Abstract
Objective
To compare resin–dentin bond strengths and the micropermeability of hydrophobic vs. hydrophilic resins bonded to acid-etched or EDTA-treated dentin, using the ethanol wet-bonding technique.
Methods
Flat dentin surfaces from extracted human third molars were conditioned before bonding with: 37% H3PO4 (15 s) or 0.1 M EDTA (60 s). Five experimental resin blends of different hydrophilicities and one commercial adhesive (SBMP: Scotchbond Multi-Purpose) were applied to ethanol wet-dentin (1 min) and light-cured (20 s). The solvated resins were used as primers (50% ethanol/50% comonomers) and their respective neat resins were used as the adhesive. The resin-bonded teeth were stored in distilled water (24 h) and sectioned in beams for microtensile bond strength testing. Modes of failure were examined by stereoscopic light microscopy and SEM. Confocal tandem scanning microscopy (TSM) interfacial characterization and micropermeability were also performed after filling the pulp chamber with 1 wt% aqueous rhodamine-B.
Results
The most hydrophobic resin 1 gave the lowest bond strength values to acid-etched dentin and all beams failed prematurely when the resin was applied to EDTA-treated dentin. Resins 2 and 3 gave intermediate bond strengths to both conditioned substrates. Resin 4, an acidic hydrophilic resin, gave the highest bond strengths to both EDTA-treated and acid-etched dentin. Resin 5 was the only hydrophilic resin showing poor resin infiltration when applied on acid-etched dentin.
Significance
The ethanol wet-bonding technique may improve the infiltration of most of the adhesives used in this study into dentin, especially when applied to EDTA-treated dentin. The chemical composition of the resin blends was a determining factor influencing the ability of adhesives to bond to EDTA-treated or 37% H3PO4 acid-etched dentin, when using the ethanol wet-bonding technique in a clinically relevant time period.
Keywords: Microtensile bond strength, Micropermeability Confocal microscopy, Ethanol-saturated dentin, Hydrophobic hybrid layer
1. Introduction
Etch-and-rinse adhesives require application of acid-conditioners to dentin, as the first step of the bonding procedure. The most commonly used acid etchant is ortho-phosphoric that modifies the smear layer and exposes the collagen fibril network [1,2]. The 50 vol% of dentin previously occupied by minerals, is replaced by water after rinsing [3]. This water is necessary to prevent the collapse of the collagen fibrils and allow the adhesive monomers to diffuse into the demineralized collagen network. The adhesive system should displace as much water as possible from the demineralized dentin in order to create a hypothetical perfect encapsulation of all the collagen fibrils with the intention of obtaining optimal and durable bonds [4–6].
Water sorption and plasticization of resins are determined by their hydrophilicity [7]. The more hydrophilic the resin, the more the water sorption and plasticization [8,9]. Nishitani et al. [10] showed that adhesive bond strengths to acid-etched dentin matrices are directly related to the hydrophilicity of the resins. It has been hypothesized that if dentin could be bonded with more hydrophobic resins, a major decrease in water sorption would result in increased longevity of the resin–dentin interfaces. It is possible to coax hydrophobic monomers into a hydrophilic matrix by saturating the matrix with ethanol [10–12] without any sign of phase change and/or micropermeability at the resin–dentin interface [13]. Even though this technique leads to better infiltration of hydrophilic and/or hydrophobic dimethacrylates into ethanol-saturated matrices [14], concerns have been raised that the ethanol saturation of demineralized dentin requires 5 min, which may not be suitable for clinical practice.
Because EDTA creates a thinner demineralized layer compared to 37% phosphoric acid, it was thought that resins could infiltrate the thinner hybrid layers faster than is possible with thicker demineralized zones. The purpose of this study was to compare resin–dentin bond strengths of hydrophobic vs. hydrophilic resins bonded to acid-etched or EDTA-treated dentin created with a modified ethanol wet-bonding technique using a shorter period of ethanol saturation (1 min). Confocal tandem scanning microscopy (TSM) was used for interfacial characterization and micropermeability evaluation, with the intention of evaluating the quality and morphology of the resin–dentin bonded interfaces. The test null hypotheses were that there is no difference in bond strength following EDTA vs. phosphoric acid pretreatment using hydrophobic vs. hydrophilic resins and there is no difference in the micropermeability of the hybrid layers produced by those same procedures.
2. Materials and methods
2.1. Specimen preparation
Ninety-six human molars (age 20–40), extracted for periodontal reasons under a protocol approved by the institutional review board of Guy’s Hospital were used in this study for the microtensile bond strength and Confocal microscopy tests. The teeth were stored in 4°C water for no more than one month. The specimens were sectioned below the dentin–enamel junction (Accutom-50, Struers, Copenhagem, Denmark) using a water-cooled diamond saw (330-CA RS-70300, Struers, Copenhagen, Denmark). The occlusal surfaces were ground flat (LaboPol-4, Struers, Copenhagem, Denmark) using an abrasive paper (180- and 500-grit) under constant water irrigation to provide standardized smear-layer covered dentin surfaces.
2.2. Experimental resins
The five comonomer blends used in this study as dentin bonding agents (DBAs) were formulated based on known concentrations of all ingredients, including 50 wt% ethanol-solvated resin mixtures used as primers (Table 1). All experimental neat resins contained 0.25 wt% camphoquinone and 1.0 wt% ethyl-dimethyl-4-aminobenzoate.
Table 1.
Composition of neat and solvated resins used in this study.
| Neat resin (pH: ~7) | Solvated primer (pH: ~7) | Total conc. (mole/L) of non-volatile ingredientsa |
|---|---|---|
|
| ||
| Resin 1 (most hydrophobic) | ||
| 70 wt% E-BisADM | 34.4 wt% E-BisADM | 2.55 |
| 28.75% TEGDMA | 14.35% TEGDMA | |
| - | 50 wt% ethanol | |
|
| ||
| Resin 2 (second most hydrophobic) | ||
| 70 wt% BisGMA | 34.4 wt% BisGMA | 2.37 |
| 28.75% TEGDMA | 14.35% TEGDMA | |
| - | 50 wt% ethanol | |
|
| ||
| Resin 3 (mildly hydrophilic) | ||
| 70 wt% BisGMA | 34.4 wt% BisGMA | 3.58 |
| 28.75% HEMA | 14.35% HEMA | |
| - | 50 wt% ethanol | |
| Neat resin (pH: ~7) | Solvated primer (pH: ~4) | |
|
| ||
| Resin 4 (more hydrophilic) | ||
| 40 wt% BisGMA | 20 wt% BisGMA | 3.57 |
| 30 wt% TDCM | 14.4 wt% BisMP | |
| 28.75% HEMA | 14.35% HEMA | |
| - | 50 wt% ethanol | |
| Neat resin (pH: ~4) | Solvated primer (pH: ~3) | |
|
| ||
| Resin 5 (most hydrophilic) | ||
| 40 wt% BisGMA | 20 wt% BisGMA | 3.92 |
| 30 wt% BisMP | 14.4 wt% BisMP | |
| 28.75% HEMA | 14.35% HEMA | |
| - | 50 wt% ethanol | |
| Neat adhesive (pH: 3) | Solvated adhesive (pH: 3) | |
|
| ||
| Scotchbond Multi-Purposeb (adhesive-bottle) (3M ESPE, St. Paul, MN, USA) | ||
| BisGMA | BisGMA | Unknown |
| HEMA | HEMA | |
| Polyalkenoic acid polymer | Polyalkenoic acid polymer | |
| Tertiary amines | Tertiary amines | |
| Photo-initiator | Photo-initiator | |
| - | 50 wt% ethanol | |
| Adhesive (pH: 8.2) | Primer (pH: 4.3) | |
|
| ||
| Scotchbond Multi-Purposeb (adhesive-bottle) (3M ESPE, St. Paul, MN, USA) | ||
| BisGMA | HEMA | Unknown |
| Polyalkenoic acid polymer | Polyalkenoic acid polymer | |
| HEMA | Water | |
| Tertiary amines | - | |
| Photo-initiator | - | |
All the experimental resin blends also contained 0.25wt% camphorquinone and 1.0wt% 2-ethyl-dimethyl-4-aminobenzoate. Abbreviations: E-BisADM = ethoxylated BisPhenol A dimethacrylate; BisGMA = 2,2-bis[4-(2-hydroxy-3-methacryloylpropoxy)]-phenyl propane; TEGDMA = triethyleneglycol dimethacrylate; HEMA = 2-hydroxyethylmethacrylate; TCDM = di-(hydroxyethyl-methacrylate) ester of 5-(2,5-dioxotetrahydrofurfuryl)-methyl-3-cyclohexane-1,2_-dicarboxylic acid; BisMP = Bis[2-(methacryloyloxy)ethyl]phosphate.
Comonomer concentrations of neat resin blends were calculated by summing the molar concentrations of the non-volatile constituents [31].
Percentage of the constituents of the primer and adhesive of the commercial Adper Scotchbond Multi-Purpose are not given by the 3M ESPE, St. Paul, MN, USA.
Resins 1 and 2 are similar to non-solvated hydrophobic resins used in the final step of three-step, etch-and-rinse, and two-step, self-priming adhesives. Resin 3 represents the formulation of typical two-step, etch-and-rinse adhesives, while resins 4 and 5 correspond to very hydrophilic one-step, self-etching adhesives, containing carboxylic- or phosphate-substituted methacrylates, respectively.
2.3. Solubility of the resins in water vs. ethanol
Most methacrylate-based monomers have poor water solubility even though they are claimed to be hydrophilic (Table 2). A standard quantity (2 mL) of each experimental comonomer mixture was mixed with 10 mL of water or ethanol. The solvent-saturated with resin was removed and transferred to a tared container and the increase in mass was gravimetrically evaluated after solvent evaporation. This provided quantitative data of the relative solubility of these resins. Note that among the experimental comonomers, the most hydrophobic blend, resin 1, was 64 times more soluble in ethanol than water (Table 2).
Table 2.
Relative solubility (g comonomer/mL solvent) of experimental comonomers and selected adhesive monomers in water vs. ethanol.
| Water | Ethanol | Ethanol/water | |
|---|---|---|---|
| Resin 1 | 0.0027 | 0.1735 | 64.3 |
| Resin 2 | 0.0038 | 0.1632 | 42.8 |
| Resin 3 | 0.0063 | 0.1268 | 20.1 |
| Resin 4 | 0.0118 | 0.1296 | 11.0 |
| Resin 5 | 0.0422 | 0.1515 | 3.6 |
| HEMA | 0.1120 | 0.1451 | 1.3 |
| TEGDMA | 0.0095 | 0.1692 | 17.8 |
| BisGMA | - | 0.1688 | - |
| BisMP | 0.1282 | 0.1890 | 1.5 |
2.4. Bonding procedures
Dentin surfaces were acid-etched for 15 s with 37% phosphoric acid (PA) or treated with 0.1 M EDTA (pH 7.8) for 60 s and then bonded using the ethanol wet-bonding technique. The ethanol wet-bonding substrate was achieved by covering the conditioned, water-rinsed dentin surfaces with absolute ethyl alcohol for 1 min. The procedure was performed by keeping the dentin specimens visibly moist with ethanol prior to the application of the resin blends [13].
Two consecutive coats of the five 50% ethanol/50% experimental primers were then applied onto the ethanol wet-dentin. Excess solvent was gentle air-dried from the primer/dentin for 3 s. Subsequently, a layer of each respective neat comonomer adhesive was applied, spread thin with moisture-free air, and light-cured for 20 s (Translux EC halogen light-curing unit, Kulzer GmBh, Bereich Dental, Werheim, Germany). The output intensity was monitored with a Demetron Radiometer (Model 100, Demetron Research, Danbury, CT, USA). A minimal light output intensity of 600 mW/cm2 was employed for the experiments. A commercial adhesive Scotchbond Multi-Purpose (SBMP) (3M ESPE, St. Paul, MN, USA) was also applied with the ethanol wet-bonding either as per manufactures’ instructions (i.e. application of the primer and adhesive layers) or applying of the 50% adhesive diluted with 50% ethanol, in two consecutive coats followed by one layer of neat adhesive. Composite build-ups (6 mm) were constructed with a light-cured flowable resin composite Tetric EvoFlow® (Ivoclar, Vivadent, Schaan, Liechtenstein – batch number: L26398) in four 1-mm-thick increments. The resin-bonded specimens were stored in de-ionized water for 24 h at 37°C.
2.5. Microtensile bond strength (JLTBS) test
Sixty teeth were used for the 1-TBS test. Each principal group (i.e. EDTA and H3PO4) was constituted by 30 teeth that were subsequently subdivided in 5 teeth for each sub-group according to the resin adhesives used in this study. The resin–dentin specimens were sectioned with a diamond wafering blade (Accutom-50, Struers, Copenhagen, Denmark) using a hard tissue saw (330-CA RS-70300, Struers, Copenhagen, Denmark) in both x and y directions across the adhesive interface to obtain beams with cross-sectional areas of 0.9 mm2. By excluding peripheral beams showing the presence of residual enamel, only the central 10 beams were used from each tooth. Thus, there were 10 beams × 4 teeth = 40 beam specimens in each subgroup (Table 3).
Table 3.
Microtensile bond strength values (MPa) to dentin when resin adhesives were applied with the ethanol wet-bonding in EDTA or H3PO4 treated dentin.
| Dentin conditioning | EDTA | H3PO4 |
|---|---|---|
| Resin 1 | 0 (0)c (40/0) [100/0/0] |
14.1 (13.3)d (18/22) [85/0/15] |
| Resin 2 | 36.5 (14.4)b (0/40) [11/19/70] |
30.9 (12.7)c (0/40) [14/25/61] |
| Resin 3 | 41.8 (10.2)b (0/40) [0/62/38] |
49.2 (12.6)b (0/40) [0/75/25] |
| Resin 4 | 48.2 (8.3)a (0/40) [0/80/20] |
51.7 (7.8)a (0/40) [0/63/33] |
| Resin 5 | 42.4 (8.3)a,b (0/40) [11/29/60] |
17.6 (14.3)d (11/29) [30/0/70] |
| Scotchbond Multi-Purpose (primer + adhesive) | 41.1 (10.0)b (0/40) [10/68/22] |
59.8 (9.9)a (0/40) [0/71/29] |
| Primer made from 50% ethanol-solvated SBMP adhesive + neat adhesive | 40.1 (12.5)b (0/40) [21/27/52] |
43.5 (14.3)b (0/40) [8/50/42] |
Values are mean (SD) in MPa. In columns, same superscripts letters indicate no differences (p > 0.05). Premature failures were included in the statistical analysis as zero values and are indicated in parentheses (for instance 18/22 means that there were 18 premature failures and 22 testable beams). The modes of failure are expressed in percentage in the brackets [adhesive/mix/cohesive].
Each beam was attached to a modified Bencor Multi-T testing apparatus (Danville Engineering Co., Danville, CA, USA) with cyanoacrylate adhesive (Zapit, Dental Ventures of America Inc., Corona, CA, USA) and stressed to failure in tension using a universal testing machine (Instron 4411, Instron Corporation, Canton, MA, USA) at a crosshead speed of 0.5 mm/min. Bond strength data were calculated in MPa. Premature failures were included in the statistical analysis as zero values. Two-way ANOVA including interactions and Student–Newman–Keuls multiple comparisons were used for the statistical analysis. Adhesive systems and dentin surface treatment were considered as independent variables and μ-TBS as the dependent variable. Statistical significance level was set in advance at α = 0.05.
Modes of failure were classified as adhesive (A), mixed (M), or cohesive (C) when the failed bonds were examined at 30× by stereoscopic light microscopy or by SEM. Ten representative fractured specimens from each group were critical-point dried and then mounted on aluminum stubs with carbon cement. They were sputter-coated with gold (SCD 004 Sputter Coater; Bal-Tec, Vaduz, Liechtenstein) and viewed using a scanning electron microscope (SEM) (S-3500; Hitachi, Wokingham, UK) with an accelerating voltage of 15 kV and a working distance of 25 mm at increasing magnifications from 60× to 5000×.
2.6. SEM observations of the failed bonds
Ten failed specimens from each sub-group were examined by SEM to classify the modes of failure into the percentage of the total surface area of failed dentin exhibiting pure adhesive, cohesive or mixed failure modes.
2.7. Confocal microscopy characterization
Three resin–dentin bonded specimens were prepared for each sub-group. The pulpal tissue was carefully removed from the exposed pulp chamber of each crown segment, without crushing the pulpal-dentin walls using thin tissue forceps. A pincer-type calliper was used for measuring the remaining dentin thickness (RDT) from the dentin surface to the highest pulpal horns (0.7–0.9 ± 0.1 mm). Each tooth section was attached to a Perspex™ (Perspex Distributions Ltd., London, UK) platform (2 cm × 2 cm × 0.5 cm) that was perforated by an 18-gauge stainless steel tube glued in place using cyanocrylate adhesive (Rocket Heavy, Dental Ventures of America, Corona, CA, USA). Each specimen was connected to a 60 cc syringe barrel filled with 1 wt% aqueous rhodamine-B solution that was connected to the pulp chamber via polyethylene tubing as described by Sauro et al. [15]. The height of the fluid was 20 cm above the dentin surface. After 3 h, the specimens were disconnected from perfusion system, separated from the Perplex platform and finally sectioned into 1 mm slabs using a slow-speed water-cooled diamond saw (Labcut, Agar Scientific, Stansted, UK). The surface of the slabs was slightly polished using 1200-grit silicon carbide paper and ultra-sonicated for 2 min at each step [15].
The dentin/adhesive interfaces were examined using a confocal tandem scanning microscope (TSM: Noran Instruments, Middleton, Wisconsin, USA) in both the fluorescence and reflection modes. Reflection and fluorescence images were recorded using a Andor iXonEM, EMCCD camera (Andor Instruments, Belfast, Northern Ireland), digitized and image processed or reconstructed with suitable computer hardware and software (iQ, Andor iXonEM, Andor Instruments).
To measure the micropermeability of the bonded interface, all the specimens were examined using a X100/1.4 NA oil immersion objective with a 10× ocular and phototube in the fluorescence mode with 546 nm excitation and 600 nm long-pass filters, and in the reflection mode. Since peripheral dentin located close to the dentin–enamel junction has a low dentin permeability due to its reduced density of dentinal tubules [16,17], only the resin-bonded dentin surfaces located at least 1 mm from the enamel were analyzed in order to avoid underestimating micropermeability [15,18]. Three representative images from resin-bonded dentin surfaces were recorded 1 mm from the dentin–enamel junction. One image was taken from the centre of the bonded interface and two additional images in proximity of the pulpal horns were obtained from each site after a complete investigation of the entire resin–dentin interface [15,18]. These images were intended to be representative of the most common features observed in each specimen. Two additional specimens for each group were bonded with the resin adhesives previously mixed with 0.1 wt% rhodamine-B in order to image monomer diffusion of the tested resin adhesives into differently pretreated dentin [18,19]. In those specimens, the pulp chamber, polyethylene tubing and syringe barrel were full of water without the presence of fluorochrome. That is, no micropermeability was performed in these specimens.
3. Results
3.1. Solubility of resins in water vs. ethanol
In the current study, resin–dentin bond strengths to water vs. ethanol-wet acid-etched dentin were not compared because this has already been done in previous studies [10,13,18,20,21]. However, the solubilities of resin blends 1–5 as well as their constituent comonomers were measured to demonstrate that many so-called hydrophilic monomers have poor solubilities in water. With the exception of HEMA and BisMP, all of the resin blends or monomers were much more soluble in ethanol than in water (Table 2).
3.2. Microtensile bond strength test
Mean bond strengths in MPa and mode of failures obtained for each group are shown in Table 3. Mean bond strength was affected by the adhesive system (F = 82.74; p < 0.001) and dentin surface treatment (F = 95.09; p < 0.001). Interactions between dentin treatment/adhesive systems were also significant (p < 0.001).
The most hydrophobic resin 1 showed the lowest 1-TBS to both EDTA and phosphoric acid-treated dentin. All of the beams bonded with resin 1 to EDTA-treated dentin failed prematurely, yielding a bond strength of zero. Resin 1 bonded to acid-etched dentin showed 18 premature failures out of 40 specimens, yielding a mean bond strength of 14.1 ± 13.3 MPa. Most of the failures were adhesive.
The hydrophobic resin 2 produced higher bond strengths to EDTA treated-dentin compared to acid-etched dentin. The SEM investigation showed most of the H3PO4 acid-etched dentin beams failed mainly in a cohesive mode.
The more hydrophilic resin 3 on either substrate showed no statistical difference in bond strengths compared to resin 2 bonded to EDTA treated-dentin. The failure mode was predominantly cohesive or mixed in both groups.
The highest bond strength in the EDTA group was obtained using resin 4 to both substrates. However, no statistical difference was found for the bond strength results in the EDTA group between resins 4 and 5. No differences in μ-TBS were found when the hydrophilic resin 4 was applied on EDTA-treated dentin or PA-etched dentin. In both groups, the failure mode was predominantly cohesive and mixed.
Dentin bond strength of resin 5 was much higher (p < 0.01) when applied on EDTA-treated dentin than in the acid-etched dentin due to 11 premature failures in the acid-etched group (Table 3). Failure modes of acid-etched bonded dentin were predominantly mixed and adhesive. When dentin was treated using EDTA, failures were frequently mixed.
The three-step etch-and-rinse adhesive system SBMP applied as per manufacturer’s instructions on the H3PO4 acid-etched dentin had the highest μ-TBS values (p < 0.05) than when applied on EDTA-treated dentin. Most of the interfaces failed in a cohesive mode in both groups. No differences were observed when the SBMP primer was omitted from the SBMP bonding procedures in EDTA-treated dentin, but there was a significant reduction in μ-TBS when the SBMP primer was omitted from the H3PO4 acid-etched dentin (p < 0.05).
3.3. SEM observations of the failed bonds
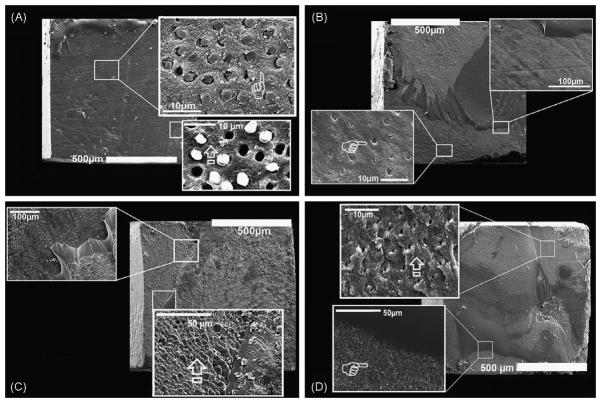
When bonded to EDTA-treated dentin, resin 1 bonds all failed adhesively due to a lack of infiltration of resin into the demineralized dentin layer. The demineralized collagen fibrils remain exposed (not shown). When resin 1 was bonded to PA-etched dentin (Fig. 1A), there was better resin infiltration into the demineralized dentin. The bonds all failed adhesively (100%). Resin tags were seen in some tubules, although many pulled out or showed gaps around the tags.
Fig. 1.
SEM micrographs of failure modes of hydrophobic resins bonded to dentin conditioned with EDTA or PA. (A) SEM micrograph of the failure mode of the resin-bonded dentin created with the most hydrophobic resin 1 on the PA-etched dentin. Note the adhesive failure mode and collapsed demineralized collagen network due to incomplete infiltration of the resin at higher magnification (arrow in lower insert). Higher magnification also shows remnant resin tags into the dentinal tubules after the failure (pointer in upper insert). (B) SEM micrograph of the failure mode of resin-bonded dentin created with resin 2 applied on PA-etched dentin. A mixed failure mode was seen with the presence of hybrid layer protected by the resin and resin tags into the dentinal tubules (pointer in upper insert). (C) SEM micrograph of the mixed failure mode of resin-bonded dentin created with resin 2 applied on the EDTA-treated dentin which shows at higher magnification that the dentinal surface was still covered by the resin with no exposure of dentinal tubules (arrow in lower insert). (D) SEM micrograph of the failure mode of resin-bonded dentin created with SBMP applied on ethanol wet-dentin conditioned using EDTA. Note the mixed failure characterized by a large presence of remnant resin on the dentin surface. At higher magnification (lower insert) part of exposed dentin is still covered by the resin (pointer) while other parts show exposed fractured dentinal tubules characterized by the presence of intact peritubular dentin and no exposed demineralized collagen fibrils (arrow in upper insert).
In contrast, resin 2 (a less hydrophobic resin), when applied to acid-etched dentin showed good infiltration of demineralized dentin. None of the resin tags were pulled out indicating that they hybridized with the surrounding intertubular dentin (Fig. 1B). The failure modes were 14% adhesive, 25% cohesive and 61% mixed (Table 3). When resin 2 was bonded to EDTA-treated dentin, the resin infiltrated the demineralized dentin which remained covered with resin after bond testing (Fig. 1C). That is, the failure modes included 11% adhesive failure, 19% cohesive failure and 70% mixed failure.
When SBMP primer and adhesive was applied to ethanol-saturated EDTA-treated dentin (Fig. 1D), the failure modes were 10% adhesive, 68% cohesive and 22% mixed. In about 10% of the failed surfaces, the failure mode was adhesive failure at the top of the hybrid layer. No exposed, uninfiltrated collagen fibrils were seen and the resin tags fractured at the top of the hybrid layer indicating that they had hybridized firmly to the surrounding dentin.
When SBMP adhesive was diluted with 50% ethanol and used as a primer, followed by neat adhesive, the failure modes were 21% adhesive, 27% cohesive and 52% mixed.
When resin 3, a more hydrophilic resin, was applied to acid-etched dentin (Fig. 2A), there were no adhesive failures, 75% cohesive failures and 25% mixed failures. The dentin remained covered by resin. When the same resin was applied to EDTA-conditioned dentin (Fig. 2B), failure mode was similar to that seen in the PA-treated dentin. That is, there were no adhesive failures, 62% cohesive and 38% mixed failures.
Fig. 2.
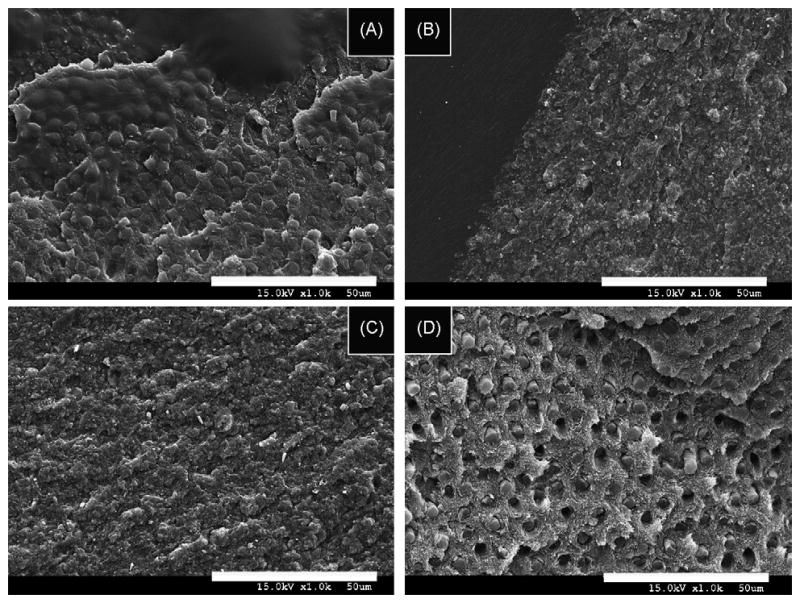
SEM micrographs of failure modes of hydrophilic resins applied to dentin conditioned with EDTA or PA. (A) SEM micrograph of the failure mode of resin-bonded dentin created with resin 3 applied to PA-etched dentin showing the bonded dentin failed in a mixed mode with the exposed dentin surface still covered by well-hybridized dentin, and no exposure of collagen fibrils or dentinal tubules. (B) SEM micrograph of the failure mode of resin-bonded dentin created with resin 3 applied on EDTA-treated dentin that failed in a mixed mode. The dentin surface remains covered with resin or hybridized dentin. No dentinal tubules are detected. (C) SEM micrograph of the failure mode of resin-bonded dentin created with resin 4 applied on EDTA-treated dentin. Note the absence of exposed dentinal tubules and the presence of resin on the dentinal surface. (D) SEM micrograph of the failure mode of resin-bonded dentin created with the most acidic and hydrophilic resin 5 applied on PA-etched dentin. Note the presence of fractured resin tags inside the dentinal tubules and collagen fibrils on the dentin surface left exposed by the cohesive failure of the hybrid layer. In the upper right portion of the image, the hybrid layer remains present. However, most of the image shows the top of the underlying sound dentin that was etched but not infiltrated.
Application of resin 4, a mildly acidic resin blend, to EDTA-treated dentin produced no adhesive failures, 80% cohesive failures in adhesive and 20% mixed failures (Fig. 2C). Similar failures were seen when resin 4 was applied to acid-etched dentin (not shown).
When resin 5, the most acidic and most hydrophilic experimental resin (Fig. 2D) was applied to EDTA-treated dentin, the failure modes were 11% adhesive, 29% cohesive and 60% mixed failures.
In the adhesive failures, although some resin tags remained in the tubules, most of the resin tags pulled out indicating that they did not hybridize with the surrounding dentin. Some of the hybrid layers failed cohesively leaving etched exposed collagen fibrils (Fig. 2D).
3.4. Confocal microscopy interfacial characterization
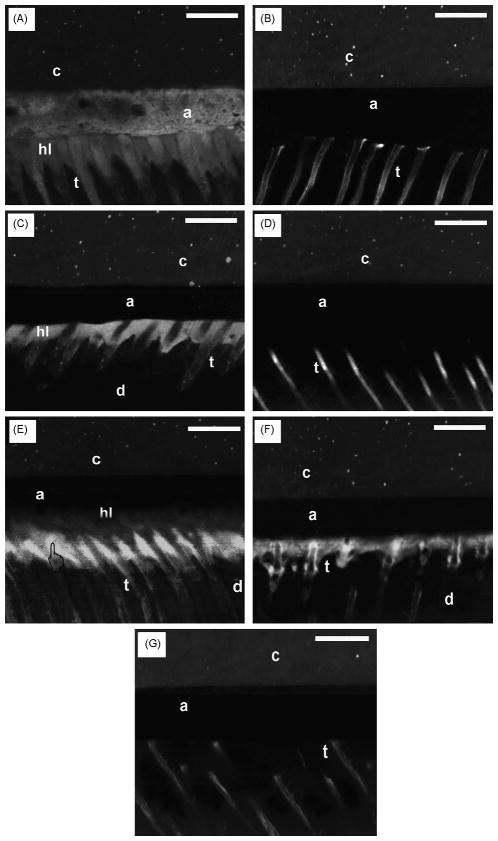
Sites of micropermeability within the resin-bonded dentin interfaces had different characteristics when the adhesives were applied on the ethanol wet-dentin treated with EDTA or with H3PO4 acid (Fig. 3). The resin-bonded dentin interfaces created with most hydrophobic resin 1 applied to both acid-etched and the EDTA-treated dentin showed severe micropermeability of rhodamine-B along the walls of the tubules and within hybrid and adhesive layers, with several voids and gaps entrapped within the resin–dentin interface (Fig. 3A).
Fig. 3.
Confocal fluorescence/reflection images of the micropermeability of resin-dentin interfaces. (A) Resin-bonded dentin interfaces created with hydrophobic resin 1 applied on acid-etched ethanol wet-dentin showed severe micropermeability of rhodamine-B along the walls of the tubules (t) and within adhesive (a) and hybrid layers (hi). Several voids were detected entrapped between the resin dentin interfaces. (B) Resin-bonded dentin interfaces created with resin 2 applied on ethanol wet-dentin that was conditioned with EDTA for 60s showed slight micropermeability of rhodamine-B only along the walls of the tubules (t). No fluorescence was detected in the adhesive layer (a). The composite layer is
The resin-bonded dentin interfaces created with the hydrophobic resin 2 applied on the ethanol EDTA-treated dentin showed rhodamine-B uptake only along the walls of the tubules with no diffusion within the hybrid or adhesive layers (Fig. 3B). When the same resin was applied onto H3PO4-etched ethanol wet-dentin, rhodamine-B diffused along the walls of the tubules and within the hybrid layer (Fig. 3C).
Resins 3, 4 and SBMP applied as per manufacturers’ instructions on H3PO4-etched dentin showed micropermeability of rhodamine-B only along the walls of the tubules (Fig. 3D). The most hydrophilic resin 5 applied on H3PO4 acid-etched dentin created a bonded interface highly affected by micropermeability within the hybrid layer and along the walls of the tubules. It was interesting to observe a region of over-etching at the bottom of the hybrid layer (Fig. 3E).
The adhesive bond of the SBMP applied onto H3PO4 acid-etched dentin created a resin–dentin interface characterized by micropermeability at the bottom of the hybrid layer and along the walls of the tubules (Fig. 3F).
In general, all the resin-bonded dentin interfaces created with the more hydrophilic resins and with the primer/adhesive of the SBMP (Fig. 3G) applied on EDTA-treated dentin showed rhodamine-B uptake only along the walls of the tubules with no sign of fluorescence within the hybrid or adhesive layers.
The results of the TSM investigation regarding the diffusion of the resin adhesives into the EDTA- or acid-etched treated ethanol wet-dentin are shown in Fig. 4. When the most hydrophobic resin 1 was applied to both EDTA and H3PO4 acid-etched dentin (Fig. 4A), it was possible to observe the presence of gaps between the adhesive and dentin. No fluorescent resin infiltrated the hybrid layer or created resin tags in the tubules.
Fig. 4.
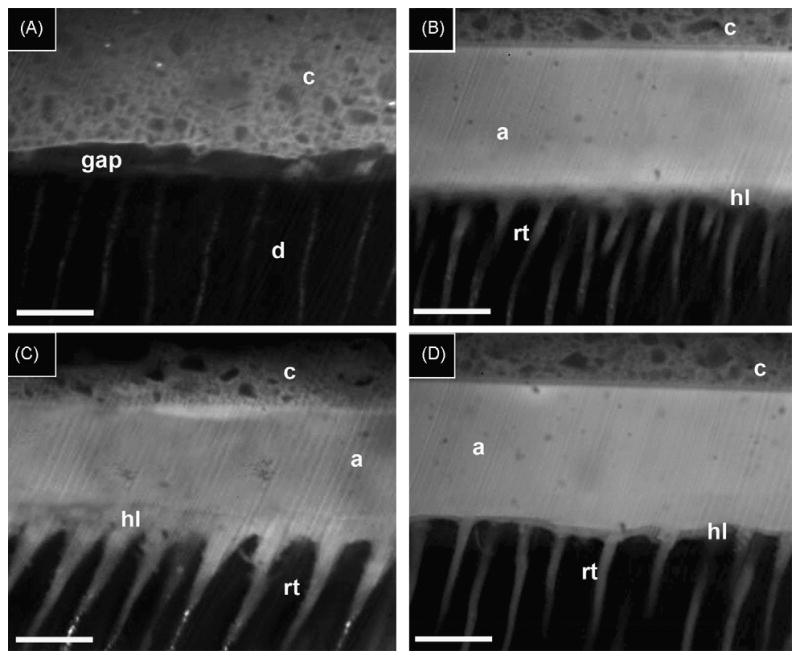
Confocal fluorescence of the resin–dentin interfaces. (A) Resin-bonded dentin interfaces created with hydrophobic resin 1 containing 0.1% rhodamine-B, applied on ethanol wet-dentin that was conditioned with EDTA for 60 s. Note that the absence of a hybrid layer and absence solid resin tags formation indicates that the primer and adhesive did not penetrate the dentin during the bonding procedures. Gaps were present between dentin (d) and composite (c). (B) Resin-bonded dentin interfaces created with resin 2 containing 0.1% rhodamine-B, applied on ethanol wet-dentin that was conditioned with EDTA for 60 s showed the presence of a very thin (ca. 1–2 t-m) hybrid layer (hl), the adhesive layer (a) and composite (c) layer. The scale bar in all the images is 10 t-m. (C) Resin-bonded dentin interfaces created with resin 2 containing 0.1% rhodamine-B, applied on acid-etched ethanol wet-dentin showed the presence of a thicker hybrid layer (hl) beneath the adhesive layer (a) and composite (c). The resin tags (rt) appeared to have larger diameters compared to those observed in the resin-bonded dentin interfaces created with resin 2 applied on EDTA/ethanol wet-dentin. (D) Resin-bonded dentin interfaces created with the primer and adhesive of SBMP containing 0.1% rhodamine-B, applied on ethanol wet-dentin that was conditioned with EDTA for 60 s. Note the presence of a thin (i.e. 1–2 t-m) hybrid layer (hl) and resin tags (rt) beneath the adhesive composite (c).
When the hydrophobic resin 2 was applied on ethanol wet-dentin conditioned with EDTA for 60 s, the presence of a very thin (ca. 1–2 μ-m) hybrid layer was seen along with long, thin resin tags penetrating the dentinal tubules several microns (Fig. 4B).
Resin 2 applied on H3PO4 acid-etched dentin created a thicker hybrid layer (i.e. 5–7 μ-m). The resin tags presented wider diameters compared to those observed in EDTA-treated dentin (Fig. 4C). The resin-bonded dentin interfaces created with the primer and adhesive of Scotchbond Multi-Purpose adhesive doped with rhodamine-B applied on ethanol wet-dentin conditioned with EDTA also showed the presence of a thin (i.e. 1–2 μ-m) hybrid layer and resin tags diffusion (Fig. 4D).
4. Discussion
The water wet-bonding technique is usually employed for bonding resins to dentin. Two main reasons have been advocated to explain the relatively poor infiltration of etch-and-rinse resins into water wet-dentin: (1) large differences between molar concentrations of water (55.6 moles/L) and that of typical dentin adhesives comonomer blends (2.3–3.9 moles/L, Table 1) making it nearly impossible for comonomers to displace all of the water from collagen fibrils [22]; (2) dental adhesives usually contain relatively hydrophobic dimethacrylates that have a low solubility in water (Table 2), that can cause macro, micro or nanophase separation when applied on acid-etched water wet-dentin [1,2,23,24]. Therefore, the wet-bonding technique has been modified by replacing water with absolute ethyl alcohol [22]. After rinsing the acid-etched dentin, the water is replaced with an excess of 100% ethyl alcohol applied on dentin for 5 min [12,13,20]. Ethanol replaces water in the interfibrillar spaces and at the top of the dentinal tubules, as ethanol is completely miscible with water. It is thought that any residual layer of ethanol on collagen fibrils will permit infiltrating monomers to dissolve in the ethanol to produce a more intimate association between collagen and resin. This is unlikely to occur if monomolecular layer of residual water covers collagen fibrils, because resin monomers are less soluble in water, even though many classify them as hydrophilic (Table 2).
However, because the ethanol wet-bonding protocol as it was first introduced, applied ethanol to acid-etched dentin for 5 min, its clinical use was questioned [13,20].
The ethanol wet-bonding technique performed in the current study was applied over a more clinically relevant period of time (1 min). This technique permitted dentin infiltration by the relatively hydrophobic resin 2 (but not resin 1), when applied on both EDTA and 37% H3PO4 acid treated dentin.
As the bond strengths obtained in the current study using the modified ethanol wet-bonding technique were related to the hydrophilicity of the resin blend and the mode and type of dentin conditioning (i.e. EDTA-treated or 37% H3PO4-etched dentin), the first test null hypothesis (that there is no difference in bond strengths following etching with EDTA vs. phosphoric acid) must be rejected.
Resin 1 (most hydrophobic) produced the lowest μ-TBS results, both when applied on EDTA-treated or on the acid-etched dentin, due to incomplete infiltration of the resin into the demineralized collagen network and dentinal tubules. Apparently, the ethanol-solvated resin 1 was unable to adequately wet the conditioned dentin. SEM investigation showed only remnant resin tags in the dentinal tubules after the μ-TBS failure with exposed demineralized collagen fibrils especially in those specimens conditioned by 37% H3PO4. Clearly, the low bond strength produced by resins to acid-etched dentin 1 is probably due only to mechanical retention of the resin tags (Fig. 1A). Confocal tandem microscopic investigation of the resin-bonded dentin interfaces created with resin 1 applied both on the H3PO4-etched and EDTA-treated ethanol wet-dentin showed severe micropermeability of rhodamine-B along the walls of the tubules and within hybrid and adhesive layers with several voids entrapped within the resin–dentin interface and gaps (Fig. 3A). Gaps were seen along the resin–dentin interface of specimens bonded with resin 1 because the demineralized collagen fibrils had not been sealed by resin (Fig. 4A).
The micropermeability studies revealed that, with the exception of resins 1 and 5, application of adhesives to phosphoric acid-etched dentin produced far more extensive micropermeability than was seen when dentin was conditioned with EDTA. In fact, micropermeability of the SBMP-bond specimens of EDTA-conditioned dentin results showed rhodamine-B diffusion only along the walls of the tubules several microns away from the hybrid layer (Fig. 3G).
We speculate that phosphoric acid-etching of dentin causes collagen fibrils to become slightly denatured and swollen compared to EDTA-treatment [29]. This may interfere with resin diffusion into these interfibrillar spaces allowing more nanovoids to exist after resin infiltration. These nanovoids may be responsible for the diffusion of rhodamine-B around resin tags and into the hybrid layer of acid-etched dentin. Such uptake of rhodamine-B does not seem to occur in EDTA-conditioned dentin. This may be because EDTA does not denature collagen and creates thinner hybrid layers that are more easily infiltrated with resin than does phosphoric acid.
It is thought that EDTA avoids the denaturation of collagen due, in part, to the presence of more residual apatite crystallites left within the collagen matrix [1,25,26]. It may also be that the lower thickness of the demineralized collagen layer and the presence of more residual apatite crystallites within the collagen matrix will prevent shrinkage during infiltration of comonomers, thus facilitating the diffusion of relatively hydrophobic resins into demineralized dentin when using the ethanol wet-bonding technique.
When water-saturated dentin is treated with a large excess of ethanol (even for just 1 min) the collagen fibrils remain expanded and do not collapse, due to the creation of matrix interpeptide H-bonds [12,22]. It may be especially evident in EDTA-treated dentin. This process will also stiffens the matrix [28,29] and prevents any further shrinkage during resin infiltration. It has also been shown that the treatment of acid-etched dentin with 100% ethanol causes a 15% shrinkage in the collagen matrix [30] by removing water from the collagen fibrils, resulting in larger interfibrillar spaces [12] that may enhance resin infiltration [21].
The success of the ethanol wet-bonding technique may be partly attributed to the higher degree of conversion of resins (DC) undergoing this procedure. Cadenaro et al. [31] have recently shown that resin 1 had the lowest residual ethanol concentration after solvent evaporation, due to the fact that its component comonomers (BisPhenol A dimethacrylate and triethyleneglycol dimethacrylae) do not form hydrogen bonds with ethanol. Although resin 2 had an even lower comonomer concentration (2.37 mole/L), BisGMA contains hydroxyl groups that can hydrogen bond with ethanol. This residual presence of ethanol decreases the initial reaction rate but enhances DC after 60 s exposure. Residual ethanol also decreases the viscosity of comonomer blends, allowing radical propagation to continue longer without the reaction being diffusion controlled [32,33].
Phase changes in resin 1 may explain differences in μ-TBS to dentin [1,11,20,23,24]. Resin 1 is based on ethoxylated BisPhenol A dimethacrylate, while resin 2 is principally constituted by BisGMA. It was shown that ethoxylated BisPhenol A dimethacrylate (resin 1) underwent phase changes when applied to dentin that is not completely saturated with ethanol [8,13], thereby weakening the hybrid and adhesive layers [1]. These phase changes were never seen in ethanol-saturated matrices using resin 2 [30], and hence no micropermeability was detected along the resin dentin interfaces using a two-photon confocal microscopy [13,35].
The resin–dentin bonds created with the hydrophilic resin 3 (pH 7, Table 1) showed no significant differences in μ-TBS when applied on EDTA or H3PO4 acid treated dentin. The failure mode was predominantly cohesive and mixed leaving resin covering the dentin surface (Fig. 2B).
The mildly acidic/hydrophilic resin 4 (pH 4), showed no significant differences in μ-TBS when applied on EDTA- or H3PO4-treated dentin, with a failure mode that was predominantly cohesive and mixed with the presence of resin adhering to the dentin surface (Fig. 2C). Rhodamine-B diffusion was only observed along the walls of the tubules in H3PO4-etched resin-bonded dentin (Fig. 3D), while EDTA-treated dentin showed rhodamine-B diffusion only into the dentinal tubules several microns away from the hybrid layer. The confocal results indicate a high quality hybrid layer due to a better diffusion of resins 3 and 4 into the EDTA-demineralized collagen network producing good sealing of dentin.
Conversely, the most acidic/hydrophilic resin 5 applied on H3PO4 acid-etched dentin created an interface that was severely affected by micropermeability of rhodamine-B within the hybrid layer and at the bottom of the hybrid layer (Fig. 3E). This resin has a pH of 3 in the presence of residual water; hence, it may be possible that a phenomenon of self-etching occurred during the application of this resin 5 (Fig. 3E). The μ-TBS results of resin 5 to acid-etched dentin gave much lower bond strengths (p < 0.05) compared to EDTA-treated dentin and correlated well with the micropermeability results. The SEM investigation showed a mixed failure mode of acid-etched resin 5 bonded dentin with the presence of exposed demineralized dentin collagen fibrils (Fig. 2D). In contrast, resin 5 bonded to EDTA-treated bonded dentin had a mode of failure predominantly mixed and cohesive, with no exposed collagen fibrils detected, and yielding a high tensile bond strength (Table 3).
The manufacturers of SBMP require the use of a water/HEMA/polyalkenoic acid primer applied to water wet-dentin etched with PA. This is followed by the application of a solvent-free adhesive. That combination gave the highest 24 h microtensile bond strength (Table 3) to acid-etched dentin (59.8 MPa) but a bond strength of only 41.1 MPa when applied to EDTA-treated dentin saturated with ethanol. Presumably, the water in the SBMP primer diluted the ethanol in both EDTA and acid-etched specimens. We cannot explain why the bond strength fell in the EDTA-treated dentin.
In the experimental resin group, the primers were made by solvating the adhesives in 50 vol% ethanol. After evaporating the solvent, the primed dentin was then covered with neat resin as the adhesive. In the SBMP group, the adhesive was also solvated in 50 vol% ethanol to serve as a substitute primer for the manufacturer’s primer that consists of water/ethanol/HEMA and polyalkenoate. When our simpler primer was used instead of the manufacturer’s primer, the bond strengths fell from 59.8 MPa to 43.5 MPa (p < 0.05) in the acid-etched group, but remained the same in the EDTA-treated dentin (Table 3). Apparently, polyalkenoate is necessary for optimal bond strength of acid-etched dentin, but not for EDTA-treated dentin.
It has been recently shown [18] that the resin–dentin bonds created using a neutral 0.1 M EDTA solution as dentin conditioner showed no interfacial micropermeability and produced similar bond strengths to those made using H3PO4. However, the higher micropermeability seen in the current study using acid-etched dentin as a bonding substrate compared to EDTA-treated dentin requires rejection of the second null hypothesis that there are no differences in micropermeability of bonds made to EDTA vs. phosphoric acid-conditioned dentin.
It is clear from the results of this study that the acidity and the presence functional monomers may influence the performance of the adhesive systems [36]. Resin 4 has a pH of 4 and contains BisGMA, HEMA (2-hydroxyethylmethacrylate) and a functional monomer known as TCDM (dihydroxyethyl-methacrylate) ester of 5-(2,5-dioxotetrahydrofurfuryl)-methyl-3-cyclohexane-1,2′-dicarboxylic acid. Resin 5 has a pH of 3 and contains BisGMA, HEMA, and an acidic functional monomer known as BisMP (Bis[2-(methacryloyloxy)ethyl]phosphate). The primer of SBMP has a pH of 4.3 and contains a polyalkenoic acid functional co-polymer as well as HEMA and water (Table 1).
The functional monomers are characterized by at least one polymerizable group and a functional group, which can serve different purposes, such as wetting or demineralizing the substrate. Van Landuyt et al. [36] showed that functional groups capable of releasing one or more protons, such as carboxyl, phosphate, and phosphonate groups, may also have the potential for chemical bonding to calcium in hydroxyapatite. This latter issue is in accordance with the concept of ‘Adhesion–Decalcification’ which states that the functional monomer either decalcifies, or bonds to the tooth substrate [37,38]. According to this concept, we speculate that the functional groups of the tested acidic/functional monomers may have interacted (at least as an ionic reaction) with calcium in hydroxyapatite left within the fibrils after EDTA conditioning offering optimal resin–dentin interfacial characteristics.
The use of EDTA-conditioning, combined with ethanol wet-bonding seems able to coax adhesive monomers into thin EDTA-demineralized dentin, more easily than with conventional acid-etched dentin. We speculate that this allows a more intimate association between resin polymers and the bio-polymers of collagen fibrils. Further research is ongoing to determine if this strategy will lead to more durable resin–dentin bonds after in vitro exposure to cyclic loading, chemical degradation or aging.
Acknowledgments
This investigation was supported by CICYT/FEDER MAT2008-02347/MAT, JA-P07-CTS-2568, and JA-P08-CTS-3944, and by R01 DE014911 from the NIDCR to DHP (PI). The authors also acknowledge support from the Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy’s & St Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust.
References
- 1.Spencer P, Wang Y. Adhesive phase separation at the dentin interface under wet bonding conditions. J Biomed Mater Res. 2002;62:447–56. doi: 10.1002/jbm.10364. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Spencer P. Hybridization of the adhesive/dentin interface with wet bonding. J Dent Res. 2003;82:141–5. doi: 10.1177/154405910308200213. [DOI] [PubMed] [Google Scholar]
- 3.Kinney JH, Marshall SJ, Marshall GW. The mechanical properties of human dentin: a critical review and re-evaluation of the dental literature. Crit Rev Oral Biol Med. 2003;14:13–29. doi: 10.1177/154411130301400103. [DOI] [PubMed] [Google Scholar]
- 4.Kanca J. Resin bonding to wet substrate. 1. Bonding to dentin. Quintessence Int. 1992;23:39–41. [PubMed] [Google Scholar]
- 5.Marshall GW, Marshall SJ, Kinney JH, Balooch M. The dentin substrate: structure and properties related to bonding. J Dent. 1997;25:441–58. doi: 10.1016/s0300-5712(96)00065-6. [DOI] [PubMed] [Google Scholar]
- 6.Nakabayashi N, Pashley DH. Hybridization of dental hard tissues. Tokyo: Quintessence Publishing; 1998. [Google Scholar]
- 7.Toledano M, Osorio R, Albaladejo A, Aguilera FS, Osorio E. Differential effect of in vitro degradation on resin–dentin bonds produced by self-etch versus total-etch adhesives. J Biomed Mater Res A. 2006;77:128–35. doi: 10.1002/jbm.a.30656. [DOI] [PubMed] [Google Scholar]
- 8.Ito S, Hashimoto M, Wadgaonkar B, Svizero N, Carvalho RM, Yiu C, et al. Effects of resin hydrophilicity on water sorption and changes in modulus of elasticity. Biomaterials. 2005;26:6449–59. doi: 10.1016/j.biomaterials.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 9.Malacarne J, Carvalho RM, de Goes MF, Svizero N, Pashley DH, Tay FR, et al. Water sorption/solubility of dental adhesive resins. Dent Mater. 2006;22:973–80. doi: 10.1016/j.dental.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 10.Nishitani Y, Yoshiyama M, Donnelly AM, Agee KA, Sword J, Tay FR, et al. Effects of resin hydrophilicity on dentin bond strength. J Dent Res. 2006;85:1016–21. doi: 10.1177/154405910608501108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agee KA, Becker TD, Joyce AP, Rueggeberg FA, Borke JL, Waller JL, et al. Net expansion of dried demineralized dentin matrix produced by monomer/alcohol saturation and solvent evaporation. J Biomed Mater Res A. 2006;79:349–58. doi: 10.1002/jbm.a.30752. [DOI] [PubMed] [Google Scholar]
- 12.Tay FR, Pashley DH, Kapur RR, Carrilho MRO, Hur TB, Garrett LV, et al. Bonding BisGMA to dentin – a proof of concept. J Dent Res. 2007;86:1034–9. doi: 10.1177/154405910708601103. [DOI] [PubMed] [Google Scholar]
- 13.Sauro S, Watson TF, Mannocci F, Miyake K, Huffman BP, Tay FR, et al. Two-photon laser confocal microscopy of micropermeability of resin–dentin bonds made with water or ethanol wet bonding. J Biomed Mater Res B: Appl Biomater. 2009;90:327–37. doi: 10.1002/jbm.b.31290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin TP, Yao X, Huenergardt R, Walker MP, Wang Y. Morphological and chemical characterization of bonding hydrophobic adhesive to dentin using ethanol wet bonding technique. Dent Mater. 2009;25:1050–7. doi: 10.1016/j.dental.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sauro S, Pashley DH, Mannocci F, Tay FR, Pilecki P, Sherriff M, et al. Micropermeability of current self-etching and etch-and-rinse adhesives bonded to deep dentin: a comparison study using a double-staining/confocal microscopy technique. Eur J Oral Sci. 2008;116:184–93. doi: 10.1111/j.1600-0722.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 16.Garberoglio R, Brännström M. Scanning electron microscopic investigation of human dentinal tubules. Arch Oral Biol. 1976;21:355–62. doi: 10.1016/s0003-9969(76)80003-9. [DOI] [PubMed] [Google Scholar]
- 17.Pashley DH, Andringa HJ, Derkson GD, Derkson ME, Kalathoor SR. Regional variability in the permeability of human dentin. Arch Oral Biol. 1987;32:519–23. doi: 10.1016/s0003-9969(87)80014-6. [DOI] [PubMed] [Google Scholar]
- 18.Sauro S, Mannocci F, Toledano M, Osorio R, Pashley DH, Watson TF. EDTA or H3PO4/NaOCl dentin treatments may increase hybrid layers’ resistance to degradation: a microtensile bond strength and confocal-micropermeability study. J Dent. 2009;37:279–88. doi: 10.1016/j.jdent.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Sidhu SK, Watson TF. Interfacial characteristics of resin-modified glass-ionomer materials: a study on fluid permeability using confocal fluorescence microscopy. J Dent Res. 1998;77:1749–59. doi: 10.1177/00220345980770091101. [DOI] [PubMed] [Google Scholar]
- 20.Sadek FT, Pashley DH, Nishitani Y, Carrilho MR, Donnelly A, Ferrari M, et al. Application of hydrophobic resin adhesives to acid-etched dentin with an alternative wet bonding technique. J Biomed Mater Res A. 2008;84A:19–29. doi: 10.1002/jbm.a.31290. [DOI] [PubMed] [Google Scholar]
- 21.Hosaka K, Nishitani Y, Tagami J, Yoshiyama M, Brackett WW, Agee KA, et al. Durability of resin–dentin bonds to water vs. ethanol-saturated dentin. J Dent Res. 2009;88:146–51. doi: 10.1177/0022034508328910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pashley DH, Tay FR, Carvalho RM, Rueggeberg FA, Agee KA, Carvalho RM, et al. From dry bonding to wet bonding to ethanol-wet bonding: a review of the interactions between dentin matrix and solvated resins using a macromodel of the hybrid layer. Am J Dent. 2007;20:7–20. [PubMed] [Google Scholar]
- 23.Eliades G, Vougiouklakis G, Palaghias G. Heterogeneous distribution of single-bottle adhesive monomers in the resin–dentin interdiffusion zone. Dent Mater. 2001;17:277–83. doi: 10.1016/s0109-5641(00)00082-8. [DOI] [PubMed] [Google Scholar]
- 24.Ye Q, Park JG, Tapp E, Wang Y, Misra A, Spencer P. In vitro performance of nano-heterogeneous dentin adhesive. J Dent Res. 2008;87:829–33. doi: 10.1177/154405910808700911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takarada K. Stable adhesion to dentin. Combination of EDTA 3-2 (NH4/Fe) pretreatment and 2% 4-META/MMA-TBB resin. Shika Zairyo Kikai. 1990;9:841–9. [PubMed] [Google Scholar]
- 26.Osorio R, Erhardt MC, Pimenta LA, Osorio E, Toledano M. EDTA treatment improves resin–dentin bonds’ resistance to degradation. J Dent Res. 2005;84:736–40. doi: 10.1177/154405910508400810. [DOI] [PubMed] [Google Scholar]
- 28.Carvalho RM, Mendonça JS, Santiago SL, Silveira RR, Garcia FC, Tay FR, et al. Effects of HEMA/solvent combinations on bond strength to dentin. J Dent Res. 2003;82:597–601. doi: 10.1177/154405910308200805. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Spencer P. Analysis of acid-treated dentin smear debris and smear layer using confocal Raman microspectroscopy. J Biomed Mater Res. 2002;60:300–8. doi: 10.1002/jbm.10108. [DOI] [PubMed] [Google Scholar]
- 30.Becker TD, Agee KA, Joyce AP, Rueggeberg FA, Borke JL, Waller JL, et al. Infiltration/evaporation induced shrinkage of demineralized dentin by solvated model adhesives. J Biomed Mater Res B: Appl Biomater. 2007;80:156–65. doi: 10.1002/jbm.b.30580. [DOI] [PubMed] [Google Scholar]
- 31.Cadenaro M, Breschi L, Rueggeberg FA, Suchko M, Grodin E, Agee K, et al. Effects of residual ethanol on the rate and degree of conversion of five experimental resins. Dent Mater. 2009;25:621–8. doi: 10.1016/j.dental.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye Q, Wang Y, Williams K, Spencer P. Characterization of photopolymerization of dental adhesives as a function of light source and irradiance. J Biomed Mater Res B: Appl Biomater. 2007;80:440–6. doi: 10.1002/jbm.b.30615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye Q, Spencer P, Wang Y, Misra A. Relationship of solvent to the photopolymerization process, properties, and structure in model dentin adhesives. J Biomed Mater Res. 2007;80:342–50. doi: 10.1002/jbm.a.30890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sauro S, Watson T, Mannocci F, Tay FR, Pashley DH. Prevention of water contamination of ethanol-saturated dentin and hydrophobic hybrid layers. J Adhes Dent. 2009;11:271–8. [PMC free article] [PubMed] [Google Scholar]
- 36.Van Landuyt KL, Yoshida Y, Hirata I, Snauwaert J, De Munck J, Okazaki M, et al. Influence of the chemical structure of functional monomers on their adhesive performance. J Dent Res. 2008;87:757–61. doi: 10.1177/154405910808700804. [DOI] [PubMed] [Google Scholar]
- 37.Yoshioka M, Yoshida Y, Inoue S, Lambrechts P, Vanherle G, Nomura Y, et al. Adhesion/decalcification mechanisms of acid interactions with human hard tissues. J Biomed Mater Res. 2002;59:56–62. doi: 10.1002/jbm.1216. [DOI] [PubMed] [Google Scholar]
- 38.Yoshida Y, Nagakane K, Fukuda R, Nakayama Y, Okazaki M, Shintani H, et al. Comparative study on adhesive performance of functional monomers. J Dent Res. 2004;83:454–8. doi: 10.1177/154405910408300604. [DOI] [PubMed] [Google Scholar]