Abstract
Glioblastoma (GBM) is one of the most highly aggressive neoplasms of the central nervous system. Extra-cranial metastases in GBM are rare. Here we present the case of a 26-year-old man with extra-cranial metastasis of a frontal lobe GBM to the parotid gland, cervical lymph nodes, and bones, with initial diagnosis made by fine needle aspiration cytology (FNAC) of the parotid gland. FNAC is a reliable technique in the study of primary and secondary parotid gland neoplasms, allowing a presumptive diagnosis in difficult cases. We correlate the cytologic, histopathologic, and immunohistochemical findings in this case and discuss previous literature reports.
Keywords: Glioblastoma, Parotid gland, Metastasis, Cytology, Histopathology
Introduction
Glioblastoma (GBM) is one of the most highly aggressive neoplasms that originates from central nervous system (CNS) glial elements [1]. This tumor is also the most common glial neoplasm in the CNS, and its incidence increase in the elderly population [1, 2]. GBM is classified according to the World Health Organization (WHO) system as grade IV tumors [3]. GBM commonly spreads by direct extension by infiltration into adjacent brain tissue or along white matter tracts and also by subarachnoid dissemination [1, 4]; intracranial metastases to the meninges or spinal cord are frequently reported [5].
Extra-cranial or extra-neural metastases outside the CNS are exceptionally rare events reported to occur in 0.2–2 % of all GBM [6]. The rarity of this phenomenon has been attributed to various aspects of pathophysiology that prevent GBM cells from infiltrating and surviving beyond the intra-cranial environment [6, 7]. The most common sites of GBM metastasis are to the lung, lymph node, bone, and liver, with rare reports to skin (6 reports found) and parotid gland (7 reports found) [8–10, 12].
Herein we present a case of a young man with extra-cranial metastasis of a frontal lobe GBM to the parotid gland, cervical lymph nodes and bones, in which the initial diagnosis of extra-cranial spread was made with fine needle aspiration cytology of the parotid gland. We correlate the cytologic, histopathologic and immunohistochemical results in this case and discuss these findings in the context of previous literature reports.
Case Report
Our patient is a 26 year old male with previous diagnosis of intra-axial glioblastoma localized to the frontal lobe 6 months prior. His symptoms on presentation were severe headaches and dysaesthesias. He was treated with surgical resection, radiation therapy, and temozolomide (140 mg/day for 42 days). He had no known significant past medical history and had never smoked.
After completion of treatment, he again presented to his physician with progressive growth of a nodule in his left cheek and generalized bone pain. A parotid gland fine needle aspiration cytology (FNAC) was performed of the nodule, which revealed several groups of highly pleomorphic cells with clumped chromatin, prominent nucleoli, and fibrillar cytoplasm. These cells were positive for glial fibrillary acid protein (GFAP) (Fig. 1). A cytologic diagnosis of metastatic high grade glioma was established. Full body gammography and computed tomography (CT) scans were performed which demonstrated disseminated metastatic involvement of the parotid gland, cervical lymph nodes (Levels IIB, III and IV), and bones (Fig. 2).
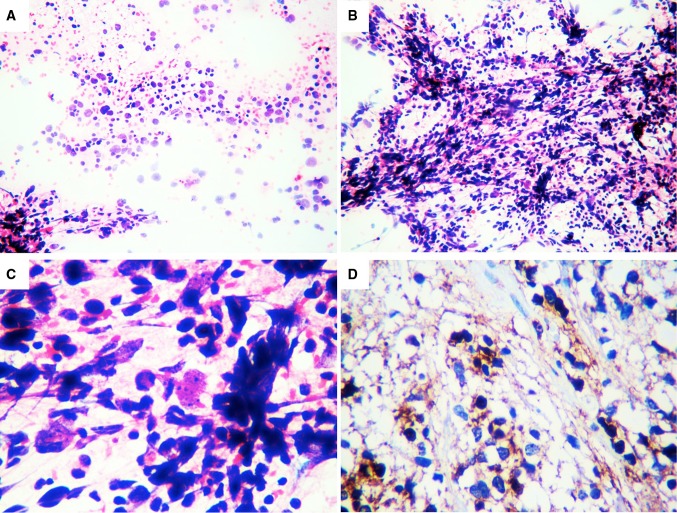
Fig. 1.
In the FNAC samples we observed a hemorrhagic background with neoplastic round and fibrillar cells with cytoplasmic processes, nuclear atypia, hyperchromatic nuclei with coarse chromatin, and some mitotic figures (a, HE 5×). These cells form clusters and tufts resembling glomeruli (b, HE 5×), Also observed were anaplastic figures, large pleomorphic cells with irregular nuclear outlines and prominent nucleoli (c, HE 40×), and immunoreactivity for GFAP (d, 40×). GFAP glial fibrillary acid protein
Fig. 2.
CT scans showed multiple heterogeneous cervical lymph nodes with uptake of contrast medium in levels II and IV (a, b). Also observed was a left frontal defect in relation to previous surgery with an intra-axial heterogeneous mass in the frontal lobe associated with vasogenic edema that crossed the midline and have invasion of extra-cranial soft tissues (c). Spinal MRIs showed in T1, T1 with medium and T2, blastic metastatic infiltration hypo-intense on T1, with vertebral bone compromise (d). Bone gammography showed generalized capitation (superscan images) (e, f)
One of the involved cervical lymph nodes was additionally biopsied to exclude a second primary neoplasm. Histopathologic evaluation of the lymph node showed effacement of normal lymph node architecture by giant pleomorphic and small malignant cells dispersed in groups or forming rosette-like structures. These cells were positive for GFAP and showed a Ki67 proliferation index of 15 % (Fig. 3). Negativity for PGP9.5, chromogranin, synaptophysin, cytokeratin, CK7, CK20, and neurofilament immunohistochemical stains was documented. The previous intracranial resection specimen was reviewed for comparison, which showed a neoplasm composed of small to medium sized cells with hyperchromatic nuclei and vacuolated, clear cytoplasm, forming discrete nodules and sheets in a myxoid stroma; rosette-like formations, endothelial proliferation, and extensive areas of coagulative necrosis were also observed. The neoplastic cells in the original specimen demonstrated strong immunoreactivity for GFAP (Figure 4).
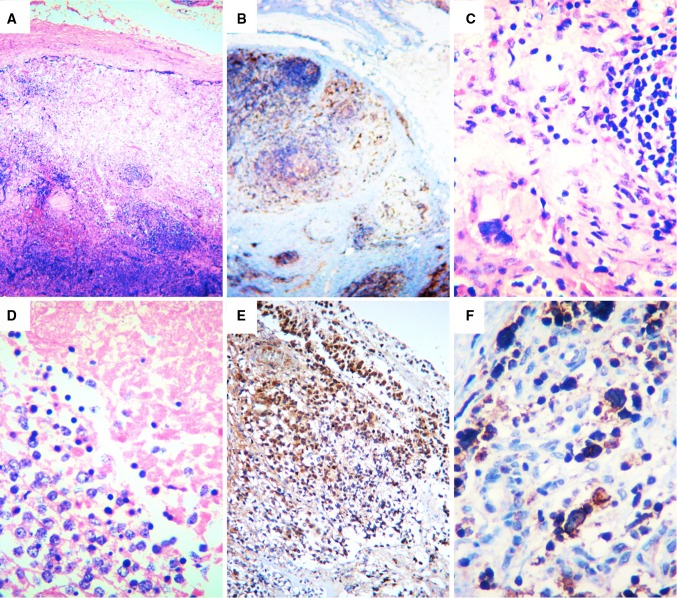
Fig. 3.
In the histopathologic study of the peripheral lymph node there was complete compromise by a neoplasm composed of hyperchromatic cells distributed in aggregates (a, HE4×). These cells showed positivity to GFAP (b, 4×), were highly polymorphic, showed bizarre formations (c, HE40×), necrosis (d, HE40×), and were present in a subcapsular lymphatic pattern (e, f, GFAP). HE hematoxylin and eosin, GFAP glial fibrilary acid protein
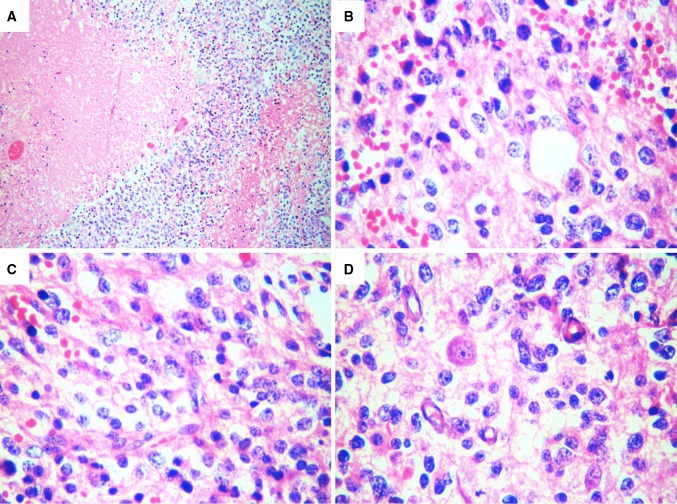
Fig. 4.
The histomorphology observed in the primary tumor was similar to that observed in the lymph node, with a highly aggressive necrotic neoplasm (a, HE-4×), composed of round and small atypical cells, with coarse chromatin, prominent nucleoli, and fibrillar processes (b, c, HE-40×). Microvascular endothelial proliferation and anaplastic cellular figures were also observed (d, HE-40×). HE hematoxylin and eosin
The patient was continued on temozolomide 300 mg/day and again underwent radiotherapy. A total survival from diagnosis of 2 years was documented.
Discussion
Davis first reported, in 1928, on a patient with GBM that had disseminated to the lung, soft tissue of an arm, and the chest wall [13]. Since this initial report of extra-cranial metastasis, the underlying mechanism of this phenomenon has yet to be completely elucidated and only few cases have been reported. However, the incidence of extra-cranial metastasis as reported in an earlier autopsy series was much higher which suggests that the true incidence of these distant metastases may be significantly higher than what has been clinically detected [2, 7, 8].
The protective factors against this phenomenon include: (1) the absence of lymphatic vessels within the brain and spinal cord, which limits lymphatic dissemination; (2) action of the blood-brain barrier; (3) the extremely shortened survival of patients with GBM; (4) the dense connective tissue barrier of dura-mater that is rarely invaded by GBM; (5) the virtual absence of collagen and fibronectin within the CNS parenchyma that would facilitate dissemination; (6) the lack of a permissive stroma in other organs that would allow survival and proliferation of GBM cells; (7) the poor affinity of glioma cells seem to have for arterial blood vessels; and (8) the lack of direct connection between the subarachnoid space witch is a close cavity and the hematogenous or lymphatic system [2, 8, 9]. However, it has been postulated by Willis, that the thin-walled and poorly supported by dura mater CNS venules may permit metastatic spread; different to the observed in CNS venous sinuses, which are much larger and buttressed by dura mater. [14].
In general, metastasis occurs via lymphatic or hematogenous spread, or through cavities that allow fluid exchange, or through direct invasion that allow further dissemination. Direct invasion via iatrogenic channels (e.g. the result of previous surgical interventions to treat the primary tumor, biopsy procedures, or ventriculo-peritoneal shunting), where defects in meningeal and parenchymal blood vessels are created has been postulated to be a major mechanism to facilitate the extra-cranial dissemination of GBM [1, 2, 10]. Other mechanisms are less common, since metastases do rarely occur in the absence of surgical manipulation. This supports the hypothesis that GBM cells need contact with lymphatic or blood vessels to metastasize outside CNS [9]. However, in a 1970 review by Anzil, more than 10 % of all cases of extra-cranial spread were found to occur in the absence of prior surgical intervention. The conclusion reached was that surgery, radiation, or long survival durations are not necessarily prerequisites for extra-cranial dissemination of GBM, suggesting instead, in these instances, early hematogenous spread may be the underlying mechanism [11].
New models of GBM tumorigenesis suggest that the classic pseudopalisading necrosis of GBM represents an actively proliferating zone wherein the tumor cells are migrating away from a central vascular insult and, in response to the hypoxia, express pro-angiogenic factors (e.g. VEGF and IL-8). The resultant microvascular proliferation in turn represents an alteration in the normal blood-brain barrier. This alteration may in fact provide GBM cells with direct access to the systemic circulation. Low levels of circulating tumor cells, with extra-cranial metastatic potential, would probably be suppressed by the peripheral immune system [15]. However, this peripheral suppression would be hindered in the milieu of a severely immunocompromised patient by adjuvant therapy and cancer which would theoretically allow for systemic metastases to develop, given enough time and a permissive microenvironment in the extra-cranial site [15, 16]. Interestingly, the development of GBM in organs transplanted from a previously treated (without history of ventriculosystemic shunt) donor with GBM have been documented [17]. This phenomenon lends credence to the theory that low levels of circulating GBM cells may be present without overt metastasis [17]. Furthermore, cytologic samples of cerebral blood taken during and after neurosurgery of gliomas demonstrated that tumor cells are present in the systemic circulation and probably produce microscopic implants in rich vascular organs such as the lungs and liver [2, 18].
Based on available case reports, the GBM cells demonstrate a predilection for the lung and pleura (60 %), lymph nodes (51 %), bone marrow (30 %), and liver (22 %) [1, 2]. Other targets for metastases less commonly reported include the kidney, spleen, adrenal glands, heart, small bowel, and parotid glands [4, 9, 10]. Regarding bone metastases, the vertebral spine (73 %) was the most common site of involvement, followed by the ribs (23 %), sternum (18 %), skull (14 %), and acetabulum (9 %) [1, 2, 6]. The timeframe of metastasis reported is also variable in relation with the organ involved. Patients with metastasis to the liver had the longest time interval between diagnosis of the intracranial GBM and detection of metastasis; and patients with metastasis to the lung had the shortest interval from diagnosis to detection of metastasis and also from detection of metastasis to death [19]. The reason for this heterogeneity is unclear, but may be related to the permissibility of the target organ to grow CNS-derived elements and capabilities for immune-surveillance [15, 19]. Battista et al. [20] have demonstrated that malignant glial cells removed from the brain, which are then experimentally implanted into other body sites, do survive, evading the immune system.
Metastastic GBM has been reported commonly in medium adult patients (median age around 40 years), youngers than in the typical elderly population of GBM. Secondary GBM, e.g. those which have progressed from a lower grade glioma, also are more common in this younger population, and it is thought that the higher risk of metastasis may correspond to the longer duration of tumor existence in these patients [1, 9]. Nevertheless, age has not been reported to be prognostic factor in patients with extra-cranial metastases [4].
The WHO classification of tumors distinguishes between primary and secondary salivary gland tumors as well as tumor-like lesions. Benign tumors mainly consist of salivary gland adenomas and Warthin tumors, while malignant tumors include salivary gland carcinomas, lymphomas and intra-glandular metastases [21, 22]. The most commonly found metastasis has a lymphatic origin from primary squamous cell carcinoma of the skin and malignant melanoma in the head and neck region. In cases of GBM when the primary lesion is invading within the cervical lymph node drainage, ipsilateral parotid lymph nodes can be compromised by tumor. Hematogenous metastases are more rare and tend to be derived from thyroid, lung, kidney, breast and colorectal cancers. Rarely, compromise by direct extension of malignant skin tumors has been reported [22].
Due to low cost and high diagnostic accuracy, the combination of ultrasound and FNAC represents a clinically valuable and reliable method of choice for diagnosis in most salivary gland tumors [23, 24]. FNAC allows for differentiation between malignant and benign neoplasms and often also for rendering an approximate diagnosis [23]. The features supporting diagnosis of involvement of a salivary gland by a glial neoplasm via FNAC are poorly defined because this presentation has been rarely reported. In our cytologic samples we found malignant fibrillary cells dispersed in hypercellular aggregates, with formation of rosette-like structures and positivity for GFAP [25–27]. These findings, along with the history of previous diagnosis of GBM, allowed for the formulation of an indicative diagnosis.
Histopathologic confirmation is mandatory in indeterminate cases where suspicious of metastatic compromise by a GBM persist. In general, the histomorphology is similar to that observed in primary GBM, e.g., a malignant neoplasm composed of fibrillar astrocytes, some of which are anaplastic (multinucleated tumor cells, bizarre nuclei, karyorrhectic cells) with hypercellular aggregates, variable number of mitotic figures (some atypical), with coagulative necrosis and/or microvascular proliferation. Classically, there is pseudopalisading necrosis. The neoplastic cells are positive for GFAP and AE1-AE3, and also demonstrate high Ki67 proliferation index [8, 28], which has been reported to be higher in extra-cranial GBM metastasis [12, 19, 28]. The morphology of the primary and metastatic GBM tissue was similar in our patient. Also, we observed a lymphatic pattern of metastasis, with compromise of multiple lymph nodes and lymphatic vessels, in accordance with the reported finding of regional lymph node involvement that can be attributed to lymphatic spread [1, 19].
Treatment of GBM includes surgical excision of the primary tumor, followed by radiation therapy. Solitary and localized metastases may be treated with surgical excision with improvement of symptoms. Chemotherapy is frequently given in combination with radiation therapy as an adjuvant treatment either before or after surgery [1, 8]. In cases of parotid gland compromise, total parotidectomy with or without resection of the facial nerve should be reserved for those few cases with extensive infiltration of the overlying skin and severe functional compromise [19]. The parotid gland resection in other cases may be only useful to confirm diagnosis, and an excisional biopsy is sufficient, [2, 19] such as in our case.
In patients with disseminated metastatic disease, palliative radiation therapy is the best available option. Survival remains dismal after the onset of metastatic disease. The increase in reported cases of extra-cranial metastases of GBM is generally attributed to the improved survival from more radical surgery, better imaging studies, a higher index of suspicion, and improvement in adjuvant treatment. However a systematic review shows that this improved survival is low despite major advancements attained in surgery, radiation, and chemotherapies [2]. For example, with the therapeutic regimen used in our patient (temozolomide plus radiotherapy), there is nearly 10 % survival 5 years after initial diagnosis, as compared to 1.9 % of those treated with radiation alone and more than 70 % of cases die within 2 years of diagnosis [2, 8, 10].
In conclusion, FNAC is a reliable technique in the study of primary and secondary parotid gland neoplasm, allowing a provisional diagnosis. However, definitive diagnosis may require an open biopsy. Given the extent of mystery in which metastatic mechanisms of GBM and other neoplasms are still shrouded in, we encourage the practice of autopsies in oncologic patients and studies which may allow for the determination of more accurate data in pathogenesis and behavior in neoplastic diseases.
Conflict of interest
There are no conflicts of interest to disclose.
References
- 1.Zhen L, Yufeng C, Zhenyu S, Lei X. Multiple extracranial metastases from secondary glioblastoma multiforme: a case report and review of the literature. J Neurooncol. 2010;97(3):451–457. doi: 10.1007/s11060-009-0044-9. [DOI] [PubMed] [Google Scholar]
- 2.Amitendu S, Mak SK, Ling JM, Ng WH. A single institution experience of the incidence of extracranial metastasis in glioma. J Clin Neurosci. 2012;19(11):1511–1515. doi: 10.1016/j.jocn.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 3.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. WHO classification of tumours of the central nervous system. Lyon: IARC; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saad AG, Sachs J, Turner CD, Proctor M, Marcus KJ, Wang L, Lidov H, Ullrich NJ. Extracranial metastases of glioblastoma in a child: case report and review of the literature. J Pediatr Hematol Oncol. 2007;29(3):190–194. doi: 10.1097/MPH.0b013e31803350a7. [DOI] [PubMed] [Google Scholar]
- 5.Shah A, Redhu R, Nadkarni T, Goel A. Supratentorial glioblastoma multiforme with spinal metastases. J Craniovertebr Junction Spine. 2010;1(2):126–129. doi: 10.4103/0974-8237.77678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mujic A, Hunn A, Taylor AB, Lowenthal RM. Extracranial metastases of a glioblastoma multiforme to the pleura, small bowel and pancreas. J Clin Neurosci. 2006;13(6):677–681. doi: 10.1016/j.jocn.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 7.Smith DR, Hardman JM, Earle KM. Metastasizing neuroectodermal tumors of the central nervous system. J Neurosurg. 1969;31(1):50–58. doi: 10.3171/jns.1969.31.1.0050. [DOI] [PubMed] [Google Scholar]
- 8.Frank S, Kuhn SA, Brodhun M, Mueller U, Romeike B, Kosmehl H, Regenbrecht CR, Ewald C, Reichart R, Kalff R. Metastatic glioblastoma cells use common pathways via blood and lymphatic vessels. Neurol Neurochir Pol. 2009;43(2):183–190. [PubMed] [Google Scholar]
- 9.Mentrikoski M, Johnson MD, Korones DN, Scott GA. Glioblastoma multiforme in skin: a report of 2 cases and review of the literature. Am J Dermatopathol. 2008;30(4):381–384. doi: 10.1097/DAD.0b013e31817532c4. [DOI] [PubMed] [Google Scholar]
- 10.Kraft M, Lang F, Braunschweig R, Janzer RC. Parotid gland metastasis from glioblastoma multiforme: a case report and review of the literature. Eur Arch Otorhinolaryngol. 2008;265(6):709–711. doi: 10.1007/s00405-007-0499-2. [DOI] [PubMed] [Google Scholar]
- 11.Anzil AP. Glioblastoma multiforme with extracranial metastases in the absence of previous craniotomy. Case report. J Neurosurg. 1970;33(1):88–94. doi: 10.3171/jns.1970.33.1.0088. [DOI] [PubMed] [Google Scholar]
- 12.Taha M, Ahmad A, Wharton S, Jellinek D. Extra-cranial metastasis of glioblastoma multiforme presenting as acute parotitis. Br J Neurosurg. 2005;19(4):348–351. doi: 10.1080/02688690500305506. [DOI] [PubMed] [Google Scholar]
- 13.Davis L. Spongioblastoma multiforme of the brain. Ann Surg. 1928;87(1):8–14. [PMC free article] [PubMed] [Google Scholar]
- 14.Willis RA. The spread of tumors in the human body. London: Butterworth; 1952. p. 101. [Google Scholar]
- 15.Rong Y, Durden DL, Van Meir EG, Brat DJ. “Pseudopalisading” necrosis in glioblastoma: a familiar morphologic feature that links vascular pathology, hypoxia, and angiogenesis. J Neuropathol Exp Neurol. 2006;65:529–539. doi: 10.1097/00005072-200606000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21(2):137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 17.Val-Bernal F, Ruiz JC, Cotorruelo JG, Arias M. Glioblastoma multiforme of donor origin after renal transplantation: report of a case. Hum Pathol. 1993;24(11):1256–1259. doi: 10.1016/0046-8177(93)90224-5. [DOI] [PubMed] [Google Scholar]
- 18.Morley TP. The recovery of tumour cells from venous blood draining cerebral gliomas; a preliminary report. Can J Surg. 1959;2(4):363–365. [PubMed] [Google Scholar]
- 19.Lun M, Lok E, Gautam S, Wu E, Wong ET. The natural history of extracranial metastasis from glioblastoma multiforme. J Neurooncol. 2011;105(2):261–273. doi: 10.1007/s11060-011-0575-8. [DOI] [PubMed] [Google Scholar]
- 20.Battista AF, Bloom W, Loffman M, Feigin I. Autotransplantation of anaplastic astrocytoma in subcutaneous tissue of man. Neurology. 1961;11:977–981. doi: 10.1212/WNL.11.11.977. [DOI] [PubMed] [Google Scholar]
- 21.Johns ME. The salivary glands: anatomy and embryology. Otolaryngol Clin North Am. 1977;10(2):261–271. [PubMed] [Google Scholar]
- 22.Eveson JW, Auclair PL, Gnepp DR, et al. Tumors of the salivary glands: introduction. In: Barnes EL, Eveson JW, Reichart P, Sidransky D, et al., editors. World Health Organization classification of tumours: pathology and genetics. Head and neck tumours. Lyon: IARC Press; 2005. pp. 221–222. [Google Scholar]
- 23.Schmidt RL, Hunt JP, Hall BJ, Wilson AR, Layfield LJ. A systematic review and meta-analysis of the diagnostic accuracy of frozen section for parotid gland lesions. Am J Clin Pathol. 2011;136(5):729–738. doi: 10.1309/AJCP2SD8RFQEUZJW. [DOI] [PubMed] [Google Scholar]
- 24.Herrera-Hernandez AA, Diaz-Perez JA, Garcia CA, Herrera LP, Aranda-Valderrama P, Orozco Vargas LC. Evaluation of fine needle aspiration cytology in the diagnosis of cancer of the parotid gland. Acta Otorrinolaringol Esp. 2008;59(5):212–216. doi: 10.1016/S0001-6519(08)73297-7. [DOI] [PubMed] [Google Scholar]
- 25.Schultz S, Pinsky GS, Wu NC, Chamberlain MC, Rodrigo AS, Martin SE. Fine needle aspiration diagnosis of extracranial glioblastoma multiforme: case report and review of the literature. Cytojournal. 2005;2:19. doi: 10.1186/1742-6413-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.González Cámpora R, Otal Salaverri C, Vázquez Ramirez F, Salguero Villadiego M, Galera Davidson H. Metastatic glioblastoma multiforme in cervical lymph nodes. Report of a case with diagnosis by fine needle aspiration. Acta Cytol. 1993;37(6):938–942. [PubMed] [Google Scholar]
- 27.Vural G, Hagmar B, Walaas L. Extracranial metastasis of glioblastoma multiforme diagnosed by fine-needle aspiration: a report of two cases and a review of the literature. Diagn Cytopathol. 1996;15(1):60–65. doi: 10.1002/(SICI)1097-0339(199607)15:1<60::AID-DC12>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 28.Ueda S, Mineta T, Suzuyama K, Furuta M, Shiraishi T, Tabuchi K. Biologic characterization of a secondary glioblastoma with extracranial progression and systemic metastasis. Neuro Oncol. 2003;5(1):14–18. doi: 10.1093/neuonc/5.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]