Abstract
Congenital melanocytic nevus (CMN) is a melanocytic proliferation that has its onset at birth or shortly thereafter and shows characteristic histopathologic features including symmetric proliferation of benign melanocytes, extension of nevus cells into the deep reticular dermis and subcutis, maturation of melanocytes with descent, tracking of melanocytes around and within adnexal structures, vessels, or nerves and splaying of collagen bundles by nevus cells arranged in single rows or cords. We report the case of a 34 year old previously healthy woman who presented with a progressively enlarging soft tissue mass in the right neck and back adjacent to a medium sized CMN. Magnetic resonance imaging showed multiple lipomatous masses within the soft tissues of the posterior superficial neck. Subsequent excision of the soft tissue mass showed a well circumscribed lipomatous lesion with diffuse infiltration by benign appearing melanocytes within the fat lobules. Excision of the mass was not accompanied by overlying skin and, thus, posed a diagnostic challenge. Sudden increase in the size of a CMN is worrisome for the development of a melanoma, however, this lesion lacked significant cytologic atypia and mitotic figures, and had a low proliferative index by Ki-67 immunohistochemistry. This case serves to illustrate the initial diagnostic dilemma as well as the plasticity of the neural crest cells.
Keywords: Neurocristic hamartoma, Neural crest, Nevus lipomatosus, Melanoma
Introduction
Congenital melanocytic nevus (CMN) is defined as a melanocytic proliferation, invariably pigmented, which is evident at birth or within the early infancy period. These lesions can be classified on the basis of size as small (<1.5 cm), medium (1.5–19.9 cm) and large (20 cm or greater). Clinically, they may be flat, elevated, verrucous or rarely gyriform (or cerebriform). They often have dense, coarse, dark hair, are evenly pigmented, and may range in color from tan to dark brown to black. Unlike the acquired melanocytic nevus, the CMN has the capacity for infiltrative growth into the depths of the reticular dermis, subcutis and fascia yet maintaining its bland cytologic features. Lymphatic, perineural and lymph node involvement are other pathologic findings. On the basis of histopathologic features, they can also be classified as junctional, compound or intradermal. The incidence among newborns ranges between 0.2 and 6 % [1], except for the giant nevi which are rare, with an estimated incidence of 0.0005 % [2]. CMNs may undergo various changes with age. There may be darkening in the first 5–6 years of life and then again at puberty. The lesion is usually flat at birth and becomes palpable with age. Terminal hair are acquired gradually. At later ages, they develop hyperkeratosis and may feel ‘doughy’ as a result of mucinous degeneration. True neurofibromas can form within a CMN. The appearance of firm nodules in a CMN should be looked upon as a finding of great concern and the differential diagnosis includes proliferative nodules and malignant melanoma. The likelihood of evolution to melanoma is proportionate to the size of the CMN, and the lifetime risk for the development of a cutaneous melanoma is between 6 and 12 % for giant nevi [3, 4].
The current report documents a deep CMN which presented as an enlarging mass in the neck with imaging studies and biopsy consistent with a lipomatous neoplasm. Given the various types of dysmorphogenetic and related processes that can occur as perturbations in neural crest-derived tissues, this case raises the possibility of a hamartomatous proliferation arising from a CMN. The findings in this case would possibly serve to provide a small modicum of insight into the ectomesenchymal properties of the neural crest cells that give rise to CMN.
Case Report
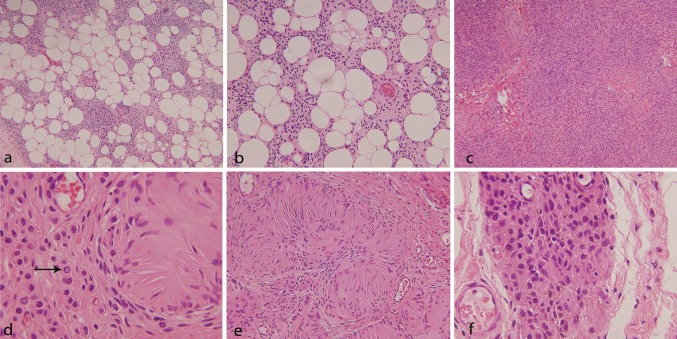
A 34 year old woman with a history of Crohn’s disease presented with multiple soft tissue masses in the posterior neck that had been present for 6 months with a recent increase in size and accompanying pain. The pain was described as dull, chronic and radiating to both arms (left greater than right). There was also tingling and numbness in both arms, one episode requiring an emergency department visit. Magnetic resonance imaging revealed multiple lipomatous masses within the posterior superficial neck (Fig. 1). The dominant mass measured 8.1 × 3.6 × 2.7 cm with an enhancing anterior soft tissue nodule measuring 1.2 cm. Because of concern for an atypical lipomatous neoplasm, an incisional biopsy was performed which revealed mature lipocytes with an accompanying spindle cell population. On subsequent resection, the mass consisted of vaguely circumscribed fibroadipose tissue whose cut surface showed lobules of yellow adipose tissue with multiple fibrotic, pale-white foci. No dominant mass was appreciated. Microscopic examination revealed infiltration by small, monotonous, ovoid to spindled cells (Fig. 2a). These small, ovoid cells with bland nuclei were found along the fibrous septae of the subcutis similar to those seen in the original biopsy. The mature appearing adipocytes were closely associated with the small monotonous cells raising the possibility of being a part of the lesion. In addition, there were more cellular areas composed of similar small monotonous cells but with a different architectural arrangement (Fig. 2b). In these areas, the growth pattern was solid and nested and the cells had moderate amounts of pale, eosinophilic cytoplasm and small nuclei with occasional intranuclear cytoplasmic inclusions (Fig. 2c). Neither pleomorphism nor mitotic figures were identified. Several fibro-hyaline bodies were identified resembling Wagner–Meissner bodies of the type seen in some neurofibromas (Fig. 2d). Focal melanin pigment was also seen (Fig. 2e). The spindle cells tracked along hair follicles in some sections, but overlying epidermis and dermis were not included in the resection.
Fig. 1.
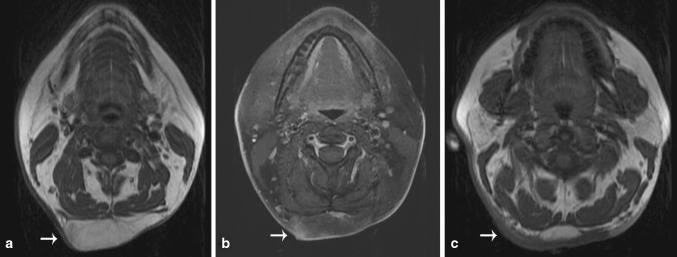
Showing T1 weighted (a) and T1 weighted post-contrast fat saturated (b) images demonstrating the presence of a lobular almost entirely fat containing lesion (arrows) in the subcutaneous tissues of the posterior neck. The lesion only minimally enhances. Cephalad to the lesion, T1 weighted image in (c) demonstrates extensive T1 hypointense skin thickening, corresponding to the patient’s melanocytic nevus
Fig. 2.
Showing HE stained sections of the lesion with diffuse infiltration by small, monotonous slightly spindled cells into the subcutaneous fat [a (×10), b (×20)]; adjacent solid area with a nested growth pattern, [c, (×10)]; presence of intra-nuclear inclusions [d, (×40)]; Wagner–Meissner bodies [e, (×20)]; and focal brown pigment [f, (×40)]
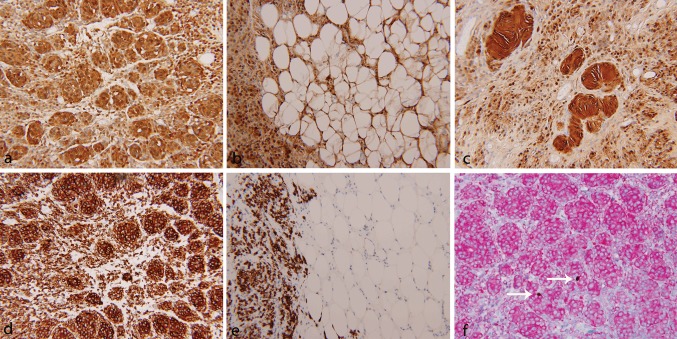
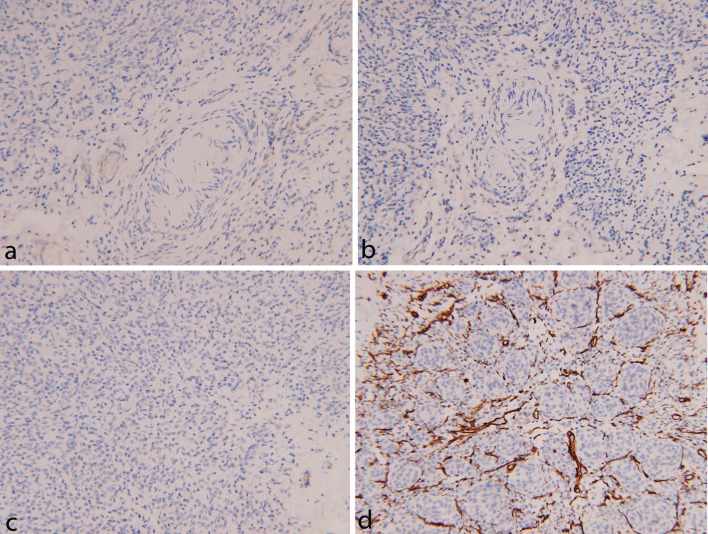
Immunohistochemical staining was performed on a Ventana NexES automated system (Ventana Medical Systems, Tucson, AZ, USA). Strong and diffuse reactivity for S100 protein was seen in the solid as well as infiltrative areas of the lesion (Fig. 3a, b). On the contrary, Melan-A immunostain highlighted the cells in the solid areas and did not show reactivity in the infiltrative areas, indicating that the infiltrative cells were more primitive than the cells in the solid areas (Fig. 3d, e). A Melan-A/Ki-67 dual immunostain showed a very low proliferative index of up to 2 % (Fig. 3f). Other immunostains including progesterone receptor (Fig. 4a), epithelial membrane antigen (Fig. 4b) and neurofilament (Fig. 4c) were negative in the solid as well the infiltrative areas. Immunostain for CD34 was negative in the solid areas and also failed to show diffuse reactivity in the stromal cells (Fig. 4d).
Fig. 3.
Shows immunostain for S100 protein with a strong and diffuse reactivity in the solid area (a, ×20), infiltrative area (b, ×20) and Wagner–Meissner bodies (c, ×20). Immunostain for Melan-A also showed strong and diffuse reactivity in the solid areas (d, ×20). But the infiltrative spindle cells were negative for Melan-A (e, ×20). Melan-A/Ki-67 dual immunostain (f, ×20) showed diffuse immunoreactivity for Melan-A (red) and rare nuclear positivity for Ki-67 (brown)
Fig. 4.
Shows immunostains with negative reaction for progesterone receptor (a, ×20), epithelial membrane antigen (b, ×20) and neurofilament (c, ×20). CD34 failed to show diffuse reactivity in the stromal cells (d, ×20)
Discussion
Further inquiries after the review of the pathologic findings revealed a history of a medium sized CMN in the skin overlying the lipomatous mass. The original biopsy contained only the infiltrating spindle cell component in the mature fat lobules and hence had raised the possibility of a spindle cell lipoma. However, once the true nature of the underlying pathology was established on the resection specimen, the appropriate question could be posed to the clinician about a pre-existing CMN. The resected specimen did not have an ellipse of skin to answer the question for ourselves.
Congenital melanocytic nevi, especially the medium and large variants, have the capacity for growth through the full thickness of the dermis and into the subcutis. One of the extreme expressions of large or multiple CMNs is neurocutaneous melanosis, with the seeding of the leptomeninges by neoplastic melanocytes. However, most CMNs are examples of compound melanocytic nevi with a nested junctional component and a dermal component which is generally nested in the superficial and mid-dermis, but is diffusely infiltrative with individual small cells in the deeper dermis and subcutis. The entire length of the hair follicles, eccrine sweat glands, lymphatic spaces and the perineural sheath are the various structures that can be enveloped by the nevus cells. It is not uncommon to identify individual melanocytes in the subcutis along the septa with infiltration into the subcutaneous fat and access to lymphvascular spaces. As such it is not surprising that nodal melanocytic nevi have been observed in regional lymph nodes sampled as sentinel lymph nodes in patients with malignant melanoma. The melanocytic nevi in these cases are often congenital in type. Other unusual features include fascial or muscle involvement and choristomatous elements including cartilaginous and osseous differentiation. Such deeply growing nevi are seen more commonly in the head and neck region but can also occur in other locations.
Congenital melanocytic nevus (CMNs) have the capacity to differentiate along several pathways, all of which are expressions of the diversity of the neural crest cells. It is known that the embryonic neural crest, a transitory structure, undergoes an epithelial-mesenchymal transition as these cells migrate along the ectoderm of the embryo or through the fetal mesodermal core [5, 6]. Within the neural crest, populations are fated to differentiate and migrate along patterned pathways. Some neural crest cells emerge as uncommitted cells whose fate is determined in transit. It should come as no surprise that the large CMN contains cells that can differentiate into tissues resembling those found in the neurofibroma, spindle cells with schwannian features and immature skeletal muscle. Some of these neoplasms are a complex collage of CMN, schwannoma, neurofibroma and rosette-like formations of primitive neuroectodermal tumor. These neoplasms have been referred to as cutaneous neurocristic hamartomas (NCHs). There is a great deal of histological overlap between NCH and CMN. In the present case, the absence of the following findings: increased CD34 positive stromal cells, thick bands of polarizable fibrillar matrix material between the melanocytes, tactoid bodies, well circumscribed schwannian nodules surrounded by EMA positive perineural cells and alopecia argues against the possibility of a NCH. Additionally, the maturation of melanocytes with depth which was typically seen in this lesion is usually not seen in a NCH. The overlying skin harboring the CMN was not available for assessment of the junctional component, presence of which would have additionally favored the interpretation of a CMN. The clinical significance of differentiating a CMN from NCH lies in the difference in biologic behavior wherein a significant number of NCHs have shown the development of a malignant melanocytic component [7, 8].
An example of ‘lipomatous melanocytic nevomatosus’ has been illustrated in a 67-year old man on the lower trunk and buttock [9], which may be an example of a collision between a large CMN and nevus lipomatosis. However, the tumor under discussion in this report illustrates another aspect of the changes seen in the CMN with respect to the pluripotential nature of the neural crest cells. In addition to being pluripotent, the neural crest cells are the source of skeletal muscle and adipose tissue of the face and neck. We would propose that our case, arising in the neck, may represent a true composite neural crest-derived tumor. This case emphasizes the importance for pathologists to be aware of the various histopathologic changes associated with melanocytic nevi, especially when they are large or deep and particularly if sampled without the overlying skin.
References
- 1.Ingordo V, Gentile C, Iannazzone SS, Cusano F, Naldi L. Congenital melanocytic nevus: an epidemiologic study in Italy. Dermatology. 2007;214(3):227–230. doi: 10.1159/000099587. [DOI] [PubMed] [Google Scholar]
- 2.Castilla EE, da Graca Dutra M, Orioli-Parreiras IM. Epidemiology of congenital pigmented naevi: I. Incidence rates, relative frequencies. Br J Dermatol. 1981;104(3):307–315. doi: 10.1111/j.1365-2133.1981.tb00954.x. [DOI] [PubMed] [Google Scholar]
- 3.Lorentzen M, Pers M, Bretteville-Jensen G. The incidence of malignant transformation in giant pigmented nevi. Scand J Plast Reconstr Surg. 1977;11(2):163–167. doi: 10.3109/02844317709025513. [DOI] [PubMed] [Google Scholar]
- 4.DeDavid M, Orlow SJ, Provost N, et al. A study of large congenital melanocytic nevi and associated malignant melanomas: review of cases in the New York University Registry and the world literature. J Am Acad Dermatol. 1997;36(3 Pt 1):409–416. doi: 10.1016/S0190-9622(97)80217-4. [DOI] [PubMed] [Google Scholar]
- 5.Reed RJ. Neuromesenchyme. The concept of a neurocristic effector cell for dermal mesenchyme. Am J Dermatopathol. 1983;5(4):385–395. doi: 10.1097/00000372-198308000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Bronner ME, LeDouarin NM. Development and evolution of the neural crest: an overview. Dev Biol. 2012;366(1):2–9. doi: 10.1016/j.ydbio.2011.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pathy AL, Helm TN, Elston D, Bergfeld WF, Tuthill RJ. Malignant melanoma arising in a blue nevus with features of pilar neurocristic hamartoma. J Cutan Pathol. 1993;20(5):459–464. doi: 10.1111/j.1600-0560.1993.tb00672.x. [DOI] [PubMed] [Google Scholar]
- 8.Pearson JP, Weiss SW, Headington JT. Cutaneous malignant melanotic neurocristic tumors arising in neurocristic hamartomas. A melanocytic tumor morphologically and biologically distinct from common melanoma. Am J Surg Pathol. 1996;20(6):665–677. doi: 10.1097/00000478-199606000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Cabrera H, Gomez ML, Garcia S. Lipomatous melanocytic nevomatosis. J Eur Acad Dermatol Venereol. 2002;16(4):377–379. doi: 10.1046/j.1468-3083.2002.00569.x. [DOI] [PubMed] [Google Scholar]