Abstract
Papillary squamous cell carcinoma (PSCCA) is a rarely occurring variant of squamous cell carcinoma (SCCA) with distinctive exophytic and papillary features and a more favorable prognosis than conventional SCCA. The larynx is the most commonly affected site in the head and neck. The oral cavity, oropharynx, sinonasal tract, and nasopharynx are also affected. Within the oral cavity cases have been reported on the alveolar ridge, oral mucosa, floor of the mouth, ventral tongue, and rarely other areas. We identified 61 cases of gingival PSCCA within the parameters of a larger study of 519 cases of gingival SCCA. We evaluated the clinical and histologic features of these lesions. The average age of the PSCCA patient was 74 years, with a very slight male predominance of 1.2:1. The mandible was affected nearly twice as often (64 %, n = 39) as the maxilla (35 %, n = 21, and 1 % of cases unspecified), and the most common location by far was the mandibular posterior region (52 %, n = 32). Most lesions were reportedly present over 2 months in duration (48 %, n = 29) prior to biopsy but a significant amount of clinicians were unsure of the duration of the lesion as well (36 %, n = 22). Only 10 cases (16 %) were reportedly present less than 2 months. The most common clinical presentation was that of an erythematous or mixed white and red exophytic mass (74 %, n = 45). 62 % (n = 38) of submitting clinicians considered a malignant or premalignant lesion in their differential diagnosis, but other clinical impressions included papillomas, reactive gingival lesions, and fungal infections. Histologically, 88 % (n = 52 of 59 cases graded) of the lesions were either well or moderately-well differentiated. PSCCA is a rare subtype of SCCA which has been reported infrequently involving the gingiva or alveolar ridges but should be considered by clinicians for the differential diagnosis of papillary gingival masses.
Keywords: Papillary squamous cell carcinoma, Oral cavity, Gingiva, Alveolar ridge, Head and neck
Introduction
Papillary squamous cell carcinoma (PSCCA) is a rare variant of squamous cell carcinoma (SCCA) with an exophytic and papillary pattern and a more favorable prognosis than conventional SCCA [1, 2]. The larynx is the most commonly affected site in the head and neck [3, 4]. Other sites of involvement include the oral cavity, oropharynx, sinonasal tract, and nasopharynx [3, 4]. In the oral cavity cases have been reported on the alveolar ridge, oral mucosa, floor of the mouth, ventral tongue, and rarely other areas [5–8]. Within head and neck PSCCA, a relationship has been established with the human papillomavirus (HPV) to varying degrees [4, 9]. Oral cavity PSCCA, including gingival PSCCA, has been reported infrequently in the literature, with clinical characteristics of these cases rarely discussed in detail.
Materials and Methods
Sixty-one PSCCA cases were identified within a larger study of 519 cases of gingival SCCA collected through an archival search at the Oral and Maxillofacial Pathology Biopsy Service at University of Florida College of Dentistry with institutional review board approval. This overall study was reported in a previous publication [10]. The original study retrospectively examined cases of verrucous carcinoma (VC) and SCCA occurring only on the gingiva or alveolar ridge identified by a search of the archives from the time period of 1994–2011. Cases were excluded if the gingival involvement was secondary to primary involvement at an adjacent site, if any question of metastatic disease was considered, and if surface involvement was not demonstrated. Only the first biopsy was included in cases of multiple biopsies to the same site within a 1 year period. Two of the study authors (I.B. and D.C.) were responsible for the histological diagnosis of all lesions included during this time period or reviewed the histological diagnosis of lesions diagnosed prior to their arrival at the University. Original biopsy submission forms and biopsy reports along with submitted images were reviewed and a database was constructed evaluating clinical and histologic characteristics including age, gender, exact location on the gingiva, duration of the lesion prior to biopsy, color, clinical appearance, the submitting clinician’s clinical impression or differential diagnosis, any pre-biopsy treatment performed, history of concurrent oral disease, and radiographic features if available. The type of SCCA and histologic grade were also recorded, along with any evidence suggestive of bony invasion. No prognostic or treatment data was included in this study as the majority of the submitted cases were from private practice contributors outside the UF system and this information was not available. Out of the overall study, cases of PSCCA were then identified and compiled into a second database. Cases were diagnosed as PSCCA if they were consistent with invasive SCCA and included a majority of the tumor exhibited exophytic, papillary growth. Histopathologic features of PSCCA according to the WHO Classification of Head and Neck Tumors are presented in Table 1 [11]. In our previously published study of 519 cases of gingival carcinoma, cases were diagnosed as PSCCA if they met the criteria listed above, otherwise they were characterized as VC (39 cases of 519), or simply SCCA, usually a very well differentiated form [10]. In some cases of very well differentiated lesions with verrucous or papillary features, cases were signed out as “atypical epithelial proliferations consistent with early verrucous carcinoma or very well differentiated squamous cell carcinoma” and these cases were not included in the PSCCA subset. Results were tabulated from the 61 cases of PSCCA for the clinical and histologic characteristics and summarized.
Table 1.
Histopathologic features of PSCCA as adapted from WHO classification [11]
| Histopathologic features of PSCCA |
|---|
| Predominantly papillary growth pattern centering around fibrovascular cores |
| Keratinization may be minimal in many cases |
| Necrosis may be a feature |
| Stromal invasion should be present as individual cells or tumor islands |
Results
The average age of the PSCCA patients was 74 years (age range 27–99 years). 33 patients (54 %) were male and 28 (46 %) were female. 39 cases (64 %) occurred on the mandible and 21 (35 %) on the maxilla with one case only designating “gingiva” (1 %). In the mandible, the posterior region (molar and premolar) comprised 32 cases together and the anterior region only involved 4 cases, with 3 cases only reporting “mandibular gingiva”. In the maxilla, however, the posterior cases (11 cases total) were more equal to the anterior cases (8 cases) with 2 cases listing “maxillary gingiva” only. In many cases the clinician and patient were unaware of the length of time the lesion was present (36 % or 22 cases), but 10 cases (16 %) were present less than 2 months with the remainder (29 cases or 48 %) present over two months. Of those cases present over 2 months, the most common time frame was 2–6 months.
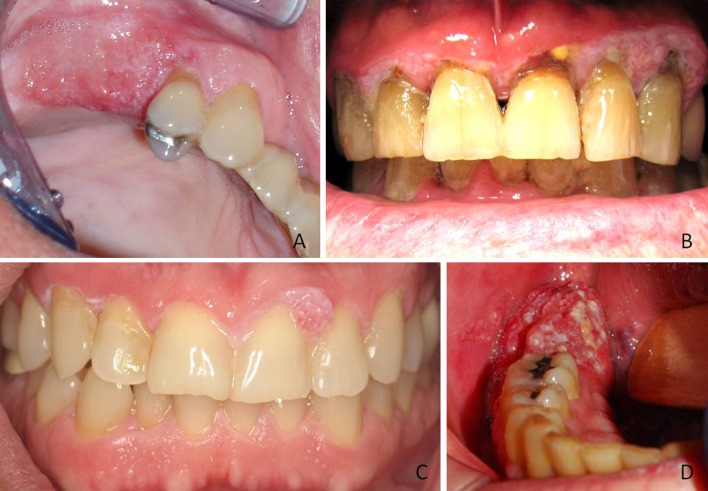
Thirty-one cases (51 %) presented as red or pink lesions, 14 (23 %) as mixed red and white, and 7 (11 %) as white, with 9 cases (15 %) either other responses or had no response given. The most common category of clinical appearance described an exophytic growth process and included the descriptors: exophytic, fungating, papillary, pebbly, verrucous, swollen, or “growth” like tissue with 36 total responses in this category. Other appearances described less frequently were ulcerated, hemorrhagic, painful, granulation tissue like, inflamed, friable, or leukoplakic. Figure 1 shows representative clinical images of the cases included in the study.
Fig. 1.
Clinical presentation of PSCCA. a Pebbly raised red and white lesion involving the alveolar ridge distal to tooth #5 (Photo courtesy Dr. Tom Kassube). b Diffuse mixed lesion with papillary areas “cuffing” the facial gingival margins of the maxillary anterior teeth (Photo courtesy Dr. Douglas Johnson). c Thin white leukoplakia of marginal gingiva from teeth #6–11 with area of PSCCA interproximally between teeth #9–10 (Photo courtesy Dr. Brian Van Aelst). d Exophytic red and white mass on the alveolar ridge distal to tooth #30 (Photo courtesy Dr. James Heit)
The clinical impressions given by the submitting clinician are summarized in Table 2. Of interest, 62 % (n = 38) of clinicians considered a malignant or premalignant lesion in their differential diagnosis (first 5 rows). The most common benign considerations were gingival reactive lesions and papillomas, followed by other less common entities.
Table 2.
Clinical impression
| Clinical impression | Number of cases (% total) |
|---|---|
| SCCA or VC | 22 (36) |
| “Cancer” or “neoplasm” | 5 (8) |
| PVL/VPHK (premalignant) | 1 (<2) |
| Reactive lesion versus SCCA | 7 (11) |
| Infection versus SCCA | 3 (5) |
| Gingival reactive lesion (PG, POF, PGCG) or other inflammatory lesion | 8 (13) |
| Papilloma | 5 (8) |
| Fungal infection | 2 (3) |
| Periodontal disease | 1 (<2) |
| Lichen planus | 1 (<2) |
| Hyperkeratosis | 1 (<2) |
| Gingival hyperplasia | 1 (<2) |
| Unknown | 4 (7) |
Differential diagnosis or clinical impressions given by the submitting clinician on the original biopsy accession form for 61 cases of PSCCA
SCCA squamous cell carcinoma, VC verrucous carcinoma, VPHK verruco-papillary hyperkeratosis, PG pyogenic granuloma, POF peripheral ossifying fibroma, PGCG peripheral giant cell granuloma
In 7 cases, the clinicians indicated that extractions had been done recently in the location of the lesion. 4 cases reported a history of prior biopsies (3 benign, 1 unknown), and pre-biopsy periodontal treatment was performed in one case, along with one case of topical medication treatment with no response prior to biopsy. 15 cases designated indicators of potential bony involvement including non healing extraction sites (3 cases), loose or self-exfoliating teeth (2 cases), severe localized periodontal disease (2 cases), radiographic evidence of bone invasion (6 cases), pathologic fracture (1 case), or evidence of bony involvement from the biopsy report (1 case). Figure 2 exhibits radiographic evidence of bone invasion in cases included in the study.
Fig. 2.
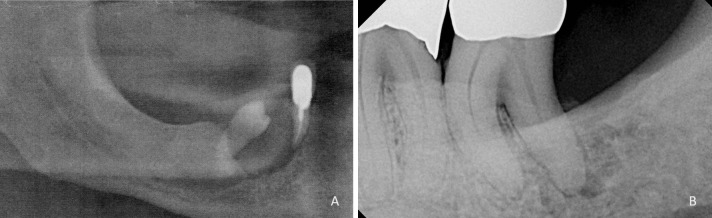
Radiographic presentation of PSCCA. a Bone loss and displacement of the mandibular right premolar in a patient with PSCCA (Radiograph courtesy Dr. Eric Fox). b Irregular radiolucency surrounding the distal root of tooth #18 mimicking a lesion of endodontic-periodontic origin (Radiograph courtesy Dr. Luis Rosario)
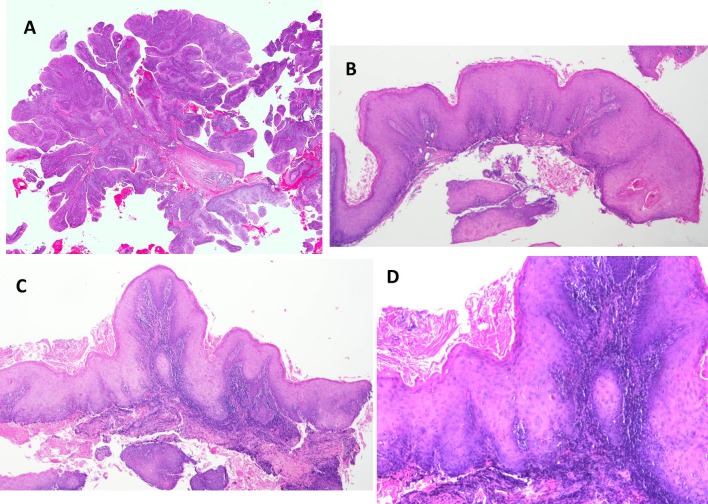
Histologically, 40 cases were graded as well differentiated, 12 cases moderately well differentiated, 6 cases moderate, 1 case moderately-poor, and 0 poorly differentiated. 2 cases did not have histologic gradings assigned from the original biopsy report. Figures 3a, b, c, d illustrate the prominent papillary nature of these lesions along with the bulbous and elongated broad pushing margins formed by the invasive epithelium. In addition, cytologic abnormalities such as increased and abnormal mitoses, individual cell keratinization and increased nuclear/cytoplasmic ratios are seen in some case (Fig. 4a, b). No HPV testing was available for any of the cases. Four clinicians noted the clinical presence of a denture sore and one additional case occurred underneath a fixed partial denture. Three patients had a history of lichen planus. An additional three patients had a history of proliferative verrucous leukoplakia (PVL).
Fig. 3.
Histologic features of PSCCA. a Low power histomicrograph demonstrating the prominent papillary nature of neoplastic proliferation surrounding fibrovascular cores and minimal surface keratinization (H&E, ×2 magnification). b Low power features demonstrating the verrucoid papillary nature with bulbous rete ridges with keratin pearl formation from a different case. (H&E, ×4 magnification). c Papillary proliferation with elongated and bulbous rete ridge formation. (H&E, ×4 magnification). d Higher magnification of case in image c demonstrating individual cell keratinization and keratin pearl formation. (H&E, ×10 magnification)
Fig. 4.
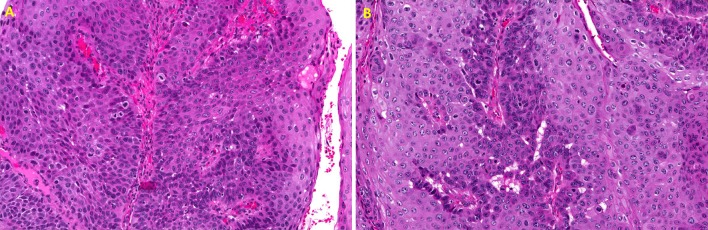
a Higher magnification demonstrating abundant atypical features in the proliferating epithelium including increased abnormal mitoses with increased nuclear/cytoplasmic ratios, crowding of cells and pleomorphism. (H&E, ×20 magnification). b Higher magnification demonstrating increased mitoses, enlarged and abnormal nuclei and numerous dyskeratotic cells (H&E, ×20 magnification)
Discussion
Papillary squamous cell carcinoma shares many features with other well-differentiated forms of SCCA such as VC. VC differs from PSCCA by exhibiting a highly keratinized neoplasm with broad pushing borders and benign appearing features showing no evidence of invasion [12]. PSCCA, on the other hand, resembles a malignant version of a squamous papilloma with minimal keratinization and a papillary architecture and atypical features (Fig. 3) [1, 4]. Two variants of PSCCA have been described in the past, a papillary form and a broad based exophytic form [12].
Head and neck PSCCA is most common in the larynx, where it also carries the best prognosis [13]. The sinonasal tract has been considered to have the worst prognosis [13]. A study in 2011 by Russell et al. [3] of 52 cases of PSCCA of the head and neck determined the frequency of head and neck sites to be affected by PSCCA to be 36.5 % larynx, followed by 34.6 % oral cavity, 15.4 % sinonasal tract, and finally 13.5 % oropharynx. Suarez et al. [13] in 2000 also reported the larynx to be most common, but reported the oral cavity and oropharynx as less commonly affected than the sinonasal tract. Specific sites in the oral cavity are rarely mentioned in many of the prior studies of PSCCA; however, two large studies have subcategorized oral cavity PSCCA. Recently, Bao et al. [14] reviewed 56 cases of PSCCA in comparison to oral squamous papillomas and found the most common site to be the gingiva, followed in descending order by the buccal mucosa, lateral tongue, palate and lower lip, then dorsal tongue, floor of the mouth, and oropharynx. Ishiyama et al. [5] in 1994, reported 52 cases of PSCCA of the oral cavity and oropharynx. Of the cases reported in this study, the most common location was on the alveolar ridge, followed in descending order by buccal mucosa, floor of mouth and ventral tongue, retromolar pad, lateral tongue, and palate [5]. Other locations reported in this study included the glottis, supraglottis, and tonsil [5]. Our cohort of 61 cases of PSCCA of the gingiva represented 12 % of overall gingival carcinoma cases in our previously published study [10]. It is difficult to find exact statistics of the percentage of PSCCA out of overall SCCA cases in the oral cavity, but as it has been described only rarely it is unlikely that it approaches this percentage. This relatively high occurrence of a rare subset of SCCA in our study may indicate that PSCCA occurs disproportionately more on the gingiva than in other sites in the oral cavity.
Many of the previously reported cases of PSCCA from the oral cavity have been described in patients with a history of clinical leukoplakia or proliferative verrucous leukoplakia (PVL) [5]. Prior research has suggested that the cases of PSCCA that have been reported in the oral cavity differ from the form typically found in other locations in the head and neck. Suarez et al. argued that the form of PSCCA reported in the Ishiyama et al. study is more consistent with exophytic SCCA arising from PVL than true PSCCA [5, 13]. Most literature describe PSCCA in the head and neck as usually arising in sites most common to squamous papilloma occurrence such as the larynx [3, 13]. In the oral cavity, the most common locations for squamous papilloma are the soft palate or labial/buccal mucosa; though in prior reports the most common location for PSCCA in the oral cavity is the gingiva or alveolar ridge when a subsite is identified [4, 5, 14–16]. Many studies of head and neck PSCCA show a history of prior benign papillary lesions in the area in at least a subset of cases [1, 3, 13]. In the study by Russell et al., 18 cases or oral PSCCA were included, most of which did not have a history of prior leukoplakia unlike the Ishiyama et al. study [3, 5]. In addition, the same study also reported two of the patients in their study to have reported a history of another concurrent condition, lichen planus [3]. In this study, only 3 patients were reported to have a history of PVL, with 3 additional cases reporting a history of lichen planus.
The relationship of HPV to PSCCA has been evaluated in several studies [17]. Jo et al. [9] found two-thirds of 31 cases of PSCCA in the upper aerodigestive tract were reactive to p16, a surrogate immunohistochemical marker for HPV. Most of the cases of PSCCA in the head and neck that are associated with HPV arise in the oropharynx, specifically base of tongue and tonsillar areas, and more rarely occur in the sinonasal and laryngeal areas [9]. In a literature review of head and neck PSCCA, Cobo et al. [4] reported that of the PSCCA cases in the literature that had been evaluated for HPV expression, 50 % were associated.
Papillary squamous cell carcinoma has been shown to have a more favorable outcome than other forms of SCCA in the head and neck, though it has a high rate of local recurrence with estimates between 25 and 45 % [2, 3, 5, 13]. Survival rates of PSCCA of the head and neck have been reported up to 90 % at 2 years and 72 % at 5 years in one recent study [3]. HPV associated SCCA has been shown to have a better prognosis in the upper aerodigestive tract than non-HPV associated SCCA [9]. Papillary SCCA cases have been found to have a better prognosis than exophytic SCCA [2].
As a subset of SCCA cases within our larger previously published study of gingival SCCA, most clinical parameters were very similar in the PSCCA to the overall group of SCCA [10]. The average age of the PSCCA patients was slightly older than the overall gingival SCCA (74 years compared to 72.3 years) and the male: female ratio was similar with slightly more males in the PSCCA cases than the overall group [10]. The location prevalence was very similar between the two groups, with the posterior mandible the most represented area [10]. A slightly higher percentage of cases of those with a known timeline were present over 2 months in duration in the PSCCA cases (74 % of known cases of PSCCA versus 72 % in the known cases of overall SCCA) [10].
The clinical appearance was also very similar between the PSCCA cases and the overall SCCA cases with erythroplakia the most common presentation in both groups [10]. PSCCA cases had a much narrower clinical presentation spectrum with the vast majority of cases falling into a category described as exophytic, papillary, or verrucous; however, this was also the most common clinical appearance of the majority of cases in the total gingival SCCA group as well, indicating a propensity for SCCA at this location to take on this particular appearance [10]. Clinicians were slightly more likely to suspect a malignancy in the overall SCCA group than the PSCCA cases (64 % compared to 62 %) in their differential diagnoses, but the benign diagnoses considered were similar between the two [10]. One big difference between the two groups, however, was seen in the histologic grading levels. Over a twofold increase in cases in the well differentiated or moderately-well differentiated groups was seen in the PSCCA group over overall SCCA (85 % of PSCCA compared to 41.5 % of overall SCCA) [10]. Only 12 % of the PSCCA were graded moderately or moderately-poor with no cases of poorly differentiated grading, while 49.5 % of overall SCCA cases were moderate, moderately-poor, or poorly differentiated [10]. No grading was available in 3 % of the PSCCA cases and 9 % of the SCCA cases overall [10].
Conclusions
In conclusion, PSCCA is a distinct variant of SCCA with a more favorable outcome profile. The recognition of this subset of SCCA in gingival carcinoma is helpful to clinicians due to the expected behavior pattern. Though rare in the oral cavity, the gingiva/alveolar ridge appears to be a common location for this particular variant of SCCA. A distinct set of clinical characteristics for PSCCA of the gingiva is evident from this study, including an older and relatively non gender-specific patient population, predilection for the posterior mandible, and presentation as a red or mixed red and white exophytic lesion. PSCCA may mimic benign lesions on the gingiva including reactive gingival lesions and papillomas, and histologically it is most often well-differentiated in nature. The lesion should be considered in the differential diagnosis of papillary gingival masses.
References
- 1.Crissman JD, Kessis T, Shah KV, Fu YS, Stoler MH, Zarbo RJ, Weiss MA. Squamous papillary neoplasia of the adult upper aero digestive tract. Hum Pathol. 1988;19:1387–1396. doi: 10.1016/S0046-8177(88)80231-4. [DOI] [PubMed] [Google Scholar]
- 2.Thompson LDR, Wenig BM, Heffner DK, Gnepp DR. Exophytic and papillary squamous cell carcinomas of the larynx: a clinicopathologic series of 104 cases. Otolaryngol Head Neck Surg. 1999;120:718–724. doi: 10.1053/hn.1999.v120.a92773. [DOI] [PubMed] [Google Scholar]
- 3.Russell JO, Hoschar AP, Scharpf J. Papillary squamous cell carcinoma of the head and neck: a clinicopathologic series. Am J Otolaryngol. 2011;32:557–563. doi: 10.1016/j.amjoto.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Cobo F, Talavera P, Concha A. Relationship of human papillomavirus with papillary squamous cell carcinoma of the upper aero digestive tract: a review. Int J Surg Pathol. 2008;16(2):127–136. doi: 10.1177/1066896908314700. [DOI] [PubMed] [Google Scholar]
- 5.Ishiyama A, Eversole LR, Ross DA, Raz Y, Kerner MM, Yao S, Blackwell K, Feneberg R, Bell TS, Calcaterra TC. Papillary squamous neoplasms of the head and neck. Laryngosope. 1994;104(12):1446–1452. doi: 10.1288/00005537-199412000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Takeda Y, Satoh M, Nakamura S, Yamamoto H. Papillary squamous cell carcinoma of the oral mucosa: immunohistochemical comparison with other carcinomas of oral mucosal origin. J Oral Sci. 2001;43(3):165–169. doi: 10.2334/josnusd.43.165. [DOI] [PubMed] [Google Scholar]
- 7.Khan SM, Gossweiler MK, Zunt SL, Edwards MD, Blanchard SB. Papillary squamous cell carcinoma presenting on the gingiva. J Periodontol. 2005;76:2316–2321. doi: 10.1902/jop.2005.76.12.2316. [DOI] [PubMed] [Google Scholar]
- 8.Terada T. Papillary squamous cell carcinoma of the oral cavity with acantholytic and pseudovascular features. Int J Clin Exp Pathol. 2011;4(8):794–796. [PMC free article] [PubMed] [Google Scholar]
- 9.Jo VY, Mills SE, Stoler MH, Stelow EB. Papillary squamous cell carcinoma of the head and neck: frequent association with human papillomavirus infection and invasive carcinoma. Am J Surg Pathol. 2009;33:1720–1724. doi: 10.1097/PAS.0b013e3181b6d8e6. [DOI] [PubMed] [Google Scholar]
- 10.Fitzpatrick SG, Neuman AN, Cohen DM, Bhattacharyya I. The clinical and histologic presentation of gingival squamous cell carcinoma: a study of 519 cases. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114:509–515. doi: 10.1016/j.oooo.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 11.Cardesa A, Zidar N, Nadal A, Ereno C. Papillary Squamous Cell Carcinoma. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization classification of tumors: pathology and genetics, head and neck tumors. Lyon: IACR Press; 2005. p. 126. [Google Scholar]
- 12.Slootweg PJ, Richardson M. Squamous cell carcinoma of the upper aerodigestive system. In: Gnepp, editor. Diagnostic surgical pathology of the head and neck. 2. Philadelphia: Saunders Elsevier; 2009. pp. 45–110. [Google Scholar]
- 13.Suarez PA, Adler-Storth K, Luna MA, El-Naggar AK, Abudul-Karim FW, Batsakis JG. Papillary squamous cell carcinomas of the upper aero digestive tract: a clinicopathologic and molecular study. Head Neck. 2000;22:360–368. doi: 10.1002/1097-0347(200007)22:4<360::AID-HED8>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 14.Bao Z, Yang X, Shi L, Feng J, Liu W, Zhou Z. Clinicopathologic features of oral squamous papilloma and papillary squamous cell carcinoma: a study of 197 patients from eastern China. Ann Diagn Pathol. 2012;16:454–458. doi: 10.1016/j.anndiagpath.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Bouquot JE, Muller S, Nikai H. Lesions of the oral cavity. In: Gnepp DR, editor. Diagnostic surgical pathology of the head and neck. 2. Philadelphia: Saunders Elsevier; 2009. pp. 191–308. [Google Scholar]
- 16.Neville BW, Damm DD, Allen CM, Bouquot JE, Chi A (revision). Epithelial pathology. In: Oral and maxillofacial pathology, 3rd edition. Saunders Elsevier, St. Louis; 2008. P. 362–452.
- 17.Cardesa A, Nadal A. Carcinoma of the head and neck in the HPV era. Acta Dermatoven APA. 2011;20(3):161–173. [PubMed] [Google Scholar]