurons were infected with lentivirus expressing control or Trib3 shRNA and examined for phospho-FoxO1a 16 h later. Phospho-FoxO1a levels fell by about 75% with NGF deprivation and control shRNA, and this was significantly restored by Trib3 knockdown (Figures 6e and f). There was a trend of elevated total FoxO1a, but this was not significant (P>0.05; Figure 6f), nor was there a significant effect of Trib3 knockdown on phospho-FoxO1a in the presence of NGF (data not shown). Together, these findings indicate that Trib3 significantly contributes to the fall in Akt phosphorylation triggered by NGF withdrawal and to the consequent dephosphorylation of FoxO1a.
Trib3 promotes and is required for FoxO1a's nuclear translocation after NGF withdrawal
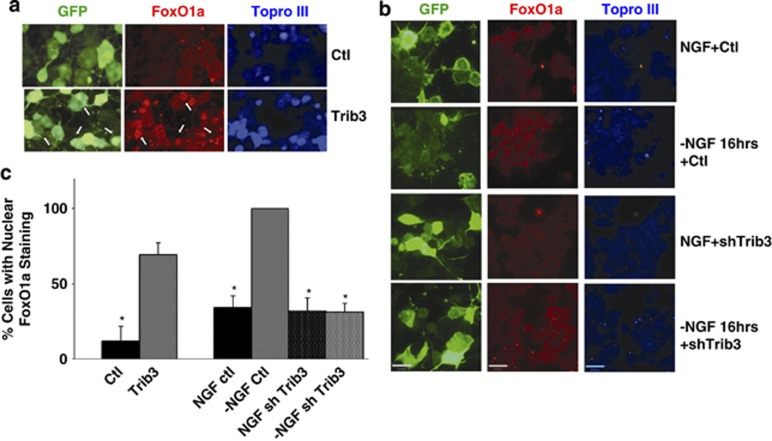
In view of Trib3's effect on FoxO1a phosphorylation, we next determined whether Trib3 affects its nuclear translocation. First, we infected PC12 cells with Trib3 lentivirus in the presence of NGF. This caused a nearly 5-fold increase in proportion of cells with detectable nuclear FoxO1a (Figures 7a and c), indicating that Trib3 expression is sufficient to drive FoxO1a translocation. We next queried whether Trib3 knockdown affects nuclear translocation of FoxO1a that occurs with NGF deprivation. Sixteen hours after NGF deprivation, there was a 4-fold increase in proportion of cells with nuclear FoxO1a, so that nearly all possessed nuclear FoxO1a (Figures 7b and c). This was fully prevented by Trib3 knockdown (Figures 7b and c). Thus, Trib3 is required for pro-apoptotic FoxO1a nuclear translocation that occurs after NGF deprivation.
Figure 7.
Trib3 regulates FoxO1a nuclear translocation. (a) Trib3 overexpression promotes nuclear translocation of FoxO1a. Neuronal PC12 cells were infected for 5 days with lentivirus expressing either Trib3 or eGFP (enhanced green fluorescent protein) only (control) while maintained with NGF and then subjected to immunostaining for eGFP and FoxO1a. White arrows indicate Trib3-expressing cells that exhibit nuclear FoxO1a staining. (b) Trib3 is required for nuclear translocation of FoxO1a in response to NGF deprivation. Neuronal PC12 cells were infected with lentivirus expressing shTrib3 or control shRNA for 72 h and were either maintained with NGF or deprived of NGF for an additional 16 h. Cells were immunostained as indicated for GFP and FoxO1a. Bar=20 μm. (c) Quantification of the effects of Trib3 and shTrib3 on nuclear translocation of FoxO1a. Bars show percentage of GFP-expressing cells with nuclear FoxO1a staining under each condition. Left-hand bars show the effects of Trib3 overexpression on nuclear localization of FoxO1a with or without Trib3 overexpression, all in the presence of NGF. Right hand bars show the effect of Trib3 knockdown on FoxO1a nuclear translocation caused by NGF deprivation. Conditions correspond to those in panels a and b, respectively. In all, 30–84 nuclei were blindly evaluated per culture. Values are means±S.E. for three independent experiments. *P<0.001, compared with either pWPI control (left hand bars) or −NGF control (right hand bars); Student's t-test
Trib3 induction after NGF withdrawal requires FoxO
Examination of the rat Trib3 gene +2000 to −100 bp 5′ from the first exon using web-based programs PROMO38 and JASPAR39 identified several predicted FoxO binding sites resembling the consensus FoxO sequence 5′-GTAAACAA-3′40, 41 as well as two putative alternate promoters. The binding motifs 5′-TGTAAACC-3′ and 5′-GTAAACTA-3′ had the highest match score to the consensus sequence and are located approximately 700 and 2000 bp, respectively, upstream of the translation initiation site and within 250 bp upstream of putative transcription start sites. These motifs are also conserved in the putative mouse, macaque and human Trib3 promoters.
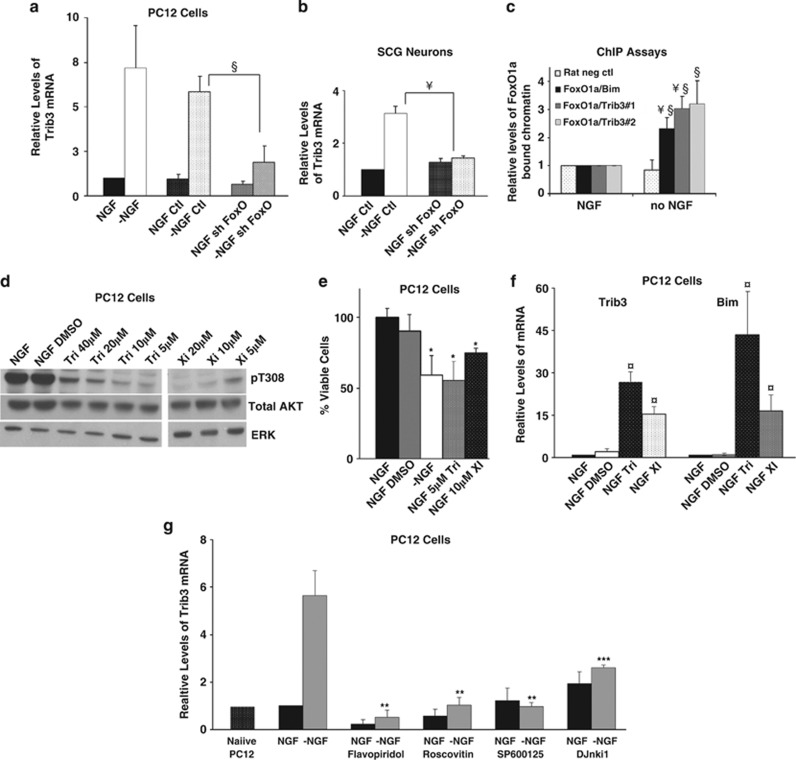
To determine whether FoxOs regulate Trib3 upon NGF deprivation, NGF-treated PC12 cells and sympathetic neurons were infected for 3 days with lentivirus expressing control or a previously described shRNA targeting FoxO family members, including FoxO1a.42 The cells were then assessed for relative Trib3 mRNA levels after an additional 16 h with or without NGF. In both systems, FoxO silencing blocked induction of Trib3 mRNA caused by NGF removal (Figures 8a and b).
Figure 8.
Regulation of Trib3 expression. (a and b) Induction of Trib3 in response to NGF deprivation requires FoxO. Neuronal PC12 cells (a) and SCG neurons (b) were infected with shFoxO or control shRNA expressing lentivirus for 72 h. Cultures were maintained with or without NGF for an additional 16 h for PC12 cells and 24 h for SCG neurons. Total mRNA was isolated and quantitative PCR was performed using Trib3 primers. Rat α-tubulin mRNA levels were used to normalize input cDNA. Values are means±S.E. of data from three independent experiments (a) and one experiment (b) each done in triplicate; §P<0.05; ¥P<0.005, (Student's t-test) compared with −NGF control. (c) ChIP assay indicates that FoxO1a occupancy of rat Trib3 promoter regions increases after NGF deprivation. Neuronal PC12 cells were either maintained with NGF or subjected to NGF withdrawal for 16 h. After crosslinking, the chromatin was sheared and immunoprecipitated using antibody against total FoxO1a or rabbit immunoglobulin G (IgG) isotype control. After reversing the crosslink, the genomic DNA was purified and subjected to quantitative PCR using two different primer sets designed to amplify promoter regions of Trib3 identified as corresponding to the putative FoxO1a binding sites. FoxO1a/Trib3#1 and FoxO1a/Trib3#2 refer to primer sets designed to amplify a region near the FoxO1a DNA located approximately 700 and 2000 bp upstream of the rat Trib3 translation initiation site, respectively, and that include the putative FoxO1a binding sites. Primers, including a previously identified FoxO binding site in the Bim promoter, were used as a positive control (FoxO1a/Bim) and commercially made primers recognizing a region of a ‘gene desert' on rat chromosome 3 were used as a negative control (‘rat neg control') for quantitative PCR. QPCR signals were normalized as bound/INPUT, and background signals obtained from rabbit IgG immunoprecipitated samples (25–50% of the values obtained with NGF and FoxO1a antibody) were subtracted. The data are normalized against NGF control samples for all primer sets and are presented as mean relative levels of FoxO1a-enriched chromatin in cells±S.E. of three independent experiments each conducted in triplicate. §P<0.05; ¥P<0.005, (Student's t-test) compared with NGF control and §P<0.05 compared with no NGF/rat negative control primers. (d) Inhibition of Akt phosphorylation by various inhibitors. Cell lysates of neuronal PC12 cells treated with various concentrations of Triciribine, and compound XI in the presence of NGF for 20 h were subjected to SDS-PAGE and immunoblotted using antisera against phospho-Akt (pT308), total Akt and ERK. (e) Inhibition of Akt activity promotes cell death. Neuronal PC12 cells were treated with the indicated concentrations of Akt inhibiting drugs for 20 h while maintained with NGF. Viable cells were then counted. Values are means±S.E. of three independent experiments each performed in triplicate. *P<0.05 (Student's t-test), compared with NGF/DMSO (dimethyl sulfoxide). (f) Akt inhibition induces Trib3 mRNA. cDNA derived from cultures treated as in panel (e) were subjected to quantitative PCR using Trib3 and Bim primers. Rat α-tubulin mRNA levels were used to normalize input cDNA. Values are means±S.E. of three independent experiments each performed in triplicate. ¤P<0.005, (Student's t-test) compared with NGF/DMSO. (g) Inhibitors of JNK and cyclin-dependent kinases suppress Trib3 mRNA induction in response to NGF deprivation. Neuronal PC12 cells were treated with the Cdk inhibiting drugs roscovitine and flavopiridol and the JNK inhibitors SP600125 and DJnkI1 at 10 μM each (g) in the presence and absence of NGF for 16 h. cDNA derived from the cultures was subjected to quantitative PCR using Trib3 primers. Rat α-tubulin mRNA levels were used to normalize input cDNA. Values are means±S.E. of four independent experiments performed in triplicate, **P<0.05 and ***P<0.05 of two independent experiments performed in triplicate (Student's t-test) compared with −NGF control
To assess whether FoxO transcription factors bind the regulatory region of the trib3 gene and whether such binding is affected by NGF deprivation, we performed chromatin immunoprecipitation (ChIP) experiments using FoxO1a antibodies. Neuronal PC12 cells were maintained with or without NGF for 16 h followed by ChIP using anti-FoxO1a or control IgG. Quantitative PCR on the immunoprecipitated chromatin was performed using primers designed to amplify the two putative alternative promoter regions of Trib3 that correspond to the above predicted FoxO binding sites, and relative occupancy of FoxO on each Trib3 promoter was determined. Because Bim is regulated by FoxOs and its promoter region undergoes enhanced FoxO3a occupancy upon NGF deprivation,7 Bim promoter occupancy by FoxO1a served as a positive control. The ChIP assays revealed a 2- to 3-fold elevation in FoxO1a occupancy of the Bim and Trib3 promoter regions after NGF withdrawal (Figure 8c).
Taken together, our findings reveal that Trib3 is essential for FoxO1a nuclear translocation after NGF deprivation and that FoxO1a in turn binds the Trib3 promoter region and is required for transcriptional induction of Trib3. This suggests that Trib3 and FoxO1a are linked in a feed-forward loop triggered by NGF withdrawal.
Akt inhibition triggers Trib3 induction and death
Loss of Akt signaling promotes neuron death, in part, by leading to dephosphorylation of FoxOs and their consequent nuclear translocation and transactivation of pro-apoptotic genes.7 Our findings indicate that Trib3 is a FoxO target, thus predicting that loss of Akt signaling should be sufficient to induce Trib3. We therefore exposed NGF-treated PC12 cells to two drugs (Triciribine and 3-formylchromone thiosemicarbazone, Cu(II)Cl2 complex) that inhibit Akt phosphorylation by different mechanisms.43, 44 Concentrations that blocked Akt phosphorylation (Figure 8d) caused death at levels similar to those with NGF deprivation (Figure 8e) and significantly induced Trib3 transcripts (Figure 8f). As a positive control, we also detected induction of Bim transcripts. These observations thus indicate that Akt signaling inhibition is sufficient to induce Trib3, even in the presence of NGF. Our findings are consistent with the idea that Trib3 expression is controlled by a pathway that includes negative regulation by Akt and positive regulation by nuclear FoxOs.
The cell cycle and JNK pathways regulate Trib3 induction after NGF deprivation
NGF deprivation activates multiple transcriptional pathways that induce pro-apoptotic genes. The most intensively studied is Bim for which induction involves at least four different transcription factors.6, 7, 8, 9, 10 Interference with any of these pathways disrupts Bim induction and neuron death by NGF deprivation. Examination of the Trib3 promoter revealed, in addition to potential FoxO binding sites, sequences for potential binding of AP1/c-Jun and Myb. Neuron death after NGF withdrawal requires activation of JNKs that in turn activate c-Jun.9 To assess whether this pathway is required for Trib3 induction, Trib3 mRNA levels were measured in PC12 cells deprived of NGF in the presence or absence of JNK inhibitors SP600125 (JNKi) and D-JNKi-1.45, 46 Both inhibitors blocked Trib3 induction caused by NGF withdrawal (Figure 8g). The ‘apoptotic cell-cycle pathway' is also required for Bim induction and neuron death after NGF deprivation and includes activation of cyclin-dependent kinase Cdk4 and consequent elevation of Myb transcription factors.12 Two different Cdk inhibitors, flavopiridol and roscovitine, fully blocked Trib3 induction triggered by NGF withdrawal (Figure 8g). These findings support the idea that Trib3 induction by NGF deprivation, as for Bim, requires simultaneous activation of at least the FoxO, c-Jun and Myb transcriptional pathways.
Discussion
We explored Trib3's role in neuron death induced by NGF deprivation. We find that NGF withdrawal increases Trib3 mRNA and protein before the onset of death. Trib3 is sufficient to promote sympathetic neuron death and is required for apoptosis induced by NGF withdrawal. Mechanistic studies reveal that Trib3 diminishes neuronal phosphorylation/activation of the survival kinase Akt. This leads to dephosphorylation of FoxO1a and promotes its nuclear translocation and enhanced occupancy of the Trib3 promoter. Additionally, FoxO transcription factors are essential for Trib3 induction after NGF deprivation. These observations suggest a self-amplifying loop involving Akt, FoxOs and Trib3 that underlies neuron death.
We found that Trib3 knockdown not only provided prolonged protection from NGF deprivation but also protected axons and maintained overall neuronal morphology. Although protection by Trib3 knockdown was substantial, it was not total. This could reflect residual Trib3 expression or contributions of additional pro-apoptotic proteins. Other transcriptionally induced pro-apoptotic proteins participate in death after NGF deprivation, and of these, Bim has been the most studied. Bim silencing partially protects sympathetic neurons from NGF deprivation in vitro, and Bim deletion delays developmental neuron death of DRG neurons in vivo.34 We confirmed partial protection by Bim knockdown in NGF-deprived sympathetic neurons, and this was less protective than Trib3 knockdown. Moreover, there was only modestly increased protection by silencing both Bim and Trib3, suggesting that they may act in the same pathway.
Studies in non-neuronal and PC12 cells indicate that Trib3 binds Akt and negatively regulates its phosphorylation and activity.31, 32, 37 In agreement, we found that Trib3 overexpression reduced neuronal phospho-Akt levels and that silencing Trib3 partially reversed the decrease in phospho-Akt caused by NGF deprivation. Moreover, Trib3 overexpression produced dephosphorylation of the Akt substrate FoxO1a, and dephosphorylation of FoxO1a caused by NGF deprivation was suppressed by silencing Trib3. Previous work underscores the requirement of Akt signaling for neuron survival.1, 2, 3 In corroboration, we found that Akt inhibitors caused sympathetic neuron death even in the presence of NGF. Together, these observations support the idea that Trib3 contributes to neuron death by reducing phospho-Akt to levels below that required for survival. Akt also regulates axonal growth and morphology, and this might contribute to the sparing effects we observed in Trib3 knockdown on processes of NGF-deprived neurons.47
PI3K and Akt activation is a key pathway by which NGF promotes survival and NGF withdrawal reduces PI3K activity and Akt phosphorylation.1, 2, 3, 5 Why then are Trib3 and its actions on Akt required for neuron death after NGF deprivation? One possibility is that PI3K can be activated by NGF-independent receptors on neurons. For instance, sympathetic neurons have receptors for IGF-I, GDNF family ligands and HGF, all of which stimulate PI3K.48, 49, 50 Akt can also be phosphorylated by PI3K-independent pathways not necessarily regulated by NGF/Trk signaling.51 Finally, when neurons compete for limited NGF supplies, residual Trk signaling may persist even in neurons that will die. Thus, NGF deprivation alone may not sufficiently depress Akt activity to a degree permitting neuron death. In this context, Trib3 may function in an amplification system in which an initial partial loss of Akt signaling promotes Trib3 induction, which in turn ultimately suppresses Akt activity below levels required for survival.
If initial decline in Akt activity after NGF deprivation inaugurates Trib3 induction, how does this occur? Our findings implicate FoxO proteins. When Akt activity falls, FoxOs become dephosphorylated and translocate to nuclei where they activate genes required for neuron death.4, 5 We observed that Trib3 induction by NGF deprivation is abolished by FoxO silencing. Thus loss of Akt signaling upon NGF deprivation activates FoxO transcriptional activity and that, in turn, induces Trib3.
The FoxO-dependent induction of Trib3 that occurs when Akt activity falls and the inhibition of Akt activity caused, in turn, by Trib3 induction imply a feed-forward amplification loop. In this mechanism, initial reduction of PI3K-Akt signaling after NGF deprivation leads to FoxO activation and Trib3 induction. Induced Trib3, in turn, further depresses Akt activity, enhancing FoxO signaling and consequently further Trib3 induction. Supporting this model, chemical Akt inhibition was sufficient to induce Trib3, even with NGF present. Moreover, not only was Trib3 induction by NGF deprivation dependent on FoxOs, but under such circumstances, FoxO activation and nuclear translocation required Trib3. Once initiated, this loop would spiral out of control until death is induced.
Neuron death caused by NGF deprivation requires de novo transcription of death-promoting genes.52 Significantly, such death requires multiple transcription factors.6, 7, 10, 12, 35 Silencing of any of these or their upstream signaling pathways represses death. On this basis, it was proposed that transcriptional regulatory regions of neuronal apoptotic genes function as ‘fail-safe' coincidence detectors, so that they are not induced unless all required transcription factors are simultaneously activated.6 Our findings support this model for Trib3. In addition to FoxO knockdown, JNK/c-Jun activity inhibition substantially blocked Trib3 induction by NGF withdrawal as did in a previous study16 the mixed lineage kinase inhibitor CEP11004, which blocks JNK activation. Another pathway required for death after NGF withdrawal includes activation of cyclin-dependent kinases and consequent induction of Myb transcription factors.12 We observed that two Cdk inhibitors blocked Trib3 induction after NGF deprivation. Although further study is needed, these findings suggest that Trib3 is subject to a regulatory mechanism that requires coincident activation of multiple transcription factors.
We examined the role of Trib3 in vivo by comparing developmental apoptosis in WT versus Trib3−/− mice SCG and did not find any differences (Supplementary Figures S1A and B). Okamoto et al.53 also observed no alterations in Akt phosphorylation or glucose metabolism in Trib3−/− mice. It may be that Trib3 has no role in developmental SCG neuron death in vivo. Alternatively, other protein(s) may compensate for Trib3. It was reported that another tribbles family member, Trib2, also binds Akt and suppresses its phosphorylation,31 and we have observed that Trib1 and Trib2 mRNA levels significantly increase by 2–3-fold in Trib3−/− brain and SCG (Supplementary Figures S2A and B). This suggests that Trib1 and Trib2 could promote developmental apoptosis in the absence of Trib3. In future, it will be of interest to assess developmental neuron death either after conditional knockdown of Trib3 or in mice null for all three trib genes.
Although the present work focused on NGF deprivation, our findings may have implications for other paradigms of neuron degeneration and death. In addition to NGF withdrawal, Trib3 is induced by a variety of cellular stresses relevant to neurons, including the unfolded protein response and ER stress, nutrient deprivation, anoxia and ethanol exposure.20, 21, 22, 23, 24, 25, 27, 28, 30, 36 Given this and Trib3's suppression of Akt signaling, it can be anticipated that Trib3 is a participant in, and therefore a potential target for treatment of, a variety of neurological disorders and insults.
Materials and Methods
Cell culture
PC12 cells were grown as previously described54 on collagen (Roche, Indianapolis, IN, USA) coated plates (Nunclone, Waltham, MA, USA) with RPMI 1640 medium (Cellgro, Manassas, VA, USA) supplemented with 10% heat inactivated horse serum and 5% fetal bovine serum. Neuronal differentiation was induced in RPMI medium supplemented with 1% horse serum and 100 ng/ml hNGF (kind gift of Genentech, Inc, Oceanside, CA, USA). Rat SCG neuron cultures were generated and maintained as previously described.55 NGF withdrawal was carried out by washing the cultures with serum-free RPMI medium three to four times and then maintaining them in serum-free RPMI plus 10 μg/ml anti-NGF antibody (R&D System, Minneapolis, MN, USA: #MAB2561). HEK293T/17 cells (ATCC, Manassas, VA, USA) were cultured in polystyrene plates (Corning, Manassas, VA, USA) with DMEM supplemented with 10% fetal bovine serum.
Reagents
An antibody against Trib3 was a kind gift from Dr. Keyong Du.31 Additional Anti-Trib3 antibodies were purchased from Santa Cruz Biotechnology, Inc (Dallas, TX, USA) (SC67122 for western blotting and SC314214 for immunostaining) and EMD Millipore (Billerica, MA, USA; ST1032). Monoclonal antibodies against total Akt, phospho-Akt (Ser473 and Thr308) and total FoxO1a and polyclonal antiserum against phospho-FoxO1a (Ser256) were obtained from Cell Signaling Technology (Danvers, MA, USA). A second polyclonal antiserum against total FoxO1a was also obtained from AbCam (Cambridge, MA, USA). Monoclonal antibodies against β-tubulin III and GFP were from Sigma-Aldrich (St. Louis, MO, USA) and Invitrogen (Grand Island, NY, USA), respectively. For ChIP assays, protein A magnetic beads (Millipore #16-661), ChIP grade polyclonal FoxO1a antibody (AbCam #ab39670), rabbit IgG isotype control (Cell Signaling #2729), protease inhibitors (Roche #11873580001) and proteinase K (Viagen, Cedar Park, TX, USA; #501-PK) were used.
Plasmids
Primers used for PCR amplification of rat Trib3 were: Fwd 5′-ACCATGCGAGCCACATCTCTG-3′ and Rvrs 5′-CTAGCCATACAGCCCCACCTC-3′. PCR products were gel purified and cloned into PmeI-digested vector pWPI (AddGene, Cambridge, MA, USA; http://tronolab.epfl.ch). Primers used for shTrib3 were based on the following Trib3 sequences: shTrib3#1 5′-GAGTGAGAGATGAGCCTG-3′, shTrib3#2 5′-CTGGAGGATGCCTGTGTG-3′ and shTrib3#3 5′-TGCTCGATTTGTCTTCAGCAA-3′. Forward and reverse oligonucleotides were 5′ phosphorylated, annealed and cloned into Hpa1-Xho1-digested pLL 3.7 vector.56 Primers used for pan shFoxO were based on the sequence 5′-GGATAAGGGCGACAGCAA-3′.42 Primers used for shBim were based on the following sequences: shBim#1 5′-GACAGAGAAGGTGGACAATTG-3′57 and shBim#2 5′-TTCGATTACCGAGAGGCGGAA-3′.
Lentiviral preparation
HEK293T/17 cells (ATCC) were co-transfected with pLL3.7 containing the desired shRNA construct and third-generation lentiviral packaging plasmids, RRE, Rsv/Rev and VSV-G (AddGene), using the Ca-phosphate method.58 For overexpression, cells were co-transfected with pWPI plasmid containing the Trib3 cDNA and second-generation lentiviral packaging plasmids, pSPAX2 and VSV-G (AddGene). Four hours later, medium containing Ca-phosphate was replaced by fresh DMEM supplemented with 10% FBS. Lentiviral medium was collected 72 h later and replaced with fresh DMEM supplemented with 10% FBS. A second lentiviral medium was collected 24 h later. All lentiviral supernatants were pooled, filtered through a 0.45-micron membrane (Nalgene, Rochester, NY, USA) and subjected to ultracentrifugation in clear tubes (Beckman Scientific, Danvers, MA, USA) at 25 000 r.p.m. using Beckman SW28 rotor in a Beckman Coulter ultracentrifuge for 1.5 h. Supernatants were discarded and viral pellets were incubated in PBS without MgCl2 or CaCl2 overnight. The dissolved pellets were stored at −80 °C. Viral titer was determined.
Lentiviral infection
A predetermined volume (approximate viral MOI between 10 and 15 as determined by viral titration) of lentivirus was added to the medium, which was then added to cultures of neuronal PC12 cells and SCG neurons. The medium was replaced with that without virus 72 h later.
Transfections
DNA was prepared with a Plasmid Maxi kit (Qiagen, Valencia, CA, USA or Sigma-Aldrich). PC12 cells were transfected with 1–2 μg of plasmid in 48-well dishes using LipofectAMINE 2000 (Invitrogen). Four hours later, medium with LipofectAMINE 2000 was supplemented with fresh RPMI medium supplemented with 1% horse serum and 100 ng/ml NGF. Transfected GFP or mCherry-positive cells were visualized under a Nikon (Melville, NY, USA) TE300 epi-fluorescent microscope.
Survival assays
Cell survival was determined as previously described.54 For Trib3 overexpression, replicate wells of control and experimentally treated cells were depleted of culture medium and harvested in a specified volume of detergent-containing solution that lyses the plasma membrane and leaves the nuclei intact. Counts of intact, non-apoptotic nuclei were determined in a hemocytometer. In order to determine cell survival after Trib3 and Bim knockdown, transfected neuronal PC12 cells or lentivirally infected SCG neurons coexpressing GFP along with either control or experimental shRNA were counted under an epi-fluorescent microscope at indicated times.
Western immunoblotting
Neuronal PC12 cells and SCG neurons were lysed with Laemmli Sample Buffer supplemented with 2-mercaptoethanol (Bio-Rad, Hercules, CA, USA), sonicated and subjected to electrophoresis using pre-cast Bis-Tris 10% or 4–12% SDS gels (Invitrogen). Protein was then transferred to HyBond PVDF membrane (GE Amersham Biosciences, Pittsburgh, PA, USA). Detection was carried out using either ECL or ECL+ chemiluminescence according to the manufacturer's instructions (GE Amersham Biosciences).
Immunostaining
Neuronal PC12 cells and SCG neurons were cultured in eight-chambered borosilicate coverglasses (Nunc LabTek cat# 155411, BioExpress, Kaysville, UT, USA) and fixed with 4% paraformaldehyde for 10 min. Cells were then washed three times with PBS and blocked in SuperBlock buffer (Pierce, Thermo Fisher Sci., Rockford, IL, USA) in PBS for 2 h. The cultures were then incubated with the appropriate antibodies or antisera diluted in blocking buffer overnight. Cultures were washed with PBS the next day, and this was followed by incubation with the appropriate secondary antibody for 2 h in room temperature. Finally, cultures were labeled with the nuclear stain Topro III at 1/2000 dilution in PBS (Molecular Probes, Invitrogen, Grand Island, NY, USA). Immunostained cells were visualized and photographed using a Nikon Eclipse TE200 spinning disk confocal microscope (Melville, NY, USA).
ChIP
Neuronal PC12 cells were cultured in 10-cm plates and were either maintained with NGF or subjected to 16 h of NGF deprivation. ChIP assays were carried out as previously described.59 Protease inhibitors (Roche #11873580001) were used in all buffers except the elution buffer. In all, 1% of each sample was set aside as INPUT material before immunoprecipitation. The INPUTs were used to quantify the amount of DNA present in each sample. A total of 20 μl of protein A magnetic beads (Millipore #16-661) were used to pre-clear all samples for 1 h at 4 °C with agitation. Overnight immunoprecipitations were carried out at 4 °C with agitation using 30 μl of fresh protein A beads and 10 μg of either FoxO1a antibody (AbCam #ab39670) or rabbit IgG isotype control (Cell Signaling #2729). All samples were subjected to reverse crosslinking in elution buffer for 2 h at 62 °C and purified using QIAquick PCR Purification kit (Qiagen #28104). Amplifications were carried out using quantitative PCR as described below, and data were normalized as bound/INPUT followed by IgG background signal subtraction and finally presented as normalized to NGF control for each set of primers. Primer sequences are given below.
Reverse transcription and quantitative PCR
Cells were lysed and total mRNA was isolated using TRI reagent (Molecular Research Center, Cincinnati, OH, USA) following the manufacturer's instruction. Whole brains and superior sympathetic ganglia from Trib3 WT and knockout mice were harvested and homogenized in TRI reagent, and mRNA was isolated. cDNA was reverse-transcribed from total RNA with a cDNA synthesis kit (Marligen, OriGene, Rockville, MD, USA) following the manufacturer's instruction. The primers used for PCR amplification of rat/mouse Trib1 were Fwd 5′-CCGAGTACCAGGACGACAAT-3′ and Rvrs 5′-AGTGCATGCTGGTCAAATCA-3′, rat/mouse Trib2 Fwd 5′-GCATGGGAACAAGATGTGTG-3′ and Rvrs 5′-TGCAATGCCAAGGTATGTGT-3′, Trib3 Fwd 5′-GTTGCGTCGATTTGTCTTCA-3′ and Rvrs: 5′-CGGGAGCTGAGTATCTCTGG-3′, Bim forward 5′-GCCCCTACCTCCCTACAGAC-3′ and reverse 5′-CCTTATGGAAGCCATTGCAC-3′, GAPDH forward 5′-AAGTGGACATTGTTGCCATC-3′ and reverse 5′-CATACTCAGCACCAGCATCA-3′ and rat/mouse α-tubulin Fwd 5′-TACACCATTGGCAAGGAGAT-3′ and reverse 5′-GGCTGGGTAAATGGAGAACT-3′. An equal amount of cDNA template was used for each PCR reaction. An OmniMix PCR kit (Cephaid, Sunnyvale, CA, USA) and a SYBR green/DNA polymerase PCR kit was used (Roche), and quantitative PCR reactions were carried out using a Cephaid SmartCycler and an Eppendorf Realplex thermocycler following the manufacturers' specifications. Values obtained from SYBR green count were recorded, transcript levels were normalized against rat GAPDH or α-tubulin signals and results were reported as times fold increase in reference to control values. For genomic DNA QPCR obtained from ChIP assay, the following primers were used: FoxO/Trib3#1 (FoxO binding location approximately 700 bp upstream of the translation initiation site) forward: 5′-GTGCTGGGACTCCGAGATAG-3′ and reverse: 5′-CAACCTTCTTGCCAGACCTC-3′ FoxO/Trib3#2 (FoxO binding location approximately 2000 bp upstream of the translation initiation site) forward: 5′-ATGGCTGAGCAGATGAAGGT-3′ and reverse: 5′-TAGACTGCGACAACCCACAG-3′ and FoxO/Bim forward: 5′-TAAGTTCCGCTCTGAGAGGT-3′ and reverse: 5′-CAGGCTGCGACAGGTAGTG-3′.60 The rat negative control primer set for ChIP was obtained from Active Motif (Carlsbad, CA, USA; #71024).
Harvest and immunohistochemistry of mouse superior cervical ganglia
WT and Trib3−/− mice pups at ages P3 and P7 were collected and killed. A disk-shaped section of the neck area containing the superior cervical ganglia was embedded in paraffin and prepared as 5-μm-thick sections. Twenty-five such sections were obtained for each neck pieces, which contained both the right and left ganglia. Four sections were mounted on a single glass slide, and every fifth section was stained with hematoxylin and eosin by the histology service providers. The remaining sections were immunostained with antibody against the apoptotic marker pH2AX protein (Cell Signaling #9718). The following kits were used in the process: blocking reagent and biotinylated secondary-antibody from the Vectastatin Elite ABC Kit (#PK6101), peroxidase substrate from ImmPACT SG (Vector Laboratories, Burlingame, CA, USA; #SK4705), and counterstaining with Nuclear Fast Red from Vector Laboratories (#H3403).
Statistics
The statistical significance of differences between means was evaluated by Student's t-test and was performed as paired, two-tailed distribution of arrays and presented as P values.
Acknowledgments
Supported in part by the NIH grant NS033689 (to LAG) and NRSA 5F31AG033477-02 (to NZ).
Glossary
- NGF
nerve growth factor
- Ser
serine
- Thr
threonine
- Trk
tropomyosin receptor kinase
- PI3K
phosphoinositide-3 kinase
- FoxO
forkhead box, class O
- ER
endoplasmic reticulum
- JNK
Jun N-terminal kinase
- ChIP
chromatin immunoprecipitation
- SCG
superior cervical ganglion
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Edited by M Deshmuck
Supplementary Material
References
- Andjelkovic M, Suidan HS, Meier R, Frech M, Alessi DR, Hemmings BA. Nerve growth factor promotes activation of the alpha, beta and gamma isoforms of protein kinase B in PC12 pheochromocytoma cells. Eur J Biochem/FEBS. 1998;251:195–200. doi: 10.1046/j.1432-1327.1998.2510195.x. [DOI] [PubMed] [Google Scholar]
- Crowder RJ, Freeman RS. Phosphatidylinositol 3-kinase and Akt protein kinase are necessary and sufficient for the survival of nerve growth factor-dependent sympathetic neurons. J Neurosci. 1998;18:2933–2943. doi: 10.1523/JNEUROSCI.18-08-02933.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C. PI3K/Akt and apoptosis: size matters. Oncogene. 2003;22:8983–8998. doi: 10.1038/sj.onc.1207115. [DOI] [PubMed] [Google Scholar]
- Tzivion G, Dobson M, Ramakrishnan G. FoxO transcription factors; Regulation by AKT and 14-3-3 proteins. Biochim Biophys. 2011;1813:1938–1945. doi: 10.1016/j.bbamcr.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Zhang X, Tang N, Hadden TJ, Rishi AK. Akt, FoxO and regulation of apoptosis. Biochim Biophys. 2011;1813:1978–1986. doi: 10.1016/j.bbamcr.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Biswas SC, Shi Y, Sproul A, Greene LA. Pro-apoptotic Bim induction in response to nerve growth factor deprivation requires simultaneous activation of three different death signaling pathways. J Biol Chem. 2007;282:29368–29374. doi: 10.1074/jbc.M702634200. [DOI] [PubMed] [Google Scholar]
- Gilley J, Coffer PJ, Ham J. FOXO transcription factors directly activate bim gene expression and promote apoptosis in sympathetic neurons. J Cell Biol. 2003;162:613–622. doi: 10.1083/jcb.200303026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene LA, Liu DX, Troy CM, Biswas SC. Cell cycle molecules define a pathway required for neuron death in development and disease. Biochim Biophys. 2007;1772:392–401. doi: 10.1016/j.bbadis.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham J, Eilers A, Whitfield J, Neame SJ, Shah B. c-Jun and the transcriptional control of neuronal apoptosis. Biochem Pharmacol. 2000;60:1015–1021. doi: 10.1016/s0006-2952(00)00372-5. [DOI] [PubMed] [Google Scholar]
- Hughes R, Kristiansen M, Lassot I, Desagher S, Mantovani R, Ham J. NF-Y is essential for expression of the proapoptotic bim gene in sympathetic neurons. Cell Death Differ. 2011;18:937–947. doi: 10.1038/cdd.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs WB, Kaplan DR, Miller FD. The p53 family in nervous system development and disease. J Neurochem. 2006;97:1571–1584. doi: 10.1111/j.1471-4159.2006.03980.x. [DOI] [PubMed] [Google Scholar]
- Biswas SC, Liu DX, Greene LA. Bim is a direct target of a neuronal E2F-dependent apoptotic pathway. J Neurosci. 2005;25:8349–8358. doi: 10.1523/JNEUROSCI.1570-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi K, Tsuda M, Imai Y, Wanaka A, Takagi T, Tohyama M. Molecular cloning of a novel polypeptide, DP5, induced during programmed neuronal death. J Biol Chem. 1997;272:18842–18848. doi: 10.1074/jbc.272.30.18842. [DOI] [PubMed] [Google Scholar]
- Kole AJ, Swahari V, Hammond SM, Deshmukh M. miR-29b is activated during neuronal maturation and targets BH3-only genes to restrict apoptosis. Genes Dev. 2011;25:125–130. doi: 10.1101/gad.1975411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscomb EA, Sarmiere PD, Freeman RS. SM-20 is a novel mitochondrial protein that causes caspase-dependent cell death in nerve growth factor-dependent neurons. J Biol Chem. 2001;276:5085–5092. doi: 10.1074/jbc.M008407200. [DOI] [PubMed] [Google Scholar]
- Kristiansen M, Menghi F, Hughes R, Hubank M, Ham J. Global analysis of gene expression in NGF-deprived sympathetic neurons identifies molecular pathways associated with cell death. BMC Genomics. 2011;12:551. doi: 10.1186/1471-2164-12-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayumi-Matsuda K, Kojima S, Suzuki H, Sakata T. Identification of a novel kinase-like gene induced during neuronal cell death. Biochem Biophys Res Commun. 1999;258:260–264. doi: 10.1006/bbrc.1999.0576. [DOI] [PubMed] [Google Scholar]
- Mata J, Curado S, Ephrussi A, Rorth P. Tribbles coordinates mitosis and morphogenesis in Drosophila by regulating string/CDC25 proteolysis. Cell. 2000;101:511–522. doi: 10.1016/s0092-8674(00)80861-2. [DOI] [PubMed] [Google Scholar]
- Seher TC, Leptin M. Tribbles, a cell-cycle brake that coordinates proliferation and morphogenesis during Drosophila gastrulation. Curr Biol. 2000;10:623–629. doi: 10.1016/s0960-9822(00)00502-9. [DOI] [PubMed] [Google Scholar]
- Corcoran CA, Luo X, He Q, Jiang C, Huang Y, Sheikh MS. Genotoxic and endoplasmic reticulum stresses differentially regulate TRB3 expression. Cancer Biol Ther. 2005;4:1063–1067. doi: 10.4161/cbt.4.10.2205. [DOI] [PubMed] [Google Scholar]
- Ord D, Ord T. Characterization of human NIPK (TRB3, SKIP3) gene activation in stressful conditions. Biochem Biophys Res Commun. 2005;330:210–218. doi: 10.1016/j.bbrc.2005.02.149. [DOI] [PubMed] [Google Scholar]
- Ryu EJ, Angelastro JM, Greene LA. Analysis of gene expression changes in a cellular model of Parkinson disease. Neurobiol Dis. 2005;18:54–74. doi: 10.1016/j.nbd.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Wennemers M, Bussink J, Grebenchtchikov N, Sweep FC, Span PN. TRIB3 protein denotes a good prognosis in breast cancer patients and is associated with hypoxia sensitivity. Radiother Oncol. 2011;101:198–202. doi: 10.1016/j.radonc.2011.05.057. [DOI] [PubMed] [Google Scholar]
- Hua F, Mu R, Liu J, Xue J, Wang Z, Lin H, et al. TRB3 interacts with SMAD3 promoting tumor cell migration and invasion. J Cell Sci. 2011;124:3235–3246. doi: 10.1242/jcs.082875. [DOI] [PubMed] [Google Scholar]
- Ord D, Meerits K, Ord T. TRB3 protects cells against the growth inhibitory and cytotoxic effect of ATF4. Exp Cell Res. 2007;313:3556–3567. doi: 10.1016/j.yexcr.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Ohoka N, Hayashi H, Sato R. TRB3 suppresses adipocyte differentiation by negatively regulating PPARgamma transcriptional activity. J Lipid Res. 2008;49:880–892. doi: 10.1194/jlr.M700545-JLR200. [DOI] [PubMed] [Google Scholar]
- Kiss-Toth E, Bagstaff SM, Sung HY, Jozsa V, Dempsey C, Caunt JC, et al. Human tribbles, a protein family controlling mitogen-activated protein kinase cascades. J Biol Chem. 2004;279:42703–42708. doi: 10.1074/jbc.M407732200. [DOI] [PubMed] [Google Scholar]
- Liew CW, Bochenski J, Kawamori D, Hu J, Leech CA, Wanic K, et al. The pseudokinase tribbles homolog 3 interacts with ATF4 to negatively regulate insulin exocytosis in human and mouse beta cells. J Clin Invest. 2010;120:2876–2888. doi: 10.1172/JCI36849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L, Heredia JE, Altarejos JY, Screaton R, Goebel N, Niessen S, et al. TRB3 links the E3 ubiquitin ligase COP1 to lipid metabolism. Science. 2006;312:1763–1766. doi: 10.1126/science.1123374. [DOI] [PubMed] [Google Scholar]
- Wu M, Xu LG, Zhai Z, Shu HB. SINK is a p65-interacting negative regulator of NF-kappaB-dependent transcription. J Biol Chem. 2003;278:27072–27079. doi: 10.1074/jbc.M209814200. [DOI] [PubMed] [Google Scholar]
- Du K, Herzig S, Kulkarni RN, Montminy M. TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science. 2003;300:1574–1577. doi: 10.1126/science.1079817. [DOI] [PubMed] [Google Scholar]
- He L, Simmen FA, Mehendale HM, Ronis MJ, Badger TM. Chronic ethanol intake impairs insulin signaling in rats by disrupting Akt association with the cell membrane. Role of TRB3 in inhibition of Akt/protein kinase B activation. J Biol Chem. 2006;281:11126–11134. doi: 10.1074/jbc.M510724200. [DOI] [PubMed] [Google Scholar]
- Cravero JD, Carlson CS, Im HJ, Yammani RR, Long D, Loeser RF, et al. Increased expression of the Akt/PKB inhibitor TRB3 in osteoarthritic chondrocytes inhibits insulin-like growth factor 1-mediated cell survival and proteoglycan synthesis. Arthritis Rheumat. 2009;60:492–500. doi: 10.1002/art.24225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putcha GV, Moulder KL, Golden JP, Bouillet P, Adams JA, Strasser A, et al. Induction of BIM, a proapoptotic BH3-only BCL-2 family member, is critical for neuronal apoptosis. Neuron. 2001;29:615–628. doi: 10.1016/s0896-6273(01)00238-0. [DOI] [PubMed] [Google Scholar]
- Whitfield J, Neame SJ, Paquet L, Bernard O, Ham J. Dominant-negative c-Jun promotes neuronal survival by reducing BIM expression and inhibiting mitochondrial cytochrome c release. Neuron. 2001;29:629–643. doi: 10.1016/s0896-6273(01)00239-2. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wen HJ, Guo ZM, Zeng MS, Li MZ, Jiang YE, et al. TRB3 overexpression due to endoplasmic reticulum stress inhibits AKT kinase activation of tongue squamous cell carcinoma. Oral Oncol. 2011;47:934–939. doi: 10.1016/j.oraloncology.2011.06.512. [DOI] [PubMed] [Google Scholar]
- Zou CG, Cao XZ, Zhao YS, Gao SY, Li SD, Liu XY, et al. The molecular mechanism of endoplasmic reticulum stress-induced apoptosis in PC-12 neuronal cells: the protective effect of insulin-like growth factor I. Endocrinology. 2009;150:277–285. doi: 10.1210/en.2008-0794. [DOI] [PubMed] [Google Scholar]
- Farre D, Roset R, Huerta M, Adsuara JE, Roselló L, Albà MM, et al. Identification of patterns in biological sequences at the ALGGEN server: PROMO and MALGEN. Nucleic Acids Res. 2003;31:3651–3653. doi: 10.1093/nar/gkg605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandelin A, Alkema W, Engstrom P, Wasserman WW, Lenhard B. JASPAR: an open-access database for eukaryotic transcription factor binding profiles. Nucleic Acids Res. 2004;32:D91–D94. doi: 10.1093/nar/gkh012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuyama T, Nakazawa T, Nakano I, Mori N. Identification of the differential distribution patterns of mRNAs and consensus binding sequences for mouse DAF-16 homologues. Biochem J. 2000;349:629–634. doi: 10.1042/0264-6021:3490629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan Z, Zhang MQ. From worm to human: bioinformatics approaches to identify FOXO target genes. Mech Ageing Dev. 2005;126:209–215. doi: 10.1016/j.mad.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Hribal ML, Nakae J, Kitamura T, Shutter JR, Accili D. Regulation of insulin-like growth factor-dependent myoblast differentiation by Foxo forkhead transcription factors. J Cell Biol. 2003;162:535–541. doi: 10.1083/jcb.200212107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barve V, Ahmed F, Adsule S, Banerjee S, Kulkarni S, Katiyar P, et al. Synthesis, molecular characterization, and biological activity of novel synthetic derivatives of chromen-4-one in human cancer cells. J Med Chem. 2006;49:3800–3808. doi: 10.1021/jm051068y. [DOI] [PubMed] [Google Scholar]
- Yang L, Dan HC, Sun M, Liu Q, Sun XM, Feldman RI, et al. Akt/protein kinase B signaling inhibitor-2, a selective small molecule inhibitor of Akt signaling with antitumor activity in cancer cells overexpressing Akt. Cancer Res. 2004;64:4394–4399. doi: 10.1158/0008-5472.CAN-04-0343. [DOI] [PubMed] [Google Scholar]
- Borsello T, Clarke PG, Hirt L, Vercelli A, Repici M, Schorderet DF, et al. A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat Med. 2003;9:1180–1186. doi: 10.1038/nm911. [DOI] [PubMed] [Google Scholar]
- Shin M, Yan C, Boyd D. An inhibitor of c-jun aminoterminal kinase (SP600125) represses c-Jun activation, DNA-binding and PMA-inducible 92-kDa type IV collagenase expression. Biochim Biophys. 2002;1589:311–316. doi: 10.1016/s0167-4889(02)00195-7. [DOI] [PubMed] [Google Scholar]
- Read DE, Gorman AM. Involvement of Akt in neurite outgrowth. Cell Mol Life Sci. 2009;66:2975–2984. doi: 10.1007/s00018-009-0057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JW, Feldman EL. Insulin-like growth factor-I prevents apoptosis in sympathetic neurons exposed to high glucose. Horm Metab Res. 1999;31:90–96. doi: 10.1055/s-2007-978704. [DOI] [PubMed] [Google Scholar]
- Runeberg-Roos P, Saarma M. Neurotrophic factor receptor RET: structure, cell biology, and inherited diseases. Ann Med. 2007;39:572–580. doi: 10.1080/07853890701646256. [DOI] [PubMed] [Google Scholar]
- Thompson J, Dolcet X, Hilton M, Tolcos M, Davies AM. HGF promotes survival and growth of maturing sympathetic neurons by PI-3 kinase- and MAP kinase-dependent mechanisms. Mol Cell Neurosci. 2004;27:441–452. doi: 10.1016/j.mcn.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Mahajan K, Mahajan NP. PI3K-independent AKT activation in cancers: a treasure trove for novel therapeutics. J Cell Physiol. 2012;227:3178–3184. doi: 10.1002/jcp.24065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DP, Schmidt RE, DiStefano PS, Lowry OH, Carter JG, Johnson EM, Jr, et al. Inhibitors of protein synthesis and RNA synthesis prevent neuronal death caused by nerve growth factor deprivation. J Cell Biol. 1988;106:829–844. doi: 10.1083/jcb.106.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H, Latres E, Liu R, Thabet K, Murphy A, Valenzeula D, et al. Genetic deletion of Trb3, the mammalian Drosophila tribbles homolog, displays normal hepatic insulin signaling and glucose homeostasis. Diabetes. 2007;56:1350–1356. doi: 10.2337/db06-1448. [DOI] [PubMed] [Google Scholar]
- Greene LA, Farinelli SE, Cunnigngham ME, Park DS.Culture and Experimental Use of the PC12 Rat Pheochromocytoma Cell LineCulturing Nerve Cells,2nd edn,MIT Press: Cambridge, MA, USA; 1998 [Google Scholar]
- Zareen N, Greene LA.Protocol for culturing sympathetic neurons from rat superior cervical ganglia (SCG) J Visualized Exp 200923: pii988. [DOI] [PMC free article] [PubMed]
- Rubinson DA, Dillon CP, Kwiatkowski AV, Sievers C, Yang L, Kopinja J, et al. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat Genet. 2003;33:401–406. doi: 10.1038/ng1117. [DOI] [PubMed] [Google Scholar]
- Biswas SC, Shi Y, Vonsattel JP, Leung CL, Troy CM, Greene LA, et al. Bim is elevated in Alzheimer's disease neurons and is required for beta-amyloid-induced neuronal apoptosis. J Neurosci. 2007;27:893–900. doi: 10.1523/JNEUROSCI.3524-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiscornia G, Singer O, Verma IM. Production and purification of lentiviral vectors. Nat Protoc. 2006;1:241–245. doi: 10.1038/nprot.2006.37. [DOI] [PubMed] [Google Scholar]
- Schlesinger S, Goff SP. Silencing of proviruses in embryonic cells: efficiency, stability and chromatin modifications. EMBO Rep. 2013;14:73–79. doi: 10.1038/embor.2012.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanphui P, Biswas SC. FoxO3a is activated and executes neuron death via Bim in response to beta-amyloid. Cell Death Dis. 2013;4:e625. doi: 10.1038/cddis.2013.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.