Abstract
The term inflammatory myofibroblastic tumor (IMT) encompasses a diverse group of spindle cell entities that traverses a clinical and histologic spectrum, extending from reactive to benign neoplastic to highly aggressive with malignant inclinations. Head and neck IMTs are rarely seen and comprise less than 5 % of tumors. Here we report a case of a 30 year old male who presented with a rapidly enlarging and extremely painful growth in the right posterior mandible, post extraction. Histopathological examination revealed a highly cellular connective tissue stroma comprised of spindle shaped cells arranged in fascicles, admixed with inflammatory cells, predominantly plasma cells. Apart from routine hematological investigations, serum protein electrophoresis was also performed. The final diagnosis was confirmed by a panel of immunomarkers consisting of MPO, CD34, CD20, CD3, CD23, CD138, SMA and ALK. To the best of our knowledge, this is the third case of oral IMT arising from an extraction socket.
Keywords: Inflammatory myofibroblastic tumour, Inflammatory pseudotumor, Gingiva, Plasma cells, Myofibroblasts, Immunohistochemistry
Case Presentation
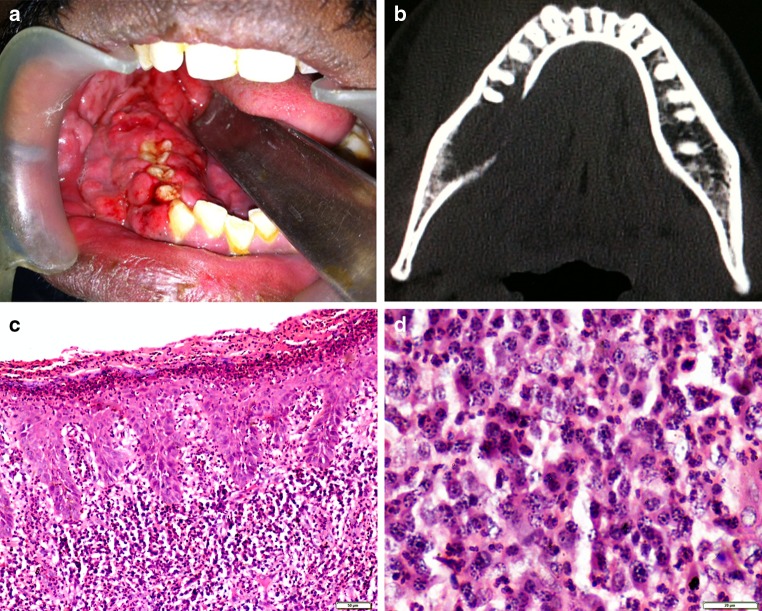
A 30-year old male presented with a painful, rapidly enlarging growth in the right posterior mandible of 1 month duration. The patient had undergone extraction of the right mandibular third molar about 4 months back, with persistence of postoperative pain in that region. The medical history was unremarkable. Clinical examination revealed a 7 × 5 cm reddish pink mass with an irregular surface in the right mandible. The mass extended from the extraction socket of the third molar to the canine and involved both buccal and lingual surfaces, almost covering the occlusal aspects of the teeth (Fig. 1a). The swelling was soft in consistency and elicited tenderness on palpation. Routine hematological investigations revealed mild leukocytosis (15,400 cells/mm3) and an elevated erythrocyte sedimentation rate (107 mm). Fine needle aspiration cytology and histopathological examination following an incisional biopsy were inconclusive. The lesion continued to display rapid growth with involvement and progressive mobility of all the posterior teeth. Computed tomography of the mandible in the transverse plane revealed a hypodense area involving the premolar and molar region, associated with a breach in the continuity of the lingual cortical plate (Fig. 1b). 3-dimensional CT reconstruction confirmed this to be an osteolytic lesion giving the impression of an aggressive neoplasm, which necessitated repeating the incisional biopsy at two representative areas of the lesion.
Fig. 1.
a Clinical photograph showing a soft tissue mass arising from extraction socket of right mandibular third molar. b Axial CT highlighting osteolytic areas with destruction of lingual cortical plate. c Highly cellular connective tissue stroma with surface erosions and psoriasiform epithelium (Haematoxylin and eosin, ×100). d Intense proliferation of plasma cells with scattered lymphocytes, neutrophils and eosinophils. (Haematoxylin and eosin, ×400)
Histopathological examination revealed a highly cellular connective tissue stroma with the overlying epithelium showing surface erosion. The cellular proliferation comprised of spindle shaped cells arranged in a fascicular growth pattern, admixed with a dense inflammatory cell infiltrate consisting of plasma cells, eosinophils, neutrophils and lymphocytes. The vascular component, present as slit-like spaces, was obscured by background cellularity. (Fig. 1c, d)
Diagnosis
The differential diagnoses of a rapidly growing soft tissue lesion causing osteolysis comprise reactive lesions, benign neoplasms, connective tissue malignancies and hematologic pathologies.
The proliferating endothelial cells, demonstrated by CD34 staining, in an abundant inflammatory background suggested a granulation tissue associated with healing of extraction wound [1]. The plasma cell gingivitis was contemplated because of the erythematous, diffuse gingival enlargement, with predominant plasma cell component and psoriasiform epithelium [1]. The aggressiveness of the lesion and destructive growth noted in the present case, along with fascicular pattern of spindle cells in a mixed inflammatory infiltrate ruled out these reactive lesions.
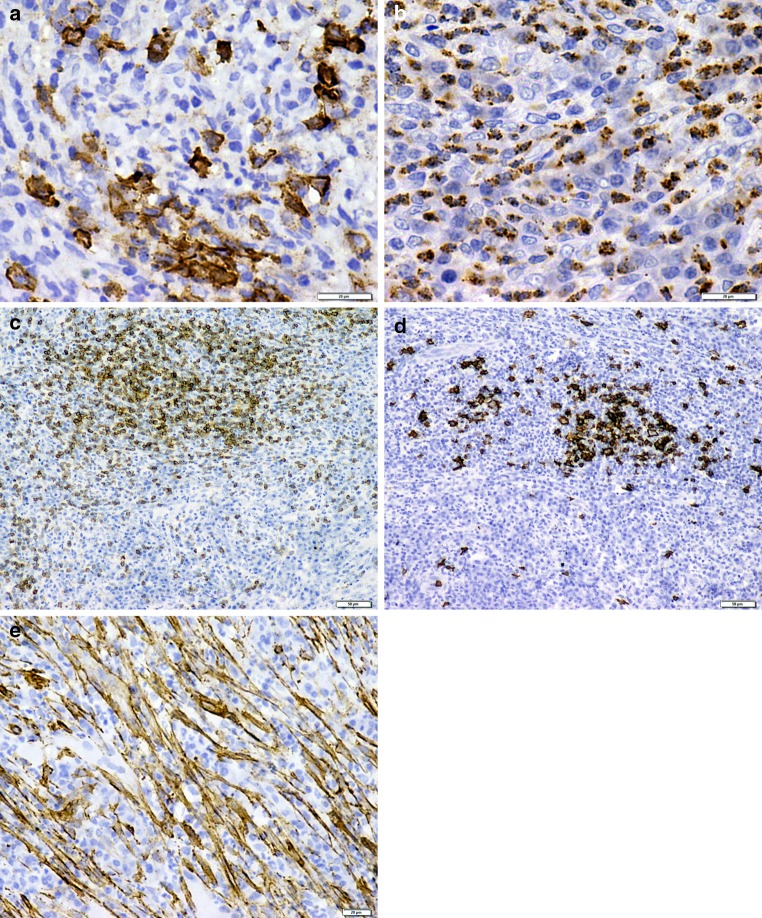
Abundance of plasma cells in an osteolytic lesion confirmed by CD138 positivity (Fig. 2a), indicated a possible diagnosis of plasmacytoma [2]. However, the admixture of polymorphs and spindle cells, absence of monoclonal gammopathy and expression of both the kappa and lambda light chains confirmed with serum protein electrophoresis, conclusively ruled out plasmacytoma.
Fig. 2.
a Plasma cells showing strong membranous and partial cytoplasmic staining for CD138 (×400). b Cytoplasmic staining of mature myeloid cells with MPO (×400). c Focal positivity for CD3 (×100). d Focal positivity for CD20 (×100). e Spindle cells expressing intense positivity for SMA (×400)
Myeloid sarcoma, the extramedullary manifestation of acute myeloid leukemia, was considered because of the conspicuous granulocyte population in our case. Despite the presence of a mixed inflammatory infiltrate dominated by plasma cells, primitive myeloid cells (blasts) were not evident in our case [4]. While the mature myeloid cells of neutrophilic and eosinophilic lineage stained positively for myeloperoxidase (MPO) (Fig. 2b), other inflammatory cells failed to express MPO, thus eliminating the diagnosis of myeloid sarcoma.
The predominant lymphocyte population and rapidly growing lesion enforced us to rule out a T or B cell lymphoma. The focal positivity of CD3 (Fig. 2c) and CD20 (Fig. 2d) along with normal hematologic laboratory values precluded the diagnosis of lymphoma.
Since the background component was spindle cell predominant, staining with SMA was performed. Strong positivity for SMA noted in our case suggested a myofibroblastic differentiation (Fig. 2e). Nodular fasciitis presents as a rapid growth with high cellularity and short linear curved fascicles of spindle cells. Follicular dendritic tumor presents as a fascicular arrangement of spindle cells along with an inflammatory infiltrate and shows strong positivity for CD21 [5]. The presence of longer fascicles of spindle cells and a plasma cell predominant inflammatory infiltrate in our case ruled out both these entities. The lack of necrosis and absence of staining for CD23 used as a substitute for CD21, further confirmed the exclusion of nodular fasciitis and follicular dendritic tumor, respectively.
Inflammatory myofibroblastic tumor (IMT) or plasma cell granuloma is a distinctive lesion composed of myofibroblastic spindle cells accompanied by an inflammatory infiltrate rich in plasma cells, lymphocytes, and eosinophils with occasional presence of neutrophils. It occurs primarily in soft tissue of children and young adults and does not demonstrate any hematological aberrations or systemic symptoms. An aggressive and rapid growth pattern is often noted [5, 6]. Our case presented with all the above features.
After considering the above differential diagnosis, the lesion was eventually signed out as an inflammatory myofibroblastic tumor. Approximately 8–60 % of IMTs exhibit chromosomal translocation of the anaplastic lymphoma kinase (ALK) gene and immunoreactivity for the ALK protein [7]. However, the present case was negative for ALK immunoexpression.
The patient was given a course of steroids following which the lesion resolved. The patient has been under regular follow-up since 7 months and there has been no evidence of recurrence.
Discussion
The term inflammatory myofibroblastic tumor (IMT) encompasses a diverse group of spindle cell lesions that traverses a clinical and histologic spectrum, spanning from reactive non neoplastic to highly aggressive lesions with malignant inclinations [7]. First reported by Brunn et al. in the lung [8], this lesion was reported under a varied nomenclature until the WHO termed it as inflammatory myofibroblastic tumor, a unique lesion comprising myofibroblastic spindle cells and an inflammatory infiltrate of plasma cells, lymphocytes, and eosinophils [3].
IMT is predominantly a tumor of the viscera, [9] and head and neck sites comprise less than 5 % of tumors, with the buccal mucosa, posterior mandible, tongue and gingiva being frequent oral locations [7, 10]. To the best of our knowledge, this is the third case of IMT arising from the extraction socket to be documented [6]. Occurrence in almost all age groups has been reported [9], but IMT shows a distinct predilection for children and young adults [3]. Oral lesions develop over a short duration of time and often exhibit a disturbingly rapid growth rate [7].
The WHO delineates three primary histological patterns of IMT—a “myxoid/vascular pattern”, a “compact spindle cell pattern” with fascicular architecture and a distinctive infiltrate of plasma cells, and a “hypocellular pattern” with heavy collagenisation, bearing resemblance to fibrous scar tissue [3, 9, 11]. The present case exhibited a compact spindle cell pattern.
The etiopathogenesis of IMT remains unresolved. These lesions were traditionally considered a benign reactive process [12], possibly as an exaggerated immune response to Epstein–Barr virus, human herpes virus-8, trauma, surgery or a foreign body [3, 6]. At least a fraction of IMTs appear to be neoplastic in nature, based on reports of recurrence [11], regional metastases [3], extensive local invasion leading to death in one case, and malignant transformation [11]. This concept has been further supported by evidence of aberrations in the anaplastic lymphoma tyrosine kinase (ALK) receptor locus on chromosome 2p23, reported in up to 50 % of IMTs [9]. Chromosomal rearrangement of the ALK gene appears to promote aberrant phosporylation and may induce tumorigenesis [7]. ALK expression was negative in our case.
The controversy over the neoplastic nature of IMT hovers over its treatment, with surgical excision [5], curettage [13], corticosteroid therapy [3], radical surgery [13] and radiotherapy [6], as possible options. Although complete surgical resection is widely reported as the treatment of choice [3, 6, 9], instances of lesional resolution achieved by conservative modalities [5] as in our case, have reinforced the ambiguity over the nature of IMT. Pankaj et al. [13] have noted no instance of recurrence reported in the head and neck or oral region so far. They hypothesized that IMT occurring in head and neck sites behave distinctly from extra-oral IMT, thus advocating a different treatment protocol. Prudent therapeutic intervention in any case is mandatory, considering the rapid growth the lesion exhibits, and postoperative follow up for at least 10 years is mandatory to exclude recurrence and metastasis [7].
Multiple entities resemble IMT and must be excluded via a complete pathologic workup, utilizing immunohistochemistry and hematology. Since the clinical behavior of IMT mimics malignancy, a thorough investigation is compulsory to avoid needlessly radical treatment modalities. A potentially aggressive course of IMT mandates regular follow up.
Footnotes
Parul Sah and Aditi Amit Byatnal have contributed equally to the manuscript.
References
- 1.Neville BW, Damm DD, Allen CM, Bouquot JE. Periodontal diseases. In: Oral and maxillofacial pathology. 2nd edn. Philadelphia: W.B. Saunders Company; 2002. p. 234–266.
- 2.Brandwein-Gensler MS, Mahadevia P, Gnepp DR. Nonsquamous pathologic diseases of the hypopharynx, larynx, and trachea. In: Gnepp DR, editor. Diagnostic surgical pathology of the head and neck. 2. Philadelphia: Saunders Elsevier; 2001. pp. 370–371. [Google Scholar]
- 3.Coffin CM, Fletcher JA. Inflammatory myofibroblastic tumour. In: Fletcher CD, Krishnan Unni K, Mertens F, editors. World Health Organization classification of tumors. Pathology and genetics of tumours of soft tissue and bone. Lyon: IARC Press; 2002. pp. 91–93. [Google Scholar]
- 4.Lee YH, Lee NJ, Choi EJ, Kim JH. Granulocytic sarcoma (chloroma) presenting as a lateral neck mass: initial manifestation of leukemia: a case report. Eur Arch Otorhinolaryngol. 2006;263:16–18. doi: 10.1007/s00405-005-0952-z. [DOI] [PubMed] [Google Scholar]
- 5.Shek AW, Wu PC, Samman N. Inflammatory pseudotumour of the mouth and maxilla. J Clin Pathol. 1996;49:164–167. doi: 10.1136/jcp.49.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eley KA, Watt-Smith SR. Intraoral presentation of inflammatory myofibroblastic tumor (pseudotumor) at the site of dental extraction: report of a case and review of the literature. J Oral Maxillofac Surg. 2010;68:2016–2020. doi: 10.1016/j.joms.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Brooks JK, Nikitakis NG, Frankel BF, Papadimitriou JC, Sauk JJ. Oral inflammatory myofibroblastic tumor demonstrating ALK, p53, MDM2, CDK4, pRb, and Ki-67 immunoreactivity in an elderly patient. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:716–726. doi: 10.1016/j.tripleo.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 8.Brunn H. Two interesting benign lung tumors of contradictory histopathology. J Thorac Surg. 1939;9:119–131. [Google Scholar]
- 9.Gleason BC, Hornick JL. Inflammatory myofibroblastic tumours: where are we now? J Clin Pathol. 2008;61:428–437. doi: 10.1136/jcp.2007.049387. [DOI] [PubMed] [Google Scholar]
- 10.Gawande PD, Sambhus M, Garde JB, Halli R, Deshmukh V, Kulkarni A, et al. Aggressive inflammatory pseudotumor of the mandible. J Craniofac Surg. 2012;23:1101–1103. doi: 10.1097/SCS.0b013e318252da65. [DOI] [PubMed] [Google Scholar]
- 11.Gale N, Zidar N, Podboj J, Volavsek M, Luzar B. Inflammatory myofibroblastic tumour of paranasal sinuses with fatal outcome: reactive lesion or tumour? J Clin Pathol. 2003;56:715–717. doi: 10.1136/jcp.56.9.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poh CF, Priddy RW, Dahlman DM. Intramandibular inflammatory myofibroblastic tumor—a true neoplasm or reactive lesion? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:460–466. doi: 10.1016/j.tripleo.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Pankaj C, Uma C. How to manage oral inflammatory myofibroblastic tumor (inflammatory pseudotumor)? Oral Dis. 2001;7:315–316. doi: 10.1034/j.1601-0825.2001.00696.x. [DOI] [PubMed] [Google Scholar]