Abstract
Systemic sclerosis (SSc) is characterized by vascular alterations, activation of the immune system and tissue fibrosis. Vascular insufficiency manifests early in the disease, and although there is evidence of an active repair process, capillaries deteriorate and regress. Factors that contribute to the failure of vascular regeneration might include persistent injury, an imbalance between proangiogenic and antiangiogenic mediators, intrinsic abnormal properties of the cellular components of the vessels, and the presence of fibroblast-derived antiangiogenic factors. In addition, circulating dysfunctional endothelial progenitor cells might further exacerbate vessel deterioration. Abnormal expression of transcription factors, including Fra2 and Fli1, has been proposed to contribute to SSc vasculopathy. Fli1 regulates genes that are involved in vessel maturation and stabilization, suggesting that reduced levels of Fli1 in SSc vasculature could contribute to the development of unstable vessels that are prone to regression. Conversely, proliferating endothelial cells and pericytes, in the presence of an appropriate stimulus, might transdifferentiate into collagen-producing cells, and thus contribute to the initiation of fibrosis. Despite progress in treating the symptoms of vascular disease in SSc, the underlying mechanisms remain poorly understood. An improved knowledge of the molecular and cellular pathways that contribute to SSc vasculopathy could help in the design of effective therapies in the future.
Introduction
Systemic sclerosis (SSc) is a connective tissue and autoimmune disease of unknown etiology that affects various organ systems, including the lungs, heart, gastrointestinal tract and kidneys.1 The three major features of SSc are systemic vascular dysfunction, the presence of mononuclear cell infiltrates and connective tissue fibrosis.1,2 Raynaud phenomenon is present in the majority of SSc patients and could precede a definite diagnosis of SSc by years or even decades.3 On the basis of diagnostic criteria proposed by LeRoy and Medsger,4 Raynaud phenomenon can be classified either as primary (idiopathic), in which the defect is primarily functional with no underlying secondary disorder, or secondary to other systemic diseases. In patients with SSc, Raynaud phenomenon is associated with structural abnormalities of the microvasculature and activation of the immune system. A large, prospective study of patients with Raynaud phenomenon concluded that progressive microvascular damage and the presence of an SSc-specific autoantibody pattern strongly predict development of definite SSc.5
The event that initiates vascular injury in patients with SSc is presently unknown. Current hypotheses include the presence of infectious agents, autoantibodies against endothelial cells, cytotoxic T cells, nitric-oxide-related free radicals or granzyme.6 The inter-relationship between structural and functional vascular abnormalities is complex, and also not fully defined. Various mechanisms, including dysfunction of endothelial cells, neural abnormalities and various intravascular defects, could contribute to impaired vascular flow.7 During disease progression, pathological changes in the vessels and excessive collagen accumulation occur in parallel, eventually leading to tissue ischemia and widespread fibrosis; however, immune infiltrates are usually only associated with the early stages of the disease. Factors that contribute to the progression of vascular disease remain poorly defined. Vascular disease in scleroderma has been comprehensively reviewed elsewhere;8 in this Review, I will focus on the cellular and molecular aspects of SSc vasculopathy.
Morphological vascular changes
Vascular disease in SSc predominantly affects the microcirculation, especially capillaries and arterioles. Capillaries consist of a layer of endothelial cells variably covered by pericytes and embedded in a shared basement membrane. Structural changes in the vasculature of SSc patients have been documented in numerous studies. In 1925, Brown and O’Leary,9 using capillary microscopy of the nailfold of the fingers, recorded the first evidence of abnormal microvasculature, which included a striking decrease in the number of capillary loops, dilatation and distortion of remaining capillaries and occasional hemorrhage. Subsequent studies performed more than 40 years ago demonstrated a decrease in the number of normal capillaries, swelling of microvascular endothelial cells and reduplication of the capillary basement membrane.10,11
The earliest detectable changes consist of loss of membrane-bound storage vesicles in endothelial cells, cytoskeletal rearrangement, and deposition of amorphous material within the endothelial basement membrane.12,13 Additional changes include cytoplasmic vacuolization and increased capillary permeability, resulting in edema. The results of a 20-year prospective study of patients with Raynaud phenomenon leading to SSc have demonstrated that microvascular damage is sequential: capillary enlargement is followed by capillary loss and then by capillary telangiectasias.5 Further disease progression is characterized by morphological changes in the remaining vessels occurring in parallel with tissue fibrosis. Besides the skin, vascular changes are present in the lungs, kidneys and other organs. SSc patients with pulmonary hypertension develop vascular lesions in small-sized and medium-sized vessels of the lung. These lesions are characterized by concentric intimal proliferation, marked luminal obstruction and a frequent presence of infiltrating immune cells; relatively few plexiform lesions, which may result from hyperproliferation of endothelial cells, occur.14-16 Similar pathological changes, comprising intimal proliferation, medial hyperplasia and obliteration of the lumen, were reported in renal vessels.17 However, the mechanisms underlying the pathological vascular changes in SSc remain unclear.
Defective angiogenesis
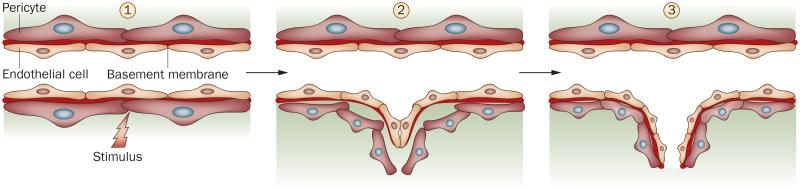
The remarkable regression of the capillaries and small vessels in patients with SSc strongly suggests a defect in the process of angiogenesis; however, specific mechanisms contributing to vessel dysfunction and subsequent deterioration have not been elucidated. Angiogenesis is defined as the process by which new vessels are formed from pre-existing ones. Under physiological conditions, this highly regulated process requires coordination of several independent steps.18,19 An initial invasive phase involves detachment of pericytes (also called periendothelial cells or mural cells) from the endothelium, degradation and remodeling of the basement membrane and surrounding connective tissue, and subsequent migration and proliferation of endothelial cells. The maturation phase of angiogenesis is characterized by lumenization of the sprout and deposition of new basement membrane components, including collagen type IV and laminin. Stabilization of the newly formed vessels is facilitated by the recruitment of pericytes (Figure 1).
Figure 1.
Schematic overview of the principal stages of angiogenesis. (1) In quiescent capillaries, endothelial cells form tight contacts with each other and with neighboring pericytes. Both cell types are embedded in the shared basement membrane. (2) Angiogenic sprouting requires local degradation of extracellular components of the basement membrane by endothelial-cell-derived proteases, loosening of the cell–cell and cell–matrix contacts, endothelial cell proliferation, migration, and formation of a nonlumenized sprout. Factors secreted by the endothelial cells attract pericytes that contribute to stabilization of the newly formed vessel. (3) Maturation of capillaries involves re-establishment of cell–cell contacts, lumen formation, secretion of new basement membrane, and recruitment of pericytes.
Evidence of angiogenesis
Several studies have examined endothelial cell proliferation in skin explants using autoradiography with 3H-thymidine in an effort to assess angiogenesis in SSc patients; they all found considerably increased labeling in endothelial cells from the explants of SSc patients compared with those individuals without the disease, with especially high uptake in telangiectasias.20-22 These observations are supported by studies in which skin explants from SSc patients were transplanted into severe combined immunodeficient (SCID) mice or chick embryo chorioallantoic membrane, the results of which showed appreciably higher neovascularization of the SSc explants compared to skin from control individuals.23,24 Evidence of endothelial cell growth has also been noted in lung tissues from SSc patients.14,25 Although concentric nonproliferative lesions predominate in SSc patients with pulmonary arterial hypertension (PAH), it has been suggested that these lesions represent a more ‘mature’ form of the highly proliferative plexiform lesions that are also occasionally seen in SSc lungs.14,16 In another study,25 hypervascularity and irregularly sized microvessels were observed in the early stages of pulmonary fibrosis, with subsequent vessel regression and development of fibrosis.
Existing studies, therefore, point to the presence of intrinsic proangiogenic factors residing in the SSc vasculature or the adjacent tissues. The inability to regenerate injured vessels might result from the failure of subsequent stages of angiogenesis, such as lumen formation or vessel maturation or stabilization, or a combination of these processes. Alternatively, persistent injury or the presence of circulating antiangiogenic factors might interfere with this process.
Imbalance of angiogenic factors
One of the striking features associated with SSc vascular disease is the presence of high circulating levels of the key angiogenic mediator vascular endothelial growth factor (VEGF), even at a very early disease stage (pre-scleroderma).26 The protein levels of other proangiogenic factors, including platelet-derived growth factor (PDGF), placental growth factor (PIGF) and fibroblast growth factor 2 (FGF-2) are also considerably elevated in the plasma of SSc patients.27 Furthermore, the expression of VEGF and its receptors VEGFR1 and VEGFR2 is increased in the skin of SSc patients in vivo.28-30 Elevated levels of VEGF have been found in the epidermis and in fibroblasts, endothelial cells and immune cells from the skin of SSc patients. Interestingly, the enhanced expression of VEGF does not seem to be driven by hypoxia, as indicated by the reduced levels of hypoxia-inducible factor 1 (HIF-1) in skin biopsies from SSc patients.29 However, the occurrence of localized hypoxia has been noted in some groups of patients—for example, those with limited cutaneous SSc with calcinosis and in some patients with diffuse cutaneous SSc—suggesting that hypoxia could be a contributing factor to increased expression of VEGF in some patients.28 Although elevated levels of VEGF are consistent with active angiogenesis, an uncontrolled, prolonged presence of VEGF might contribute to a disorganized vascular network and eventually lead to vessel degeneration.31
Evidence also exists for the enhanced presence of circulating antiangiogenic factors, including angiostatin, CXC chemokine ligand 4 (CXCL4, also known as platelet factor 4), thrombospondin and interleukin (IL)-4.32,33 However, these factors are present only in some subsets of patients, and although they might contribute to overall vessel dysfunction, it is unlikely that they represent a primary mechanism responsible for SSc vasculopathy. Furthermore, in addition to VEGF, elevated levels of other proangiogenic mediators, including endothelin 1, adhesion molecules, and some chemokines were found in the circulation of some SSc patients,32 which is consistent with active neoangiogenesis. Whether dysregulated levels of circulating angiogenic factors or angiostatic factors (or both) are a cause or a consequence of an ongoing vascular disease is presently unknown.
Endothelial progenitor cells
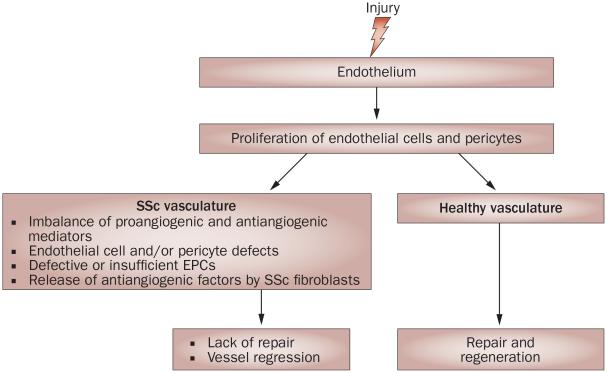
Although a number of studies have implicated endothelial progenitor cells (EPCs) in the process of postnatal vasculogenesis, the role of these cells in the formation of new blood vessels is still controversial. Furthermore, owing to the scarcity of specific markers and the reliance on cell culture methods for their isolation, defining EPCs is still a matter of scientific debate.34,35 A variety of cells with endothelial cell characteristics have been identified that could contribute in different capacities to vessel repair and regeneration, but the concept that circulating cells can form new vessels is not supported by current evidence.36 Interestingly, results from a 2009 study have suggested that mechanical tension can give rise to new endothelium during wound healing through the recruitment of pre-existing vessels into granulation tissue.37 Thus, although it is well documented that circulating cells are mobilized and recruited into damaged or ischemic tissues, their contribution to vascular repair might be indirect, through the production of proangiogenic cytokines and chemokines. Circulating levels of EPCs in SSc patients have been reported to be either increased or decreased; this discrepancy might reflect different stages of the disease38 or different methodologies used to isolate these cells. Apoptosis of EPCs caused by a circulating factor present in the serum has been proposed to account for their reduced numbers in patients with SSc.39 Additionally, EPCs obtained from the bone marrow of SSc patients show a reduced angiogenic potential.40 Given the existing uncertainty regarding the role of EPCs in neovascularization, it is currently difficult to assess the specific contributions of defects in these cells to SSc vasculopathy. Interestingly, however, a pilot study has suggested that statins could be effective in improving Raynaud phenomenon by increasing the number of circulating EPCs,41 thus implicating EPCs in restoring endothelial dysfunction in SSc (Figure 2).
Figure 2.
Schematic overview of the potential mechanisms involved in impaired neoangiogenesis in SSc. In healthy individuals, injury to the endothelium triggers a repair process that leads to vessel regeneration. In patients with SSc, there is evidence that endothelial cells and pericytes proliferate during the early stages of the disease, suggesting an initiation of the angiogenic process in response to injury. However, the repair process fails, ultimately leading to regression of small vessels. Studies have revealed several potential mechanisms that could account for the impairment of the angiogenic process in SSc, but the specific contribution of each of these mechanisms to SSc vasculopathy requires further evaluated. Abbreviations: EPCs, endothelial progenitor cells; SSc, systemic sclerosis.
SSc vessels: Intrinsic abnormalities
In addition to circulating factors, intrinsic properties of the cellular components of the capillaries and arterioles have been proposed to contribute to vascular dysfunction in SSc patients.42 Previous studies have identified proteins that are expressed at abnormal levels in endothelial cells and pericytes from SSc patients,42-44 as well as altered patterns of gene expression in cultured endothelial cells derived from SSc patients,45 suggesting a potential direct contribution of these cellular alterations to the disease process.
Endothelial cells
Fleming et al.42 have reported a highly unusual feature of dermal vessels from SSc patients in vivo. The vasculature in the SSc dermis is characterized by the absence of vascular endothelial (VE)-cadherin,42 a main constituent of adherens junctions that has a key role in regulating endothelial barrier function.46 Furthermore, dermal endothelial cells from SSc patients showed evidence of activated interferon (IFN)-α signaling,42 possibly reflecting the presence of endoplasmic reticulum stress and the unfolded protein response in these cells.47,48 Although IFN-α has been shown to have antiangiogenic and proapoptotic effects on endothelial cells, Fleming et al.42 failed to detect apoptotic endothelial cells in their study, contrary to a previous report by Sgonc et al.49
Studies performed in endothelial cells cultured from the dermis of SSc patients have revealed additional abnormal characteristics. A comparison of global gene expression in endothelial cells from SSc and control skin demonstrated the differential expression of 3.2% of total gene transcripts.45 Although the SSc endothelial cells exhibited a proangiogenic gene expression pattern, which is consistent with the studies discussed above, these cells showed reduced angiogenic properties in in vitro functional studies. This reduced capability was linked to the cleavage (by matrix metalloproteinase 12 [MMP12]) of urokinase-type plasminogen activator receptor (uPAR) and a resulting loss of uPar connection with β2 integrin, which is required for endothelial-cell-mediated angiogenesis.50 Furthermore, SSc fibroblasts were shown to inhibit angiogenesis in vitro through the MMP12–uPAR-dependent mechanism,51 further supporting the potential pathological role of MMP12 in SSc vasculopathy.
Pericytes
Pericytes are key mediators of vascular maturation and stabilization during angiogenesis.52 Dysregulation of pericyte function is associated with several pathological processes, including tumorigenesis and diabetic nephropathy.53 Abnormal characteristics of microvascular pericytes have also been reported in patients with early SSc and autoimmune Raynaud phenomenon.44,54 In SSc lesions, pericytes are characterized by the expression of PDGF receptor β (PDGFRβ) and high molecular weight melanoma-associated antigen (HMW-MAA), which are markers of pericyte activation. Another marker of angiogenic pericytes, regulator of G protein signaling 5 (RGS5), is also highly expressed in SSc vasculature.42 Although the specific functions of RGS5 are not well understood, current studies indicate that it is a negative regulator of vessel maturation.55 Studies in mice have shown that pericytes that lack RGS5 predominantly express markers associated with mature pericytes, such as α-smooth muscle actin (α-SMA) and the NG2 proteoglycan, whereas RGS5-positive pericytes show features of immature pericytes, characterized by the expression of PDGFRβ and low levels of α-SMA.56 Consistent with these observations, pericyte hyperplasia in the peripheral zones of active disease that correlated with increased capillary density has been observed in cases of diffuse and limited SSc.57 In addition, expression of α-SMA has been shown to be reduced in the vasculature of SSc patients, especially in the capillaries and venules, further supporting the concept of the presence of immature or activated pericytes in SSc lesions, which is consistent with angiogenesis.43 Together, these studies suggest that endothelial cells and pericytes both undergo changes during early stages of SSc.
Basement membrane
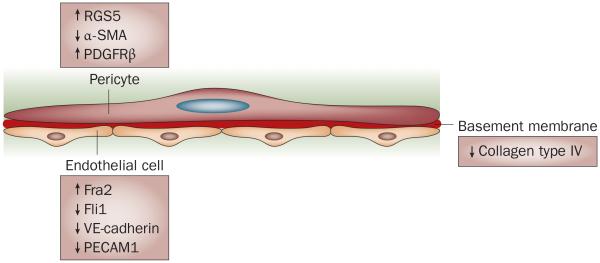
Vascular basement membrane is an important structural and functional component of blood vessels, and is composed of type IV collagen, laminin, heparan sulfate proteoglycans, nidogen, perlecan and other minor proteins. Vascular basement membrane components have an important role in angiogenesis, especially during initiation and resolution stages.58 As discussed above, structural changes in the basement membrane are observed early in SSc. Furthermore, analyses of the components of the vascular basement membrane in the skin of patients with SSc suggest possible alterations in the distribution of type IV collagen, but not fibronectin or laminin.59 In comparison to vessels from the skin of healthy individuals, the levels of type IV collagen are decreased in SSc vascular basement membrane.43,59 Presently, however, alterations in the basement membrane in the microvasculature of SSc patients remain poorly characterized (Figure 3).
Figure 3.
Molecular alterations of components of the SSc vasculature. Pericytes exhibit markers of immaturity or proliferation, including upregulation of RGS5 and PDGFRβ and downregulation of α-SMA. Endothelial cells are characterized by an increased level of the proapoptotic transcription factor Fra2, and decreased levels of the Fli1 transcription factor, VE-cadherin and PECAM1, which are indicative of impaired cell–cell contact, indicating a potential contribution to vessel leakiness. The levels of collagen type IV are reduced, suggesting alterations in the composition of the basement membrane. Abbreviations: α-SMA, α-smooth muscle actin; PDGFRβ, platelet-derived growth factor β receptor; PECAM1, platelet endothelial cell adhesion molecule 1; RGS5, regulator of G protein signaling 5; SSc, systemic sclerosis; VE-cadherin, vascular endothelial cadherin.
Dysregulation of transcription factors
Transcription factors have a key role in regulating cellular responses to extracellular signals and environmental stimuli as final effectors of signaling cascades. Dysregulated expression of several transcription factors has been observed in the SSc vasculature, and might offer new clues to the disease pathogenesis.
Fos related antigen 2
Fos related antigen 2 (Fra2) is a member of the multifunctional activator protein 1 (AP-1) family; this family includes the bZip group of transcription factors, which are characterized by a DNA-binding domain which contains clustered basic amino acids next to a leucine-zipper motif that is responsible for mediating dimerization of the transcription factors.60
Strong expression of Fra2 was detected in lung samples of patients with idiopathic pulmonary fibrosis or nonspecific interstitial pneumonia, as well as from the dermis of patients with SSc,61,62 suggesting that inappropriately elevated expression of Fra2 might contribute to disease pathogenesis. Transgenic mice overexpressing Fra2 underwent vascular remodeling and obliteration, and developed prominent pulmonary and dermal fibrosis.61,62 Interestingly, in both organs vascular changes preceded the development of fibrosis, suggesting a possible inter-relationship between these two pathological processes (see below). Furthermore, enhanced apoptosis of endothelial cells was demonstrated in the skin, but not in the lung vasculature, indicating organ-specific manifestations of Fra2 overexpression.
The factors leading to tissue fibrosis in mice that overexpress Fra2 are not well established, but might involve an increased presence of fibrogenic mediators such as osteopontin. Fra2 does not directly influence collagen synthesis, as collagen production was not increased in cultured fibroblasts ectopically expressing Fra2. Further studies of Fra2 should clarify the mechanism that underlies the vasculopathic and fibrogenic function of this transcription factor in vivo.
Friend leukemia virus integration 1
Friend leukemia virus integration 1 (Fli1) belongs to the Ets family of transcription factors, members of which are characterized by the presence of the evolutionarily conserved DNA-binding ETS domain.63 Fli1 has an essential role in the regulation of genes encoding components of the extracellular matrix, including type I collagen64-67 and the multifunctional matricellular factor connective tissue growth factor (CTGF, also known as CCN2).68 Importantly, Fli1 potently inhibits collagen biosynthesis in dermal fibroblasts and its aberrant expression might have a role in the pathogenesis of cutaneous fibrosis in SSc.64,68 Fli1 is expressed in the skin microvasculature of healthy individuals; however, its presence is greatly reduced in endothelial and periendothelial cells from SSc skin.67 Mice with a conditional knockout of Fli1 in endothelial cells show abnormal skin vasculature, with greatly compromised vessel integrity and markedly increased vessel permeability.43
Further studies have demonstrated that Fli1 directly regulates a number of genes that are important in the maintenance of vascular homeostasis. Specifically, Fli1 deficiency in endothelial cells resulted in a considerable decrease in the expression (at the messenger RNA and protein levels) of molecules involved in regulating endothelial cell–cell interactions, including VE-cadherin and platelet endothelial cell adhesion molecule 1 (PECAM1), and endothelial-cell–pericyte interactions, including PDGFB, sphingosine-1-phosphate receptor 1 and the Tie2 receptor. Furthermore, Fli1 deficiency led to a marked decrease in the protein levels of type IV collagen and an increase in MMP9 mRNA and protein expression, suggesting that Fli1 might also contribute to alterations in the composition of the basement membrane.43 Thus, reduced expression of Fli1 in endothelial cells is consistent with the initial invasive stage of angiogenesis, and the failure to re-express Fli1 during the subsequent stages of angiogenesis could prevent proper vessel maturation and stabilization. Notably, vascular changes resulting from Fli1 deficiency overlap to a great extent with the abnormalities observed in the vasculature of SSc patients (Table 1), suggesting that persistently reduced levels of Fli1 in endothelial cells in SSc patients could be important in disease pathogenesis.
Table 1.
Vascular changes in SSc*
| Protein | SSc patients | Fli1-knockout mice |
|---|---|---|
| Cell–cell junction | ||
| VE-cadherin | Decreased | Decreased |
| PECAM1 | Decreased | Decreased |
| BM–ECM | ||
| Type IV collagen | Decreased | Decreased |
| MMP9 | Increased | Increased |
| Pericyte marker | ||
| α-SMA | Decreased | Decreased |
| RGS5 | Increased | ND |
| S1P1 receptor | ND | Decreased |
The table compares vascular changes in the skin of SSc patients and mice with an endothelial-specific conditional knockout of Fli1. Abbreviations: α-SMA, α smooth muscle actin; MMP, matrix metalloproteinase; ND, not determined; PECAM1, platelet endothelial cell adhesion molecule 1; RGS5, regulator of G protein signaling 5; S1P1 receptor, sphingosine 1-phosphate receptor 1; SSc, systemic sclerosis; VE-cadherin, vascular endothelial cadherin.
Peroxisome proliferator-activated receptor γ
Peroxisome proliferator-activated receptor γ (PPAR-γ), a member of the nuclear hormone receptor superfamily, is a ligand-inducible transcription factor and an important regulator of glucose and lipid homeostasis.69 The expression of PPAR-γ is reportedly reduced in SSc fibroblasts.70 Treatment with the PPAR-γ agonist rosiglitazone alleviated profibrotic features of cultured fibroblasts from SSc patients and effectively prevented bleomycin-induced fibrosis in an animal model.70,71 PPAR-γ expression is also reduced in the pulmonary vasculature of patients with idiopathic PAH.72 The critical role of PPAR-γ in maintaining pulmonary vascular homeostasis was demonstrated in mouse models in which PPAR-γ was conditionally knocked out in smooth muscle cells (SMCs) or in endothelial cells.73,74 Spontaneous pulmonary hypertension developed in both models, but the symptoms were more pronounced in mice with targeted deletion of PPAR-γ in smooth muscle cells.
Although the function of PPAR-γ in the pulmonary vasculature is not yet fully characterized, it has a well-established role downstream of bone morphogenetic protein 2 (BMP2) in mediating antiproliferative and proapoptotic effects in arterial SMCs.74 It has also been shown that the antiproliferative effects of PPAR-γ are in part mediated through upregulation of apolipo-protein E (apoE) and adiponectin (APN), both of which were shown to inhibit PDGF-BB-induced proliferation of cultured SMCs.75 The pathogenic role of adiponectin deficiency in PAH is further supported by a study that showed development of spontaneous PAH in APN–/– mice.76
GATA-binding protein 6
The transcription factors GATA-1 to GATA-6 belong to a family of six related zinc-finger proteins. GATA-6 has an important role in lung development and morphogenesis and has been linked to lung fibrosis as a mediator of myofibroblast differentiation induced by transforming growth factor β (TGF-β).77 Previous studies have shown that GATA-6 has a critical role in the response of vascular SMCs to injury. Reduced levels of GATA-6 promoted the proliferation and dedifferentiation of SMCs, whereas re-expression of GATA-6 in balloon-injured carotid arteries prevented the formation of vessel lesions associated with vascular SMC phenotypic modulation.78,79 Markedly reduced levels of GATA-6 have been reported in occluded and nonoccluded pulmonary vessels in patients with idiopathic PAH and SSc-associated PAH.80 Further studies using cultured human endothelial cells demonstrated that the absence of GATA-6 leads to the abnormal expression of a number of genes involved in vascular homeostasis; endothelial nitric oxide synthase, VE-cadherin and the TGF-β pseudoreceptor BMP and activin membrane-bound inhibitor (BAMBI) are down-regulated, whereas the metalloproteinases MMP1 and MMP10 are upregulated.80 Thus, loss of endothelial GATA-6 might contribute to enhanced vasoconstriction and increased permeability of pulmonary vessels.
Linking vasculopathy and fibrosis
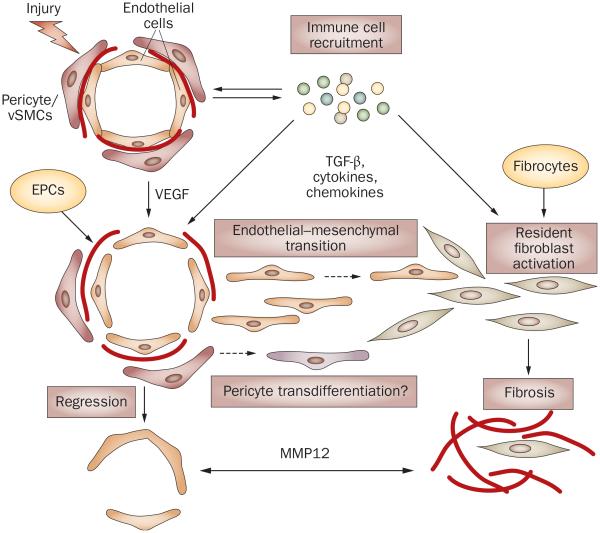
One of the key unresolved questions in SSc pathogenesis is the relationship between vasculopathy and fibrosis. Previous studies using animal models of organ fibrosis strongly suggested that activated fibroblasts in fibrotic tissues could originate from several sources, including resident fibroblasts, circulating fibroblast precursors (fibrocytes), epithelial cells, endothelial cells and pericytes. Whereas epithelial–mesenchymal transition has been extensively studied in various experimental models, endothelial–mesenchymal transition has only been recognized in the last 5 years as an important source of fibroblasts in cancer, as well as in cardiac and kidney fibrosis.81,82 Although endothelial–mesenchymal transition has yet to be demonstrated in an animal model of SSc, analysis of SSc lesional fibroblasts suggests that a subset of activated fibroblasts might have originated from endothelial cells. The characteristic features of endothelial cells, such as the presence of endoglin and constitutive activation of ALK1–Smad1 signaling, characterize these SSc fibroblasts.83,84 As the number of capillaries in SSc decreases despite the existence of actively proliferating endothelial cells and pericytes, it is tempting to speculate that these cells migrate into the surrounding tissues and acquire the ability to produce collagen and other extracellular matrix proteins.85,86 Consistent with this notion, collagen-producing cells are found around the vessels in the early inflammatory stage of SSc.87 Such activated fibroblasts might, in turn, negatively affect neoangiogenesis, for example, by secreting MMP12 (Figure 4).51
Figure 4.
Possible links between vasculopathy and fibrosis in SSc. Vascular injury due to an unknown trigger and the influx of the immune cells are the early events in SSc. High levels of VEGF and other proangiogenic mediators derived from the activated immune cells facilitate proliferation of endothelial cells and pericytes in an attempt to restore injured vessels. For reasons that are still not clearly understood, but which may involve imbalance of proangiogenic and antiangiogenic mediators, intrinsic properties of endothelial cells, and the fibroblast-secreted antiangiogenic factors (for example, MMP12) this process fails, leading to vessel regression. In the presence of TGF-β or other immune mediators (such as cytokines and chemokines), or both, endothelial cells could acquire a migratory phenotype through endothelial–mesenchymal transition and enter the surrounding tissue, where they further differentiate into collagen-producing cells. Likewise, pericytes could transdifferentiate into fibroblasts or myofibroblasts and produce collagen. In SSc, intrinsic abnormal properties of endothelial cells and pericytes might render vascular cells particularly susceptible to undergoing these transitions. Activated resident fibroblasts and fibrocytes that enter the injured tissue from the circulation are likely to represent additional source of collagen producing cells that contribute to fibrosis in SSc lesion. Abbreviations: EPCs, endothelial progenitor cells; VEGF, vascular endothelial growth factor; MMP12, matrix metalloproteinase 12; SSc, systemic sclerosis; TGF-β, transforming growth factor β.
Conclusions
Although fibrosis is the most prominent feature of SSc, knowledge of the underlying vascular disease might be pivotal not only to the understanding of how fibrosis develops, but also in providing new insights into treatment. The vascular repair process in SSc is abnormal and does not lead to vessel regeneration; instead, vascular cells might give rise to collagen-producing fibroblasts and contribute to the initiation of the fibrotic process. The main vascular defect has been proposed to reside within endothelial cells, which display many abnormal characteristics. Furthermore, abnormally low expression levels of Fli1 in the vasculature of SSc patients, possibly owing to epigenetic factors,88 could contribute to microvessel instability and degeneration. Despite progress in the delineation of factors contributing to SSc vasculopathy and new advances in the treatment of vascular disease in SSc, the mechanism underlying vascular disease and the relationship between vasculopathy and fibrosis remain to be elucidated. A great need exists for new animal models of SSc that would combine vascular and fibrotic aspects of the disease. An improved understanding of the relationship between these processes could help in designing more-effective therapies to treat this disease.
Key points.
Systemic sclerosis (SSc) is initiated by endothelial cell injury; this event leads to progressive structural defects in microvessels
There is evidence of enhanced angiogenic activity early in the disease
Multiple factors might contribute to defective vascular repair and regeneration
Intrinsic pathogenic alterations in endothelial cells might be a key factor that interferes with the vascular repair process
Aberrant expression of endothelial transcription factors, including Fra2, Fli1, peroxisome proliferator-activated receptor γ and GATA-6, has been associated with SSc, and might contribute to vascular defects
Endothelial cells and pericytes could represent a source of activated fibroblasts early in the disease
Review criteria.
A search of MEDLINE and PubMed for original articles focusing on SSc vascular disease published between 1925 and 2010 was performed. The search terms used were “systemic sclerosis”, “endothelial”, “vascular” and “angiogenesis”. All papers identified were English-language full text papers. The reference lists of identified articles were also searched for further papers.
Footnotes
Competing interests
The author declares no competing interests.
References
- 1.Sapadin AN, Esser AC, Fleischmajer R. Immunopathogenesis of scleroderma—evolving concepts. Mt Sinai J. Med. 2001;68:233–242. [PubMed] [Google Scholar]
- 2.Varga J, Abraham D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J. Clin. Invest. 2007;117:557–567. doi: 10.1172/JCI31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakst R, Merola JF, Franks AG, Jr, Sanchez M. Raynaud’s phenomenon: pathogenesis and management. J. Am. Acad. Dermatol. 2008;59:633–653. doi: 10.1016/j.jaad.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 4.LeRoy EC, Medsger TA., Jr. Raynaud’s phenomenon: a proposal for classification. Clin. Exp. Rheumatol. 1992;10:485–488. [PubMed] [Google Scholar]
- 5.Koenig M, et al. Autoantibodies and microvascular damage are independent predictive factors for the progression of Raynaud’s phenomenon to systemic sclerosis: a twenty-year prospective study of 586 patients, with validation of proposed criteria for early systemic sclerosis. Arthritis Rheum. 2008;58:3902–3912. doi: 10.1002/art.24038. [DOI] [PubMed] [Google Scholar]
- 6.Kahaleh B. Vascular disease in scleroderma: mechanisms of vascular injury. Rheum. Dis. Clin. North Am. 2008;34:57–71. doi: 10.1016/j.rdc.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Herrick AL. Pathogenesis of Raynaud’s phenomenon. Rheumatology (Oxford) 2005;44:587–596. doi: 10.1093/rheumatology/keh552. [DOI] [PubMed] [Google Scholar]
- 8.Wigley FM. Vascular disease in scleroderma. Clin. Rev. Allergy Immunol. 2009;36:150–175. doi: 10.1007/s12016-008-8106-x. [DOI] [PubMed] [Google Scholar]
- 9.Brown GE, O’Leary PA. Skin capillaries in scleroderma. Arch. Intern. Med. 1925;36:73–88. [Google Scholar]
- 10.Campbell PM, LeRoy EC. Pathogenesis of systemic sclerosis: a vascular hypothesis. Semin. Arthritis Rheum. 1975;4:351–368. doi: 10.1016/0049-0172(75)90017-7. [DOI] [PubMed] [Google Scholar]
- 11.Michalowski R, Kudejko J. Electron microscopic observations on skeletal muscle in diffuse scleroderma. Br. J. Dermatol. 1966;78:24–28. doi: 10.1111/j.1365-2133.1966.tb12129.x. [DOI] [PubMed] [Google Scholar]
- 12.Freemont AJ, Hoyland J, Fielding P, Hodson N, Jayson MI. Studies of the microvascular endothelium in uninvolved skin of patients with systemic sclerosis: direct evidence for a generalized microangiopathy. Br. J. Dermatol. 1992;126:561–568. doi: 10.1111/j.1365-2133.1992.tb00100.x. [DOI] [PubMed] [Google Scholar]
- 13.Prescott RJ, Freemont AJ, Jones CJ, Hoyland J, Fielding P. Sequential dermal microvascular and perivascular changes in the development of scleroderma. J. Pathol. 1992;166:255–263. doi: 10.1002/path.1711660307. [DOI] [PubMed] [Google Scholar]
- 14.Cool CD, Kennedy D, Voelkel NF, Tuder RM. Pathogenesis and evolution of plexiform lesions in pulmonary hypertension associated with scleroderma and human immunodeficiency virus infection. Hum. Pathol. 1997;28:434–442. doi: 10.1016/s0046-8177(97)90032-0. [DOI] [PubMed] [Google Scholar]
- 15.Dorfmuller P, et al. Fibrous remodeling of the pulmonary venous system in pulmonary arterial hypertension associated with connective tissue diseases. Hum. Pathol. 2007;38:893–902. doi: 10.1016/j.humpath.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 16.Nagai Y, et al. Autopsy case of systemic sclerosis with severe pulmonary hypertension. J. Dermatol. 2007;34:769–772. doi: 10.1111/j.1346-8138.2007.00381.x. [DOI] [PubMed] [Google Scholar]
- 17.Cannon PJ, et al. The relationship of hypertension and renal failure in scleroderma (progressive systemic sclerosis) to structural and functional abnormalities of the renal cortical circulation. Medicine (Baltimore) 1974;53:1–46. doi: 10.1097/00005792-197401000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 19.Papetti M, Herman IM. Mechanisms of normal and tumor-derived angiogenesis. Am. J. Physiol. Cell Physiol. 2002;282:C947–C970. doi: 10.1152/ajpcell.00389.2001. [DOI] [PubMed] [Google Scholar]
- 20.Fleischmajer R, Perlish JS. [3H]Thymidine labeling of dermal endothelial cells in scleroderma. J. Invest. Dermatol. 1977;69:379–382. doi: 10.1111/1523-1747.ep12510308. [DOI] [PubMed] [Google Scholar]
- 21.Kazandjian S, Bruneval P, Fiessinger JN, Camilleri JP, Housset E. Active proliferation of telangiectases in skin of patients with progressive systemic sclerosis (PSS) Arch. Dermatol. Res. 1986;279:8–11. doi: 10.1007/BF00404350. [DOI] [PubMed] [Google Scholar]
- 22.Kazandjian S, Fiessinger JN, Camilleri JP, Dadoune JP, Housset E. Endothelial cell renewal in skin of patients with progressive systemic sclerosis (PSS): an in vitro autoradiographic study. Acta Derm. Venereol. 1982;62:425–429. [PubMed] [Google Scholar]
- 23.Dong C, et al. Deficient Smad7 expression: a putative molecular defect in scleroderma. Proc. Natl Acad. Sci. USA. 2002:3908–3913. doi: 10.1073/pnas.062010399. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Ribatti D, et al. Systemic sclerosis stimulates angiogenesis in the chick embryo chorioallantoic membrane. Clin. Rheumatol. 1998;17:115–120. doi: 10.1007/BF01452256. [DOI] [PubMed] [Google Scholar]
- 25.Beon M, Harley RA, Wessels A, Silver RM, Ludwicka-Bradley A. Myofibroblast induction and microvascular alteration in scleroderma lung fibrosis. Clin. Exp. Rheumatol. 2004;22:733–742. [PubMed] [Google Scholar]
- 26.Distler O, et al. Angiogenic and angiostatic factors in systemic sclerosis: increased levels of vascular endothelial growth factor are a feature of the earliest disease stages and are associated with the absence of fingertip ulcers. Arthritis Res. 2002;4:R11. doi: 10.1186/ar596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hummers LK, Hall A, Wigley FM, Simons M. Abnormalities in the regulators of angiogenesis in patients with scleroderma. J. Rheumatol. 2009;36:576–582. doi: 10.3899/jrheum.080516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davies CA, Jeziorska M, Freemont AJ, Herrick AL. The differential expression of VEGF, VEGFr-2, and GLUT-1 proteins in disease subtypes of systemic sclerosis. Hum. Pathol. 2006;37:190–197. doi: 10.1016/j.humpath.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Distler O, et al. Uncontrolled expression of vascular endothelial growth factor and its receptors leads to insufficient skin angiogenesis in patients with systemic sclerosis. Circ. Res. 2004;95:109–116. doi: 10.1161/01.RES.0000134644.89917.96. [DOI] [PubMed] [Google Scholar]
- 30.Mackiewicz Z, et al. Increased but imbalanced expression of VEGF and its receptors has no positive effect on angiogenesis in systemic sclerosis skin. Clin. Exp. Rheumatol. 2002;20:641–646. [PubMed] [Google Scholar]
- 31.Dor Y, et al. Conditional switching of VEGF provides new insights into adult neovascularization and pro-angiogenic therapy. EMBO J. 2002;21:1939–1947. doi: 10.1093/emboj/21.8.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koch AE, Distler O. Vasculopathy and disordered angiogenesis in selected rheumatic diseases: rheumatoid arthritis and systemic sclerosis. Arthritis Res. Ther. 2007;9(Suppl. 2):S3. doi: 10.1186/ar2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mulligan-Kehoe MJ, et al. Antiangiogenic plasma activity in patients with systemic sclerosis. Arthritis Rheum. 2007;56:3448–3458. doi: 10.1002/art.22861. [DOI] [PubMed] [Google Scholar]
- 34.Urbich C, Dimmeler S. Endothelial progenitor cells functional characterization. Trends Cardiovasc. Med. 2004;14:318–322. doi: 10.1016/j.tcm.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Yoder MC. Defining human endothelial progenitor cells. J. Thromb. Haemost. 2009;7(Suppl. 1):49–52. doi: 10.1111/j.1538-7836.2009.03407.x. [DOI] [PubMed] [Google Scholar]
- 36.Purhonen S, et al. Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proc. Natl Acad. Sci. USA. 2008;105:6620–6625. doi: 10.1073/pnas.0710516105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kilarski WW, Samolov B, Petersson L, Kvanta A, Gerwins P. Biomechanical regulation of blood vessel growth during tissue vascularization. Nat. Med. 2009;15:657–664. doi: 10.1038/nm.1985. [DOI] [PubMed] [Google Scholar]
- 38.Westerweel PE, Verhaar MC. Endothelial progenitor cell dysfunction in rheumatic disease. Nat. Rev. Rheumatol. 2009;5:332–340. doi: 10.1038/nrrheum.2009.81. [DOI] [PubMed] [Google Scholar]
- 39.Zhu S, et al. Transcriptional regulation of Bim by FOXO3a and Akt mediates scleroderma serum-induced apoptosis in endothelial progenitor cells. Circulation. 2008;118:2156–2165. doi: 10.1161/CIRCULATIONAHA.108.787200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cipriani P, et al. Impairment of endothelial cell differentiation from bone marrow-derived mesenchymal stem cells: new insight into the pathogenesis of systemic sclerosis. Arthritis Rheum. 2007;56:1994–2004. doi: 10.1002/art.22698. [DOI] [PubMed] [Google Scholar]
- 41.Kuwana M, et al. Increase in circulating endothelial precursors by atorvastatin in patients with systemic sclerosis. Arthritis Rheum. 2006;54:1946–1951. doi: 10.1002/art.21899. [DOI] [PubMed] [Google Scholar]
- 42.Fleming JN, et al. Capillary regeneration in scleroderma: stem cell therapy reverses phenotype? PLoS ONE. 2008;3:e1452. doi: 10.1371/journal.pone.0001452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Asano Y, et al. Endothelial Fli1 deficiency impairs vascular homeostasis: a role in scleroderma vasculopathy. Am. J. Pathol. 2010;176:1983–1998. doi: 10.2353/ajpath.2010.090593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rajkumar VS, et al. Shared expression of phenotypic markers in systemic sclerosis indicates a convergence of pericytes and fibroblasts to a myofibroblast lineage in fibrosis. Arthritis Res. Ther. 2005;7:r1113–r1123. doi: 10.1186/ar1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giusti B, et al. A model of anti-angiogenesis: differential transcriptosome profiling of microvascular endothelial cells from diffuse systemic sclerosis patients. Arthritis Res. Ther. 2006;8:r115. doi: 10.1186/ar2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taddei A, et al. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-Nat. Cell Biol. 2008;10:923–934. doi: 10.1038/ncb1752. [DOI] [PubMed] [Google Scholar]
- 47.Gargalovic PS, et al. The unfolded protein response is an important regulator of inflammatory genes in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2006;26:2490–2496. doi: 10.1161/01.ATV.0000242903.41158.a1. [DOI] [PubMed] [Google Scholar]
- 48.Lenna S, et al. HLA-B35 upregulates endothelin-1 and downregulates endothelial nitric oxide synthase via endoplasmic reticulum stress response in endothelial cells. J. Immunol. 2010;184:4654–4661. doi: 10.4049/jimmunol.0903188. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Sgonc R, et al. Endothelial cell apoptosis is a primary pathogenetic event underlying skin lesions in avian and human scleroderma. J. Clin. Invest. 1996;98:785–792. doi: 10.1172/JCI118851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Margheri F, et al. Domain 1 of the urokinase-type plasminogen activator receptor is required for its morphologic and functional, β2 integrin-mediated connection with actin cytoskeleton in human microvascular endothelial cells: failure of association in systemic sclerosis endothelial cells. Arthritis Rheum. 2006;54:3926–3938. doi: 10.1002/art.22263. [DOI] [PubMed] [Google Scholar]
- 51.Serrati S, et al. Systemic sclerosis fibroblasts inhibit in vitro angiogenesis by MMP-12-dependent cleavage of the endothelial cell urokinase receptor. J. Pathol. 2006;210:240–248. doi: 10.1002/path.2048. [DOI] [PubMed] [Google Scholar]
- 52.Jain RK. Molecular regulation of vessel maturation. Nat. Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 53.Kutcher ME, Herman IM. The pericyte: cellular regulator of microvascular blood flow. Microvasc. Res. 2009;77:235–246. doi: 10.1016/j.mvr.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rajkumar VS, Sundberg C, Abraham DJ, Rubin K, Black CM. Activation of microvascular pericytes in autoimmune Raynaud’s phenomenon and systemic sclerosis. Arthritis Rheum. 1999;42:930–941. doi: 10.1002/1529-0131(199905)42:5<930::AID-ANR11>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 55.Manzur M, Ganss R. Regulator of G protein signaling 5: a new player in vascular remodeling. Trends Cardiovasc. Med. 2009;19:26–30. doi: 10.1016/j.tcm.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 56.Hamzah J, et al. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature. 2008;453:410–414. doi: 10.1038/nature06868. [DOI] [PubMed] [Google Scholar]
- 57.Helmbold P, Fiedler E, Fischer M, Marsch W. Hyperplasia of dermal microvascular pericytes in scleroderma. J. Cutan. Pathol. 2004;31:431–440. doi: 10.1111/j.0303-6987.2004.00203.x. [DOI] [PubMed] [Google Scholar]
- 58.Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat. Rev. Cancer. 2003:422–433. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 59.Hoyland JA, Newson L, Jayson MI, Freemont AJ. The vascular basement membrane in systemic sclerosis skin: heterogeneity of type IV collagen. Br. J. Dermatol. 1993;129:384–388. doi: 10.1111/j.1365-2133.1993.tb03162.x. [DOI] [PubMed] [Google Scholar]
- 60.Zenz R, et al. Activator protein 1 (Fos/Jun) functions in inflammatory bone and skin disease. Arthritis Res. Ther. 2008;10:201. doi: 10.1186/ar2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eferl R, et al. Development of pulmonary fibrosis through a pathway involving the transcription factor Fra-2/AP-1. Proc. Natl Acad. Sci. USA. 2008;105:10525–10530. doi: 10.1073/pnas.0801414105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maurer B, et al. Transcription factor fos-related antigen-2 induces progressive peripheral vasculopathy in mice closely resembling human systemic sclerosis. Circulation. 2009;120:2367–2376. doi: 10.1161/CIRCULATIONAHA.109.855114. [DOI] [PubMed] [Google Scholar]
- 63.Oikawa T, Yamada T. Molecular biology of the Ets family of transcription factors. Gene. 2003;303:11–34. doi: 10.1016/s0378-1119(02)01156-3. [DOI] [PubMed] [Google Scholar]
- 64.Asano Y, et al. Transcription factor Fli1 regulates collagen fibrillogenesis in mouse skin. Mol. Cell. Biol. 2009;29:425–434. doi: 10.1128/MCB.01278-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Czuwara-Ladykowska J, Shirasaki F, Jackers P, Watson DK, Trojanowska M. Fli-1 inhibits collagen type I production in dermal fibroblasts via an Sp1-dependent pathway. J. Biol. Chem. 2001;276:20839–20848. doi: 10.1074/jbc.M010133200. [DOI] [PubMed] [Google Scholar]
- 66.Jinnin M, et al. α2(I) collagen gene regulation by protein kinase C signaling in human dermal fibroblasts. Nucleic Acids Res. 2005;33:1337–1351. doi: 10.1093/nar/gki275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kubo M, et al. Persistent down-regulation of Fli1, a suppressor of collagen transcription, in fibrotic scleroderma skin. Am. J. Pathol. 2003;163:571–581. doi: 10.1016/S0002-9440(10)63685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakerakanti SS, Kapanadze B, Yamasaki M, Markiewicz M, Trojanowska M. Fli1 and Ets1 have distinct roles in connective tissue growth factor/CCN2 gene regulation and induction of the profibrotic gene program. J. Biol. Chem. 2006;281:25259–25269. doi: 10.1074/jbc.M600466200. [DOI] [PubMed] [Google Scholar]
- 69.Glass CK, Ogawa S. Combinatorial roles of nuclear receptors in inflammation and immunity. Nat. Rev. Immunol. 2006;6:44–55. doi: 10.1038/nri1748. [DOI] [PubMed] [Google Scholar]
- 70.Shi-wen X, et al. Rosiglitazone alleviates the persistent fibrotic phenotype of lesional skin scleroderma fibroblasts. Rheumatology (Oxford) 2010;49:259–263. doi: 10.1093/rheumatology/kep371. [DOI] [PubMed] [Google Scholar]
- 71.Wu M, et al. Rosiglitazone abrogates bleomycin-induced scleroderma and blocks profibrotic responses through peroxisome proliferator-activated receptor-gamma. Am. J. Pathol. 2009;174:519–533. doi: 10.2353/ajpath.2009.080574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ameshima S, et al. Peroxisome proliferator-activated receptor gamma (PPArγ) expression is decreased in pulmonary hypertension and affects endothelial cell growth. Circ. Res. 2003;92:1162–1169. doi: 10.1161/01.RES.0000073585.50092.14. [DOI] [PubMed] [Google Scholar]
- 73.Guignabert C, et al. Tie2-mediated loss of peroxisome proliferator-activated receptor-γ in mice causes PDGF receptor-β-dependent pulmonary arterial muscularization. Am. J. Physioi Lung Cell. Mol. Physiol. 2009;297:L1082–L1090. doi: 10.1152/ajplung.00199.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hansmann G, et al. An antiproliferative BMP-2/ PPAry/apoE axis in human and murine SMCs and its role in pulmonary hypertension. J. Clin. Invest. 2008;118:1846–1857. doi: 10.1172/JCI32503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hansmann G, et al. Pulmonary arterial hypertension is linked to insulin resistance and reversed by peroxisome proliferator-activated receptor-y activation. Circulation. 2007;115:1275–1284. doi: 10.1161/CIRCULATIONAHA.106.663120. [DOI] [PubMed] [Google Scholar]
- 76.Summer R, et al. Adiponectin deficiency: a model of pulmonary hypertension associated with pulmonary vascular disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009;297:L432–L438. doi: 10.1152/ajplung.90599.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lepparanta O, et al. Transcription factor GATA-6 is expressed in quiescent myofibroblasts in idiopathic pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2010;42:626–632. doi: 10.1165/rcmb.2009-0021OC. [DOI] [PubMed] [Google Scholar]
- 78.Mano T, Luo Z, Malendowicz SL, Evans T, Walsh K. Reversal of GATA-6 downregulation promotes smooth muscle differentiation and nhibits intimal hyperplasia in balloon-injured rat carotid artery. Circ. Res. 1999;84:647–654. doi: 10.1161/01.res.84.6.647. [DOI] [PubMed] [Google Scholar]
- 79.Perlman H, Suzuki E, Simonson M, Smith RC, Walsh K. GATA-6 induces p21(Cip1) expression and G1 cell cycle arrest. J. Biol. Chem. 1998;273:13713–13718. doi: 10.1074/jbc.273.22.13713. [DOI] [PubMed] [Google Scholar]
- 80.Ghatnekar AV, et al. The role of GATA-6 in pulmonary arterial hypertension in scleroderma patients. Arthritis Rheum. 2009;60:1266. [Google Scholar]
- 81.Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007;67:10123–10128. doi: 10.1158/0008-5472.CAN-07-3127. [DOI] [PubMed] [Google Scholar]
- 82.Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J. Am. Soc. Nephrol. 2008;19:2282–2287. doi: 10.1681/ASN.2008050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Leask A, et al. Dysregulation of transforming growth factor β signaling in scleroderma: overexpression of endoglin in cutaneous scleroderma fibroblasts. Arthritis Rheum. 2002;46:1857–1865. doi: 10.1002/art.10333. [DOI] [PubMed] [Google Scholar]
- 84.Pannu J, et al. Smad1 pathway is activated in systemic sclerosis fibroblasts and is targeted by matinib mesylate. Arthritis Rheum. 2008;58:2528–2537. doi: 10.1002/art.23698. [DOI] [PubMed] [Google Scholar]
- 85.Lin SL, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am. J. Pathol. 2008;173:1617–1627. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sundberg C, Ivarsson M, Gerdin B, Rubin K. Pericytes as collagen-producing cells in excessive dermal scarring. Lab. Invest. 1996;74:452–466. [PubMed] [Google Scholar]
- 87.Kulozik M, Hogg A, Lankat-Buttgereit B. Krieg, T Co-localization of transforming growth factor β2 with α1(I) procollagen mRNA in tissue sections of patients with systemic sclerosis. J. Clin. Invest. 1990;86:917–922. doi: 10.1172/JCI114793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Y, Fan PS, Kahaleh B. Association between enhanced type I collagen expression and epigenetic repression of the FLI1 gene in scleroderma fibroblasts. Arthritis Rheum. 2006;54:2271–2279. doi: 10.1002/art.21948. [DOI] [PubMed] [Google Scholar]