Abstract
Mammary analogue secretory carcinoma (MASC) is a recently described salivary gland tumor characterized by ETV6 translocation. It appears that prior studies have identified MASC by reviewing salivary gland carcinomas, such as acinic cell carcinoma and adenocarcinoma, not otherwise specified. To address the possibility of MASC mimicking benign salivary neoplasms we reviewed 12 salivary gland (cyst)adenomas diagnosed prior to the discovery of MASC. One encapsulated (cyst)adenoma of the parotid gland demonstrated features of MASC. The diagnosis was confirmed by fluorescence in situ hybridization with an ETV6 break-apart probe. An unusual complex pattern of ETV6 rearrangement with duplication of the telomeric/distal ETV6 probe was identified. This case illustrates that MASC may mimic salivary (cyst)adenomas. To more accurately assess true clinical and morphologic spectrum of MASC, future studies may have to include review of salivary (cyst)adenomas. The differential diagnosis of MASC may have to be expanded to include cases resembling salivary (cyst)adenomas.
Keywords: Mammary analogue secretory carcinoma, ETV6, Salivary adenoma
Introduction
Since the first description of mammary analogue secretory carcinoma (MASC) of the salivary glands [1], about 70 additional cases have been reported [2]. The clinicopathologic features of MASC are now well recognized and the distinctive morphology of MASC (i.e., lobular architecture with microcystic, tubular or solid growth patterns and monomorphic cells with low-grade, vesicular nuclei and eosinophilic vacuolated cytoplasm) has now been confirmed by numerous series and case reports. As reported initially, virtually all cases of MASC are characterized by the ETV6 (ets variant 6) rearrangement, [1] while all of the other tested salivary gland tumors showed intact ETV6.
While in most recent case reports and smaller series MASC was identified prospectively (likely as a part of clinical care), the majority of the larger series represent retrospective studies of archived cases [2–5]. In an attempt to bridge MASC to more established diagnostic entities, most, but not all, series explicitly indicated the original diagnoses for cases that were re-categorized as MASC. To the best of what can be determined from the literature, only malignant salivary tumors were included in such reviews—adenocarcinomas, not otherwise specified, acinic cell carcinomas, etc [1, 3, 4, 6–8]. This study design certainly yielded the highest number of newly identified MASC; however, such a biased approach may narrow the clinical and morphologic spectrum of recognized MASC. For instance, it may lead to an overestimation of how aggressive this tumor actually is. Since MASC can be very small, well-circumscribed and are usually composed of low-to-intermediate grade cells, we hypothesized that some salivary neoplasms historically diagnosed as salivary (cyst)adenomas may in fact represent MASC. The aim of this study was to re-evaluate salivary (cyst)adenomas in the light of the discovery of MASC. To assess the possibility that some of the cases previously diagnosed as salivary (cyst)adenomas may actually represent MASC, we reviewed salivary (cyst)adenomas diagnosed from 1991 to 2008.
Materials and Methods
Studied Patients
This study was approved by the University of Pittsburgh Institutional Review Board (IRB# PRO07050360). The surgical pathology archives of the University of Pittsburgh Medical Center were searched for the terms “salivary adenoma” and “salivary cystadenoma” from 1991 to 2008. Specimens diagnosed as other named salivary gland adenomas (e.g., basal cell adenoma, pleomorphic adenoma, canalicular adenoma, myoepithelioma, Warthin tumor) were excluded. The histologic features were re-evaluated with the knowledge of MASC.
ETV6 Fluorescence In Situ Hybridization (FISH)
FISH for ETV6 was performed with commercial break-apart ETV6 probes (Abbott Molecular, Des Plaines, IL, USA) as previously described [7]. The break-apart ETV6 (12p13) probe consists of a Spectrum Green labeled proximal (centromeric) probe and a Spectrum Orange labeled distal (telomeric) probe. The presence of >20 % of analyzed cells with split green and orange signals was considered indicative of ETV6 rearrangement. Imaging analysis was performed with a fluorescence microscope (Nikon Eclipse E600) and CytoVision Workstation (Applied Imaging, Santa Clara, CA, USA). Slides were counterstained with 4′-6-diamidino-2-phenylindole (DAPI) (Abbott Molecular, Des Plaines, IL, USA). FISH was performed on the destained H&E slide.
Literature Review
All publications in the English medical literature that referenced the original paper by A. Skálová and colleagues as of November 2012 were reviewed; 76 total cases of MASC had been reported at that time [1–15]. These reports were reviewed for comments on MASC encapsulation, benign or malignant nature of re-classified tumors, and pattern of ETV6 rearrangement, as detected by FISH.
Results
A total of 12 salivary (cyst)adenomas were identified. There were 6 female and 6 male patients with an average age of 58 years (range, 17–83 years). All 12 (cyst)adenomas involved the parotid gland and were 1.8 cm on average (range 0.5–4 cm). Five occurred on the left and 6 on the right (the laterality was not specified in one case). Surgical procedures ranged from excisions to superficial parotidectomies. Available clinical presentations included that of a mass (neck, parotid, facial or preauricular), some of which were specified to be painless and/or slow growing. Ten cases were received in consultation from outside institutions (including the case presented below) and 2 were in-house. In 9 cases the diagnosis of cystadenoma (including 6 cases of oncocytic cystadenoma) was confirmed. In one case the original diagnosis of “hybrid salivary gland adenoma with components of oncocytoma and basal cell adenoma” was confirmed and another case is still best categorized as “salivary adenoma, not otherwise specified.” The only re-classified case is described below.
Case Report
A 37-year-old female presented with a left facial mass in January of 2001. Excisional biopsy revealed a 0.5 cm encapsulated cystic tumor surrounded by atrophic parotid tissue (Fig. 1a). The cyst contents consisted of microfollicles and delicate papillae lined by a monomorphic population of cuboidal and columnar cells (Fig. 1b). The cells had pale cytoplasm and bland nuclei with fine chromatin and occasional small nucleoli. No mitotic figures were appreciated. Periodic acid-Schiff (PAS) stain with diastase digestion showed no cytoplasmic zymogen granules. Both the PAS and mucicarmine stains highlighted intracystic and intraluminal mucin (Fig. 1b). The diagnosis of “consistent with cystadenoma of parotid gland” was rendered. The possibility of papillary-cystic acinic cell carcinoma was excluded due to the lack of zymogen granules and tumor encapsulation. Clinical follow-up is not available.
Fig. 1.
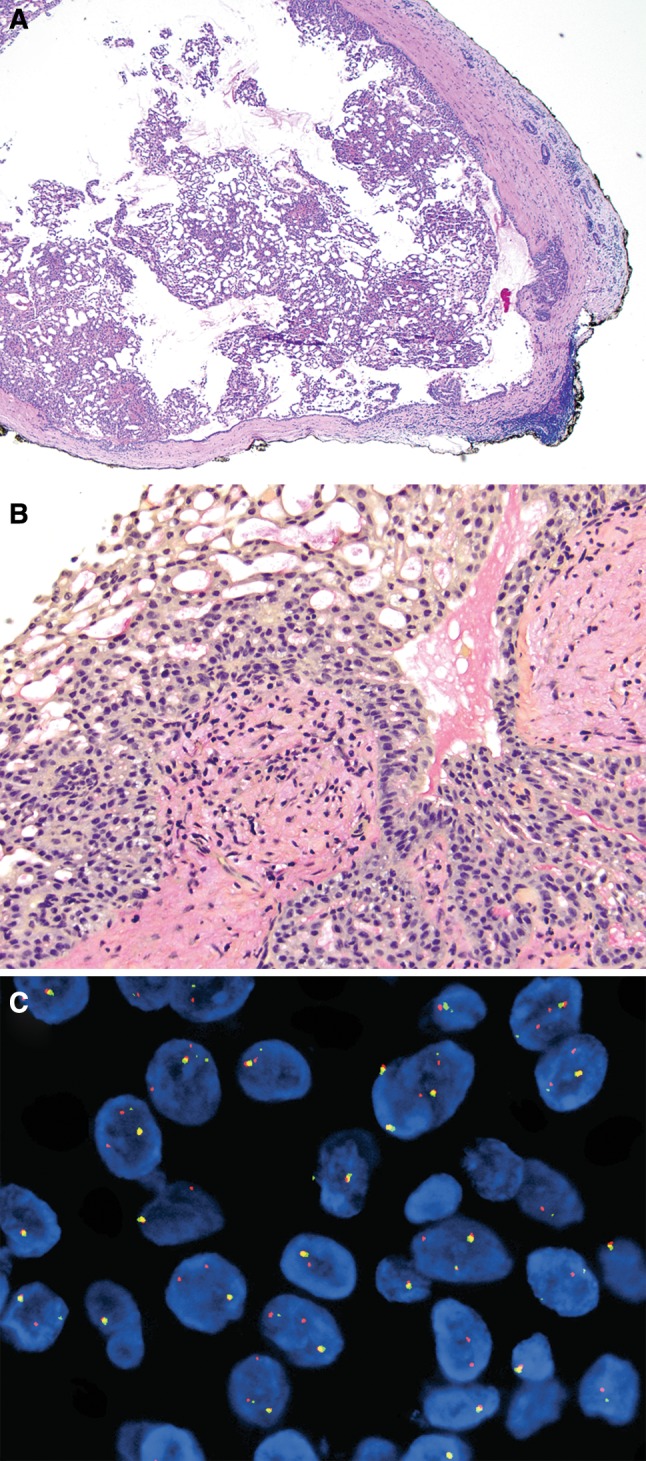
a Encapsulated cystic tumor, Periodic acid-Schiff stain, 40× magnification. b Mucicarmine, 200×, highlights intraluminal mucin. c ETV6, fluorescence in situ hybridization showing nuclei with classic rearrangement pattern (one yellow fusion intact signal accompanied by a single split orange and green signal indicative of ETV6 rearrangement). Many nuclei showed a complex rearrangement pattern (two or more orange signals in addition to one green signal and one yellow fusion signal)
At present, the morphologic features of this case are more consistent with a diagnosis of MASC. To confirm this impression, FISH for ETV6 rearrangement was performed and demonstrated that 45 of 60 (75 %) analyzed cells harbored ETV6 gene breaks (Fig. 1c). Eight of 60 (13.3 %) analyzed cells showed a classic rearrangement pattern—one fusion signal accompanied by separate single orange and single green (i.e., split) signals indicative of ETV6 rearrangement. Interestingly, 37 of 60 (61.7 %) analyzed cells showed a complex rearrangement pattern (one fusion signal, two or more orange signals, and one green signal).
Discussion
Since its first description in late 2010, numerous additional publications from several institutions have reported 76 cases of MASC [2]. These reports comprise both cases diagnosed prospectively as part of clinical care and retrospectively, through focused review of archived material. To the best of our knowledge, retrospective studies have not included re-evaluation of benign salivary neoplasms. Here we show that a minority (perhaps about 8 %) of salivary (cyst)adenomas may represent MASC. Exclusion of (cyst)adenomas from retrospective studies may lead to overestimation of the aggressiveness of MASC. For example, in prior reports on the clinicopathologic features of MASC, the rate of lymph node involvement appeared to be higher than that in acinic cell carcinoma [3]. When compared to acinic cell carcinoma, MASC was also found to have a somewhat shorter disease-free survival. None of these trends reached statistical significance; however, inclusion of a MASC discovered by review of salivary (cyst)adenomas may eliminate such trends altogether. Although clinical follow-up is not available for the case presented here and lymph nodes were not histologically examined, given the morphologic presentation, it seems unlikely that this patient will ever present with disease progression. Future studies may uncover additional small and indolent cases of MASC.
In addition, the morphologic spectrum of MASC may not be fully appreciated without considering the most bland appearing cases. For instance, among 38 published cases of MASC with detailed descriptions of the tumor periphery and interface with normal adjacent tissue, the majority were well-circumscribed (29/38, 76 %) and about one-quarter demonstrated infiltrative growth or invasion (at least 9/38, 23.7 %) [2, 4–6, 10–13, 15]. Of note, none of the MASC previously reported by our group were encapsulated. Only 3 other published cases were encapsulated: capsular invasion was identified in 1 case, while the other two showed no capsular invasion. Although follow-up was limited, no evidence of recurrence or metastasis was identified in these three patients (4–8 months follow-up) [2, 10].
Also, the case presented here demonstrates an unusual pattern of ETV6 rearrangement by FISH. In addition to the classic rearrangement, numerous neoplastic cells demonstrated a complex pattern with an apparent duplication of the distal/telomeric (Spectrum Orange) ETV6 probe. To our knowledge, such ETV6 rearrangement pattern was not reported previously and its significance remains to be determined.
In conclusion, to more accurately assess the true clinical and morphologic spectrum of MASC, retrospective studies may have to include review of salivary (cyst)adenomas. Practically, the differential diagnosis of MASC may have to be expanded to include encapsulated tumors that mimic salivary (cyst)adenomas.
Acknowledgments
We are grateful to Mrs. Robyn Roche for outstanding secretarial support and to the In Situ and Developmental Laboratory, Department of Pathology, University of Pittsburgh Medical Center for excellent technical support.
Conflict of interests
None.
References
- 1.Skalova A, Vanecek T, Sima R, Laco J, Weinreb I, Perez-Ordonez B, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol. 2010;34(5):599–608. doi: 10.1097/PAS.0b013e3181d9efcc. [DOI] [PubMed] [Google Scholar]
- 2.Kratochvil FJ, 3rd, Stewart JC, Moore SR. Mammary analog secretory carcinoma of salivary glands: a report of 2 cases in the lips. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114(5):630–635. doi: 10.1016/j.oooo.2012.07.480. [DOI] [PubMed] [Google Scholar]
- 3.Chiosea SI, Griffith C, Assaad A, Seethala RR. Clinicopathological characterization of mammary analogue secretory carcinoma of salivary glands. Histopathology. 2012;61(3):387–394. doi: 10.1111/j.1365-2559.2012.04232.x. [DOI] [PubMed] [Google Scholar]
- 4.Connor A, Perez-Ordonez B, Shago M, Skalova A, Weinreb I. Mammary analog secretory carcinoma of salivary gland origin with the ETV6 gene rearrangement by FISH: expanded morphologic and immunohistochemical spectrum of a recently described entity. Am J Surg Pathol. 2012;36(1):27–34. doi: 10.1097/PAS.0b013e318231542a. [DOI] [PubMed] [Google Scholar]
- 5.Fehr A, Loning T, Stenman G. Mammary analogue secretory carcinoma of the salivary glands with ETV6-NTRK3 gene fusion. Am J Surg Pathol. 2011;35(10):1600–1602. doi: 10.1097/PAS.0b013e31822832c7. [DOI] [PubMed] [Google Scholar]
- 6.Bishop JA, Yonescu R, Batista DA, Westra WH, Ali SZ. Cytopathologic features of mammary analogue secretory carcinoma. Cancer Cytopathol. 2012 doi: 10.1002/cncy.21245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffith C, Seethala R, Chiosea SI. Mammary analogue secretory carcinoma: a new twist to the diagnostic dilemma of zymogen granule poor acinic cell carcinoma. Virchows Arch. 2011;459(1):117–118. doi: 10.1007/s00428-011-1098-6. [DOI] [PubMed] [Google Scholar]
- 8.Lei Y, Chiosea SI. Re-evaluating historic cohort of salivary acinic cell carcinoma with new diagnostic tools. Head Neck Pathol. 2012;6(2):166–170. doi: 10.1007/s12105-011-0312-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiosea SI, Griffith C, Assaad A, Seethala RR. The profile of acinic cell carcinoma after recognition of mammary analog secretory carcinoma. Am J Surg Pathol. 2012;36(3):343–350. doi: 10.1097/PAS.0b013e318242a5b0. [DOI] [PubMed] [Google Scholar]
- 10.Ito S, Ishida E, Skalova A, Matsuura K, Kumamoto H, Sato I. Case report of Mammary Analog Secretory Carcinoma of the parotid gland. Pathol Int. 2012;62(2):149–152. doi: 10.1111/j.1440-1827.2011.02759.x. [DOI] [PubMed] [Google Scholar]
- 11.Levine P, Fried K, Krevitt LD, Wang B, Wenig BM. Aspiration biopsy of mammary analogue secretory carcinoma of accessory parotid gland: another diagnostic dilemma in matrix-containing tumors of the salivary glands. Diagn Cytopathol. 2012 doi: 10.1002/dc.22886. [DOI] [PubMed] [Google Scholar]
- 12.Petersson F, Lian D, Chau YP, Yan B. Mammary analogue secretory carcinoma: the first submandibular case reported including findings on fine needle aspiration cytology. Head Neck Pathol. 2012;6(1):135–139. doi: 10.1007/s12105-011-0283-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pisharodi L. Mammary analog secretory carcinoma of salivary gland: cytologic diagnosis and differential diagnosis of an unreported entity. Diagn Cytopathol. 2012 doi: 10.1002/dc.21766. [DOI] [PubMed] [Google Scholar]
- 14.Rastatter JC, Jatana KR, Jennings LJ, Melin-Aldana H. Mammary analogue secretory carcinoma of the parotid gland in a pediatric patient. Otolaryngol Head Neck Surg. 2012;146(3):514–515. doi: 10.1177/0194599811419044. [DOI] [PubMed] [Google Scholar]
- 15.Sams RN, Gnepp DR. P63 Expression can be used in differential diagnosis of salivary gland acinic cell and mucoepidermoid carcinomas. Head Neck Pathol. 2012 doi: 10.1007/s12105-012-0403-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


