Abstract
It has been shown previously that phosphorylation of the endothelial nitric oxide synthase (eNOS) at serine 116 (S116) under basal conditions suppresses eNOS enzymatic activity in endothelial cells. It has also been shown that vascular endothelial growth factor (VEGF) treatment of endothelial cells produces a rapid S116 dephosphorylation, which is blocked by the calcineurin inhibitor, cyclosporin A (CsA). In this study, we show that activation of eNOS in response to a variety of other eNOS-activating agonists and the cytosolic calcium-elevating agent, thapsigargin also involves CsA-inhibitable S116 dephosphorylation. Studies with the purified eNOS enzyme also demonstrate that neither mimicking phosphorylation at S116 nor phosphorylation of the purified enzyme at S116 in vitro has any effect on enzymatic activity. Phospho-mimicking, however, does interfere with the interaction of eNOS with c-Src, an interaction which is known to activate eNOS by phosphorylation at tyrosine 83 (Y83). Agonist-stimulated eNOS-Src complex formation, as well as agonist-stimulated Y83 phosphorylation, are blocked by calcineurin inhibition by CsA and by a cell-permeable calcineurin inhibitory peptide. Taken together, these data suggest a mechanism of eNOS regulation whereby calcineurin-mediated dephosphorylation of eNOS at S116 affects eNOS enzymatic activity indirectly, rather than directly, by facilitating c-Src binding and Y83 phosphorylation.
Keywords: endothelial nitric oxide synthase (eNOS), calcineurin, phosphorylation, dephosphorylation, cyclosporin A
1. Introduction
Regulation of the endothelial nitric oxide synthase (eNOS) by phosphorylation is a highly complex process. Seven regulatory phosphorylation sites have been identified in bovine eNOS at Y83, S116, T497, S617, S635, Y659, and S1179 (bovine numbering of eNOS residues will be used throughout this manuscript). Equivalent, functional sites are also found in human eNOS at Y81, S114, T495, S615, S633, Y657, and S1177 (Fleming, 2010). Phosphorylation of eNOS at any one of these sites can have either a positive or a negative influence on eNOS enzymatic activity (Fleming, 2010; Mount et al., 2007). Agonist- and flow-stimulated phosphorylation at S1179, for example, increases eNOS activity (Fulton et al., 1999; Dimmeler et al., 1999) whereas phosphorylation at T497 reduces enzyme activity (Harris et al., 2001b; Fleming et al., 2001; Michell et al., 2001). Tyrosine phosphorylation of eNOS at Y83 also appears to have a requisite role in the agonist-stimulated eNOS activation process (Fulton et al., 2005; Fulton et al., 2007). Site-specific phosphorylation of eNOS at S116 appears to have an inhibitory role in eNOS regulation (Li et al., 2007; Bauer et al., 2003), although evidence has also been presented that argues against a role for S116 influencing eNOS activity under Ca2+-stimulated conditions in intact cells (Boo et al., 2003). Specifically, phosphorylation at S116 appears to have a role in long-term, sustained suppression of eNOS activity under basal conditions while vascular endothelial growth factor (VEGF)-stimulated S116 dephosphorylation has a role in short-term, transient eNOS activation. For example, we have shown recently that, under basal conditions in endothelial cells, eNOS is subjected to proline-directed phosphorylation at S116 by the ERK 1/2 protein kinases. This phosphorylation event produces a docking site in eNOS for the Pin1 prolyl isomerase. Subsequent Pin1-catalyzed prolyl isomerization of eNOS produces a conformational change in the enzyme that suppresses its catalytic activity in the long-term and consequently reduces the vascular reactivity of blood vessels (Ruan et al., 2011). In addition, Kou et al. (Kou et al., 2002) reported a decade earlier, that VEGF induces a rapid dephosphorylation of eNOS at S116. Dephosphorylation in this case is transient in nature, occurring within a time-frame between 10 and 30 min of VEGF exposure. This is followed by a rephosphorylation of S116 in eNOS that is complete by 60 min after initial VEGF exposure. Dephosphorylation is associated with an increase in NO release and appears to be mediated by the Ca2+-calmodulin (CaM)-dependent protein phosphatase, calcineurin, because it is inhibited by the immunosuppressive drug and calcineurin inhibitor, cyclosporin A (CsA). Consequently, the suggestion has been made that inhibition of S116 dephosphorylation by CsA may contribute to the known effect of the drug to induce endothelial dysfunction (Kou et al., 2002). Based on a lack of detection in the study by Kou et al. of S116 dephosphorylation in response to certain other agonists, these authors concluded that involvement of S116 dephosphorylation in agonist activation of eNOS is specific for VEGF signaling and does not occur in response to other stimuli.
In this study, we have investigated the possibility that agonist-induced, rapid dephosphorylation of eNOS at S116 may be a common feature of agonist activation of eNOS and that it is not restricted to VEGF alone. We have further investigated whether agonist-induced dephosphorylation in response to other agonists is also mediated by calcineurin. In addition, we have examined whether phosphorylation or mimicking of phosphorylation of the purified eNOS enzyme at S116 reduces its enzymatic activity. Surprisingly, we have found that, for the isolated enzyme, neither phosphorylation nor mimicking of phosphorylation at S116 has any effect on eNOS maximal activity or Ca2+-calmodulin (CaM) sensitivity, indicating an indirect mechanism of dephosphorylation-induced eNOS activation. We present evidence here for such a potential indirect mechanism of activation of eNOS in which dephosphorylation at S116 promotes eNOS interaction with the c-Src tyrosine kinase and subsequent eNOS activation through Src-mediated phosphorylation at Y83.
2. Materials and Methods
2.1 Materials
Anti-eNOS antibody and all materials for protein expression in the baculovirus system were obtained from BD Biosciences. Anti-phospho-S116 eNOS antibody and anti-Src antibody were purchased from Upstate-Cell Signaling Solutions. Preparation of the anti-phospho-Y83 eNOS antibody has been described previously (Fulton et al., 2007). Bradykinin, thapsigargin, angiopoietin, ATP, cyclosporin A, purified constitutively active ERK2, FK506, calmodulin, FAD, FMN, NADPH, arginine, and citrulline were obtained from Sigma-Aldrich. Tetrahydrobiopterin was purchased from Cayman Chemical. L-[14C]Arginine was from Perkin Elmer and AG-50W-X8 was from Bio-Rad. 2’,5’-ADP Sepharose came from GE Healthcare. Calcineurin autoinhibitory peptide (CAIP, sequence: RRRRRRRRRRRGGGRMAPPRDAMPSDA) and the scrambled control peptide (sequence: RRRRRRRRRRPDAGAPMGPGPARSMD) were purchased from Biomatik.
2.2 Cell Culture
Primary cultures of bovine aortic endothelial cells (BAECs) were purchased from VEC Technologies Inc. All endothelial cell experiments were performed on BAECs in passages 2 to 6. Cells were maintained in medium 199 supplemented with 10% FBS, 5% iron-supplemented calf serum, 0.6 µg/ml thymidine, 2.2 mg/ml sodium bicarbonate, 500 IU/ml penicillin, and 500 µg/ml streptomycin. COS-7 cells were maintained in DMEM supplemented with 10% FBS, 500 IU penicillin, and 500 µg/ml streptomycin. Sf9 insect cells were maintained in Grace’s medium supplemented with 10% FBS, 500 IU penicillin, and 500 µg/ml streptomycin.
2.3 Immunoprecipitation and Immunoblotting
Cell lysates were prepared by lysis of cells in ice-cold lysis buffer containing 50 mM Tris-HCl, pH 7.4, 100 mM NaF, 15 mM Na4P2O7, 1 mM Na3VO4, 1% Triton X-100, and 1 mM PMSF. Immunoprecipitation and immunoblotting were then carried out as described previously (Fulton et al., 2005). All data shown are representative of a minimum of at least 3 separate experiments and are also reported as means +/− S.E. Differences were analyzed by 2-way ANOVA and were considered significant at P<0.05.
2.4 Expression and Purification of eNOS
Bovine wild-type and S116D eNOS were expressed and purified in a baculovirus/Sf9 insect cell system as described in detail previously (Venema et al., 1995). Briefly, baculovirus expression of the two forms of eNOS enzyme was carried out in Sf9 insect cells. Cells were lysed and eNOS was purified to homogeneity (as judged by a single band on a Coomassie-stained SDS polyacrylamide gel) by affinity chromatography on 2’-5’-ADP-Sepharose.
2.5 Assay of eNOS Enzymatic Activity
Purified eNOS activity was measured by the method of Bredt and Snyder (Bredt and Snyder, 1990) which determines the rate of formation of L-[14C]citrulline from L-[14C]arginine (100 µM) in the presence of excess cofactors including Ca2+ (2 mM), calmodulin (200 units), NADPH (1 mM), FAD (4 µM), FMN (4 µM), and tetrahydrobiopterin (40 µM). Product (L-[14C]citrulline) was separated from substrate (L-[14C]arginine) on Bio-Rad AG 50W-X8 cation exchange columns.
2.6 In Vitro Phosphorylation
Purified wild-type eNOS (1µg) was incubated with purified constitutively active ERK2 (1µg) for 1 h at 30°C in buffer containing 50 mM HEPES, pH 7.5, 10 mM MgCl2, 1 mM EGTA, 0.001% TWEEN 20, in the absence and presence of 3.3 mM ATP. The reaction samples were placed on ice before being either analyzed by immunoblotting or being assayed for arginine-to-citrulline conversion activity.
3. Results
3.1 Dephosphorylation of eNOS at S116 is a Common Feature of Agonist Stimulation of Endothelial Cells
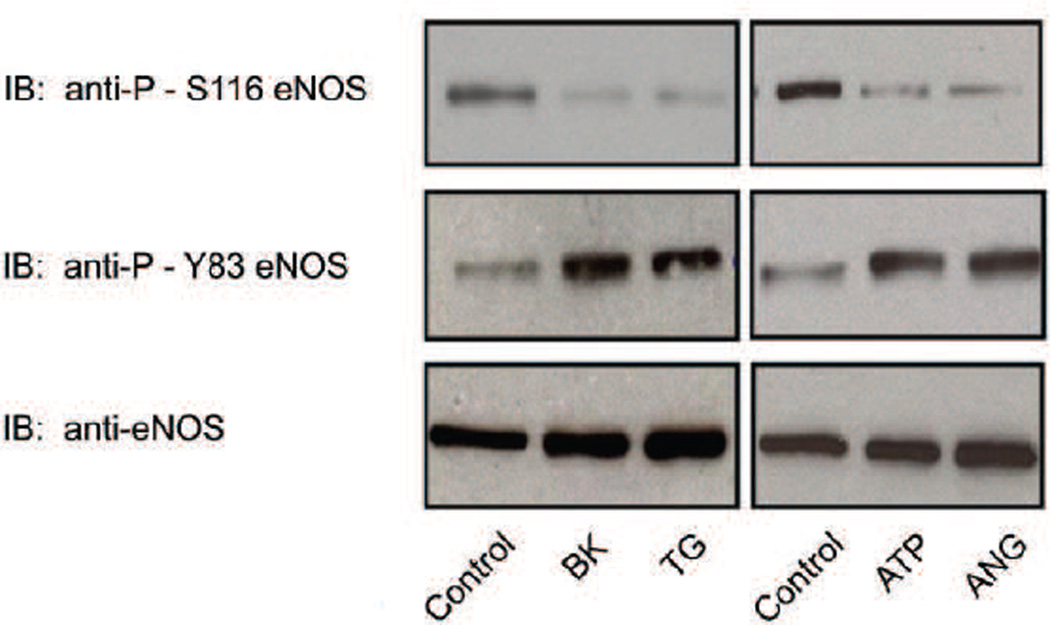
It has been reported previously that VEGF treatment of cultured bovine aortic endothelial cells (BAECs) induces dephosphorylation of eNOS at S116 whereas other agonists such as bradykinin (BK) do not (Kou et al., 2002). It has thus been concluded that S116 dephosphorylation in response to endothelial cell stimulation is specific for VEGF. We have also tested this hypothesis and have found that agonist stimulation of eNOS dephosphorylation at S116 is a common feature of the actions of many other eNOS-activating agonists and is not exclusive for VEGF. BAECs were either not treated or treated for 10 min with BK (10 µM), the intracellular Ca2+ elevating agent, thapsigargin (TG, 100 nM), ATP (10 µM), or angiopoietin (ANG, 50 ng/ml). Equal amounts of protein from lysates were analyzed by immunoblotting with non-phospho-specific and phospho-specific antibodies for eNOS phosphorylated at either S116 or Y83. Phosphorylation of Y83 was analyzed both as a positive control (to confirm that signaling to eNOS had occurred in response to agonist stimulation), and to confirm that dephosphorylation at S116 occurs during the same rapid time-frame as does phosphorylation at Y83. As shown in Fig. 1, all of the agonists tested (BK, TG, ATP, and ANG) induced a significant dephosphorylation of eNOS at S116 within 10 min. Densitometric and statistical analysis of blots from repeat experiments showed that BK reduced phosphorylation of S116 by 92 +/− 12 % (mean +/− S.E., n=6, P<0.05), TG reduced phosphorylation by 95 +/− 10% (mean +/− S.E., n=6, P<0.05), ATP reduced phosphorylation by 82 +/− 8% (mean +/− S.E., n=3, P<0.05), and ANG reduced phosphorylation by 77 +/− 6% (mean +/− S.E., n=3, P<0.05). Concurrently, Y83 in eNOS underwent a significant increase (approximately 3-fold in each case, determined by densitometry) in phosphorylation, consistent with the results for Y83 phosphorylation in response to agonist stimulation that we have previously reported (Fulton et al., 2007). No changes were detected in levels of total eNOS.
Fig. 1.
Agonist-stimulated dephosphorylation of eNOS at S116 and phosphorylation of eNOS at Y83 in BAECs. BAECs were either not treated (control) or treated for 10 min with bradykinin (BK, 1µM), thapsigargin (TG, 100 nM), ATP (10 µM), or angiopoietin (ANG, 50 ng/ml). Cells were lysed and equal quantities of lysate protein from each condition were immunoblotted (IB) with anti-phospho-S116 eNOS, anti-phospho-Y83 eNOS, and non-phospho-specific anti-eNOS antibodies, Results shown are representative of at least 3 separate experiments.
3.2 Identification of Calcineurin as the Protein Phosphatase Responsible for Agonist-induced eNOS Dephosphorylation at S116
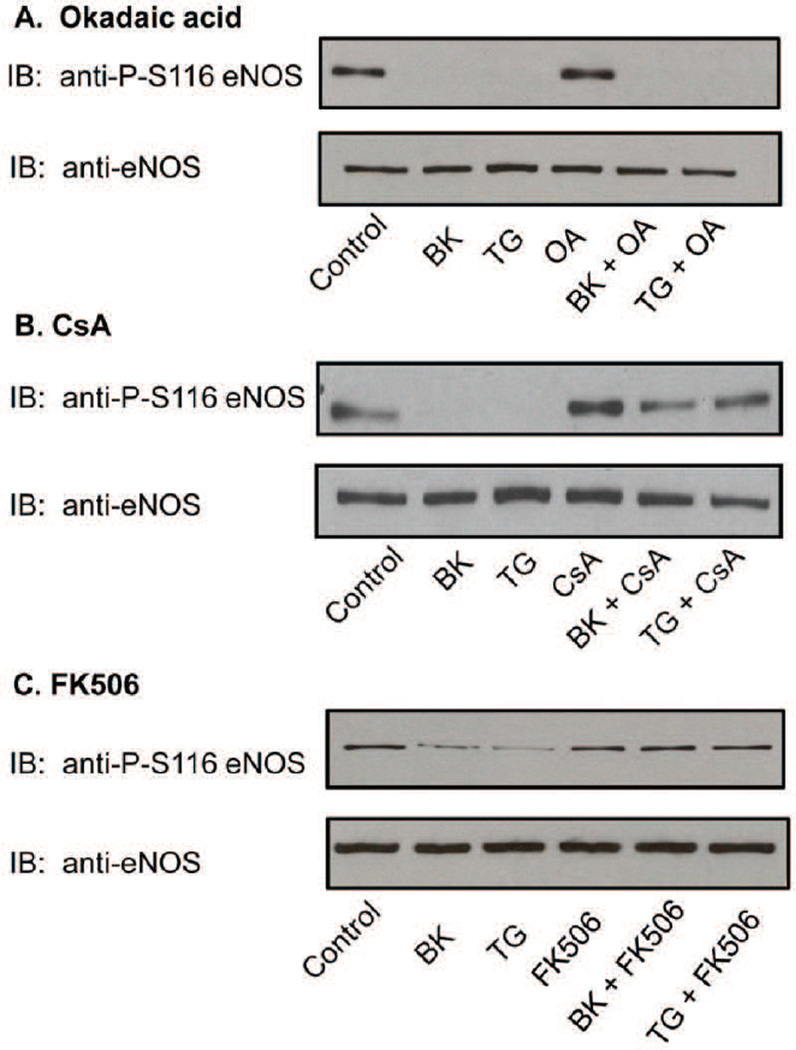
Four major serine/threonine-specific protein phosphatases are found in mammalian cells termed protein phosphatase-1 (PP1), protein phosphatase-2A (PP2A), protein phosphatase-2C (PP2C), and protein phosphatase-2B (PP2B). PP2B is a Ca2+-calmodulin (CaM)-dependent enzyme that is more commonly known by the name, calcineurin (Cohen and Cohen, 1989). PP1 and PP2A are potently inhibited by okadaic acid, whereas PP2C and calcineurin are not (Cohen et al., 1990). To test whether PP1 or PP2A might be responsible for S116 dephosphorylation, BAECs were pretreated with okadaic acid (100 nM for 30 min) prior to BK (1µM) and TG (100 nM) stimulation. eNOS dephosphorylation at S116 was analyzed as described above. Okadaic acid had no effect on either BK- or TG-stimulated dephosphorylation at S116 (Fig. 2A) suggesting that neither PP1 nor PP2A is responsible for catalyzing the dephosphorylation reaction. We next utilized cyclosporin A (CsA), an immunosuppressive drug that is commonly used as a specific inhibitor for calcineurin (Liu et al., 1991). BAECs were either not pretreated or pretreated with CsA (100 nm for 30 min) prior to treatment with BK (1µM) or TG (100 nM) for 10 min, followed by immunoblotting of equal amounts of protein from cell lysates. As shown in Fig. 2B, CsA completely blocked BK- and TG-stimulated dephosphorylation of eNOS at S116 without affecting levels of total eNOS. Confirmatory results showing complete inhibition of agonist-induced dephosphorylation were also obtained when cells were treated with a structurally distinct calcineurin inhibitor, FK506 (100 nM) (Liu et al., 1991) (Fig. 2C).
Fig. 2.
Effects of calcineurin inhibition on agonist-induced dephosphorylation of eNOS at S116 in BAECs. BAECs were either not pretreated or pretreated with okadaic acid (OA, 100nM), cyclosporin A (CsA, 100 nM), or FK506 (100 nM) for 30 min and then either not treated or treated with bradykinin (BK, 1uM) or thapsigargin (TG, 100 nM) for 10 min. Cells were lysed an equal quantities of lysate protein from each condition were immunoblotted (IB) with anti-phospho-S116 eNOS or non-phospho-specific anti-eNOS antibodies. A, okadaic acid. B, CsA. C, FK506. Similar results were obtained in at least 3 different experiments.
3.3 Phosphorylation or Mimicking of Phosphorylation of eNOS at S116 Does Not Alter Either the Ca2+-CaM-dependence or Maximal Enzymatic Activity of the Purified eNOS Enzyme
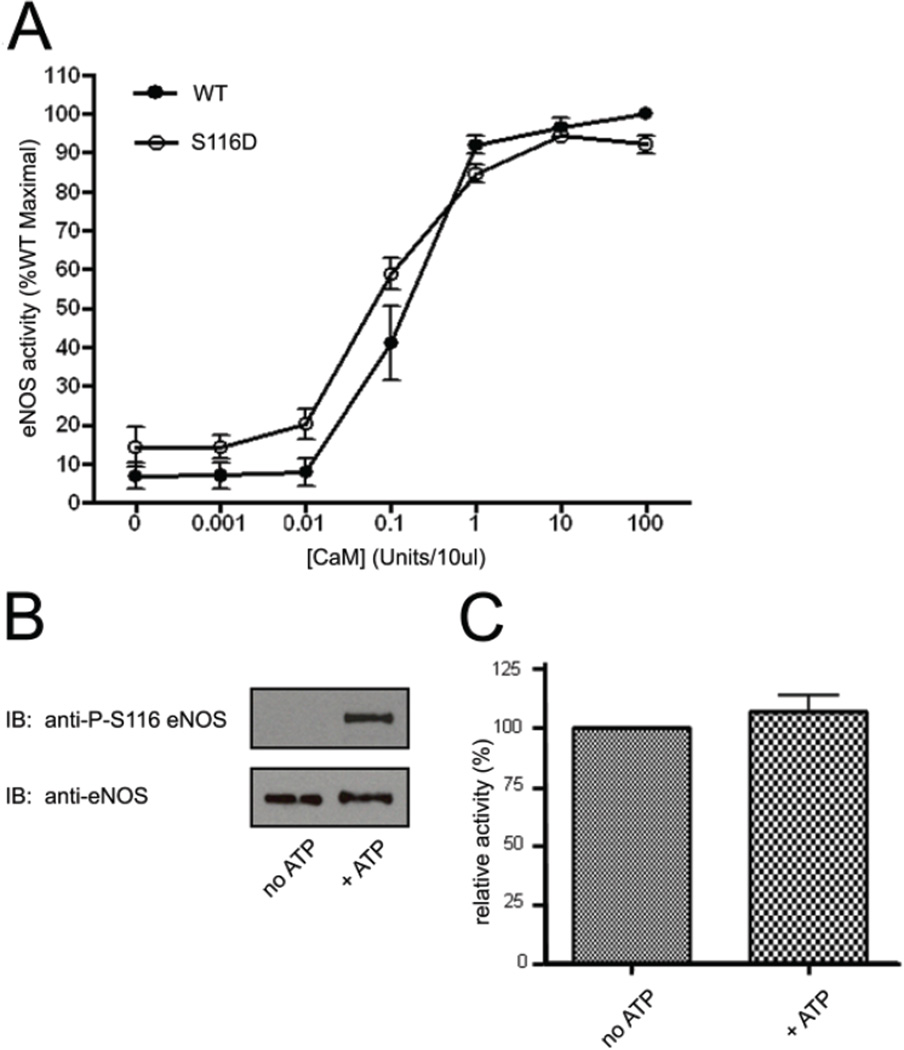
Agonist-stimulated and calcineurin-mediated dephosphorylation of eNOS in intact endothelial cells is accompanied by an increase in eNOS activity (Kou et al., 2002). Furthermore, we and others have also shown that an ectopically expressed phospho-mimetic S116D form of eNOS has reduced activity compared to the wild-type enzyme (Li et al., 2007; Bauer et al., 2003). We have now tested whether phosphorylation of the purified eNOS enzyme at S116 with purified ERK2 kinase results in a reduction of eNOS enzymatic activity. In addition, we have tested whether mimicking S116 phosphorylation by creation of an S116D eNOS mutant enzyme also results in an enzyme that has a lower specific activity. Bovine wild-type (WT) eNOS and S116D phospho-mimetic eNOS were expressed in a baculovirus/Sf9 insect cell system and purified to homogeneity as active enzymes by single-step, affinity chromatography on 2’, 5’-ADP-Sepharose as described previously (Venema et al., 1995). The relative enzymatic activities of equal quantities of freshly (never frozen) and highly purified WT eNOS and S116D eNOS were then determined by the method of Bredt and Snyder (Bredt and Snyder, 1990) which monitors the rate of formation of L-[14C] citrulline from L-[14C] arginine in the presence of excess cofactors including Ca2+, NADPH, FAD, FMN, tetrahydrobiopterin and, in this experiment, in the presence of increasing amounts of CaM. As shown in Fig. 3A, mimicking of phosphorylation of eNOS at S116 by substitution of a negatively charged aspartate for phosphoserine, had no effect on either the maximal activity or the Ca2+-CaM-dependence (no significant differences in EC50 values for Ca2+-CaM determined) of eNOS. The effects of phosphorylation in vitro of purified WT eNOS by purified ERK2 were also tested. Equal quantities of purified WT eNOS were incubated with equal quantities of a constitutively active form of ERK2 in the absence and presence of ATP. Immunoblotting with anti-phospho-S116 eNOS antibody confirmed that the purified enzyme was not phosphorylated as isolated and that phosphorylation of eNOS at S116 occurred in vitro, but only in the presence of the ERK2 phospho-donor substrate ATP (Fig. 3B). Arginine-to-citrulline conversion assay of eNOS enzymatic activity in the presence of a saturating concentration of Ca2+-CaM, however, showed no statistically significant effect of S116 phosphorylation on eNOS maximal activity (Fig. 3C). Changes were also not detected in Ca2+-CaM dependence of eNOS following S116 phosphorylation by ERK2.
Fig. 3.
Effects of mimicking phosphorylation of eNOS at S116 or of ERK2-catalyzed phosphorylation of eNOS at S116 on the Ca2+-CaM-dependence and maximal activity of purified eNOS. A, wild-type (WT) and S116D forms of eNOS were expressed and purified from a baculovirus system. The relative specific activities of equal quantities of the two purified enzymes were then determined by arginine-to-citrulline conversion assay in the presence of excess cofactors and Ca2+ and in the presence of either no CaM or increasing concentrations of CaM. Results shown are expressed as percent of maximal activity of the wild-type enzyme from 3 separate, fresh (never frozen) preparations of each enzyme (means ± S.E.). B, equal quantities of purified WT eNOS were incubated in vitro with active ERK2 in the absence and presence of added ATP. S116 phosphorylation was determined by immunoblotting with anti-phospho-S116 eNOS antibody. C, relative activities of the non-phosphorylated (no ATP) and phosphorylated enzymes in the presence of excess Ca2+-CaM were determined by arginine-to-citrulline conversion assay. Results shown are means ± S.E. from measurements of 3 separate, fresh preparations of eNOS incubated with active ERK2 without and with ATP.
3.4 Mimicking of Phosphorylation of eNOS at S116 Disrupts Interactions of eNOS with c-Src
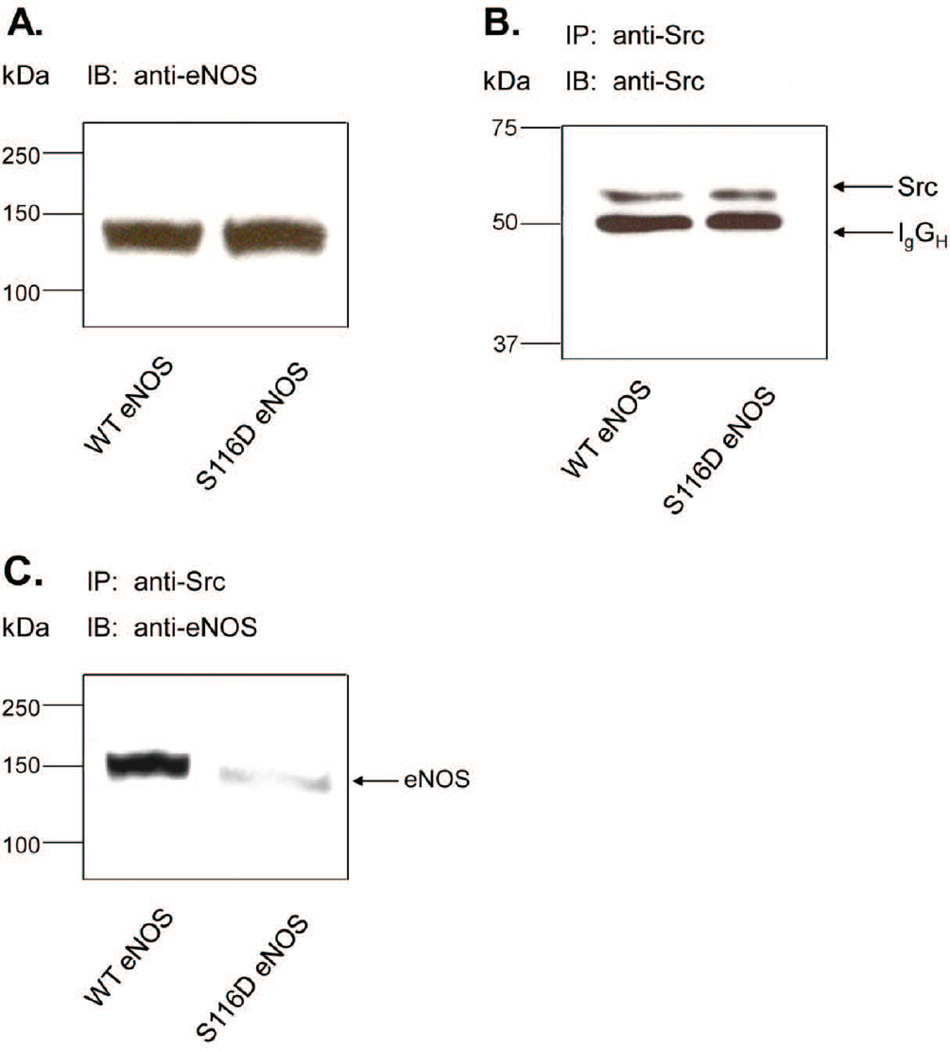
Because agonist-induced dephosphorylation of eNOS at S116 does not appear to directly affect eNOS enzymatic activity, we tested whether dephosphorylation might activate eNOS indirectly by reducing its interaction with another protein that we have previously shown to be essential in the agonist-stimulated eNOS activation process. This protein is the c-Src tyrosine kinase which activates eNOS by phosphorylation of the enzyme at Y83 (Fulton et al., 2005; Fulton et al., 2007). COS-7 cells were co-infected for 48 h with adenoviruses for either WT eNOS or S116D eNOS together with an adenovirus described previously that expresses human constitutively active c-Src (Fulton et al., 2005). Cells were lysed and equal quantities of lysate protein were immunoblotted with anti-eNOS antibody. Blotting showed that each of the two proteins were expressed at the same level (Fig. 4A). Src protein was then immunoprecipitated from lysates with anti-Src antibody. Src immunoprecipitates were them immunoblotted with anti-Src and anti-eNOS antibodies. As shown in Fig. 4B, equal amounts of Src protein were immunoprecipitated in each condition. WT eNOS (130 KDa) was also co-immunoprecipitated with Src. Co-immunoprecipitation was markedly reduced, however, with the phospho-mimetic S116D eNOS mutant (88 +/− 8% reduction, mean +/− S.E, n=3, P<0.05).
Fig. 4.
Effects of mimicking phosphorylation of S116 in eNOS on eNOS interactions with c-Src. Adenoviruses expressing either wild-type (WT) or phospho-mimetic S116D eNOS together with an adenovirus expressing constitutively active c-Src were used to transduce COS-7 cells. A, cell lysates were immunoblotted (IB) with anti-eNOS antibody. B, cell lysates were immunoprecipitated (IP) with anti-Src antibody and immunoblotted with the same anti-Src antibody. C, cell lysates were immunoprecipitated (IP) with anti-Src antibody and immunoblotted (IB) with anti-eNOS antibody. Results shown are representative of at least 3 separate experiments.
3.5 Agonist Stimulation of Endothelial Cells Results in a Transient Increase in Calcineurin-dependent eNOS-c-Src Complex Formation
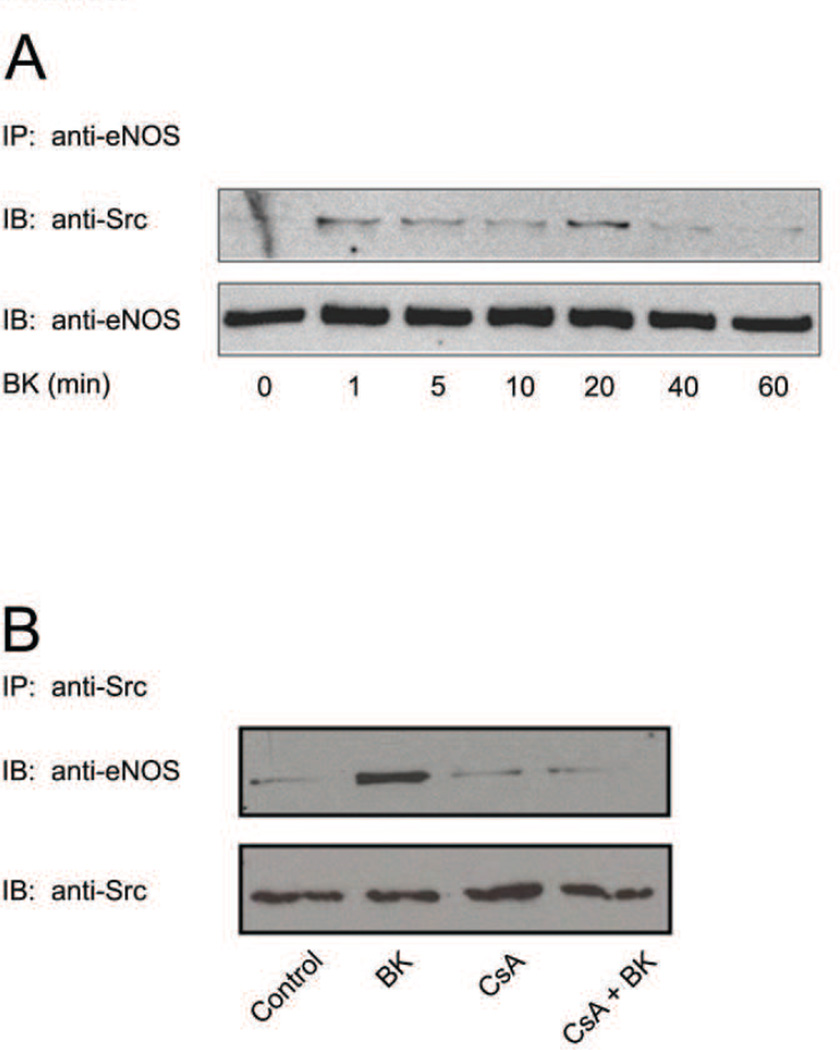
Under basal conditions, at least a subset of endothelial cell eNOS molecules exists in a stable binary complex with c-Src. Thus, we have demonstrated previously that the two proteins can be co-immunoprecipitated from BAEC lysates by an antibody directed against only one of the two proteins (Fulton et al., 2005). Because eNOS binding of Src appears to be negatively modulated by S116 phosphorylation, we sought to determine whether eNOS-Src interactions might be increased in BAECs during the time-frame of agonist-induced S116 dephosphorylation. Such an event would be consistent with a potential mechanism of eNOS activation by which agonist stimulation could facilitate increased eNOS-Src interaction resulting in subsequent or simultaneous phosphorylation of eNOS at Y83. BAECs were treated for either 0, 1, 5, 10, 20, 40, or 60 min with BK (1 µM). eNOS was immunoprecipitated with anti-eNOS antibody and immunoprecipitates were immunoblotted with anti-Src antibody in order to quantify the relative extent of eNOS-Src complex formation in each condition. Immunoprecipitates were also blotted for total eNOS with non-phospho-specific anti-eNOS antibody to confirm equal immunoprecipitation of eNOS in each condition. As shown in Fig. 5A, BK treatment of BAECs induces a rapid and transient binding of Src to eNOS that was maximal between 1 and 20 min (4.8 +/− 0.8-fold increase in eNOS-Src association (mean +/− S.E., n=5, P<0.05). Co-immunoprecipitation experiments to determine whether eNOS-Pin1 association was affected by BK treatment for these treatment times showed that there was no effect on eNOS-Pin1 binding. Co-immunoprecipiation experiments were also carried out in which S116 dephosphorylation was blocked by CsA. BAECs were either not treated or treated with BK (1 µM) for 10 min following either no pretreatment or pretreatment with CsA (100 nM for 30 min). Src was then immunoprecipitated followed by blotting of precipitated proteins with anti-eNOS and anti-Src antibodies. Calcineurin inhibition with CsA completely blocked BK-stimulated eNOS-Src complex formation in these experiments (Fig. 5B). No changes, however, were observed under any of the conditions in the amounts of immunoprecipitated Src.
Fig. 5.
Effects of agonist stimulation without and with calcineurin inhibition on eNOS-Src complex formation in BAECs. A, BAECs were treated for the times indicated with bradykinin (BK, 1 µM). Cell lysates were then subjected to immunoprecipitation (IP) with anti-eNOS antibody and immunoprecipitates were immunoblotted (IB) with either anti-Src or anti-eNOS antibodies. B, BAECs were either not pretreated or pretreated with cyclosporin A (CsA, 100 nM) and then either not stimulated or stimulated with bradykinin (BK) for 10 min. Cells were lysed and lysates were subjected to immunoprecipitation (IP) with anti-Src antibody and immunoblotted (IB) with either anti-eNOS or anti-Src antibodies. Results shown are similar to those obtained in at least 3 separate experiments.
3.6 Phosphorylation of eNOS at Y83 in Endothelial Cells is Dependent on Dephosphorylation at S116
Because agonist-stimulated dephosphorylation of eNOS at S116 appears to facilitate c-Src binding to eNOS and because c-Src activates eNOS by phosphorylating Y83 in the enzyme, we next investigated whether blockade of S116 dephosphorylation with CsA prevents or reduces BK-stimulated Y83 phosphorylation. BAECs were either not treated or treated with CsA (100 nM for 30 min) and then treated with BK (1µM for 10 min). Cells were lysed and lysate proteins were immunoblotted with phospho-Y83-specific and nonphospho-specific anti-eNOS antibodies. As shown in Fig. 6A, BK induced a clear phosphorylation of eNOS at Y83 which was completely blocked by calcineurin inhibition by CsA. Levels of total eNOS protein were not affected by any of the treatments. Experiments were also carried out using the same protocol except that calcineurin inhibition was achieved by utilizing a cell-permeable calcineurin autoinhibitory peptide (CAIP) first described by Terada et al. (Terada et al., 2003). This peptide contains an 11 arginine protein transduction domain (to facilitate cell permeability) in tandem with a 17 amino acid autoinhibitory domain found in calcineurin that potently inhibits calcineurin activity. BAECs were either not treated or treated for 2 h with the CAIP peptide or with a different cell-permeable peptide of the same length in which the autoinhibitory domain was scrambled (50µM of each). Cells were then lysed and lysates were immunoblotted with phospho-Y83-specific and nonphospho-specific anti-eNOS antibodies. As shown in Fig 6B, BK treatment of cells for 10 min stimulated a robust phosphorylation of Y83. CAIP treatment for 2 h increased the basal level of Y83 phosphorylation in BAECs that was equivalent to 30 +/− 5% (mean +/− S.E., n=3, P<0.05) of that achieved by 10 min of BK treatment. Addition of BK for 10 min following CAIP treatment, however, produced no further increase in phosphorylation above that produced by CAIP treatment alone. Pretreatment with the scrambled (inactive) peptide, on the other hand, had no effect on eNOS phosphorylation at Y83 relative to that seen with BK stimulation alone.
Fig. 6.
Effects of calcineurin inhibition on agonist-induced phosphorylation of eNOS at Y83 in BAECs. BAECs were either not pretreated or pretreated with: Panel A, cyclosporin A (CsA, 100 nM for 30 min), or Panel B, calcineurin autoinhibitory peptide (CAIP, 50 µM for 2 h) or a peptide in which the autoinhibitory sequence was scrambled (50 µM for 2h). Cells were then either not stimulated or stimulated with bradykinin (BK, 1 µM for 10 min). Cell lysates were immunoblotted (IB) with either anti-phospho-Y83 eNOS antibody or non-phospho-specific anti-eNOS antibody. Similar results to those shown were obtained in at least 3 separate experiments.
4. Discussion
The results of the present study provide several new insights into the role of reversible S116 phosphorylation of eNOS in regulation of NO production by endothelial cells. A first important finding of our investigation is that agonist activation of eNOS in response to a variety of different eNOS-activating agonists is associated with S116 dephosphorylation. Thus, this event appears to be a common feature of agonist stimulation of endothelial cells and is not restricted to VEGF signaling as previously suggested by Kou et al. (Kou et al., 2002). Among the agonists tested in this study were bradykinin (BK), ATP, angiopoietin, and thapsigargin (TG). Kou et al. tested only VEGF and BK and failed to detect an effect of BK on S116 phosphorylation. It is conceivable that the reported negative results of Kou et al. for BK stimulation may relate to the use of later passage cultured endothelial cells in that particular study, as it is known that cultured endothelial cells lose their responsiveness to certain agonists in later passage cells. Furthermore, we also tested for a rapid induction of S116 dephosphorylation by TG. This compound works to activate eNOS by increasing intracellular calcium levels (Thastrup, 1990). It is therefore likely that other agonists that elevate cytosolic endothelial cell calcium, including acetylcholine, thrombin, endothelin-1, and others also promote dephosphorylation of eNOS at S116. Furthermore, concurrent agonist stimulation in endothelial cells of eNOS-Src association, as shown in the present study, of S116 dephosphorylation (Kou et al., 2002), of Y83 phosphorylation (Fulton et al., 2007), and of NO release (He et al., 1999) are all transient in nature and are highly correlated in time (maximal between 10 and 30 min with each returning to baseline within 60 minutes). This suggests the possibility that these events all contribute in a coordinated fashion to the agonist-induced eNOS activation process in endothelial cells.
We also present evidence that Ca2+- and agonist-induced dephosphorylation of eNOS at S116 is mediated in each case by the Ca2+-CaM-dependent protein phosphatase, calcineurin. Dephosphorylation is potently blocked by cyclosporin A (CsA). We have shown previously that CsA blocks BK-stimulated dephosphorylation of T497 in eNOS and also blocks BK-stimulated NO release from endothelial cells (Harris et al., 2001b). In this previous study, we postulated that the known effect of CsA to induce hypertension and endothelial dysfunction (ie. in post-transplant patients) (Morris et al., 2000) might be mediated in part through prevention of agonist-stimulated and calcineurin-catalyzed dephosphorylation of eNOS at the eNOS-inhibitory T497 phosphorylation site. The present study suggests a more prominent dual role for calcineurin in eNOS activation that occurs through reversal of the inhibitory actions of phosphorylation of eNOS at both the T497 and S116 phosphorylation sites.
Our results demonstrate further that phosphorylation/dephosphorylation of eNOS at S116 by itself is not sufficient to alter the enzymatic activity of the isolated, purified enzyme. When baculovirus-expressed, purified bovine eNOS was used as a substrate for constitutively active ERK2, S116 in eNOS was phosphorylated. However, no change in enzyme activity was observed. Furthermore, phospho-mimetic S116D eNOS was found to have identical Ca2+-CaM-dependence and maximal activity to that of wild-type eNOS. Bernier et al. (Bernier et al., 2000) have shown previously that, when eNOS is immunoprecipitated from BAECs and immunoprecipitated proteins are subsequently phosphorylated in vitro by ERK2, that there is a significant reduction in eNOS enzyme activity. These authors suggested that ERK2 probably phosphorylates an unknown site in eNOS that suppresses that activity of the enzyme. At the time of that study, the only eNOS phosphorylation site that had yet been identified was S1179 and the only eNOS phospho-specific antibody available was one that was specific for the S1179 phosphorylation site. S1179, however, was ruled out by Bernier et al. as the site of ERK2 phosphorylation. In the present study, and in many of our previous studies (Ju et al., 1997; Ju et al., 1998; Harris et al., 2001a; Venema et al., 2003; Li et al., 2005; Harris et al., 2006; Ruan et al., 2011), we have found that eNOS immunoprecipitation from BAECs results in co-precipitation of a large number of other eNOS-interacting accessory proteins, including (as shown here) c-Src. It is conceivable that in the investigation by Bernier et al., ERK2 phosphorylation of S116 in eNOS immune complexes, with consequent reduction in eNOS activity, may have occurred by an indirect mechanism via prevention of Y83 phosphorylation, potentially catalyzed by co-immunoprecipitating c-Src.
A final important conclusion that follows from the results that we have presented here is that eNOS regulation by phosphorylation/dephosphorylation is a very complex process. By far the best-studied eNOS phosphorylation site is S1179 and, when the eNOS protein is phosphorylated at this site, it is often referred to simply as “phospho-eNOS”. Focusing only on S1179 phosphorylation, however, is an over-simplication because many different regulatory phosphorylation sites are involved in eNOS regulation (7 have been identified thus far and there may be others that are yet to be identified). In addition, cross-talk exists between different sites of eNOS phosphoregulation. Moreover, phosphorylation or dephosphorylation does not always involve a direct effect on eNOS enzymatic activity. Indirect effects of eNOS phosphorylation at one specific site include modulation of eNOS interactions with other eNOS-interacting accessory proteins. Phosphorylation/dephosphorylation at one site can also influence the state of phosphorylation/dephosphorylation at a completely different site. Our data demonstrate an important role of this phenomenon in regulation of eNOS activity in endothelial cells. In the case presented here, calcineurin-mediated dephosphorylation of eNOS at S116 affects eNOS activity indirectly, rather than directly, by facilitating c-Src binding and Y83 phosphorylation. This will serve to increase the activity of the eNOS enzyme, given that we have shown previously (Fulton et al., 2005; Fulton et al., 2007) that Y83 phosphorylation of eNOS positively modulates enzyme activity.
Acknowledgements
This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute [Grants HL085827, HL108719]; and the American Heart Association [Grant 0655250B].
Abbreviations
- eNOS
endothelial nitric oxide synthase
- VEGF
vascular endothelial growth factor
- ERK
extracellular signal regulated kinase
- Pin
protein interacting with never in mitosis A
- CAIP
calcineurin autoinhibitory peptide
- BAECs
bovine aortic endothelial cells
- BK
bradykinin
- TG
thapsigargin
- ANG
angiopoietin
- PP1
protein phosphatase-1
- PP2A
protein phosphatase-2A
- PP2C
protein phosphatase-2C
- PP2B
protein phosphatase-2B
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bauer PM, Fulton D, Boo YC, Sorescu GP, Kemp BE, Jo H, Sessa WC. Compensatory Phosphorylation and Protein-Protein Interactions Revealed by Loss of Function and Gain of Function Mutants of Multiple Serine Phosphorylation Sites in Endothelial Nitric-Oxide Synthase. J. Biol. Chem. 2003;278:14841–14849. doi: 10.1074/jbc.M211926200. [DOI] [PubMed] [Google Scholar]
- Boo YC, Sorescu GP, Bauer PM, Fulton D, Kemp BE, Harrison DG, Sessa WC, Jo H. Endothelial NO Synthase Phosphorylated at Ser635 Produces NO Without Requiring Intracellular Calcium Increase. Free Radic. Biol. Med. 2003;35:729–741. doi: 10.1016/s0891-5849(03)00397-6. [DOI] [PubMed] [Google Scholar]
- Bernier SG, Haldar S, Michel T. Bradykinin-Regulated Interactions of the Mitogen-Activated Protein Kinase Pathway With the Endothelial Nitric-Oxide Synthase. J. Biol. Chem. 2000;275:30707–30715. doi: 10.1074/jbc.M005116200. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Snyder SH. Isolation of Nitric Oxide Synthetase, a Calmodulin-Requiring Enzyme. Proc. Natl. Acad. Sci. USA. 1990;87:682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, Cohen PTW. Protein Phosphatases Come of Age. J. Biol. Chem. 1989;264:21435–21438. [PubMed] [Google Scholar]
- Cohen P, Holmes CFB, Tsukitani Y. Okadaic Acid: a New Probe for the Study of Cellular Regulation. Trends Biochem. Sci. 1990;15:98–102. doi: 10.1016/0968-0004(90)90192-e. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of Nitric Oxide Synthase in Endothelial Cells by Akt-Dependent Phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R. Phosphorylation of Thr495 Regulated Ca2+/Calmodulin-Dependent Endothelial Nitric Oxide Synthase Activity. Circ. Res. 2001;88:e68–e75. doi: 10.1161/hh1101.092677. [DOI] [PubMed] [Google Scholar]
- Fleming I. Molecular mechanisms underlying the activation of eNOS. Pflugers Arch. 2010;459:793–806. doi: 10.1007/s00424-009-0767-7. [DOI] [PubMed] [Google Scholar]
- Fulton D, Church JE, Ruan L, Li C, Sood SG, Kemp BE, Jennings IG, Venema RC. Src Kinase Activates Endothelial Nitric-Oxide Synthase by Phosphorylating Tyr-83. J. Biol. Chem. 2005;280:35943–35952. doi: 10.1074/jbc.M504606200. [DOI] [PubMed] [Google Scholar]
- Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of Endothelium-Derived Nitric Oxide Production by the Protein Kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton D, Ruan L, Sood SG, Li C, Zhang Q, Venema RC. Agonist-Stimulated Endothelial Nitric Oxide Activation and Vascular Relaxation: Role of eNOS Phosphorylation at Tyr-83. Circ. Res. 2007;102:497–504. doi: 10.1161/CIRCRESAHA.107.162933. [DOI] [PubMed] [Google Scholar]
- Harris MB, Bartoli M, Sood SG, Matts RL, Venema RC. Direct Interaction of the Cell Division 37 Homolog Inhibits Endothelial Nitric Oxide Synthase Activity. Circ. Res. 2006;98:335–341. doi: 10.1161/01.RES.0000203564.54250.0b. [DOI] [PubMed] [Google Scholar]
- Harris MB, Ju H, Venema VJ, Blackstone M, Venema RC. Role of Heat Shock Protein 90 in Bradykinin-Stimulated Endothelial Nitric Oxide Release. Gen. Pharmacol. 2001a;35:165–170. doi: 10.1016/s0306-3623(01)00104-5. [DOI] [PubMed] [Google Scholar]
- Harris MB, Ju H, Venema VJ, Liang H, Zou R, Michell BJ, Chen ZP, Kemp BE, Venema RC. Reciprocal Phosphorylation and Regulation of Endothelial Nitric-Oxide Synthase in Response to Bradykinin Stimulation. J. Biol. Chem. 2001b;276:16587–16591. doi: 10.1074/jbc.M100229200. [DOI] [PubMed] [Google Scholar]
- He H, Venema VJ, Gu X, Venema RC, Marrero MB, Caldwell RB. Vascular Endothelial Growth Factor Signals Endothelial Cell Production of Nitric Oxide and Prostacyclin through Flk-1/KDR Activation of c-Src. J. Biol. Chem. 1999;274:25130–25135. doi: 10.1074/jbc.274.35.25130. [DOI] [PubMed] [Google Scholar]
- Ju H, Venema VJ, Marrero MB, Venema RC. Inhibitory Interactions of the Bradykinin B2 Receptor With Endothelial Nitric-Oxide Synthase. J. Biol. Chem. 1998;273:24025–24029. doi: 10.1074/jbc.273.37.24025. [DOI] [PubMed] [Google Scholar]
- Ju H, Zou R, Venema VJ, Venema RC. Direct Interaction of Endothelial Nitric-Oxide Synthase and Caveolin-1 Inhibits Synthase Activity. J. Biol. Chem. 1997;272:18522–18525. doi: 10.1074/jbc.272.30.18522. [DOI] [PubMed] [Google Scholar]
- Kou R, Greif D, Michel T. Dephosphorylation of Endothelial Nitric Oxide Synthase by Vascular Endothelial Growth Factor: Implications for the Vascular Responses to Cyclosporin A. J. Biol. Chem. 2002;277:29669–29673. doi: 10.1074/jbc.M204519200. [DOI] [PubMed] [Google Scholar]
- Li C, Huang W, Harris MB, Goolsby JM, Venema RC. Interaction of the Endothelial Nitric Oxide Synthase With the CAT-1 Arginine Transporter Enhances NO Release by a Mechanism Not Involving Arginine Transport. Biochem. J. 2005;386:567–574. doi: 10.1042/BJ20041005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Ruan L, Sood SG, Papapetropoulos A, Fulton D, Venema RC. Role of eNOS Phosphorylation at Ser-116 in Regulation of ENOS Activity in Endothelial Cells. Vasc. Pharmacol. 2007;47:257–264. doi: 10.1016/j.vph.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Farmer JD, Jr, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin Is a Common Target of Cyclophilin-Cyclosporin A and FKBP-FK506 Complexes. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Michell BJ, Chen ZP, Tiganis T, Stapleton D, Katsis F, Power DA, Sim AT, Kemp BE. Coordinated Control of Endothelial Nitric-Oxide Synthase Phosphorylation by Protein Kinase C and the CAMP-Dependent Protein Kinase. J. Biol. Chem. 2001;276:17625–17628. doi: 10.1074/jbc.C100122200. [DOI] [PubMed] [Google Scholar]
- Morris ST, McMurray JJ, Rodgers RS, Farmer R, Jardine AG. Endothelial Dysfunction in renal transplant recipients maintained on cyclosporine. Kidney Int. 2000;57:1100–1106. doi: 10.1046/j.1523-1755.2000.00937.x. [DOI] [PubMed] [Google Scholar]
- Mount PF, Kemp BE, Power DA. Regulation of Endothelial and Myocardial NO Synthesis by Multi-Site ENOS Phosphorylation. J. Mol. Cell. Cardiol. 2007;42:271–279. doi: 10.1016/j.yjmcc.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Ruan L, Torres CM, Qian J, Chen F, Mintz JD, Stepp DW, Fulton D, Venema RC. Pin1 Prolyl Isomerase Regulates Endothelial Nitric Oxide Synthase. Arterioscler. Thromb. Vasc. Biol. 2011;31:392–398. doi: 10.1161/ATVBAHA.110.213181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada H, Matsushita M, Lu YF, Shirai T, Li ST, Tomizawa K, Moriwaki A, Nishio S, Date I, Ohmoto T, Matsui H. Inhibition of Excitatory Neuronal Cell Death by Cell-Permeable Calcineurin Autoinhibitory Peptide. J. Neurochem. 2003;87:1145–1151. doi: 10.1046/j.1471-4159.2003.02098.x. [DOI] [PubMed] [Google Scholar]
- Thastrup O. Role of Ca2(+)-ATPases in regulation of cellular Ca2+ signalling, as studied with the selective microsomal Ca2(+)-ATPase inhibitor, thapsigargin. Agents Actions. 1990;29:8–15. doi: 10.1007/BF01964706. [DOI] [PubMed] [Google Scholar]
- Venema RC, Sayegh HS, Arnal JF, Harrison DG. Role of the Enzyme Calmodulin-Binding Domain in Membrane Association and Phospholipid Inhibition of Endothelial Nitric Oxide Synthase. J. Biol. Chem. 1995;270:14705–14711. doi: 10.1074/jbc.270.24.14705. [DOI] [PubMed] [Google Scholar]
- Venema RC, Venema VJ, Ju H, Harris MB, Snead C, Jilling T, Dimitropoulou C, Maragoudakis ME, Catravas JD. Novel Complexes of Guanylate Cyclase With Heat Shock Protein 90 and Nitric Oxide Synthase. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H669–H678. doi: 10.1152/ajpheart.01025.2002. [DOI] [PubMed] [Google Scholar]