Abstract
Purpose
To describe the evaluation of optical coherence tomography (OCT) scans in the Muliticenter Uveitis Steroid Treatment (MUST) trial and report baseline OCT features of enrolled participants.
Methods
Time domain OCTs acquired by certified photographers using a standardized scan protocol were evaluated at a Reading Center. Accuracy of retinal thickness data was confirmed with quality evaluation and caliper measurement of centerpoint thickness (CPT) was performed when unreliable. Morphological evaluation included cysts, subretinal fluid,epiretinal membranes (ERMs),and vitreomacular traction.
Results
Of the 453 OCTs evaluated, automated retinal thickness was accurate in 69.5% of scans, caliper measurement was performed in 26%,and 4% were ungradable. Intraclass correlation was 0.98 for reproducibility of caliper measurement. Macular edema (centerpoint thickness ≥ 240um) was present in 36%. Cysts were present in 36.6% of scans and ERMs in 27.8%, predominantly central. Intergrader agreement ranged from 78 − 82% for morphological features.
Conclusion
Retinal thickness data can be retrieved in a majority of OCT scans in clinical trial submissions for uveitis studies. Small cysts and ERMs involving the center are common in intermediate and posterior/panuveitis requiring systemic corticosteroid therapy.
Introduction
Optical coherence tomography (OCT) provides cross-sectional images of the retina with differential reflectivity characterizing the retinal layers and various morphological features. OCT scans have been widely used to objectively assess retinal thickening in clinical trials in various diseases.1-4 Macular edema traditionally has been assessed using stereoscopic color photographs with graders assessing the percentage of elevation within the subfields of the ETDRS grid. 5 OCT scans have changed macular edema assessment options by providing an objective measure of the retinal thickening using a similar grid with subfields.6 In addition,OCT allows identification of intraretinal fluid in the form of cysts and subretinal fluid. The cross-sectional imaging also is advantageous for assessment of vitreoretinal interface abnormalities. Retinal distortion due to epiretinal membrane (ERM),and adherence of the posterior vitreous surface can be well characterized. 7
The Multicenter Uveitis Steroid Treatment (MUST) Trial is an ongoing study to compare standardized systemic therapy versus fluocinolone acetonide implant therapy for the treatment of active or recently active (within 60 days) cases of non-infectious intermediate uveitis, posterior uveitis or panuveitis.8 One of the goals of the study is to evaluate the incidence and outcomes of major ocular complications of uveitis. In the MUST Trial, imaging modalities including color photographs, fluorescein angiography, lens photographs and time-domain OCTs are used in a standardized fashion to assess the ocular complications. Here,we report the reproducibility of the evaluation method and baseline OCT features in the MUST Trial.
Methods
Imaging protocol
OCT images are obtained using the Stratus OCT-3 (Carl Zeiss Meditec, Dublin, California). All OCT operators and OCT machines are certified by the University of Wisconsin Reading Center. OCT scans are acquired using the fast macular scan protocol (128 A scans/B scans; 6.0 mm line length; 6 radial scans) and processed through the ‘retinal map (single eye)’ and ‘retinal thickness (single eye)’ analysis programs in the Stratus software (Carl Zeiss Meditec, Dublin, California). Additional high resolution scans are captured using the cross hair scan protocol ( 512 A scans/B scan) and processed to generate a vertical and horizontal B scan using the ‘align process’ algorithm in the Stratus software. The operators performed a preliminary quality check on the OCT scans before submitting to the Reading Center. Due to the lack of commercially available standalone review software at the time of this study, paper prints of the fast macular retinal map thickness report—the six retinal thickness reports showing the six radial B scans and two align process reports with the vertical and horizontal cross hair B scans—were submitted to the Reading Center. To ensure consistent, standardized data capture throughout the life of the study, the study leadership decided not to shift to digital or to spectral domain OCT evaluation during the course of the study. Subject identifiers other than a study number were removed from all images in compliance with the Health Insurance Portability and Accountability Act (HIPAA).
Grading methodology
At the Reading Center, OCT evaluation is performed by trained and certified non-physician ocular disease evaluators (graders). All OCT scans are evaluated independently with no reference to any other visit or the results of any other imaging modality from the same visit. Each OCT scan is evaluated by a single evaluator. The evaluation procedures consisted of 3 parts; the check-in process, quality scoring and morphological assessment. The check-in process consists of a careful verification that the images had been taken in accordance with the scan protocol and that all required paper prints had been submitted. If verification indicates a problem, queries are sent to the clinical sites either for a repeat scan or a repeat submission, if possible.
Quality scoring of the OCT scan involves a detailed assessment of the map report and the B scans for evidence of image artifacts that could affect the retinal thickness data.9 B scans are reviewed to look for artifacts that could affect central subfield thickness; e.g.,errors in the segmentation algorithm (boundary line errors) and inaccurate centering of the macula within the B scan (decentration). A score of Good, Fair, Borderline or Ungradable is given based on this assessment. A score of Good indicates that no artifacts are present and all the automatically generated retinal thickness data can be used. In order for an OCT scan to be considered of Good quality,the additional features required are: signal strength of 5 or greater and a standard deviation of less than 10% for the center point thickness. Fair indicates that one or more of the non-central subfields contains inaccurate data. In these cases, the central subfield thickness and centerpoint thickness are utilized and the unreliable non central subfields are discarded. Borderline indicates that the central subfield and automated centerpoint thickness are unreliable and a manual measurement for the centerpoint is required (Figure 1). Ungradable score is given when the central subfield and automated centerpoint are unreliable and a manual measurement of centerpoint thickness cannot be performed. The reason for the borderline or ungradable quality, such as poor signal strength or presence of a confounding abnormality, is noted. Manual measurement of centerpoint thickness is performed on the paper prints of the retinal thickness reports using a handheld digital caliper (Product 9900; Precision Graphic Instruments Inc, Spokane, Washington) after computing a scale factor. 9
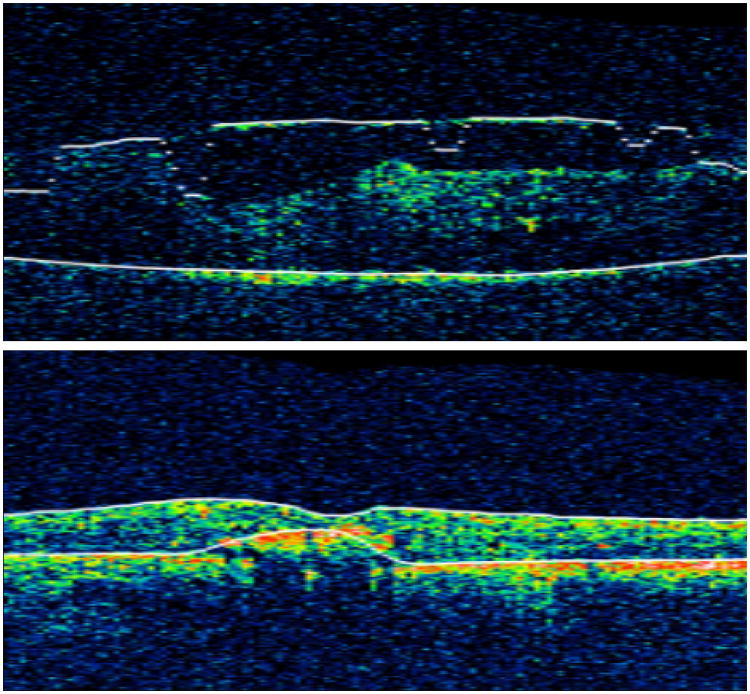
Figure 1.
Inner boundary line errors in an OCT with epi-retinal membranes and poor signal strength (above) and outer boundary line errors in an OCT with intra-retinal hyper-reflectivity (below).
Morphological assessment includes assessment of presence and lateral extent of cysts, and subretinal fluid. Cysts are defined as rounded, non-reflective or minimally reflective spaces withinin the intraretinal layers and include the following features: at least 2mm in size on the B scan, rounded in shape,well defined walls with at least 75% of the wall discernable, with reflectivity comparable to the vitreous. Subretinal fluid is a well defined, non –reflective,bell shaped space between the posterior boundary of the neurosensory retina and the RPE. Lateral extent is categorized into involvement of central 1mm (central subfield) alone, involvement of central 2 mm or extension of cysts beyond central 2mm. In addition, the axial diameter of the cyst at the centerpoint is categorized as small (≤ 200 μ),medium(201 – 400μ) or large (> 400μ). The height of subretinal fluid at the centerpoint is measured with calipers similar to centerpoint thickness. The type of vitreoretinal interface abnormality is identified; epiretinal membrane (ERM) with and without vitreomacular traction and macular hole. If present,their location within and outside the central subfield is categorized.
Intergrader agreement is assessed on a quarterly basis to ensure good quality control. Approximately 5% of images are randomly selected and re-graded every quarter by all four graders participating in the MUST Trial. Intergrader agreement is assessed as the percentage of agreement using kappa statistics for categorical variables and intraclass correlation coefficients for continuous variables.
Results
Of the 479 baseline eyes,OCT images were not available for 26(5.4%) eyes. OCT scanning was not performed in eyes with poor dilation secondary to synechiae, dense cataracts or vitreous opacities. Of the 453 OCT images, 315 (69%) were of good or fair quality (see Table 1). Caliper measurement of centerpoint could be performed in 119 (26%). Boundary line errors and decentration were the primary contributors to automated centerpoint thickness errors. Although this study of severe uveitis cases included many patients with media opacities, which interfered with photographic grading more frequently,8 center point thickness data were measureable in all but 19 (4%) of the 453 scans performed. Poor signal strength was the most common cause for ungradable quality. Confounding abnormalities such as macular hole (2 eyes) and vitreomacular traction (2 eyes) were the reason for missing data in 4 scans.
Table 1. Quality evaluation of baseline Optical Coherence Tomography (OCT) scans.
| Quality score | N=453 | % |
|---|---|---|
|
| ||
| Good | 282 | 62.2% |
|
| ||
| Fair | 33 | 7.3% |
|
| ||
| Borderline | 119 | 26.3% |
| Decentered alone | 23 | 19.3% |
| Boundary line error | 58 | 48.7% |
| Both of the above | 15 | 12.6% |
| Others | 23 | 19.3% |
|
| ||
| Ungradable | 19 | 4.2% |
| Poor signal strength | 12 | 63.2% |
| Scan protocol issue | 3 | 15.8% |
| Confounding lesion | 4 | 21.0% |
The mean centerpoint thickness was 268 (SD 185) um with 36 % of eyes qualifying for the definition of macular edema (centerpoint thickness ≥ 240 um). 8
Morphological features are tabulated in Table 2. Cystoid spaces were present in 166 (37%), absent in 264 (58%), and ungradable in 23 (5%). Almost 86% of cysts involved the central subfield and most eyes had small cysts (<200 microns in diameter). Subretinal fluid was seen in <1% of scans. ERMs were seen in 126 (28%) of eyes, mostly involving the central subfield.
Table 2. Morphological evaluation of baseline OCT images.
| Morphological characteristics | N=453 | % |
|---|---|---|
|
| ||
| Cystoid spaces present | 166 | 36.6% |
| Within central subfield alone | 43 | 25.9% |
| Within and outside central subfield | 100 | 60.2% |
| Outside central subfield | 23 | 13.9% |
|
| ||
| Cyst diameter at centerpoint | 108 | 23.8% |
| Small ≤200 μ | 55 | 50.9% |
| Medium 201-400 μ | 27 | 25.0% |
| Large >400 μ | 26 | 24.1% |
|
| ||
| Subretinal fluid | 4 | <1% |
|
| ||
| Epiretinal membranes | 126 | 27.8% |
| Involving central subfield | 83 | 65.8% |
| Vitreo macular traction | 3 | 2.4% |
Regrades were performed on 78 baseline OCT scans. Intergrader agreement for the categories of quality and morphological evaluation was substantial (Table3). Agreement on quality score was 88% (k =0.78). Of the 78 images, 16 required caliper measurement. The intraclass correlation between the original grade and the regrade for caliper measured centerpoint thickness was 0.98.
Table 3. Intergrader agreement for OCT evaluation variables (n= 78).
| OCT variable | Agreement n (%) | Kappa (95% confidence interval) |
|---|---|---|
| Quality Score | 69 (88%) | 0.78( 0.65 –0.91) |
| Manual measurement of centerpoint | 72(92%) | 0.83( 0.7 – 0.96) |
| Cystoid spaces | 64(82%) | 0.66 ( 0.5 – 0.81) |
| Epiretinal membrane | 61(78%) | 0.67 (0.49–0.85) |
Discussion
Central retinal thickness is an important endpoint for various clinical trials with macular edema, and is an important parameter in the clinical management of macular edema. Image artifacts affecting automated retinal thickness measurements are not disease-specific, but the frequency of the artifacts varies with the disease.10,11,12 In a large series of 3,794 OCTs with diverse retinal pathology, automated central retinal thickness was erroneous in 16% of scans in diabetic retinopathy,24% in vein occlusion and 55% in wet macular degeneration. 10 Evaluation for structural changes showed that 10% of scans could not be interpreted in eyes with uveitis. 13 Detailed evaluation of OCT quality has not been reported previously for eyes with uveitis, which is known to potentially result in macular edema, ERM and tractional changes,14 and a propensity towards media opacity. OCT Evaluation in the MUST Trial using a standardized protocol with evaluation at a central Reading Center showed that automated retinal thickness measurements were inaccurate in more than 30% of scans, demonstrating that simple use of the automated measurements is not adequate for research or clinical practice. However, manual measurement of thickness in cases with decentration or boundary line errors resulted in measurable thickness in nearly all cases, despite a high prevalence of cataract and vitreous haze in this uveitic population. 8 Our estimate that approximately 5% of eyes could not be scanned and 96% of eyes can be graded is useful in calculating the expected sample size when OCT-measured central retinal thickness data will serve as a key endpoint in clinical studies.15
Understanding the extent and pattern of artifacts observed in the setting of uveitis also is important to improve future iterations of the OCT software. In the time-domain OCT dataset from the MUST Trial, artifacts affecting the central retinal thickness measurement algorithm were more frequent than in diabetic macular edema and retinal vein occlusions.10 Boundary line errors accounted for almost 50% of scans with inaccurate automated central subfield thickness. Errors in inner boundary lines might be attributable to the higher frequency of ERMs, retinal traction and poor signal strength resulting from media opacity in severe uveitis cases (Figure 1 top). Errors in the outer boundary lines were due to changes at the level of the retinal pigment epithelium (RPE) such as scars, hard exudates, and fibrosis (Figure 1 bottom). The frequency of decentration also was higher than in clinical trials of diabetic retinopathy or retinal vein occlusion10, perhaps because a number of participants had poor visual acuity, non dilating pupils, lens opacities, vitritis, and/or disruption in macular anatomy due to pigment changes, scarring and media opacities, all of which could hinder centration.
The quality scoring system represents the accuracy of the automated central retinal measurements. A B scan with poor quality can still be evaluated for morphological changes. Despite the media problems described above, the number of scans with ungradable morphological data was <5% in the MUST Trial. The mean retinal thickness for the baseline OCT scans in the MUST trial was 268(+/−185)μ for intermediate and posterior/panuveitis cases.8 36% of eyes had macular edema at baseline, defined as a center point thickness ≥ 240 um. Characterization of the edema showed predominantly small cysts in the center. This is contrary to the distribution of edema described by Castellano et al for cases of iridocyclitis, where the edema was located predominantly in a ring around the center, perhaps reflecting the different sites of inflammation studied. In contrast to the report of Markomichelakis et al, in which 20% of 84 uveitic eyes with macular edema had subretinal fluid,14 fewer than 1% of MUST Trial cases had subretinal fluid. The higher frequency of subretinal fluid compared to the MUST trial could be attributed to the more severe baseline macular edema with a mean retinal thickness of 333(+/−171) μ. ERMs were also slightly lower in the MUST dataset (28% vs.40%). A difference in the methodology, specifically inclusion of globally adherent ERMs could attribute to this.7 The Reading Center methodology defines ERM as a hyper-reflective layer with a bridging effect over the inner retinal layers. This definition potentially excludes globally adherent ERMs if their reflectivity merges with that of the nerve fiber layer. Corrugation of inner retinal layers is also considered insufficient to identify an ERM. These detailed definitions are essential to maintain good intergrader agreement in clinical trials where evaluation is masked to other imaging modalities; i.e. OCTs are graded without information from color photographs. The agreement was substantial (k=0.67) for the presence and location of ERMs in the MUST trial.
Although the MUST trial employed time domain OCT, the data derived from this technology is still useful in the era of spectral domain OCT (SDOCT). Standardized data from such a large number of uveitis cases with a two year follow-up period is not yet available with SDOCTs. Studies have shown a lower frequency of artifacts with SDOCT but the types of artifacts largely remain the same16, 17. Thus,our results indicating that macular thickening can be imaged in the large majority of severe uveitis cases suggest that SDOCT is likely to be highly effective in imaging macular thickening in the uveitis setting. The reduced sampling of the macular area with time domain OCT may limit the evaluation of ERMs, requiring input from other imaging modalities to accurately diagnose this condition, whereas the high scan density, three dimensional viewing and higher resolution of SDOCT provide improved ability to visualize vitreoretinal surface abnormalities. 18
In summary, the time-domain OCT evaluation method used in the MUST trial is reproducible. Quality determination, with manual measurement in cases with boundary line errors or defective centration, is an integral part of OCT evaluation when retinal thickness data are used as clinical trial endpoints. In the majority of cases, retinal thickness data can be accurately obtained, even when a large number of eyes with compromised media and inflammatory macular injury are included. Macular edema in recently active intermediate and posterior/panuveitis most commonly is characterized by small central cystoid spaces.
Acknowledgments
Financial support: Supported by cooperative agreements from the National Eye Institute to Mount Sinai School of Medicine (U10 EY 014655), The Johns Hopkins University Bloomberg School of Public Health (U10 EY 014660), and the University of Wisconsin, Madison, School of Medicine (U10 EY 014656).
Credit Roster
Participating Clinical Centers
Duke University, Durham, NC
Glenn J. Jaffe, MD (Director); Shelley Day, MD; Annie Lee, MD; Cindy Skalak. Former Members: Claxton Baer, MD; Sai Chavala, MD; Michael Cusick,MD; Pouya Dayani, MD; Justis Ehlers, MD; Muge Kesen,MD Alex Melamud,MD; Jawad A. Qureshi,MD; Adrienne Williams Scott; Robert F. See, MD; Robert K. Shuler,MD.
Emory University, Atlanta, GA
Steven Yeh, MD (Director); Alicides Fernandes, MD. Former Members: Deborah Gibbs, COMT; Daniel F. Martin, MD; Sunil Srivastava, MD.
Johns Hopkins University, Baltimore, MD
James P. Dunn, MD (Director); Ellen Arnold, BS; Jeff Boring, COA; Diane M. Brown, MSN, RN; Alison G. Livingston, BSN, RN; Terry Reed,COA. Former Members: Marie-Lynn Belair, MD; Stephen G. Bolton, CRNP; Joseph B. Brodine; Lisa M. Brune, BSN, RN; Anat Galor, MD; Adam Jacobowitz, MD; Meera Kapoor; Sanjay Kedhar, MD; Stephen Kim, MD; Henry A. Leder, MD; Yavette Morton; Kisten Nolan, BSN, MPH, RN; George B. Peters, MD, MBA; Priscilla Soto; Ricardo Stevenson, MD; Michelle Tarver-Carr, MD, PhD; Yue Wang, MD.
Massachusetts Eye Research and Surgery Institute, Cambridge, MA
C. Stephen Foster, MD (Director); Linda Bruner; David M. Hinkle, MD; Jyothir Johnson; Danielle Marvell; Sana S. Siddique. Former Members: Sarah Acevedo,MD; Fahd Anzaar, MD; Tom Cesca; Angelica Contero; Kayleigh Fitzpatrick; Karina Q. Lebron, MD; Chandra Morgan; Nita Patel, MSW; Jennifer Pinto; Janet Sprague; Taygan Yilmaz.
National Eye Institute, Bethesda, MD
H. Nida Sen, MD, MHSc (Director); Michael Bono; Denise Cunningham, CRA; Darryl Hayes, COA; Dessie Koutsandreas, BS, COA; Nupura Krishnadev, MD; Theresa Larson, MD; Annal D. Meleth, MD; Robert B. Nussenblatt, MD; Patti R. Sherry, BSN; Gregory L. Short, COMT; Wendy Smith, MD; Alana Temple, BSN. Former Members: Allison Bamji, RN; Hanna Coleman, MD; Geetaniali Davuluri, MD; Lisa Faia, MD; Chloe Gottlieb, MD; Guy V. Jirawuthiworavong, MD; Julie C. Lew, MD; Richard Mercer, COT; Dominic Obiyor, BSN; Cheryl H. Perry, BSN; Natalia Potapova, MD; Eric Weichel, MD; Keith J. Wroblewski, MD; Steven Yeh, MD.
New York Eye & Ear Infirmary, New York, NY
Paul A. Latkany, MD (Director); Jason Badamo; Jenny Gallardo; Monica Lorenzo-Latkany, MD; Robert Masini; Susan Morell; Ann Nour; Meredith Sanchez; Kate Steinberg. Former Members: Kenneth M. Boyd; Jacek Jarczynski; Mirjana McGrosky.
Royal Victorian Eye & Ear Hospital, East Melbourne, Australia
Richard J. Stawell, MD (Director); Lisa Breayley; Carly D'Sylva; Julie Ewing; Lauren Hodgson; Ignatios Koukouras; Lyndell Lim; Cecilia Ling; Rachel McIntosh; Andrew Newton; Richard Smallwood; Ehud Zamir. Former Members: Nicola Hunt; Lisa Jones; Suzanne Williams.
Rush University Medical Center, Chicago, IL
Pauline T. Merrill, MD (Director); Pam Hulvey; Elaine Kernbauer; Scott Toennessen; Denise L. Voskuil-Marre , BS. Former Members: Bruce Gaines; Christina Giannoulis; Heena S. Khan, BA; Sarah J. Levine.
Texas Retina Associates, Dallas, TX
Robert C. Wang, MD (Director); Hank Aguado; Sally Arceneaux; Kimberly Cummings; Gary E. Fish, MD; Keith Gray; Nick Hesse; Susie Howden; Diana Jaramillo; Michael Mackens; Karin Mutz; Brenda Sanchez. Former Members: Jean Arnwine; David Callanan, MD.
United Kingdom Institute of Ophthalmology,London, UK
Susan Lightman, PhD (Director); Lavanish Joshi; Simon Taylor; Hamish Towler; Rebecca Tronnberg. Former Members: Kate Edwards; Timothy Stubbs.
University of California at Los Angeles, Los Angeles, CA
Gary N. Holland, MD (Director); Robert D. Almanzor, COA; Jose Castellanos, COT; Jean Pierre Hubschman, MD; Ann K. Johiro, NP; Parthu Kalyani; Michael A. Kapamajian, MD; Ralph D. Levinson, MD; Susan S. Ransome, MD. Former Members: Christine R. Gonzales, MD; Anurag Gupta, MD; Peter J. Kappel, MD.
University of California at San Diego, San Diego, CA
William R. Freeman, MD (Director); Igor Kozak, MD; Vivian Nguyen; Debbie Powell, BBA. Former Members: Tom Clark,BSc, CRA; Denine E. Cochran, COT, CCRC,Joshua Hedaya, MD; Tiara Kemper; Jacqueline M. LeMoine; Megan E. Loughran, BA; Luzandra Magana; Francesca Mojana,MD; Victoria Morrison, MD; Stephen F. Oster, MD.
University of California at San Francisco, San Francisco, CA
Ira G. Wong, MD (Director); Nisha Acharya, MD; David Clay; Claire M. Khouri, BA; Mary Lew, BA; Todd P. Margolis, MD, Jay Stewart, MD. Former Members: Salena Lee, BA, OD.
University of Illinois at Chicago, Chicago, IL
Debra A. Goldstein, MD (Director); Marcia Niec; Anna Castro-Malek; Howard H. Tessler, MD. Former Members: Catherine E. Crooke; Dimitry Pyatetsky, MD; Misel Ramirez.
University of Miami, Miami, FL
Janet L. Davis, MD (Director); Thomas Albini, MD; David A. Pinto. Former Members: Daniela Castaño; Marie Chin; Macy Ho; Jaclyn L. Kovach, MD; Richard C-S Lin, MD; Efrem Mandelcorn; Jackie K-DNguyen, MD; Aura Pacini; Susan Pineda; Jose Rebimbas; Kimberly E. Stepien, MD; Claudia Teran.
University of Michigan, Ann Arbor, MI
Susan G. Elner, MD (Director); Rebecca Brown, COA; Linda Fournier, COA; Julie R. Gothrup, COA; Richard Hackel; Moella Hesselgrave, COA; Robert Prusak; Stephen J. Saxe, MD. Former Members: Melissa Bergeron, COA; Reneé Blosser, COMT; Carrie Chrisman-McClure; Deanna Sizemore, COA.
University of Pennsylvania, Philadelphia, PA
John H. Kempen, MD, PhD (Director); James Berger; Sheri Drossner; Joan C. DuPont; Albert M. Maguire, MD; Dawn McCall; Janice Petner; Laurel Weeney. Former Members: Tim Hopkins, MD; Monique McRay; Daniel Will, MD; Wei Xu.
University of South Florida, Tampa, FL
Peter Reed Pavan, MD (Director); JoAnn Leto; Lori Mayor; Kim McDonald; Scott E. Pautler, MD; Wyatt Saxon; Judy Soto. Former Members: Maria Ortiz; Dee Dee Szalay.
University of Southern California, Los Angeles, CA
Amani Fawzi, MD; (Director); Lupe Cisneros, COA; Elizabeth Corona; Jackie Douglass; Dean Eliott, MD; Margaret Padilla; Narsing A. Rao, MD. Former Members: Alexia Aguirre; Lawrence Chong, MD; Rahul Khurana, MD; Jennifer Lim, MD; Rachel Mead; Sylvia Ramos; Julie H. Tsai, MD.
University of Utah, Salt Lake City, UT
Albert Vitale, MD (Director); Paul S. Bernstein, MD, PhD; Bonnie Carlstrom, COA; James Gilman, CRA; Sandra Hanseen, COA; Paula Morris, CRA; Diana Ramirez; Kimberley Wegner, BS, CRC.
Virginia Eye Consultants, Norfolk, VA
John D. Sheppard, MD, MMSc (Director); Brianne Anthony; Amber Casper; Lisa Felix-Kent, COA; Jeanette Fernandez, COMT; Stephen V. Scoper, MD. Former Members: R. Denise Cole; Nancy Crawford; Rebecca De La Garza; Lisa Franklin; Krista Hamelin; Jen Martin; Rebecca Marx; Gregory Schultz, DD; Joseph Webb, BS; Pamela Yeager.
Vitreoretinal Consultants, Houston, TX
Rosa Y. Kim, MD (Director); Matthew S. Benz, MD; David M. Brown, MD; Eric Chen, MD; Richard H. Fish, MD; Shayla Hay; Jame Major, MD, PhD; Laura Shawver; Tien P. Wong, MD.
Washington University, St. Louis, MO
P. Kumar Rao, MD (Director); Rhonda Weeks. Former Members: Rajendra S. Apte, MD; Kevin J. Blinder, MD; Ashley Hartz; Pam Light; Gaurav K. Shah, MD; Russell VanGelder, MD, PhD.
Committees
Executive Committee
Douglas A. Jabs, MD, MBA (Chair); Michael M. Altaweel, MD; Janet T. Holbrook, PhD; John H. Kempen, MD, PhD; Natalie Kurinij, PhD.
Steering Committee
Douglas A. Jabs, MD, MBA (Chair); Robert D. Almanzor, COA; Michael M. Altaweel MD; Diane Brown MD; James P. Dunn, MD; James Gilman MD; Janet T. Holbrook, PhD; Gary N. Holland, MD; John H. Kempen, MD, PhD; Rosa Y. Kim, MD; Natalie Kurinij, PhD; Nancy Prusakowski, MS; Jennifer E. Thorne, MD, PhD. Former Members: Stephen G. Bolton, RN, BSN; Lisa M. Brune, RN, BSN; Tom Clark, CRA; Larry Hubbard, MAT; Daniel F. Martin, MD; Robert B. Nussenblatt, MD.
Data, Safety and Monitoring Committee
Janet Wittes, PhD (Chair); Michael M. Altaweel, MD; William E. Barlow, PhD; Marc Hochberg, MD; Janet T. Holbrook, PhD; Douglas A. Jabs, MD, MBA; Natalie Kurinij, PhD; Alice T. Lyon, MD; Alan G. Palestine, MD; Lee S. Simon, MD; Harmon Smith, PhD. Former Members: James T. Rosenbaum, MD.
Surgical Quality Assurance Committee
John H. Kempen, MD, PhD (Chair); James P. Dunn, MD; Glenn J. Jaffe, MD. Former Members: Daniel F. Martin, MD.
Medical Therapy Quality Assurance Committee
Jennifer E. Thorne, MD, PhD (Chair); Nisha Acharya, MD; Douglas A. Jabs, MD, MBA; John H. Kempen, MD, PhD; Paul A. Latkany, MD; Robert B. Nussenblatt, MD; Albert Vitale, MD. Former Members: Russell VanGelder, MD, PhD.
Visual Function Quality Assurance Committee
Robert D. Almanzor, COA (Chair); Judith Alexander, BA, CCRP; Jeffrey A. Boring, COA; Deborah Gibbs, COMT; Salena Lee, OD, FAAO; Jennifer E. Thorne, MD, PhD (Advisor). Former Members: Wai Ping Ng, BS.
Glaucoma Committee
David S. Friedman, MD (Chair); Anna Adler, MS; Judith Alexander, BA, CCRP; Alyce Burke, MPH; Janet T. Holbrook, PhD; Joanne Katz, ScD; John H. Kempen, MD, PhD; Nancy Prusakowski, MS; Susan Reed; Jennifer E. Thorne, MD, PhD. Former Members: Nicholas Cohen; Sanjukta Modak, MS; Wai Ping Ng, BS.
Statistical Analysis Committee
Elizabeth A. Sugar, PhD (Chair); Lea Drye, PhD; Kevin Frick, PhD; Janet T. Holbrook, PhD; JoAnn Katz, ScD; Thomas Louis, PhD; Mark L. Van Natta, MHS. Former Members: Sanjukta Modak, MS; David Shade, JD.
Resource Centers
Chairman's Office: Mount Sinai School of Medicine, New York, NY
Douglas A. Jabs, MD, MBA (Study Chairman); Yasmin Hilal, MHS; Melissa A. Nieves, BA; Karen Pascual, MBA; Jill S. Slutsky, MPA. Former Members: Colby Glomp; Maria Stevens, CM.
University of Pennsylvania, Philadelphia, PA
John H. Kempen, MD, PhD (Study Vice-Chairman).
Coordinating Center, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD
Janet T. Holbrook, PhD, MPH (Director); Anna L. Adler, MS; Judith Alexander, BA; Jeff A. Boring, COA; Alyce E. Burke, MPH; Karen Collins; John D. Dodge; Lea T. Drye, PhD; Cathleen S. Ewing; Kevin D. Frick, PhD; David S. Friedman, MD, PhD; Rosetta Jackson; Joanne Katz, ScD; Andrea T. Lears, BS; Hope Livingston; Thomas A. Louis, PhD; Curtis L. Meinert, PhD; Jill L. Meinert; Vinnette Morrison, BS; Deborah J. Nowakowski; Nancy Prusakowski, MS; Dave M. Shade, JD; Rochelle E. Smith, BS; Karen Steuernagle; Elizabeth A. Sugar, PhD; Jennifer E. Thorne, MD, PhD; James A. Tonascia, PhD; Mark L. Van Natta, MHS; Richard Zheng, BS. Former Members: Paul Chen; Nicholas Cohen, MS; Sanjukta Modak, MS; Wai Ping Ng, BS; Weijiang Shen, BS; Charles Shiflett, BS; Ada Tieman, MBA.
University of Wisconsin Reading Center, Madison, WI
Michael M. Altaweel, MD (Director); Wendy K. Benz, PhD; Geoffrey Chambers, MS; Debra J. Christianson, BS; Amitha Domalpally, MD; Jacquelyn Freund, MS; Vonnie Gama; Sapna Gangaputra, MD, MPH; Kathleen E. Glander, BBA; Anne Goulding, BA; Jeffrey M. Joyce, MS; Christina N. Kruse, BA; Dawn J. Myers, BS; Susan Reed, BS; James L. Reimers, BA; Amy Remm, BS; Ruth A. Susman, BS; Dennis Thayer; Erika Treichel, DVM; Kelly J. Warren, RN, BS, MSES; Sheila M. Watson, BS, DVM; James K. White, BME; Tara Wilhelmson, BS. Former Members: Margaret A. Fleischli, AB, DVM; Dennis Hafford; Susan E. Harris, MS; Larry D. Hubbard, MAT; Kristine A. Johnson; Lauren Nagle; Gwyn E. Padden-Lechten, BS; Alyson Pohlman, BA; Peggy Sivesind; Mary K. Webster, BS; Grace Zhang, BA.
National Eye Institute, Bethesda, MD
Natalie Kurinij, PhD.
Footnotes
Conflict of interest: Dr Kempen is a consultant/advisor for Alcon, Allergan, Lux Biosciences, Sanofi Pasteur, Xoma and Dr Srivastava has received research grants from Bausch and Lomb, Novartis and Allergan. Drs. Domalpally, Altaweel,Myers,Davis,Foster,Latkany and Stawell have no conflicts to declare.
References
- 1.Scott IU, Edwards AR, Beck RW, Bressler NM, Chan CK, Elman MJ, et al. A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology. 2007;114(10):1860–7. doi: 10.1016/j.ophtha.2007.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scott IU, Ip MS, VanVeldhuisen PC, Oden NL, Blodi BA, Fisher M, et al. SCORE Study Report 6 - A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular Edema secondary to branch retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 6. Arch Ophthalmol. 2009;127(9):1115–28. doi: 10.1001/archophthalmol.2009.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ip MS, Scott IU, VanVeldhuisen PC, Oden NL, Blodi BA, Fisher M, et al. SCORE Study Report 5: A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5. Arch Ophthalmol. 2009;127(9):1101–14. doi: 10.1001/archophthalmol.2009.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fung AE, Lalwani GA, Rosenfeld PJ, Dubovy SR, Michels S, Feuer WJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007;143(4):566–83. doi: 10.1016/j.ajo.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 5.Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs--an extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology. 1991;98(5 Suppl):786–806. [PubMed] [Google Scholar]
- 6.Davis MD, Bressler SB, Aiello LP, Bressler NM, Browning DJ, Flaxel CJ, et al. Comparison of time-domain OCT and fundus photographic assessments of retinal thickening in eyes with diabetic macular edema. Invest Ophthalmol Vis Sci. 2008;49(5):1745–52. doi: 10.1167/iovs.07-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilkins JR, Puliafito CA, Hee MR, Duker JS, Reichel E, Coker JG, et al. Characterization of epiretinal membranes using optical coherence tomography. Ophthalmology. 1996;103(12):2142–51. doi: 10.1016/s0161-6420(96)30377-1. [DOI] [PubMed] [Google Scholar]
- 8.Multicenter Uveitis Steroid Treatment (MUST) Trial Research Group. The Multicenter Uveitis Steroid Treatment Trial: Rationale, Design, and Baseline Characteristics. American Journal of Ophthalmology. 2010;149(4):550–61.e10. doi: 10.1016/j.ajo.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Domalpally A, Blodi BA, Scott IU, Ip MS, Oden NL, Lauer AK, et al. SCORE Study Report 4 - The Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study system for evaluation of optical coherence tomograms: SCORE study report 4. Arch Ophthalmol. 2009;127(11):1461–7. doi: 10.1001/archophthalmol.2009.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Domalpally A, Danis RP, Zhang B, Myers D, Kruse CN. Quality issues in interpretation of optical coherence tomograms in macular diseases. Retina. 2009;29(6):775–81. doi: 10.1097/IAE.0b013e3181a0848b. [DOI] [PubMed] [Google Scholar]
- 11.Hee MR. Artifacts in optical coherence tomography topographic maps. Am J Ophthalmol. 2005;139(1):154–5. doi: 10.1016/j.ajo.2004.08.066. [DOI] [PubMed] [Google Scholar]
- 12.Ray R, Stinnett SS, Jaffe GJ. Evaluation of image artifact produced by optical coherence tomography of retinal pathology. Am J Ophthalmol. 2005;139(1):18–29. doi: 10.1016/j.ajo.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 13.Gupta V, Gupta P, Singh R, Dogra MR, Gupta A. Spectral-domain Cirrus high-definition optical coherence tomography is better than time-domain Stratus optical coherence tomography for evaluation of macular pathologic features in uveitis. Am J Ophthalmol. 2008;145(6):1018–22. doi: 10.1016/j.ajo.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 14.Markomichelakis NN, Halkiadakis I, Pantelia E, Peponis V, Patelis A, Theodossiadis P, et al. Patterns of macular edema in patients with uveitis: qualitative and quantitative assessment using optical coherence tomography. Ophthalmology. 2004;111(5):946–53. doi: 10.1016/j.ophtha.2003.08.037. [DOI] [PubMed] [Google Scholar]
- 15.Glassman AR, Beck RW, Browning DJ, Danis RP, Kollman C. Comparison of optical coherence tomography in diabetic macular edema, with and without reading center manual grading from a clinical trials perspective. Invest Ophthalmol Vis Sci. 2009;50(2):560–6. doi: 10.1167/iovs.08-1881. Epub 2008 Jun 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han IC, Jaffe GJ. Evaluation of artifacts associated with macular spectral-domain optical coherence tomography. Ophthalmology. 2010;117(6):1177–89. doi: 10.1016/j.ophtha.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 17.Domalpally A, Gangaputra S, Peng Q, Danis RP. Repeatability of retinal thickness measurements between spectral-domain and time-domain optical coherence tomography images in macular disease. Ophthalmic Surg Lasers Imaging. 2010;41(6):S34–41. doi: 10.3928/15428877-20100325-01. [DOI] [PubMed] [Google Scholar]
- 18.Chang LK, Fine HF, Spaide RF, Koizumi H, Grossniklaus HE. Ultrastructural correlation of spectral-domain optical coherence tomographic findings in vitreomacular traction syndrome. Am J Ophthalmol. 2008;146(1):121–7. doi: 10.1016/j.ajo.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]